Abstract
Background.
Ductal carcinoma in situ (DCIS) with microinvasion (DCISM) can be challenging in balancing the risks of overtreatment versus undertreatment. We compared DCISM, pure DCIS, and small volume (T1a) invasive ductal carcinoma (IDC) as related to histopathology, treatment patterns, and survival outcomes.
Methods.
Women ages 18–90 years who underwent breast surgery for DCIS, DCISM, or T1a IDC were selected from the SEER Database (2004–2015). Multi-variate logistic regression and Cox proportional hazards models were used to estimate the association of diagnosis with treatment and survival, respectively.
Results.
A total of 134,569 women were identified: 3.2% DCISM, 70.9% DCIS, and 25.9% with T1a IDC. Compared with invasive disease, DCISM was less likely to be ER? or PR? and more likely to be HER2?. After adjustment, DCIS and invasive patients were less likely to undergo mastectomy than DCISM patients (DCIS: OR 0.53, 95% CI 0.49–0.56; invasive: OR 0.86, CI 0.81–0.92). For those undergoing lumpectomy, the likelihood of receiving radiation was similar for DCISM and invasive patients but lower for DCIS patients (OR 0.57, CI 0.52–0.63). After adjustment, breast-cancer-specific survival was significantly different between DCISM and the other two groups (DCIS: HR 0.59, CI 0.43–0.8; invasive: HR 1.43, CI 1.04–1.96). However, overall survival was not significantly different between DCISM and invasive disease, whereas patients with DCIS had improved OS (HR 0.83, CI 0.75–0.93).
Conclusions.
Although DCISM is a distinct entity, current treatment patterns and prognosis are comparable to those with small volume IDC. These findings may help providers counsel patients and determine appropriate treatment plans.
Ductal carcinoma in situ (DCIS) is considered a preinvasive breast cancer, because it is confined to the milk ducts and, theoretically, lacks the ability to spread to distant sites. In comparison, DCIS with microinvasion (DCISM) is comprised mostly of noninvasive disease (DCIS) plus a small component of invasive disease and presumably has a small, but plausible potential to metastasize. While surgery serves as the foundation of treatment for both DCIS and the majority of invasive disease, additional treatment options vary widely between the two entities. Importantly, chemotherapy is included in the national treatment guidelines for many invasive breast cancers but is not recommended for DCIS.1 Surgical management also may differ according to the presence or absence of invasion, because axillary nodal evaluation is routinely performed for invasive disease but rarely for pure DCIS patients undergoing lumpectomy, given the low rates of positivity.2 Furthermore, omission of surgery and/or adjuvant radiotherapy is the subject of ongoing investigation for select individuals with low-risk DCIS.3–5
Given that DCISM is significantly less common than pure DCIS and most invasive ductal carcinomas, there is limited data regarding its biology and prognosis to guide patient counseling and management recommendations. Several single-institution retrospective studies have evaluated the clinical features, management, prognostic implications, and outcomes for DCISM, yielding conflicting results.6–8 Although there have been two larger studies evaluating DCISM, they had different objectives and both had several study limitations.9,10 Thus, we compared DCISM to DCIS and T1a invasive ductal carcinoma (IDC) in terms of histopathology, treatment patterns, and survival outcomes.
METHODS
Women aged 18–90 years diagnosed with nonmetastatic breast cancer between 2004 and 2015 who underwent surgery were selected from the Surveillance, Epidemiology, and End Results (SEER) database according to the criteria established by Wang et al.9 The cohort was divided into three groups based on histology and tumor size: (1) DCIS, (2) DCISM, and (3) T1a IDC. DCISM and T1a IDC patients were separated based on tumor size (DCISM ≤ 1 mm; T1a IDC > 1 mm and ≤ 5 mm). Per SEER guidelines, if the clinical and pathological stages were discordant, the collaborative stage was determined using the recorded disease-specific data (e.g., if the tumor size was recorded as 1.5 cm, stage T1c was selected).11
Patient characteristics were summarized with N (%) for categorical variables and median (interquartile range, IQR) for continuous variables. Chi square tests or Fisher’s exact tests were used to compare categorical variables, and Wilcoxon rank-sum tests or t tests were used to compare continuous variables, as appropriate. Overall survival (OS) was defined as the time from diagnosis to death or last follow-up. Cancer-specific survival (CSS) was defined as time from diagnosis to death due to currently diagnosed cancer or last follow-up (including death due to other causes). The Kaplan–Meier (KM) method was used to estimate unadjusted OS and CSS; the log-rank test was used to test for differences between groups. Cox proportional hazards regression analyses were utilized to estimate the association between OS/CSS and diagnosis, after adjustment for known covariates. Multivariate logistic regression models were used to estimate the association of diagnosis with type of surgery (mastectomy vs. lumpectomy) among all patients and receipt of radiation (yes vs. no) among patients who underwent lumpectomy, after adjustment for known covariates.
