Abstract
Study Objective:
The potential for maternal antidepressant use to influence the risk of spontaneous abortion, one of the most important adverse pregnancy outcomes, is not clear. We aimed to assess whether first trimester antidepressant exposure was associated with an increased risk of spontaneous abortion.
Design:
Community-based prospective cohort study (Right from the Start).
Setting:
Eight metropolitan areas in North Carolina, Tennessee, and Texas.
Participants:
A total of 5451 women (18 years of age or older) who were planning to conceive or were pregnant (before 12 weeks of completed gestation) and were enrolled in the study between 2000 and 2012; of those women, 223 used antidepressants (selective serotonin reuptake inhibitors [SSRIs] only [n=170], SSRIs and non-SSRIs [n=9], and non-SSRIs only [n=44]) during their first trimester, and 5228 did not (never users).
Measurements and Main Results:
First trimester antidepressant use was determined during a first trimester telephone interview. Spontaneous abortion was self-reported and verified by medical records. The association of first trimester antidepressant use and spontaneous abortion was assessed by using Cox proportional hazard regression. Among the 5451 women enrolled, 223 (4%) reported first trimester antidepressant use, and 659 (12%) experienced a spontaneous abortion. SSRIs were the most common class of antidepressants used (179 [80%]). Compared with women who never used antidepressants during first trimester of pregnancy, women who reported antidepressant use were 34% (adjusted hazard ratio [aHR] 1.34, 95% confidence interval [CI] 0.97–1.85) more likely to experience a spontaneous abortion after adjusting for covariates. Women who reported ever using SSRIs were 45% (aHR 1.45, 95% CI 1.02–2.06) more likely to experience a spontaneous abortion compared with never users. When time of loss relative to the time of interview was taken into consideration, the association between first trimester SSRI use and spontaneous abortion was significant only among those with losses before the interview (aHR 1.49, 95% CI 1.04–2.13) but was not significant among those with losses after the interview (aHR 0.43, 95% CI 0.06–3.15).
Conclusion:
The association between use of first trimester antidepressants, particularly SSRI use, and spontaneous abortion was significant only among women whose exposure status was assessed after loss. In this instance, reporting bias may create a spurious association. Future studies should take the timing of data collection relative to the timing of loss into consideration.
Keywords: antidepressant use, early pregnancy loss, first trimester, selective serotonin reuptake inhibitors, SSRI, spontaneous abortion
Depression is common in pregnancy, affecting up to 13% of women.1, 2 Untreated or undertreated antenatal depression has been linked with poor maternal self-care during pregnancy, poor obstetric and neonatal health outcomes, and lasting developmental, behavioral, and psychological sequelae in exposed offspring.3–7 Antidepressants are widely used in pregnancy and are considered the primary treatment, or an important adjunct, for moderate to severe depression and other complications.8, 9 About 4% of pregnant women will use antidepressants at some point during the first trimester, and up to 13% of all pregnant women use at least one antidepressant during pregnancy.8, 9 Use of antidepressants reduces depressive symptoms and improves womens’ ability to function well in their roles, whereas discontinuation of antidepressants during pregnancy has been associated with relapse of depression and withdrawal symptoms.10–13 Several different classes of antidepressant medications are currently available (Supplementary Table S1).14 Each one has a different putative mechanism of action and adverse effect profile. Selective serotonin reuptake inhibitors (SSRIs) are the most commonly prescribed antidepressants.9, 15 They are believed to alleviate symptoms of depression by blocking the reabsorption or reuptake of the neurotransmitter serotonin, resulting in greater availability in the synapse.15, 16 Non-SSRI antidepressants, including serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), monoamine oxidase inhibitors (MAOIs), and atypical antidepressants, work either similarly by blocking the reabsorption of the neurotransmitters (SNRIs, TCAs), or affecting the levels of dopamine, serotonin, and norepinephrine in a different manner (Supplementary Table S1).14, 16
Spontaneous abortion occurs in 10–15% of clinically recognized pregnancies.17 Several studies and recent meta-analyses have reported a modest elevation in the risk of spontaneous abortion with antenatal antidepressant use.18–24 However, in studies in which all women have depression, the significant association between antenatal antidepressant use and the risk of spontaneous abortion becomes null.25, 26 Studies also often fail to control for important confounding factors such as prior pregnancy loss, obesity, and maternal smoking and alcohol use that may distort the association.22, 27 Thus, more information from large, population-based prospective studies with accurate identification of spontaneous abortion is needed.
We aimed to determine the association between first trimester antidepressant use and the risk of spontaneous abortion in this community-based prospective cohort study of women who were enrolled either prior to pregnancy or early in pregnancy. We further assessed the association between first trimester SSRI and other non-SSRI antidepressant use with spontaneous abortion. We hypothesized that women who take antidepressants early in pregnancy have an increased risk of experiencing a spontaneous abortion, which is affected by the type of antidepressants taken. Understanding the relationship will aid in the development of clinical guidelines for safe use of antidepressants and enhance decision-making for patients and clinicians.
