Abstract
Objective:
Assess oral gargle-tumor human papillomavirus (HPV) agreement among oropharyngeal squamous cell carcinoma (OPSCC) cases by several disease characteristics.
Materials and Methods:
171 treatment naïve OPSCC were enrolled 2014–2017. Tumors were categorized as early or late disease with early disease defined as T1–2 with no nodal involvement or at most a single ipsilateral positive node <3 cm. Oral gargle samples were obtained via a 30-second rinse and gargle. The RHA Kit HPV SP10-LiPA25 was utilized for HPV genotyping of tumor (FFPE) and oral gargle specimens. Sensitivity, specificity, positive and negative predictive value, percent agreement, and 95% exact binomial confidence intervals were estimated. Multivariable logistic regression models were fit to predict agreement.
Results:
83.0% and 93.0% of oral gargle and tumor specimens were HPV positive. Oral gargle-tumor agreement for any oncogenic HPV type and HPV 16 was 73.7%. High oncogenic HPV oral gargle-tumor agreement was observed for late disease presentation, p16 positive cases, and tumors at the tonsils (74.5–80.8%). Similar trends were observed for HPV 16. Agreement for any oncogenic HPV and HPV 16 was significantly higher for late vs. early disease (77.9% vs 57.1%, p=0.01). Oral gargle-tumor oncogenic HPV and HPV 16 agreement was independently associated with age ≥50 years and late disease presentation.
Conclusion:
Overall, oral-tumor HPV agreement among OPSCC was relatively high. However, oral-tumor HPV agreement was significantly lower among younger cases and those diagnosed with earlier disease. Additional biomarkers are needed to improve oral HPV test characteristics to identify OPSCC early.
Keywords: HPV, oropharyngeal cancer, oral gargle, tumor
Introduction
Oropharyngeal squamous cell carcinoma (OPSCC) is the only cancer of the head and neck whose incidence has been steadily increasing.[1, 2] Traditionally thought to be caused mainly by tobacco and alcohol consumption, human papillomavirus (HPV) infection has become the predominant cause of OPSCC in the United States (US).[3, 4] The proportion of OPSCCs caused by HPV varies worldwide with estimates ranging from 10–70% of cases.[5] In the US, the proportion of OPSCC attributed to HPV rose from 16.3% in the 1980s to 72.7% during the 2000s.[1, 6] In addition, OPSCC incidence [7] has steadily increased over the past couple of decades in contrast with the decreasing rates of non-OPSCC head and neck cancers.[5, 8] Given these trends, OPSCC is expected to surpass cervical cancer as the most frequent HPV-related cancer in high-income countries by the year 2020.[1] In the US, the OPSCC incidence rate among men was higher than the cervical cancer incidence rate among women in 2015.[9]
Many parallels can be drawn between HPV driven carcinogenesis of the oropharynx and of the cervix. However, there is a key component that differs between these two diseases: our current inability to detect (and consequently treat) premalignant lesions in the oropharynx. Most OPSCCs go undetected until they have involved regional lymph nodes, often requiring more aggressive multi-modality treatments associated with high morbidity. Unfortunately there are no reliable screening methods or routine check-ups – “Pap smear” equivalents – for detecting precancerous lesions or early stage tumors in the oropharynx. Moreover, these tumors arise in tonsillar crypts within the tonsils or other hard-to visualize or inaccessible locations, thus making a visual inspection method challenging. Recent changes to the American Joint Committee on Cancer (AJCC) staging system (8th edition) reflect the prognostic and higher overall survival rates that have been established for HPV-driven OPSCC compared to their HPV-negative counterparts.[10–12] Given that survival is better for HPV-driven cancers, there are multiple ongoing trials aiming to determine treatment de-escalation strategies and the optimal follow-up of these cases.[13–15] Meanwhile, OPSCC treatment protocols continue to be based on extent of disease such that monotherapy (less aggressive treatment) is only provided to patients diagnosed with small tumors (T1–2) with at most a single ipsilateral positive node <3 cm. Therefore, methods facilitating diagnosis of OPSCC tumors at earlier points when monotherapy can be safely delivered may reduce treatment associated morbidity and increase survival while maintaining high cure rates.
Several studies evaluating whether HPV detected in oral exfoliated cells accurately reflects HPV status in the OPSCC tumor have produced mixed results.[16] The aim of our study was to assess the agreement and performance characteristics of a sensitive oral HPV test relative to tumor status, among OPSCC cases overall and by several tumor characteristics including p16 status, TNM stage, and disease burden.
Materials and Methods
Study Participants
One-hundred and seventy-one men with newly diagnosed, histologically confirmed and treatment naïve OPSCC of all stages were enrolled to an ongoing biomarker study at the Moffitt Cancer Center (Tampa, Florida) between May 2014 and October 2017. At the time of recruitment, all participants completed a health and risk factor questionnaire and provided an oral gargle sample. Formalin-fixed paraffin-embedded (FFPE) tumor samples were retrieved for all participants and staging and treatment data were accessed through the review of electronic medical records. This study was approved by the Liberty IRB, Chesapeake IRB, and Moffitt’s Scientific Review Committee, and written informed consent was obtained from all enrolled participants.
Data and Sample Collection
All participants completed a computer assisted self-administered interview (CASI) questionnaire at the time of enrollment. Information was obtained on general demographics, medical and family history of cancer, sexual history, oral health, and risk behavior. Tumors were divided into two categories – early or late extent of disease according to the tumor burden at the time of diagnosis; whereby, early disease included tumors diagnosed as T1–2 with no nodal involvement or at most a single ipsilateral positive node <3 cm, while all other cases were considered late disease. This classification is used to determine treatment – either monotherapy or multiple modality therapy (e.g., chemoradiation ± surgery).