As supplementary analyses, all DCISM patients were stratified, tabulated, and analyzed univariately across age groups (18–39 year, 40–55 year, 56–70 year, and > 70 year). A sensitivity analysis also was performed on the entire study cohort by stratifying patients according to hormone receptor (HR) status and examining OS/CSS through KM curves. HR-positive was defined as estrogen receptor (ER)-positive and/or progesterone receptor (PR)-positive; HR-negative was defined as ER-negative and PR-negative. For the T1a IDC patients, overall stage was derived according to the American Joint Committee on Cancer (AJCC) Staging Manual 8th edition, excluding the Oncotype Dx scores due to data restrictions in SEER.12 Survival for the T1a IDC patients was then analyzed using KM curves.
Only patients with available data for all covariates were included in each model, and effective sample sizes are reported for each table/figure. A p value < 0.05 was considered statistically significant; no adjustments were made for multiple comparisons. All statistical analyses were conducted with R 3.5.0 and SAS, version 9.4 (SAS Institute; Cary, NC). This study was exempt from Institutional Review Board review.
RESULTS
Study Population
Application of the defined inclusion and exclusion criteria resulted in the final study population of 134,569 women (Supplemental Fig. 1), including 4361 with DCISM (3.2%), 95,393 with DCIS (70.9%), and 34,815 with T1a IDC (25.9%). Median follow-up for the entire study population was 66 months. Patient, disease, and treatment characteristics for the study population are summarized in Table 1. Women with invasive disease were slightly older than those with DCIS and DCISM (median age: invasive 62 year vs. DCISM and DCIS 59 year, p < 0.001).
TABLE 1.
Cohort patient, disease, and treatment characteristics for select women with breast cancer in the SEER database from 2004 to 2015, stratified by diagnosis: DCISM, DCIS, and T1a IDC
| All patients N = 134,569 | DCISM N = 4361 (3.2%) | DCIS N = 95,393 (70.9%) | T1a IDC N = 34,815 (25.9%) | Overall p value | |
|---|---|---|---|---|---|
| Age | < 0.001 | ||||
| Median (IQR) | 60 (51–69) | 59 (51–68) | 59 (51–69) | 62 (52–71) | |
| Race/ethnicity | < 0.001 | ||||
| American Indian/Alaska Native | 603 (0.4%) | 18 (0.4%) | 432 (0.5%) | 153 (0.4%) | |
| Asian or Pacific Islander | 12,720 (9.5%) | 465 (10.7%) | 9140 (9.6%) | 3115 (8.9%) | |
| Black | 13,567 (10.1%) | 484 (11.1%) | 10,232 (10.7%) | 2851 (8.2%) | |
| Hispanic | 12,013 (8.9%) | 417 (9.6%) | 8730 (9.2%) | 2866 (8.2%) | |
| Unknown | 703 (0.5%) | 15 (0.3%) | 559 (0.6%) | 129 (0.4%) | |
| White | 94,963 (70.6%) | 2962 (67.9%) | 66,300 (69.5%) | 25,701 (73.8%) | |
| Invasive tumor size (mm) | < 0.001 | ||||
| Median (IQR) | 4 (2–5) | 1 (1–1) | N/A | 4 (3 – 5) | |
| No. of positive LNs | < 0.001 | ||||
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | |
| N stage | < 0.001 | ||||
| N0 | 131,758 (97.9%) | 3920 (89.9%) | 95,389 (100%) | 32,449 (93.2%) | |
| N1 | 2312 (1.7%) | 328 (7.5%) | 0 (0%) | 1984 (5.7%) | |
| N2 | 239 (0.2%) | 56 (1.3%) | 0 (0%) | 183 (0.5%) | |
| N3 | 110 (0.1%) | 36 (0.8%) | 0 (0%) | 74 (0.2%) | |
| NX | 150 (0.1%) | 21 (0.5%) | 4 (0%) | 125 (0.4%) | |
| Grade | < 0.001 | ||||
| 1 | 26,306 (19.5%) | 938 (21.5%) | 12,337 (12.9%) | 13,031 (37.4%) | |
| 2 | 48,521 (36.1%) | 1458 (33.4%) | 32,617 (34.2%) | 14,446 (41.5%) | |
| 3 | 44,662 (33.2%) | 937 (21.5%) | 37,852 (39.7%) | 5873 (16.9%) | |
| Unknown | 15,080 (11.2%) | 1028 (23.6%) | 12,587 (13.2%) | 1465 (4.2%) | |
| ER status | < 0.001 | ||||
| Positive | 96,258 (71.5%) | 3087 (70.8%) | 64,691 (67.8%) | 28,480 (81.8%) | |
