Abstract
The adrenal cortex governs fundamental metabolic processes though synthesis of glucocorticoid, miner-alocorticoids and androgens. Studies in rodents have demonstrated that the cortex undergoes a self-renewal process and that capsular/subcapsular stem/progenitor cell pools differentiate towards functional steroidogenic cells supporting the dynamic centripetal streaming of adrenocortical cells throughout life. We previously demonstrated that the Notch atypical ligand Delta-like homologue 1 (DLK1)/preadipocyte factor 1 (PREF1) is expressed in subcapsular Sf1 and Shh-positive, CYP11B1-negative and CYP11B2-partially positive cortical progenitor cells in rat adrenals, and that secreted DLK1 can modulate GLI1 expression in H295R cells. Here we show that the human adrenal cortex remodels with age to generate clusters of relatively undifferentiated cells expressing DLK1. These clusters (named DLK1-expressing cell clusters or DCCs) increased with age in size and were found to be different entities to aldosterone-producing cell clusters, another well-characterized and age-dependent cluster structure. DLK1 was markedly overexpressed in adrenocortical carcinomas but not in aldosterone-producing adenomas. Thus, this data identifies a novel cell population in the human adrenal cortex and might suggest a yet-to be identified role of DLK1 in the pathogenesis of adrenocortical carcinoma in humans.
Keywords: DLK1, Adrenal cortex, Remodeling, Steroidogenesis, Adrenocortical carcinoma
1. Introduction
The adrenal cortex is the primary site of steroid synthesis, producing glucocorticoids under the control of the hypothalamic-pituitary axis and mineralocorticoids under the control of the renin-angiotensin system. Glucocorticoids affect carbohydrate metabolism and mediate the mammalian stress response, while mineralocorticoids control blood volume and salt homeostasis, and hence influence blood pressure. The adrenal cortex undergoes self-renewal and important paracrine effectors underlying this phenomenon, both in the embryo and adult, have been identified with the use of specific mouse transgenic models. For example, we have shown that sonic hedgehog (Shh) is expressed in relatively undifferentiated cells throughout life in the subcapsular region of the mouse [1] and rat [2] adrenal starting from e12.5 and e13.5, respectively. Capsule cells transduce the Shh signal, and lineage-tracing studies showed that Gli1+ capsular cells delaminate into the cortex, lose their responsiveness to Shh, and become fully mature steroidogenic cells forming the distinct histological and functional layers; zona glomerulosa (ZG, secreting aldosterone and expressing CYP11B2/aldosterone synthase) and zona fasciculata (ZF, secreting glucocorticoids, expressing CYP11B1/11βhydroxylase) [1]. Capsular Gli1+ cells are therefore one source of new steroidogenic cells during development, as well as during postnatal ZF regeneration [3], and we have shown that paternally expressed delta-like homologue 1 (Dlk1), a cleavable single-pass transmembrane protein and a member of the Notch/Delta/Serrate family [4] (and co-expressed with Shh secreting cells in rat adrenals) tightly regulates Gli1 levels in H295R in a β1-integrin and ERK1/2 dependent fashion [2]. Interestingly, Dlk1 is preferentially expressed in embryonic tissues and immature cells [5], is regulated by hypoxia and facilitates the maintenance of an undifferentiated phenotype while promoting clonogenicity [6]. In adults, expression is low in most healthy tissues, however, expression is reported in hepatocellular, colonic, pancreatic and breast carcinomas at a high frequency [7]. Recently, higher expression of DLK1 has been shown to predict worse clinical outcome in hepatocellular carcinoma [8].
We show here that the human adrenal cortex remodels in regard to DLK1 expression during our lifetime, generating clusters of incompletely differentiated cortical cells. In addition, we show that DLK1 is overexpressed in ACC but not in benign adrenal tumors.
2. Material and methods
2.1. Tissues collection and processing
Specimens were collected from patients undergoing surgery at each of St Bartholomew’s, University College and Hammersmith Hospitals, London, after written consent obtained from participants and under the study protocol Genetics of endocrine tumors (REC: 06/Q0104/133). A series of FFPE histological sections of normal adrenals, benign and malignant adrenocortical tumors included in this study were obtained from tissue Biobanks (C.H.U.S, Consejeria de Salud, Fundacion MDAnderson, Fundacion Ramon Domínguez, HCB-IDIBAPS, IMIB, IMIM, IRBLleida, i+12, Sant Joan de Deu) integrated in the Spanish National Biobanks Network (www.redbiobancos.es): they were processed following standard operating procedures with the appropriate approval of the Ethics and Scientific Committees. No ENSAT/Weiss scoring as well as hormonal status was available for sections obianed from Biobanks.
Freshly collected adrenal glands were fixed in 4% paraformaldehyde (PFA) (Acros Organics) overnight at 4°C and embedded into paraffin (VWR) using standard procedures. Sections were cut at 6–8 μm using a rotary microtome (Thermo scientific) and transferred onto superfrost plus glass slides (VWR).
Cryo-sections were used for immunofluorescence and in situ hybridization experiments: briefly, adrenals were fixed in 4% PFA overnight at 4°C, washed in PBS for 1 h and then incubated in filtered 30% sucrose solution (Fisher Scientific) until the tissue sunk to the bottom of the tube. Specimens were then transferred in a container filled with liquid optimal cutting temperature compound (OCT) (VWR), oriented and placed on dry ice until the OCT solidified. Embedded specimens were stored at −80°C. Sections of OCT were cut at 12–14 μm using a cryostat (Leica GM1510S) and mounted on superfrost plus glass slides. Sections were dried at room temperature (RT) overnight and then stored at −80°C.
