Abstract
Background:
The long-term cardiovascular risk of isolated elevated office blood pressure is unclear.
Purpose:
To summarize risk of cardiovascular events and all-cause mortality associated with untreated white coat hypertension (WCH) and treated white coat effect (WCE).
Data Sources:
PubMed and Embase without language restriction from inception to December 2018.
Study Selection:
Observational studies with at least three years of follow-up evaluating the cardiovascular risk of white coat hypertension and/or white coat effect compared to normotension.
Data Extraction:
Two investigators independently extracted study data and assessed study quality.
Data Synthesis:
27 studies were included, comprising 25,786 individuals with untreated WCH or treated WCE and 38,487 individuals with normal blood pressure, followed over a mean duration of 3 to 19 years. Compared to normotension, untreated WCH was associated with an increased risk of cardiovascular events (hazard ratio [HR] 1.36, 95% confidence interval [CI] 1.03–2.00), all-cause mortality (HR 1.33, 95% CI 1.07–1.67), and cardiovascular mortality (HR 2.09, 95% CI 1.23–4.48); the risk of WCH was attenuated in studies that included stroke in the definition of cardiovascular events (HR 1.26, 95% CI 1.00–1.54). There was no significant association of treated WCE with cardiovascular events (HR 1.12, 95% CI 0.91–1.39), all-cause mortality (HR 1.11, 95% CI 0.89–1.46), or cardiovascular mortality (1.04, 95% CI 0.65–1.66). The findings persisted across multiple sensitivity analyses.
Limitation:
Sparse studies evaluating isolated cardiac outcomes or reporting participant race and ethnicity.
Conclusion:
Untreated WCH, but not treated WCE, is associated with increased risk of cardiovascular events and all-cause mortality. Out-of-office blood pressure monitoring is critical in the diagnosis and management of hypertension.
Primary Funding Source:
National Institutes of Health
INTRODUCTION
Hypertension is the foremost preventable cause of disability and premature mortality worldwide (1). Hypertension is most commonly diagnosed using in-office blood pressure (BP) measurements. However, recent guidelines strongly recommend out-of-office BP monitoring (including ambulatory BP monitoring [ABPM] and self or home BP monitoring [SBPM or HBPM]) for the diagnosis and management of hypertension (2–4). Increased use of out-of-office BP monitoring in recent decades has led to the identification of several BP phenotypes with different prognostic implications regarding long-term cardiovascular risk (5–7). These BP phenotypes, which require a combination of in-office and out-of-office BP readings to ascertain, include sustained normotension (i.e. normal in-office BP and out-of-office BP in individuals not on antihypertensive treatment), controlled hypertension (normal in-office BP and out-of-office BP in individuals on antihypertensive treatment), masked hypertension (normal in-office BP but elevated out-of-office BP), white coat hypertension (WCH; elevated in-office but normal out-of-office BP, described as WCH in individuals not on antihypertensive treatment, and as white coat effect [WCE] or white coat uncontrolled hypertension in individuals on antihypertensive treatment), and uncontrolled hypertension (elevated in-office and out-of-office BP).
Despite guideline recommendations, real-world practice has been slow to adopt out-of-office BP monitoring (8). The clinical inertia surrounding out-of-office BP monitoring seems to be driven by multiple provider-, patient-, and policy-related factors (9, 10). A major barrier to out-of-office BP measurement is skepticism over the utility of screening for isolated office hypertension (i.e. untreated WCH and treated WCE) due to unclear evidence (9). The burden and risks of WCH, in particular, differ across studies. In a systematic review for the United States Preventive Services Taskforce, Piper et al. reported that the prevalence of WCH ranges from 5% to 65% in studies using ABPM and 16% to 55% in studies using HBPM (11). Piper et al. also described that the cardiovascular risk of WCH is elevated compared to normotension in several studies, but that these findings are not consistent across studies (11); furthermore, the authors noted that studies of treated WCE show no increased risk of adverse cardiovascular outcomes. Correspondingly, previous meta-analyses demonstrated weak associations of WCH with cardiovascular risk, and no association with all-cause mortality (12, 13). However, these meta-analyses did not adequately explore factors contributing to the inconsistent findings across studies. Moreover, multiple additional studies evaluating the association between WCH and adverse cardiovascular outcomes have been subsequently published.
In this meta-analysis, we aimed to thoroughly assess the association of untreated WCH and treated WCE with future cardiovascular events and all-cause mortality. This information could promote more widespread adoption of out-of-office BP monitoring as standard of care, and could inform policy changes to provide greater reimbursement and support for out-of-office BP monitoring in routine practice.
METHODS
Data Sources and Searches
All steps of the review and meta-analysis were performed using a predefined protocol (Supplemental Methods) completed on July 5, 2018, in accordance with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (14). Publications were identified by searching PubMed and Embase from inception to 10 December 2018, without language restriction. Search algorithms incorporated “hypertension,” “blood pressure,” and several terms related to WCH, in-office BP, out-of-office BP monitoring, and cardiovascular outcomes (Supplemental Methods). Additional publications were identified by manual review of reference lists of relevant studies, reviews, and meta-analyses.
Study Selection
Publications were eligible for inclusion if they were studies of adult humans that 1) reported associations of WCH or WCE with development of non-fatal cardiovascular events (including incident coronary artery disease, myocardial infarction, angina, stroke, transient ischemic attack, peripheral artery disease, revascularization procedure, and hospitalization for congestive heart failure), fatal cardiovascular events, or all-cause mortality; 2) had a mean duration of follow-up of at least three years; and 3) provided a reference group of individuals with normotension or controlled hypertension. Two investigators independently screened abstracts and reviewed full texts to determine eligibility. Any discrepancies were resolved by a third reviewer.