Oral gargle samples were obtained from each participant by use of a 30-second rinse and gargle method with 15 mL of locally available mouthwash as described previously.[17–20] Samples were then centrifuged at 3000 × g for 15 minutes at 4°C three times. The final cell pellet was resuspended in phosphate buffered saline (PBS) and frozen at −80 °C within 24 hours of collection.
DNA extraction from oral gargle and tumor samples
DNA was extracted from the oral gargle cell pellets using the automated BioRobot MDx (Qiagen, Inc.) following the manufacturer’s instructions. For the extraction of DNA from FFPE tumor samples the QIAamp® DNA FFPE Tissue Kit (Qiagen, Inc.) was used according to the manufacturer’s instructions.
HPV genotyping
The RHA Kit HPV SP10-LiPA25 (DDL Diagnostic Laboratory, Rijswijk, The Netherlands), an in vitro reverse hybridization assay (RHA) for the qualitative identification of HPV DNA, was utilized for HPV genotyping of tumor and oral gargle specimens. The LiPA25 test targets a 65 base pair fragment of the L1 region of the HPV genome. This assay requires a three step process: 1) qPCR that determines sample adequacy; 2) a DNA enzyme immunoassay (DEIA) or ELISA method that detects the presence of the following HPV types: 2, 3, 4, 5, 6, 7, 8, 11, 13, 14, 16, 18, 20, 26, 27, 28, 30, 31, 32, 33, 34, 35, 37, 39, 40, 42, 43, 44, 45, 55, 56, 57, 58, 59, 61, 62, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 81, 82, 83, 84, 85, 86, 87, 89, 90, 91, 95, 97, 102, 106, 114 and 115; and 3) a LiPA25 genotyping multiplex PCR that selectively identifies the following HPV types by reverse hybridization: 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74.[21] Oncogenic HPV types included HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. The LiPA25 assay has proven to detect up to 20% more HPV types than other marketed assays, making it the most sensitive assay for samples with low copy number and/or mixed-type HPV samples like those obtained in oral gargles.[21, 22] Using this method, our group has reliably identified HPV types in ano-genital mucosa [23, 24] as well as oral gargle specimens.[20, 25]
Pathology review and Immunohistochemistry
Slides from FFPE blocks were stained with hematoxylin and eosin (H&E) and p16INK4a (p16). p16 staining was missing for 5 of the 171 cases. Two expert pathologists independently classified and graded the tumors and completed the visual morphometric analysis blinded to HPV genotyping results. p16 was considered positive when >70% of the cells had strong and diffuse nuclear and cytoplasmic staining.[26]
Statistical Analysis
Participant characteristics were summarized using descriptive statistics including median and range for continuous measures, and proportions and frequencies for categorical measures. Agreement between oral gargle and tumor HPV status were considered for any oncogenic HPV (type specific grouped infection) and HPV 16. Considering tumor status as gold standard or truth, sensitivity, specificity, positive and negative predictive value (PPV and NPV) were calculated along with percent agreement, with the 95% exact binomial confidence interval estimated, and p values from the McNemar’s test.
Individual logistic regression models were fit to predict agreement, followed by multivariable logistic regression model using stepwise selection. Of note, race and ethnicity were not considered in multivariable models due to sparse sampling, along with their unimportance in univariate modeling. Tumor subsite was not considered in any modeling, due to its sparse sampling, specifically in the soft palate category. Due to the exploratory nature of this analysis, p-values were not adjusted for multiple comparisons. All statistical analyses were performed using R version 3.5.1 (The R Foundation).
Results
OPSCC participant demographic and behavioral characteristics
The majority of OPSCC participants were white (93.0%), non-Hispanic (92.4%) with a median age of 61 years. Seventy-four percent were either married or cohabiting at the time of enrollment and 73.6% had at least some college education. Fifty-eight percent of OPSCC participants were either current or former smokers. Most cases (59.2%) reported having any alcohol consumption in the previous month with 45.5% reporting consumption of 1–4 alcoholic drinks per drinking occasion, and 12.6% reported having ≥5 alcoholic drinks per drinking occasion within the past month. Approximately 40% reported giving oral sex in the prior 6 months. Nearly half (45.8%) reported undergoing a tonsillectomy prior to diagnosis, and of the men that had a tonsillectomy, 81.6% had the procedure completed more than 10 years prior to cancer diagnosis (Table 1).