2.2. Immunohistochemistry
Sections were deparaffinised in xylene, washed in 100% ethanol and incubated in 3% H2O2 (Sigma) diluted in methanol for 30 min at RT to block endogenous peroxidase activity. After dehydration in a descending ethanol series, sections were treated with 10% goat serum in PBS-Triton 0.1% (T-PBS) for 1 h and then incubated overnight with the primary antibody (Table 1) at RT. Slides were washed with T-PBS and incubated with biotinylated goat anti-rabbit secondary antibody (Table 1) diluted in T-PBS for 2 h at RT. After further washes in T-PBS slides were incubated at RT for 1 h with the Avidin-Biotin Complex (Vector labs, PK-6100) according to the manufacturer’s instructions. Following washes sections were developed with 3,3′-diaminobenzidine (Vector Labs) and counter-stained with Gill hematoxylin (Sigma). Slides were dehydrated, incubated with xylene and mounted using Vecta-mount mounting medium (Vector Labs).
Table 1.
Primary and secondary antibodies.
| Antibody | Host | Source | Dilution | Application | Tissue processing |
|---|---|---|---|---|---|
| SF1 (A1) | Mouse | Santa Cruz Biotechnology sc-393592 | 1:200 | IF (AUM) | PFSCOE |
| CYP11A1 (D8F4F) | Rabbit | Cell Signaling | 1:400 | IF | PFSCOE |
| CYP11B1 | Rat | Celso Gomez-Sanchez | 1:100 | IF (AUM) | PFSCOE |
| CYP11B2 | Mouse | Celso Gomez-Sanchez | 1:100 | IF (AUM) | PFSCOE |
| CYP17A1 | Rabbit | A gift from Ian Bird, University of Wisconsin | 1:500 | IF | PFSCOE |
| DLK1 (H-118) | Rabbit | Santa Cruz Biotechnology sc-25437 | 1:500 | IF, IHC, WB | PFPE + PFSCOE |
| DLK1 (B-7) | Mouse | Santa Cruz Biotechnology sc-376755 | 1:200 | IF (AUM) | PFPE + PFSCOE |
| DAB2 (H-110) | Rabbit | Santa Cruz Biotechnology sc-13982 | 1:200 | IF | PFSCOE |
| VILIP1 | Rabbit | Abcam ab151741 | 1:100 | IF | PFSCOE |
| SOAT1 (H-125) | Rabbit | Santa Cruz Biotechnology sc-20951 | 1:300 | IHC | PFPE |
| Anti-mouse IgG Alexa Fluor 488 | Goat | Invitrogen A11029 | 1:1000 | IF | |
| Anti-rabbit IgG Alexa Fluor 568 | Goat | Invitrogen A11036 | 1:1000 | IF | |
| Anti-rat IgG Alexa Fluor 488 | Goat | Invitrogen A11006 | 1:1000 | IF | |
| Biotinylated Anti-Rabbit | Goat | Vector Labs BA-1000 | 1:500 | IHC | |
| RDye 800 Anti-Rabbit | Goat | LICOR 925–32211 | 1:10000 | WB |
Abbreviations: AUM, required antigen unmasking; IF, immunofluorescence; IHC, immunohistochemistry; PFPE: paraformaldehyde-fixed paraffin-embedded; PFSCOE, paraformaldehyde-fixed sucrose-cryoprotected, OCT-embedded; WB, western blotting.
2.3. Immunofluorescence
Sections were fixed in 4% PFA in PBS for 10 min on ice and then washed in T-PBS. If needed, sections were incubated in 10 mM Citrate Buffer pH 6.0 for 30 min in water bath at 95°C (antigen unmasking) and then allowed to cool for 20 min at RT before blocking with 10% goat serum in T-PBS for 1 h at RT. They were then incubated with primary antibodies (Table 1) overnight at RT, washed in T-PBS, incubated with fluorescently -labelled secondary antibodies (Table 1) and reacted with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) before mounting.
2.4. Non-radioactive in situ hybridization
A cDNA fragment of human DLK1 was amplified via polymerase chain reaction (PCR) using human adrenal cDNA as template with the following primers FW:5′-AAATGGATTCTGCGAGGATG-3′; REV:5′-CAGGCCCGAACATCTCTATC-3′. The amplicon was cloned into pGEM-T easy (Promega) and sequenced. Probe preparation and in situ protocol was performed as previously described [9].
2.5. Image acquisition and analysis
Images were acquired using a Leica DM5500B microscope (Leica, Nussloch, Germany), equipped with a DCF295 (for bright field) and DCF365FX cameras (for fluorescence) (Leica) and Leica Application Suite (LAS) software (Leica), and then processed with Abode Photoshop CS6 and Adobe Illustrator CS6. Analysis of DLK1 staining (DLK1 stained area/total area of adrenal cortex or ACC), change in expression pattern (layered-continuous vs clustered) and DCC size was performed on acquired panoramic images (Olympus BX61) using the HALO™ image analysis software (Indica Labs).