Data Extraction and Quality Assessment
Two investigators independently extracted data from each eligible publication using a standardized form (Supplemental Methods). Extracted data included cohort name; year of publication; country and location of the study; study design; inclusion and exclusion criteria; type and duration of out-of-office BP measurement; criteria for diagnosis of WCH or WCE; number of study participants overall and with WCH or WCE; number of participants on antihypertensive treatment at baseline; number of participants with a history of diabetes, cardiovascular disease, and chronic kidney disease; number of participants who were current smokers and male sex; mean age, body mass index, and duration of follow-up; covariates included in statistical adjustment; outcomes reported and outcome definitions; adjusted risk estimates, separated by antihypertensive treatment status (i.e. treated, untreated, or treated and untreated combined) and type of outcome (i.e. fatal and non-fatal cardiovascular event, fatal cardiovascular event, or all-cause mortality). Any discrepancies were resolved by a third reviewer. Study authors were contacted directly by the lead author if a publication met all inclusion criteria but did not report the outcomes in a way that could be extracted for meta-analysis (e.g. 95% confidence intervals not reported).
Quality of the evidence was evaluated by two investigators using a modified Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool to assess individual study bias for each outcome (Supplemental Methods). The QUADAS-2 tool assesses if there is a low, high, or unclear risk of bias in a study across four domains: patient selection, index test (modified to reflect the quality of the ABPM or HBPM assessment), reference standard (modified to reflect the quality of the in-office BP), and flow and timing (15). The modified tool incorporated quality of the statistical analyses, handling of confounding, and outcome assessment. Confounding was considered to be adequately addressed if there was adjustment for age, sex, previous cardiovascular events, antihypertensive medication, and at least two additional covariates among smoking status, lipids, diabetes mellitus, body mass index, kidney function, left ventricular hypertrophy, clinic BP, and alcohol use. Studies were determined to have a high risk of bias in the handling of confounding if the same covariates were used for analyzing cardiovascular events and all-cause mortality without adequate justification (e.g., subject exclusion for important risk factors for all-cause mortality, such as malignancy or high infectious risk). The primary analyses were restricted to studies determined to have a low risk of bias across at least five out of seven domains of the modified QUADAS-2 assessment.
Data Synthesis and Analysis
Meta-analyses were performed by calculating pooled log hazard ratios using random-effects inverse-variance models, with profile likelihood estimation (16–18) and Bartlett’s correction (in analyses of more than five studies) (19) to address heterogeneity across the relatively small number of studies. All analyses incorporated multivariable adjusted hazard ratios to quantify the association between WCH or WCE and each of the outcomes, with normotension or controlled hypertension as the reference group. The primary analyses were stratified by baseline antihypertensive treatment status reported in each study (WCH [untreated], WCE [treated], or combined). The primary outcomes evaluated were 1) fatal and non-fatal cardiovascular events and 2) all-cause mortality. Heterogeneity was assessed by Cochran’s Q test and was quantified with the I2 index (20) in analyses of three or more studies. Begg’s rank correlation test (21) and Egger’s weighted linear regression test (22) were planned to assess for small study effects (i.e. publication bias). However, these tests do not perform well with less than ten studies contributing to a given estimate, and were consequently omitted.
In instances with multiple publications from the same cohort, data from the most recent and applicable publication were used for the primary analyses; other publications from that cohort were included in pertinent subgroup analyses where the data were not available from the most recent publication.
Analyses were performed using packages admetan and metabias in STATA version 15.1 (Statacorp LP, College Station, TX).
RESULTS
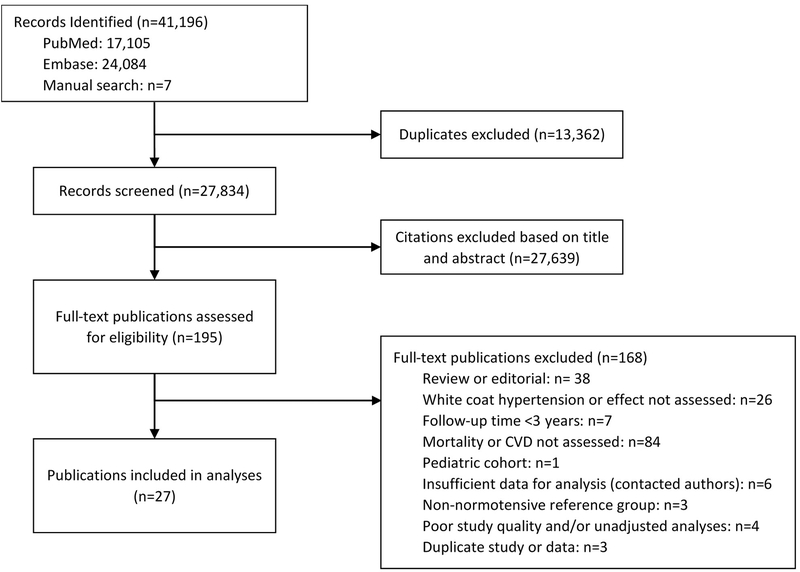
The search strategy identified 27 publications that were eligible for inclusion from 29 unique cohorts, involving 25,786 individuals with WCH or WCE and 38,487 individuals with normotension or controlled hypertension (Figure 1, Appendix Table 1). Two studies were based in North America, 13 in Europe, seven in Asia, and five across multiple regions. Fourteen studies reported funding from government, university, medical society, and/or research foundation grants; three studies reported only industry sponsorship; four studies reported a combination of industry and government or foundation funding; six studies (encompassing four distinct cohorts) did not report any source of funding. Six studies were population-based studies, 11 studies involved subject recruitment from outpatient clinics, and ten studies included subjects who were referred for ABPM or to a specialized hypertension clinic. Eighteen studies assessed out-of-office BP with ABPM, seven studies with HBPM, and two studies with both methods. Fifteen studies used a daytime out-of-office BP threshold of <135/85 to determine a diagnosis of WCH or WCE, seven studies used a 24-hour threshold of <130/80, and five studies used a different threshold (e.g. 125/80) or the combination of both thresholds.
