Abstract
BACKGROUND:
Neoadjuvant chemotherapy (NACT) is often recommended for patients with node-positive invasive lobular carcinoma (ILC) despite unclear benefit in this largely hormone receptor-positive (HR+) group. We sought to compare overall survival (OS) between patients with node-positive ILC who received neoadjuvant endocrine therapy (NET) and those who received NACT.
METHODS:
Women with cT1-4c, cN1-3 HR+ ILC in the National Cancer Data Base (2004-2014) who underwent surgery following neoadjuvant therapy were identified. Kaplan-Meier curves and Cox proportional hazards modeling were used to estimate unadjusted and adjusted OS, respectively.
RESULTS:
Of the 5,942 patients in the cohort, 855 received NET, and 5,087 received NACT. NET recipients were older (70 vs 54), had more comorbidities (Charlson-Deyo score≥1: 21.1% vs 11.5%), lower cT classification (cT3-4: 44.2% vs 51.0%), lower rates of mastectomy (72.5% vs 82.2%), lower rates of pathologic complete response (0% vs 2.5%), and lower rates of post-lumpectomy (73.2% vs 91.0%) and post-mastectomy (60.0% vs 80.8%) radiation vs NACT recipients (all p<0.001). NACT recipients had higher unadjusted 10-year OS vs NET recipients (57.9% vs. 36.0%), but after adjustment, there was no significant difference in OS between the two groups (p=0.10).
CONCLUSION:
Patients with node-positive ILC who received NET presented with smaller tumors, older age, and greater burden of comorbidities vs NACT recipients but had similar adjusted OS. While there is evidence from clinical trials supporting efficacy of NET in HR+ breast cancer, our findings suggest the need for further, histology-specific investigation regarding the optimal inclusion and sequence of endocrine therapy and chemotherapy in ILC.
Keywords: breast cancer, chemotherapy, endocrine therapy, invasive lobular carcinoma, locally advanced, neoadjuvant
INTRODUCTION
The use of neoadjuvant chemotherapy (NACT) in patients with non-metastatic, resectable breast cancer was initially investigated in the National Surgical Adjuvant Breast and Bowel Project (NSABP) trials B-18 and B-27 to establish whether preoperative systemic therapy could be a means through which to potentially improve rates of breast conserving surgery for estrogen receptor-negative (ER-) tumors without compromising disease-free and overall survival.1 In contemporary breast cancer care, NACT continues to be administered for the purpose of facilitating breast conservation by tumor downstaging, but it is also used to decrease nodal burden in patients with node-positive (LN+) disease and to allow for preoperative, in vivo assessment of tumor response to NACT.
Invasive lobular carcinoma (ILC), which comprises ~10-15% of all breast cancers, has distinct clinical characteristics including a high proportion of low-grade and estrogen receptor-positive (ER+) tumors that influence both the types of treatment recommended and response to treatment.2,3 Several studies have demonstrated a poor response to NACT among ILC patients, with lower rates of pathologic complete response (pCR) following NACT in ILC patients as compared to patients with invasive ductal carcinoma (IDC).4–9 Nevertheless, NACT continues to be recommended for many node-positive ILC patients.
Few ILC patients have been included in clinical trials of neoadjuvant endocrine therapy (NET), despite evidence from clinical trials supporting efficacy of NET in ER+ breast cancer.10,11 The American College of Surgeons Oncology Group (ACOSOG) Z1031 trial demonstrated that NET significantly improved rates of breast conservation in patients with ER+ invasive carcinoma, but <20% of the trial cohort had lobular histology.12 A 2011 study by Dixon et al. specifically examined the effectiveness of neoadjuvant letrozole in post-menopausal women with ER+ ILC. NET was found to be successful in reducing tumor size in this patient population, with a mean tumor volume reduction of 66% in 3 months. As with Z1031, over half of the women in this study became candidates for breast conserving surgery following NET.2 Nonetheless, NET for HR+ breast cancer continues to be underutilized in the United States, with a previous examination of the National Cancer Data Base (NCDB) demonstrating that only 3% of potentially eligible patients received it. 13 Furthermore, because NET is typically recommended for elderly breast cancer patients who are not considered candidates for chemotherapy or up-front surgery, the potential benefit of NET in younger, healthier patients with locally advanced ILC remains unknown.14,15 Accordingly, we sought to compare outcomes following NET and NACT in patients with LN+ ILC.
METHODS
Women diagnosed with cT1-4c, LN+ (cN1-3), hormone receptor-positive (HR+) ILC between 2004 and 2015 and who received either NET or NACT and underwent surgery were selected from the NCDB. Patients who received both NET and NACT and those with pertinent unknown or missing information were excluded. Per NCDB reporting guidelines, survival data is unavailable for patients diagnosed during the last reporting year, so patients diagnosed in 2015 were ultimately excluded.
Patient characteristics – including type of surgery received (lumpectomy and mastectomy) and response to neoadjuvant systemic therapy (overall pCR [ypT0N0], breast-only pCR, node-only pCR, upstage, discordant, downstage, and no change; see Supplemental Table 1 for response definitions) – were summarized with N (%) for categorical variables and median (interquartile range, IQR) for continuous variables. Chi-square and t-tests were used to compare study groups on categorical and continuous variables, respectively.
Overall survival (OS) was defined as time from diagnosis to death or last follow-up. Unadjusted OS was estimated using the Kaplan-Meier method. Using the log-rank test, 5- and 10-year unadjusted OS rates were compared among four groups of LN+ ILC patients who received different combinations of endocrine therapy and chemotherapy: (1) NET only, (2) NACT only, (3) NET + adjuvant chemotherapy (ACT), and (4) NACT + adjuvant endocrine therapy (AET). Continuation of NET or NACT in the adjuvant setting cannot be discerned in the NCDB, as only the start date of each therapy is available; therefore, patients who received both NACT and ACT or both NET and AET were included in the study but could not be distinguished from those who received all of their chemotherapy or all of their endocrine therapy in the neoadjuvant setting.