2.6. Western blotting
Freshly isolated adrenals (n = 5) and ACC (n = 5) were minced using a Precellys 24 homogenizer (Bertin Instruments) with Precellys Lysis kit in RIPA buffer. Samples were kept on ice for 20 min before centrifugation at 13,000 rpm at 4°C. The concentration of the super-natant was assessed using the BCA Assay kit (Thermo Fisher). Equal amounts of protein were run on a 10% acrylamide gel, before blotting onto nitrocellulose membranes (Protran), blocking with 5% nonfat dry milk in PBS containing 0.1% Tween-20 and incubating with anti DLK1 antibody (H118, Table 1) overnight at 4°C. After washes membranes were incubated with secondary antibodies (Table 1). Immunoblots were scanned using the Odyssey Fc Imaging System (LI-COR). Ponceau red staining of membranes was used to assess equal loading.
2.7. Analysis of public genomic data sets
Affymetrix Human Gene 2.0 ST gene expression array, performed on 44 adrenocortical carcinomas and deposited by the European Network for the Study of Adrenal Tumors (ENSAT), was downloaded from Gene Expression Omnibus (GSE49278). After batch correction, one-way ANOVA analysis with Fisher’s Least Significant Difference (LSD) the Partek Genomics Suite 6.6 software was used to examine differentially expressed transcripts with a stringent false discovery rate (FDR) less than 0.05. Unsupervised clustering of ACC gene expression was performed as previously reported, with tumors clustering in two groups C1A and C1B [10,11].
RNAseq FASTQ data from The Cancer Genome Atlas (TCGA-phs000178, http://cancergenome.nih.gov/) (n = 79 ACC) and Genotype-Tissue Expression project (GTEx-phs000424, https://www.gtexportal.org/home/) (n = 45 normal adrenals), both performed on Illumina HiSeq platform, were downloaded from dbGaP. Raw FASTQ RNA sequencing reads were analyzed concurrently and mapped to the human genome sequence (hg19) using GSNAP. Transcript assembly and expression was calculated by Cufflinks as the fragments per kilobase of exon per million mapped reads (FPKM) [12] and analyzed for differential gene expression by ANOVA in R [10,12–14]
2.8. Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Group differences were analyzed by one-way or two-way ANOVA, Pearson’s correlation test, using GraphPad Prism software (GraphPad Software Inc).
3. Results
3.1. The human adrenal cortex remodels to generate clusters of cells expressing DLK1 (DCCs) with age
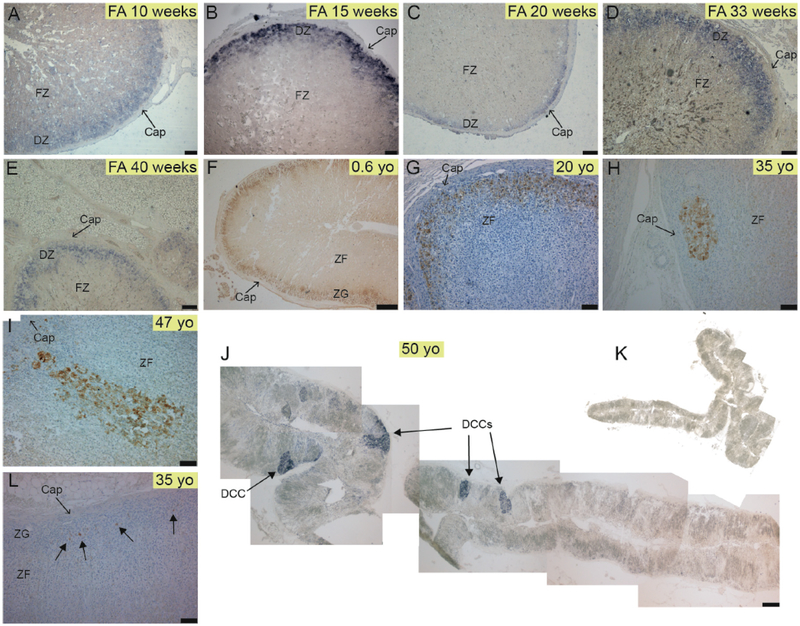
DLK1 protein and DLK1 mRNA expression were assessed in normal human adrenal samples through immunohistochemistry (IHC) and non-radioactive in situ hybridization (NR-ISH), respectively. IHC and NRISH on consecutive sections yielded exactly the same pattern (see Fig. 2 I–K). Samples ranged from fetal adrenal (FA) to late life. Like in rat [2], DLK1 was expressed in the outer part of the cortex in a layered-continuous manner in FAs (Fig. 1A–E) and adrenals from younger donors (Fig. 1F and G). However, starting from the third decade DLK1 expression was also present as clusters of cells (Fig. 1H–J); we named these structures DLK1-expressing Cell Clusters (DCCs). DCCs were mainly subcapsular with a round shape (Fig. 1H) although occasionally could be located deeper in the ZF (Fig. 1I). We have not previously encountered structures resembling DCCs in young or aging (> 2 yo) rodent adrenals (not shown). In adult humans (other than DCCs), large areas of the cortex were either devoid of DLK1+ cells or expressed DLK1 sparingly (Fig. 1L).
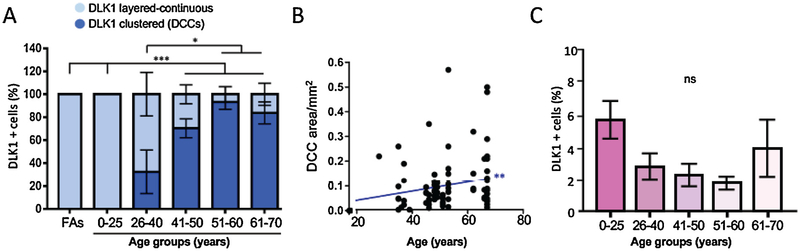
Fig. 2. DCCs appear in the third decade of life and increase in size with age.