Methods
Study Design, Study Population, and Data Collection
Right from the Start (RFTS) is a community-based pregnancy cohort study that prospectively enrolled women who were either planning to conceive or were pregnant (before 12 wks of completed gestation) between 2000 and 2012.28 The RFTS enrolled participants from eight metropolitan areas in North Carolina, Tennessee, and Texas. Women who were at least 18 years old, were English or Spanish speaking, and did not use assisted reproductive technologies were eligible for recruitment. The rationale and methods for the RFTS cohort have been reported previously.28 The institutional review board of Vanderbilt University (Nashville, TN) approved this analysis.
At enrollment (mean ± SD 49 ± 14 days’ gestation), participants provided basic demographic information. Selected risk factors for adverse pregnancy outcomes were also provided at enrollment and were included in the study. An extensive telephone interview was conducted near the end of the first trimester of pregnancy (mean ± SD 85 ± 21 days’ gestation). Data on prior reproductive history, medical history, symptoms and events during the current pregnancy, health behaviors (caffeine, tobacco, and alcohol use), medication and supplement use, and physical activity were collected at the first trimester interview.
This analysis was limited to the participant’s first study pregnancy if she enrolled in the study for more than one pregnancy. By excluding womens’ subsequent pregnancies, all pregnancies studied were independent and represented unique women. Women with induced abortions and ectopic pregnancies were also excluded.
First Trimester Antidepressant Use
All women were assessed for antidepressant use in the first trimester interview. Questions were asked separately for medications treating depression and anxiety. Only medications that are classified as antidepressants were considered in this analysis (Supplementary Table S1). Participants were considered exposed if they reported any use of antidepressants since becoming pregnant and before the interview. Women exposed to a first trimester antidepressant were then asked slightly different questions depending on their pregnancy status at the time of the interview. For women who had not experienced a loss at the time of the telephone interview and reported ever using an antidepressant during the first trimester of pregnancy, we further asked whether they were taking an antidepressant at the time of the interview. If so, we asked whether they began taking the antidepressants before getting pregnant. Women who had a loss before the interview, in contrast, were not asked whether they were using an antidepressant at the time of the interview. They were no longer considered as pregnant, and thus use of antidepressants at the time of the interview had no effect on the risk of spontaneous abortion. We further classified antidepressants as SSRIs or non-SSRIs (Supplementary Table S1). Women were considered SSRI users if they reported ever using SSRIs (including those who used only SSRIs and those who used both SSRIs and non-SSRIs) during the first trimester of pregnancy; they were considered non-SSRI users otherwise.
Pregnancy Outcome
Pregnancy was verified by ultrasonography or repeat pregnancy test. Gestational age was calculated based on self-reported date of the last menstrual period (LMP), which has been documented to be reliable for a subset of this population (n=1867) with a mean and median difference between LMP- and ultrasound-estimated gestational age of less than 1 day.29 Pregnancy outcomes were self-reported by participants and verified by medical or vital records. We defined spontaneous abortion as loss of a clinically recognized pregnancy at or before 20 completed weeks’ gestation from LMP. All spontaneous abortion cases were clinically confirmed, and all participants had research ultrasounds to assist with accurate dating. Women confirmed to have pregnancies extending past 20 weeks of gestation were censored at the end of 20 weeks and were considered as having no spontaneous abortion.
Statistical Analysis
We used Cox proportional hazard survival models to characterize the risk of spontaneous abortion in relation to early pregnancy antidepressant use. Participants were followed from the time of enrollment in the study and contributed to gestational age-specific analysis risk sets until the occurrence of spontaneous abortion, loss to follow-up, or the end of the 20 weeks’ gestation, whichever came first.30, 31 Four women with pregnancy loss did not record timing of loss. Their loss time was considered to be interval censored and set as median time in between the last follow-up date and the end of 20 weeks’ gestation. We further conducted adjusted analysis to estimate the association between early pregnancy antidepressant use and the risk of spontaneous abortion with the consideration of confounders. We identified important confounders following a backward elimination strategy. A change in estimate of at least 10% for the exposure of interest was used to classify a variable as a confounder. Candidate confounders consisted of known and strongly suspected influences on spontaneous abortion, including maternal age, race-ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and other), smoking (current and not current smoker), alcohol use (current and not current drinker), caffeine consumption per day (in milligrams) in the first trimester, vitamin use (yes/no), first trimester progesterone use (yes/no), diabetes mellitus status (yes/no), first trimester body mass index (BMI), prior termination of pregnancy (yes/no), parity (primipara, one, two or greater), marital status (married/cohabiting and other), maternal education (high school or less, some college, college and more), maternal employment status (yes/no), household income (≤ $40,000, $40,001-$80,000, and > $80,000), and pregnancy intention (unintended and intended). Calendar year of conception and study site were also evaluated as candidate confounders as they might be markers of medical and socioeconomic change over time and/or across different geographic areas. To optimize fit, we applied restricted cubic splines with four knots in the model to allow for nonlinear relationship of subjects’ age and BMI at study entry as well as total daily caffeine consumption (mg/day) in first trimester and spontaneous abortion risk.32 The potential effect modification of age, BMI, smoking status, alcohol consumption status, and race-ethnicity was assessed separately with a likelihood ratio test. No effect modification was detected (p > 0.11). Prior history of spontaneous abortion was considered as a risk factor of spontaneous abortion. We conducted sensitivity analyses additionally adjusting for women’s prior history of spontaneous abortion (yes/no). All analyses were repeated to assess the potential differential effect of type of antidepressant use (SSRIs and non-SSRIs) on the risk of spontaneous abortion. Assumptions of proportional hazards for the final models were met. Women who used both SSRI and non-SSRI medications were classified as SSRI users. A sensitivity analysis was conducted excluding women who reported both SSRI and non-SSRI antidepressant use.