Table 1:
Study participant characteristics
| N (%) | |
|---|---|
| Race | |
| White | 159 (93.0%) |
| Black | 7 (4.1%) |
| Other | 5 (2.9%) |
| Ethnicity: | |
| Hispanic | 13 (7.6%) |
| Non-Hispanic | 158 (92.4%) |
| Median Age, years (range) | 61 [38;87] |
| Age (years): | |
| 35–49 | 19 (11.1%) |
| 50–59 | 53 (31.0%) |
| 60–69 | 64 (37.4%) |
| 70+ | 35 (20.5%) |
| Marital Status: | |
| Single, divorced, separated, widowed | 41 (24.0%) |
| Married or cohabiting | 127 (74.3%) |
| Refused | 3 (1.8%) |
| Education: | |
| 12 years/high school | 42 (24.6%) |
| Some college/vocational school | 56 (32.7%) |
| College graduate | 43 (25.1%) |
| Postgraduate/professional school | 27 (15.8%) |
| Refused | 3 (1.8%) |
| Smoking Status: | |
| Never | 71 (42.0%) |
| Former | 86 (50.9%) |
| Current | 12 (7.1%) |
| Ever Smoked: | |
| No | 71 (42.0%) |
| Yes | 98 (58.0%) |
| Cigarette Pack-Years, median (range) | 20.0 [0.0;84.0] |
| Cigarette Pack-Years: | |
| 0–5 | 19 (19.6%) |
| 6–29 | 43 (44.3%) |
| 30+ | 35 (36.1%) |
| Any Alcohol in Past Month: | |
| No | 69 (40.8%) |
| Yes | 100 (59.2%) |
| Alcohol Drinks per Occasion in Past Month | 1.0 [0.0;34.0] |
| Alcohol Drinks per Occasion in Past Month: | |
| No Alcohol | 70 (41.9%) |
| 1–4 Drinks | 76 (45.5%) |
| 5+ Drinks | 21 (12.6%) |
| Giving Oral Sex in Past 6 Months: | |
| No | 99 (59.6%) |
| Yes | 67 (40.4%) |
| Tonsillectomy: | |
| No | 91 (54.2%) |
| Yes | 77 (45.8%) |
| Time Since Tonsillectomy: | |
| <10 years | 14 (18.4%) |
| 10+ years | 62 (81.6%) |
| Gingivitis: | |
| No | 129 (76.8%) |
| Yes | 39 (23.2%) |
| Tumor Subsite: | |
| Base of Tongue | 84 (49.1%) |
| Tonsil | 78 (45.6%) |
| Soft Palate | 9 (5.3%) |
| *p16 (by IHC): | |
| Negative | 13 (7.8%) |
| Positive | 153 (92.2%) |
| *Stage at Presentation (AJCC 8th Edition.): | |
| I (p16+) | 85 (51.2%) |
| II/III (p16+) | 68 (41.0%) |
| I/II (p16−) | 4 (2.4%) |
| III/IV (p16−) | 9 (5.4%) |
| Early or Late Disease Presentation: | |
| Early | 35 (20.5%) |
| (T1–2 with only a single ipsilateral positive node <3 cm or less) | |
| Late | 136 (79.5%) |
p16 was evaluated in 166/171 OPSCC participants
Tumor and pathological characteristics
The majority of OPSCC cases had tumors located in either the base of tongue (49.1%) or the palatine tonsils or tonsillar fossa (45.6%). The remaining 5.3% had tumors located at other sites within the oropharynx including the soft palate. p16 was evaluated in 166/171 OPSCC participants. Of these, 92.2% had tumors that stained positive for p16. Staging was assessed at the time of diagnosis using the AJCC-7th edition and converted to AJCC-8th edition according to p16 involvement for this study. Among the p16 positive cases, 85 (51.2%) were stage I and 68 (41.0%) were either stage II or III at diagnosis. Of the thirteen p16 negative cases, four were stage I/II and nine were stage III/IV at diagnosis. When grouped according to the definition of early vs late disease, nearly 80% presented as late disease at the time of diagnosis (Table 1).
HPV genotype profile
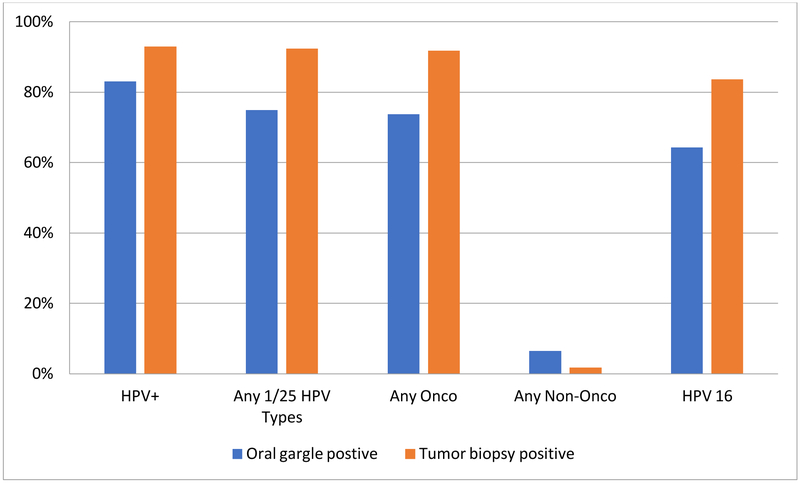
HPV DNA (any genotype) was detected in 83.0% and 93.0% of the oral gargle and tumor biopsy specimens, respectively. Among the oral gargle specimens, 74.9% were positive for one or more of 25 HPV types genotyped by the LiPA25 assay. Overall, 73.7% were positive for an oncogenic HPV type (64.3% were HPV 16 positive) and 6.4% for a non-oncogenic HPV type. Among the tumor specimens, 92.4% were positive for one or more of 25 HPV types genotyped, 91.8% were positive for an oncogenic HPV type (83.6% HPV 16 positive), and 1.8% for a non-oncogenic type (Figure 1). In addition to HPV 16, HPV 18 (4.1% for both specimens), HPV 33 (4.7% and 7.0%, oral and tumor specimens respectively), and HPV 35 (2.9% and 4.1%, oral and tumor specimens respectively) were detected. All other oncogenic HPV types were either not detected or detected at <3% in both specimen types (data not shown). Due to the exceedingly low sample size for individual HPV types other than HPV 16, we do not report tumor-oral gargle agreement.
