Abstract
Cardiomyopathy is a manifestation of transthyretin amyloid (ATTR) amyloidosis, which is an underrecognized systemic disease whereby the transthyretin protein misfolds to form fibrils that deposit in various tissues and organs. ATTR amyloidosis is debilitating and associated with poor life expectancy, especially in those with cardiac dysfunction, but a variety of treatment options have recently become available. Considered a rare disease, ATTR amyloidosis may be more prevalent than thought, particularly in older persons. Diagnosis is often delayed because of a lack of disease awareness and the heterogeneity of symptoms at presentation. Given the recent availability of effective treatments, early recognition and diagnosis are especially critical because treatment is likely more effective earlier in the disease course. The Amyloidosis Research Consortium recently convened a group of experts in ATTR amyloidosis who, through an iterative process, agreed on best practices for suspicion, diagnosis, and characterization of disease. This review describes these consensus recommendations for ATTR associated with cardiomyopathy (ATTR-CM) as a resource to aid cardiologists and others in the recognition and diagnosis of ATTR-CM. Included in this review is an overview of red flag signs and symptoms and a recommended diagnostic approach, including testing for monoclonal protein, scintigraphy, or biopsy and, if ATTR-CM is identified, TTR genotyping.
Keywords: ATTR amyloidosis, ATTR-CM, transthyretin, cardiomyopathy, diagnosis, Computerized Tomography (CT), Diagnostic Testing, Echocardiography, Electrocardiology (ECG), Magnetic Resonance Imaging (MRI), Nuclear Cardiology and PET, Prognosis
INTRODUCTION
Transthyretin amyloid (ATTR) amyloidosis is a disease caused by abnormal fibrils derived from transthyretin (TTR), a protein produced mainly by the liver, that aggregate and deposit in tissues and organs.1 Cardiomyopathy (CM) is a common manifestation of ATTR amyloidosis (ATTR-CM) and is associated with a particularly poor life expectancy of 2-6 years after diagnosis.2 Patients with ATTR-CM experience debilitating physical symptoms common to heart failure (HF), such as exercise intolerance and fatigue, which result in decreased functional capacity, diminished quality of life, and eventual death.3 ATTR-CM can be acquired through aggregation of wild-type TTR (ATTRwt) or inherited from a variety of genetic variants of TTR (ATTRm; also known as hereditary ATTR or hATTR).
Epidemiology
ATTRm is considered rare and is transmitted in an autosomal-dominant manner and with variable penetrance. Certain variants typically result in CM, whereas others typically result in polyneuropathy (PN), although CM and PN manifestations may overlap (Figure 1). The prevalence of CM among persons with ATTRm is estimated at approximately 40,000 of the 50,000 persons with ATTRm globally,4 but this may be an underestimate.
Figure 1.
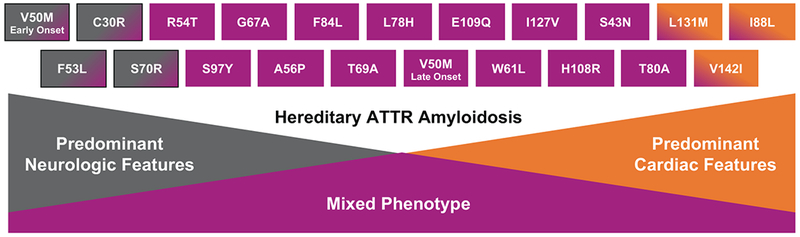
Genotype–phenotype correlations in ATTRm amyloidosis. ATTR, transthyretin amyloidosis.
The most common worldwide TTR variant, Val122Ile (or pV142I), occurs in approximately 3%-4% of African Americans, with undefined phenotypic penetrance.5,6 This Val122Ile TTR variant manifests predominantly as CM,7 and one estimate shows 10% of African Americans with HF who are older than 60 are carriers of the Val122Ile TTR variant.8 Thr60Ala, another common TTR variant, often manifests as a mixed phenotype, including CM, PN, and gastrointestinal (GI) dysfunction, and is present in approximately 1% of persons in northwest Ireland.9 The Val30Met variant is the most common cause of ATTRm with PN; however, late-onset ATTRm in patients of the Val30Met variant typically manifests as CM. Phenotypic penetrance of ATTRm is clearly age dependent; thus, ascertainment of population prevalence varies depending on age.
The true prevalence of ATTRwt is unknown; it may be relatively high compared with the prevalence of ATTRm. In autopsy studies, approximately 25% of the hearts of persons 80 years or older contained wild-type TTR fibrils, regardless of the presence of symptoms.4,10 Studies using non-biopsy approaches to diagnosis demonstrate a TTR prevalence of 16% among patients undergoing percutaneous aortic valve replacement for severe aortic stenosis,11 13% among patients with heart failure with a preserved ejection fraction (HFpEF),12 5% among patients with presumed hypertrophic cardiomyopathy,13 and 7-8% among patients with carpal tunnel syndrome upon biopsy of the tenosynovial tissue.14 Furthermore, approximately 1%-3% of persons older than 75 showed myocardial retention of the DPD, which is indicative of TTR cardiac amyloidosis.15,16
Diagnosis of ATTR Amyloidosis
Delays in diagnosis of ATTR-CM amyloidosis commonly occur because of physician- and disease-related reasons, including fragmented knowledge among different specialists and subspecialists, shortage of centers and specialists dedicated to disease management, erroneous belief that it is an incurable disease, perceived rarity of the condition, intrinsic phenotypic and genotypic heterogeneity, and, in some cases, the necessity of target organ tissue histologic diagnosis.12,17,18
The Amyloidosis Research Consortium recently led the development of a comprehensive set of consensus recommendations for the suspicion and diagnosis of ATTR amyloidosis. These recommendations were developed in collaboration with companies conducting research in ATTR amyloidosis (GSK, Ionis, Pfizer, and Alnylam) and through an iterative process with key specialists in amyloidosis. They also reflect collaboration and consensus among key amyloidosis experts of best practices for diagnosis and characterization of the disease.
This review describes the consensus recommendations for ATTR-CM amyloidosis with a goal of providing clinicians with an overview of key aspects of ATTR-CM diagnosis to help facilitate rapid and accurate identification of the disease. Focus is placed on disease presentation, characterization, and challenges for early and accurate diagnosis.
MISDIAGNOSIS AND RAISING SUSPICION
Misdiagnosis
Because they are considered rare and typically manifest with heterogeneous symptoms similar to those of other more common diseases, ATTRm and ATTRwt amyloidosis can be difficult to diagnose. Unexplained sensorimotor neuropathy or autonomic symptoms, such as orthostasis, erectile dysfunction, sweating abnormalities, and diarrhea, may lead to many lengthy and unfocused medical evaluations before amyloid is discovered. Depending on the mutation, patients with ATTR-CM show common signs and symptoms of HF, such as dyspnea, orthopnea, paroxysmal nocturnal dyspnea, edema, fatigue, exercise intolerance, dizziness/syncope, palpitations, electrical conduction abnormalities, and arrhythmias. Therefore, ATTR-CM is sometimes mistakenly diagnosed as hypertrophic cardiomyopathy13 or as generic, undifferentiated HFpEF rather than as amyloidosis.12
It is significant that in addition to symptoms of CM, other systemic phenotypes such as PN and GI disorders may be present. Because of the age-dependent development of ATTR-CM, many patients have true comorbid conditions including hypertension, diabetes, ischemic heart disease, and/or aortic stenosis (particularly low flow-low gradient) before amyloidosis develops. In this context, a high degree of clinical suspicion is necessary to identify incident ATTR-CM.