To further examine whether there was benefit from receiving any neoadjuvant therapy in this patient population, unadjusted OS was also assessed for three groups of patients with non-metastatic LN+ ILC who underwent surgery but only received adjuvant therapy and were otherwise excluded from the overall study: (5) AET only, (6) ACT only, and (7) ACT + AET. Trends in rates of NET and NACT receipt over time were calculated out of this larger cohort of patients plus the patients who received both NET and NACT (who were otherwise excluded from all analyses), with p<0.05 defined as significant for the Cochran-Armitage trend test.
Cox proportional hazards modeling was used to estimate the effect of NET vs NACT on OS after adjustment for known covariates, including treatment group, age, race, ethnicity, Charlson-Deyo comorbidity score, pathological T and N classification, grade, breast surgery type, receipt of complementary adjuvant therapy following neoadjuvant therapy (i.e., ACT after NET or AET after NACT), receipt of radiation, income, insurance status, education level, facility type, and facility location. Year of diagnosis, stratified into 2004-2009 and 2010-2014, was also included in the model, with this division being used to account for greater inclusion of biomarker data after 2010, when the NCDB began routinely collecting HER2 status information. Receipt of radiation and receipt of complementary adjuvant therapy were allowed to be time-varying in the model to address the time-dependent effects of tumor and treatment characteristics on long-term survival.16 Interactions were examined and found to be insignificant for both type of neoadjuvant therapy*receipt of complementary adjuvant therapy and type of breast surgery*receipt of radiation so both interaction terms were excluded from the final model. A robust sandwich covariance estimator was used for the adjusted model to account for the correlation of patients treated at the same facility. We report hazard ratios (HRs) and 95% confidence intervals (CIs). Two-tailed p<0.05 was considered significant for all analyses.
Patients with missing or unavailable data were excluded from each model, and effective sample sizes are included in all tables and figures. No adjustments were made for multiple comparisons. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). Our institutional review board granted the study exempt status due to use of de-identified data.
RESULTS
Patient, Tumor, and Treatment Characteristics
A cohort of 5,942 patients receiving neoadjuvant systemic therapy was identified, including 855 women (14.4%) who received NET and 5,087 women (85.6%) who received NACT (Figure 1). The median age among all patients was 55 (IQR 47-64, Table 1). Compared to NACT patients, those who received NET were older (70 vs 54) and had more comorbidities (Charlson-Deyo comorbidity [CDC] score≥1: 21.1% vs 11.5%), lower cT classification (cT3-4: 44.2% vs 51.0%), lower rates of mastectomy (72.5% vs 82.2%), and lower rates of both post-lumpectomy (73.2% vs 91.0%) and post-mastectomy (60.0% vs 80.8%) radiation (all p<0.001). About 1/3 of patients who received NET received adjuvant chemotherapy (n=280, 32.7%), while a majority of NACT recipients went on to receive adjuvant endocrine therapy (n=4,380, 86.1%). Despite similar cN classification at presentation (p=0.26), patients who received NET had higher rates of upstage in the breast (NET 10.5% vs NACT 7.0%), nodes (NET 35.6% vs NACT 29.4%), and overall (NET 31.0% vs NACT 20.6%, all p<0.001, Supplemental Table 2), however, these findings may be due to breast and nodal understaging at diagnosis rather than true progression during treatment.17 Overall pCR was observed in only 2.1% of all patients and was not observed in any NET patients (NET 0% vs NACT 2.5%, p<0.001, Supplemental Table 2).
Figure 1. Patient Flow Diagram, Women with cT1-4c, cN1-3, HR+ Invasive Lobular Carcinoma, National Cancer Data Base, 2004-2014.
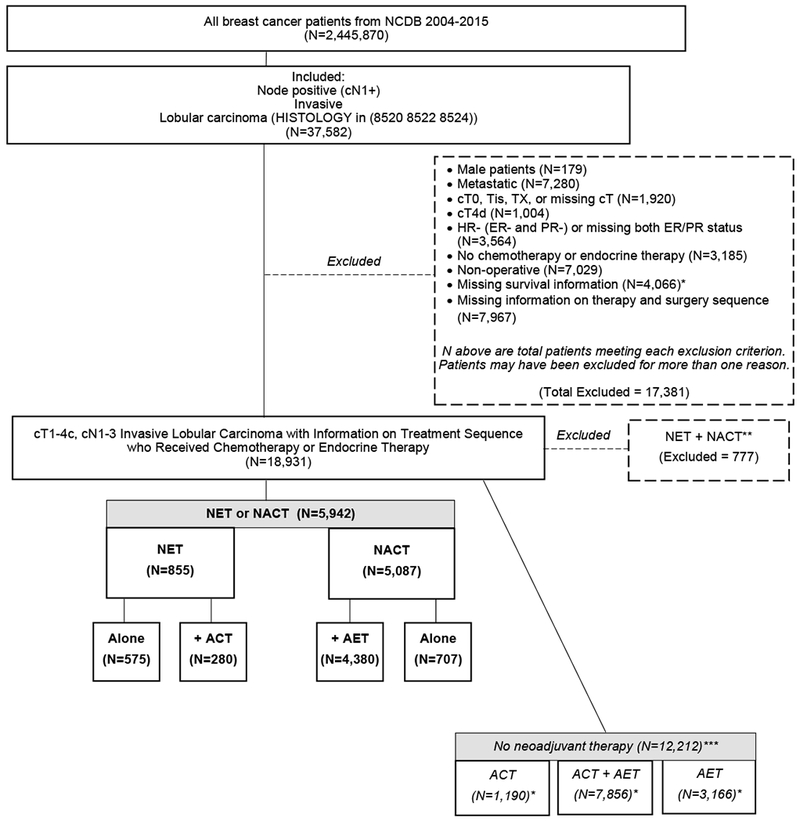
ACT, adjuvant chemotherapy. AET, adjuvant endocr ine therapy. NACT, neoadjuvant chemotherapy. NET, neoadjuva nt endocrine therapy. Boldface indicates inclusion in fina l study cohort (n=5,942). * Includes patients diagnosed in 2015. **Only included in calculation of NACT and NET rates over time (N=18,931). ***Only included in calculation of NACT and NET rates over time (N=18,931) as well as unadjusted overall survival analyses (N=18,154).
Table 1.