| Negative/borderline | 18,923 (14.1%) | 997 (22.9%) | 12,848 (13.5%) | 5078 (14.6%) | |
| Unknown | 19,388 (14.4%) | 277 (6.4%) | 17,854 (18.7%) | 1257 (3.6%) | |
| PR status | < 0.001 | ||||
| Positive | 79,606 (59.2%) | 2457 (56.3%) | 53,005 (55.6%) | 24,144 (69.3%) | |
| Negative/borderline | 30,489 (22.7%) | 1550 (35.5%) | 19,802 (20.8%) | 9137 (26.2%) | |
| Unknown | 24,474 (18.2%) | 354 (8.1%) | 22,586 (23.7%) | 1534 (4.4%) | |
| HER2 status | < 0.001 | ||||
| Positive | 5016 (3.7%) | 456 (10.5%) | 1902 (2%) | 2658 (7.6%) | |
| Negative/borderline | 21,231 (15.8%) | 1269 (29.1%) | 4437 (4.7%) | 15,525 (44.6%) | |
| Diagnosed before 2010* | 64,695 (48.1%) | 2124 (48.7%) | 47,169 (49.4%) | 15,402 (44.2%) | |
| Unknown | 43,627 (32.4%) | 512 (11.7%) | 41,885 (43.9%) | 1230 (3.5%) | |
| Prognostic stage groups (AJCC 8th edition) | < 0.001* | ||||
| Stage 0 | 95,393 (70.9%) | 0 | 95,393 (100%) | 0 | |
| Stage IA | 26,882 (20.0%) | 2094 (48.0%) | 0 | 24,788 (71.2%) | |
| Stage IB | 7671 (5.7%) | 916 (21.0%) | 0 | 6701 (19.2%) | |
| Stage II | 216 (0.2%) | 36 (0.8%) | 0 | 180 (0.5%) | |
| Stage III | 78 (0.1%) | 17 (0.4%) | 0 | 61 (0.2%) | |
| Unable to determine | 4383 (3.3%) | 1298 (29.8%) | 0 | 3085 (8.9%) | |
| Chemotherapy | < 0.001 | ||||
| Yes | 5925 (4.4%) | 476 (10.9%) | 1355 (1.4%) | 4094 (11.8%) | |
| No/unknown | 128,644 (95.6%) | 3885 (89.1%) | 94,038 (98.6%) | 30,721 (88.2%) | |
| Radiation | < 0.001 | ||||
| Yes | 61,391 (45.6%) | 1985 (45.5%) | 42,068 (44.1%) | 17,338 (49.8%) | |
| No | 71,204 (52.9%) | 2311 (53%) | 51,992 (54.5%) | 16,901 (48.5%) | |
| Unknown | 1974 (1.5%) | 65 (1.5%) | 1333 (1.4%) | 576 (1.7%) | |
| Breast surgery type | < 0.001 | ||||
| Lumpectomy | 90,326 (67.1%) | 2464 (56.5%) | 65,649 (68.8%) | 22,213 (63.8%) | |
| Mastectomy | 44,243 (32.9%) | 1897 (43.5%) | 29,744 (31.2%) | 12,602 (36.2%) | |
| Axillary surgery (no. of lymph nodes removed) | < 0.001 | ||||
| 1–5 | 57,667 (42.8%) | 2924 (67%) | 29,265 (30.7%) | 25,478 (73.2%) | |
| 6–9 | 5765 (4.3%) | 347 (8%) | 2598 (2.7%) | 2820 (8.1%) | |
| ≥ 10 | 4654 (3.46%) | 449 (10.3%) | 1359 (1.4%) | 2846 (8.2%) | |
| None | 65, 376 (48.6%) | 605 (13.9%) | 61,325 (64.3%) | 3446 (9.9%) | |
| Breast surgery ± radiation | < 0.001 | ||||
| Lumpectomy alone | 28,953 (21.5%) | 580 (13.3%) | 23,228 (24.3%) | 5145 (14.8%) | |
| Lumpectomy + radiation | 59,799 (44.4%) | 1845 (42.3%) | 41,337 (43.3%) | 16,617 (47.7%) | |
| Mastectomy alone | 42,251 (31.4%) | 1731 (39.7%) | 28,764 (30.2%) | 11,756 (33.8%) | |
| Mastectomy + radiation | 1592 (1.2%) | 140 (3.2%) | 731 (0.8%) | 721 (2.1%) | |
| Other/unknown | 1974 (1.5%) | 65 (1.5%) | 1333 (1.4%) | 576 (1.7%) | |
DCIS ductal carcinoma in situ; DCISM DCIS with microinvasion; IDC invasive ductal carcinoma. LN lymph nodes; ER estrogen receptor; PR progesterone receptor; HER2 human-epidermal-growth-factor-receptor-2
This statistical comparison is only for DCISM versus T1a IDC
Tumor Histopathology
On univariate analysis, DCISM was less likely to be ER-positive (70.8% vs. 81.8%) or PR-positive (56.3% vs. 69.3%) and more likely to be human-epidermal-growth-factor-receptor-2 (HER2)-positive (10.5% vs. 7.6%) compared with T1a tumors. Rates of ER-positivity and PR expression were comparable between DCIS and DCISM (Table 1). After excluding patients with missing biomarker data, similar trends were noted (Supplemental Table 1). Patients with DCISM had higher-grade tumors than patients with invasive disease (Table 1). Although the majority of patients were node-negative for all groups, patients with DCISM were slightly more likely to have a higher N stage than those with invasive disease (7.5% vs. 5.7% N1 disease, 1.3% vs. 0.5% N2 disease; Table 1).