A) Clustered (DCCs) and layered-continuous DLK1 expression in physiologically normal human adrenals are expressed as percentage of total cortical DLK1 staining in each age group. Error bars represent standard error of the mean, two-way ANOVA: * (p < 0.05), *** (p < 0.001) B) Quantitative analysis of the size of DCCs with age. Pearson correlation test. C) Percentage of total DLK1 staining expressed as percentage of DLK1 staining/total cortical area showing a non-significant trend of reduction of DLK1 positivity up to approximately 60 yo. ns, not significant. For A–C (also applicable to Fig. 1), n = 11 (FAs), 7 (0–25 yo), 6 (26–40 yo), 5 (41–50 yo), 6 (51–60 yo), 8 (61–70 yo).
Hadjidemetriou et al., Fig 3 (2 columns fitting).
Fig. 1. The human adrenal cortex remodels to generate clusters of cells expressing DLK1.
A-E) Expression of DLK1 mRNA in human fetal adrenals showing a subcapsular layered-continuous pattern in the definitive zone (DZ). F-G) DLK1 protein expression in a 0.6 yo and 20 yo adrenal showing a subcapsular layered-continuous pattern. H) A round-shaped subcapsular DCC in a 35 yo adrenal. I) A columnar-shaped DCC in a 47 yo adrenal. J) Expression of DLK1 mRNA in a 50 yo adrenal showing multiple DCCs and absence of a layered-continuous pattern of DLK1 expression. K) Control sense probe in an adjacent section to J. L) From the third decade onwards, large areas of the cortex were devoid of either clustered or layered-continuous DLK1 staining, and have single cells sparingly expressing DLK1 or no DLK1 expression at all. Abbreviations: Cap, capsule; DCC, DLK1 cells cluster; DZ, Definitive Zone; FZ, fetal Zone; ZG, Zona Glomerulosa; ZF, Zona Fasciculata. Scale bars A, B = 75 μm; C = 200 μm; D, E, G, H, I, L = 100 μm; F = 500 μm; J = 1500 μm.
Hadjidemetriou et al., Fig 2 (1.5 columns fitting).
DCCs comprised 28% of total DLK1 staining in the cortex in the 26–40 yo group (ranging from 0 to 71% as shown in Fig. 2A). An increase in DCC appearance was observed in the age group 41–50 yo, where an average of 70% DLK1+ cells were arranged in clusters (range of 50–100%). In age groups of 51–60 and 61–70 yo, DCCs accounted for approximately 80–95% of total DLK1 staining. We observed a significant positive correlation between DCC area and age, with a Pearson’s correlation coefficient of r = 0.5852 (Fig. 2B). However, there was a trend of decreased DLK1 staining/total cortex area (both clustered and layered-continuous) with age (Fig. 2C). We did not find gender differences regarding DLK1 expression and DCC appearance (not shown).
3.2. DCCs are different entities to APCCs and are poorly steroidogenic
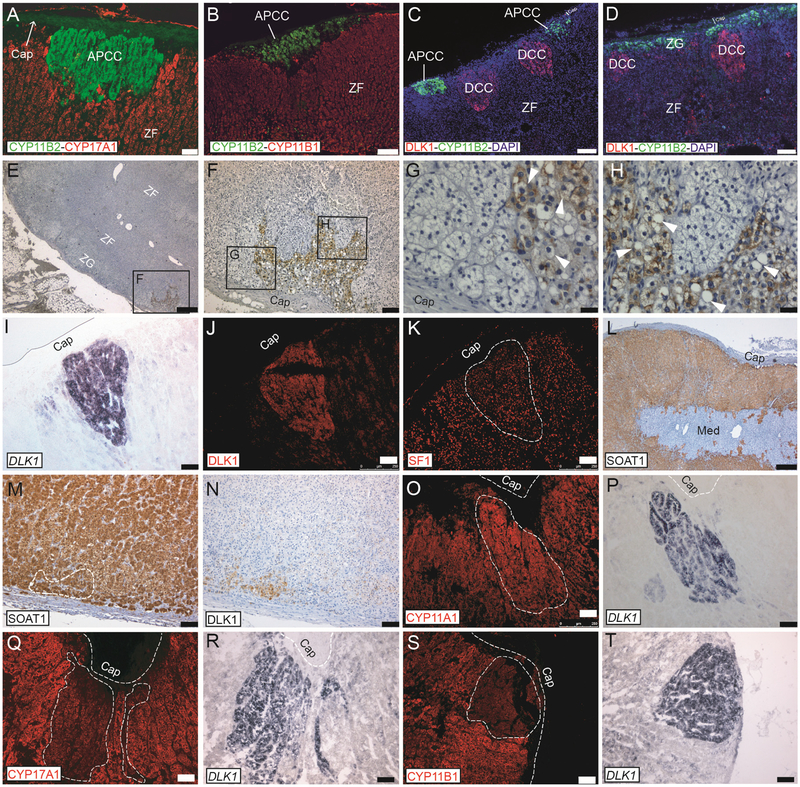
The dual pattern of clustered and layered-continuous is remarkably similar to that of CYP11B2 (Aldosterone Synthase), a marker of functional ZG cells. Aldosterone Producing Cell Clusters (APCCs), which strongly express CYP11B2, possibly in a renin-independent manner, are a common finding in physiologically normal post-pubertal human adrenals [15]. APCCs may be a precursor of benign Aldosterone Producing Adenomas (APAs), after a notable study discovered that nearly half of APCCs (but not neighboring cortical tissue) harbor mutations in ion channels/pumps found in clinically manifesting APAs [15]. As per our observation with DLK1, the staining pattern of CYP11B2 is layered-continuous, and clusters of CYP11B2 (APCCs) develop in adulthood, with their incidence increasing with age [16]. APCCs were easily detectable in our adrenal samples (Fig. 3A and B). Remarkably, APCCs and DCCs were found to be completely separate entities (Fig. 3C) in all adrenals where double staining was performed (n = 7, post 40 yo adrenals). Even DCCs located adjacent to layered-continuous “classical” ZG never expressed CYP11B2 (Fig. 3D).