Women with cT1-4c, cN1-3, HR+ Invasive Lobular Carcinoma Who Received Neoadjuvant Endocrine Therapy or Chemotherapy, National Cancer Data Base, 2004-2014 (N=5942)
| All patients (N=5942) n (%)a | Neoadjuvant endocrine therapy (N=855) n (%)a | Neoadjuvant chemotherapy (N=5087) n (%)a | P-Valuef | |
|---|---|---|---|---|
| Age (years) | <0.001 | |||
| ≥50 | 3978 (66.9%) | 783 (91.6%) | 3195 (62.8%) | |
| <50 | 1964 (33.1%) | 72 (8.4%) | 1892 (37.2%) | |
| Median (IQR) | 55.0 (47.0-64.0) | 70.0 (59.0-78.0) | 54.0 (46.0-62.0) | <0.001 |
| Race | 0.79 | |||
| White | 5018 (84.4%) | 729 (85.3%) | 4289 (84.3%) | |
| Black | 682 (11.5%) | 97 (11.3%) | 585 (11.5%) | |
| Other | 188 (3.2%) | 24 (2.8%) | 164 (3.2%) | |
| Ethnicity | 0.007 | |||
| Hispanic | 364 (6.1%) | 35 (4.1%) | 329 (6.5%) | |
| Non-Hispanic | 5324 (89.6%) | 783 (91.6%) | 4541 (89.3%) | |
| Charlson-Deyo comorbidity score | <0.001 | |||
| 0 | 5175 (87.1%) | 674 (78.8%) | 4501 (88.5%) | |
| 1 | 638 (10.7%) | 137 (16.0%) | 501 (9.8%) | |
| ≥2 | 129 (2.2%) | 44 (5.1%) | 85 (1.7%) | |
| Clinical T classification | <0.001 | |||
| 1 | 715 (12.0%) | 139 (16.3%) | 576 (11.3%) | |
| 2 | 2254 (37.9%) | 338 (39.5%) | 1916 (37.7%) | |
| 3 | 2344 (39.4%) | 261 (30.5%) | 2083 (40.9%) | |
| 4 | 629 (10.6%) | 117 (13.7%) | 512 (10.1%) | |
| Pathological T classification | <0.001 | |||
| 0 | 184 (3.1%) | 1 (0.1%) | 183 (3.6%) | |
| 1 | 1311 (22.1%) | 137 (16.0%) | 1174 (23.1%) | |
| 2 | 1802 (30.3%) | 337 (39.4%) | 1465 (28.8%) | |
| 3 | 1320 (22.2%) | 220 (25.7%) | 1100 (21.6%) | |
| 4 | 210 (3.5%) | 55 (6.4%) | 155 (3.0%) | |
| X | 947 (15.9%) | 87 (10.2%) | 860 (16.9%) | |
| Clinical N classification | 0.26 | |||
| 1 | 4626 (77.9%) | 683 (79.9%) | 3943 (77.5%) | |
| 2 | 919 (15.5%) | 117 (13.7%) | 802 (15.8%) | |
| 3 | 397 (6.7%) | 55 (6.4%) | 342 (6.7%) | |
| Pathological N classification | <0.001 | |||
| 0 | 753 (12.7%) | 69 (8.1%) | 684 (13.4%) | |
| 1 | 1771 (29.8%) | 266 (31.1%) | 1505 (29.6%) | |
| 2 | 1416 (23.8%) | 208 (24.3%) | 1208 (23.7%) | |
| 3 | 1027 (17.3%) | 193 (22.6%) | 834 (16.4%) | |
| X | 858 (14.4%) | 99 (11.6%) | 759 (14.9%) | |
| Grade | <0.001 | |||
| 1 | 924 (15.6%) | 211 (24.7%) | 713 (14.0%) | |
| 2 | 3209 (54.0%) | 467 (54.6%) | 2742 (53.9%) | |
| 3 | 999 (16.8%) | 77 (9.0%) | 922 (18.1%) | |
| Surgery type | <0.001 | |||
| Lumpectomy | 1143 (19.2%) | 235 (27.5%) | 908 (17.8%) | |
| Mastectomy | 4799 (80.8%) | 620 (72.5%) | 4179 (82.2%) | |
| Median no. of nodes examined (IQR) | 13.0 (8.0-18.0) | 13.0 (7.0-18.0) | 13.0 (8.0-18.0) | 0.07 |
| Median no. of positive nodes (IQR) | 3.0 (1.0-8.0) | 4.0 (1.0-9.0) | 3.0 (1.0-8.0) | <0.001 |
| Treated with radiation post-lumpectomyb | <0.001 | |||
| No | 145 (12.7%) | 63 (26.8%) | 82 (9.0%) | |
| Yes | 998 (87.3%) | 172 (73.2%) | 826 (91.0%) | |
| Treated with radiation post-mastectomyc | <0.001 | |||
| No | 1051 (21.9%) | 248 (40.0%) | 803 (19.2%) | |
| Yes | 3748 (78.1%) | 372 (60.0%) | 3376 (80.8%) | |
| Treated with adjuvant endocrine therapyd | - | |||
| No | 536 (9.0%) | - | 536 (10.5%) | |
| Yes | 4380 (73.7%) | - | 4380 (86.1%) | |
| Treated with adjuvant chemotherapye | - | |||
| No | 563 (9.5%) | 563 (65.8%) | - | |
| Yes | 280 (4.7%) | 280 (32.7%) | - | |
| Year of diagnosis | <0.001 | |||
| 2004 | 247 (4.2%) | 21 (2.5%) | 226 (4.4%) | |
| 2005 | 265 (4.5%) | 21 (2.5%) | 244 (4.8%) | |
| 2006 | 328 (5.5%) | 40 (4.7%) | 288 (5.7%) | |
| 2007 | 392 (6.6%) | 50 (5.8%) | 342 (6.7%) | |
| 2008 | 460 (7.7%) | 57 (6.7%) | 403 (7.9%) | |
| 2009 | 521 (8.8%) | 88 (10.3%) | 433 (8.5%) | |
| 2010 | 685 (11.5%) | 103 (12.0%) | 582 (11.4%) | |
| 2011 | 654 (11.0%) | 121 (14.2%) | 533 (10.5%) | |
| 2012 | 725 (12.2%) | 116 (13.6%) | 609 (12.0%) | |
| 2013 | 762 (12.8%) | 112 (13.1%) | 650 (12.8%) | |
| 2014 | 903 (15.2%) | 126 (14.7%) | 777 (15.3%) | |
| Income level ($) | 0.001 | |||
| <$35,000 | 1429 (24.0%) | 241 (28.2%) | 1188 (23.4%) | |
| ≥$35,000 | 4287 (72.1%) | 574 (67.1%) | 3713 (73.0%) | |
| Insurance status | <0.001 | |||
