Abstract
IgD−CD27− double negative (DN) B cells with pro-inflammatory characteristics are abnormally elevated in a proportion of multiple sclerosis (MS) patients. Here, the origin and selection characteristics of DN B cells were studied in MS patients and healthy controls (HC).
Expression of developmental markers on peripheral blood DN, IgD−CD27+ class-switched memory (CSM) and IgD+CD27− naïve B cells of HC (n = 48) and MS patients (n = 96) was determined by flow cytometry. High-throughput adaptive immune receptor repertoire (AIRR) sequencing was performed on peripheral blood DN and CSM B cells of HC and MS patients (n = 3 each).
DN B cells from HC and MS patients showed similar phenotypic and Ig repertoire characteristics. Phenotypic analysis indicated a mature state of DN B cells by low CD5, CD10 and CD38 expression. However, the frequency of CD95+ and IgA+ cells was lower in DN versus CSM B cells. DN B cells are antigen-experienced, as shown by somatic hypermutation of their Ig genes in AIRR sequencing, although they showed a lower mutation load than CSM B cells. Shared clones were found between DN and CSM B cells, although > 95 % of the clones were unique to each population and differences in V(D)J usage and CDR3 physicochemical properties were found.
Thus, DN B cells arise in HC and MS patients via a common developmental pathway that is probably linked to immune aging. However, DN and CSM B cells develop through unique differentiation pathways with most DN B cells representing an earlier maturation state.
Keywords: double negative B cells, immune aging, multiple sclerosis, adaptive immune receptor repertoire sequencing, antibody repertoire, memory B cells, autoimmunity, flow cytometry
1. Introduction
Multiple sclerosis (MS) is known as an inflammatory autoimmune demyelinating disease of the CNS. Although T cells have been regarded as the principal effectors, an important role of B cells in MS pathogenesis is now widely accepted. B cells are found at sites of tissue injury in the CNS. They are also found in the cerebrospinal fluid (CSF), white matter lesions, gray matter and in the meninges, where they form lymphoid-like tissue aggregates (1) that associate with proximal tissue damage (2). The B cells that populate distinct compartments of the CNS are clonally related (3) and link to populations in the periphery (4). Furthermore, they are responsible for the production of the oligoclonal Ig bands (OCB) in the CSF that remain a hallmark of the disease (5, 6). The strongest evidence supporting their role comes from B cell depleting therapy, which demonstrates remarkable efficacy in relapsing-remitting (RR) MS and even primary progressive (PP) MS (7–9). These collective findings notwithstanding, the mechanisms underpinning the pathogenic contribution of B cells require further understanding.
Abnormalities in specific subsets of the B cell lineage have been increasingly identified in autoimmune diseases, including MS (10). One such B cell subset is the age-associated IgD−CD27− double negative (DN) B cell. We recently reported an increased proportion of young MS patients < 60 years old (20 % of subjects) with increased peripheral frequencies of DN B cells compared with age-matched healthy controls (HC, 3 % of subjects) (11). The proportion of DN B cells was also increased in MS CSF. Their potential to induce (pro-inflammatory) T cell responses was indicated by expression of MHC-II, CD80 and CD86 (11). Further, DN B cells produced pro-inflammatory and cytotoxic cytokines following ex vivo stimulation. These results, together with the finding of clonal relations between Ig class-switched DN B cells in the peripheral blood of MS patients and intrathecal Ig repertoires (4), point towards the possible involvement of DN B cells in MS pathogenesis. However, the origin and developmental pathway of DN B cells in MS patients remain unknown.
DN B cells are elevated in aged individuals (11, 12), in rotavirus (13) and HIV infection (14) and in several autoimmune diseases such as systemic lupus erythematosus (SLE) (15, 16) and rheumatoid arthritis (RA) (17, 18). In SLE, their frequency was associated with more severe disease status and increased titers of disease-specific autoantibodies (15, 19), indicating that they may contribute to autoimmune pathology. DN B cells show similarities with the recently described CD21lowCD11c+T-bet+ age-associated B cells (ABCs) in aged and autoimmune mice and autoimmune individuals (20–22). Further, DN B cells constitute a heterogeneous population of IgG+, IgA+ and IgM+ isotypes (11, 15, 17, 23). They resemble IgD−CD27+ class-switched memory (CSM) B cells in their shortened telomeres (12), their expression of somatically mutated Ig H chain V region genes (15) and their inability to extrude rhodamine or similar dyes (15) due to the lack of the transmembrane protein ATP-binding-cassette-B1 (ABCB1) transporter expression (12). The absence of ABCB1 expression was previously indicated as a characteristic of CSM B cells compared with CD27− B cells (24). There furthermore appears to be a clonal relationship between DN and CD27+ memory B cells in HC (23). In addition, DN B cells demonstrated a decreased Ig H chain mutation frequency compared with CSM B cells (17, 23, 25, 26).
In this study, we further investigated the origin and selection characteristics of DN B cells in MS patients and HC. First, we determined the expression of several Ig isotype and developmental markers on peripheral blood DN, naïve and CSM B cells of MS patients and HC using flow cytometry. Next, we examined the H and L chain Ig repertoire of both DN and IgD−CD27+ memory B cells of MS patients and HC using high-throughput adaptive immune receptor repertoire (AIRR) sequencing. This analysis focused on clonality, V region segment usage, mutational profiles and CDR3 physicochemical properties.
2. Materials and methods
2.1. Study subjects
The study was approved by both the Human Research Protection Program at Yale School of Medicine and Hasselt University Commissions of Medical Ethics. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. MS patients were recruited at the Rehabilitation & MS-Center (Pelt, Belgium) or Hospital Ramón y Cajal (Madrid, Spain) and were diagnosed according to the McDonald criteria (27). HC were recruited at Hasselt University (Hasselt, Belgium). Samples were cryopreserved at the University Biobank Limburg.
For flow cytometry, peripheral blood was collected from 63 RRMS patients, 20 secondary progressive (SP) MS patients, 13 PPMS patients and 48 HC (Table I). All PPMS patients and 54 RRMS patients were treatment-naïve, while 9 RRMS patients were previously being treated with first-line MS therapy. Of the SPMS patients, 17 were untreated and 3 were treated with IFN-β within 6 months prior to sampling. For AIRR sequencing, peripheral blood was collected from 5 untreated RRMS patients and 4 HC (Table II) representing the earliest subject enrollment of the study. Of the MS patients, 4 were treatment-naïve and 1 was untreated for at least 3 months after a short IFN-β treatment regimen.
Table I.
Characteristics of MS patients and HC for flow cytometry
| Number | Agea | Gender % F | EDSSb | Previous treatmentc | % DN B cellsd | |
|---|---|---|---|---|---|---|
| HC | 48 | 45.7±15.6 | 68.8 % | N.A. | N.A. | 4.4±2.0 |
| MS total | 96 | 44.1±13.4 | 72.9 % | 3.4±2.2 | UT: 84, IFN: 9, GA: 2, TF: 1 | 6.1±3.8 |
| RRMS | 63 | 38.8±11.6 | 69.8 % | 2.3±1.4 | UT: 54, IFN: 6, GA: 2, TF: 1 | 6.1±3.5 |
| PPMS | 13 | 52.1±8.7 | 61.5 % | 5.4±2.0 | UT: 13 | 6.0±4.0 |
| SPMS | 20 | 55.9±11 | 90.0 % | 5.8±1.6 | UT: 17, IFN: 3 | 5.9±4.5 |
in years, presented as mean ± SD
presented as mean ± SD
all previous treatments are shown for RRMS and PPMS patients, previous treatments ≤ 6 months prior to sampling are shown for SPMS patients
within CD19+ B cells, presented as mean ± SD; Abbreviations: HC, healthy controls; MS, multiple sclerosis; RR, relapsing-remitting; PP, primary progressive; SP, secondary progressive; F, female; EDSS, expanded disability status scale; N.A., not applicable; UT, untreated; IFN, interferon-β; GA, glatiramer acetate, TF: teriflunomide
Table II.
Characteristics of MS patients and HC for AIRR sequencing
| Subject ID | Disease state | Age (years) | Gender | EDSS | Treatment | Disease duration | % DN B cellsa | NGS |
|---|---|---|---|---|---|---|---|---|
| MS | ||||||||
| MS085 | RRMS | 49 | F | 5.0 | None | 23 y | 3.4 | + |
| MS469 | RRMS | 32 | F | 2.5 | None | < 1 y | 4.2 | − |
| MS495 | RRMS | 20 | F | 1.5 | None | < 1 y | 7.0 | − |
| MS537 | RRMS | 41 | F | 4.5 | None | < 1 y | 2.5 | + |
| MS560 | RRMS | 23 | F | 6.0 | None | 1.5 y | 4.3 | + |
| HC | ||||||||
| HC024 | / | 54 | F | / | / | / | 2.1 | + |
| HC038 | / | 24 | F | / | / | / | 4.9 | + |
| HC209 | / | 23 | F | / | / | / | 2.2 | − |
| HC263 | / | 39 | F | / | / | / | 3 | + |
within CD19+ B cells; Abbreviations: RRMS, relapsing-remitting multiple sclerosis; F, female; EDSS, expanded disability status scale; NGS, next-generation sequencing
All RRMS patients were younger than 60 years in order to exclude findings related to aging. Individuals older than 60 years were included in the PPMS and SPMS groups as the progressive forms of MS are rare in younger individuals. HC reported no history of autoimmune disease or malignancies and were matched to MS patients as closely as possible with regard to age and gender. Relevant demographic and clinical data (Table I–II) were collected from all study subjects.
2.2. Cell isolation
PBMC were isolated from whole blood by Ficoll density gradient centrifugation (Lympholyte; Cedarlane Laboratories, SanBio B.V., Uden, The Netherlands). Cryopreserved PBMC were used for flow cytometry. After thawing, PBMC were immediately recovered in 20% FBS in RPMI 1640 (Lonza, Basel, Switzerland) and 0.5 mg/ml DNAse. For AIRR sequencing, fresh samples were used except for MS469, MS495 and HC209. B cells were purified from the PBMC using negative magnetic selection (STEMCELL Technologies SARL, Grenoble, France) according to the manufacturer’s instructions. Purity of the isolated B cells was routinely ≥ 99.5% as determined by flow cytometry on a FACSAria II flow cytometer (BD Biosciences, Erembodegem, Belgium) following the staining procedure prior to sorting described below.