Figure 1. Evidence search and selection.
Abbreviations: CVD = Cardiovascular disease
Mean study-specific participant age ranged from 43 to 72 years (median 56 years, Appendix Table 2), with a mean duration of follow-up of 3 to 19 years (median 8 years). Twenty-four studies were included in the primary analyses after excluding three studies due to overlapping cohort-specific data with regard to the primary outcomes. All studies included in the primary analyses demonstrated a low risk of bias in at least five out of seven domains of the modified QUADAS-2 assessment (Appendix Table 3). All multivariable models, at minimum, accounted for age, sex, and prior cardiovascular events (Appendix Table 4); 25 studies incorporated antihypertensive medication in the models, and all studies adjusted for at least two additional covariates among smoking status, lipids, diabetes mellitus, body mass index, kidney function, and left ventricular hypertrophy. Nine studies that evaluated both cardiovascular events and all-cause mortality used the same models for both outcomes without clear justification.
Cardiovascular Events
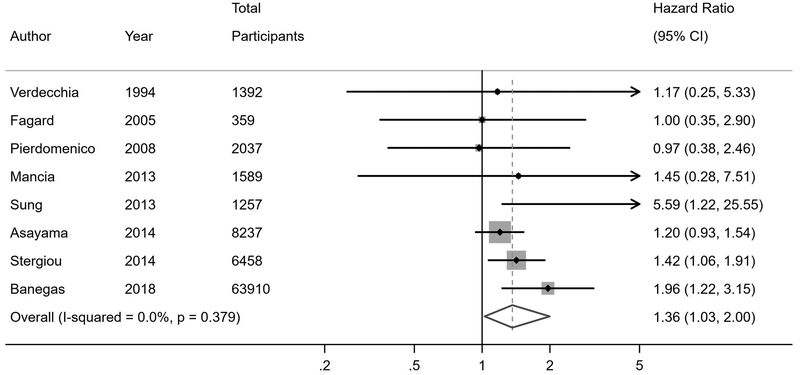
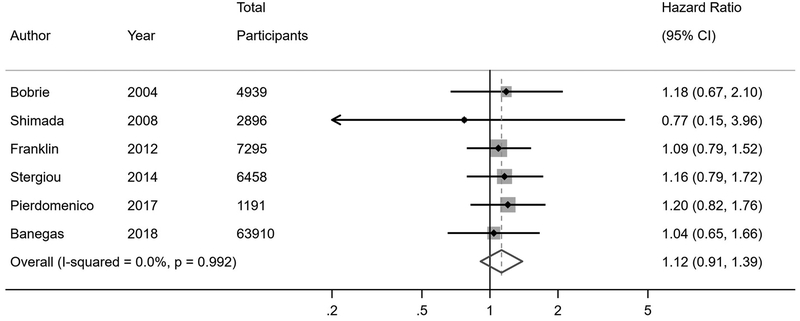
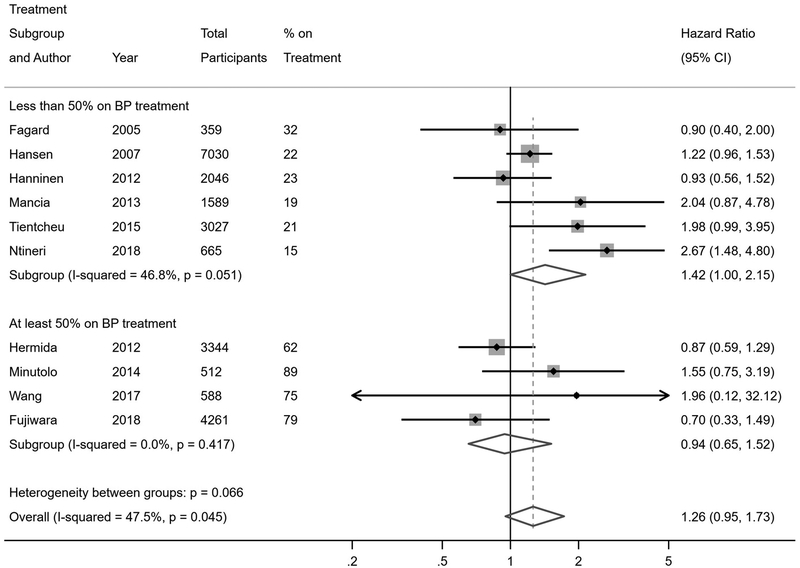
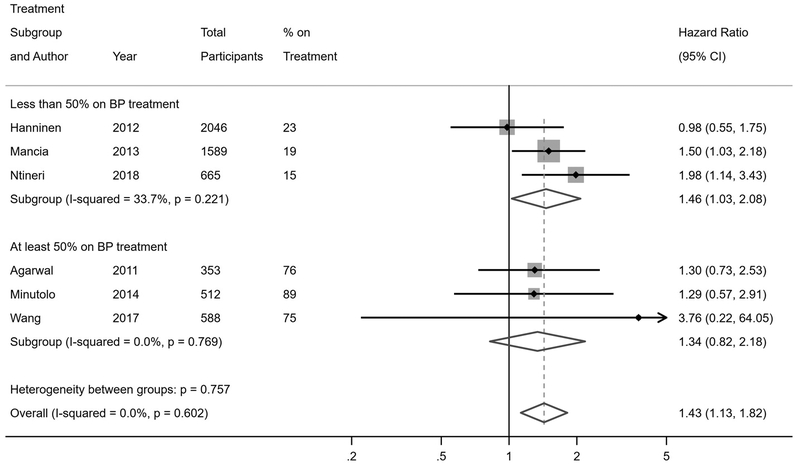
Twenty-one studies reported risk of fatal and non-fatal cardiovascular events among individuals with WCH or WCE compared to individuals with normotension or controlled hypertension (Figure 2). In the primary analyses of studies that were stratified by antihypertensive treatment status, individuals with WCH had a higher risk of cardiovascular events compared to normotensives (hazard ratio [HR] 1.36, 95% confidence interval [CI] 1.03–2.00), while individuals with WCE had no increased risk of cardiovascular events (HR 1.12, 95% CI 0.91–1.39). In the primary analyses of studies that did not stratify by antihypertensive treatment status, WCH or WCE was not associated with increased risk of cardiovascular events overall compared to normotensives or controlled hypertensives (HR 1.26, 95% CI 0.95–1.73); however, upon restricting the analyses to unstratified studies in which less than half of participants were on antihypertensive treatment, there was an increased risk of cardiovascular events associated with WCH or WCE (HR 1.42, 95% CI 1.00–2.15). These findings were more robust when restricting to studies with less than 20% of participants on antihypertensive treatment (HR 2.45, 95% CI 1.31–4.30).