Signs and Symptoms
The spectrum of clinical presentations in patients with ATTR amyloidosis obliges all clinicians to be aware of common disease patterns (Table 1, Figure 2), additional clues, and commonly affected populations. Suspicion of ATTR-CM should be triggered in older persons who have been hospitalized for HF, elevated troponin levels, or levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) that are out of proportion to the clinical context. Other hints of ATTR amyloidosis include hypertension that resolves over time and an intolerance of angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), or beta blockers. In addition, though not infrequent in the general population, carpal tunnel syndrome occurs particularly frequently among males with ATTR.19 Lumbar spinal stenosis,20,21 previous orthopedic procedures,22 and spontaneous biceps tendon rupture23 may also be early indicators of ATTR-CM.
Table 1.
Diagnostic Clues to ATTR-CM
| History/examination clues |
| • Evidence of right-sided heart failure (eg, hepatomegaly, ascites, and lower extremity edema) • HFpEF, particularly in men • Intolerance to ACE inhibitors or beta blockers • Bilateral carpal tunnel syndrome • Lumbar spinal stenosis • Biceps tendon rupture • Unexplained peripheral neuropathy (eg, loss of warm/cold discrimination), particularly if associated with autonomic dysfunction (eg, postural hypotension, alternating bowel pattern) • Unexplained atrial arrhythmias or conduction system disease/need for a pacemaker |
| Imaging clues |
| • Myocardial uptake on PYP/DPD or HMDP imaging • “Infiltrative phenotype” (eg, biventricular hypertrophy pericardial effusion, valve thickening, interatrial septal thickening) • Diffuse subendocardial or transmural LGE or increased ECV fraction on cardiac MRI • Apical sparing on longitudinal strain imaging • Low myocardial contraction fraction • Restrictive LV filling with RV wall thickening |
| Combined clues |
| • HF with unexplained increased LV wall thickening and nondilated LV • Concentric LV wall thickening, possibly with an abnormal QRS voltage-to-LV thickness ratio • Depressed longitudinal LV function despite normal EF • Aortic stenosis with RV thickening, particularly if paradoxical low flow/low gradient |
ACE, angiotensin-converting enzyme; ATTR-CM, transthyretin amyloidosis with predominant cardiomyopathy; DPD, diphosphono-1,2-propanodicarboxylic acid; ECV, extracellular volume; EF, ejection fraction; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HMDP, hydroxymethylene diphosphonate; LGE, late gadolinium enhancement; LV, left ventricular; MRI, magnetic resonance imaging; PYP, pyrophosphate; RV, right ventricular.
The sensitivity and specificity of these “clues” has not been delineated in population-based samples with heart failure.
Figure 2.
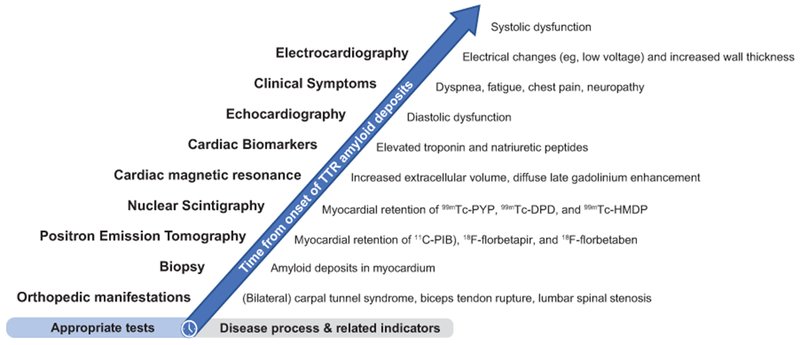
Proposed timeline of appropriate diagnostic tests based on typical disease process. 11C-PIB, Pittsburgh compound B; 99mTc-DPD, 99mtechnetium-3,3-diphosphono-1,2-propanodicarboxylic acid; 99mTc-HMDP, hydroxymethylene diphosphonate; 99mTc-PYP, technetium pyrophosphate; ATTR-CM, transthyretin amyloidosis with predominant cardiomyopathy (either wild-type or hereditary); CA, cardiac amyloidosis; ECG, electrocardiography; LVST, left ventricular septal thickness.
Biomarkers
No plasma or urinary biomarker is available for the diagnosis of ATTR. Nevertheless, in the clinical arena, the combination of very high plasma levels of NT-proBNP (disproportionate compared with the degree of HF) and elevated troponin levels in a patient with echocardiographic hypertrophic phenotype is strongly suggestive of amyloidotic cardiomyopathy and can prompt the diagnostic workup. NT-proBNP is a biomarker that is elevated early in ATTRm amyloidosis before cardiac symptoms appear, especially among asymptomatic carriers of a TTR gene mutation or patients with neurologic symptoms only.24 In addition, the usefulness of circulating retinol binding protein 4 in conjunction with electrocardiographic and echocardiographic measures to identify patients with HF who have ATTR cardiac amyloidosis from the Val122Ile mutation has recently been reported.25
Electrocardiography and Cardiac Imaging
Electrocardiography (ECG) is a broadly available screening test, and findings may reveal abnormalities associated with ATTR-CM (eg, low voltage) that are classically described in patients with cardiac amyloidosis.26–28 However, low voltage is less common in cardiac amyloidosis than a pseudoinfarct pattern of Q waves unrelated to prior myocardial infarctions.27 Given that low QRS voltage has been seen in approximately 50% of patients with AL amyloidosis and in approximately 25% of patients with ATTR amyloidosis, its usefulness as a screening test is limited by low sensitivity.27 More commonly, cardiac amyloidosis is hallmarked by QRS voltages that are disproportionate to the thickness of the left ventricular (LV) wall, which can be assessed using a ratio of QRS voltage to LV wall thickness.29,30 The presence of left ventricular hypertrophy on ECG does not exclude ATTR-CM.
Echocardiography (ECHO) is cost-effective, commonly available, relatively quick to perform, and better than most other imaging techniques at identifying diastolic dysfunction. Although all are not invariably present, classic ECHO findings of infiltrative disease include LV wall thickening, small LV cavity size, biatrial enlargement, thickened valves, elevated right ventricular systolic pressure and atrial septum thickness, granular sparkling appearance of the myocardial wall, pericardial effusion, restrictive transmitral Doppler filling pattern, and reduced ventricular strain, apical-to-basal strain ratio >2.1, LV ejection fraction-to-strain ratio >4 (Figures 2, 3).26,31,32 Combining ECG (low voltage) and ECHO (LV septal thickness above the upper limit of normal, especially >12 mm) is particularly useful for increased clinical suspicion of ATTR-CM33 (Table 1, Figure 2).