Fig. 3. DCCs are different entities to APCCs and are incompletely differentiated.
A–B) APCCs of different shapes and sizes can be detected by specific anti-CYP11B2 antibodies and were subcapsular in CYP17A1 and CYP11B1 negative cells. C) Double staining with DLK1 and CYP11B2 showing that DCCs and APCCs are different cellular entities in the adrenal cortex. Two APCCs and two DCCs are seen. D) DCCs can be located adjacent to CYP11B2 positive cells with the latter organized in a classical ZG manner (subcapsular layered-continuous); even in this case double-positive cells are not visible. Age of adrenal in A–D is 50 yo. E–H) A DCC in the adrenal of a 28 yo donor stained with anti DLK1 antibodies and counterstained with hematoxylin. Structural features of DCC are in G and H, where vacuoles are indicated by white arrowheads. I–J) Three consecutive sections were processes for DLK1 in situ hybridization (I) or DLK1 IF (J), showing an identical pattern of DLK1 mRNA and protein expression. The third section was stained with anti SF1 antibodies (K), showing DCCs to be SF1 positive. L–N) SOAT1 is expressed throughout the cortex but not in the capsule and in the medulla (L). Consecutive adrenal sections were processes for SOAT1 (M) and DLK1 (N) IHC showing DCCs to be SOAT1 positive. O–P) Consecutive adrenal sections were processed for CYP11A1 IF (O) and DLK1 in situ hybridization (P), showing DCCs to be CYP11A1 positive. Q–R) Consecutive adrenal sections were processes for CYP17A1 IF (Q) and DLK1 in situ hybridization (R), showing DCCs to express low/nil levels of CYP17A1. S–T) Consecutive adrenal sections were processes for CYP11B1 IF (S) and DLK1 in situ hybridization (T), showing DCCs to express low/nil levels of be CYP11B1. Abbreviations: APCC: Aldosterone-Producing Cell Cluster; Cap, capsule; DCCs, DLK1 Cells Clusters; ZG, Zona Glomerulosa; ZF, Zona Fasciculata; Med., medulla. Scale bars A–D = 75 μm; E and L = 500 μm; F, I–K and M–T = 100 μm; G, H = 25 μm. n = 3 or > 3 each experiment.
Hadjidemetriou et al., Fig 4 (2 columns fitting).
Moreover, known markers of ZG in rodents, such as disabled-2 (DAB2, a mitogen-regulated phosphoprotein and possible modulator of aldosterone synthesis [17]) and Visinin-like 1 (VILIP1, a neuronal calcium sensing protein and maker of ZG in rats [18]) did not localize to DCCs, nor for APCCs, and showed a layered-continuous pattern irrespective of donor’s age (Fig S1). To our knowledge, APCC-like structures, like DCCs, have not been described in rodents or non-human adrenals.
DCCs were irregularly packed and lacked the typical bundle and arches structure of the ZG or near-parallel columns structure of the ZF. Cells within DCCs lacked typical polygonal (ZG) or polyhedral (ZF) cell shape and had bigger nuclei, more evident nucleoli, and varying degree of nuclear/cytoplasm ratios (Fig. 3 G and H). DLK1 positive cells within DCCs had both cytoplasmic and membranous staining and had no apparent lipid droplets or multi-vacuolar aspect. Around half of DCCs contained instead larger uni-vacuolar structures (arrowheads in Fig. 3 G and H), and within DCCs there might be cells not expressing DLK1. Interestingly, whilst DCCs cells were clearly SF1 positive (Fig. 3 I–K) and expressed both Sterol O-Acyltransferase 1 (SOAT1, Fig. 3 L–N) and CYP11A1 (Fig. 3 O and P), they expressed low to nil levels of ZF markers such as CYP17A1 (Fig. 3 Q and R) and CYP11B1 (Fig. 3S and T). GATA4 is a transcription factor required for mouse adrenal gland development [19] and accumulation of GATA4 cells is frequently observed in clusters of hyperplastic capsular/subcapsular spindle-like cells observed in aging mice as well as in some transgenic or gonadectomized mice who later develop adrenal tumors [20,21]. We could not detect an enrichment of GATA4 in DCCs (which were indeed GATA4 negative), as GATA4 itself was not expressed or only detectable in sparse ZF cells in postnatal adrenals (Fig S2) in agreement with published data [22].
3.3. DLK1 is overexpressed in ACCs but not in APAs
DLK1 has been shown to be overexpressed in hepatocellular, colonic, pancreatic and breast carcinomas [7], neuroendocrine tumors [23] and gliomas [24].