Figure 2. Cardiovascular event risk in white coat hypertension and white coat effect.
A) Untreated white coat hypertension
B) Treated white coat effect
C) Results not stratified by antihypertensive treatment
All-Cause Mortality
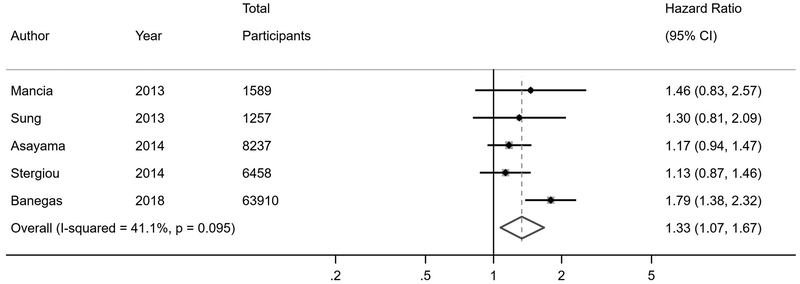
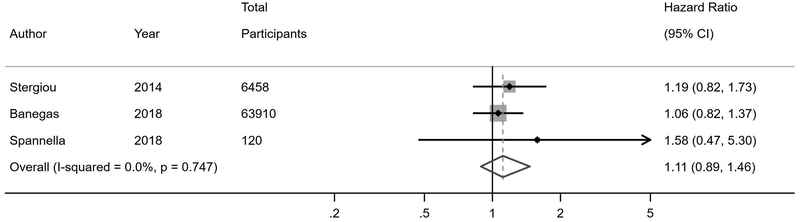
Eleven studies reported on all-cause mortality risk in WCH or WCE relative to normotension or controlled hypertension (Figure 3). The primary analyses of studies stratified by antihypertensive treatment status demonstrated an increased mortality risk in individuals with WCH (HR 1.33, 95% CI 1.07–1.67) compared to normotension or controlled hypertension. There was no increased mortality risk in WCE (HR 1.11, 95% CI 0.89–1.46). In studies that did not stratify by antihypertensive treatment status, WCH or WCE was associated with an increased risk of mortality (HR 1.46, 95% CI 1.03–2.08) if less than half of participants were on treatment, but not if at least half of participants were on treatment (HR 1.34, 95% CI 0.82–2.18); these findings were corroborated after restricting to studies with less than 20% of participants on treatment (HR 2.00, 95% CI 1.16–3.47).
Figure 3. All-cause mortality risk in white coat hypertension and white coat effect.
A) Untreated white coat hypertension
B) Treated white coat effect
C) Results not stratified by antihypertensive treatment
Sensitivity Analyses by Outcome Definitions
In sensitivity analyses evaluating differential reporting of cardiovascular events (Table 1), WCH was associated with increased cardiovascular mortality (HR 2.09, 95% CI 1.23–4.48), while WCE was not associated with increased cardiovascular mortality (HR 1.04, 95% CI 0.65–1.66). There was attenuated risk from WCH in a limited number of studies that reported fatal and non-fatal stroke (WCH HR 1.15, 95% CI 0.61–2.16; combined WCH or WCE HR 1.27, 95% CI 0.53–2.31). Studies that included stroke in the definition of cardiovascular events also demonstrated lower risk from WCH (HR 1.26, 95% CI 1.00–1.54) than studies that did not include stroke in the definition of cardiovascular events (HR 2.09, 95% CI 1.23–4.48).
Table 1.
