Abstract
Objective:
To identify predictors of desmoid progression during observation.
Summary Background Data:
Untreated desmoids can grow, remain stable, or regress, but reliable predictors of behavior have not been identified.
Methods:
Primary or recurrent desmoid patients were identified retrospectively from an institutional database. In those managed with active observation who underwent serial MRIs with T2-weighted sequences, baseline tumor size was recorded, and two radiologists independently estimated the percentage of tumor volume showing hyperintense T2 signal at baseline. Associations of clinical or radiographic characteristics with progression-free survival (PFS; by RECIST) were evaluated by Cox regression and Kaplan-Meier statistics.
Results:
Among 160 patients with desmoids, 72 were managed with observation, and 37 of these had serial MRI available for review. Among these 37 patients, median age was 35 years and median tumor size was 4.7 cm; all tumors were extra-abdominal (41% in abdominal wall). While PFS was not associated with size, site, or age, it was strongly associated with hyperintense T2 signal in ≥90% vs <90% of baseline tumor volume (as defined by the “test” radiologist; hazard ratio=11.3, P=0.003). For patients in the ≥90% group (n=20), 1-year PFS was 55%, compared to 94% in the <90% group (n=17). The percentage of baseline tumor volume with hyperintense T2 signal defined by a validation radiologist correlated with results of the test radiologist (ρ=0.75).
Conclusions:
The percent tumor volume characterized by hyperintense T2 signal is associated with desmoid progression during observation and may help distinguish patients who would benefit from early intervention from those who may be reliably observed.
MINI-ABSTRACT
This study tested the association between T2 signal intensity on magnetic resonance imaging (MRI) of desmoid fibromatosis and progression during active observation. Among 37 patients, MRI signal was strongly associated with progression (HR 11.3; p=0.003), whereas clinical factors were not predictive.
INTRODUCTION
Desmoid-type fibromatosis is a mesenchymal neoplasm with unique properties that make its clinical management challenging.1–3 Although these tumors lack metastatic potential, they may be locally aggressive and can create significant morbidity.2 For example, extremity tumors can cause debilitating pain, and intraabdominal desmoids may lead to intestinal obstruction or fistulization. Historically, the accepted treatment for the majority of desmoid tumors was complete surgical resection with wide margins. Yet the aftermath of resection can be morbid as well; definitive resection of some extremity lesions may require amputation, and resection of mesenteric tumors has the potential to cause short bowel syndrome.2, 4, 5 Moreover, the rates of local recurrence after resection are high, ranging from 25% to 70% depending on the series.2, 5–11 As a result, clinicians are increasingly accepting a non-operative approach to initial management.12–14 In addition to surgery, the indication for use of systemic therapy in treatment of desmoid-type fibromatosis is evolving. A number of systemic agents may alleviate symptoms and arrest growth; in certain series, up to 70% of patients derived benefit.15, 16 Algorithms for how to best incorporate systemic therapy and surgery in the management of desmoids are currently being refined.
Adding to the complexity of clinical decision-making for desmoid fibromatosis, the course of these tumors is difficult to predict. Some tumors rapidly progress while others can remain asymptomatic and stable for long periods.2, 12, 17, 18 Some untreated tumors even slowly regress over time. Given this fact, along with the morbidity associated with aggressive operations often required to achieve R0 resections and the lack of metastatic potential of these lesions, several groups have proposed a conservative initial management strategy of observation. At Memorial Sloan Kettering Cancer Center (MSKCC), we created a prognostic nomogram to predict recurrence of resected desmoids.4 Patient age and tumor site and size were the features used to estimate recurrence risk after resection, in concordance with other large series.19 These predictors apply specifically to resected desmoids, but reproducible clinical or radiological prognostic predictors for progression of desmoid tumors managed with active observation have yet to be defined. In a series of 142 desmoid tumors managed non-operatively, no clinical features were associated with progression,12 making it difficult to determine whether a program of observation is appropriate for an individual patient.
On MRI, desmoids have shown a wide spectrum of T2 signal intensity.20 T2 signal intensity reflects water content within the tissue, and is therefore thought to be a surrogate for cellular content (versus fibrous, extracellular matrix components).21 Response to systemic therapies, in fact, is associated with decrease in T2 signal intensity.16 We hypothesized that desmoids with a large percentage of hyperintense T2 signal at baseline would be more likely to progress under observation, and that this imaging feature could be used to more reliably select patients for intervention versus observation.
In the current study, we examine clinicopathologic characteristics for association with eventual intervention (surgery, systemic therapy, or cryoablation) in a retrospective analysis of a series of patients managed with active observation at our institution. In a subset with serial imaging, we had the opportunity to analyze PFS by RECIST criteria and to rigorously test our hypothesis on hyperintense T2 signal. Accordingly, we analyzed the percentage of tumor volume characterized by high T2 signal intensity at baseline, and analyzed the associations of this factor with clinical characteristics, time to eventual intervention, and PFS.