2.3. Flow cytometry
DN B cells were analyzed using anti-human CD19 Brilliant Violet (BV)421 (clone HIB19), IgM PerCP-Cy5.5 (clone MHM-88), IgD PE-Cy7 (clone IA6–2), CD95 PE-Dazzle594 (clone DX2), CD5 BV605 (clone L17F12), CD38 BV711 (clone HIT2), CD20 allophycocyanin (APC)-Cy7 (clone 2H7) (all from Biolegend, London, United Kingdom), CD27 APC (clone M-T271), IgG FITC (clone G18–145), CD10 BV786 (clone H110a) (all from BD Biosciences) and IgA PE (clone IS118E10) (Miltenyi Biotec, Leiden, The Netherlands). Viable cells were selected using the Fixable Viability Dye eFluor 506 (eBioscience, San Diego, USA). Fluorescence minus one (FMO) controls were used for gating. The gating strategy is depicted in Supplemental Fig. 1A. All flow cytometry was performed on a LSRFortessa flow cytometer (BD Biosciences) and analysis was executed using FACSDiva (BD Biosciences) or FlowJo software (FlowJo, LLC, Oregon, USA). The cut-off to identify donors with expanded DN B cells was the mean percentage of DN B cells from healthy donors < 60 years plus 2 times the SD (cut-off: 7 %).
2.4. Sorting
For the isolation of DN and IgD−CD27+ memory B cells, enriched B cells were stained with anti-human CD27 APC (clone M-T271), IgD PE-CF594 (clone IA6–2) (both from BD Biosciences) and CD19 PE-Cy7 (clone HIB19; BioLegend) during 30 min at 4°C. At least 2 × 104 DN or IgD−CD27+ memory B cells were then sorted on a FACSAria II flow cytometer (BD Biosciences) according to the gating strategy shown in Supplemental Fig. 1C. Sorted cells were pelleted or suspended in RNAlater® Stabilization Solution (ThermoFisher Scientific, Erembodegem, Belgium) before storage at −80°C.
2.5. RNA isolation and BCR library preparation
Total RNA was extracted from the sorted cells with RNeasy kits (QIAGEN, Germantown MD) according to the manufacturer’s protocol. Amplicons for sequencing on the Illumina MiSEQ platform were synthesized using reagents and a protocol provided by the manufacturer (New England Biolabs, Ipswich MA). Briefly, RNA was reverse-transcribed into cDNA using a biotinylated oligo dT primer. An adaptor sequence, containing a universal priming site and a 17-nucleotide unique molecular identifier (UMI), was added to the 3’ end of all cDNA. Following purification using streptavidin-coated magnetic beads, PCR was performed to enrich for Ig sequences using a pool of primers targeting the IGHA, IGHD, IGHE, IGHG, IGHM, IGKC and IGLC regions. This Ig-specific primer pool contained tailed sequences with a priming site for a secondary PCR step. The second primer was specific to the adaptor sequence added during the RT step, and contained a sample index for downstream pooling of samples prior to sequencing. Following purification of PCR products using AMPure XP beads, the secondary PCR was performed in order to add the full-length Illumina P5 Adaptor sequence to the end of each Ig amplicon. Final products were purified using AMPure XP beads, then quantified with a TapeStation (Agilent Genomics, Santa Clara CA).
2.6. BCR sequencing and data analysis
Raw read quality control and assembly was performed as we have previously described with pRESTO version 0.5.2.999–2017.01.18 (28). For each sequence, germline segments were identified with IMGT/HighV-QUEST (http://imgt.org) using the February 2, 2017 version of the site (29). Subsequent analyses on the annotated sequences were performed using the immcantation tool suite (http://www.immcantation.org) version 1.8.0, which contains Change-O (version 0.3.9.999–2018.01.15) (30), alakazam (version 0.2.10) and shazam (version 0.1.9). Non-functional sequences, as defined by IMGT, as well as sequences for which the constant region primer and the V or J gene calls were inconsistent (suggesting a chimera) were excluded from further analysis. Sequences with more than 20 Ns distributed in more than 15 intervals of Ns in the V region were removed. Duplicated sequences were collapsed. For each subject, sequences were assigned into clones using the DefineClones imgt function of Change-O by single-linkage clustering excluding sequences with non-ACGT characters in the junction (--maxmiss 0) and requiring the same junction region length, common IGHV and IGHJ gene annotations and a maximum length normalized nucleotide Hamming distance of 0.19 (--model ham –dist 0.19) between their junction regions (31). For each clone, a germline sequence was reconstructed from the alignment data masking the N/P nucleotides and D-segments of the junction. Mutation frequencies for the V region were calculated as the number of mismatches from the germline sequence. Sequencing data were deposited in the Sequence Read Archive (https://www.ncbi.nlm.nih.gov/sra) under BioProject accession PRJNA429427. Processed AIRR sequencing data from naïve B cell sorts of HC were obtained from a previously published study (32) to be used as a reference control.
2.7. Statistical analysis
For flow cytometry, statistical analyses were performed using Graphpad Prism version 8. Multiple groups were compared by Kruskal-Wallis with Tukey post-hoc testing. B cell subsets were compared within one study group using the Friedman test with Dunn’s multiple comparisons correction and between two study groups using Mann-Whitney U test. Fisher’s exact test was used for differences in proportions. For AIRR sequencing, statistics was done using the two-sided t-Test, using the paired version when comparing HC DN B cells versus HC IgD−CD27+ memory B cells or MS DN B cells versus MS IgD−CD27+ memory B cells. The Benjamini-Hochberg False Discovery Rate (FDR) was used to correct for multiple hypothesis testing. To assess the significance of the D frame usage between populations, a sign test was used.
3. Results
3.1. DN B cells resemble CSM B cells in the expression of developmental surface markers
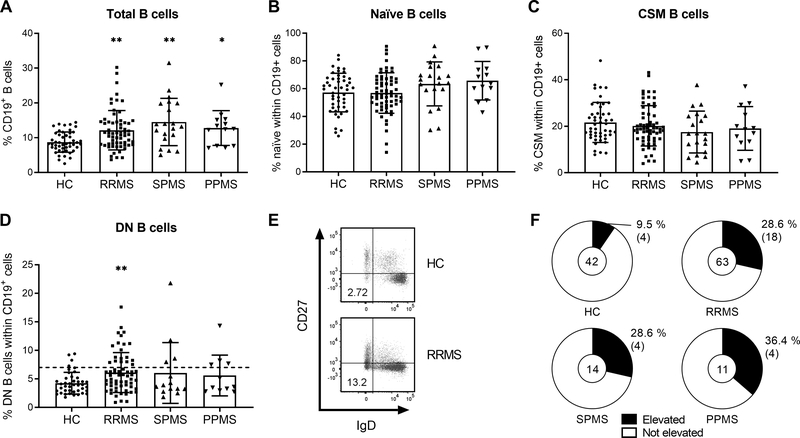
First, frequencies of IgD+CD27− naïve B cells, IgD−CD27+ CSM B cells and IgD−CD27− DN B cells were measured in the peripheral blood of HC (n = 48), RRMS patients (n = 63), SPMS patients (n = 20) and PPMS patients (n = 13). The frequency of total B cells within the PBMC population was significantly increased in all MS patient groups when compared to HC (p = 0.004 RRMS; p = 0.002 SPMS; p = 0.04 PPMS; Fig 1A). Frequencies of naïve and CSM B cells were similar between HC and MS patient groups (Fig. 1B–C). Interestingly, the frequency of DN B cells was significantly elevated in the peripheral blood of RRMS patients (6.1 ± 3.5 %) when compared with HC (4.3 ± 1.9 %; p = 0.004), all younger than 60 years (Fig. 1D). Moreover, the proportion of RRMS patients (18/63; 28.6 %) and PPMS patients (4/11; 36.4 %) younger than 60 years who presented with increased peripheral blood frequencies of DN B cells (> 7 % of CD19+ B cells, Fig. 1E) was significantly elevated compared with age-matched HC (4/42; 9.5 %; p = 0.027 and p = 0.048, respectively; Fig. 1F). In total, 26/88 (29.5 %) MS patients younger than 60 years showed an elevated frequency of DN B cells in the peripheral blood.
Figure 1. Frequency of B cell subsets in the peripheral blood of HC and MS patients.
(A-C) The percentage of total CD19+ B cells (A), IgD+CD27− naïve B cells (B) and IgD−CD27+ CSM B cells (C) in the peripheral blood of HC (n = 48), RRMS (n = 63), SPMS (n = 20) and PPMS (n = 13) patients. Mean (bars) ± SD is depicted. (D) The percentage of IgD−CD27− DN B cells in the peripheral blood of HC (n = 42), RRMS (n = 63), SPMS (n = 14) and PPMS (n = 11) patients younger than 60 years. Black dashed line represents the cut-off. Mean (bars) ± SD is depicted. (E) Representative plots of a HC without and a RRMS patient with an elevated frequency of DN B cells. (F) The percentage of individuals with and without an elevated DN B cell frequency (> 7 % of CD19+ B cells) in HC, RRMS, SPMS and PPMS patients younger than 60 years. Central oval “n” indicates the number of individuals examined. The percentage and number ( ) of individuals with an increased DN B cell frequency are depicted. * p < 0.05, ** p < 0.01
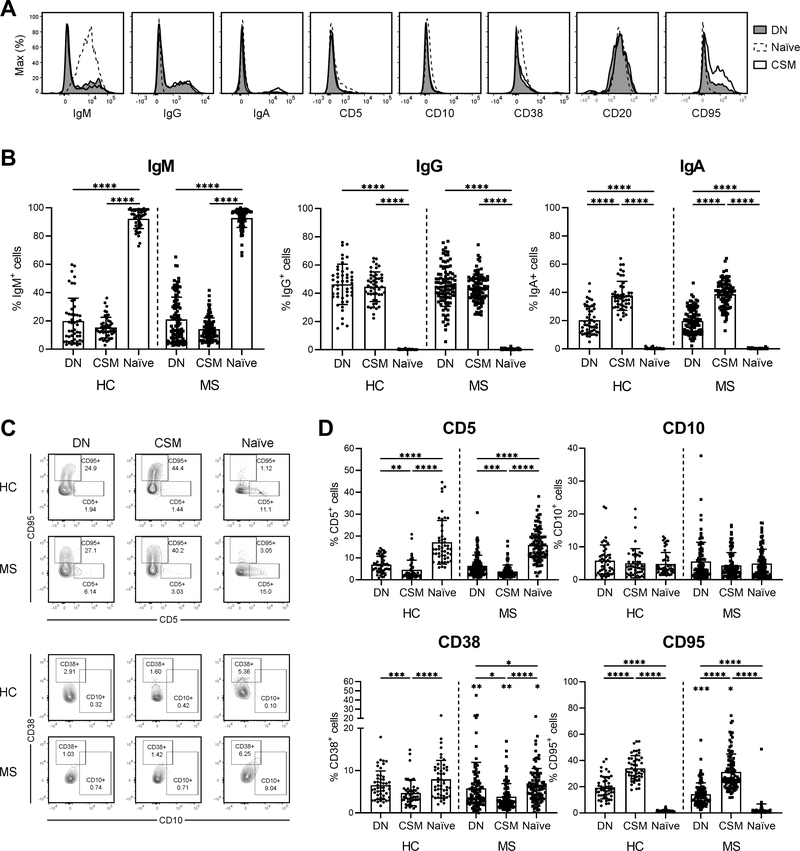
To evaluate the expression of well-described B cell developmental markers, we measured the distribution of Ig isotypes and the developmental markers CD5, CD10, CD38, CD20 and CD95 on naïve, CSM and DN B cells (Fig. 2A). In all B cell subsets, > 93 % of the cells were CD20+. As there was no statistically significant difference in the distribution of Ig isotypes in DN, CSM or naïve B cells between HC, RRMS, SPMS and PPMS patients (Supplemental Fig. 1B), B cell subsets were compared between HC and total MS patients. On average, the largest proportion of DN B cells was IgG+ in both HC and MS patients (46.4 ± 14.3 % and 44.6 ± 14.2 %, respectively), followed by an equal proportion of IgM+ cells (20 ± 16.1 % and 21.5 ± 15.9 %, respectively) and IgA+ cells (20.3 ± 9.8 % and 19.8 ± 8.9 %, respectively) (Fig. 2B). Nevertheless, a high inter-donor variability was observed in the frequency of isotype-specific DN and CSM B cells. DN B cells resembled CSM B cells in the frequency of IgM+ and IgG+ cells, although the frequency of IgA+ cells was significantly lower in the DN versus CSM B cell population, both for HC and MS patients (p < 0.0001).