| Private | 3682 (62.0%) | 256 (29.9%) | 3426 (67.3%) | |
| Government | 1966 (33.1%) | 564 (66.0%) | 1402 (27.6%) | |
| Not Insured | 181 (3.0%) | 15 (1.8%) | 166 (3.3%) | |
| Education level | 0.73 | |||
| >80% High School Graduation Rate | 3789 (63.8%) | 536 (62.7%) | 3253 (63.9%) | |
| ≤80% High School Graduation Rate | 1927 (32.4%) | 279 (32.6%) | 1648 (32.4%) | |
| Facility type | 0.72 | |||
| Academic | 2333 (39.3%) | 334 (39.1%) | 1999 (39.3%) | |
| Integrated Network | 747 (12.6%) | 116 (13.6%) | 631 (12.4%) | |
| Comprehensive | 2450 (41.2%) | 351 (41.1%) | 2099 (41.3%) | |
| Community | 412 (6.9%) | 54 (6.3%) | 358 (7.0%) | |
| Facility location | 0.18 | |||
| Midwest | 1540 (25.9%) | 215 (25.1%) | 1325 (26.0%) | |
| Northeast | 1293 (21.8%) | 199 (23.3%) | 1094 (21.5%) | |
| South | 2224 (37.4%) | 299 (35.0%) | 1925 (37.8%) | |
| West | 885 (14.9%) | 142 (16.6%) | 743 (14.6%) |
Percentages are out of total population counts unless otherwise indicated and may not add up to 100 due to rounding or missing values.
Percentages represent rates of radiation receipt among patients receiving lumpectomy.
Percentages represent rates of radiation receipt among patients receiving mastectomy.
Adjuvant endocrine therapy rates are only summarized for patients who did not receive neoadjuvant endocrine therapy.
Adjuvant chemotherapy rates are only summarized for patients who did not receive neoadjuvant chemotherapy.
P-values for categorical variables are from chi-square tests. P-values from continuous variables are from pooled t-tests.
HR+: hormone receptor-positive. IQR: interquartile range.
As only 72 (8.4%) of the 855 patients who received NET were under 50, we compared NET recipients<50 to NACT recipients<50 (Supplemental Table 3). As compared to women <50 who received NACT (n=1892), NET recipients<50 had more T3 and less T4 disease both at presentation (cT3: NET 47.2% vs NACT 43.0%; cT4: NET 1.4% vs NACT 8.9%, p=0.002) and following systemic therapy (ypT3: NET 27.8% vs NACT 13.2%; ypT4: NET 0% vs NACT 2.4%, p<0.001). Young patients with NET also had higher rates of mastectomy (94.4% vs 85%, p=0.03) and lower rates of post-mastectomy radiation (PMRT, 64.7% vs 81.2%, p<0.001) compared to young patients undergoing NACT. Notably, there was no significant difference in comorbidities between young NET and NACT recipients (p=0.08).
Among NET patients (Supplemental Table 4), patients under 50 (n=72) had more c/ypT3 disease (p<0.01) and higher rates of both mastectomy (94.4% vs 70.5%) and adjuvant chemotherapy (56.9% vs 30.5%, both p<0.001) compared to NET recipients ≥50 (n=783), but there was no difference in rates of PMRT (p=0.40).
Notably, among all non-metastatic LN+ ILC patients who underwent surgery and received all combinations of neoadjuvant and adjuvant endocrine therapy and chemotherapy including both NET and NACT (n=18,931), annual rates of both NET and NACT increased over time (Supplemental Table 5, both p<0.001).
Unadjusted Overall Survival Analyses
10-year unadjusted OS favored patients who received NACT compared to those who received NET (NACT: 57.9%, 95% CI 55.2%-60.5% vs NET: 36%, 95% CI 27.4%-44.6%, p<0.001, Supplemental Figure 1). Among NET patients, those who received ACT had improved survival vs those who did not, and likewise, among NACT patients, those who received AET had improved survival as compared to those who did not take AET (NET+ACT: 53.4%, 95% CI 38.4%-66.2%; NACT+AET: 59.9%, 95% CI 56.8%-62.8%, p<0.001 Supplemental Figure 2). When we examined non-metastatic LN+ILC patients who underwent surgery and received all combinations of neoadjuvant and adjuvant endocrine therapy and chemotherapy except both NACT and NET (n=18,154), we found that patients treated with NET alone (n=575) had worse OS compared to all other groups, with a 10-year survival rate of 26.5% (95% CI 16.1-38.1%) while patients treated with ACT+AET (n=7856) had the best survival (68.4%, 95% CI 66.4%-70.4%, p<0.001, Figure 2).
Fig. 2.