METHODS
This study was approved by the Institutional Review Board at MSKCC. Patients with surgical pathology specimens from biopsy or resection confirming diagnosis of desmoid-type fibromatosis between January 1, 2008, and December 31, 2015, were identified retrospectively from a prospectively maintained institutional database. For directed image analysis, we identified a subset of these patients initially managed with a course of active observation after presenting at MSKCC with gross disease as determined on imaging. This subset of cases included patients in a program of active observation and who, during that time period, had serial MRI imaging available that included T2-weighted images, either with or without fat saturation.
Clinicopathologic variables and outcomes were recorded retrospectively. Anatomic relationships were defined as previously reported.4 Tumors in axilla, shoulder, buttock, and neck were classified as extremity lesions. Chest and abdominal wall lesions were differentiated based on location above and below the 10th rib, respectively; intrathoracic tumors were classified as chest wall tumors. Tumors characterized as visceral or retroperitoneal in origin were considered intra-abdominal tumors.
For radiologic analysis, an expert musculoskeletal radiologist (the test radiologist) determined tumor size at baseline and on follow-up imaging, by RECIST criteria (20% increase in long-axis measurement; only the largest mass was measured if more than one). On the baseline MRI only, this radiologist estimated percentage of tumor volume showing hyperintense T2 signal. This percentage estimate was performed after all the images of the tumor were viewed on the baseline T2-weighted sequence, but before the tumor sizes were measured on follow-up MR imaging in order to avoid bias in assessing progression. Areas were deemed to be hyperintense on T2 weighted images if they were brighter than adjacent muscles on the same image, and the percentage of tumor volume occupied by this hyperintense signal was assessed subjectively. This percentage will be referred to as the hyperintense T2 score. For the validation analysis, a second musculoskeletal radiologist (the validation radiologist) evaluated the scans independently. For statistical analyses, patients were dichotomized by the median of the hyperintense T2 scores as assigned by the test radiologist (90%).
Time to intervention was defined as the time between initiation of active observation for gross disease on imaging to the date at which intervention (surgery, systemic therapy or cryoablation) was initiated. PFS was calculated from the date of baseline MRI to the date of radiographic progression using RECIST criteria or the date of last MRI; patients for whom intervention was prescribed in the absence of RECIST progression were censored on the date of intervention.
PFS and time to intervention were estimated using Kaplan-Meier analysis and statistical significance determined by log-rank tests. Correlation of hyperintense T2 scores between radiologists was determined using Cohen’s Kappa statistic. Hazard ratios (HRs) and corresponding p-values were determined by Cox proportion hazards; age and tumor size were considered as categorical values as described in the results section. To describe variation in clinical characteristics between subsets of the patient cohort, the Wilcoxon test was used for continuous variables and Fisher’s exact or χ2 tests for categorical variables.
RESULTS
The initial study cohort consisted of 160 patients who presented for evaluation of primary, recurrent, or residual (margins grossly positive after surgical resection) desmoid-type fibromatosis at MSKCC (Table 1). Of these, 88 were managed with immediate intervention (most commonly surgery), while 72 enrolled in a program of initial observation. Patients with abdominal wall tumors were commonly managed with an initial course of active observation (19 of 31 patients; 61%), while chest wall and intra-abdominal lesions more commonly underwent immediate intervention (80% and 60% of tumors, respectively). Compared with tumors treated with immediate intervention, tumors selected for active observation tended to be asymptomatic (54% vs. 38%; p=0.039) and smaller (83% vs. 76% of lesions were ≤10 cm in largest diameter; p=0.098) and occurred in younger patients (64% vs. 56% were 45 years or younger; p=0.079). Presentation status (primary vs recurrent), date of presentation, and gender were not associated with the decision to intervene at presentation.
Table 1.
Characteristics of patients with desmoid-type fibromatosis managed with either immediate intervention or an initial trial of active observation.