Figure 1-.
Tumor-oral gargle HPV status among oropharyngeal cancer cases
HPV+ = positive for one or more HPV types 2, 3, 4, 5, 6, 7, 8, 11, 13, 14, 16, 18, 20, 26, 27, 28, 30, 31, 32, 33, 34, 35, 37, 39, 40, 42, 43, 44, 45, 55, 56, 57, 58, 59, 61, 62, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 81, 82, 83, 84, 85, 86, 87, 89, 90, 91, 95, 97, 102, 106, 114 and 115
Any 1/25 HPV types = HPV types 6, 11, 16, 18, 31, 33, 34, 35, 39, 40, 42, 43, 44, 45, 51, 52, 53, 54, 56, 58, 59, 66, 68/73, 70, and 74
Any Oncogenic HPV types = HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68
Any Non-Oncogenic HPV types = HPV types 6, 11, 34, 40, 42, 43, 44, 53, 54, 66, 70, 74
HPV agreement between oral gargle and tumor specimens
The overall oral gargle-tumor agreement for any oncogenic HPV type was 73.7% (Table 2). Agreement was highest among late (77.9%) disease presentation cases, for p16 positive tumors (74.5%), and tonsillar tumors (80.8%). The percent agreement was significantly higher for late disease cases compared to early disease cases (p=0.01).
Table 2:
Tumor-Oral HPV Status Agreement Overall and By Clinical Disease Presentation, p16 Status, and Tumor Location
| N | %Oral HPV Positive | %TumorHPV Positive | % Agreement (95% CI)a | P-valueb | |
|---|---|---|---|---|---|
| Any Onco HPV | |||||
| Overall | 171 | 73.7 | 91.8 | 73.7(66.4, 80.1) | <0.0001 |
| *Early Disease | 35 | 57.1 | 88.6 | 57.1 (39.4, 73.7) | 0.0098 |
| Late Disease | 136 | 77.9 | 92.6 | 77.9 (70.0, 84.6) | 0.0005 |
| p16 Positive | 153 | 78.4 | 94.8 | 74.5 (66.8, 81.2) | 0.0001 |
| p16 Negative | 13 | 15.4 | 53.8 | 61.5 (31.6, 86.1) | 0.0736 |
| Base of Tongue | 84 | 66.7 | 91.7 | 67.9 (56.8, 77.6) | 0.0001 |
| Tonsil | 78 | 84.6 | 93.6 | 80.8 (70.3, 88.8) | 0.1213 |
| Soft Palate | 9 | 44.4 | 77.8 | 66.7 (29.9, 92.5) | 0.2482 |
| HPV 16 | |||||
| Overall | 171 | 64.3 | 83.6 | 73.7 (66.4, 80.1) | <0.0001 |
| *Early Disease | 35 | 48.6 | 85.7 | 57.1 (39.4, 73.7) | 0.0019 |
| Late Disease | 136 | 68.4 | 83.1 | 77.9 (70.0, 84.6) | 0.0005 |
| p16 Positive | 153 | 69.3 | 87.6 | 73.9 (66.1, 80.6) | <0.0001 |
| p16 Negative | 13 | 7.7 | 38.5 | 69.2 (38.6, 90.9) | 0.1336 |
| Base of Tongue | 84 | 57.1 | 83.3 | 71.4 (60.5, 80.8) | <0.0001 |
| Tonsil | 78 | 75.6 | 84.6 | 78.2 (67.4, 86.8) | 0.1456 |
| Soft Palate | 9 | 33.3 | 77.8 | 55.6 (21.2, 86.3) | 0.1336 |
Early Disease = T1–2 with only a single ipsilateral positive node <3 cm
Confidence intervals estimated using exact binomial method
McNemar’s test of agreement. Low p-value indicates that there are statistically significantly more positives in one of the two groups.
The overall oral gargle-tumor HPV 16 agreement was 73.7% (Table 2). As with oncogenic HPV oral-tumor agreement, HPV 16 agreement was highest among late (77.9%) disease presentation cases, among p16 positive tumors (73.9%), and tonsillar tumors (78.2%). Similarly, the percent HPV 16 agreement was significantly higher for late disease cases compared to early disease cases (p=0.01).
Diagnostic performance of oral gargle HPV detection
Using oncogenic HPV detection in tumor specimens as the gold standard, the sensitivity and specificity were 75.8% and 50.0%, respectively (Table 3). The PPV and NPV for oral oncogenic HPV were 94.4% and 15.6%, respectively. Sensitivity was higher for late disease cases (80.2% vs 58.1%), p16 positive cases (77.9% vs. 28.6%), and tumors originating in the tonsils (84.9% vs 68.8% for base of tongue, and 57.1% for soft palate); whereas, specificity did not differ by early or late disease (50%), was highest among p16 negative tumors (100% vs. 12.5%) and tumors originating in the soft palate (100% vs. 57.1% base of tongue, and 20% tonsil) although the number of p16 negative and soft palate cases was small. Similar results were observed for HPV 16 analyses with the exception that specificity (78.6% HPV 16 vs. 50.0% oncogenic HPV) and the NPV (36.1% HPV 16 vs. 15.6% oncogenic HPV) were higher for oral HPV 16 compared to oral oncogenic HPV.