Figure 3.
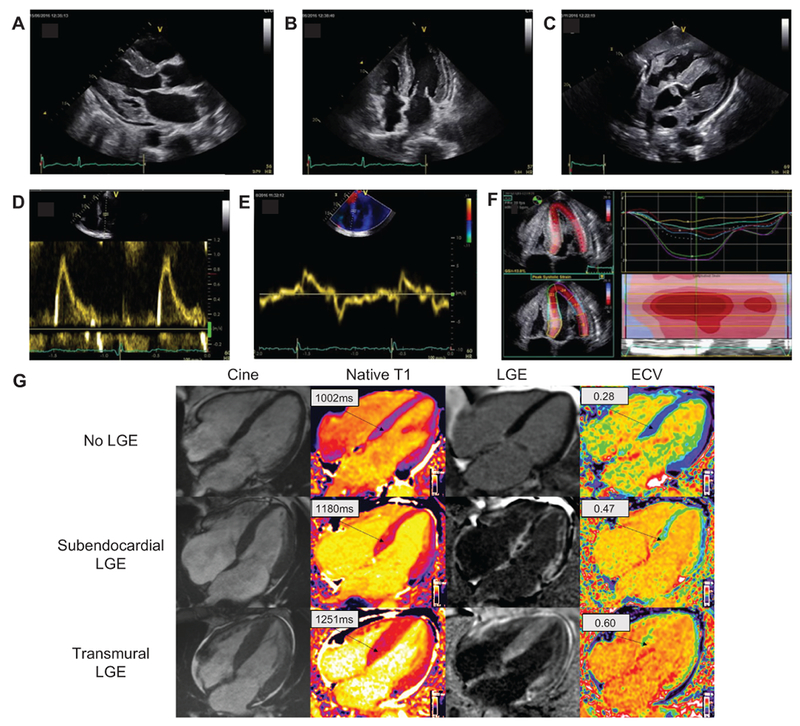
Typical ECHO and CMR findings in a patient with cardiac amyloidosis. Parasternal longitudinal axis (A) and apical 4-chamber (B) ECHO views show considerably increased LVWT in the absence of ventricular dilation; the myocardial walls appear hyperechogenic. Other characteristic findings include biatrial enlargement and thickening of valve leaflets (A, B) of the interatrial septum (B) and RV free wall (C). A generalized small pericardial effusion is also noticeable (A-C). The profile of LV filling (D) is restrictive, with markedly elevated E wave, reduced A wave, and decreased deceleration time. A decreased E’ wave measurement can be observed on lateral wall tissue Doppler imaging (E). The longitudinal systolic function is impaired with decreased S’ measurement on lateral wall tissue Doppler imaging (E) and markedly reduced longitudinal strain evident on the apical 4-chamber view (F). LV longitudinal strain (F) is preserved at the LV apex but is significantly impaired at the midbasal segments. Each colored curve shows longitudinal strain at 1 of the 6 LV measured segments. Dotted line is the mean. Color map represents the 6 LV segments, with time corresponding to the x-axis. The “bulls-eye” appearance (with apex at the center of the color-coding map) is typical of cardiac amyloidosis. Cardiac magnetic resonance images (G) include 4-chamber cine, corresponding native T1 maps, LGE image with phase-sensitive reconstruction and ECV maps in a patient with no cardiac amyloidosis (upper row) and 2 patients with cardiac amyloidosis (middle row and bottom row). In the upper row, the patient with no cardiac amyloidosis has no LGE and normal native T1 and ECV maps; in the middle row, the patient with cardiac amyloidosis has subendocardial LGE, elevated T1 values and elevated ECV values; in the bottom row, the patient with cardiac amyloidosis has a very high cardiac amyloid load, with transmural LGE, very high native T1 values, and very high ECV values. CMR, cardiac magnetic resonance; ECHO, echocardiography; ECV, extracellular volume; LGE, late gadolinium enhancement; LV, left ventricle; LVWT, left ventricular wall thickness; RV, right ventricle.
Similarly, cardiac magnetic resonance (CMR) can show detailed information about systolic function and cardiac structure (Figure 3). The advantage of CMR is its unique ability to enable tissue characterization,34,35 allowing it to differentiate amyloidosis from nonamyloid wall-thickening disorders. On tissue characterization, the typical CMR findings of cardiac amyloidosis include diffuse subendocardial or transmural late gadolinium enhancement on late gadolinium imaging with nulling of the blood pool and elevated native T1 and extracellular volume (ECV) on T1 mapping sequences. T1 mapping, a relatively new and quantitative CMR technique, with native T1 and ECV, has the potential to longitudinally monitor disease progression.35,36
Myocardial scintigraphy with bone avid tracers 99mtechnetium pyrophosphate (99mTc-PYP), 99mtechnetium 3,3-diphosphono-1,2-propanodicarboxylic acid (99mTc-DPD), and 99mtechnetium hydroxymethylene diphosphonate (99mTc-HMDP) has high sensitivity and specificity for ATTR-CM and may help with the early diagnosis of ATTR-CM (Figures 4, 5).37–43 Ease of access, simplicity of imaging, relatively low cost, and specificity for cardiac ATTR amyloid deposits are some of the advantages of myocardial scintigraphy compared with echocardiography, CMR, and endomyocardial biopsy. In addition, the utility of these agents in identifying ATTR-CM before increases in wall thickness are observed or electrocardiographic voltage is reduced15,41,44,45 suggests that they may be useful for early identification of affected individuals. Molecular imaging with targeted amyloid-binding positron emission tomography radiotracers 11C-Pittsburgh compound B (11C-PIB), 18F-florbetapir, and 18F-florbetaben is an emerging quantitative diagnostic approach that may distinguish cardiac amyloidosis from other forms of heart disease.46–49
Figure 4.
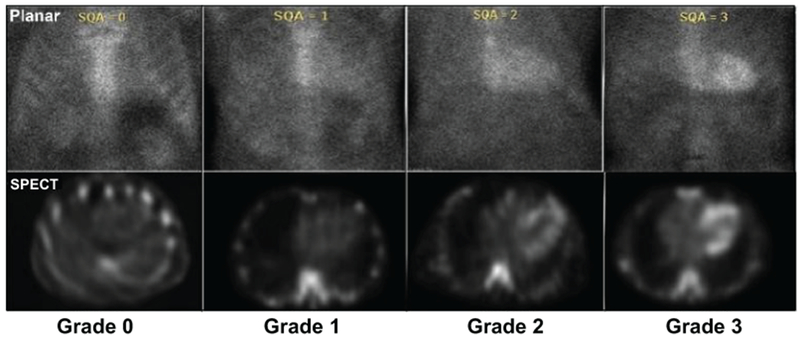
99mTechnetium imaging procedures for cardiac amyloidosis. Adapted with permission from Dorbala S et al. ASNC Practice Points. SPECT imaging to identify myocardial retention of technetium-based isotopes is particularly useful in discriminating blood pool on planar scans that result in in a false-positive test from myocardial uptake of the isotope indicative of ATTR-CM. 99mTechnetium-pyrophosphate imaging for transthyretin cardiac amyloidosis. ASNC, American Society of Nuclear Cardiology. https://www.asnc.org/files/Practice%20Resources/Practice%20Points/ASNC%20Practice%20Point-99mTechnetiumPyrophosphateImaging2016.pdf. Accessed March 6, 2019.