Additional subgroup analyses of spontaneous abortion before and after the interview were conducted to assess potential recall bias. As women who planned to become pregnant might have different patterns of antidepressant use, we also conducted a subgroup analysis with women whose pregnancies were planned/intended. Pregnancy intention was assessed at enrollment using the intendedness of pregnancy items developed by the Centers for Disease Control and Prevention.33 A pregnancy was considered planned/intended if the participant affirmed that she desired to have a baby at some time in her life and became pregnant in what she deemed was “about the right time” or later than desired.34
All analyses were conducted by using R-software version 3.1.2 (www.r-project.org) and STATA version 13 (StataCorp, College Station, TX). We used a two-sided 5% significance level for all statistical inferences.
Results
We enrolled women with a total of 6105 pregnancies. We excluded 325 pregnancies of women who were enrolled in the study for subsequent pregnancies. We excluded 304 women without first trimester interview information. We also excluded 14 women with induced abortions and 10 women with ectopic pregnancies. Lastly, we excluded one woman who had a missing gestational age. This left 5451 pregnancies for our analysis (Figure 1).
Figure 1.
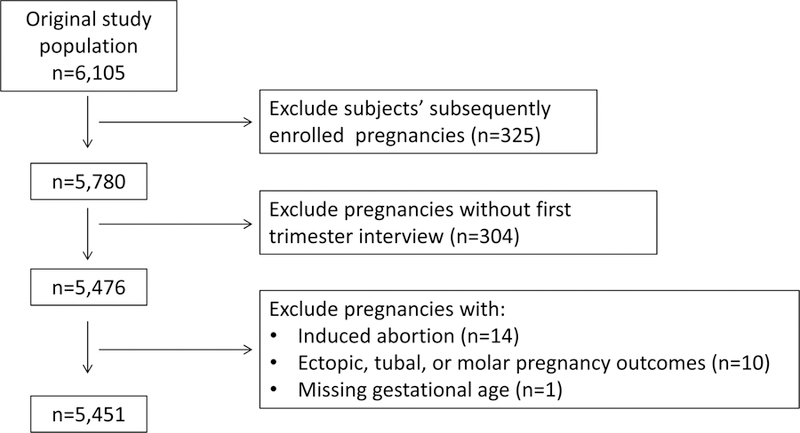
Flow diagram showing selection of the study population, sample size, and exclusions from women enrolled in the Right from the Start study from 2000–2012.
Among 5451 women enrolled in the RFTS between 2000 and 2012, 659 (12%) women experienced spontaneous abortion before the end of 20 weeks of gestation. The mean ± SD gestational age at loss was 72 ± 21 days. A total of 86% (564/659) occurred in the first trimester of pregnancy. Overall, 4% (223) of the 5451 women used at least one kind of antidepressant during the first trimester of pregnancy. Compared with women who never used antidepressants during the first trimester of pregnancy (n=5228), women with first trimester antidepressant exposure (n=223) were more likely to be older, non-Hispanic White, married/cohabiting, have a greater educational attainment, have a higher household income, and have an intended pregnancy. They were also more likely to drink alcohol and caffeine, and consume vitamins in the first trimester of pregnancy (Table 1).
Table 1.