To further define the DCISM cohort specifically, patients were stratified by age: 18–39 year (N = 160), 40–55 year (N = 1556), 56–70 year (N = 1786), and >70 year (N = 859). Younger women with DCISM had a higher percentage of grade 3 (18–39 year: 30% vs. > 70 year: 15.6%) and HER2-positive tumors (18–39 year: 9.4% vs. > 70 year: 5.6%), whereas older women had higher proportions of node-negative (18–39 year: 80% vs. > 70 year: 92.8%), ER-positive (18–39 year: 65.6% vs. > 70 year: 74.9%), and PR-positive tumors (18–39 year: 53.1% vs. > 70 year: 58.8%; Supplemental Table 2). Given the substantial number of patients with missing biomarker and tumor grade data, additional comparisons were made excluding those with missing data, and similar trends were noted (Supplemental Table 3).
Treatment Patterns
Compared with patients with DCIS and invasive disease, a higher proportion of DCISM patients underwent mastectomy (DCISM 43.5% vs. 31.2% for DCIS and 36.2% for invasive; p < 0.001; Table 1). After adjustment, DCISM patients were more likely to undergo mastectomy than DCIS or invasive patients (DCISM: Reference; DCIS: OR 0.53, 95% CI 0.49–0.56, p < 0.001; T1a IDC: OR 0.86, CI 0.81–0.92, p < 0.001; Table 2). For those undergoing lumpectomy, the probability of radiation receipt was similar for DCISM and invasive patients (OR 1.04, CI 0.94–1.15, p = 0.40), whereas lower for DCIS compared with DCISM patients (OR 0.57, CI 0.52–0.63, p < 0.001; Table 3). Rates of chemotherapy receipt were similar for patients with DCISM and invasive disease (10.9% vs. 11.8%, respectively; Table 1).
TABLE 2.
Logistic regression predicting mastectomy receipt (N = 133,461; events = 43,881) for select women with breast cancer in the SEER database from 2004 to 2015. Model adjusted for patient age, race/ethnicity, insurance status, and history of other cancers
| OR (95% CI) | p value | Overall p value | |
|---|---|---|---|
| Diagnosis | < 0.001 | ||
| DCISM | -REF- | ||
| DCIS | 0.53 (0.49–0.56) | < 0.001 | |
| T1a IDC | 0.86 (0.81–0.92) | < 0.001 | |
| Grade | < 0.001 | ||
| 1 | -REF- | ||
| 2 | 1.28 (1.23–1.32) | < 0.001 | |
| 3 | 1.75 (1.69–1.82) | < 0.001 | |
| Unknown | 1.38 (1.31–1.45) | < 0.001 | |
| ER status | < 0.001 | ||
| Positive | -REF- | ||
| Negative/borderline | 1.25 (1.20–1.31) | < 0.001 | |
| Unknown | 1.38 (1.29–1.48) | < 0.001 | |
| PR status | < 0.001 | ||
| Positive | -REF- | ||
| Negative/borderline | 1.23 (1.18–1.28) | < 0.001 | |
| Unknown | 0.99 (0.93–1.06) | 0.82 | |
| HER2 status | < 0.001 | ||
| Positive | -REF- | ||
| Negative/borderline | 0.75 (0.70–0.81) | < 0.001 | |
| Unknown | 0.89 (0.83–0.95) | < 0.001 | |
| Diagnosed before 2010* | 0.83 (0.77–0.88) | < 0.001 |
DCIS ductal carcinoma in situ; DCISM DCIS with microinvasion; IDC invasive ductal carcinoma; ER estrogen receptor; PR progesterone receptor; HER2 human-epidermal-growth-factor-receptor-2
Patients diagnosed before 2010 did not routinely have HER2 status reported in the database
TABLE 3.