Figure 2. Phenotype of DN B cells in the peripheral blood of HC and MS patients.
(A) Representative surface staining of MS B cells for the indicated markers. DN B cells (shaded) are compared to naïve B cells (dashed line) and CSM B cells (solid line). (B) Percentages of IgM+, IgG+ and IgA+ cells within the DN, CSM and naïve B cell subsets in the peripheral blood of HC (n = 48) and MS patients (n = 96). Mean (bars) ± SD is depicted. (C) Representative flow cytometry from a HC and MS patient. The expression pattern of CD95 versus CD5 and CD38 versus CD10 is shown for DN, CSM and naïve B cells. (D) Percentages of CD5+, CD10+, CD38+ and CD95+ cells within the DN, CSM and naïve B cell subsets in the peripheral blood of HC (n = 48) and MS patients (n = 96). The significance levels shown without bars indicate differences in 1 B cell subset between HC and MS patients. Mean (bars) ± SD is depicted. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
In addition, DN B cells resembled CSM B cells in the expression of CD5, CD95, CD10 and CD38 (Fig. 2C), although quantitative analyses indicated several differences. The frequency of CD5+, CD38+ and CD95+ cells in the DN B cell population was in between that of CSM and naïve B cells (Fig. 2D). In this regard, naïve B cells demonstrated the highest frequency of CD5+ and CD38+ cells while CSM B cells presented with the highest frequency of CD95+ cells. In general, CD38+ and CD95+ cells were less frequent in all included B cell subsets from MS patients when compared to HC (Fig. 2D). No difference was observed in the frequency of CD10+ cells between DN, CSM and naïve B cells.
Thus, DN B cells resemble CSM B cells in Ig and developmental marker expression.
3.2. IgM+, IgG+ and IgA+ DN B cells differ in the expression of developmental markers
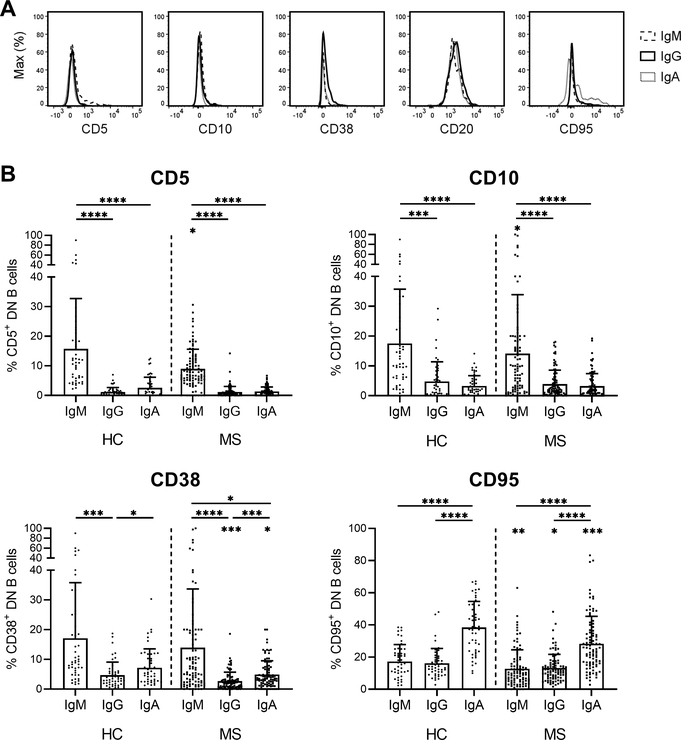
Differences in the expression of the developmental markers CD5, CD10, CD38 and CD95 were observed between IgM+, IgG+ and IgA+ DN B cells (Fig. 3A). IgM+ DN B cells differed from both IgG+ and IgA+ DN B cells in their significantly increased frequency of CD5+, CD10+ or CD38+ cells (Fig. 3B). In this regard, IgM+ DN B cells resembled naïve B cells (Fig. 2D). IgM+ and IgG+ DN B cells showed a similar frequency of CD95+ cells, which was significantly lower than that of IgA+ DN B cells (p < 0.0001, Fig. 3B). The frequency of CD95+ cells within the IgA+ DN B cell population (Fig. 3B) was comparable to that of the CSM B cell population (Fig. 2D). IgG+ DN B cells further differed from IgA+ DN B cells in the frequency of CD38+ cells, which was significantly lower for IgG+ DN B cells (p = 0.02 HC and p = 0.0002 MS).
Figure 3. Phenotype of IgM+, IgG+ and IgA+ DN B cells in the peripheral blood of HC and MS patients.
(A) Representative surface staining of MS DN B cells for the indicated markers. IgM+ DN B cells (dashed line), IgG+ DN B cells (solid line) and IgA+ DN B cells (dotted line) are depicted. (B) Percentages of CD5+, CD10+, CD38+ and CD95+ cells within IgM+, IgG+ and IgA+ DN B cells in the peripheral blood of HC (n = 48) and MS patients (n = 96). Mean (bars) ± SD is depicted. The significance levels shown without bars indicate differences in 1 B cell subset between HC and MS patients. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001
When comparing DN B cells of HC and MS patients, HC presented with a higher frequency of CD5+ (p = 0.03), CD10+ (p = 0.04) and CD95+ (p = 0.002) IgM+ DN B cells (Fig. 3B). Furthermore, higher frequencies of CD38+ and CD95+ IgG+ DN B cells (p = 0.0002 and p = 0.02, respectively) and IgA+ DN B cells (p = 0.03 and p = 0.0002, respectively) were observed in HC compared with MS patients (Fig. 3B).
Together, these data demonstrate that IgM+ DN B cells have the closest resemblance to naïve B cells while IgA+ DN B cells are most similar to CSM B cells considering the expression of developmental surface markers.
3.3. B cell subset isolation and repertoire overview following AIRR sequencing
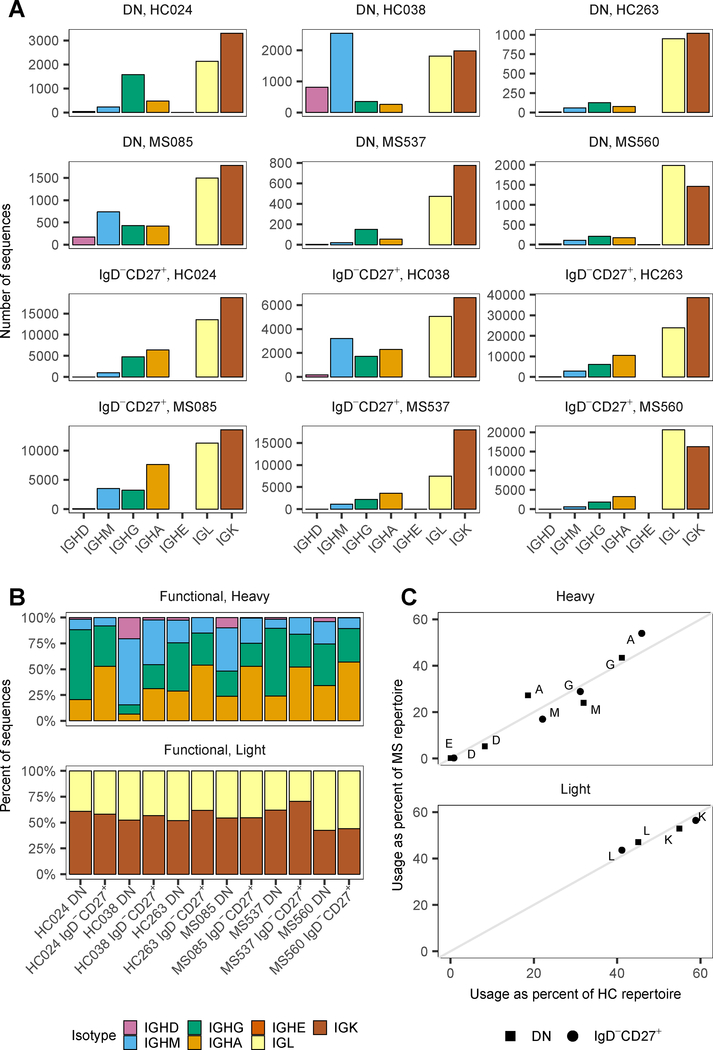
To further look into the developmental pathways of DN B cells, we performed AIRR sequencing of DN B cells and compared their Ig repertoires to those of IgD−CD27+ memory (CSM) B cells. B cell subpopulations of IgD−CD27− DN and IgD−CD27+ memory B cells were sorted from PBMC of HC (n = 4) and RRMS patients (n = 5) (Table II, Supplemental Fig. 1C). DN B cells were again elevated in the MS group relative to the HC group, although not statistically significant (Supplemental Fig 1D), due to the limited sample size. We performed deep sequencing of the Ig repertoire of sorted DN and IgD−CD27+ memory B cells in MS patients (n = 5) and HC (n = 4). Specimens from 2 MS patients and 1 HC were excluded from this analysis due to low RNA quality, which can adversely affect accurate representation of the repertoire diversity. Therefore, sequencing of the VH and VL from sorted B cell subsets from MS patients (n = 3) and HC (n = 3) produced a total of 10,427,119 raw reads. After quality control and processing, a high-fidelity data set was generated that comprised 75,098 unique error-corrected H chain sequences and 212,846 unique error-corrected IgL and IgK sequences (Table III). As expected, the number of sequences from the DN population was lower than that of the CSM (Table III), however in several instances the DN/CSM sequence ratio was lower than that measured by flow cytometry (Supplemental Fig. 1D). This difference may be a consequence of several variables that affect sequencing output, such as RNA quality and non-linear sequence recovery. Focusing on unique sequences equalizes the contribution of cells expressing different levels of mRNA, but does not differentiate between BCR sequences from different B cells expressing identical receptors. It represents a sampling of the total cell population that can be used to estimate Ig repertoire features as described below.