Figure 5.
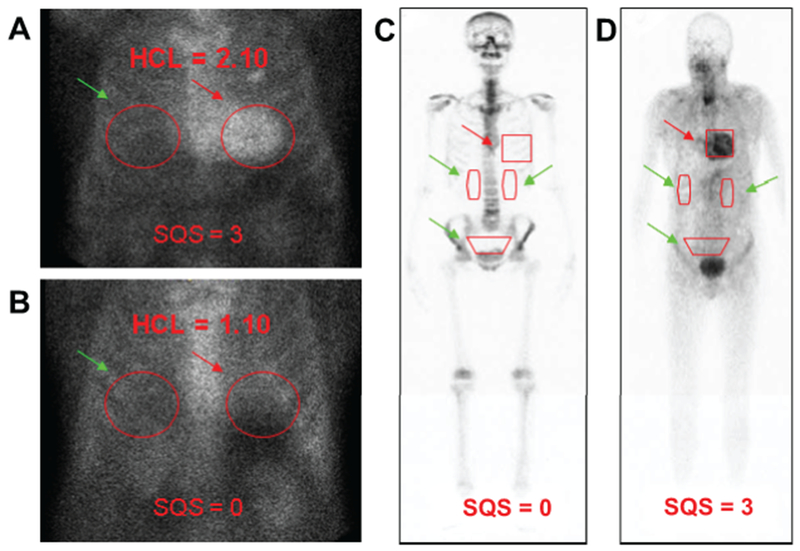
Semiquantitative approach to 99mTc-PYP/DPD/HMDP imaging in cardiac amyloidosis. Semiquantitative methods to generate HCL ratios with a target ROI over the heart (A, B, red arrows) mirrored over the contralateral chest for a background ROI (A, B, green arrows). An HCL ratio of >1.5 on 1-hour imaging is diagnostic of ATTR-CM. Comparatively, 99mTc DPD includes a whole-body scan, 25–30 mCi of radiotracer, 200 minutes of study time, with heart-to-whole-body ratios generated by a target ROI over the heart (C, D, red arrows) as well as background ROIs over the kidneys and bladder (C, D, green arrows). 99mTc HMDP was validated for diagnosing ATTR CA, and representative scans show diffuse myocardial uptake in a patient with cardiac transthyretin amyloidosis at baseline. 99mTc-DPD, 99mtechnetium-3,3-diphosphono-1,2-propanodicarboxylic acid; 99mTc-HMDP, hydroxymethylene diphosphonate; 99mTc-PYP, technetium pyrophosphate; ATTR-CM, transthyretin amyloidosis with predominant cardiomyopathy (either WT or hereditary); HCL, heart-to-contralateral; ROI, region of interest. Panels C and D are modified with permission from Perugini E et al. J Am Coll Cardiol. 2005;6:1076–1084.
DIAGNOSIS AND ASSESSMENT
A diagnostic approach for patients with suspected cardiac amyloidosis should include testing for monoclonal protein followed by scintigraphy or biopsy (Figure 6).50 Clinicians should note that up to 40% of patients with ATTR-CA can have a monoclonal gammopathy of unknown significance (MGUS)51 and in this setting scintigraphy alone cannot ensure a diagnosis with 100% specificity. Nuclear imaging can also be performed concurrent to AL assessment, even in the case of a detected monoclonal gammopathy, for additive information. However, in the context of MGUS, endomyocardial biopsy is necessary to definitively diagnose ATTR-CM. If no monoclonal protein is detected and a diagnosis of AL cardiac amyloidosis is excluded, radionuclide scintigraphy alone, without myocardial biopsy, can be used to diagnose ATTR-CM.37 Among all radiotracers that have been tested, DPD, PYP, and HMDP are recommended for the diagnosis of amyloidosis (Table 2).52,53 The radiotracer 123I-metaiodobenzylguanidine (MIBG) can detect sympathetic innervation of the heart and may indicate cardiac amyloid,63–66 although MIBG imaging is also abnormal in other cardiac conditions and is not specific enough to diagnose ATTR-CM. If ATTR-CM is identified, TTR genotyping should be performed.
Figure 6. Diagnostic algorithm for patients with suspected cardiac amyloidosis.50.
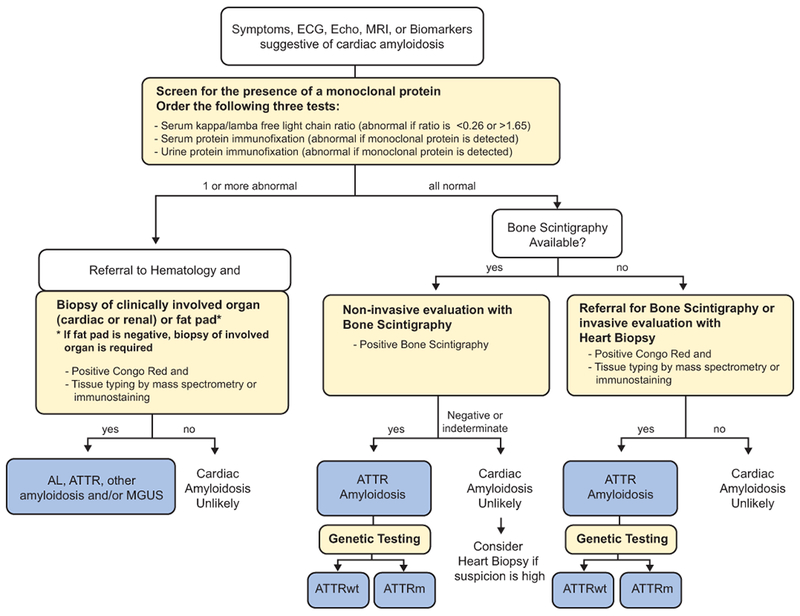
Note that urine protein electrophoresis with immunofixation can be performed on spot or 24-hour urine collection. AL, light chain amyloidosis; ATTR, transthyretin amyloidosis; ATTRm, mutant transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; ECG, electrocardiography; Echo, echocardiogram; MGUS, monoclonal gammopathy of undetermined significance; MRI, magnetic resonance imaging. Figure modified with permission from Nativi-Nicolau and Maurer. Curr Opin Cardiol. 2018;33:571–579.
Table 2.