| Total (n=160) | Active observation (n=72) | Immediate intervention (n=88) | p-value | |
|---|---|---|---|---|
| Gender | 0.38* | |||
| Male | 52 (32%) | 22 (31%) | 30 (34%) | |
| Female | 108 (68%) | 50 (69%) | 58 (66%) | |
| Age, yrs | 0.08 | |||
| ≤25 | 23 (14%) | 13 (18%) | 10 (11%) | |
| ˃25, ≤45 | 73 (46%) | 33 (46%) | 40 (45%) | |
| ˃45, ≤65 | 50 (31%) | 24 (33%) | 26 (29%) | |
| >65 | 14 (8.8%) | 2 (2.7%) | 12 (14%) | |
| Location of primary tumor | 0.02 | |||
| Abdominal wall | 31 (19%) | 19 (26%) | 12 (14%) | |
| Chest wall | 25 (16%) | 5 (6.9%) | 20 (23%) | |
| GI/intra-abdominal | 53 (33%) | 21 (29%) | 32 (36%) | |
| Extremity | 42 (26%) | 21 (29%) | 21 (24%) | |
| Other | 9 (5.6%) | 6 (8.3%) | 3 (3.4%) | |
| Size of treated tumor | 0.08 | |||
| ≤5 cm | 48 (30%) | 28 (39%) | 20 (23%) | |
| 5–10 cm | 79 (49%) | 32 (44%) | 47 (53%) | |
| ≥10 cm | 33 (21%) | 12 (17%) | 21 (24%) | |
| Presentation status | 0.16 | |||
| Primary | 118 (74%) | 50 (69%) | 68 (77%) | |
| Local recurrence | 37 (23%) | 21 (29%) | 16 (18%) | |
| Residual disease | 5 (3.1%) | 1 (1.4%) | 4 (4.5%) | |
| Symptoms | 0.04* | |||
| Yes | 72 (45%) | 33 (46%) | 55 (63%) | |
| No | 88 (55%) | 39 (54%) | 33 (38%) | |
| Date of presentation | 0.36* | |||
| 2008 – 2010 | 77 (48%) | 33 (46%) | 44 (50%) | |
| 2011 – 2015 | 83 (52%) | 39 (54%) | 44 (50%) | |
| Initial therapy | ||||
| Surgical resection | n/a | n/a | 61 (76%) | n/a |
| Radiation | 1 (1.1%) | |||
| Systemic therapy | 25 (28%) | |||
| Cryoablation | 1 (1.1%) |
Calculated using Fisher’s exact test, all other values represent results of χ2 analysis.
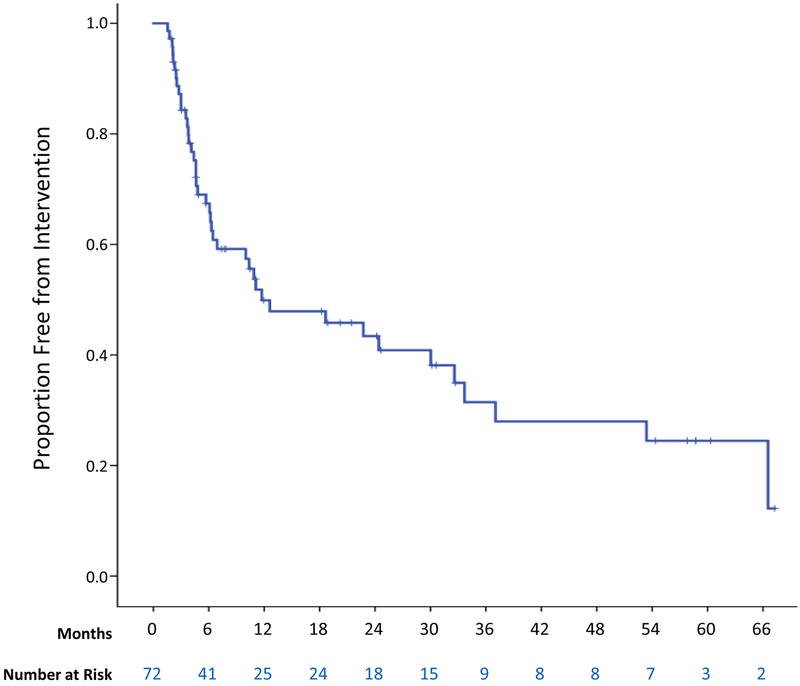
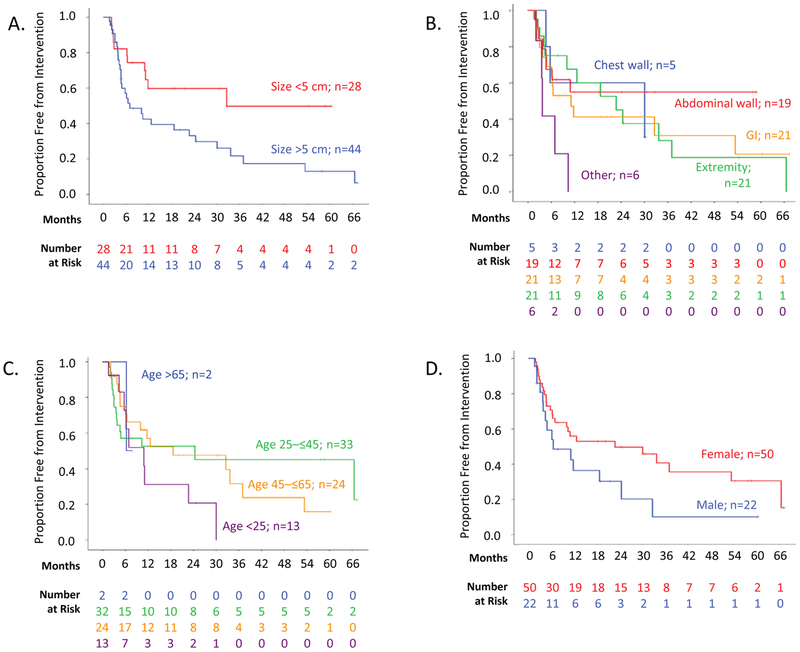
In our initial attempt to identify clinical predictors of progression during active observation, we used time to intervention as a surrogate for tumor progression. Time to intervention was examined in the cohort of 72 patients initially managed with active observation. Their median follow-up was 25.1 months (range 1.8–177), and 15.1 months in the subset of patients not undergoing intervention. Median time to any type of intervention was 11.7 months (±6.5 months), with 42 patients undergoing treatment during follow-up (Figure 1). In univariate analysis of the association of clinical characteristics with time to intervention, only larger tumor size was clearly associated with shorter time to intervention. Median time to intervention was 32.6 months in patients with tumors 5 cm or smaller in largest diameter vs. 6.9 months in patients with tumors larger than 5 cm (p=0.02; Figure 2A and Supplementary Table 1). The small number of tumors located in the paraspinal region or flank (“other”) were more commonly associated with ultimate intervention (5 of 6) than abdominal wall tumors (median 11.7 months in the “other” group, not reached in the abdominal wall group; p=0.01), but extremity, intraabdominal, and chest wall tumors did not require treatment more often than these abdominal wall tumors (Figure 2B). There was no statistically significant association between intervention and age (p=0.22; Figure 2C), gender (p= 0.07; Figure 2D), and documentation of symptoms at presentation (p=0.35) when analyzed by log-rank tests or univariate Cox regression analysis (Supplementary Table 1).