Table III.
Sequencing processing results
| Subject ID | B cell subtype | Input cells | Raw reads | Final analyzed unique sequencesa | |
|---|---|---|---|---|---|
| H chain | L chain | ||||
| MS | |||||
| MS085 | |||||
| DN | 20,553 | 949,032 | 1,760 | 3,277 | |
| IgD−CD27+ | 196,358 | 1,691,899 | 14,384 | 24,785 | |
| MS537 | |||||
| DN | 30,025 | 472,136 | 229 | 1,248 | |
| IgD−CD27+ | 433,266 | 480,386 | 6,922 | 25,499 | |
| MS560 | |||||
| DN | 111,100 | 696,888 | 517 | 3,447 | |
| IgD−CD27+ | 747,620 | 696,237 | 5,716 | 36,886 | |
| HC | |||||
| HC024 | |||||
| DN | 33,600 | 934,434 | 2,330 | 5,439 | |
| IgD−CD27+ | 238,000 | 565,745 | 12,134 | 32,330 | |
| HC038 | |||||
| DN | 51,517 | 471,249 | 3,971 | 3,794 | |
| IgD−CD27+ | 154,643 | 594,295 | 7,405 | 11,680 | |
| HC263 | |||||
| DN | 42,304 | 1,298,653 | 270 | 1,968 | |
| IgD−CD27+ | 370,125 | 1,576,165 | 19,460 | 62,493 | |
Refers to the count of unique, error-corrected sequences which passes all quality control and filtering steps
The DN VH repertoire was composed of 21.7 [8.7, 64.1] % IgM, 43.6 [9.0, 67.8] % IgG and 23.9 [6.5, 33.9] % IgA sequences, while the IgD−CD27+ memory VH repertoire consisted of 15.5 [8.1, 43.3] % IgM, 31.3 [22.4, 39.1] % IgG and 52.1 [31.1, 56.9] % IgA sequences (Fig. 4). A significantly higher proportion of IgA sequences was present in the IgD−CD27+ memory compared to the DN B cell repertoire of MS patients (p = 0.005 and FDR = 0.067) and HC (p = 0.008 and FDR = 0.067). For the L chain, IgK made up 53.3 [42.4, 62.1] % of the DN and 57.4 [44.1, 70.6] % of the IgD−CD27+ memory B cell repertoire, followed by IgL in 46.7 [37.9, 57.6] % of the DN and 42.6 [29.4, 55.9] % of the IgD−CD27+ repertoire. No significant differences were observed between HC and MS patients in terms of isotype usage. Comparing DN and IgD−CD27+ memory B cells, the only difference was an increased proportion of IgA+ sequences in the IgD−CD27+ memory B cell population.
Figure 4. Repertoire size and isotype composition of DN and IgD−CD27+ memory B cells.
(A) Number of sequences of the different isotypes in each sample. (B) Isotype composition of the repertoire expressed as percent of sequences of each isotype. (C) Comparison of isotype usage in healthy and MS repertoires. Each dot represents the mean of the isotype frequency (top panel) or kappa (K) and lambda (L) usage in the DN or IgD−CD27+ B cell population.
3.4. Donor-specific clonal expansion in DN B cells of HC and MS patients
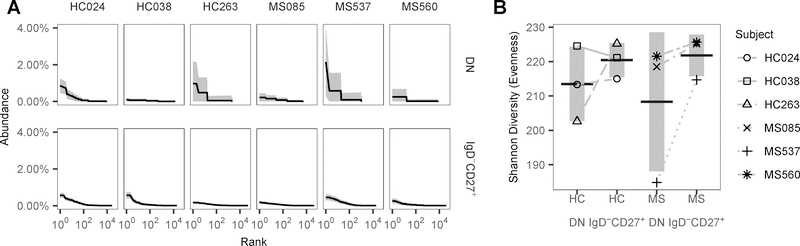
To test whether the DN and IgD−CD27+ B cell repertoires of MS patients and HC were characterized by conspicuous clonal expansions, sequences were clustered into clonal groups, with each member of the group representing one unique variant (including variants that differed only by isotype). In the IgD−CD27+ memory B cell population, expanded clones were identified in all MS patients and HC but even the most abundant clones did not represent more than 1 % of the repertoire (Fig. 5A). DN B cells, however, showed a small number of expanded clones in 1 out of 3 MS patients and 1 out of 3 HC. The largest identified clone size was 2 % of the repertoire. Repertoire diversity of IgD−CD27+ and DN B cells was further explored by comparison of the evenness of both B cell populations. Evenness quantifies the extent to which an abundance distribution deviates from a uniform distribution. Diversity was higher in the IgD−CD27+ B cell samples when compared with DN B cells in both HC and MS patients, although this was not statistically significant (Fig. 5B).
Figure 5. Clone size and diversity of DN and IgD−CD27+ memory B cells in HC and MS patients.
(A) The rank-abundance distribution of DN and IgD−CD27+ memory VH clones with clone size (y-axis) as a percent of the repertoire against the size rank of the clone on a log10 scale (x-axis). Each dark line represents the estimated clonal abundance curve, with shaded areas representing 95 % confidence intervals derived via bootstrap (2000 realizations). (B) Evenness at diversity order q = 1 (Shannon Diversity) of the VH DN and IgD−CD27+ compartment clone size distributions. Each point represents the estimated evenness score for a subject from the clonal abundance distributions in (A). The vertical shading represents the SD of the mean evenness scores and the horizontal bar represents the mean of the mean evenness scores.
Overall, repertoire diversity was not significantly different between the IgD−CD27+ and DN B cell populations. Nevertheless, in some donors belonging to both the HC and MS groups, DN B cells displayed more expansion and less repertoire diversity suggesting that DN B cell oligoclonality could be donor-specific.
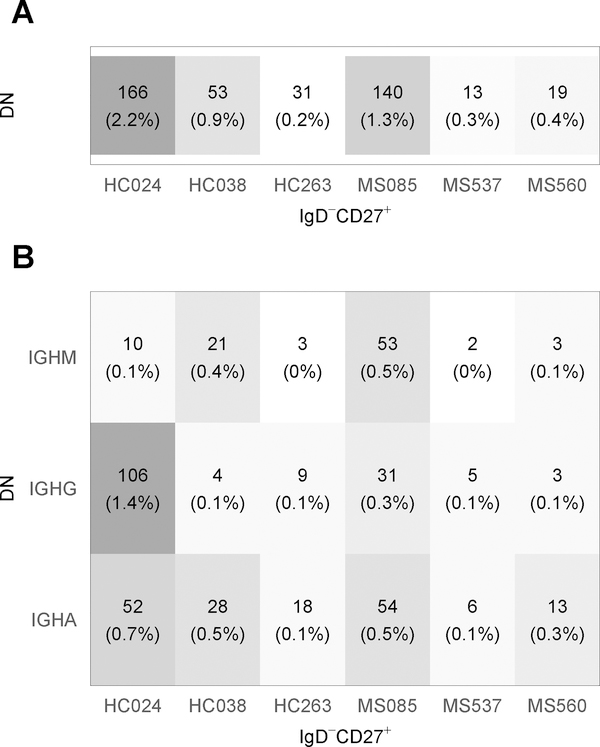
3.5. Clonal overlap between DN and IgD−CD27+ memory B cells
We next examined whether DN B cells are related to IgD−CD27+ memory B cells by analysis of clonal overlap between both populations. Focusing on the H chain locus, overlap of IgD−CD27+ memory B cell clones with DN B cell clones ranged between 0.2 and 2.2 % in HC and between 0.3 and 1.3 % in MS patients (Fig. 6A). Interestingly, all isotypes of DN B cell sequences showed a connection to the IgD−CD27+ memory B cell compartment, although IgD−CD27+ memory B cell clones more frequently contained IgA+ DN B cell sequences than IgM+ DN B cell sequences (Fig. 6B, Supplemental Fig. 2). Thus, a small fraction of clones connected the DN and IgD−CD27+ memory B cell populations although overall, these populations were clonally distinct.
Figure 6. Clone overlap between DN and IgD−CD27+ memory B cells in HC and MS patients.
(A) H chain clone overlap across samples of the same subject. The number in the cells shows the number of unique clones shared between the two samples of the same subject. The percent is relative to the number of clones in IgD−CD27+. (B) Clone overlap by DN B cell H chain isotype, not considering the IgD−CD27+ memory B cell isotypes.
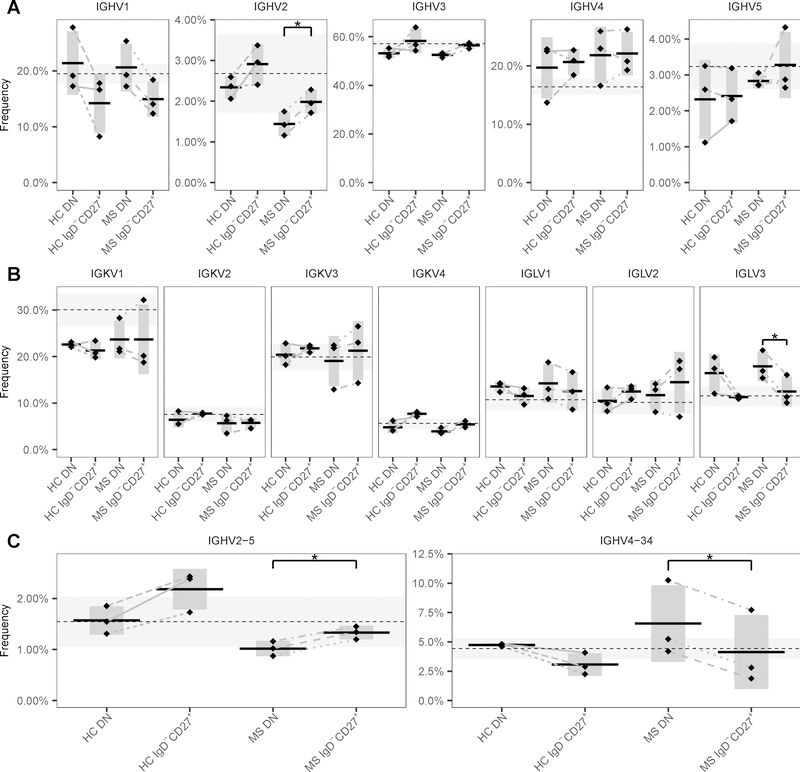
3.6. DN B cells show differential V(D)J gene usage
Biased usage of IGHV, IGHD or IGHJ genes has been demonstrated in the B cells present in some autoimmune diseases including those present in the CNS of MS patients (3, 33–36). Therefore, the V(D)J family and gene usage was compared between DN and IgD−CD27+ memory B cells of HC and MS patients. While the relative rankings of gene usage frequencies in both subsets were similar to naïve B cells from a set of HC (Fig. 7), significant differences were apparent between them. IGHV2 family (p = 1.460×10−4 and FDR = 0.011) sequences were decreased in DN B cells (Fig. 7A) while IGLV3 (p = 2.810×10−4 and FDR = 0.028) was increased (Fig. 7B) when compared to IgD−CD27+ memory B cells of MS patients. Similar results were observed in DN B cells of HC, although not statistically significant. Frequencies of IGHV6 and IGHV7 family usage did not exceed 1.5 % (not shown). No significant differences were present in IGHD and IGHJ family usage between DN and IgD−CD27+ memory B cells of HC and MS patients (not shown). When considering V(D)J gene usage (Fig 7C), DN B cells of MS patients showed decreased usage of IGHV2–5 (p = 0.001 and FDR = 0.163) and increased usage of IGHV4–34 (p = 0.001 and FDR = 0.163) genes when compared to IgD−CD27+ memory B cells. No significant differences in V(D)J family or gene usage were found between HC and MS patients.