Table 3:
Diagnostic performance of HPV detection in oral gargles compared to the tumor biopsy reference
| aSensitivity, % | bSpecificity, % | cPPV, % | dNPV, % | |
|---|---|---|---|---|
| Any Onco HPV | ||||
| Overall | 75.8% (119/157) | 50.0% (7/14) | 94.4% (119/126) | 15.6% (7/45) |
| *Early Disease | 58.1% (18/31) | 50.0% (2/4) | 90.0% (18/20) | 13.3% (2/15) |
| Late Disease | 80.2% (101/126) | 50.0% (5/10) | 95.3% (101/106) | 16.7% (5/30) |
| p16 Positive | 77.9% (113/145) | 12.5% (1/8) | 94.2% (113/120) | 3.0% (1/33) |
| p16 Negative | 28.6% (2/7) | 100.0% (6/6) | 100.0% (2/2) | 54.5% (6/11) |
| Base of Tongue | 68.8% (53/77) | 57.1% (4/7) | 94.6% (53/56) | 14.3% (4/28) |
| Tonsil | 84.9% (62/73) | 20.0% (1/5) | 93.9% (62/66) | 8.3% (1/12) |
| Soft Palate | 57.1% (4/7) | 100.0% (2/2) | 100.0% (4/4) | 40.0% (2/5) |
| HPV 16 | ||||
| Overall | 72.7% (104/143) | 78.6% (22/28) | 94.5% (104/110) | 36.1% (22/61) |
| *Early Disease | 53.3% (16/30) | 80.0% (4/5) | 94.1% (16/17) | 22.2% (4/18) |
| Late Disease | 77.9% (88/113) | 78.3% (18/23) | 94.6% (88/93) | 41.9% (18/43) |
| p16 Positive | 74.6% (100/134) | 68.4% (13/19) | 94.3% (100/106) | 27.7% (13/47) |
| p16 Negative | 20.0% (1/5) | 100.0% (8/8) | 100.0% (1/1) | 66.7% (8/12) |
| Base of Tongue | 67.1% (47/70) | 92.9% (13/14) | 97.9% (47/48) | 36.1% (13/36) |
| Tonsil | 81.8% (54/66) | 58.3% (7/12) | 91.5% (54/59) | 36.8% (7/19) |
| Soft Palate | 42.9% (3/7) | 100.0% (2/2) | 100.0% (3/3) | 33.3% (2/6) |
Early Disease = T1–2 with only a single ipsilateral positive node <3 cm or less
Sensitivity: Percentage of positive tumor biopsy samples that were also positive oral gargle samples
Specificity: Percentage of negative tumor biopsy samples that were also negative oral gargle samples
Positive Predictive Value (PPV): Percentage of positive oral gargle samples that were also positive tumor biopsy samples
Negative Predictive Value (NPV): Percentage of negative oral gargle samples that were also negative tumor biopsy samples
Variables contributing to oral gargle-tumor specimen HPV status agreement
In the final multivariable logistic model only three variables were independently associated with oral gargle-tumor biopsy agreement for any oncogenic HPV type. Age 50 or older at diagnosis (multivariable adjusted odds ratio [aOR] for ages 50–59, 60–69, and ≥70 years was 5.78, 11.97, and 4.46, respectively, compared to ages 35–49 years) and late disease at presentation (aOR 2.81; 95% CI 1.14, 6.95) were both significantly associated with higher odds of agreement. In contrast, tonsillectomy was associated with significantly lower odds of oral gargle-tumor biopsy oncogenic HPV agreement (aOR 0.40; 95% CI 0.17, 0.91) (Table 4). Only ages 60–69 years (aOR 6.69 compared to age 35–49 years) and late disease at presentation (aOR 2.95; 95% CI 1.23, 7.11) were significantly associated with oral gargle-tumor biopsy HPV 16 agreement in the final multivariable logistic model (Table 5).
Table 4:
Factors independently associated with tumor biopsy-oral gargle oncogenic HPV type agreement
| Variable | ORa | aOR(95% CI)b | |
|---|---|---|---|
| Race | White | 1.0(Ref) | |
| Non-White | 4.2 | ||
| Ethnicity | Hispanic | 1.0(Ref) | |
| Non-Hispanic | 1.8 | ||
| Age (Years)c | 35–49 | 1.0(Ref) | 1.0(Ref) |
| 50–59 | *3.4 | 5.78 (1.75,20.48) | |
| 60–69 | *6.0 | 11.97 (3.44,45.78) | |
| 70+ | 2.1 | 4.46 (1.22,17.73) | |
| Smoking Status | Never | 1.0(Ref) | |
| Former | 1.3 | ||
| Current | 0.8 | ||
| Alcohol Drinks per Occasion in Past Month | No Alcohol | 1.0(Ref) | |
| 1–4 Drinks | 1.1 | ||
| 5+ Drinks | 0.9 | ||
| Oral Sex in Past 6 Months | No | 1.0(Ref) | |
| Yes | 1.5 | ||
| Tonsillectomy | No | 1.0(Ref) | 1.0(Ref) |
| Yes | *0.5 | 0.40 (0.17,0.91) | |
| Gingivitis | No | 1.0(Ref) | |
| Yes | 1.2 | ||
| Stage at Presentation (AJCC 8th Ed.) | I(p16+) | 1.0(Ref) | |
| Il/lll(p16+) | *3.6 | ||
| I/II(p16−) | 0.6 | ||
| III/IV(p16−) | 1.1 | ||
| p16 (by IHC) | Negative | 1.0(Ref) | |
| Positive | 1.8 | ||
| **Early or Late Disease | Early | 1.0(Ref) | 1.0(Ref) |
| Late | *2.7 | 2.81 (1.14,6.95) |
p-value <0.05 in univariate analyses
Early disease=T1–2 with only a single ipsilateral positive node <3 cm or less
Results from univariate logistic regression model, odds ratio for any oncogenic HPV type concordance
Results from multivariable logistic regression model (stepwise model selection used, only variables with significant results in the univariate logistic regression model were included). Odds ratio for any oncogenic
HPV type concordance, model sample size is n=159.