Logistic regression predicting radiation receipt for patients undergoing lumpectomy (N = 89,580; events = 59,331) for select women with breast cancer in the SEER database from 2004–2015
| OR (95% CI) | p value | Overall p value | |
|---|---|---|---|
| Diagnosis | < 0.001 | ||
| DCISM | -REF- | ||
| DCIS | 0.57 (0.52–0.63) | < 0.001 | |
| T1a IDC | 1.04 (0.94–1.15) | 0.40 | |
| Grade | < 0.001 | ||
| 1 | -REF- | ||
| 2 | 1.27 (1.22–1.32) | < 0.001 | |
| 3 | 2.03 (1.94–2.13) | < 0.001 | |
| Unknown | 1.03 (0.98–1.09) | 0.21 | |
| ER status | < 0.001 | ||
| Positive | -REF- | ||
| Negative/borderline | 1.03 (0.97–1.10) | 0.35 | |
| Unknown | 0.55 (0.51–0.59) | < 0.001 | |
| PR status | < 0.001 | ||
| Positive | -REF- | ||
| Negative/borderline | 1.12 (1.07–1.18) | < 0.001 | |
| Unknown | 0.81 (0.75–0.87) | < 0.001 | |
| HER2 status | < 0.001 | ||
| Positive | -REF- | ||
| Negative/borderline | 1.20 (1.09–1.32) | < 0.001 | |
| Unknown | 0.99 (0.70–1.08) | 0.77 | |
| Diagnosed before 2010* | 1.18 (1.07–1.29) | < 0.001 |
Patients with unknown radiation receipt were combined with those not receiving radiation therapy. Model adjusted for patient age, race/ethnicity, insurance status, and history of other cancers. DCIS ductal carcinoma in situ; DCISM DCIS with microinvasion; IDC invasive ductal carcinoma; ER estrogen receptor; PR progesterone receptor; HER2 human-epidermal-growth-factor-receptor-2
Patients diagnosed before 2010 did not routinely have HER2 status reported in the database
Survival Outcomes
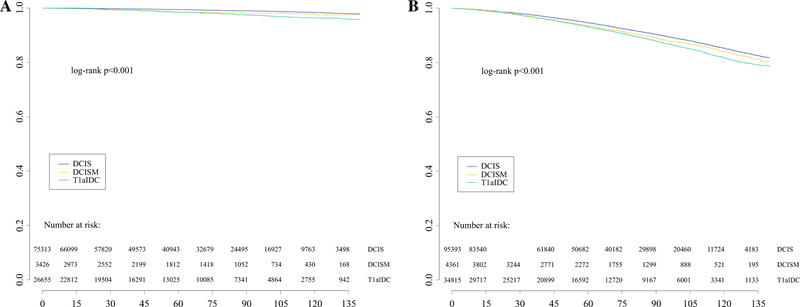
Overall, the unadjusted CSS and OS were high and similar for all groups: 5-year CSS 99% for all groups (Fig. 1a) and 5-year OS 95% for DCIS versus 93% for DCISM and T1a IDC (Fig. 1b). After adjustment, CSS was significantly different between DCISM and the other two cohorts (DCISM: Reference; DCIS: HR 0.59, CI 0.43–0.80, p < 0.001; T1a IDC: HR 1.43, CI 1.04–1.96, p = 0.03; Table 4). However, the adjusted OS was not significantly different between DCISM and invasive disease (HR 0.98, CI 0.87–1.09, p = 0.66), whereas patients with DCIS had improved OS compared with DCISM (HR 0.84, CI 0.75–0.93, p < 0.001; Table 4).
FIG. 1.
Unadjusted survival for select women with breast cancer in the SEER database from 2004 to 2015, stratified by diagnosis: cancer-specific survival (a) and overall survival (b). DCIS ductal carcinoma in situ; DCISM DCIS with microinvasion; IDC invasive ductal carcinoma
TABLE 4.
Cox proportional hazards regression analyses of cancer-specific survival (N = 105,394) and overall survival (N = 134,568) for select women with breast cancer in the SEER database from 2004 to 2015
| Cancer-specific survival |
Overall survival |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Group | ||||
| DCISM | -REF- | ** | -REF- | ** |
| DCIS | 0.59 (0.43–0.80) | < 0.001 | 0.84 (0.75–0.93) | 0.001 |
| T1a IDC | 1.43 (1.04–1.98) | 0.03 | 0.98 (0.87–1.09) | 0.66 |
| Grade | ||||
| 1 | -REF- | ** | -REF- | ** |
| 2 | 1.45 (1.19–1.77) | < 0.001 | 1.06 (1.00–1.12) | 0.05 |
| 3 | 2.06 (1.66–2.55) | < 0.001 | 1.09 (1.03–1.16) | 0.005 |
| Unknown | 1.56 (1.21–2.012) | < 0.001 | 1.09 (1.02–1.17) | 0.02 |
| ER | ||||
| Positive | -REF- | -REF- | ** | |
| Negative/borderline | 1.08 (0.87–1.33) | 0.48 | 1.10 (1.02–1.19) | 0.009 |
| Unknown | 1.05 (0.73–1.53) | 0.78 | 1.25 (1.10–1.41) | < 0.001 |
| PR | ||||
| Positive | -REF- | ** | -REF- | |
| Negative/borderline | 1.33 (1.09–1.61) | 0.004 | 1.05 (0.98–1.11) | 0.16 |
| Unknown | 1.25 (0.88–1.78) | 0.21 | 0.92 (0.82–1.04) | 0.18 |
| HER2 | ||||
| Positive | -REF- | -REF- | ||
| Negative/borderline | 1.78 (1.06–2.97) | 0.03 | 1.02 (0.85–1.23) | 0.81 |
| Unknown | 1.27 (0.75–2.16) | 0.37 | 0.96 (0.80–1.15) | 0.68 |
| Diagnosed before 2010* | 1.45 (0.90–2.36) | 0.13 | 1.02 (0.86–1.22) | 0.79 |
| Local-regional treatment | ||||
| Mastectomy alone | -REF- | ** | -REF- | ** |
| Lumpectomy + radiation | 0.61 (0.52–0.70) | < 0.001 | 0.73 (0.69–0.77) | < 0.001 |
| Lumpectomy alone | 1.17 (0.99–1.39) | 0.06 | 1.10 (1.05–1.16) | < 0.001 |
| Mastectomy + radiation | 2.36 (1.73–3.21) | < 0.001 | 1.31 (1.10–1.56) | 0.003 |
| Other/unknown | 1.08 (0.65–1.79) | 0.76 | 0.92 (0.76–1.12) | 0.41 |
| Chemotherapy | ||||
| No/unknown | -REF- | ** | -REF- | ** |
| Yes | 2.58 (2.09–3.20) | < 0.001 | 1.67 (1.51–1.85) | < 0.001 |
Model adjusted for patient age, race/ethnicity, insurance status, and history of other cancers. DCIS ductal carcinoma in situ; DCISM DCIS with microinvasion; IDC invasive ductal carcinoma; ER estrogen receptor; PR progesterone receptor; HER2 human-epidermal-growth-factor-receptor-2
Patients diagnosed before 2010 did not routinely have HER2 status reported in the database
Overall p value <0.05
All patients were then stratified by hormone receptor (HR) status: HR-positive or HR-negative. For patients with HR-positive disease, the 5-year CSS and OS were high for all three groups (CSS: DCIS 100%, DCISM 99%, T1a IDC 99%; OS: DCIS 95%, DCISM 94%, T1a IDC 93%) (Supplemental Figs. 2A–B). For patients with HR-negative disease, the 5-year CSS and OS were slightly lower but still quite high for all three groups (CSS: DCIS 99%, DCISM 98%, T1a IDC 97%; OS: DCIS 93%, DCISM 92%, T1a IDC 91%; Supplemental Figs. 2C–D).