Subgroup analyses by reporting of outcome events in individuals with white coat hypertension and white coat effect compared to normotension or controlled hypertension
| White coat hypertension (untreated) | White coat effect (treated) | Combined white coat hypertension and white coat effect | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome definition | N | HR (95% CI) | I2 (P-value) | N | HR (95% CI) | I2 (P-value) | N | HR (95% CI) | I2 (P-value) | |
| Fatal CVD events (i.e. cardiovascular mortality) | 3 | 2.09 (1.23–4.48) | 0% (0.393) | 1 | 1.04 (0.65–1.66) | 2 | 1.74 (0.90–3.43) | |||
| Only fatal and non-fatal stroke reported | 1 | 1.15 (0.61–2.16) | 2 | 1.27 (0.53–2.31) | ||||||
| Included stroke in CVD definition | 5 | 1.26 (1.00–1.54) | 0% (0.866) | 5 | 1.14 (0.94–1.39) | 0% (0.984) | 9 | 1.22 (0.90–1.68) | 48.0% (0.046) | |
| Excluded stroke from CVD definition | 3 | 2.09 (1.23–4.48) | 0% (0.393) | 1 | 1.04 (0.65–1.66) | 1 | 2.04 (1.87–4.78) | |||
| Included CHF in CVD definition | 3 | 1.27 (1.00–1.59) | 0% (0.587) | 4 | 1.15 (0.94–1.40) | 0% (0.984) | 7 | 1.33 (0.96–1.98) | 54.1% (0.040) | |
| Excluded CHF from CVD definition | 5 | 1.82 (1.08–2.85) | 0% (0.432) | 2 | 1.02 (0.49–1.88) | 3 | 1.05 (0.52–2.21) | 13.8% (0.166) | ||
I2 value was not reported in analyses of less than 3 studies due to insufficient statistical power to assess for heterogeneity
Abbreviations: CI = Confidence interval; CHF = Congestive heart failure; CVD = Cardiovascular disease; HR = Hazard ratio; N = Number of studies
Sensitivity Analyses by Study Design Characteristics
Multiple analyses were performed to explore potential sources of heterogeneity and differences in outcomes across the treatment groups. In subgroup analyses of study design characteristics (Appendix Table 5), the overall results were similar regardless of level of bias (based on the modified QUADAS-2 tool). Results were also similar to the primary analyses when restricted to studies with ABPM (as opposed to HBPM) used to determine WCH or WCE, mean participant age ≥55 years, validated BP monitors, daytime threshold of <135/85 mmHg for defining WCH or WCE, participants recruited for the study (as opposed to referred for indication-specific ABPM), ≥2,000 participants, at least five years of mean follow-up time, study publication year after 2012, and inclusion of individuals with a previous history of cardiovascular disease, chronic kidney disease, or diabetes. The elevated risk of cardiovascular events associated with WCH dissipated in the one study that did not use validated BP monitors (HR 1.20, 95% CI 0.93–1.54) and in studies with referred participants (HR 1.31, 95% 0.92–1.98), less than 2,000 participants (HR 1.56, 95% CI 0.71–4.01), less than five years of follow-up time (HR 1.87, 95% CI 0.84–3.36), study year on or before 2012 (HR 1.01, 95% CI 0.53–1.97), HBPM (HR 1.42, 95% CI 0.88–2.31), WCH defined using 24-hour BP <130/80 mmHg (HR 1.36, 95% CI 0.91–2.33), mean participant age <55 years (HR 1.21, 95% CI 1.00–1.51), and exclusion of individuals with previous cardiovascular disease (HR 0.98, 95% CI 0.44–2.20).
Influence analyses demonstrated no meaningful differences in the HRs for cardiovascular events upon omission of each individual study from the primary analyses (Appendix Table 6).
DISCUSSION
Our findings from 27 studies involving over 64,000 individuals who underwent in-office and out-of-office BP monitoring demonstrate that untreated WCH is associated with increased risk of cardiovascular events and all-cause mortality compared to normotension, while treated WCE is not associated with elevated risk. These results persisted across a multitude of sensitivity analyses.
Our literature review identified several previous systematic reviews and meta-analyses that evaluated WCH and longitudinal cardiovascular risk (12, 13, 45). These reviews reported data from fewer studies than the current review, and performed limited sensitivity analyses to explore differences across the studies. Several impactful studies evaluating the longitudinal association of WCH and adverse cardiovascular outcomes and mortality have been published since the previous reviews (30, 34, 37, 38, 40–43, 46), providing more robust data and greater opportunity for detailed sensitivity analyses. Moreover, previous meta-analyses used fixed-effects modeling as the analytic approach, which does not adequately address differences in study design and participant characteristics observed across the studies (47). In contrast to previous meta-analyses, we used random-effects modeling with profile likelihood estimation, which is particularly suited to address the presence of these types of dissimilarities (16, 17). We also included studies of individuals with diabetes and chronic kidney disease, which were previously excluded (12). We instead performed sensitivity analyses that showed no meaningful differences in studies that included these groups.
The current review supports and expands upon earlier findings in multiple ways. Similar to previous reviews (12, 13), we identified that WCH is associated with an increased risk of cardiovascular events. Unlike in previous meta-analyses, we had sufficient statistical power to also demonstrate an increased risk of all-cause mortality and cardiovascular mortality in WCH. We were also able to explore the impact of cause-specific outcomes on the findings across studies. Most notably, WCH did not seem to be associated with increased risk of stroke (42, 46, 48, 49). To further support this observation, the cardiovascular risk of WCH was attenuated in studies that included stroke in the definition of cardiovascular events (23–25, 28, 29). We also evaluated potential factors contributing to inconsistent outcomes in studies that combined subjects with untreated WCH and treated WCE. There was an increased risk of cardiovascular events and mortality associated with WCH or WCE in studies with less than half of participants on antihypertensive treatment at baseline (24, 26, 35–38), corroborated when restricting to studies with less than 20% of participants on treatment as baseline (26, 38). There was no increased risk in studies with at least half of participants on treatment at baseline (39–42, 44). These findings suggest that the risk of cardiovascular events and mortality in studies combining WCH and WCE is likely driven by the proportion of participants with untreated WCH. We conclude that future studies evaluating the association of WCH with adverse cardiovascular outcomes would benefit from stratifying by baseline antihypertensive treatment status and reporting stroke outcomes separately from other cardiovascular outcomes.
We found that differences in study design characteristics may explain many discrepancies in findings across studies. For example, studies with mean participant age <55 years or exclusion of individuals with previous cardiovascular disease were associated with mitigated risk of cardiovascular events in WCH. These findings are consistent with detailed subgroup analyses by Franklin et al. in the International Database of Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes (50), which suggest that the long-term cardiovascular risk of WCH is largely associated with older age and higher baseline cardiovascular risk. However, these findings may be related to insufficient follow-up time to observe events in younger, lower risk populations. Correspondingly, the cardiovascular risk of WCH was attenuated in studies with shorter follow up time (<5 years, which was also correlated with earlier study year). In the two studies that reported rates of progression to sustained hypertension, individuals with WCH had an approximately 3- to 4-fold increased risk of developing sustained hypertension compared to normotensives over seven to ten years of follow up (26, 37). Longer follow up time may be associated with increased risk of cardiovascular events in WCH due to greater conversion to sustained hypertension.