Overall P value for age 0.002
Table 5:
Factors independently associated with tumor biopsy-oral gargle HPV 16 agreement
| Variable | Level | ORa | aOR (95% CI)b |
|---|---|---|---|
| Race | White | 1.0(Ref) | |
| Non-White | 1.9 | ||
| Ethnicity | Hispanic | 1.0(Ref) | |
| Non-Hispanic | 1.3 | ||
| Age (Years)c | 35–49 | 1.0(Ref) | 1.0(Ref) |
| 50–59 | 1.8 | 2.26 (0.71,7.16) | |
| 60–69 | *4.4 | 6.69 (1.94,24.42) | |
| 70+ | 1.2 | 1.85 (0.54,6.41) | |
| Smoking Status | Never | 1.0(Ref) | |
| Former | 1.2 | ||
| Current | 1.2 | ||
| Alcohol Drinks per Occasion in Past Month | No Alcohol | 1.0(Ref) | |
| 1–4 Drinks | 1.1 | ||
| 5+ Drinks | 1.6 | ||
| Oral Sex in Past 6 Months | No | 1.0(Ref) | |
| Yes | 1.1 | ||
| Tonsillectomy | No | 1.0(Ref) | |
| Yes | 0.6 | ||
| Gingivitis | No | 1.0(Ref) | |
| Yes | 1.2 | ||
| Stage at Presentation (AJCC 8th Ed.) | I(p16+) | 1.0(Ref) | |
| II/III(p16+) | *2.7 | ||
| I/II(p16−) | 0.5 | ||
| III/IV(p16−) | 1.8 | ||
| p16 (by IHC) | Negative | 1.0(Ref) | |
| Positive | 1.3 | ||
| Early or Late Disease | Early | 1.0(Ref) | 1.0(Ref) |
| Late | *2.7 | 2.95 (1.23,7.11) |
p-value <0.05 in univariate analyses
Early disease=T1–2 with only a single ipsilateral positive node <3 cm or less
Results from univariate logistic regression model, odds ratio for HPV16 concordance
Results from multivariable logistic regression model (stepwise model selection used, only variables with significant results in the univariate logistic regression model were included). Odds ratio for HPV16 concordance, model sample size is n=159.
Overall P value for age 0.02
Discussion
In this study of OPSCC cases, a high prevalence of HPV DNA was detected in both tumor (93.0%) and oral gargle (83.0%) specimens. HPV 16 was the predominant type detected with 83.6% of tumors positive for this one HPV type with a minority of other oncogenic HPV types detected, including HPV 18, 33, and 35. The observed tumor HPV prevalence is higher than the 70% described in previous US studies [27] and higher than studies that compared oral-tumor HPV agreement [16], likely due to a growing proportion of tumors attributable to HPV over time [28] and utilization of a method that has optimal performance characteristics for HPV detection in samples with fragmented DNA (i.e., FFPE) and low viral load (i.e., oral gargle specimens) [29]. Similar to other studies we observed high agreement between oral gargle and tumor biopsy specimens for any oncogenic HPV and HPV 16 (73.7% in both), high sensitivity (75.8% and 72.7% respectively), and PPV (94.4 and 94.5% respectively). However, specificity was relatively high for HPV 16 only (78.6% compared to 50% for oncogenic HPV) and NPV was relatively low for both oncogenic HPV (15.6%) and HPV 16 (36.1%). Ours is the first study to identify factors associated with oral gargle-tumor biopsy HPV agreement which indicated that older age, tumor location (tonsil vs. base of tongue), and no prior tonsillectomy were associated with higher oral gargle-tumor biopsy HPV agreement. Importantly, results from this study indicate that oral gargle-tumor biopsy agreement was significantly lower for early compared to late disease presentation cases.
A recent systematic review and meta-analysis [16] found that among studies evaluating HPV detection in oral rinses from head and neck squamous cell carcinoma (HNSCC) cases, sensitivity for any oncogenic HPV type was similar to what we observed (77%), but specificity was higher (95%).[16] After restricting analyses to the studies of OPSCC cases only, the sensitivity of any oncogenic HPV type in the oral rinse or swab was lower (55%), although the specificity remained unchanged (94%).[16] A significant limitation of the recent meta-analysis is the heterogeneity in the HPV detection methods utilized across studies which included classifying cases based on either in situ hybridization (ISH), PCR, or p16 methods.
In the current study, one of the most sensitive methods available for detecting HPV DNA, the SPF10PCR-DEIA-LiPA25 assay, was used for both the oral gargle and tumor specimens. The higher HPV DNA detection may have led to the lower specificity of oral HPV 16 observed in our study compared to that reported in other studies as 21.4% (n=6/28) of cases with HPV 16 negative tumors were positive for oral HPV 16 (false positives). As a result of such high oral HPV prevalence, we found a high PPV (94.5%) but low NPV (36.1%) for HPV 16 detection in oral gargles.