For both the DCISM and T1a IDC cohorts, prognostic stages were calculated, and the majority were noted to have stage IA disease (DCISM 48.0% vs T1a IDC 71.2%), although stage assignment was not possible for a large proportion of DCISM patients (29.8%) due to missing prognostic variables (Table 1). For patients with stage IA disease in both groups, survival outcomes were similarly high at 5 years (CSS: DCISM 0.99 vs. T1a IDC 0.99; OS: DCISM 0.94 vs. T1a IDC 0.94; Supplemental Table 4). When comparing outcomes between those with DCISM and T1a IDC, those with stage IA (Supplemental Figs. 3A–B) or stages II/III (Supplemental Figs. 3E–F) disease did not have significantly different CSS or OS (all p > 0.05). Although there was a slight difference in CSS for those with stage IB disease (p = 0.002), OS was not significantly different (p = 0.12; Supplemental Figs. 3C–D).
DISCUSSION
In a contemporary cohort of women with breast cancer, we demonstrate that the treatment patterns and prognosis for DCISM more closely reflect that of invasive breast cancer than pure in situ disease, although the prognoses for all three disease processes were excellent. In addition, DCISM appears to have some distinct biologic characteristics that may distinguish it from both entities. As such, pathological upstaging of DCIS to DCISM has the potential to meaningfully change treatments and prognosis in a diagnosis overwhelming made through screening mammography.
In our study, DCISM was more likely to be ER-negative, PR-negative, HER2-positive, and of a higher grade than invasive disease, perhaps suggesting a more aggressive process. DCISM was also more likely than DCIS to be ER-negative and PR-negative. Similar results were found in a recent single-institution retrospective review of 219 patients, which also suggested that DCISM may have a more aggressive biological nature than DCIS due to a higher proportion of triple-negative and HER2-enriched tumors.13 Furthermore, one of the largest studies comparing DCIS and DCISM demonstrated that DCISM was associated with more aggressive tumor biology.9
Notably, DCISM patients in our study cohort had higher mastectomy rates than those with invasive or pure in situ disease. This may be related to the extent of in situ disease surrounding the microinvasive component, which is not reported in the database. Furthermore, previous studies have shown that DCIS on core needle biopsy is upstaged to invasive disease at the time of surgical excision in 15–20% of cases, and this may be related to the extent of disease on preoperative imaging.14–17 For example, women with large or multifocal areas of disease on imaging may have been more likely to undergo mastectomy, and these same women may have only had one or two biopsies of these large areas, which may or may not have been truly representative of the disease process. In other words, the true extent of disease may have been undersampled at the time of biopsy, and we assume that microinvasive disease is more likely to be present in a larger background of DCIS than a small area.
Regarding prognosis, we found that survival outcomes were quite good for all three subgroups and often varied within 1–3% from each other. Furthermore, OS was not significantly different between DCISM and invasive disease, but OS for DCISM was slightly worse than DCIS. Our findings align with those of two prior studies evaluating DCISM patients in SEER.9,10 In a large cohort of 87,695 DCIS and 8863 DCISM patients, those with DCISM had a worse OS (HR 1.263, p < 0.001).9 In contrast to our work, this study did not include invasive disease (vs. T1a included), restricted patient ages to 20–69 year (vs. ages 18–90 year), and included more historic data starting in 1990 (vs. starting in 2004). In another large cohort of 525,395 women with DCIS or small (≤ 2 cm) node-negative invasive breast cancer, Sopik et al. similarly found that DCISM more closely resembled small invasive breast cancers than pure DCIS.10 However, this study excluded node-positive disease (9.6% of DCISM patients had positive nodes in our study) and HER2 status (10.5% of DCISM patients were HER2-positive in our study), and it also included older data (1990–2013 vs. 2004–2015 in our study). In contrast to these studies, one of the significant highlights of our work is the comparison of invasive disease using the latest prognostic staging groups from the AJCC 8th edition, which again demonstrated similar survival outcomes for those with DCISM and T1a IDC. Irrespective of the differences, all three studies suggest that DCISM prognosis is more similar to invasive disease than pure DCIS. Given these findings, the name ‘‘DCIS with microinvasion’’ may need to be reconsidered, because it may suggest to some that the DCIS component is the most important aspect of the disease process (particularly as it relates to prognosis) and may be misleading. Furthermore, DCISM may be more appropriately included in the T1a subgroup in future revisions of the AJCC’s staging manual, as opposed to being listed as a separate entity.