We also determined that the risk from WCH was diminished in studies that used a mean 24-hour BP of <130/80 mmHg rather than a daytime BP of <135/85 mmHg to define WCH or WCE (23, 28, 30). The differences in findings across BP thresholds are supported by a study by Asayama et al. (28), which demonstrated that using 24-hour BP to define WCH, compared to using daytime or nighttime BP alone, eliminated the increased risk of cardiovascular events associated with WCH. Additionally, the adverse cardiovascular risks of WCH were attenuated in studies that included individuals who were referred for ABPM rather than actively recruited from a broader population. We suspect that this finding represents the effects of selection bias. Particularly, control groups identified as having an indication to undergo ABPM may have greater underlying risk at baseline than normotensives in the community, biasing the results towards the null. Use of HBPM, as opposed to ABPM, was also associated with attenuation in the cardiovascular risk of WCH, consistent with recent studies suggesting that ABPM may be superior to HBPM as an indicator of cardiovascular risk in masked hypertension (51, 52). Moreover, we found that use of an unvalidated BP monitor mitigated the association of WCH with adverse cardiovascular events, potentially reflecting measurement error (53).
The recent study by Banegas et al. (30), included in this meta-analysis, was a Spanish registry study of 63,910 individuals who underwent 24-hour ABPM, with a median follow-up of 4.7 years. This was by far the largest study included in the meta-analysis, and the results of the study paralleled our overall findings. The mean age and frequency of prior cardiovascular events and smoking in the Spanish registry study approximated the medians across the included cohorts, although there was a higher proportion of individuals with diabetes mellitus in the Spanish study (20%, compared to a median of 11%). This study subjectively appeared to heavily impact the results of our meta-analysis; however, influence analyses demonstrated no objective difference in the overall results when this study was excluded. While the Spanish registry study had several limitations (e.g., referral for diagnostic ABPM, rather than study-specific recruitment), we infer that the large sample size and careful attention to antihypertensive treatment status by the authors contributed to highly generalizable results. Specifically, individuals were stratified by antihypertensive treatment status, with further adjustment by number (and in sensitivity analyses, type) of antihypertensives among individuals with treated hypertension. Additionally, the authors separately evaluated cardiovascular mortality and all-cause mortality, which was only performed in a minority of the studies included in our meta-analysis.
There are several important limitations of the current meta-analysis to consider. Much like previous meta-analyses (12, 13, 45), our review is limited by the use of observational cohort studies, which are prone to unmeasured confounding that may not be adequately addressed by robust study-specific adjustment and meta-analytic methods. Additionally, several subgroup analyses were limited to a very small number of studies. For example, only one study reported the association of untreated WCH alone (i.e. not combined with treated WCE) with fatal and non-fatal stroke (48), and no studies reported the association between untreated WCH and other distinct cardiovascular endpoints (such as ischemic coronary disease). We suspect the dearth of publications evaluating the association of WCH with stroke may be related to consistently negative findings in studies in which it was assessed. Finally, only a small number of studies reported race and ethnicity (37, 44), precluding examination of risk differences in potentially high-risk minorities.
Findings from this review have important clinical and public health implications. In conjunction with the markedly elevated cardiovascular risk previously associated with masked hypertension (54), the elevated risk associated with WCH underscores the importance of recent guidelines recommending out-of-office BP screening for the diagnosis of hypertension (2, 3). These findings advocate developing policy to support broader implementation of out-of-office BP monitoring in routine clinical practice. In order to promote more widespread use of out-of-office BP monitoring, there needs to be more comprehensive insurance reimbursement and provider training (10). Furthermore, this review supports the need for additional studies, specifically evaluating cardiovascular risk of WCH in ethnic minorities, risk of isolated cardiac endpoints (e.g., stroke, ischemic heart disease) in WCH, and approaches to reduce cardiovascular risk in individuals with WCH.
In conclusion, individuals with untreated WCH, but not treated WCE, have markedly increased risk of cardiovascular events and all-cause mortality compared to individuals with normal BPs. The cardiovascular risk of WCH was particularly evident in studies of older individuals, studies that used ABPM with daytime BP <135/85 as the threshold for BP control, studies with ≥5 years of follow up, and studies that excluded stroke from the definition of cardiovascular events. These findings support more widespread use of out-of-office BP monitoring in the diagnosis and management of hypertension. Untreated individuals with isolated office hypertension should be closely monitored for transition to sustained hypertension (26, 37), while treated individuals could be harmed by overly aggressive management (11, 55). Taking into account recommendations from the recent American College of Cardiology/American Heart Association hypertension guideline (2) and the increased cardiovascular risk associated with WCH, we encourage lifestyle modifications (including improved diet, exercise, weight loss, reduction in alcohol use, and smoking cessation) in all individuals found to have WCH. This systematic review and meta-analysis highlights the importance of future trials to evaluate interventions to reduce cardiovascular risk in WCH.
Supplementary Material
Financial Support:
Jordana B. Cohen: National Institutes of Health (K23-HL133843)
Role of the Funding Source
This research was supported in part by the National Institutes of Health grant number K23-HL133843 (NHLBI, PI: Cohen). The funding source had no role in the study design or implementation. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the National Institutes of Health.