Figure 1.
Proportion of desmoid-type fibromatosis patients (n=72) free from intervention after the start of active observation.
Figure 2.
Proportion of desmoid-type fibromatosis patients (n=72) free from intervention after the initiation of active observation, stratified by (A) tumor size, (B) tumor location, (C) patient age, and (D) gender.
Time to intervention was thought initially to represent a surrogate for tumor progression, but, as described below, a substantial number of patients initially on observation proceeded to therapeutic intervention without imaging documentation of tumor growth. To perform a more rigorous analysis of clinicopathologic factors associated with desmoid progression, we undertook a formal analysis of imaging studies performed on desmoid patients during active observation. From the 72 desmoid patients managed with initial observation, we identified 37 who had baseline MRI and serial imaging available for RECIST analysis; 35 were excluded from this portion of the study because they lacked baseline MRIs with T2-weighted sequences or follow-up MRI for RECIST calculation. Mean interval between scans was 5.9 months and patients underwent median of 2 follow-up scans (range 1–8) during follow-up. Median age of the MRI-evaluable group was 35 years (range 8–69), and median tumor size was 4.7 cm (range 1.2–9.1 cm). All tumors were extra-abdominal, as intra-abdominal lesions are not routinely evaluated by MRI at our institution (because bowel motion can interfere with accurate assessment). In univariate analysis of the 37 patients with imaging, we found no clinical characteristics significantly associated with PFS (Supplemental Table 2). Notably, PFS was not clearly associated with tumor size (hazard ratio [HR] 1.027, p=0.13), tumor site (p=0.54 for extremities and p=0.38 for all other sites compared to abdominal wall), or patient age (HR 0.99 when analyzed as a continuous variable, p=0.31).
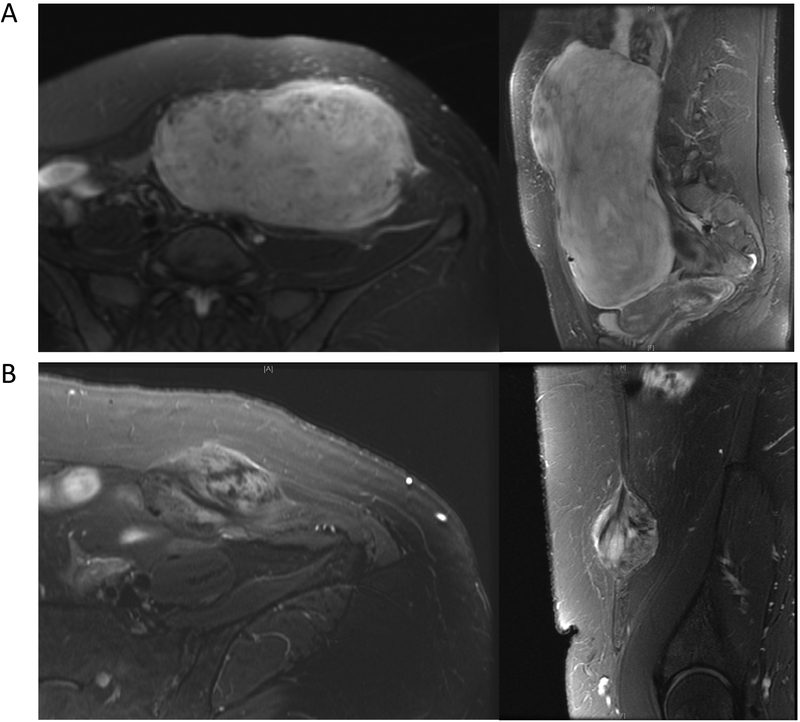
T2 signal intensity varied significantly among tumors at baseline (Fig. 3). To determine whether this characteristic is associated with PFS, we assessed the percent tumor volume that was hyperintense on baseline T2 sequences. Table 2 shows clinical characteristics for this study group as a whole and stratified by median hyperintense T2 score (i.e. <90% vs. ≥90% of tumor volume with hyperintense T2 signal at baseline evaluation). Patients with tumors characterized by hyperintense T2 scores <90% tended to be female (94% vs 75% of patients with hyperintense T2 score ≥90%; p=0.19) and to have abdominal wall lesions (59% vs 25%) as opposed to extremity tumors (23% vs 50%; p=0.099), but these differences were not statistically significant, nor did the groups differ significantly in the other clinicopathologic characteristics: presentation status, patient age, or tumor size.