Radiotracers for Imaging of Cardiac Amyloidosis
| Radiotracer | Imaging Modality | Mechanism of Uptake | Amyloid Subtype Uptake | Imaging Capability | Considerations |
|---|---|---|---|---|---|
| Bone avid tracers primarily used in the USA | |||||
| 99mTc-PYP44, 54–57 | Planar/SPECT | Bone tracer | ATTR-CM | Diagnostic; possibly early detection | Some uptake in patients with AL amyloidosis, less than ATTR amyloidosis |
| Bone avid tracers primarily used outside the USA | |||||
| 99mTc-DPD39–41 and 99mTc-HMDP38, 58, 59 | Planar/SPECT | Bone tracer | ATTR-CM | Diagnostic; possibly early detection and disease monitoring | Some uptake in patients with AL amyloidosis, less than ATTR amyloidosis |
| Amyloid-binding radiotracers | |||||
| 11C-PIB47, 60, 61 | PET | Amyloid deposits | ATTR-CM, AL amyloidosis | Possibly quantitation of amyloid burden, disease monitoring | Short half-life, expensive isotope relative to SPECT tracers |
| 18F-florbetapir48, 62 | PET | Amyloid deposits | ATTR-CM, AL amyloidosis | Possibly early detection, quantitation, and disease monitoring | Expensive isotope relative to SPECT tracers |
| 18F-florbetaben49 | PET | Amyloid deposits | ATTR-CM, AL amyloidosis | Possibly early detection, quantitation, and disease monitoring | Expensive isotope relative to SPECT tracers |
| 18F-NaF52, 53 | PET | Bone tracer | Equivocal ATTR-CM | Possibly early detection, quantitation, and disease monitoring | No uptake in patients with AL amyloidosis, equivocal uptake in patients with ATTR-CM |
11C-PIB, Pittsburgh compound B; 18F-NaF, sodium fluoride; 123I, iodine-123; 99mTc-DPD, 99mtechnetium-3,3-diphosphono-1,2-propanodicarboxylic acid; 99mTc-HMDP, hydroxymethylene diphosphonate; 99mTc-PYP, technetium pyrophosphate; AL, amyloid light chain; ATTR-CM, transthyretin amyloidosis with predominant cardiomyopathy (either wild-type or hereditary); PET, positron emission tomography; SPECT, single-photon emission computed tomography.
Note: The tracers 99mTc-MDP and 99mTc-aprotinin are not recommended.
If monoclonal protein is detected, assessments should include amyloid typing of a tissue biopsy from a clinically affected organ (eg, endomyocardial biopsy if the heart is clinically affected), abdominal fat, or bone marrow, depending on availability and expertise at the clinic. TTR genotyping should be performed if a diagnosis of ATTR amyloidosis is made on biopsy. Endomyocardial biopsy is invasive, carries a small risk for serious complications, and requires technical expertise, whereas fat pad biopsy is less invasive and poses little risk but has varying sensitivity in ATTR-CM (with roughly 45% sensitivity for ATTRm and roughly 15% sensitivity for ATTRwt).67 Given the high false-negative rate from biopsies of nonclinically involved sites (eg, fat pad, bone marrow), further evaluation is warranted even in the presence of a negative biopsy from such sites if clinical suspicion remains elevated. In such cases, biopsy of a clinically affected organ (eg, endomyocardial biopsy) is imperative. Endomyocardial biopsy assessment with Congo red staining has approximately 100% specificity and sensitivity for detecting amyloid deposits and is still considered the gold standard in situations with equivocal noninvasive findings.68 Regardless of the site of biopsy, amyloid deposits must then undergo either immunofluorescence or mass spectrometry to confirm the amyloidosis subtype (eg, AL or ATTR).
In patients with confirmed ATTR amyloidosis, TTR gene sequencing is necessary even if they do not have a family history of amyloidosis or evidence of PN because the penetrance of ATTRm varies among the variants and families. If a TTR variant is detected, genetic counseling for relatives of the affected patient is indicated.
Prognostic Stratification Using Biomarkers
Natriuretic peptides and cardiac troponins, though not specific markers of ATTR-CM, are well established to assess risk (eg, Mayo staging) and to evaluate response to treatment in patients with AL amyloidosis.69–71 However, evidence in AL amyloidosis may not apply to ATTR amyloidosis because the two diseases have substantially different biologies.29,72,73 Different staging systems for ATTRwt amyloidosis and ATTR-CM have been proposed. A proposed system in patients with ATTRwt includes NT-proBNP (>3000 pg/mL) and troponin T (>0.05 ng/mL).74 The most recently proposed system for staging ATTR-CM (including ATTRwt and ATTRm) uses NT-proBNP (>3000 pg/mL) and estimated glomerular filtration rate (<45 mL/min).75 Staging in both systems is defined such that stage 1 does not meet either threshold, stage 2 meets 1 of the 2 thresholds, and stage 3 meets both thresholds.
FUTURE DIRECTIONS
In order to reduce the delays in diagnosis of this important health problem, specific programs for screening or early identification should be studied that leverage techniques having appropriate sensitivity and specificity as well as favorable cost/benefit. Implementation of appropriate screening programs for ATTR-CM will need to include factors such as whether there are methods and facilities available for diagnosis, whether certain diagnostic tests are acceptable to those at risk, and the particular test characteristics, as well as cost and benefits of treatments and when appropriate timing would be for the intervention. Given that prognosis is highly dependent on the underlying cardiac dysfunction coupled with the recently available treatments for ATTR amyloidosis, screening programs may become important.
CONCLUSIONS
ATTR amyloidosis is a progressive disease associated with increased morbidity and mortality and occurs in inherited (ATTRm or hATTR) or acquired (ATTRwt) forms. Disease-related cardiac dysfunction in patients with ATTR amyloidosis is associated with particularly poor outcomes and is a manifestation of many of the genetic variants and the wild-type form of ATTR amyloidosis.
Diagnosis of ATTR-CM is often missed or mistaken as hypertrophic cardiomyopathy or HFpEF of unknown cause. Although considered a rare disease, the true prevalence of ATTR-CM is unclear and is likely higher than appreciated. Physicians should consider systemic signs and symptoms along with evidence from biomarkers and imaging to build suspicion for ATTR-CM. To facilitate early diagnosis of ATTR-CM, evaluation of myocardial uptake on bone scintigraphy should be considered in patients with HF, unexplained neuropathy, family history of amyloidosis, or unexplained increased LV wall thickness. Appropriate evidence on echocardiography or cardiac MRI—combined with no light chain clone, grade ≥2 myocardial uptake of 99mTc-PYP, DPD, and HMDP—is diagnostic of ATTR-CM, in which case endomyocardial biopsy is unnecessary. Genetic testing should be performed to differentiate ATTRm from ATTRwt causes of ATTR-CM.
The consensus recommendations described in this review were developed with the goal of providing clinicians with an overview of key aspects of ATTR-CM diagnosis. We hope these recommendations facilitate early, rapid, and accurate identification of ATTR-CM to allow implementation of targeted, disease-modifying treatment and improved outcomes for patients.
Acknowledgments
Funding support for these recommendations was received from GSK, Ionis, Pfizer, and Alnylam; these companies were not involved in writing this manuscript. Medical writing and editorial assistance was provided by ApotheCom (San Francisco, CA, USA).
Sources of Funding
This review was sponsored by the Amyloidosis Research Consortium.