Figure 7. H and L chain V family and gene usage of DN and IgD−CD27+ memory B cells of HC and MS patients.
IGHV (A), IGKV and IGLV (B) family usage is depicted for the DN and IgD−CD27+ memory B cell compartments. (C) V gene segment usage is shown for DN and IgD−CD27+ memory B cells. Usage is shown as a percent of the total unique IGHV, IGKV or IGLV sequences (y-axis) for HC and MS patients. Horizontal bars indicate the mean abundance over all subjects of a given status and compartment, while vertical shading indicates ± 1 SD about the mean. As a reference, gene usage in naïve B cells from HC is indicated by the dashed lines (mean) and horizontal gray shaded areas (± 1 SD). Families IGHV6 and IGHV7 are not shown. * FDR < 0.20 and p < 0.05
We next focused on IgM+ and IgG+ DN B cells separately. In MS patients, IgM+ DN B cells showed significantly higher usage of IGHV1 compared with IgM+ IgD−CD27+ memory B cells (p = 7.503×10−5 and FDR = 0.033, Supplemental Fig. 3A). Interestingly, IGHV4–34 gene usage was non-significantly elevated in IgM+ DN B cells compared to IgG+ DN B cells of HC and also in 2 out of 3 MS patients (Supplemental Fig. 3B).
Thus, (IgM+) DN and IgD−CD27+ memory B cells showed differences in V(D)J family and gene usage both in HC and MS patients.
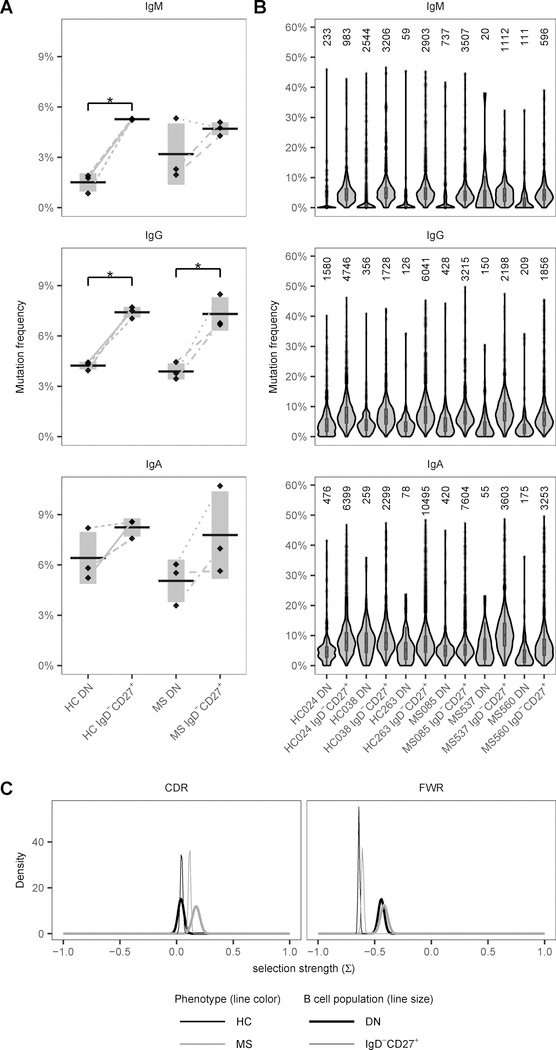
3.7. Decreased mutation loads in DN B cell BCR sequences
Somatic hypermutation (SHM) is associated with affinity maturation during the germinal center reaction where B cells are exposed to antigen and T cell help, and undergo multiple rounds of proliferation. Our data demonstrated SHM in both DN and IgD−CD27+ memory B cell sequences (Fig. 8). However, mean mutation loads of DN B cells were significantly lower than those of IgD−CD27+ memory B cells both in the IgM+ (2 % and 5 %, respectively, p = 0.008 and FDR = 0.056) and IgG+ (4 % and 7 %, respectively, p = 0.009 and FDR = 0.056) isotype in HC (Fig. 8A). In MS patients, the same SHM pattern was observed in IgG+ sequences (p = 0.041 and FDR = 0.165). The higher SHM in IgD−CD27+ memory B cell sequences was consistent in all tested subjects except one (MS537, which had a very low number of sequences, Fig. 8B). There was no difference in IGHV mutation frequency of DN or IgD−CD27+ memory B cells between HC and MS patients.
Figure 8. Mutational load of DN and IgD−CD27+ memory B cells of HC and MS patients.
(A) Distribution of the mean mutation frequency for the V region of IgM+, IgG+ and IgA+ sequences of the DN and IgD-CD27+ compartments is shown per HC or MS status. Mutation frequency for each sequence was calculated as the number of base changes from germline in the V region. Horizontal bars indicate the mean of the mean mutation rates with the vertical shading indicating ± 1 SD about the mean of means. (B) Distribution of the mutation frequency in the V region for the IgM, IgG and IgA isotypes is shown in the individual HC and MS patients. The number on top of the violin plots is the number of sequences in each sample. (C) BASELINe probability density functions (PDFs) from selection analysis are shown for DN and IgD-CD27+ repertoires of HC and MS patients, with density shown on the y-axis and the selection strength (∑) shown on the x-axis. PDFs for each status were determined via convolution of the individual PDFs for subjects within each status group, resulting in a single aggregate PDF for each status. * FDR < 0.20 and p < 0.05
Antigen-selected Ig sequences usually have a higher frequency of mutations that lead to the replacement of amino acids in the CDR and a low frequency of such mutations in the framework region (FWR) that confers structural stability (37, 38). As expected, both DN and IgD−CD27+ memory B cells showed negative selection in the FWR and neutral to positive selection in the CDR according to BASELINe analysis of mutation patterns (Fig. 8C) (39).
The mutational load within clonal populations was also examined. Although we found that IgD−CD27+ memory B cells were in general more mutated than DN B cells, this was not the case within clones that included members from both subsets (Supplemental Fig. 4). The average mutation frequency was similar when comparing sequences in the same clone spanning the DN and IgD−CD27+ compartments in both HC and MS patients. Clones exclusive of the DN B cells showed the lowest average clonal mutation frequency (Supplemental Fig. 4).
Collectively, these results indicate that DN B cells are antigen-experienced cells but differ from IgD−CD27+ memory B cells when considering mutation load, except for clonally related DN and IgD−CD27+ memory B cells.
3.8. DN B cells differ from IgD−CD27+ memory B cells in CDR3 amino acid sequence physicochemical properties
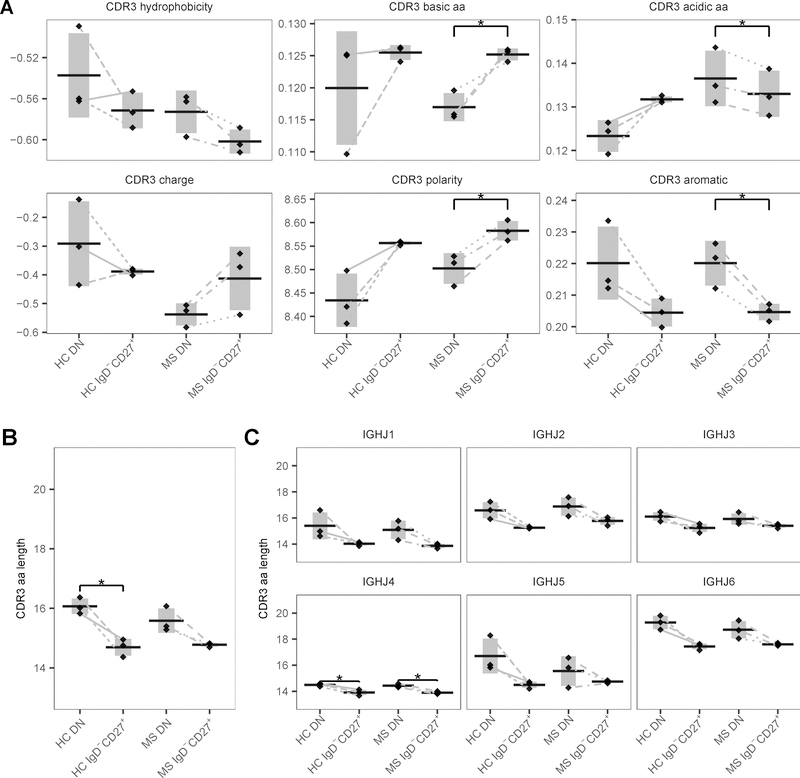
The hypervariable region CDR3 makes major contributions to B cell repertoire diversity as it is generated by random selection and recombination of V, D and J gene segments in the H chain. Consequently, the physicochemical properties of CDR3 can reflect the function of the repertoire. Differences in CDR3 H chain properties are found between different B cell subsets with smaller, more hydrophilic and more basic CDR3 for memory B cells compared to naïve B cells (40). Longer CDR length, hydrophobicity and positive charge of antibodies have been associated with autoimmunity (40–43). Here, differences were observed in polarity (p = 0.013 and FDR = 0.05), aromatic (p = 0.027 and FDR = 0.109), basic (p = 0.048 and FDR = 0.194) and acidic (p = 0.039 and FDR = 0.101) amino acid residue content between DN and IgD−CD27+ memory B cells of MS patients (Fig. 9A). DN B cells showed longer CDR3 (p = 0.032 and FDR = 0.128) in HC than IgD−CD27+ memory B cells (Fig. 9B). As DN B cell sequences showed a non-significant higher usage of the longer IGHJ6 family genes (not shown), CDR3 length was analyzed in DN B cell sequences utilizing the different IGHJ families. However, CDR3 regions of DN B cells were longer than that of IgD−CD27+ memory B cells irrespective of the contiguous IGHJ family used (Fig. 9C).