Agreement between oral rinses and tumor HPV 16 DNA reported in previous studies has been variable, ranging between 59% [30] and 96% [18], with most studies reporting values of approximately 70–80%.[30–35] Chai et al. reported the highest oral-tumor agreement (96.3%) [18] which could be attributed to the “type-specific” primers used in their study. Conversely, Ahn et al. reported in 2014 the lowest agreement of 59.0%.[30] However, similar to the challenges of comparing sensitivity and specificity across studies, agreement cannot be directly compared as different methods were used to detect HPV in both oral and tumor specimens. Furthermore, most studies included a variety of HNSCC sites in their analysis, while only a few were exclusive of the oropharynx. In addition to the different HPV assays utilized across studies, two different tumor staging systems (AJCC 7th and 8th Editions) were used, as the change to the AJCC 8th Edition occurred recently. Thus, test characteristics by tumor stage cannot be adequately compared across studies.
To our knowledge, this is the first study to examine the agreement of HPV DNA detected in oral and tumor specimens by extent of disease at presentation. This is clinically relevant as treatment options differ according to disease presentation; tumors diagnosed as T1–2 with at most a single ipsilateral positive node <3 cm can safely be treated with monotherapy. Unfortunately, results from this study suggest that agreement between oral gargle and tumor specimens was significantly lower for tumors presenting earlier compared to later and for tumors diagnosed at the base of tongue. It is possible that oral gargles do not retrieve epithelial cells from deep areas of the oropharynx, like the base of the tongue, as effectively as from the tonsils, a finding that has been noted by prior studies.[33, 34, 36] Moreover, HPV may be harder to detect in oral gargle specimens of smaller tumors, which may contribute smaller numbers of shed epithelial cells. If this is indeed correct, detection of HPV DNA in oral gargles might not allow early detection of OPSCC tumors, at least not as a single biomarker. However, as this study was conducted among OPSCC cases only, its diagnostic performance as a screening tool cannot be assessed.
Conclusion:
We observed high oral and tumor HPV prevalence among OPSCC cases and relatively high agreement, sensitivity, specificity, PPV, and NPV for HPV 16. However, the performance of the oral HPV 16 test was significantly lower among younger cases and those diagnosed with earlier disease that can be treated with monotherapy. Additional biomarkers are needed to improve oral HPV 16 test characteristics to identify OPSCC early. More research is needed to develop methods to anatomically locate small primary tumors and post-biomarker assessment for appropriate treatment.
HIGHLIGHTS.
Assessed HPV status agreement between oral gargle vs. tumor biopsy among OPSCC
83% of oral gargle and 93% of tumor biopsy specimens were HPV positive
73.7% oral-tumor agreement for oncogenic and HPV 16
Oral-tumor agreement was higher among older and cases with late disease
Acknowledgements
Financial Support: This work was supported by funding from the National Institute of Dental and Cranial Research (NIDCR) at the National Institutes of Health [R21 DE024816 to A.R.G.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
A.R.G. is a member of the Merck Advisory Board. A.R.G. currently receives funding through a Merck investigator initiated studies program. L.M-G. is currently employed by GlaxoSmithKline. For the remaining authors, no conflicts of interest were declared.
References:
- [1].Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, Kim E, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:4294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, et al. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus(HPV)-associated cancers and HPV vaccination coverage levels. Journal of the National Cancer Institute. 2013;105:175–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. Journal of the National Cancer Institute. 2000;92:709–20. [DOI] [PubMed] [Google Scholar]
- [4].Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90. [PMC free article] [PubMed] [Google Scholar]
- [5].Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:4550–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. The Lancet Oncology. 2010;11:781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Castellsague X, Mena M, Alemany L. Epidemiology of HPV-Positive Tumors in Europe and in the World. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2017;206:27–35. [DOI] [PubMed] [Google Scholar]
- [8].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [9].Van Dyne EA, Henley SJ, Saraiya M, Thomas CC, Markowitz LE, Benard VB. Trends in Human Papillomavirus-Associated Cancers - United States, 1999–2015. MMWR Morbidity and mortality weekly report. 2018;67:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Muller S Update from the 4th Edition of the World Health Organization of Head and Neck Tumours: Tumours of the Oral Cavity and Mobile Tongue. Head and neck pathology. 2017;11:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lydiatt WM, Patel SG, O’Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, et al. Head and Neck cancers—major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA: A Cancer Journal for Clinicians. 2017;67:122–37. [DOI] [PubMed] [Google Scholar]
- [12].Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (Eds.). AJCC Cancer Staging Manual 8th Edition 2017. [Google Scholar]
- [13].Kelly JR, Park HS, An Y, Contessa JN, Yarbrough WG, Burtness BA, et al. Comparison of Survival Outcomes Among Human Papillomavirus-Negative cT1–2 N1–2b Patients With Oropharyngeal Squamous Cell Cancer Treated With Upfront Surgery vs Definitive Chemoradiation Therapy: An Observational Study. JAMA oncology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bratman SV, Bruce JP, O’Sullivan B, Pugh TJ, Xu W, Yip KW, et al. Human Papillomavirus Genotype Association With Survival in Head and Neck Squamous Cell Carcinoma. JAMA oncology. 2016;2:823–6. [DOI] [PubMed] [Google Scholar]