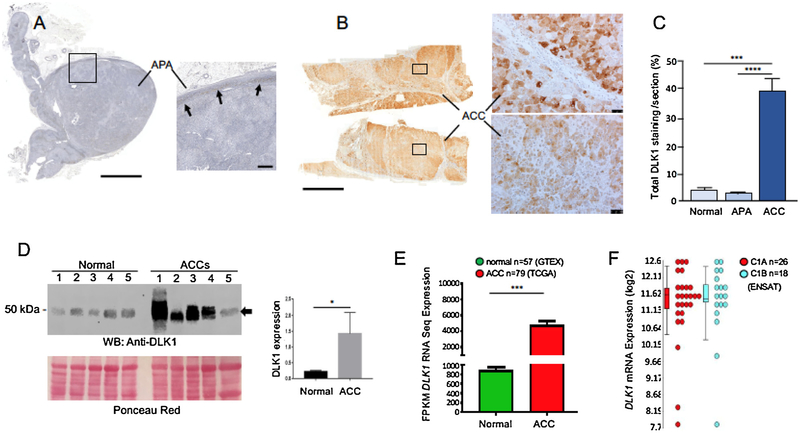
We assessed expression of DLK1 in 9 ACCs (5 females, 4 males), 10 aldosterone-producing adenoma (APA) (8 females, 2 males) and compared it to that of normal adrenals (Fig. 4). While parenchymal cells within APAs expressed very low levels of DLK1 or none at all (Fig. 4A), we observed a heterogeneous pattern of DLK1 expression in ACC in all samples analyzed (Fig. 4B). Measurement of DLK1 staining as a percentage of the total cortical area showed a significant overexpression of DLK1 in ACC samples compared to both normal and APA tissues (Fig. 4C). No significant difference in DLK1 expression was observed in APAs as compared to normal adrenal gland. Western blot analysis of DLK1 expression in a different cohort of 5 normal adrenals and 5 ACC samples corroborated this data (Fig. 4D). We also confirmed this finding using next generation RNA Seq data sets from TCGA (n = 79 ACC) and GTEx (n = 45 normal adrenals), where DLK1 transcript expression was4.6-fold upregulated in ACC samples compared to normal adrenals (p < 0.001) (Fig. 4E). By analyzing the ENSAT gene expression microarray data, no difference in DLK1 expression was observed between aggressive C1A and less aggressive C1B subtypes (Fig. 4F). Moreover, the log2 expression levels suggest that DLK1 is present in ACCs at high levels.
Fig. 4. DLK1 is overexpressed in adrenocortical carcinomas.
Immunohistochemical detection of DLK1 protein in APA (A) and ACC sections (cohort 1, B). In APAs, detectable levels of DLK1 staining are seen in the subcapsular region only (arrows). Note the widespread DLK1 staining in ACC compared to APA. However, in each section analyzed, DLK1 staining was not homogeneous within the ACC parenchyma, with areas of high (top right panel in B) and low (bottom right panel in B) DLK1 expression. C) Quantification of DLK1 staining in ACC, APA and normal adrenal sections (cohort 1) showed a significant higher expression of DLK1 in ACC compared to both normal adrenals and APAs. No ENSAT/Weiss scoring as well as hormonal status was available for these samples. D) Western blot analysis of DLK1 expression in 5 normal adrenals and 5 ACC lysates (cohort 2) showing up-regulation of DLK1 (arrow) in ACC samples. ACCs were as follow: 1) score 2 (ENSAT), score 4/9 (Weiss), cortisol secreting; 2) score 2 (ENSAT), score 6/9 (Weiss), no secreting; 3) score 3 (ENSAT), score 8/9 (Weiss), androgens secreting (plus cortisol from urine steroid profiling); 4) score 3 (ENSAT), score 9/9 (Weiss), androgens and cortisol secreting; 5) score 2 (ENSAT), score 8/9 (Weiss), androgens secreting (plus cortisol from urine steroid profiling). Densitometric analysis of western blot bands is provided in the inset. E) DLK1 transcript expression is significantly higher in ACC compared to normal adrenal, ***(p < 0.001). Green – normal adrenal transcript GTeX (n = 45), Red – ACC transcript TCGA (n = 79) F) DLK1 level is consistently upregulated on mRNA level using ENSAT microarray gene expression data.
Error bars in C represent +/− SEM; One-way ANOVA, with statistical significance denoted as ***(p < 0.001) and ****(p < 0.0001). Error bars in panel to D represent +/− SEM; Mann–Whitney–Wilcoxon test, *(p < 0.05). Analysis of E and F as in Methods. Scale bars A and B = 5000 μm; inset to A = 500 μm; insets to B = 50 μm.
In summary, these results demonstrated that DLK1 is significantly overexpressed in ACC compared to normal adrenal tissue and adenomas.
4. Discussion
The understanding of the complex mechanisms underlying adreno-cortical self-renewal and homeostasis has increased exponentially over the last ten years, mainly due to the generation of specific transgenic mouse models. These studies have uncovered important cell fate regulators and signaling pathways which act by either promoting or inhibiting differentiation events at the capsular/subcapsular region and within steroidogenic zones of the gland (i.e. [1,25–28], and reviewed in [29–32]). It is essential to translate these findings (and pinpoint potential differences) to humans in order to gain a better understanding of both adrenocortical physiology and pathology. Similarly, mouse models are instrumental in dissecting the pathogenic mechanisms in adreno-cortical tumorigenesis and to establish the functional significance of the mutations and alterations identified in genomic studies of adrenal tumors (i.e [28,33]. for ACC, and reviewed in [20,34]). However, it is recognized that some models do not completely phenocopy the human disease in terms of metastatic properties or penetrance [34].