Additional research is needed to better understand the underlying biology and relationship between in situ and invasive disease. While DCIS is a known nonobligate precursor to invasive disease, the exact progression is poorly understood. Some have proposed a sequential model of progression from in situ to invasive disease, whereas others have suggested that DCIS may already possess metastatic potential from inception.18–20 Previous research has shown that DCIS and IDC have a similar degree of chromosomal alterations, and DCIS associated with invasive disease is genetically similar to the invasive component.21–24 However, studying the genomics of DCISM may further advance our understanding of breast cancer progression.
Controversy exists around the risks associated with DCIS and the appropriate standard therapy. As such, the breast cancer community is evaluating de-escalation of locoregional therapy for pure DCIS.3–5 However, our study suggests that outcomes for women with DCISM more closely reflect those of small invasive cancers and thus may warrant comparable treatment. Locoregional therapy, particularly as it relates to surgical margins and radiation, should likely be tailored accordingly. Furthermore, it is critical that breast cancer patients understand the potential implications of microinvasion, including the possible need for more aggressive treatments. When diagnosed, clinicians must be alerted to this potential change in prognosis and tailor treatment recommendations accordingly, while also educating patients about this unique disease entity.
Study Limitations
The limitations of our study include those inherent in any retrospective analysis of a large national database. As previously discussed, there were several limitations related to extent of disease, which may have affected treatment planning. Additionally, we were unable to identify patients who received neoadjuvant chemotherapy and may have been downstaged. However, the TNM stages entered into SEER are based on the collaborative stage, which relies on the specific tumor size and number of positive nodes to determine the T and N stages, respectively.11 Regarding nodal status, any DCIS patient in SEER with positive lymph nodes is recorded as an ‘‘invasive’’ tumor, and it is uncertain whether these patients may have been included in the DCISM or T1a IDC cohorts instead. Per national guidelines, the grading criteria for in situ and invasive disease are notably different and, as such, precludes meaningful comparisons of grade assignments between in situ and invasive disease. Furthermore, grade was missing in a large proportion of DCISM patients (23.6%), possibly because the invasive component was too small to adequately grade. HER2 status was missing for a large proportion of DCIS patients (43.9%), as were ER and PR status (18.7% and 23.7%, respectively). The SEER database does not include data on receipt of endocrine therapy or type of facility (e.g., academic, community, etc.), which may have affected outcomes for all of subgroups.
CONCLUSIONS
DCIS, DCISM, and T1a IDC all have excellent prognoses with high rates of CSS and OS. However, within the spectrum of DCIS, there is marked heterogeneity, with some DCIS at very low risk of progression to invasive disease and others that are similar to small-volume invasive disease. Our data suggest that DCISM in particular may be more similar to invasive disease and that many practitioners are treating it accordingly. These findings related to the histopathology and prognosis of DCISM may help providers to counsel patients more accurately and to determine the best management strategy.
Supplementary Material
ACKNOWLEDGMENT
The Surveillance, Epidemiology, and End Results (SEER) database is supported by the Surveillance Research Program in the National Cancer Institute’s Division of Cancer Control and Population Sciences. The SEER database is the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors. Dr. R. Greenup is supported by the National Institutes of Health Office of Women’s Research Building Interdisciplinary Research Careers in Women’s Health K12HD043446 (PI: Andrews). Dr. O. Fayanju is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number Award Number 1KL2TR002554 (PI: Svetkey). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Presentation: Oral presentation at the 20th Annual American Society of Breast Surgeons’ Meeting; Dallas, TX, May 2019.
Electronic supplementary material The online version of this article (https://doi.org/10.1245/s10434-019-07556-9) contains supplementary material, which is available to authorized users.
DISCLOSURES None.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
REFERENCES
- 1.Gradishar WJ, Anderson BO, Aft R, et al. NCCN guidelines: breast cancer, version 1. 2018 20 March 2018.