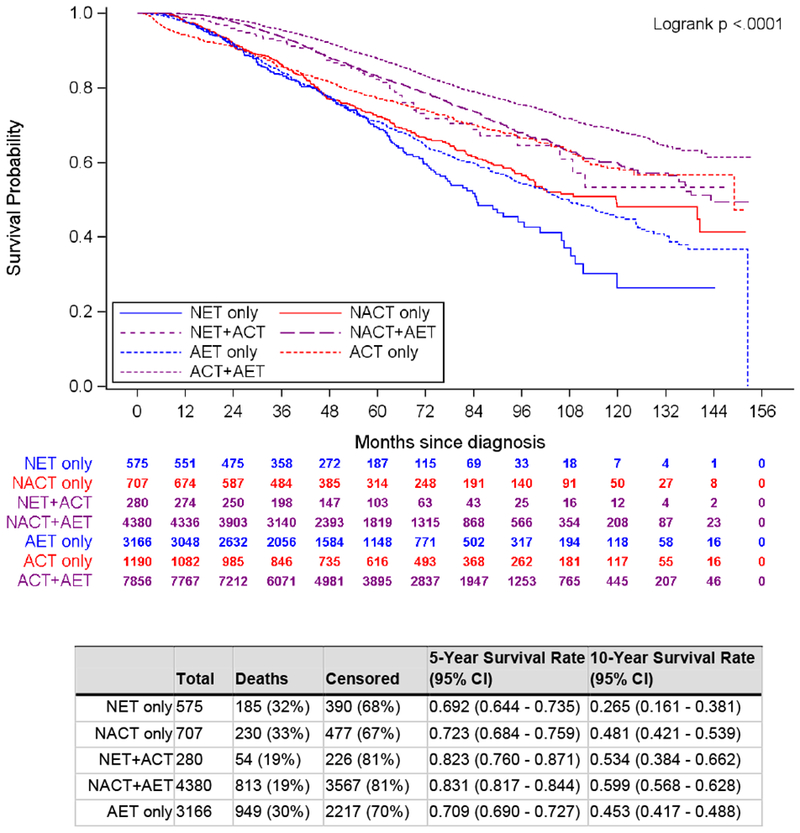
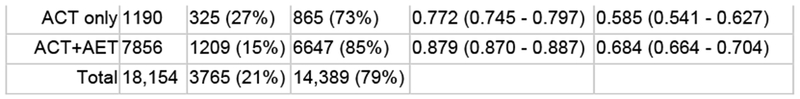
Unadjusted overall survival, women with cT1-4c, cN1-3, HR+ invasive lobular carcinoma who received endocrine therapy or chemotherapy, National Cancer Data Base, 2004–2014 (N = 18,154). ACT adjuvant chemotherapy, AET adjuvant endocrine therapy, NACT neoadjuvant chemotherapy, NET neoadjuvant endocrine therapy
Adjusted Overall Survival Analyses
After adjustment for covariates, there was no significant difference in OS between patients who received NET and those who received NACT (HR 0.84, 95% CI 0.68-1.03, p=0.10, Table 2). Factors associated with worse OS included having government vs private insurance (HR 1.39, 95% CI 1.20-1.61) and having higher grade, pT classification, and pN classification (all p<0.001). Higher CDC scores were also associated with worse OS (vs CDC=0, CDC=1: HR 1.16, 95% CI 0.93-1.43; CDC≥2: HR 1.71, 95% CI 1.19-2.44; p=0.009), suggesting that the inferior survival outcomes observed after NET in our unadjusted analysis may reflect a greater burden of comorbidities among NET recipients. Improved survival was associated with age<50 (HR 0.76, 95% CI 0.65-0.88) and with receipt of both adjuvant radiation (HR 0.77, 95% CI 0.65-0.02) and complementary adjuvant systemic therapy (HR 0.60, 95% CI 0.50-0.72, all p<0.01).
Table 2.
Adjusted Overall Survival, Women with cT1-4c, cN1-3, HR+ Invasive Lobular Carcinoma Who Received Neoadjuvant Endocrine Therapy or Chemotherapy, National Cancer Data Base, 2004-2014 (N=4478)
| HR (95% CI) | P-Value | Overall P-Value | |
|---|---|---|---|
| Treatment group | 0.10 | ||
| Neoadjuvant chemotherapy | REF | ||
| Neoadjuvant endocrine therapy | 0.84 (0.68 - 1.03) | 0.10 | |
| Adjuvant therapya | <0.001 | ||
| No | REF | ||
| Yes | 0.60 (0.50-0.72) | <0.001 | |
| Age (years) | <0.001 | ||
| ≥50 | REF | ||
| <50 | 0.76(0.65-0.88) | <0.001 | |
| Race | 0.05 | ||
| White | REF | ||
| Black | 1.17 (0.95 - 1.45) | 0.14 | |
| Other | 0.61 (0.37 - 1.03) | 0.06 | |
| Ethnicity | 0.01 | ||
| Hispanic | REF | ||
| Non-Hispanic | 1.67 (1.12-2.48) | 0.01 | |
| Charlson/Deyo comorbidity score | 0.009 | ||
| 0 | REF | ||
| 1 | 1.16 (0.93 - 1.43) | 0.18 | |
| ≥2 | 1.71 (1.19-2.44) | 0.003 | |
| Pathological T classification | <0.001 | ||
| 1 | REF | ||
| 0 | 1.78 (1.11 - 2.86) | 0.02 | |
| 2 | 1.33 (1.07 - 1.65) | 0.010 | |
| 3 | 1.54 (1.24-1.92) | <0.001 | |
| 4 | 2.01 (1.47-2.75) | <0.001 | |
| X | 1.30 (0.82-2.04) | 0.26 | |
| Pathological N classification | <0.001 | ||
| 0 | 0.87 (0.65 - 1.15) | 0.33 | |
| 1 | REF | ||
| 2 | 1.51 (1.25 - 1.83) | <0.001 | |
| 3 | 2.75 (2.28-3.32) | <0.001 | |
| X | 0.60 (0.38-0.95) | 0.03 | |
| Grade | <0.001 | ||
| 1 | REF | ||
| 2 | 1.30 (1.08 - 1.57) | 0.006 | |
| 3 | 1.69 (1.35-2.10) | <0.001 | |
| Surgery type | 0.001 | ||
| Lumpectomy | REF | ||
| Mastectomy | 1.37 (1.13 - 1.67) | 0.001 | |
| Treated with radiationb | 0.003 | ||
| No | REF | ||
| Yes | 0.77 (0.65-0.92) | 0.003 | |
| Income level ($) | 0.87 | ||
| <$35,000 | REF | ||
| ≥$35,000 | 0.99 (0.82 - 1.18) | 0.87 | |
| Insurance status | <0.001 | ||
| Private | REF | ||
| Government | 1.39 (1.20 - 1.61) | <0.001 | |
| Not Insured | 1.45 (0.99-2.12) | 0.05 | |
| Education level | 0.06 | ||
| ≤80% High School Graduation Rate | REF | ||
| >80% High School Graduation Rate | 0.85 (0.72 - 1.01) | 0.06 | |
| Facility type | 0.01 | ||
| Academic | REF | ||
| Integrated Network | 1.36 (1.08 - 1.71) | 0.009 | |
| Comprehensive | 1.26 (1.07 - 1.48) | 0.005 | |
| Community | 1.06 (0.81 - 1.39) | 0.66 | |
| Facility location | 0.14 | ||
| South | REF | ||
| Midwest | 1.12 (0.93 - 1.34) | 0.24 | |
| Northeast | 0.97 (0.80 - 1.19) | 0.79 | |
| West | 0.85 (0.67 - 1.07) | 0.16 | |
Hazard ratios, confidence intervals, and p-values are from a Cox proportional hazards model, stratified by year of diagnosis (grouped as 2004-2009 and 2010-2014). A robust sandwich covariance estimator was used to account for correlation of patients treated at the same facility.