Figure 9. CDR3 physicochemical properties of DN and IgD−CD27+ memory B cells of HC and MS patients.
H-CDR3 mean physicochemical properties for each subject are shown as a single point for both the DN and IgD−CD27+ memory B cell populations of HC and MS patients. Horizontal bars indicate the mean of the mean property scores with the vertical shading indicating ± 1 SD about the mean of means. (A-B) CDR3 hydrophobicity, basic aa, acidic aa, aromatic aa, charge, polarity and aa length are depicted. (C) CDR3 aa length is shown for the different IGHJ families. * FDR < 0.20 and p < 0.05
We next considered CDR3 properties for different isotypes (Supplemental Fig. 3C). For HC, IgM+ B cells showed longer CDR3 in DN B cells compared to IgD−CD27+ memory B cells (p = 0.017 and FDR = 0.185). In MS, IgG+ B cells showed longer CDR3 in DN B cells compared to IgD−CD27+ memory B cells (p = 0.04 and FDR = 0.185). Further, the acidic and aromatic residue content was significantly higher for IgG+ DN B cells compared with IgG+ IgD−CD27+ memory B cells of MS patients (p = 0.005 and FDR = 0.114; p = 0.01 and FDR = 0.16, respectively). For HC, mean side-chain bulkiness was significantly higher for IgM+ DN B cells compared with IgD−CD27+ memory B cells (p = 0.004 and FDR = 0.048). When comparing IgM+ and IgG+ DN B cells, IgM+ DN B cells displayed a higher CDR3 charge compared to IgG+ DN B cells of HC (p = 0.003 and FDR = 0.081) (Supplemental Fig. 3C). No differences were observed in DN B cells between HC and MS patients for any of the CDR3 physicochemical properties. Thus, CDR3 sequences of DN B cells differ in length, side-chain bulkiness, acidic and aromatic residue content from those of IgD−CD27+ memory B cells. IgM+ DN B cells further differ from IgG+ DN B cells in CDR3 charge.
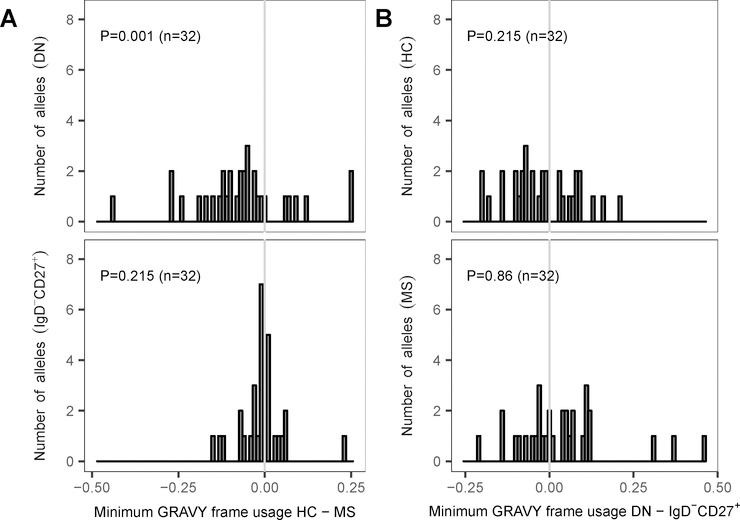
As part of the H chain CDR3, the D segment is positioned in the center of the antigen binding site. D segments can be used in three different reading frames because of the addition and deletion of non-templated nucleotides at the junctions between the recombining gene segments. Hydrophobic reading frames were previously reported to favor antibody self-reactivity and are normally counter-selected in B cells from HC (44). For each D allele germline sequence in IMGT, we identified the D reading frames with the highest and lowest GRAVY or hydropathy index to examine whether these were utilized differently between DN and IgD−CD27+ memory B cells in HC and MS patients. When analyzing the distribution of the frame usage difference between HC and MS for all the alleles, MS samples showed an increased usage of the frame with lower GRAVY index for DN B cells (p = 0.001, sign test), but not IgD−CD27+ memory B cells (Fig. 10A). D frame usage associated with hydrophobicity was similar for DN and IgD−CD27+ memory B cells in both HC and MS patients (Fig. 10B). Thus, the DN B cell population tended to utilize D frames with a lower GRAVY index in MS patients compared to HC.
Figure 10. D reading frame usage based on the GRAVY index.
For each sequence in the experiment data, we determined if it was using the D segment reading frame that encoded for the lowest GRAVY index in the reference germline. For each subject and set of DN B cells and IgD−CD27+ sequences, the frequency of usage of the minimum GRAVY frame for each D allele was calculated as the number of positive events over the total number of calls of the D allele. (A) For DN and IgD−CD27+ memory B cells, mean usages for HC and MS patients were retrieved for each allele and these values were used to calculate differences HC minus MS. The figure shows the distribution of the difference in mean usage of the minimum GRAVY frame for all the alleles (n = 32), in the DN and IgD−CD27+ memory B cell compartments. (B) A similar comparison method was used to explore differences between DN and IgD−CD27+ memory B cells of HC (top) and MS patients (bottom). The figure shows the distribution of the difference (DN minus IgD−CD27+) in mean usage of the minimum GRAVY frame for all the alleles in HC (top) and MS (bottom).
4. Discussion
In this study, we report the absence of major differences in both the developmental marker expression and the Ig repertoires of DN B cells in HC and MS patients. This leads us to suggest that these cells have a similar origin in both cohorts. Elevated frequencies of DN B cells could be triggered because of the aging process that is common in the immune system of the elderly (45) and occurs prematurely in a proportion of MS patients (46). Aging of the immune system is accompanied by a low grade chronic inflammation, termed “inflammaging”, that is characterized by an increase in pro-inflammatory cytokines (IL-6, IL-15, IL-8), an increase in other inflammatory mediators such as coagulation factors and sub-clinical infections with common viruses (47). This chronic inflammatory environment combined with genetic predisposition could result in the expansion of DN B cells in aged individuals and a proportion of MS patients.
Flow cytometric analysis confirmed the finding of abnormal elevations in the frequency of DN B cells in the peripheral blood of MS patients. The proportion of MS patients (29.5 %) and HC (9.5 %) younger than 60 years with an elevated frequency of DN B cells was higher compared to our previous study (20 % MS, 3 % HC) (11), which could be explained by the higher number of included individuals in the current study.
The isotype distribution of DN B cells from HC and MS patients was similar in our flow cytometry and AIRR sequencing analyses. IgG+ cells made up the largest proportion of DN B cells, which is in agreement with our previous results (11) and with studies in aged healthy individuals (12, 48) and RA patients (17). The finding of a significantly lower frequency of IgA+ cells and sequences in DN versus CSM B cells is also in line with findings in healthy individuals (26) and RA patients (17). It has previously been suggested that IgA+CD27− and IgA+CD27+ memory B cells are generated via separate response pathways due to molecular differences between these cell populations (49). Thereby, IgA+CD27− B cells demonstrated a germinal center-independent origin and were suggested to be a blood counterpart of IgA+ cells from the gut lamina propria. Another report, however, indicated a high level of clonal relationships between the IgA+CD27− and IgA+CD27+ B cell pool with only few clonal relationships between germinal center-independent IgD+CD27+ B cells and IgA+CD27− B cells (50).
DN B cells are mature antigen-experienced B cells with an expression profile of developmental markers more similar to CSM than naïve B cells. Their mature state was demonstrated by the low expression of CD38 and the immature markers CD5 and CD10. Previously, the majority of DN B cells in SLE patients were also shown to be CD5− and CD10− (19). The absence of CD38 is further characteristic of CD21lowCD11c+T-bet+ age-associated B cells (21, 51) and of DN B cells in SLE (16). The most prominent difference between DN and CSM B cells, however, was the lower mutation load of DN B cell Ig repertoires in HC and MS patients. This is in agreement with previous reports in HC (23, 25, 26, 48), SLE (16, 19) and RA patients (17). Overall, DN and CSM B cell populations were clonally distinct with differences in V(D)J family and gene usage, which could indicate differential activation pathways or differences in the antigens driving the response. Furthermore, the intermediate frequency of CD5+, CD38+ and CD95+ cells in the DN B cell population between that of the naïve and CSM B cell population could point to an intermediate developmental state. Our results are in line with a previous report of increased CD95 transcripts in CD27+ versus CD27− memory B cells in a gene expression profile analysis (52). Nevertheless, a small fraction of clonally related cells with similar mutation loads was demonstrated in DN and IgD−CD27+ memory B cells. Therefore, part of DN B cells could be exhausted memory B cells that have lost CD27 expression because of chronic antigen stimulation, as previously suggested (48, 53). Taken together, our results indicate that the majority of DN B cells show earlier maturation features compared to CSM B cells and are formed via differential activation pathways while a small part of DN B cells are clonally related to CSM B cells and could be exhausted memory B cells.
IgG+ and IgA+ DN B cells displayed several characteristics of memory B cells, including the absence of CD5 and CD10 surface expression, a class-switched state and SHM. Given that the Ig mutation frequency was lower in class-switched CD27− versus CD27+ B cells and that isotype switching occurs before SHM during the germinal center response (54), we propose that class-switched DN B cells are involved in (primary) T cell dependent immune responses that prematurely exit the germinal center response. In line with this, a similar number of cell divisions and Ig mutation level was reported for DN and germinal center B cells (49). In addition, the lower mutation load of DN B cells could be due to counterselection mechanisms. AIRR sequencing provided some suggestive data for autoreactivity of a subset of the DN B cell population. IGHV4–34 gene segments were increased in DN B cells of MS patients when compared with IgD−CD27+ memory B cells. Previous reports demonstrated the expression of 9G4 (VH4–34), which encodes intrinsically autoreactive B cells and antibodies, by DN B cells in SLE (15), and by B cells and plasma cells in MS brain and CSF (33–35, 55, 56). The longer CDR3 length of DN B cells compared to IgD−CD27+ memory B cells could further point to autoreactivity of a proportion of DN B cells (40, 41, 43). Contradictory to these postulations is the tendency of DN B cells and IgD−CD27+ memory B cells of MS patients to select for D frames with a lower hydrophobicity when compared with those of HC. Hydrophobic D reading frames have previously been described to favor antibody self-reactivity, although low patient numbers were used (44).
IgG+ DN B cells could further be discriminated from IgA+ DN B cells due to their lower frequency of CD38+ and CD95+ cells, and thus their decreased activation status. Although IgA+ DN B cell sequences showed the highest clonal overlap with IgD−CD27+ memory B cells, overall, these populations were clonally distinct. It thus remains unclear whether IgA+ DN B cells represent germinal center-independent or -dependent cells.