Characteristics of the Study Participants
| Characteristic | Participants with first trimester antidepressant use (n=223) |
Participants without first trimester antidepressant use (n=5228) |
Odds ratio (95% confidence interval) | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Maternal age (yrs) | |||||
| < 25 | 19 | 8.5 | 1,068 | 20.4 | Reference |
| 25–29 | 57 | 25.6 | 1,806 | 34.5 | 1.77 (1.05, 3.00) |
| 30–34 | 91 | 40.8 | 1,645 | 31.5 | 3.11 (1.88, 5.13) |
| ≥ 35 | 56 | 25.1 | 709 | 13.6 | 4.44 (2.62, 7.53) |
| Missing data | 0 | 0 | 0 | 0 | |
| Race-ethnicity | |||||
| White, non-Hispanic | 200 | 89.7 | 3,612 | 69.1 | Reference |
| Black, non-Hispanic | 10 | 4.5 | 1,021 | 19.5 | 0.18 (0.09, 0.34) |
| Hispanic | 6 | 2.7 | 360 | 6.9 | 0.30 (0.13, 0.68) |
| Other | 7 | 3.1 | 232 | 4.4 | 0.54 (0.25, 1.17) |
| Refused to answer | 0 | 0 | 3 | 0.1 | |
| Education | |||||
| High school or less | 16 | 7.2 | 960 | 18.4 | Reference |
| Some college | 37 | 16.6 | 944 | 18.1 | 2.35 (1.30, 4.26) |
| College or more | 170 | 76.2 | 3,323 | 63.6 | 3.07 (1.83, 5.15) |
| Missing data | 0 | 0 | 1 | 0 | |
| Marital status | |||||
| Married/cohabiting | 216 | 96.9 | 4,643 | 88.8 | Reference |
| Other | 7 | 3.1 | 585 | 11.2 | 0.26 (0.12, 0.55) |
| Missing data | 0 | 0 | 0 | 0 | |
| Employment | |||||
| No | 74 | 33.2 | 1,535 | 29.4 | Reference |
| Yes | 149 | 66.8 | 3,679 | 70.4 | 0.84 (0.63, 1.12) |
| Missing data | 0 | 0 | 14 | 0.3 | |
| Household income ($) | |||||
| ≤ 40,000 | 36 | 16.1 | 1,623 | 31.0 | Reference |
| 40,001–80,000 | 96 | 43.1 | 1,854 | 35.5 | 2.33 (1.58, 3.44) |
| > 80,000 | 89 | 39.9 | 1,550 | 29.7 | 2.59 (1.75, 3.84) |
| Missing data | 2 | 0.9 | 201 | 3.8 | |
| Parity | |||||
| 0 | 91 | 40.8 | 2,481 | 47.5 | Reference |
| 1 | 93 | 41.7 | 1,763 | 33.7 | 1.44 (1.07, 1.93) |
| ≥ 2 | 39 | 17.5 | 897 | 17.2 | 1.19 (0.81, 1.74) |
| Missing data | 0 | 0 | 87 | 1.7 | |
| Miscarriage history | |||||
| No prior pregnancy loss | 169 | 75.8 | 3,985 | 76.2 | Reference |
| ≥ 1 prior losses | 54 | 24.2 | 1,156 | 22.1 | 1.10 (0.81, 1.51) |
| Missing data | 0 | 0 | 87 | 1.7 | |
| Prior termination of pregnancy | |||||
| None | 185 | 83.0 | 4,389 | 84.0 | Reference |
| Any | 38 | 17.0 | 752 | 14.4 | 1.20 (0.84, 1.71) |
| Missing data | 0 | 0 | 87 | 1.7 | |
| Body mass index category | |||||
| Underweight | 9 | 0.5 | 134 | 2.6 | 0.18 (0.03, 1.32) |
| Normal weight | 113 | 50.7 | 2,766 | 52.9 | Reference |
| Overweight | 49 | 22.0 | 1,227 | 23.5 | 0.98 (0.69, 1.38) |
| Obese | 60 | 26.9 | 1,036 | 19.8 | 1.42 (1.03, 1.95) |
| Missing data | 0 | 0 | 65 | 1.2 | |
| Any type of diabetes mellitus† | |||||
| No | 217 | 97.3 | 5,052 | 96.6 | Reference |
| Yes | 6 | 2.7 | 150 | 2.9 | 0.93 (0.00, +∞) |
| Missing data | 0 | 0 | 26 | 0.5 | |
| Progesterone use in first trimester | |||||
| No | 216 | 96.9 | 5,076 | 97.1 | Reference |
| Yes | 7 | 3.1 | 152 | 2.9 | 1.08 (0.50, 2.34) |
| Missing data | 0 | 0 | 0 | 0 | |
| Cigarette smoking status | |||||
| Not current | 210 | 94.2 | 5,023 | 96.1 | Reference |
| Current smoker | 13 | 5.8 | 187 | 3.6 | 1.66 (0.93, 2.97) |
| Missing data | 0 | 0 | 18 | 0.3 | |
| Alcohol use status | |||||
| Not current | 191 | 85.7 | 4,947 | 94.6 | 1 |
| Current drinker | 32 | 14.4 | 261 | 5.0 | 3.18 (2.14, 4.71) |
| Missing data | 0 | 0 | 20 | 0.4 | |
| Any use of vitamins in first trimester | |||||
| No | 0 | 0 | 186 | 3.6 | Reference |
| Yes | 223 | 100.0 | 5,012 | 95.8 | NA‡ |
| Missing data | 0 | 0 | 33 | 0.6 | |
| Caffeine use in first trimester (mg/day) | |||||
| No | 44 | 19.7 | 1,635 | 31.3 | Reference |
| Quartile 1: [38.4, 137.5) | 40 | 17.9 | 878 | 16.8 | 1.32 (0.83, 2.09) |
| Quartile 2: [137.5, 309.0) | 50 | 22.4 | 889 | 17.0 | 1.69 (1.09, 2.62) |
| Quartile 3: [309.0, 584.9) | 33 | 14.8 | 929 | 17.8 | 2.09 (1.38, 3.16) |
| Quartile 4: [584.9, 7705.6] | 56 | 25.1 | 883 | 16.9 | 2.36 (1.57, 3.53) |
| Missing data | 0 | 0 | 14 | 0.3 | |
| Pregnancy intention | |||||
| Unintended | 46 | 20.6 | 1,427 | 27.3 | Reference |
| Intended | 155 | 69.5 | 3,408 | 65.2 | 1.41 (1.01, 1.97) |
| Missing data | 22 | 9.9 | 393 | 7.5 | |
| Study site | |||||
| North Carolina | 136 | 61.0 | 2,947 | 56.4 | Reference |
| Tennessee | 86 | 38.6 | 1,875 | 35.9 | 0.99 (0.75, 1.31) |
| Texas | 1 | 0.5 | 406 | 7.8 | 0.05 (0.01, 0.38) |
| Missing data | |||||
Women with diabetes included 18 with type 1 diabetes, 18 with type 2 diabetes, 119 with gestational diabetes, and 1 with both type 1 and gestational diabetes, respectively.