Footnotes
Reproducible Research Statement:
Study protocol: see Supplemental Methods
Statistical code: Fully available upon request from Jordana B. Cohen, MD, MSCE (jco@pennmedicine.upenn.edu)
Data: see Appendix tables. Additional data fully available upon request from Jordana B. Cohen, MD, MSCE (jco@pennmedicine.upenn.edu)
REFERENCES
- 1.Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1923–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–e115. [DOI] [PubMed] [Google Scholar]
- 3.Siu AL, Force USPST. Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163(10):778–86. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, et al. Measurement of Blood Pressure in Humans: A Scientific Statement From the American Heart Association. Hypertension. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimbo D, Abdalla M, Falzon L, Townsend RR, Muntner P. Role of Ambulatory and Home Blood Pressure Monitoring in Clinical Practice: A Narrative Review. Ann Intern Med. 2015;163(9):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen JB, Cohen DL. Integrating Out-of-Office Blood Pressure in the Diagnosis and Management of Hypertension. Curr Cardiol Rep. 2016;18(11):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abolbashari M White Coat Hypertension and Cardiovascular Diseases: Innocent or Guilty. Curr Cardiol Rep. 2018;20(4):25. [DOI] [PubMed] [Google Scholar]
- 8.Shimbo D, Kent ST, Diaz KM, Huang L, Viera AJ, Kilgore M, et al. The use of ambulatory blood pressure monitoring among Medicare beneficiaries in 2007–2010. J Am Soc Hypertens. 2014;8(12):891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronish IM, Kent S, Moise N, Shimbo D, Safford MM, Kynerd RE, et al. Barriers to conducting ambulatory and home blood pressure monitoring during hypertension screening in the United States. J Am Soc Hypertens. 2017;11(9):573–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giles TD. Apathy towards ambulatory blood pressure monitoring use in hypertension: is it due to cognitive dissonance, money, or both? Hypertension. 2015;65(6):1156–7. [DOI] [PubMed] [Google Scholar]
- 11.Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(3):192–204. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Huang W, Mai W, Cai X, An D, Liu Z, et al. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J Hypertens. 2017;35(4):677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briasoulis A, Androulakis E, Palla M, Papageorgiou N, Tousoulis D. White-coat hypertension and cardiovascular events: a meta-analysis. J Hypertens. 2016;34(4):593–9. [DOI] [PubMed] [Google Scholar]
- 14.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 15.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. [DOI] [PubMed] [Google Scholar]
- 16.Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160(4):267–70. [DOI] [PubMed] [Google Scholar]
- 17.Veroniki AA, Jackson D, Viechtbauer W, Bender R, Bowden J, Knapp G, et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardy RJ, Thompson SG. A likelihood approach to meta-analysis with random effects. Stat Med. 1996;15(6):619–29. [DOI] [PubMed] [Google Scholar]
- 19.Noma H Confidence intervals for a random-effects meta-analysis based on Bartlett-type corrections. Stat Med. 2011;30(28):3304–12. [DOI] [PubMed] [Google Scholar]
- 20.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 21.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. [PubMed] [Google Scholar]
- 22.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25(20):3443–57. [DOI] [PubMed] [Google Scholar]
- 23.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension 1994;24(6):793–801. [DOI] [PubMed] [Google Scholar]
- 24.Fagard RH, Van Den Broeke C, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19(10):801–7. [DOI] [PubMed] [Google Scholar]
- 25.Pierdomenico SD, Lapenna D, Di Mascio R, Cuccurullo F. Short- and long-term risk of cardiovascular events in white-coat hypertension. J Hum Hypertens. 2008;22(6):408–14. [DOI] [PubMed] [Google Scholar]
- 26.Mancia G, Bombelli M, Brambilla G, Facchetti R, Sega R, Toso E, et al. Long-term prognostic value of white coat hypertension: an insight from diagnostic use of both ambulatory and home blood pressure measurements. Hypertension. 2013;62(1):168–74. [DOI] [PubMed] [Google Scholar]
- 27.Sung SH, Cheng HM, Wang KL, Yu WC, Chuang SY, Ting CT, et al. White coat hypertension is more risky than prehypertension: important role of arterial wave reflections. Hypertension. 2013;61(6):1346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asayama K, Thijs L, Li Y, Gu YM, Hara A, Liu YP, et al. Setting thresholds to varying blood pressure monitoring intervals differentially affects risk estimates associated with white-coat and masked hypertension in the population. Hypertension. 2014;64(5):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stergiou GS, Asayama K, Thijs L, Kollias A, Niiranen TJ, Hozawa A, et al. Prognosis of white-coat and masked hypertension: International Database of HOme blood pressure in relation to Cardiovascular Outcome. Hypertension. 2014;63(4):675–82. [DOI] [PubMed] [Google Scholar]
- 30.Banegas JR, Ruilope LM, de la Sierra A, Vinyoles E, Gorostidi M, de la Cruz JJ, et al. Relationship between Clinic and Ambulatory Blood-Pressure Measurements and Mortality. N Engl J Med. 2018;378(16):1509–20. [DOI] [PubMed] [Google Scholar]
- 31.Bobrie G, Chatellier G, Genes N, Clerson P, Vaur L, Vaisse B, et al. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA. 2004;291(11):1342–9. [DOI] [PubMed] [Google Scholar]
- 32.Shimada K, Fujita T, Ito S, Naritomi H, Ogihara T, Shimamoto K, et al. The importance of home blood pressure measurement for preventing stroke and cardiovascular disease in hypertensive patients: a sub-analysis of the Japan Hypertension Evaluation with Angiotensin II Antagonist Losartan Therapy (J-HEALTH) study, a prospective nationwide observational study. Hypertens Res. 2008;31(10):1903–11. [DOI] [PubMed] [Google Scholar]