IgM+ DN B cells showed similarity to naïve B cells in their frequency of CD5+, CD10+ and CD38+ cells. Although there was no significant difference in Ig mutation load of IgM+ versus IgG+ DN B cells, the lower frequency trend we observed in IgM+ was consistent with previous findings (23). The increased IGHV4–34 usage in IgM+ versus IgG+ DN B cells was not significant, but the trend we observed could be explained by the negative selection that naïve VH4–34+ B cells tend to undergo, which results in their underrepresentation in the memory compartment (57). Further, naïve B cells were previously reported to express more negatively charged H-CDR3s than memory B cells (32). The increased CDR3 charge of IgM+ versus IgG+ DN B cells of HC could therefore point to an alternative developmental pathway. IgM+ DN B cells could display characteristics of IgD+CD27+ NCSM B cells which have been termed “innate-like” B cells as they develop independent of germinal centers and can respond to T cell independent antigens (58). This is in line with the described repertoire similarity of IgD−IgM+CD27− or IgD−IgM+CD27+ memory B cells and IgD+CD27+ B cells (23). Nevertheless, a proportion of the IgM+ DN B cells showed clonal overlap with IgD−CD27+ memory B cells. These results lead us to suggest that part of the IgM+ DN B cells could be precursors of CD27+ memory B cells in T cell-dependent immune responses. They could be involved in the extrafollicular B cell response that produces an initial burst of low-affinity antibodies early after B cell activation. This response can occur with or without T cell help and does not induce SHM (59).
A strength of this study is the combination of protein expression of developmental surface markers and Ig repertoire analysis to study the origin and selection characteristics of DN B cells in HC and MS patients. Results obtained in the flow cytometry analysis were further explored in the AIRR sequencing. Although our Ig repertoire dataset, which included 287,944 unique sequences, was large, the number of included HC and MS patients was limited. Inclusion of a larger cohort of MS patients, including subjects that represent the different clinical subtypes, is necessary to study the selection and differentiation mechanisms of DN B cells in relation to disease subtype and severity, age and response to treatment.
DN B cells of MS patients resemble DN B cells described in SLE and RA patients in several characteristics: surface expression of IgM, IgG, CD10, CD38 (15, 60), Ig mutation load (15, 16) and expression of VH4–34 encoded antibodies (16). In RA, the distribution of IgM+, IgG+ and IgA+ cells within the DN B cell population differed from our results (17, 18), although IgG again was the predominant isotype (17). Positive correlations between DN B cell frequency and disease activity or autoantibody titers have been demonstrated in SLE (15), but not in RA (18), and remain to be determined in MS. In RA, DN B cells correlated with a clinical response to IL-6 receptor inhibition therapy, namely tocilizumab (17). It remains unknown whether DN B cells resemble the T-bet driven CD21low B cells. CD21low B cells in mice are activated by TLR7/9 stimulation in combination with IFN-γ and IL-21, which can be amplified by BCR ligation (61, 62). Therefore, it was proposed that antigens with a capacity to engage TLRs, including viral particles containing nucleic acids or self antigens associated with RNA or DNA, are able to drive T-bet expression in B cells in which the BCR could play a role by amplifying T-bet expression through delivery of nucleic acids containing antigens (61, 62). Recently, DN B cell expansions in SLE were assigned to a subset of CXCR5−CD21−CD11c+T-bet+ cells (16). These “DN2” cells differentiated into autoantibody producing plasma cells driven by TLR7, which led to their characterization as extra-follicular B cells responding to innate stimuli. As our previous study indicated, about 20% of DN B cells could be retraced to the CD21lowCD11c+ B cell gate (11), we expect that the majority of DN B cells in MS patients are not DN2 cells. This could be explained by the difference in pathological mechanisms in SLE and MS that center around antibody-mediated and cell-mediated immune mechanisms, respectively. Moreover, a subset of DN B cells with cytoplasmic FOXO1, a transcription factor involved in B cell development, was found to be increased in SLE patients and positively correlated with disease activity (60). Whether this DN B cell subset corresponds with DN2 cells is not clear. Involvement of the T-bet driven pathway in the abnormal elevations of DN B cells in MS remains to be determined.
In conclusion, developmental surface marker expression and Ig repertoire characteristics suggest that DN B cells share a common developmental pathway in HC and MS patients that leads to expansion of these cells because of “inflammaging”. We postulate that DN B cells consist of B cells with different origins dependent on the isotype. IgG+ DN B cells are more related to CSM B cells while IgM+ DN B cells are more similar to naïve and non-class switched IgD+CD27+ memory B cells. Further study is warranted to examine the downstream pathways that lead to expansion of DN B cells in a proportion of MS patients and whether DN B cells play a role in MS pathology.
Supplementary Material
Key points.
DN B cells are abnormally elevated in MS patients but have the same origin as in HC
DN B cells resemble CSM B cells but appear to be at an earlier maturation state
DN and CSM B cells undergo unique differentiation pathways
5. Acknowledgments
The authors thank Igna Rutten, Kim Ulenaers (Hasselt University and UBiLim, Hasselt) and Anita Knevels (Revalidation & MS Center, Pelt) for patient recruitment and sample collection. The authors also thank Drs. Song Chen and Eileen Dimalanta (New England Biolabs, Massachusetts) for providing technical guidance during the B cell sequencing library production.
This work was supported by the National Multiple Sclerosis Society through a Grant to KCO, under award number PP-1509–06370 and the Belgian Charcot Foundation. JF is a postdoctoral fellow of the Fund for Scientific Research, Flanders. SHK and SM were funded in part by National Institutes of Health (NIH) award number R01AI104739.
2. Abbreviations used in this manuscript
- AIRR
adaptive immune receptor repertoire
- CSF
cerebrospinal fluid
- CSM
class-switched memory
- DN
double negative
- FWR
framework region
- HC
healthy controls
- MS
multiple sclerosis
- PPMS
primary progressive MS
- RA
rheumatoid arthritis
- RRMS
relapsing-remitting MS
- SHM
somatic hypermutation
- SLE
systemic lupus erythematosus
- SPMS
secondary progressive MS
Footnotes
6 Conflict of interests/financial disclosures
The authors have no conflicts to report.
8 References
- 1.Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, and Aloisi F. 2004. Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol 14: 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howell OW, Reeves CA, Nicholas R, Carassiti D, Radotra B, Gentleman SM, Serafini B, Aloisi F, Roncaroli F, Magliozzi R, and Reynolds R. 2011. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 134: 2755–2771. [DOI] [PubMed] [Google Scholar]
- 3.Stern JN, Yaari G, Vander Heiden JA, Church G, Donahue WF, Hintzen RQ, Huttner AJ, Laman JD, Nagra RM, Nylander A, Pitt D, Ramanan S, Siddiqui BA, Vigneault F, Kleinstein SH, Hafler DA, and O’Connor KC. 2014. B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med 6: 248ra107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palanichamy A, Apeltsin L, Kuo TC, Sirota M, Wang S, Pitts SJ, Sundar PD, Telman D, Zhao LZ, Derstine M, Abounasr A, Hauser SL, and von Budingen HC. 2014. Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci. Transl. Med 6: 248ra106.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabat EA, Glusman M, and Knaub V. 1948. Quantitative estimation of the albumin and gamma globulin in normal and pathologic cerebrospinal fluid by immunochemical methods. Am J Med 4: 653–662. [DOI] [PubMed] [Google Scholar]
- 6.Obermeier B, Mentele R, Malotka J, Kellermann J, Kumpfel T, Wekerle H, Lottspeich F, Hohlfeld R, and Dornmair K. 2008. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med 14: 688–693. [DOI] [PubMed] [Google Scholar]
- 7.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, and Group HT. 2008. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 358: 676–688. [DOI] [PubMed] [Google Scholar]
- 8.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, Traboulsee A, Wolinsky JS, Arnold DL, Klingelschmitt G, Masterman D, Fontoura P, Belachew S, Chin P, Mairon N, Garren H, Kappos L, Opera I, and Investigators OIC. 2017. Ocrelizumab versus Interferon Beta-1a in Relapsing Multiple Sclerosis. N Engl J Med 376: 221–234. [DOI] [PubMed] [Google Scholar]
- 9.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS, and Investigators OC. 2017. Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis. N Engl J Med 376: 209–220. [DOI] [PubMed] [Google Scholar]
- 10.Niino M, Hirotani M, Miyazaki Y, and Sasaki H. 2009. Memory and naive B-cell subsets in patients with multiple sclerosis. Neurosci Lett 464: 74–78. [DOI] [PubMed] [Google Scholar]
- 11.Claes N, Fraussen J, Vanheusden M, Hellings N, Stinissen P, Van Wijmeersch B, Hupperts R, and Somers V. 2016. Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients. J Immunol 197: 4576–4583. [DOI] [PubMed] [Google Scholar]
- 12.Colonna-Romano G, Bulati M, Aquino A, Pellicano M, Vitello S, Lio D, Candore G, and Caruso C. 2009. A double-negative (IgD-CD27-) B cell population is increased in the peripheral blood of elderly people. Mech. Ageing Dev 130: 681–690. [DOI] [PubMed] [Google Scholar]
- 13.Rojas OL, Narvaez CF, Greenberg HB, Angel J, and Franco MA. 2008. Characterization of rotavirus specific B cells and their relation with serological memory. Virology 380: 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rinaldi S, Pallikkuth S, George VK, de Armas LR, Pahwa R, Sanchez CM, Pallin MF, Pan L, Cotugno N, Dickinson G, Rodriguez A, Fischl M, Alcaide M, Gonzalez L, Palma P, and Pahwa S. 2017. Paradoxical aging in HIV: immune senescence of B Cells is most prominent in young age. Aging (Albany NY) 9: 1307–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei C, Anolik J, Cappione A, Zheng B, Pugh-Bernard A, Brooks J, Lee EH, Milner EC, and Sanz I. 2007. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J. Immunol 178: 6624–6633. [DOI] [PubMed] [Google Scholar]