Receipt of complementary adjuvant systemic therapy that was not received in neoadjuvant setting (i.e., ACT for patients receiving NET, AET for patients receiving NACT).
Interaction with type of breast surgery received was tested and not significant.
HR: Hazard ratio. CI: Confidence interval.
DISCUSSION
After adjusting for patient and treatment characteristics, we found that OS did not differ between patients with LN+ ILC who received NET and those who received NACT. While many clinical trials, including Z1031, show benefit for NET in ER+ invasive carcinoma, ILC remains underrepresented in these studies. To date, there has been no direct comparison of NET and NACT among patients with LN+ILC. Most of these patients are potentially eligible for chemotherapy, and most will receive adjuvant endocrine therapy, which has been shown to be beneficial in ILC.18–22 Accordingly, it is important to examine which of these two forms of systemic therapy should be prioritized in the treatment sequence of patients who could potentially benefit from receiving both.
Several studies, including ours, suggest that NET is disproportionately administered to older, sicker patients. Notably, a majority of the patients in our study who received NET were ≥50 years old at diagnosis (91.6%). Accordingly, it is unclear what, if any, role NET might play in the care of younger, healthier patients. Furthermore, we must question the benefit of current practice, in which NACT is administered to approximately 1/3 of node-positive ILC patients (Supplemental Table 5), the vast majority of whom are strongly HR+, and traditionally experience less robust responses to NACT than patients with HER2+ and triple-negative tumors.7
The administration of neoadjuvant therapy in patients with resectable, non-metastatic breast cancer has historically been reserved for facilitating breast conservation, downstaging the axilla, and providing information about the effectiveness of chemotherapy on particular breast cancer subtypes. Accordingly, success has been gauged by extent of tumor and nodal downstaging – as evidenced by rates of pCR – or improved opportunity to perform breast-conserving surgery. Given low rates of pCR in both our NET and NACT cohorts, it is important to consider which ILC patients are likely to benefit most from neoadjuvant therapy of any kind. Previous work from our group comparing the impact of neoadjuvant to adjuvant chemotherapy on long-term outcomes in LN+ ILC demonstrated no difference in receipt of mastectomy between these two groups.2,1 In our current study, the overall pCR rate of 2.1% in all ILC patients receiving neoadjuvant therapy supports data from previous studies demonstrating that pCR is not commonly achieved after neoadjuvant therapy in ILC patients.4–6 Thus, although some previous studies have demonstrated higher rates of lumpectomy following NACT and NET in HR+ patients, neither forms of neoadjuvant treatment appear to have had this effect in the patients with lobular histology in our study.
Nevertheless, despite evidence that tumor downstaging is typically less dramatic in patients with ILC as compared to patients with IDC, rates in excess of 50% have been reported.24,25 Furthermore, it is important that we not simply assume that high rates of mastectomy necessarily represent failed attempts to downstage the breast and facilitate BCT; there are many reasons why women choose to undergo mastectomy, even with tumors that are small to begin with or with tumors that shrink significantly with neoadjuvant treatment, including concerns for cosmesis, peace of mind, and a desire to potentially avoid adjuvant radiation.26–28 These reasons are not routinely captured in large databases such as the NCDB, and their potential contribution to overall mastectomy rates in our patient cohort should not be discounted.
Collectively, our findings raise the possibility that the best sequence of treatment for many patients with ILC may involve receipt of all systemic therapy after surgery, but neoadjuvant therapy could still be an important option for particular groups of patients including those who are strongly HR+ and low-grade but unresectable at presentation due to local invasion and those who are unable to proceed with surgery immediately due, for example, to having a second synchronous tumor or anticoagulation that cannot be interrupted. For patients unwilling or unable to receive NACT but who seek to improve candidacy for BCT, pre-treatment counseling regarding NET must include information as to the likelihood of success given available data; these patients must also be counseled that NET may require upwards of 3-4 months for a significant treatment effect to be observed.25 Furthermore, neoadjuvant therapy can play an important role in the “window trial” space as treatment strategies for CDK4/6 inhibitors and other new therapies are developed and continue to evolve.
While our findings also suggest that neoadjuvant therapy may not obviate the need for additional systemic therapy in the adjuvant setting, definitive clinical recommendations cannot be made until results from the ALTERNATE trial assessing need for chemotherapy following response to NET are available.29 The results of this trial will be especially welcome given our finding that rates of both NET and NACT administration have increased over time (Supplemental Table 5), even as our unadjusted survival analyses showed that patients who received all of their chemotherapy and endocrine therapy in the adjuvant setting had the highest OS (Figure 2). While it is important to prioritize adjusted analyses, there was also evidence in a recently published paper from our group that receipt of adjuvant chemotherapy was associated with improved survival in ILC patients as compared to neoadjuvant chemotherapy.23
Molecular genomic testing, such as Oncotype DX and Mammaprint, may be useful in determining which patients may safely avoid chemotherapy altogether; however, further investigation into the predictive and prognostic value of these tests for node-positive invasive lobular cancer is needed to determine how they should be interpreted and incorporated into systemic treatment decisions.30,31 A 2016 study specifically analyzing the prognostic value of MammaPrint in ILC patients demonstrated an association between having a high-risk MammaPrint score and having worse survival after a diagnosis of early-stage ILC.32 Although Mammaprint has also been prospectively validated in node-positive patients, Oncotype DX will not be validated in node-positive patients until results from the RxPonder trial are available.33 Several studies have reported a significant difference between ductal and lobular patients in distribution of Oncotype DX recurrence scores (RS), suggesting that the clinical applicability of the test for ILC patients may be different from that in IDC. Felts et al. found that almost all ILC patients in their cohort fell within the low/intermediate RS categories (97.8%).31 Similarly, in their study of node-negative patients, Kelly et al. reported no ILC patients with a high Oncotype RS: 67.5% of patients were classified as low-risk, and 32.5% were classified as intermediate-risk.34 But as our study on ILC suggests, chemotherapy may still have significant benefit in locally advanced ILC patients, even if some would have been predicted to derive little or no benefit from chemotherapy using current methods and thresholds for genomic testing.