Figure 3.
Varying hyperintense T2 scores. Desmoid tumors with hyperintense signals in high (A) and low (B) hyperintense T2 scores are pictured.
Table 2.
Characteristics of the 37 patients with desmoid tumors under observation with serial MRI results available for analysis.
| All (n=37) | Hyperintense T2 score < 90% (n=17) | Hyperintense T2 score ≥ 90% (n=20) | p value | |
|---|---|---|---|---|
| Follow-up in months, median (range) | 28 (3.1–89) | 32 (4.2–79) | 23 (3.1–89) | 0.94† |
| Hyperintense T2 score,* median (range) | 90 (20–100) | 75 (20–80) | 95 (90–100) | <0.001† |
| Gender | 0.19‡ | |||
| Male | 6 (16%) | 1 (6%) | 5 (25%) | |
| Female | 31 (84%) | 16 (94%) | 15 (75%) | |
| Age | 0.15 | |||
| ≤25 | 8 (22%) | 2 (12%) | 6 (30%) | |
| ˃25, ≤45 | 19 (51%) | 11 (65%) | 8 (40%) | |
| ˃45 | 10 (27%) | 4 (24%) | 6 (30%) | |
| Baseline tumor size | 0.72‡ | |||
| ≤5 cm | 26 (70%) | 11 (65%) | 15 (75%) | |
| >5 cm | 11 (30%) | 6 (35%) | 5 (25%) | |
| Tumor type | 0.72‡ | |||
| Primary | 27 (73%) | 13 (76%) | 14 (70%) | |
| Recurrent | 10 (27%) | 4 (24%) | 6 (30%) | |
| Site | 0.099 | |||
| Abdominal wall | 15 (41%) | 10 (59%) | 5 (25%) | |
| Extremity | 14 (38%) | 4 (23%) | 10 (50%) | |
| Other§ | 8 (22%) | 3 (18%) | 5 (25%) |
Hyperintense T2 score is defined as percentage of tumor volume with hyperintense signal on T2-weighted images at baseline.
Calculated using Mann-Whitney U test or
Fisher’s exact test; all other values represent results of χ2 analysis.
Includes chest wall lesions; this subset of the patient cohort included no patients with intra-abdominal lesions.
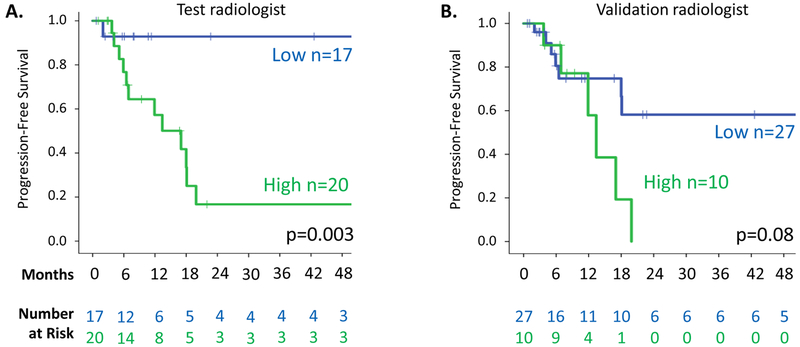
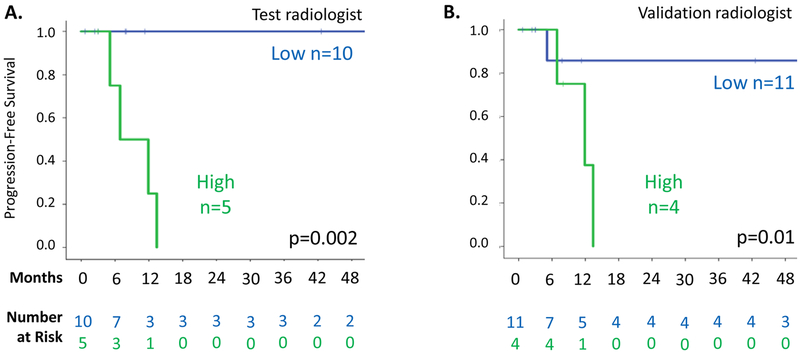
While clinical characteristics did not differ between the two groups of patients, PFS was significantly lower among patients with baseline hyperintense T2 score of ≥90% as determined by the test radiologist (Figure 4A, HR=11.5, p=0.003). The 1-year PFS for the 20 desmoid patients with hyperintense T2 score of ≥90% was 55%, compared to 94% for the 17 patients with score of <90%. By 5 years, only one of 17 patients (6%) with hyperintense T2 score <90% versus 12 of 20 patients (60%) with score ≥90% had disease progression. Median time to progression was 13 months in the group with baseline hyperintense T2 score of ≥90% and not reached for the <90% group.