4.1. Dlk1 in humans, rats and mice
We and others previously revealed a possible role for Dlk1 in the adrenal cortex by showing i) its expression in cortical Sf1/Shh-positive, Cyp11b1-negative, Cyp11b2-partially positive progenitor cells in rats [2], ii) its involvement in the regulation of Gli1 levels in H295R cells, possibly through the secreted ectodomain [2], iii) its modulation, which appeared to be inversely correlated to the differentiation status of the ZG following remodeling experiments in rats [2], iv) its potential crosstalk with subcapsular Fgf signaling, as Fgfr2IIIb knock-out mice showed hypertrophic capsule and absence of capsular Dlk1 expression [35], andv) its rapid disappearance after adrenal enucleation in rats and reappearance once zonation is restored [36]. Overall, these results suggest that Dlk1 might be a negative regulator of differentiation in the adrenal cortex, a role that has been well established in adipogenesis [37].
In this study we showed that the human adrenal remodels to generate clusters of cells expressing DLK1, named DCCs. Cells within these clusters were histologically different from neighboring ZG and ZF. Similar to the observations described above in rats, they were poorly steroidogenic, although some differences between species were observed, as DCCs were CYP11B2 negative whilst expressing low/nil levels of ZF markers CYP17A1 and CYP11B1. Another difference between remodeling of the cortex in humans vs rodents is a lack in the latter of cluster-like structures resembling either DCCs or APCCs, even in aging rodents.
Interestingly, while Dlk1 is subcapsular in the rat and human adrenal, it is expressed in capsular cells in mice [35,38]. We do not currently know i) whether capsular Dlk1 cells represent a population of resident stem cells capable of delaminating into the cortex and undergo steroidogenic differentiation and ii) whether capsular Dlk1 cells correspond to Gli1 cells and, if so, to what extent. We believe lineage tracing experiment using Dlk1Cre lines will provide a useful model to answer these questions.
4.2. Are DCCs involved in ACC biology?
The properties of DLK1-expressing cells described in this study: i) poorly steroidogenic in physiologically normal adrenals; ii) appearance of clusters in life through adrenocortical remodeling, similarly to APCCs and iii) overexpression of DLK1 in ACCs compared to APAs and normal adrenals, together with the cancer stem cells properties described for Dlk1 expressing cells in hepatoblastoma and neuronal tumor [6,39] highlight a potential role of DLK1 in the pathogenesis of ACCs, which will need to be proved using appropriate in vivo and in vitro models.
Supplementary Material
Acknowledgements
We are grateful for the generous contribution of the patients and the collaboration of several Biobanks integrated in the Spanish Biobanks Network.
Funding
This work was supported by Biotechnology and Biological Sciences Research Council (BBSRC New Investigator BB/L002671/1, to LG), Rosetrees Trust (M335-F1, to LG), Barts Charity (MGU0436 to LG). IH was supported by an MRC-QMUL PhD studentship. CEGS was supported by R01 HL27255 (NHLBI) and U54 GM115428 (NIGMS). KKV was supported by Cancer League of Colorado Award and K08 CA222620.
Abbreviations:
- APCC
Aldosterone-Producing Cell Cluster
- DCC
Dlk1 cell cluster
- Dlk1
delta-like homologue 1
- ZG
Zona Glomerulosa
- ZF
Zona Fasciculata
- NRISH
non-radioactivein situ hybridization
Footnotes
Declaration of competing interests
The author declare no conflict of interest.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.jsbmb.2019.105422.
References
- [1].King P, Paul A, Laufer E, Shh signaling regulates adrenocortical development and identifies progenitors of steroidogenic lineages, Proc. Natl. Acad. Sci. U. S. A 106 (50) (2009) 21185–21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Guasti L, et al. , Dlk1 up-regulates Gli1 expression in male rat adrenal capsule cells through the activation of beta1 integrin and ERK1/2, Endocrinology 154 (12) (2013) 4675–4684. [DOI] [PubMed] [Google Scholar]
- [3].Finco I, Lerario AM, Hammer GD, Sonic hedgehog and WNT signaling promote adrenal gland regeneration in male mice, Endocrinology 159 (2) (2018) 579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Falix FA, et al. , Possible roles of DLK1 in the Notch pathway during development and disease, Biochim. Biophys. Acta 1822 (6) (2012) 988–995. [DOI] [PubMed] [Google Scholar]
- [5].Floridon C, et al. , Does fetal antigen 1 (FA1) identify cells with regenerative, endocrine and neuroendocrine potentials? a study of FA1 in embryonic, fetal, and placental tissue and in maternal circulation, Differentiation 66 (1) (2000) 49–59. [DOI] [PubMed] [Google Scholar]
- [6].Kim Y, et al. , Hypoxia-regulated delta-like 1 homologue enhances cancer cell stemness and tumorigenicity, Cancer Res 69 (24) (2009) 9271–9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nishina H, hDlk-1: a cell surface marker common to normal hepatic stem/progenitor cells and carcinomas, J. Biochem 152 (2) (2012) 121–123. [DOI] [PubMed] [Google Scholar]
- [8].Seino S, et al. , Clinical outcome of hepatocellular carcinoma can be predicted by the expression of hepatic progenitor cell markers and serum tumour markers, Oncotarget 9 (31) (2018) 21844–21860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Guasti L, et al. , Localization of sonic hedgehog secreting and receiving cells in the developing and adult rat adrenal cortex, Mol. Cell. Endocrinol 336 (1–2) (2011) 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kiseljak-Vassiliades K, et al. , Elucidating the role of the maternal embryonic leucine zipper kinase in adrenocortical carcinoma, Endocrinology 159 (7) (2018) 2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Assie G, et al. , Integrated genomic characterization of adrenocortical carcinoma, Nat. Genet 46 (6) (2014) 607–612. [DOI] [PubMed] [Google Scholar]