Odds ratio and corresponding confidence interval were not calculated because of low number of subjects in one of the groups.
The majority (179 [80%]) of the women who ever used antidepressants during the first trimester of pregnancy used SSRIs (Table 2). Nineteen women reported having two antidepressants, three used two SSRI medications, nine used one SSRI and one non-SSRI medication, and seven used two non-SSRI medications. Among the 183 who answered the question whether they continued taking the particular antidepressants at the time of the first trimester interview, 75% (138) reported continued use at the time of the first trimester interview. Of these women who were taking antidepressants at the time of interview, we further asked about timing of use in relation to the timing of conception: 92% (127/138) started antidepressant therapy prior to the LMP. SSRI use and non-SSRI use did not statistically significantly differ by when treatment started or the pattern of quitting therapy.
Table 2.
Antidepressant Use Patterns of the 223 Women Who Ever Used Antidepressants in the First Trimester
| Antidepressant use pattern | SSRI users | Non-SSRI only users (n=44 [19.7%]) | |
|---|---|---|---|
| SSRI only users (n=170 [76.2%]) | SSRI and non-SSRI users (n=9 [4.0%]) | ||
| No. (%) | No. (%) | No. (%) | |
| Use of antidepressants at time of first trimester interview | |||
| No | 28 (16.5) | 5 (55.6) | 12 (27.3) |
| Yes | 111 (65.3) | 3 (33.3) | 24 (54.6) |
| Skippeda | 31 (18.2) | 0 (0.0) | 7 (15.91) |
| Did not answerb | 0 (0.0) | 1 (11.1) | 1 (2.3) |
| Use of antidepressants prior to the last menstrual period (n=138)c | |||
| No | 10 (8.3) | 0 (0.0) | 1 (4.2) |
| Yes | 101 (83.5) | 3 (100.0) | 23 (95.8) |
Women with loss before interview were not asked about current use as they were no longer pregnant.
Women were asked but did not answer the question.
Only women who answered yes to “use of antidepressants” in the first trimester interview were asked whether their use of antidepressants started prior to the last menstrual period.
Risk of spontaneous abortion was associated with first trimester antidepressant exposure in the univariate analysis. Women who ever used antidepressants (n=223) had a 51% (hazard ratio [HR] 1.51, 95% confidence interval [CI] 1.10–2.06) greater risk of having a spontaneous abortion when compared with women who never used antidepressants during the first trimester of pregnancy (Table 3). After adjusting for maternal age, alcohol use, vitamin use, and calendar year of pregnancy, variables that were considered as confounders, the risk in the full cohort was 34% higher (adjusted HR [aHR] 1.34, 95% CI 0.97–1.85) (Supplementary Figure S1). When we further classified women into SSRI users and non-SSRI only users, women who ever used SSRIs (n=179) during the first trimester of pregnancy had a 45% higher risk of spontaneous abortion (aHR 1.45, 95% CI 1.02–2.06) in the adjusted analysis. Non-SSRI antidepressants (n=44) were not linked to the risk of spontaneous abortion (aHR 0.96, 95% CI 0.45–2.04) (Table 3). The relationship between first trimester antidepressant use and spontaneous abortion was not influenced by prior history of spontaneous abortion (Table 3). Sensitivity analysis comparing women who used SSRIs only with women who did not use antidepressants yielded a consistent result (aHR 1.50, 95% CI 1.05–2.14).
Table 3.