- [15].D’Souza G, Anantharaman D, Gheit T, Abedi-Ardekani B, Beachler DC, Conway DI, et al. Effect of HPV on head and neck cancer patient survival, by region and tumor site: A comparison of 1362 cases across three continents. Oral oncology. 2016;62:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gipson BJ, Robbins HA, Fakhry C, D’Souza G. Sensitivity and specificity of oral HPV detection for HPV-positive head and neck cancer. Oral oncology. 2018;77:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bottalico D, Chen Z, Dunne A, Ostoloza J, McKinney S, Sun C, et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. The Journal of infectious diseases. 2011;204:787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chai RC, Lim Y, Frazer IH, Wan Y, Perry C, Jones L, et al. A pilot study to compare the detection of HPV-16 biomarkers in salivary oral rinses with tumour p16(INK4a) expression in head and neck squamous cell carcinoma patients. BMC cancer. 2016;16:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].D’Souza G, Gross ND, Pai SI, Haddad R, Anderson KS, Rajan S, et al. Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32:2408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kreimer AR, Pierce Campbell CM, Lin HY, Fulp W, Papenfuss MR, Abrahamsen M, et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet (London, England). 2013;382:877–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Geraets DT, Struijk L, Kleter B, Molijn A, van Doorn LJ, Quint WG, et al. The original SPF10 LiPA25 algorithm is more sensitive and suitable for epidemiologic HPV research than the SPF10 INNO-LiPA Extra. Journal of virological methods. 2015;215–216:22–9. [DOI] [PubMed] [Google Scholar]
- [22].Cornall AM, Quint WH, Garland SM, Tabrizi SN. Evaluation of an automated SPF10-LiPA25 assay for detection and typing of human papillomavirus in archival samples. Journal of virological methods. 2014;199:116–8. [DOI] [PubMed] [Google Scholar]
- [23].Giuliano AR, Lee JH, Fulp W, Villa LL, Lazcano E, Papenfuss MR, et al. Incidence and clearance of genital human papillomavirus infection in men (HIM): a cohort study. Lancet (London, England). 2011;377:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sudenga SL, Ingles DJ, Pierce Campbell CM, Lin H-Y, Fulp WJ, Messina JL, et al. Genital HPV infection progression to external genital lesions: The HIM Study. European urology. 2016;69:166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pierce Campbell CM, Kreimer AR, Lin HY, Fulp W, O’Keefe MT, Ingles DJ, et al. Long-term persistence of oral human papillomavirus type 16: the HPV Infection in Men (HIM) study. Cancer prevention research (Philadelphia, Pa). 2015;8:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Seethala RWI, Carlson D, Harrison L, Richardson M, Shah J, Ferris R, Wenig B, Thompson. Protocol for the Examination of Specimens From Patients With Carcinomas of the Pharynx Based on AJCC/UICC TNM, 7th edition ed. College of American Pathologist: College of American Pathologist; 2013. [Google Scholar]
- [27].Lu DJ, Luu M, Mita A, Scher K, Shiao SL, Yoshida EP, et al. Human papillomavirus-associated oropharyngeal cancer among patients aged 70 and older: Dramatically increased prevalence and clinical implications. European journal of cancer (Oxford, England: 1990). 2018;103:195–204. [DOI] [PubMed] [Google Scholar]
- [28].Windon MJ, D’Souza G, Rettig EM, Westra WH, van Zante A, Wang SJ, et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer. 2018;124:2993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kocjan BJ, Hosnjak L, Poljak M. Detection of alpha human papillomaviruses in archival formalin-fixed, paraffin-embedded (FFPE) tissue specimens. Journal of clinical virology the official publication of the Pan American Society for Clinical Virology. 2016;76 Suppl 1:S88–s97. [DOI] [PubMed] [Google Scholar]
- [30].Ahn SM, Chan JY, Zhang Z, Wang H, Khan Z, Bishop JA, et al. Saliva and plasma quantitative polymerase chain reaction-based detection and surveillance of human papillomavirus-related head and neck cancer. JAMA otolaryngology-- head & neck surgery. 2014;140:846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhao M, Rosenbaum E, Carvalho AL, Koch W, Jiang W, Sidransky D, et al. Feasibility of quantitative PCR-based saliva rinse screening of HPV for head and neck cancer. International journal of cancer. 2005;117:605–10. [DOI] [PubMed] [Google Scholar]
- [32].Agrawal Y, Koch WM, Xiao W, Westra WH, Trivett AL, Symer DE, et al. Oral human papillomavirus infection before and after treatment for human papillomavirus 16-positive and human papillomavirus 16-negative head and neck squamous cell carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2008;14:7143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Koslabova E, Hamsikova E, Salakova M, Klozar J, Foltynova E, Salkova E, et al. Markers of HPV infection and survival in patients with head and neck tumors. International journal of cancer. 2013;133:1832–9. [DOI] [PubMed] [Google Scholar]
- [34].Yoshida H, Murono S, Ueno T, Nakanishi Y, Tsuji A, Hatano M, et al. Usefulness of human papillomavirus detection in oral rinse as a biomarker of oropharyngeal cancer. Acta oto-laryngologica. 2017:1–5. [DOI] [PubMed] [Google Scholar]
- [35].Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. Journal of the National Cancer Institute. 2008;100:407–20. [DOI] [PubMed] [Google Scholar]
- [36].Nordfors C, Vlastos A, Du J, Ahrlund-Richter A, Tertipis N, Grun N, et al. Human papillomavirus prevalence is high in oral samples of patients with tonsillar and base of tongue cancer. Oral oncology. 2014;50:491–7. [DOI] [PubMed] [Google Scholar]