- 33.Franklin SS, Thijs L, Hansen TW, Li Y, Boggia J, Kikuya M, et al. Significance of white-coat hypertension in older persons with isolated systolic hypertension: a meta-analysis using the International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes population. Hypertension. 2012;59(3):564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierdomenico SD, Pierdomenico AM, Coccina F, Porreca E. Prognosis of Masked and White Coat Uncontrolled Hypertension Detected by Ambulatory Blood Pressure Monitoring in Elderly Treated Hypertensive Patients. Am J Hypertens. 2017;30(11):1106–11. [DOI] [PubMed] [Google Scholar]
- 35.Hansen TW, Kikuya M, Thijs L, Bjorklund-Bodegard K, Kuznetsova T, Ohkubo T, et al. Prognostic superiority of daytime ambulatory over conventional blood pressure in four populations: a meta-analysis of 7,030 individuals. J Hypertens. 2007;25(8):1554–64. [DOI] [PubMed] [Google Scholar]
- 36.Hanninen MR, Niiranen TJ, Puukka PJ, Johansson J, Jula AM. Prognostic significance of masked and white-coat hypertension in the general population: the Finn-Home Study. J Hypertens. 2012;30(4):705–12. [DOI] [PubMed] [Google Scholar]
- 37.Tientcheu D, Ayers C, Das SR, McGuire DK, de Lemos JA, Khera A, et al. Target Organ Complications and Cardiovascular Events Associated With Masked Hypertension and White-Coat Hypertension: Analysis From the Dallas Heart Study. J Am Coll Cardiol. 2015;66(20):2159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ntineri A, Kalogeropoulos PG, Kyriakoulis KG, Aissopou EK, Thomopoulou G, Kollias A, et al. Prognostic value of average home blood pressure and variability: 19-year follow-up of the Didima study. J Hypertens. 2018;36(1):69–76. [DOI] [PubMed] [Google Scholar]
- 39.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Sleep-time blood pressure and the prognostic value of isolated-office and masked hypertension. Am J Hypertens. 2012;25(3):297–305. [DOI] [PubMed] [Google Scholar]
- 40.Minutolo R, Gabbai FB, Agarwal R, Chiodini P, Borrelli S, Bellizzi V, et al. Assessment of achieved clinic and ambulatory blood pressure recordings and outcomes during treatment in hypertensive patients with CKD: a multicenter prospective cohort study. Am J Kidney Dis. 2014;64(5):744–52. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Zhang J, Li Y, Ma X, Ye Z, Peng H, et al. Masked hypertension, rather than white-coat hypertension, has a prognostic role in patients with non-dialysis chronic kidney disease. Int J Cardiol. 2017;230:33–9. [DOI] [PubMed] [Google Scholar]
- 42.Fujiwara T, Yano Y, Hoshide S, Kanegae H, Kario K. Association of Cardiovascular Outcomes With Masked Hypertension Defined by Home Blood Pressure Monitoring in a Japanese General Practice Population . JAMA Cardiol. 2018;3(7):583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spannella F, Filipponi A, Giulietti F, Balietti P, Bernardi B, Rosettani G, et al. Prognostic role of masked and white-coat hypertension: 10-Year mortality in treated elderly hypertensives. J Hum Hypertens. 2018;0:1–7. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal R, Sinha AD, Light RP. Toward a definition of masked hypertension and white-coat hypertension among hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(8):2003–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25(11):2193–8. [DOI] [PubMed] [Google Scholar]
- 46.Satoh M, Asayama K, Kikuya M, Inoue R, Metoki H, Hosaka M, et al. Long-Term Stroke Risk Due to Partial White-Coat or Masked Hypertension Based on Home and Ambulatory Blood Pressure Measurements: The Ohasama Study. Hypertension. 2016;67(1):48–55. [DOI] [PubMed] [Google Scholar]
- 47.Serghiou S, Goodman SN. Random-Effects Meta-analysis: Summarizing Evidence With Caveats. JAMA. 2019;321(3):301–2. [DOI] [PubMed] [Google Scholar]
- 48.Verdecchia P, Reboldi GP, Angeli F, Schillaci G, Schwartz JE, Pickering TG, et al. Short- and long-term incidence of stroke in white-coat hypertension. Hypertension. 2005;45(2):203–8. [DOI] [PubMed] [Google Scholar]
- 49.Kario K, Shimada K, Schwartz JE, Matsuo T, Hoshide S, Pickering TG. Silent and clinically overt stroke in older Japanese subjects with white-coat and sustained hypertension. J Am Coll Cardiol. 2001;38(1):238–45. [DOI] [PubMed] [Google Scholar]
- 50.Franklin SS, Thijs L, Asayama K, Li Y, Hansen TW, Boggia J, et al. The Cardiovascular Risk of White-Coat Hypertension. J Am Coll Cardiol. 2016;68(19):2033–43. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Li Y, Wei FF, Thijs L, Kang YY, Wang S, et al. Strategies for classifying patients based on office, home, and ambulatory blood pressure measurement. Hypertension. 2015;65(6):1258–65. [DOI] [PubMed] [Google Scholar]
- 52.Anstey DE, Muntner P, Bello NA, Pugliese DN, Yano Y, Kronish IM, et al. Diagnosing Masked Hypertension Using Ambulatory Blood Pressure Monitoring, Home Blood Pressure Monitoring, or Both? Hypertension. 2018;72(5):1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen JB, Padwal RS, Gutkin M, Green BB, Bloch MJ, Germino FW, et al. History and Justification of a National Blood Pressure Measurement Validated Device Listing. Hypertension. 2019;73(2):258–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierdomenico SD, Pierdomenico AM, Coccina F, Clement DL, De Buyzere ML, De Bacquer DA, et al. Prognostic Value of Masked Uncontrolled Hypertension. Hypertension. 2018;72(4):862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mancia G, Bombelli M, Facchetti R, Madotto F, Quarti-Trevano F, Polo Friz H, et al. Long-term risk of sustained hypertension in white-coat or masked hypertension. Hypertension. 2009;54(2):226–32. [DOI] [PubMed] [Google Scholar]
- 56.Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, et al. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46(3):508–15. [DOI] [PubMed] [Google Scholar]
- 57.Pierdomenico SD, Lapenna D, Bucci A, Di Tommaso R, Di Mascio R, Manente BM, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18(11):1422–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.