- [12].Trapnell C, et al. , Transcript assembly and quantification by RNA-Seq reveals un-annotated transcripts and isoform switching during cell differentiation, Nat. Biotechnol 28 (5) (2010) 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roberts A, et al. , Improving RNA-Seq expression estimates by correcting for fragment bias, Genome Biol 12 (3) (2011) R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roberts A, et al. , Identification of novel transcripts in annotated genomes using RNA-Seq, Bioinformatics 27 (17) (2011) 2325–2329. [DOI] [PubMed] [Google Scholar]
- [15].Nishimoto K, et al. , Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands, Proc. Natl. Acad. Sci. U. S. A 112 (33) (2015) E4591–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nishimoto K, et al. , Case report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism, J. Clin. Endocrinol. Metab 101 (1) (2016) 6–9. [DOI] [PubMed] [Google Scholar]
- [17].Romero DG, et al. , Disabled-2 is expressed in adrenal zona glomerulosa and is involved in aldosterone secretion, Endocrinology 148 (6) (2007) 2644–2652. [DOI] [PubMed] [Google Scholar]
- [18].Trejter M, et al. , Visinin-like peptide 1 in adrenal gland of the rat. Gene expression and its hormonal control, Peptides 63 (2015) 22–29. [DOI] [PubMed] [Google Scholar]
- [19].Tevosian SG, et al. , Adrenal development in mice requires GATA4 and GATA6 transcription factors, Endocrinology 156 (7) (2015) 2503–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Basham KJ, et al. , Mouse models of adrenocortical tumors, Mol. Cell. Endocrinol 421 (2016) 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bielinska M, et al. , Mouse strain susceptibility to gonadectomy-induced adreno-cortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor, Endocrinology 144 (9) (2003) 4123–4133. [DOI] [PubMed] [Google Scholar]
- [22].Kiiveri S, et al. , Differential expression of GATA-4 and GATA-6 in fetal and adult mouse and human adrenal tissue, Endocrinology 143 (8) (2002) 3136–3143. [DOI] [PubMed] [Google Scholar]
- [23].Jensen CH, et al. , Protein structure of fetal antigen 1 (FA1). A novel circulating human epidermal-growth-factor-like protein expressed in neuroendocrine tumors and its relation to the gene products of dlk and pG2, Eur. J. Biochem 225 (1) (1994) 83–92. [DOI] [PubMed] [Google Scholar]
- [24].Yin D, et al. , DLK1: increased expression in gliomas and associated with oncogenic activities, Oncogene 25 (13) (2006) 1852–1861. [DOI] [PubMed] [Google Scholar]
- [25].Walczak EM, et al. , Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms, Mol. Endocrinol 28 (9) (2014) 1471–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Drelon C, et al. , PKA inhibits WNT signalling in adrenal cortex zonation and prevents malignant tumour development, Nat. Commun 7 (2016) 12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Basham KJ, et al. , A ZNRF3-dependent Wnt/beta-catenin signaling gradient is required for adrenal homeostasis, Genes Dev 33 (3–4) (2019) 209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mathieu M, et al. , Steroidogenic differentiation and PKA signaling are programmed by histone methyltransferase EZH2 in the adrenal cortex, Proc. Natl. Acad. Sci. U. S.A 115 (52) (2018) E12265–E12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Finco I, et al. , Hedgehog signaling and steroidogenesis, Annu. Rev. Physiol 77 (2015) 105–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Pihlajoki M, et al. , Adrenocortical zonation, renewal, and remodeling, Front. Endocrinol. (Lausanne) 6 (2015) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yates R, et al. , Adrenocortical development, maintenance, and disease, Curr. Top. Dev. Biol 106 (2013) 239–312. [DOI] [PubMed] [Google Scholar]
- [32].Drelon C, et al. , Adrenal cortex tissue homeostasis and zonation: a WNT perspective, Mol. Cell. Endocrinol 408 (2015) 156–164. [DOI] [PubMed] [Google Scholar]
- [33].Berthon A, et al. , Constitutive beta-catenin activation induces adrenal hyperplasia and promotes adrenal cancer development, Hum. Mol. Genet 19 (8) (2010) 1561–1576. [DOI] [PubMed] [Google Scholar]
- [34].Leccia F, et al. , Mouse models recapitulating human adrenocortical tumors: what is lacking? Front. Endocrinol. (Lausanne) 7 (2016) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guasti L, et al. , FGF signalling through Fgfr2 isoform IIIb regulates adrenal cortex development, Mol. Cell. Endocrinol 371 (1–2) (2013) 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Halder SK, et al. , Cloning of a membrane-spanning protein with epidermal growth factor-like repeat motifs from adrenal glomerulosa cells, Endocrinology 139 (7) (1998) 3316–3328. [DOI] [PubMed] [Google Scholar]
- [37].Hudak CS, Sul HS, Pref-1, a gatekeeper of adipogenesis, Front. Endocrinol. (Lausanne) 4 (2013) 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Heikkila M, et al. , Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production, Endocrinology 143 (11) (2002) 4358–4365. [DOI] [PubMed] [Google Scholar]
- [39].Xu X, et al. , DLK1 as a potential target against cancer stem/progenitor cells of hepatocellular carcinoma, Mol. Cancer Ther 11 (3) (2012) 629–638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.