First Trimester Antidepressant Use and Risk of Spontaneous Abortion
| Antidepressant use | Spontaneous abortion |
Unadjusted HR (95% CI) | Adjusted HR (95% CI)a | Adjusted HR (95% CI)b | |||
|---|---|---|---|---|---|---|---|
| Yes (n=659) | No (n=4792) | ||||||
| No. | % | No. | % | ||||
| First trimester antidepressant use | |||||||
| No | 617 | 93.6 | 4,611 | 96.2 | Reference | Reference | Reference |
| Any | 42 | 6.4 | 181 | 3.8 | 1.51 (1.10–2.06) | 1.34 (0.97–1.85) | 1.34 (0.97–1.85) |
| First trimester type of antidepressant use | |||||||
| No | 617 | 93.6 | 4,611 | 96.2 | Reference | Reference | Reference |
| Ever SSRIs | 35 | 5.3 | 144 | 3.0 | 1.59 (1.13–2.23) | 1.45 (1.02–2.06) | 1.44 (1.01–2.05) |
| Non-SSRIs only | 7 | 1.1 | 37 | 0.8 | 1.21 (0.57–2.56) | 0.96 (0.45–2.04) | 0.98 (0.46–2.07) |
HR = hazard ratio; CI = confidence interval.
Multivariable Cox proportional hazard models adjusted for maternal age, alcohol use, vitamin use, and calendar year of pregnancy
Sensitivity analysis additionally adjusting for prior history of spontaneous abortion.
Of the 659 women who experienced spontaneous abortion, 437 (66%) had the loss prior to their first trimester interview and thus prior to the ascertainment of antidepressant exposure. The mean ± SD gestational age at loss was 65 ± 17 days for women who had losses before the interview and 86 ± 23 days for women whose losses were after the interview. A total of 9% (41/437) of women reported antidepressant use at the interview after they experienced a pregnancy loss. In contrast, less than 1% (1/222) and 4% (181/4792) of women reported antidepressant use at the first trimester interview when their losses were after the interview or when they did not experience loss during pregnancy, respectively. The mean ± SD gestational age at the interview was 64 ± 19 days for women who experienced loss after the interview (mean gestational age at the loss 86 ± 23 days) and 86 ± 21 days for women who did not experience loss. Analysis of losses before the interview yielded a consistent result as presented in the overall cohort. The positive association between antidepressant exposure and the risk of spontaneous abortion in the unadjusted (HR 2.15, 95% CI 1.56–2.96) analysis is attenuated after adjusting for covariates (aHR 1.37, 95% CI 0.98–1.91). First trimester SSRI use, but not non-SSRI only use, was associated with an increased risk of spontaneous abortion for women interviewed before the loss (SSRI: aHR 1.49, 95% CI 1.04–2.13; non-SSRI: aHR 0.99, 95% CI 0.47–2.11). Analysis of risk among those who had losses after the interview, on the other hand, found that antidepressant use was not associated with spontaneous abortion (aHR 0.43, 95% CI 0.06–3.15).
Lastly, 3563 women had planned/intended pregnancies. Among them, 455 women (13%) experienced a spontaneous abortion, and 155 (4%) reported use of antidepressants in the first trimester. First trimester antidepressant use increased the risk of spontaneous abortion (aHR 1.58, 95% CI 1.10–2.28) among women who planned to become pregnant.
Discussion
In this large community-based prospective cohort study, 4% of pregnant women reported antidepressant use in the first trimester, consistent with previous reports.8 SSRIs were the main antidepressant medication reported. A total of 75% of women who ever used antidepressants in the first trimester of pregnancy continued their therapy after they realized they were pregnant. This first trimester antidepressant use, particularly SSRIs, was associated with increased risk of spontaneous abortion and is consistent with two recent meta-analyses.21, 22
Interpretation of this association, however, should take potential biases into consideration. One potential source of error is confounding by indication bias related to the disease itself. Maternal depression and anxiety, if left untreated, are associated with an increased risk of spontaneous abortion.35, 36 We did not collect direct information about womens’ mental health status and disease severity. Therefore, the only indicator of a woman having depression was her reported use of antidepressants. At the same time, women who reported no use of antidepressants during first trimester of pregnancy may represent a mixture of women with no history of depression and women who had depression but were not treated with antidepressants. Most of the women who used antidepressants during the first trimester of pregnancy reported continued use at the time of the interview, and most started therapy before pregnancy, an indication of having preexisting depressive disorders or often, although not always, more severe forms of depressive disorders. Therefore, the observed increase in risk of spontaneous abortion could be partially due to depression instead of use of antidepressants that treat depression. In a large population-based cohort study of over one million pregnancies, Kjaersgaard and colleagues noted that antidepressant exposure during pregnancy was associated with a 14% increased risk of loss (adjusted relative risk [aRR] 1.14, 95% CI 1.10–1.18).25 However, this risk for spontaneous abortion was null (aRR 1.00, 95% CI 0.80–1.24) when the analysis was limited to women with a diagnosis of depression. Another study, in contrast, showed that the increased risk of spontaneous abortion associated with first trimester antidepressant use was significant even when compared with depressed women who were not exposed to antidepressants.23
Recall bias may also be in play. In our study, pregnancy loss preceded the interview about antidepressant use for 66% of participants. We previously have demonstrated that maternal recall of prescription medications such as SSRIs is specific (99.0% specificity) but less sensitive (77.8% sensitivity) in this cohort of women.37 However, the possibility of differential reporting of use by women who are considering what might have contributed to causing a loss could drive the association away from null. This opens up the literature about associations of antidepressants and miscarriage to risk of reporting a spurious association. Indeed, the increased risk of spontaneous abortion is only present among women who were interviewed about antidepressant use after their spontaneous abortion. Women who were interviewed before their loss had no increased risk.