- 16.Jenks SA, Cashman KS, Zumaquero E, Marigorta UM, Patel AV, Wang X, Tomar D, Woodruff MC, Simon Z, Bugrovsky R, Blalock EL, Scharer CD, Tipton CM, Wei C, Lim SS, Petri M, Niewold TB, Anolik JH, Gibson G, Lee FE, Boss JM, Lund FE, and Sanz I. 2018. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 49: 725–739 e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmood Z, Muhammad K, Schmalzing M, Roll P, Dorner T, and Tony HP. 2015. CD27-IgD- memory B cells are modulated by in vivo interleukin-6 receptor (IL-6R) blockade in rheumatoid arthritis. Arthritis Res Ther 17: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moura RA, Quaresma C, Vieira AR, Goncalves MJ, Polido-Pereira J, Romao VC, Martins N, Canhao H, and Fonseca JE. 2017. B-cell phenotype and IgD-CD27- memory B cells are affected by TNF-inhibitors and tocilizumab treatment in rheumatoid arthritis. PLoS One 12: e0182927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobi AM, Reiter K, Mackay M, Aranow C, Hiepe F, Radbruch A, Hansen A, Burmester GR, Diamond B, Lipsky PE, and Dorner T. 2008. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: delineation by expression of CD27, IgD, and CD95. Arthritis Rheum 58: 1762–1773. [DOI] [PubMed] [Google Scholar]
- 20.Hao Y, O’Neill P, Naradikian MS, Scholz JL, and Cancro MP. 2011. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 118: 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, and Marrack P. 2011. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c(+) B-cell population is important for the development of autoimmunity. Blood 118: 1305–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubtsova K, Rubtsov AV, van Dyk LF, Kappler JW, and Marrack P. 2013. T-box transcription factor T-bet, a key player in a unique type of B-cell activation essential for effective viral clearance. Proc Natl Acad Sci U S A 110: E3216–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu YC, Kipling D, and Dunn-Walters DK. 2011. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol 2: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirths S, and Lanzavecchia A. 2005. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol 35: 3433–3441. [DOI] [PubMed] [Google Scholar]
- 25.Fecteau JF, Cote G, and Neron S. 2006. A new memory CD27-IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation. J Immunol 177: 3728–3736. [DOI] [PubMed] [Google Scholar]
- 26.Martin V, Wu YC, Kipling D, and Dunn-Walters DK. 2015. Age-related aspects of human IgM(+) B cell heterogeneity. Ann N Y Acad Sci 1362: 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, and Wolinsky JS. 2011. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vander Heiden JA, Yaari G, Uduman M, Stern JN, O’Connor KC, Hafler DA, Vigneault F, and Kleinstein SH. 2014. pRESTO: a toolkit for processing high-throughput sequencing raw reads of lymphocyte receptor repertoires. Bioinformatics 30: 1930–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alamyar E, Duroux P, Lefranc MP, and Giudicelli V. 2012. IMGT((R)) tools for the nucleotide analysis of immunoglobulin (IG) and T cell receptor (TR) V-(D)-J repertoires, polymorphisms, and IG mutations: IMGT/V-QUEST and IMGT/HighV-QUEST for NGS. Methods Mol Biol 882: 569–604. [DOI] [PubMed] [Google Scholar]
- 30.Gupta NT, Vander Heiden JA, Uduman M, Gadala-Maria D, Yaari G, and Kleinstein SH. 2015. Change-O: a toolkit for analyzing large-scale B cell immunoglobulin repertoire sequencing data. Bioinformatics 31: 3356–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta NT, Adams KD, Briggs AW, Timberlake SC, Vigneault F, and Kleinstein SH. 2017. Hierarchical Clustering Can Identify B Cell Clones with High Confidence in Ig Repertoire Sequencing Data. J Immunol 198: 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vander Heiden JA, Stathopoulos P, Zhou JQ, Chen L, Gilbert TJ, Bolen CR, Barohn RJ, Dimachkie MM, Ciafaloni E, Broering TJ, Vigneault F, Nowak RJ, Kleinstein SH, and O’Connor KC. 2017. Dysregulation of B Cell Repertoire Formation in Myasthenia Gravis Patients Revealed through Deep Sequencing. J Immunol 198: 1460–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baranzini SE, Jeong MC, Butunoi C, Murray RS, Bernard CC, and Oksenberg JR. 1999. B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J. Immunol 163: 5133–5144. [PubMed] [Google Scholar]
- 34.Owens GP, Kraus H, Burgoon MP, Smith-Jensen T, Devlin ME, and Gilden DH. 1998. Restricted use of VH4 germline segments in an acute multiple sclerosis brain. Ann. Neurol 43: 236–243. [DOI] [PubMed] [Google Scholar]
- 35.Qin Y, Duquette P, Zhang Y, Olek M, Da RR, Richardson J, Antel JP, Talbot P, Cashman NR, Tourtellotte WW, Wekerle H, and van den NS. 2003. Intrathecal B-cell clonal expansion, an early sign of humoral immunity, in the cerebrospinal fluid of patients with clinically isolated syndrome suggestive of multiple sclerosis. Lab Invest 83: 1081–1088. [DOI] [PubMed] [Google Scholar]
- 36.Tipton CM, Fucile CF, Darce J, Chida A, Ichikawa T, Gregoretti I, Schieferl S, Hom J, Jenks S, Feldman RJ, Mehr R, Wei C, Lee FE, Cheung WC, Rosenberg AF, and Sanz I. 2015. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 16: 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang B, and Casali P. 1994. The CDR1 sequences of a major proportion of human germline Ig VH genes are inherently susceptible to amino acid replacement. Immunol Today 15: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lossos IS, Tibshirani R, Narasimhan B, and Levy R. 2000. The inference of antigen selection on Ig genes. J Immunol 165: 5122–5126. [DOI] [PubMed] [Google Scholar]
- 39.Yaari G, Uduman M, and Kleinstein SH. 2012. Quantifying selection in high-throughput Immunoglobulin sequencing data sets. Nucleic Acids Res 40: e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meffre E, Milili M, Blanco-Betancourt C, Antunes H, Nussenzweig MC, and Schiff C. 2001. Immunoglobulin heavy chain expression shapes the B cell receptor repertoire in human B cell development. J Clin Invest 108: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguilera I, Melero J, Nunez-Roldan A, and Sanchez B. 2001. Molecular structure of eight human autoreactive monoclonal antibodies. Immunology 102: 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson C, Chida AS, Adlowitz D, Silver L, Fox E, Jenks SA, Palmer E, Wang Y, Heimburg-Molinaro J, Li QZ, Mohan C, Cummings R, Tipton C, and Sanz I. 2013. Molecular basis of 9G4 B cell autoreactivity in human systemic lupus erythematosus. J Immunol 191: 4926–4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, and Nussenzweig MC. 2003. Predominant autoantibody production by early human B cell precursors. Science 301: 1374–1377. [DOI] [PubMed] [Google Scholar]
- 44.Ng YS, Wardemann H, Chelnis J, Cunningham-Rundles C, and Meffre E. 2004. Bruton’s tyrosine kinase is essential for human B cell tolerance. J Exp Med 200: 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunn-Walters DK, and Ademokun AA. 2010. B cell repertoire and ageing. Curr Opin Immunol 22: 514–520. [DOI] [PubMed] [Google Scholar]
- 46.Thewissen M, Linsen L, Somers V, Geusens P, Raus J, and Stinissen P. 2005. Premature immunosenescence in rheumatoid arthritis and multiple sclerosis patients. Ann N Y Acad Sci 1051: 255–262. [DOI] [PubMed] [Google Scholar]
- 47.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, and Salvioli S. 2007. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128: 92–105. [DOI] [PubMed] [Google Scholar]
- 48.Buffa S, Bulati M, Pellicano M, Dunn-Walters DK, Wu YC, Candore G, Vitello S, Caruso C, and Colonna-Romano G. 2011. B cell immunosenescence: different features of naive and memory B cells in elderly. Biogerontology 12: 473–483. [DOI] [PubMed] [Google Scholar]
- 49.Berkowska MA, Driessen GJ, Bikos V, Grosserichter-Wagener C, Stamatopoulos K, Cerutti A, He B, Biermann K, Lange JF, van der Burg M, van Dongen JJ, and van Zelm MC. 2011. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways. Blood 118: 2150–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bagnara D, Squillario M, Kipling D, Mora T, Walczak AM, Da Silva L, Weller S, Dunn-Walters DK, Weill JC, and Reynaud CA. 2015. A Reassessment of IgM Memory Subsets in Humans. J Immunol 195: 3716–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang S, Wang J, Kumar V, Karnell JL, Naiman B, Gross PS, Rahman S, Zerrouki K, Hanna R, Morehouse C, Holoweckyj N, Liu H, Autoimmunity Molecular Medicine T, Manna Z, Goldbach-Mansky R, Hasni S, Siegel R, Sanjuan M, Streicher K, Cancro MP, Kolbeck R, and Ettinger R. 2018. IL-21 drives expansion and plasma cell differentiation of autoreactive CD11c(hi)T-bet(+) B cells in SLE. Nat Commun 9: 1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Jong BG, H IJ, Marques L, van der Burg M, van Dongen JJ, Loos BG, and van Zelm MC. 2017. Human IgG2- and IgG4-expressing memory B cells display enhanced molecular and phenotypic signs of maturity and accumulate with age. Immunol Cell Biol 95: 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frasca D, Diaz A, Romero M, and Blomberg BB. 2016. Human peripheral late/exhausted memory B cells express a senescent-associated secretory phenotype and preferentially utilize metabolic signaling pathways. Exp Gerontol 87: 113–120. [DOI] [PubMed] [Google Scholar]
- 54.Kurosaki T, Kometani K, and Ise W. 2015. Memory B cells. Nat Rev Immunol 15: 149–159. [DOI] [PubMed] [Google Scholar]
- 55.Owens GP, Winges KM, Ritchie AM, Edwards S, Burgoon MP, Lehnhoff L, Nielsen K, Corboy J, Gilden DH, and Bennett JL. 2007. VH4 gene segments dominate the intrathecal humoral immune response in multiple sclerosis. J. Immunol 179: 6343–6351. [DOI] [PubMed] [Google Scholar]
- 56.Ritchie AM, Gilden DH, Williamson RA, Burgoon MP, Yu X, Helm K, Corboy JR, and Owens GP. 2004. Comparative analysis of the CD19+ and CD138+ cell antibody repertoires in the cerebrospinal fluid of patients with multiple sclerosis. J. Immunol 173: 649–656. [DOI] [PubMed] [Google Scholar]
- 57.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, and Sanz I. 2001. Regulation of inherently autoreactive VH4–34 B cells in the maintenance of human B cell tolerance. J. Clin. Invest 108: 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, Plebani A, Kumararatne DS, Bonnet D, Tournilhac O, Tchernia G, Steiniger B, Staudt LM, Casanova JL, Reynaud CA, and Weill JC. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 104: 3647–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zuniga E, Cook MC, and Vinuesa CG. 2003. Extrafollicular antibody responses. Immunol Rev 194: 8–18. [DOI] [PubMed] [Google Scholar]
- 60.Ahye Hritzo, K. M, and Golding A. 2018. Cytoplasmic FOXO1 identifies a novel disease-activity associated B cell phenotype in SLE. Lupus Sci Med 5: e000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myles A, Gearhart PJ, and Cancro MP. 2017. Signals that drive T-bet expression in B cells. Cell Immunol 321: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rubtsov AV, Marrack P, and Rubtsova K. 2017. T-bet expressing B cells - Novel target for autoimmune therapies? Cell Immunol 321: 35–39. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.