Disclosures
Dr. Maurer: Served on the steering committee of the ATTR-ACT trial. His institution received research support for clinical studies from Pfizer and Alnylam. Has served on advisory boards or DSMBs for Akcea, Ionis, Prothena, Pfizer, Alnylam, Eidos, GSK
Dr Bokhari: Honoraria from Pfizer
Dr Damy: His institution received research support for clinical studies from Pfizer and Alnylam. He has served on advisory boards or DSMBs for Akcea, Ionis, Prothena, Pfizer, Alnylam, GSK
Dr Dorbala: Consulting fees from Pfizer, GEHC, AAA
Dr Drachman: Consulting fees from Pfizer and Alnylam (not significant)
Dr Fontana: Research grants from GSK. Salary from BHF FS/18/21/33447. Honoraria from Pfizer, Alnylam, Prothena (modest)
Dr Grogan: Research funding from Alnylam, Eidos, Pfizer, Prothena
Dr Kristen: Consulting fees from Akcea, EIDOS. Honoraria/travel support from Pfizer, Akcea, Alnylam
Ms Lousada: Honorarium from Akcea
Dr Nativi-Nicolau: Research funding for clinical studies from Pfizer, Akcea, Eidos. Advisory boards for Alnylam, Ionis, Akcea, Pfizer
Dr Quarta: Honoraria from Alnylam
Dr Rapezzi: Research grants from Pfizer. Honoraria from Pfizer, Alnylam
Dr Ruberg: Consulting fees from Pfizer, GSK. Grant support from Eidos (significant)
Dr Witteles: Advisory board honoraria from Pfizer, Alnylam, Eidos. Clinical trial research support from Pfizer, Eidos
Dr Merlini: Honoraria from Janssen. Honoraria and travel support from Prothena. Travel support from Celgene. Consulting fees from Millennium, Pfizer, Janssen, Prothena, Ionis
REFERENCES
- 1.Castano A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20:163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel KS, Hawkins PN. Cardiac amyloidosis: where are we today? J Intern Med. 2015;278:126–144. [DOI] [PubMed] [Google Scholar]
- 3.Brunjes DL, Castano A, Clemons A, Rubin J, Maurer MS. Transthyretin cardiac amyloidosis in older Americans. J Cardiac Fail. 2016;22:996–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins PN, Ando Y, Dispenzeri A, Gonzalez-Duarte A, Adams D, Suhr OB. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47:625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson DR, Pastore R, Pool S, Malendowicz S, Kane I, Shivji A, Embury SH, Ballas SK, Buxbaum JN. Revised transthyretin Ile 122 allele frequency in African-Americans. Hum Genet. 1996;98:236–238. [DOI] [PubMed] [Google Scholar]
- 6.Quarta CC, Buxbaum JN, Shah AM, Falk RH, Claggett B, Kitzman DW, Mosley TH, Butler KR, Boerwinkle E, Solomon SD. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015;372:21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobson DR, Pastore RD, Yaghoubian R, Kane I, Gallo G, Buck FS, Buxbaum JN. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med. 1997;336:466–473. [DOI] [PubMed] [Google Scholar]
- 8.Buxbaum J, Jacobson DR, Tagoe C, Alexander A, Kitzman DW, Greenberg B, Thaneemit-Chen S, Lavori P. Transthyretin V122I in African Americans with congestive heart failure. J Am Coll Cardiol. 2006;47:1724–1725. [DOI] [PubMed] [Google Scholar]
- 9.Reilly MM, Staunton H, Harding AE. Familial amyloid polyneuropathy (TTR ala 60) in north west Ireland: a clinical, genetic, and epidemiological study. J Neurol Neurosurg Psychiatr. 1995;59:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru-Enari S, Paetau A, Tienari PJ, Myllykangas L. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: a population-based autopsy study. Ann Med. 2008;40:232–239. [DOI] [PubMed] [Google Scholar]
- 11.Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, George I, Kodali S, Leon MB, Hahn R, Bokhari S, Maurer MS. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. [DOI] [PubMed] [Google Scholar]
- 13.Damy T, Costes B, Hagege AA, Donal E, Eicher JC, Slama M, Guellich A, Rappeneau S, Gueffet JP, Logeart D, Plante-Bordeneuve V, Bouvaist H, Huttin O, Mulak G, Dubois-Rande JL, Goossens M, Canoui-Poitrine F, Buxbaum JN. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J. 2016;37:1826–1834. [DOI] [PubMed] [Google Scholar]
- 14.Sperry BW, Reyes BA, Ikram A, Donnelly JP, Phelan D, Jaber WA, Shapiro D, Evans PJ, Maschke S, Kilpatrick SE, Tan CD, Rodriguez ER, Monteiro C, Tang WHW, Kelly JW, Seitz WH, Hanna M. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol. 2018;72:2040–2050. [DOI] [PubMed] [Google Scholar]
- 15.Longhi S, Guidalotti PL, Quarta CC, Gagliardi C, Milandri A, Lorenzini M, Potena L, Leone O, Bartolomei I, Pastorelli F, Salvi F, Rapezzi C. Identification of TTR-related subclinical amyloidosis with 99mTc-DPD scintigraphy. JACC Cardiovasc Imaging. 2014;7:531–532. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed-Salem L, Santos-Mateo JJ, Sanchez-Serna J, Hernandez-Vicente A, Reyes-Marle R, Castellon Sanchez MI, Claver-Valderas MA, Gonzalez-Vioque E, Haro-Del Moral FJ, Garcia-Pavia P, Pascual-Figal DA. Prevalence of wild type ATTR assessed as myocardial uptake in bone scan in the elderly population. Int J Cardiol. 2018;270:192–196. [DOI] [PubMed] [Google Scholar]
- 17.Galant NJ, Westermark P, Higaki JN, Chakrabartty A. Transthyretin amyloidosis: an under-recognized neuropathy and cardiomyopathy. Clin Sci. 2017;131:395–409. [DOI] [PubMed] [Google Scholar]
- 18.Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Patient experience with hereditary and wild-type transthyretin amyloidosis: a survey from the Amyloidosis Research Consortium. Presented at: First European Congress on Hereditary ATTR Amyloidosis; November 2–3, 2015; Paris, France. [Google Scholar]
- 19.Sekijima Y, Uchiyama S, Tojo K, Sano K, Shimizu Y, Imaeda T, Hoshii Y, Kato H, Ikeda S. High prevalence of wild-type transthyretin deposition in patients with idiopathic carpal tunnel syndrome: a common cause of carpal tunnel syndrome in the elderly. Hum Pathol. 2011;42:1785–1791. [DOI] [PubMed] [Google Scholar]
- 20.Yanagisawa A, Ueda M, Sueyoshi T, Okada T, Fujimoto T, Ogi Y, Kitagawa K, Tasaki M, Misumi Y, Oshima T, Jono H, Obayashi K, Hirakawa K, Uchida H, Westermark P, Ando Y, Mizuta H. Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015;28:201–207. [DOI] [PubMed] [Google Scholar]