Limitations
There were several limitations to our study. The NCDB does not report breast cancer-specific survival or recurrence rates, thus OS was the only long-term outcome studied. Future studies may consider capturing survival outcomes past the 10-year mark, as there is evidence that recurrence may be a late event in ILC patients.35,36 Although we were unable to determine cause-specific mortality, we were partially able to account for burden of non-malignant disease using the Charlson-Deyo comorbidity score. Our study specifically examined patients with clinically node-positive disease, thus our results may not be applicable to node-negative patients with ILC. Because ILC is often understaged at presentation, we recognize that findings of tumor and axillary upstaging may not truly reflect progression during neoadjuvant therapy but rather underappreciation of extent of disease at presentation.17 Similarly, we recognize that downstaging cannot be equated with successfully enabling BCT, given that a patient’s tumor may shrink significantly enough to allow for BCT without experiencing a change in T classification. The NCDB determines neoadjuvant and adjuvant status based on the start date of therapy as compared to the date of surgery, so we were unable to distinguish patients who received all of their chemotherapy or endocrine therapy in the neoadjuvant setting from patients who received the same treatment in both the neoadjuvant and adjuvant setting (i.e., NACT+ACT or NET+AET, respectively). Finally, we could not account for patients who did not complete recommended treatments, and information on duration of adjuvant chemotherapy or endocrine therapy is also unavailable in the NCDB.
CONCLUSIONS
After adjustment for known covariates, there was no significant difference in overall survival between node-positive ILC patients receiving NET and those receiving NACT. In this patient population, overall survival was improved in patients who received a combination of both endocrine therapy and chemotherapy, but given low rates of in-breast and nodal downstaging following both NET and NACT as well as evidence of improved survival among patients who received all systemic therapy in the adjuvant setting, it may be preferable to defer all systemic therapy to the post-operative setting if patients can safely proceed to up-front surgery. But in patients who are unable to undergo surgery first, NET may represent a safe, less morbid, but, as yet, minimally explored alternative to NACT. While further investigation is warranted, our findings call into question the overall benefit of neoadjuvant systemic therapy for node-positive ILC and point toward the need for large, prospective cohort studies with balanced populations to investigate the optimal inclusion and sequence of endocrine therapy and chemotherapy in the management of locally advanced ILC.
Supplementary Material
Synopsis:
Among patients with node-positive invasive lobular breast cancer, recipients of neoadjuvant endocrine therapy had similar adjusted survival rates to those with neoadjuvant chemotherapy despite having more comorbidities, suggesting an opportunity to reconsider current neoadjuvant treatment utilization in this clinical setting.
Acknowledgements:
Portions of this manuscript were presented as part of a podium presentation at the 2019 Annual Meeting of the American Society of Breast Surgeons (ASBrS) on May 4, 2019. The National Cancer Data Base (NCDB) is a joint project of the American Cancer Society and the Commission on Cancer (CoC) of the American College of Surgeons. Established in 1989, the NCDB is a nationwide, facility-based, comprehensive clinical surveillance resource oncology data set that currently captures ~70% of all newly diagnosed malignancies in the US annually. The data used in this study are derived from a de-identified NCDB Participant User File and have not been verified by the CoC or the American Cancer Society. The American College of Surgeons has established a data use agreement with each of its CoC accredited hospitals; these overhead groups are not responsible for the analytic or statistical methodology employed or the conclusions drawn by the authors.
Funding: Dr. R. Greenup is supported by the National Institutes of Health (NIH) Office of Women’s Research Building Interdisciplinary Research Careers in Women’s Health Award Number K12HD043446 (PI: Andrews). Dr. O. Fayanju is supported by the National Center for Advancing Translational Sciences of the NIH under Award Number 1KL2TR002554 (PI: Svetkey). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Author Disclosures: None
Conflict of Interest: The authors declare that they have no conflicts of interest.
Ethical Approval: This article does not contain any studies with human participants or animals performed by any of the authors. The study used de-identified information and was given exempt status by our Institutional Review Board (IRB).