- 2.Shapiro-Wright HM, Julian TB. Sentinel lymph node biopsy and management of the axilla in ductal carcinoma in situ. J Natl Cancer Inst Monogr 2010;2010(41):145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang ES, Hyslop T, Lynch T, et al. The COMET (Comparison of Operative to Monitoring and Endocrine Therapy) Trial: a phase III randomized trial for low-risk ductal carcinoma in situ (DCIS). BMJ 2018;9:e026797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elshof LE, Tryfonidis K, Slaets L, et al. Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ - The LORD study. Eur J Cancer 2015;51(12):1497–510. [DOI] [PubMed] [Google Scholar]
- 5.Francis A, Fallowfield L, Rea D. The LORIS Trial: Addressing overtreatment of ductal carcinoma in situ. Clin oncol (R Coll Radiol (Great Britain)) 2015;27(1):6–8. [DOI] [PubMed] [Google Scholar]
- 6.Parikh RR, Haffty BG, Lannin D, Moran MS. Ductal carcinoma in situ with microinvasion: prognostic implications, long-term outcomes, and role of axillary evaluation. Int J Radiat Oncol Biol Phys 2012;82(1):7–13. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Zhang S, Wei X, Zhang J. The clinical features and management of women with ductal carcinoma in situ with microinvasion: a retrospective cohort study. Int J Surg (London, Engl) 2015;19:91–4. [DOI] [PubMed] [Google Scholar]
- 8.Fang Y, Wu J, Wang W, et al. Biologic behavior and long-term outcomes of breast ductal carcinoma in situ with microinvasion. Oncotarget 2016;7(39):64182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Zhu W, Du F, Luo Y, Xu B. The demographic features, clinicopathological characteristics and cancer-specific outcomes for patients with microinvasive breast cancer: a SEER database analysis. Sci. Rep 2017;7:42045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sopik V, Sun P, Narod SA. Impact of microinvasion on breast cancer mortality in women with ductal carcinoma in situ. Breast Cancer Res Treat 2018;167(3):787–95. [DOI] [PubMed] [Google Scholar]
- 11.Adjusted AJCC 6th ed. T, N, M, and Stage. National Cancer Institute’s Surveillance, Epidemiology, and End Results Program https://seer.cancer.gov/seerstat/variables/seer/ajcc-stage/6th/#cs. Accessed 16 Apr 2019.
- 12.Hortobagyi GN, Connolly JL, D’Orsi CJ, et al. Breast. In: Amin MB, Gress DM, Meyer Vega LR, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Compton CC, editors. AJCC cancer staging manual 8th edn. New York, NY: Springer; 2016. [Google Scholar]
- 13.Wan ZB, Gao HY, Wei L, et al. Expression of estrogen receptor, progesterone receptor, human epidermal growth factor receptor 2, and Ki-67 in ductal carcinoma in situ (DCIS) and DCIS with microinvasion. Medicine (Baltimore) 2018;97(44):e13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm LJ, Ryser MD, Partridge AH, et al. Surgical upstaging rates for vacuum assisted biopsy proven DCIS: implications for active surveillance trials. Ann Surg Oncol 2017;24(12): 3534–3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Paz Villanueva CC, Bonev V, Senthil M, et al. Factors associated with underestimation of invasive cancer in patients with ductal carcinoma in situ: precautions for active surveillance. JAMA Surg 2017;152(11):1007–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurniawan ED, Rose A, Mou A, et al. Risk factors for invasive breast cancer when core needle biopsy shows ductal carcinoma in situ. Arch Surg 2010;145(11):1098–104. [DOI] [PubMed] [Google Scholar]
- 17.Chin-Lenn L, Mack LA, Temple W, et al. Predictors of treatment with mastectomy, use of sentinel lymph node biopsy and upstaging to invasive cancer in patients diagnosed with breast ductal carcinoma in situ (DCIS) on core biopsy. Ann Surg Oncol 2014;21(1):66–73. [DOI] [PubMed] [Google Scholar]
- 18.Burstein HJ, Polyak K, Wong JS, Lester SC, Kaelin CM. Ductal carcinoma in situ of the breast. N Engl J Med 2004;350(14):1430–41. [DOI] [PubMed] [Google Scholar]
- 19.Narod SA, Sopik V. Is invasion a necessary step for metastases in breast cancer? Breast Cancer Res Treat 2018;169(1):9–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannakeas V, Sopik V, Narod SA. A comparison of two models for breast cancer mortality for women with ductal carcinoma in situ: an SEER-based analysis. Breast Cancer Res Treat 2018;169(3):587–94. [DOI] [PubMed] [Google Scholar]
- 21.Hwang ES, DeVries S, Chew KL, et al. Patterns of chromosomal alterations in breast ductal carcinoma in situ. Clin Cancer Res 2004;10(15):5160–7. [DOI] [PubMed] [Google Scholar]
- 22.Abba MC, Gong T, Lu Y, et al. A molecular portrait of high-grade ductal carcinoma in situ. Cancer Res 2015;75(18):3980–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellsworth RE, Vertrees A, Love B, Hooke JA, Ellsworth DL, Shriver CD. Chromosomal alterations associated with the transition from in situ to invasive breast cancer. Ann Surg Oncol 2008;15(9):2519–25. [DOI] [PubMed] [Google Scholar]
- 24.Iakovlev VV, Arneson NC, Wong V, et al. Genomic differences between pure ductal carcinoma in situ of the breast and that associated with invasive disease: a calibrated aCGH study. Clin Cancer Res 2008;14(14):4446–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.