- 21.Westermark P, Westermark GT, Suhr OB, Berg S. Transthyretin-derived amyloidosis: probably a common cause of lumbar spinal stenosis. Ups J Med Sci. 2014;119:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubin J, Alvarez J, Teruya S, Castano A, Lehman RA, Weidenbaum M, Geller JA, Helmke S, Maurer MS. Hip and knee arthroplasty are common among patients with transthyretin cardiac amyloidosis, occurring years before cardiac amyloid diagnosis: can we identify affected patients earlier? Amyloid. 2017;24:226–230. [DOI] [PubMed] [Google Scholar]
- 23.Geller HI, Singh A, Alexander KM, Mirto TM, Falk RH. Association between ruptured distal biceps tendon and wild-type transthyretin cardiac amyloidosis. JAMA. 2017;318:962–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damy T, Deux JF, Moutereau S, Guendouz S, Mohty D, Rappeneau S, Guellich A, Hittinger L, Loric S, Lefaucheur JP, Plante-Bordeneuve V. Role of natriuretic peptide to predict cardiac abnormalities in patients with hereditary transthyretin amyloidosis. Amyloid. 2013;20:212–220. [DOI] [PubMed] [Google Scholar]
- 25.Arvanitis M, Koch CM, Chan GG, Torres-Arancivia C, LaValley MP, Jacobson DR, Berk JL, Connors LH, Ruberg FL. Identification of transthyretin cardiac amyloidosis using serum retinol-binding protein 4 and a clinical prediction model. JAMA Cardiol. 2017;2:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapezzi C, Quarta CC, Riva L, Longhi S, Gallelli I, Lorenzini M, Ciliberti P, Biagini E, Salvi F, Branzi A. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat Rev Cardiol. 2010;7:398–408. [DOI] [PubMed] [Google Scholar]
- 27.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126:1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quarta CC, Kruger JL, Falk RH. Cardiac amyloidosis. Circulation. 2012;126:e178–e182. [DOI] [PubMed] [Google Scholar]
- 29.Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, Gagliardi C, Milandri A, Rapezzi C, Falk RH. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation. 2014;129:1840–1849. [DOI] [PubMed] [Google Scholar]
- 30.Carroll JD, Gaasch WH, McAdam KP. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol. 1982;49:9–13. [DOI] [PubMed] [Google Scholar]
- 31.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. [DOI] [PubMed] [Google Scholar]
- 32.Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135:1357–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Bella G, Minutoli F, Piaggi P, Casale M, Mazzeo A, Zito C, Oreto G, Baldari S, Vita G, Pingitore A, Khandheria BK, Carerj S. Usefulness of combining electrocardiographic and echocardiographic findings and brain natriuretic peptide in early detection of cardiac amyloidosis in subjects with transthyretin gene mutation. Am J Cardiol. 2015;116:1122–1127. [DOI] [PubMed] [Google Scholar]
- 34.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. [DOI] [PubMed] [Google Scholar]
- 35.Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, Kotecha T, Francis R, Hutt DF, Rezk T, Rosmini S, Quarta CC, Whelan CJ, Kellman P, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol. 2017;70:466–477. [DOI] [PubMed] [Google Scholar]
- 36.Martinez-Naharro A, Kotecha T, Norrington K, Boldrini M, Rezk T, Quarta C, Treibel TA, Whelan CJ, Knight DS, Kellman P, Ruberg FL, Gillmore JD, Moon JC, Hawkins PN, Fontana M. Native T1 and extracellular volume in transthyretin amyloidosis. JACC Cardiovasc Imaging. 2019;12:810–819. [DOI] [PubMed] [Google Scholar]
- 37.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. [DOI] [PubMed] [Google Scholar]
- 38.Galat A, Rosso J, Guellich A, Van Der Gucht A, Rappeneau S, Bodez D, Guendouz S, Tissot CM, Hittinger L, Dubois-Rande JL, Plante-Bordeneuve V, Itti E, Meignan M, Damy T. Usefulness of (99m)Tc-HMDP scintigraphy for the etiologic diagnosis and prognosis of cardiac amyloidosis. Amyloid. 2015;22:210–220. [DOI] [PubMed] [Google Scholar]
- 39.Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, Leone O, Farsad M, Ciliberti P, Bacchi-Reggiani L, Fallani F, Branzi A, Rapezzi C. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46:1076–1084. [DOI] [PubMed] [Google Scholar]
- 40.Rapezzi C, Quarta CC, Guidalotti PL, Longhi S, Pettinato C, Leone O, Ferlini A, Salvi F, Gallo P, Gagliardi C, Branzi A. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging. 2011;38:470–478. [DOI] [PubMed] [Google Scholar]
- 41.Rapezzi C, Quarta CC, Guidalotti PL, Pettinato C, Fanti S, Leone O, Ferlini A, Longhi S, Lorenzini M, Reggiani LB, Gagliardi C, Gallo P, Villani C, Salvi F. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging. 2011;4:659–670. [DOI] [PubMed] [Google Scholar]
- 42.Pilebro B, Suhr OB, Naslund U, Westermark P, Lindqvist P, Sundstrom T. (99m)Tc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Ups J Med Sci. 2016;121:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falk RH, Quarta CC, Dorbala S. How to image cardiac amyloidosis. Circ Cardiovasc Imaging. 2014;7:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haq M, Pawar S, Berk JL, Miller EJ, Ruberg FL. Can (99m)Tc-pyrophosphate aid in early detection of cardiac involvement in asymptomatic variant TTR amyloidosis? JACC Cardiovasc Imaging. 2017;10:713–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glaudemans AW, van Rheenen RW, van den Berg MP, Noordzij W, Koole M, Blokzijl H, Dierckx RA, Slart RH, Hazenberg BP. Bone scintigraphy with (99m)technetium-hydroxymethylene diphosphonate allows early diagnosis of cardiac involvement in patients with transthyretin-derived systemic amyloidosis. Amyloid. 2014;21:35–44. [DOI] [PubMed] [Google Scholar]
- 46.Park MA, Padera RF, Belanger A, Dubey S, Hwang DH, Veeranna V, Falk RH, Di Carli MF, Dorbala S. 18F-Florbetapir binds specifically to myocardial light chain and transthyretin amyloid deposits: autoradiography study. Circ Cardiovasc Imaging. 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Antoni G, Lubberink M, Estrada S, Axelsson J, Carlson K, Lindsjo L, Kero T, Langstrom B, Granstam SO, Rosengren S, Vedin O, Wassberg C, Wikstrom G, Westermark P, Sorensen J. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med. 2013;54:213–220. [DOI] [PubMed] [Google Scholar]
- 48.Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR Jr, Di Carli MF, Moore SC, Falk RH. Imaging cardiac amyloidosis: a pilot study using (1)(8)F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging. 2014;41:1652–1662. [DOI] [PubMed] [Google Scholar]
- 49.Law WP, Wang WY, Moore PT, Mollee PN, Ng AC. Cardiac amyloid imaging with 18F-florbetaben PET: a pilot study. J Nucl Med. 2016;57:1733–1739. [DOI] [PubMed] [Google Scholar]