Figure 4.
Kaplan-Meier analyses of progression-free survival in 37 desmoid tumors under observation with serial MRI sequences available. A. Patients stratified by hyperintense T2 score (<90% versus ≥90%) from the test radiologist. B. Patients stratified by hyperintense T2 score from the validation radiologist.
Of note, although hyperintense T2 score was strongly correlated with progression, it was only marginally associated with time to intervention (Supplemental Figure 1, p=0.62). Median time to intervention was 9.8 months in the ≥90% group and 11.3 months in the <90% group. Twenty-four of the 37 patients went on to active treatment; in only 10 cases was this preceded by RECIST progression.
Hyperintense T2 scores defined by the validation radiologist correlated well with scores of the test radiologist (when examined on a continuous scale; ρ=0.75), although the validation radiologist’s scores were lower on average (73%±20% vs 83%±16%, p=0.02; Supplemental Figure 2). In the validation analysis, all tumors with hyperintense T2 score of ≥90% had progressed by 20 months after initial diagnosis, but median PFS was not reached in the group with score <90%; this finding was marginally significant (Figure 4B; HR 2.6; p=0.08). In the subset of patients with abdominal wall desmoids, PFS was still associated with hyperintense T2 score group, as assessed by the test radiologist and by the validation radiologist as well (p=0.002 and p=0.01, respectively; Figure 5). As assessed by the test radiologist, 0 of 10 abdominal wall tumors with hyperintense T2 score of <90% but 4 of 5 (80%) with score of ≥90% had disease progression, at median 9.4 months. In the analysis of abdominal wall scoring in the validation radiologist (Figure 5B), patients with hyperintense T2 score ≥90% again had a shorter PFS than those with <90% (median PFS 11.9 months vs 2 year PFS of 86%), and all patients with hyperintense T2 score (≥90%) had progressed 13.4 months after diagnosis.
Figure 5.
Kaplan-Meier analyses of progression-free survival in 15 patients with abdominal wall desmoids managed with active observation and with serial MRI sequences available. A. Patients stratified by hyperintense T2 score from the test radiologist. B. Patients stratified by score from the validation radiologist.
DISCUSSION
In this analysis, we have examined clinical outcomes in a series of 72 patients presenting to MSKCC with either primary or recurrent desmoid-type fibromatosis, initially managed with active observation for gross disease. While patients with small tumors (<5 cm) less often went on to therapeutic intervention compared with patients presenting with larger lesions, when we formally analyzed PFS (by RECIST criteria), neither tumor size nor any other clinical factor clearly predicted progression. However, among 37 patients who had undergone serial MRIs with T2-weighted sequences while on active observation, their baseline hyperintense T2 score (i.e. hyperintense T2 signal in ≥90% of tumor volume) strongly associated with radiographic RECIST desmoid progression during active observation. A desmoid with hyperintense T2 signal on MRI that occupies 90 percent or more of the tumor volume suggests a more cellular, biologically active desmoid and suggests that the patient is more likely to benefit from early intervention. Hyperintense T2 score is a novel marker in this clinical scenario, and it appears to be the most reliable predictor described to date for desmoid progression during observation. We performed our analysis using RECIST criteria to correlate with the seminal analysis of active observation of desmoid tumors published by Fiore et al.12 Recognizing that debate exists regarding optimal method for defining PFS, outcomes based on RECIST 1.1 criteria were performed in parallel based on the test radiologists findings; T2 hyperintense signal was again associated with PFS in the cohort of 37 patients (p=0.02) and the abdominal wall subset (p=0.073; data not shown).