Other limitations should be considered. First, although we explored the timing of antidepressant initiation relative to onset of pregnancy, we did not collect data on the exact duration of antidepressant exposure. We therefore could not assess the cumulative effect of antidepressants. Second, although our cohort included 5451 pregnant women, the sample sizes in certain subgroups were small. Among the 42 women who were exposed to antidepressants and experienced spontaneous abortion, only one woman reported her antidepressant use prior to the loss. Caution should be taken in interpreting results from these subgroup analyses with wide confidence intervals. Finally, although depression is the main indication for use of antidepressants, these medications are also prescribed to treat other indications (e.g., generalized anxiety disorder). Women who are prescribed antidepressants for other indications may have a differential baseline risk for spontaneous abortion compared to those who receive antidepressants for depression.
Conclusion
The association between increased risk of first trimester antidepressant use, mainly SSRIs, and spontaneous abortion should be interpreted with caution. Reporting bias, when women are more likely to report their antidepressant use after experiencing loss, is a major concern and may create a spurious association. Future studies must take the timing of the exposure ascertainment relative to the spontaneous abortion into consideration. Before a randomized clinical trial of pregnant women with depression randomly assigned to receive pharmacotherapy is conducted, important gaps remain in disentangling the separate effect of maternal depression itself, the effectiveness of treatment modalities received (both pharmacologic and others), recall bias in reporting, and antidepressant treatment on spontaneous abortion.
Supplementary Material
Acknowledgments
Funding: Field research was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH; R01HD043883 and R01HD049675) and the American Water Works Association Research Foundation (2579). Salary support for DRV was provided in part by the NIH Building Interdisciplinary Research Careers in Women’s Health career development program (K12HD04383) with additional infrastructure resources provided by UL1TR000445, a grant from the Clinical and Translational Science Award Program of the NIH National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the views of the funding agencies.
Footnotes
Conflict of interest statment: Dr. Hartmann reports receiving grants from National Institutes of Health and the American Water Works Association Research Foundation during the conduct of the study. All other authors report no conflicts of interest.
References
- 1.Le Strat Y, Dubertret C, Le Foll B. Prevalence and correlates of major depressive episode in pregnant and postpartum women in the United States. J Affect Disord 2011;1–3:128–38. [DOI] [PubMed] [Google Scholar]
- 2.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 2005;5 Pt 1:1071–83. [DOI] [PubMed] [Google Scholar]
- 3.Orr ST, Blazer DG, James SA, Reiter JP. Depressive symptoms and indicators of maternal health status during pregnancy. J Womens Health (Larchmt) 2007;4:535–42. [DOI] [PubMed] [Google Scholar]
- 4.Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Implications of antenatal depression and anxiety for obstetric outcome. Obstet Gynecol 2004;3:467–76. [DOI] [PubMed] [Google Scholar]
- 5.Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry 2013;4:e321–41. [DOI] [PubMed] [Google Scholar]
- 6.Capron LE, Glover V, Pearson RM, et al. Associations of maternal and paternal antenatal mood with offspring anxiety disorder at age 18 years. J Affect Disord 2015;20–6. [DOI] [PMC free article] [PubMed]
- 7.Epstein RA, Moore KM, Bobo WV. Treatment of nonpsychotic major depression during pregnancy: patient safety and challenges. Drug Healthc Patient Saf 2014;109–29. [DOI] [PMC free article] [PubMed]
- 8.Ramos E, Oraichi D, Rey E, Blais L, Berard A. Prevalence and predictors of antidepressant use in a cohort of pregnant women. BJOG 2007;9:1055–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes RM, Wu P, Shelton RC, et al. Maternal antidepressant use and adverse outcomes: a cohort study of 228,876 pregnancies. Am J Obstet Gynecol 2012;1:49 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry 2009;5:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006;5:499–507. [DOI] [PubMed] [Google Scholar]
- 12.Einarson A Abrupt discontinuation of psychotropic drugs following confirmation of pregnancy: a risky practice. J Obstet Gynaecol Can 2005;11:1019–22. [DOI] [PubMed] [Google Scholar]