REFERENCES
- 1.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(5):778–785. [DOI] [PubMed] [Google Scholar]
- 2.Dixon JM, Renshaw L, Dixon J, Thomas J. Invasive lobular carcinoma: response to neoadjuvant letrozole therapy. Breast Cancer Res Treat. 2011;130(3):871–877. [DOI] [PubMed] [Google Scholar]
- 3.Reed AEM, Kutasovic JR, Lakhani SR, Simpson PT. Invasive lobular carcinoma of the breast: morphology, biomarkers and ‘omics. Breast Cancer Res. 2015; 17:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petruolo OA, Pilewskie M, Patil S, et al. Standard Pathologic Features Can Be Used to Identify a Subset of Estrogen Receptor-Positive, HER2 Negative Patients Likely to Benefit from Neoadjuvant Chemotherapy. Ann Surg Oncol. 2017;24(9):2556–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cristofanilli M, Gonzalez-Angulo A, Sneige N, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23(1):41–48. [DOI] [PubMed] [Google Scholar]
- 6.Petrelli F, Barni S. Response to neoadjuvant chemotherapy in ductal compared to lobular carcinoma of the breast: a meta-analysis of published trials including 1,764 lobular breast cancer. Breast Cancer Res Treat. 2013;142(2):227–235. [DOI] [PubMed] [Google Scholar]
- 7.Fayanju OM, Ren Y, Thomas SM, et al. The Clinical Significance of Breast-only and Node-only Pathologic Complete Response (pCR) After Neoadjuvant Chemotherapy (NACT): A Review of 20,000 Breast Cancer Patients in the National Cancer Data Base (NCDB). Annals of surgery. 2018;268(4):591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boughey JC, Wagner J, Garrett BJ, et al. Neoadjuvant chemotherapy in invasive lobular carcinoma may not improve rates of breast conservation. Ann Surg Oncol. 2009;16(6):1606–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lips EH, Mukhtar RA, Yau C, et al. Lobular histology and response to neoadjuvant chemotherapy in invasive breast cancer. Breast Cancer Res Treat. 2012;136(1):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikora MJ, Jankowitz RC, Dabbs DJ, Oesterreich S. Invasive lobular carcinoma of the breast: patient response to systemic endocrine therapy and hormone response in model systems. Steroids. 2013;78(6):568–575. [DOI] [PubMed] [Google Scholar]
- 11.Barbie TU, Ma C, Margenthaler JA. Management of Premenopausal Women with Neoadjuvant Endocrine Therapy: A Single-Institution Experience. Ann Surg Oncol. 2015;22(12):3861–3865. [DOI] [PubMed] [Google Scholar]
- 12.Ellis MJ, Suman VJ, Hoog J, et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype--ACOSOG Z1031. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(17):2342–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiba A, Hoskin TL, Heins CN, Hunt KK, Habermann EB, Boughey JC. Trends in Neoadjuvant Endocrine Therapy Use and Impact on Rates of Breast Conservation in Hormone Receptor-Positive Breast Cancer: A National Cancer Data Base Study. Annals of surgical oncology. 2017;24(2):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barroso-Sousa R, Silva DD, Alessi JV, Mano MS. Neoadjuvant endocrine therapy in breast cancer: current role and future perspectives. Ecancermedicalscience. 2016; 10:609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spring LM, Gupta A, Reynolds KL, et al. Neoadjuvant Endocrine Therapy for Estrogen Receptor-Positive Breast Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2(11):1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Natarajan L, Pu M, Parker BA, et al. Time-varying effects of prognostic factors associated with disease-free survival in breast cancer. American journal of epidemiology. 2009;169(12):1463–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vriens IJH, Keymeulen K, Lobbes MBI, et al. Breast magnetic resonance imaging use in patients undergoing neoadjuvant chemotherapy is associated with less mastectomies in large ductal cancers but not in lobular cancers. European journal of cancer (Oxford, England: 1990). 2017;81:74–80. [DOI] [PubMed] [Google Scholar]
- 18.Rakha EA, El-Sayed ME, Powe DG, et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. Eur J Cancer. 2008;44(1):73–83. [DOI] [PubMed] [Google Scholar]
- 19.Arpino G, Bardou VJ, Clark GM, Elledge RM. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tubiana-Hulin M, Stevens D, Lasry S, et al. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006;17(8):1228–1233. [DOI] [PubMed] [Google Scholar]
- 21.Silverstein MJ, Lewinsky BS, Waisman JR, et al. Infiltrating lobular carcinoma. Is it different from infiltrating duct carcinoma? Cancer. 1994;73(6):1673–1677. [DOI] [PubMed] [Google Scholar]
- 22.Coradini D, Pellizzaro C, Veneroni S, Ventura L, Daidone MG. Infiltrating ductal and lobular breast carcinomas are characterised by different interrelationships among markers related to angiogenesis and hormone dependence. Br J Cancer. 2002;87(10):1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamirisa NP, Vernia H, Thomas SM, et al. The impact of chemotherapy sequence on survival in node-positive invasive lobular carcinoma. Journal of Surgical Oncology. 2019. May 6 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truin W, Vugts G, Roumen RM, et al. Differences in Response and Surgical Management with Neoadjuvant Chemotherapy in Invasive Lobular Versus Ductal Breast Cancer. Ann Surg Oncol. 2016;23(1):51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charehbili A, Fontein DBY, Kroep JR, et al. Neoadjuvant hormonal therapy for endocrine sensitive breast cancer: A systematic review. Cancer Treatment Reviews. 2014;40(1):86–92. [DOI] [PubMed] [Google Scholar]
- 26.Bellavance EC, Kesmodel SB. Decision-Making in the Surgical Treatment of Breast Cancer: Factors Influencing Women’s Choices for Mastectomy and Breast Conserving Surgery. Front Oncol. 2016;6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storm-Dickerson T, Das L, Gabriel A, Gitlin M, Farias J, Macarios D. What Drives Patient Choice: Preferences for Approaches to Surgical Treatments for Breast Cancer Beyond Traditional Clinical Benchmarks. Plast Reconstr Surg Glob Open. 2018;6(4):e1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA. Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol. 2007;25(33):5203–5209. [DOI] [PubMed] [Google Scholar]
- 29.Suman VJ, Ellis MJ, Ma CX. The ALTERNATE trial: assessing a biomarker driven strategy for the treatment of postmenopausal women with ER+/Her2- invasive breast cancer. Chin Clin Oncol. 2015;4(3):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conlon N, Ross DS, Howard J, Catalano JP, Dickler MN, Tan LK. Is There a Role for Oncotype Dx Testing in Invasive Lobular Carcinoma? Breast J. 2015;21(5):514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felts JL, Zhu J, Han B, Smith SJ, Truica CI. An Analysis of Oncotype DX Recurrence Scores and Clinicopathologic Characteristics in Invasive Lobular Breast Cancer. Breast J. 2017;23(6):677–686. [DOI] [PubMed] [Google Scholar]
- 32.Beumer IJ, Persoon M, Witteveen A, et al. Prognostic Value of MammaPrint((R)) in Invasive Lobular Breast Cancer. Biomark Insights. 2016;11:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cardoso F, van’t Veer LJ, Bogaerts J, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375(8):717–729. [DOI] [PubMed] [Google Scholar]
- 34.Kelly CM, Krishnamurthy S, Bianchini G, et al. Utility of oncotype DX risk estimates in clinically intermediate risk hormone receptor-positive, HER2-normal, grade II, lymph node-negative breast cancers. Cancer. 2010;116(22):5161–5167. [DOI] [PubMed] [Google Scholar]
- 35.Anwar IF, Down SK, Rizvi S, et al. Invasive lobular carcinoma of the breast: should this be regarded as a chronic disease? Int J Surg. 2010;8(5):346–352. [DOI] [PubMed] [Google Scholar]
- 36.Pestalozzi BC, Zahrieh D, Mallon E, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26(18):3006–3014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