A nonoperative approach to desmoid tumor management is being increasingly accepted,12, 13, 19 due in part to the morbidity of resection and the propensity of desmoids for local recurrence after resection. As untreated desmoid tumors may progress, remain stable, or regress,12, 19 reliable prognostic markers of desmoid tumor biology are needed to optimize treatment decisions for patients presenting with desmoid fibromatosis. Clinical features do not fill this need, as shown by results from the largest series of desmoid tumors managed with active observation, in which clinical features (size, site, and patient age) failed to predict which tumors would progress while under nonsurgical management; published series identifying associations with progression free survival often define the event to include recurrence after complete surgical resection.12, 19 This lack of association between clinical features and progression held largely true in our own cohort of patents. When considering all 72 patients who were managed with initial active observation at MSKCC, only size was significantly associated with time to intervention in univariate analysis. When considering the smaller cohort of 37 patients managed with initial observation who had appropriate MRI sequences for formal analysis of PFS, even size was not significantly associated with PFS. However, in this group, despite the small number of patients, progression was robustly associated with baseline hyperintense T2 score, with a dramatic difference in absolute risk of progression in patients with scores of ≥90% versus <90%. In the absence of other reliable predictors, this imaging characteristic may become the most valuable tool to select patients for immediate intervention versus observation.
Of note, it is not clear that this finding will be of utility in managing patients with intraabdominal tumors as our practice has been to follow these lesions with CT; no patients with intraabdominal tumors were included in the portion of our study examining the predictive value of MRI. However, the cohort did have a particularly high number of patients with abdominal wall tumors as observation is frequently prescribed in this setting (61% of our cohort), and association between hyperintense T2 score and progression was significant in these patients. For both the test radiologist and the validation radiologist, all abdominal wall desmoid patients with hyperintense T2 score of ≥90% who were followed for at least nine months had objective tumor progression while under observation. In the test radiologist analysis, none of those with hyperintense T2 score of <90% progressed during follow-up. Therefore, this feature of tumor reliably predicts clinical behavior and could be critically important in clinical-decision making.
Interestingly, although baseline hyperintense T2 score was strongly associated with progression, this variable was not associated with time to intervention. Among the patients on initial observation for whom we had serial MRI, only 10 of 24 (42%) who eventually underwent intervention had tumor growth by RECIST criteria. The reasons for this are difficult to ascertain, but it suggests that clinicians were not incorporating T2 signal characteristics into their clinical decisions; instead, the decision may be driven by patient and physician preference. This highlights the lack of concrete evidence indicating which subsets of patients are ideal candidates for management with active observation.
Although the test radiologist’s analysis showed clear and strong association of hyperintense T2 score with progression of desmoid tumors under observation, the validation radiologist’s analysis approached but did not reach statistical significance for desmoid tumors across all sites. Hyperintense T2 score defined by the second radiologist correlated with scores of the first (with a Ρearson correlation=0.75); however, the validation radiologist tended to score tumors lower. This variation may account for the fact that significance was only approached in this analysis. While analyses using cutoffs ranging from 80% to 90% did not yield more significant associations (not shown), the limited size of this cohort prevents a more formal analysis of the optimal cutoff (e.g., with recursive partitioning). The optimal cutoff value deserves further study in subsequent planned prospective trials and may, in fact, be shown to be operator dependent.
Major limitations of this study are the small study size and retrospective nature of the data. The latter poses a particular difficulty in that clear selection criteria defining a cohort of patients managed with initial active observation were difficult to discern. We also note the slight discrepancy between the test radiologist and the validation radiologist analyses. Intra-observer variability has the potential to limit the utility of subjective testing. However, the hyperintense T2 score was strongly predictive of progression, especially in abdominal wall desmoids. Almost all tumors with a score over 90% progressed during follow-up. This finding requires further validation. Based on our results, we plan a prospective trial with multiple blinded radiologists to evaluate reproducibility of these results. The trial will incorporate both semi-quantitative results based on determining mean signal intensity in regions of interest within a tumor and quantitative results using histogram and voxel-based analyses.
In summary, the percentage of baseline tumor volume showing hyperintense T2 MRI signal is a useful tool for clinical decision-making for patients with desmoid tumors considering active observation. Those tumors with hyperintense T2 score of ≥90% have a high likelihood of progression while under observation, while those with score of <90% are less likely to undergo progression and may represent an ideal subset of tumors to be managed without active intervention.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the American Cancer Society (Mentored Research Grant in Applied and Clinical Research MRSG-15-064-01-TBG), Alicia and Corey Pinkston, the Award of Courage Foundation, the Kristen Ann Carr Foundation, the National Cancer Institute Institutional Cancer Center Core Grant (P30-CA008748) and NIH/NCI Cancer Center Support Grant (P30 CA008748). We thank Janet Novak, PhD, of MSKCC for editing the manuscript.