- 13.Einarson A, Selby P, Koren G. Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counselling. J Psychiatry Neurosci 2001;1:44–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Sartorius N, Baghai TC, Baldwin DS, et al. Antidepressant medications and other treatments of depressive disorders: a CINP Task Force report based on a review of evidence. Int J Neuropsychopharmacol 2007;S1–207. [DOI] [PubMed]
- 15.Koenig AM, Thase ME. First-line pharmacotherapies for depression - what is the best choice? Pol Arch Med Wewn 2009;7–8:478–86. [PubMed] [Google Scholar]
- 16.Davidson JR. Major depressive disorder treatment guidelines in America and Europe. J Clin Psychiatry 2010;e04. [DOI] [PubMed]
- 17.Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med 1988;4:189–94. [DOI] [PubMed] [Google Scholar]
- 18.Nakhai-Pour HR, Rey E, Berard A. Discontinuation of antihypertensive drug use during the first trimester of pregnancy and the risk of preeclampsia and eclampsia among women with chronic hypertension. Am J Obstet Gynecol 2009;2:180 e1–8. [DOI] [PubMed] [Google Scholar]
- 19.Einarson A, Choi J, Einarson TR, Koren G. Rates of spontaneous and therapeutic abortions following use of antidepressants in pregnancy: results from a large prospective database. J Obstet Gynaecol Can 2009;5:452–6. [DOI] [PubMed] [Google Scholar]
- 20.Hemels ME, Einarson A, Koren G, Lanctot KL, Einarson TR. Antidepressant use during pregnancy and the rates of spontaneous abortions: a meta-analysis. Ann Pharmacother 2005;5:803–9. [DOI] [PubMed] [Google Scholar]
- 21.Nikfar S, Rahimi R, Hendoiee N, Abdollahi M. Increasing the risk of spontaneous abortion and major malformations in newborns following use of serotonin reuptake inhibitors during pregnancy: A systematic review and updated meta-analysis. Daru 2012;1:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross LE, Grigoriadis S, Mamisashvili L, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry 2013;4:436–43. [DOI] [PubMed] [Google Scholar]
- 23.Almeida ND, Basso O, Abrahamowicz M, Gagnon R, Tamblyn R. Risk of Miscarriage in Women Receiving Antidepressants in Early Pregnancy, Correcting for Induced Abortions. Epidemiology 2016;4:538–46. [DOI] [PubMed] [Google Scholar]
- 24.Evans-Hoeker EA, Eisenberg E, Diamond MP, et al. Major depression, antidepressant use, and male and female fertility. Fertil Steril 2018;5:879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kjaersgaard MI, Parner ET, Vestergaard M, et al. Prenatal antidepressant exposure and risk of spontaneous abortion - a population-based study. PLoS One 2013;8:e72095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sorensen MJ, Kjaersgaard MI, Pedersen HS, et al. Risk of Fetal Death after Treatment with Antipsychotic Medications during Pregnancy. PLoS One 2015;7:e0132280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yonkers KA, Blackwell KA, Glover J, Forray A. Antidepressant use in pregnant and postpartum women. Annu Rev Clin Psychol 2014;369–92. [DOI] [PMC free article] [PubMed]
- 28.Promislow JH, Makarushka CM, Gorman JR, Howards PP, Savitz DA, Hartmann KE. Recruitment for a community-based study of early pregnancy: the Right From The Start study. Paediatr Perinat Epidemiol 2004;2:143–52. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman CS, Messer LC, Mendola P, Savitz DA, Herring AH, Hartmann KE. Comparison of gestational age at birth based on last menstrual period and ultrasound during the first trimester. Paediatr Perinat Epidemiol 2008;6:587–96. [DOI] [PubMed] [Google Scholar]
- 30.Cox DR, Oakley A. Analysis of survival data London: Chapman & Hall, 1984. [Google Scholar]
- 31.Dupont WD. Statistical Modeling for Biomedical Researchers Cambridge: Cambridge University Press, 2009. [Google Scholar]
- 32.Harrell J, Frank E. Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis, 2nd Edition. New York: Springer, 2015. [Google Scholar]
- 33.Prevention CfDCa. Unintended pregancy prevention. Reproductive health “Online Database”
- 34.Pryor J, Patrick SW, Sundermann AC, Wu P, Hartmann KE. Pregnancy Intention and Maternal Alcohol Consumption. Obstet Gynecol 2017;4:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugiura-Ogasawara M, Furukawa T, Nakano Y, Hori S, Aoki K, Kitamura T. Depression as a potential causal factor in subsequent miscarriage in recurrent spontaneous aborters. Hum Reprod 2002;10:2580–84. [DOI] [PubMed] [Google Scholar]
- 36.Hoirisch-Clapauch S, Brenner B, Nardi AE. Adverse obstetric and neonatal outcomes in women with mental disorders. Thromb Res 2015;S60–3. [DOI] [PubMed]
- 37.Sundermann AC, Hartmann KE, Jones SH, Torstenson ES, Velez Edwards DR. Validation of maternal recall of early pregnancy medication exposure using prospective diary data. Ann Epidemiol 2017;2:135–39 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