- 50.Nativi-Nicolau J, Maurer MS. Amyloidosis cardiomyopathy: update in the diagnosis and treatment of the most common types. Curr Opin Cardiol. 2018;33:571–579. [DOI] [PubMed] [Google Scholar]
- 51.Phull P, Sanchorawala V, Connors LH, Doros G, Ruberg FL, Berk JL, Sarosiek S. Monoclonal gammopathy of undetermined significance in systemic transthyretin amyloidosis (ATTR). Amyloid. 2018;25:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Der Gucht A, Galat A, Rosso J, Guellich A, Garot J, Bodez D, Plante-Bordeneuve V, Hittinger L, Dubois-Rande JL, Evangelista E, Sasanelli M, Chalaye J, Meignan M, Itti E, Damy T. [18F]-NaF PET/CT imaging in cardiac amyloidosis. J Nucl Cardiol. 2016;23:846–849. [DOI] [PubMed] [Google Scholar]
- 53.Gagliardi C, Tabacchi E, Bonfiglioli R, Diodato S, Nanni C, Guidalotti P, Lorenzini M, Lodi F, Milandri A, Rapezzi C, Fanti S. Does the etiology of cardiac amyloidosis determine the myocardial uptake of [18F]-NaF PET/CT? J Nucl Cardiol. 2017;24:746–749. [DOI] [PubMed] [Google Scholar]
- 54.Bokhari S, Morgenstern R, Weinberg R, Kinkhabwala M, Panagiotou D, Castano A, DeLuca A, Andrew K, Jin Z, Maurer MS. Standardization of (99m)technetium pyrophosphate imaging methodology to diagnose TTR cardiac amyloidosis. J Nucl Cardiol. 2018;25:181–190. [DOI] [PubMed] [Google Scholar]
- 55.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, Pozniakoff T, Ruberg FL, Miller EJ, Berk JL, Dispenzieri A, Grogan M, Johnson G, Bokhari S, Maurer MS. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1:880–889. [DOI] [PubMed] [Google Scholar]
- 57.Castano A, DeLuca A, Weinberg R, Pozniakoff T, Blaner WS, Pirmohamed A, Bettencourt B, Gollob J, Karsten V, Vest JA, Chiuzan C, Maurer MS, Bokhari S. Serial scanning with technetium pyrophosphate ((99m)Tc-PYP) in advanced ATTR cardiac amyloidosis. J Nucl Cardiol. 2016;23:1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wadhwa SS, Nour R. Tc-99m HDP uptake in cardiac amyloidosis. Clin Nucl Med. 1999;24:156–158. [DOI] [PubMed] [Google Scholar]
- 59.Kulhanek J, Movahed A. Uptake of technetium 99m HDP in cardiac amyloidosis. Int J Cardiovasc Imaging. 2003;19:225–227. [DOI] [PubMed] [Google Scholar]
- 60.Lee SP, Lee ES, Choi H, Im HJ, Koh Y, Lee MH, Kwon JH, Paeng JC, Kim HK, Cheon GJ, Kim YJ, Kim I, Yoon SS, Seo JW, Sohn DW. 11C-Pittsburgh B PET imaging in cardiac amyloidosis. JACC Cardiovasc Imaging. 2015;8:50–59. [DOI] [PubMed] [Google Scholar]
- 61.Kero T, Lindsjo L, Sorensen J, Lubberink M. Accurate analysis and visualization of cardiac (11)C-PIB uptake in amyloidosis with semiautomatic software. J Nucl Cardiol. 2016;23:741–750. [DOI] [PubMed] [Google Scholar]
- 62.Osborne DR, Acuff SN, Stuckey A, Wall JS. A routine PET/CT protocol with streamlined calculations for assessing cardiac amyloidosis using (18)F-florbetapir. Front Cardiovasc Med. 2015;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakata T, Shimamoto K, Yonekura S, Kobayashi N, Sugiyama T, Imai K, Iimura O. Cardiac sympathetic denervation in transthyretin-related familial amyloidotic polyneuropathy: detection with iodine-123-MIBG. J Nucl Med. 1995;36:1040–1042. [PubMed] [Google Scholar]
- 64.Delahaye N, Dinanian S, Slama MS, Mzabi H, Samuel D, Adams D, Merlet P, Le Guludec D. Cardiac sympathetic denervation in familial amyloid polyneuropathy assessed by iodine-123 metaiodobenzylguanidine scintigraphy and heart rate variability. Eur J Nucl Med. 1999;26:416–424. [DOI] [PubMed] [Google Scholar]
- 65.Hongo M, Urushibata K, Kai R, Takahashi W, Koizumi T, Uchikawa S, Imamura H, Kinoshita O, Owa M, Fujii T. Iodine-123 metaiodobenzylguanidine scintigraphic analysis of myocardial sympathetic innervation in patients with AL (primary) amyloidosis. Am Heart J. 2002;144:122–129. [DOI] [PubMed] [Google Scholar]
- 66.Noordzij W, Glaudemans AW, van Rheenen RW, Hazenberg BP, Tio RA, Dierckx RA, Slart RH. (123)I-labelled metaiodobenzylguanidine for the evaluation of cardiac sympathetic denervation in early stage amyloidosis. Eur J Nucl Med Mol Imaging. 2012;39:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quarta CC, Gonzalez-Lopez E, Gilbertson JA, Botcher N, Rowczenio D, Petrie A, Rezk T, Youngstein T, Mahmood S, Sachchithanantham S, Lachmann HJ, Fontana M, Whelan CJ, Wechalekar AD, Hawkins PN, Gillmore JD. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J. 2017;38:1905–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adams D, Suhr OB, Hund E, Obici L, Tournev I, Campistol JM, Slama MS, Hazenberg BP, Coelho T, European Network for T-F. First European consensus for diagnosis, management, and treatment of transthyretin familial amyloid polyneuropathy. Curr Opin Neurol. 2016;29(suppl 1):S14–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merlini G, Lousada I, Ando Y, Dispenzieri A, Gertz MA, Grogan M, Maurer MS, Sanchorawala V, Wechalekar A, Palladini G, Comenzo RL. Rationale, application and clinical qualification for NT-proBNP as a surrogate end point in pivotal clinical trials in patients with AL amyloidosis. Leukemia. 2016;30:1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lavatelli F, Albertini R, Di Fonzo A, Palladini G, Merlini G. Biochemical markers in early diagnosis and management of systemic amyloidoses. Clin Chem Lab Med. 2014;52:1517–1531. [DOI] [PubMed] [Google Scholar]
- 71.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, Laumann K, Zeldenrust SR, Leung N, Dingli D, Greipp PR, Lust JA, Russell SJ, Kyle RA, Rajkumar SV, Gertz MA. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cappelli F, Baldasseroni S, Bergesio F, Perlini S, Salinaro F, Padeletti L, Attana P, Paoletti Perini A, Moggi Pignone A, Grifoni E, Fabbri A, Marchionni N, Gensini GF, Perfetto F. Echocardiographic and biohumoral characteristics in patients with AL and TTR amyloidosis at diagnosis. Clin Cardiol. 2015;38:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, Wechalekar A, Gibbs SD, Venner CP, Wassef N, McCarthy CA, Gilbertson JA, Rowczenio D, Hawkins PN, Gillmore JD, Lachmann HJ. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2:e000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68:1014–1020. [DOI] [PubMed] [Google Scholar]
- 75.Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez-Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez-Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799–2806. [DOI] [PubMed] [Google Scholar]