Sources of funding: American Cancer Society (Mentored Research Grant in Applied and Clinical Research MRSG-15-064-01-TBG), Alicia and Corey Pinkston, the Award of Courage Foundation, the Kristen Ann Carr Foundation, the National Cancer Institute SPORE in Soft Tissue Sarcoma (P50 CA140146), and the NIH/NCI Cancer Center Support Grant (P30 CA008748).
Footnotes
Conflicts of interest: The authors have no conflicts of interest to report
Presented at the Society of Surgical Oncology 70th Annual Cancer Symposium March 18, 2017, Seattle, Washington
REFERENCES
- 1.Wu C, Amini-Nik S, Nadesan P, et al. Aggressive fibromatosis (desmoid tumor) is derived from mesenchymal progenitor cells. Cancer Res 2010; 70(19):7690–8. [DOI] [PubMed] [Google Scholar]
- 2.Lewis JJ, Boland PJ, Leung DH, et al. The enigma of desmoid tumors. Ann Surg 1999; 229(6):866–72; discussion 872–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M, Cordon-Cardo C, Gerald WL, et al. Desmoid fibromatosis is a clonal process. Hum Pathol 1996; 27(9):939–43. [DOI] [PubMed] [Google Scholar]
- 4.Crago AM, Denton B, Salas S, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg 2013; 258(2):347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merchant NB, Lewis JJ, Woodruff JM, et al. Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer 1999; 86(10):2045–52. [PubMed] [Google Scholar]
- 6.Easter DW, Halasz NA. Recent trends in the management of desmoid tumors. Summary of 19 cases and review of the literature. Ann Surg 1989; 210(6):765–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higaki S, Tateishi A, Ohno T, et al. Surgical treatment of extra-abdominal desmoid tumours (aggressive fibromatoses). Int Orthop 1995; 19(6):383–9. [DOI] [PubMed] [Google Scholar]
- 8.Lopez R, Kemalyan N, Moseley HS, et al. Problems in diagnosis and management of desmoid tumors. Am J Surg 1990; 159(5):450–3. [DOI] [PubMed] [Google Scholar]
- 9.Markhede G, Lundgren L, Bjurstam N, et al. Extra-abdominal desmoid tumors. Acta Orthop Scand 1986; 57(1):1–7. [DOI] [PubMed] [Google Scholar]
- 10.Plukker JT, van Oort I, Vermey A, et al. Aggressive fibromatosis (non-familial desmoid tumour): therapeutic problems and the role of adjuvant radiotherapy. Br J Surg 1995; 82(4):510–4. [DOI] [PubMed] [Google Scholar]
- 11.Rock MG, Pritchard DJ, Reiman HM, et al. Extra-abdominal desmoid tumors. J Bone Joint Surg Am 1984; 66(9):1369–74. [PubMed] [Google Scholar]
- 12.Fiore M, Rimareix F, Mariani L, et al. Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol 2009; 16(9):2587–93. [DOI] [PubMed] [Google Scholar]
- 13.Kasper B, Baumgarten C, Garcia J, et al. An update on the management of sporadic desmoid-type fibromatosis: a European Consensus Initiative between Sarcoma PAtients EuroNet (SPAEN) and European Organization for Research and Treatment of Cancer (EORTC)/Soft Tissue and Bone Sarcoma Group (STBSG). Ann Oncol 2017; 28(10):2399–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penel N, Le Cesne A, Bonvalot S, et al. Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: A nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer 2017; 83:125–131. [DOI] [PubMed] [Google Scholar]
- 15.de Camargo VP, Keohan ML, D’Adamo DR, et al. Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer 2010; 116(9):2258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gounder MM, Lefkowitz RA, Keohan ML, et al. Activity of Sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res 2011; 17(12):4082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith AJ, Lewis JJ, Merchant NB, et al. Surgical management of intra-abdominal desmoid tumours. Br J Surg 2000; 87(5):608–13. [DOI] [PubMed] [Google Scholar]
- 18.Burtenshaw SM, Cannell AJ, McAlister ED, et al. Toward Observation as First-line Management in Abdominal Desmoid Tumors. Ann Surg Oncol 2016; 23(7):2212–9. [DOI] [PubMed] [Google Scholar]
- 19.Salas S, Dufresne A, Bui B, et al. Prognostic factors influencing progression-free survival determined from a series of sporadic desmoid tumors: a wait-and-see policy according to tumor presentation. J Clin Oncol 2011; 29(26):3553–8. [DOI] [PubMed] [Google Scholar]
- 20.Vandevenne JE, De Schepper AM, De Beuckeleer L, et al. New concepts in understanding evolution of desmoid tumors: MR imaging of 30 lesions. Eur Radiol 1997; 7(7):1013–9. [DOI] [PubMed] [Google Scholar]
- 21.Sundaram M, McGuire MH, Herbold DR, et al. High signal intensity soft tissue masses on T1 weighted pulsing sequences. Skeletal Radiol 1987; 16(1):30–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.