Abstract
Non-alcoholic fatty liver disease (NAFLD) is becoming the most common chronic liver disease globally. NAFLD—which can develop into liver fibrosis, nonalcoholic steatohepatosis, cirrhosis, and hepatocellular carcinoma—is defined as an excess accumulation of fat caused by abnormal lipid metabolism and excessive reactive oxygen species (ROS) generation in hepatocytes. Recently, we reported that Peroxiredoxin 5 (Prx5) plays an essential role in regulating adipogenesis and suggested the need to further investigation on the potential curative effects of Prx5 on obesity-induced fatty liver disease. In the present study, we focused on the role of Prx5 in fatty liver disease. We found that Prx5 overexpression significantly suppressed cytosolic and mitochondrial ROS generation. Additionally, Prx5 regulated the AMP-activated protein kinase pathway and lipogenic gene (sterol regulatory element binding protein-1 and FAS) expression; it also inhibited lipid accumulation, resulting in the amelioration of free fatty acid-induced hepatic steatosis. Silence of Prx5 triggered de novo lipogenesis and abnormal lipid accumulation in HepG2 cells. Concordantly, Prx5 knockout mice exhibited a high susceptibility to obesity-induced hepatic steatosis. Liver sections of Prx5-deletion mice fed on a high-fat diet displayed Oil Red O-stained dots and small leaky shapes due to immoderate fat deposition. Collectively, our findings suggest that Prx5 functions as a protective regulator in fatty liver disease and that it may be a valuable therapeutic target for the management of obesity-related metabolic diseases.
Keywords: Peroxiredoxin 5, Hepatic steatosis, NAFLD, ROS, AMPK, SREBP-1
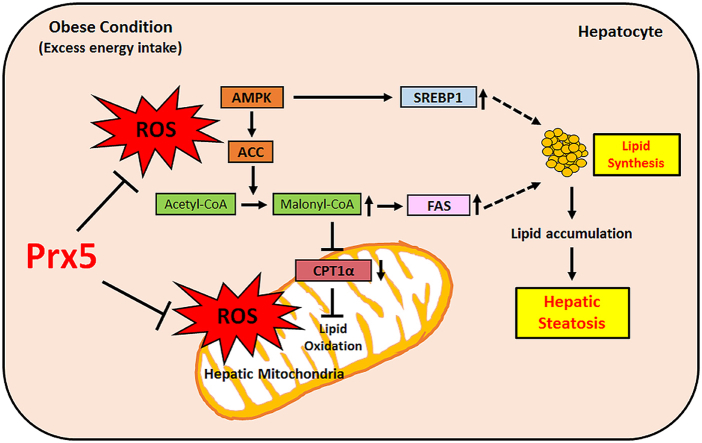
Graphical abstract
Highlights
-
•
Prx5 decreased the FFA-induced intracellular and mitochondrial ROS generation.
-
•
Prx5 improved hepatic steatosis via regulation of AMP-activated protein kinase.
-
•
Knockout of Prx5 aggravated obesity related fatty liver disease.
-
•
Prx5 has a crucial role in hepatic lipid metabolism.
Abbreviations
- NAFLD
Non-alcoholic fatty liver disease
- Prx
Peroxiredoxin
- ROS
Reactive oxygen species
- FFA
Free fatty acids
- AMPK
AMP-activated protein kinase
- ACC
Acetyl-CoA carboxylase
- SREBP-1
Sterol regulatory element binding protein-1
- HFD
High-fat-diet
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- MAPK
Mitogen-activated protein kinase
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is among the most prevalent metabolic diseases accompanying obesity, hyperlipidemia, and diabetes [1,2]. In general, unusual lipid metabolism and subsequent lipid accumulation in hepatocytes leads to hepatic steatosis, the first step of NAFLD. Insulin resistance and inflammation aggravate NAFLD. Additionally, NAFLD can progress to non-alcoholic steatohepatitis (NASH), advanced hepatic fibrosis and cirrhosis [3]. There are several studies that have investigated the pathogenetic mechanism of NAFLD, but the process remains unclear.
NAFLD is characterized by abnormal hepatocellular fat deposits, hepatic steatosis, a severely damaged histological appearance, liver enlargement, and hepatic inflammation reactions [4,5]. The development of NAFLD is often summarized using the “two-hit hypothesis.” The first hypothesis posits that steatosis stems from immoderate hepatocellular lipid accumulation, which results from hepatic de novo lipogenesis and damaged fatty acid oxidation [6]. The enzyme AMP-activated protein kinase (AMPK) is a crucial regulator of energy metabolisms in the liver, adipose tissue, and muscle. In particular, AMPK is closely associated with hepatic lipogenesis and fatty acid oxidation [7,8]. In healthy hepatocytes, AMPK controls the activity of enzymes involved in lipogenesis, such as acetyl-CoA carboxylase (ACC), sterol regulatory element binding protein (SREBP-1), and FAS, through phosphorylation and dephosphorylation. However, in NAFLD, AMPK dephosphorylation causes fatty acid synthesis (lipogenesis) through the dephosphorylation of ACC and up-regulation of SREBP-1. Following that, ACC transforms acetyl-CoA to malonyl-CoA. Elevated malonyl-CoA inhibits CPT1 activity (related with fatty acid oxidation), resulting in the development of NAFLD [9].
The second hypothesis is related to redox balance. Excessive food intake stimulates reactive oxygen species (ROS) generation, and high levels of ROS can promote NAFLD [10,11]. Immoderate ROS generation represents an imbalance between oxidant and antioxidant agents. Redox imbalance leads to hepatic mitochondrial dysfunction, inflammatory responses, and a breakdown of lipid metabolism [12]. Mitochondria are the major organs involved in ROS production, and they can easily be damaged by ROS [13]. Previous studies have been focused on the effect of ROS derived from mitochondria, linking them to the development of NAFLD [14]. In NAFLD, ROS can induce damaged mitochondrial membrane potentials and changes in mitochondrial structure. This hypothesis supports the theory ROS-induced mitochondrial dysfunction triggers ROS overproduction, causing a breakdown of lipid homeostasis. The imbalance of lipid metabolism leads to excessive lipid accumulation in hepatocyte [15]. Several studies have suggested that antioxidative treatments have therapeutic effects on NAFLD by inhibiting de novo lipogenesis and scavenging mitochondrial ROS [16,17]. Therefore, we focused on the protective effect of mitochondria-targeting antioxidant enzymes against NAFLD.
Peroxiredoxins (Prxs), a family of cysteine-dependent peroxidase enzymes, have a superior ability to scavenge peroxide and peroxynitrite in mammalian cells. There are 6 isoforms (Prx1-6) of Prxs, which are categorized according to their subcellular positions [18]. More so than the other Prxs, Prx3 and Prx5 are mitochondrial-target antioxidant enzymes, because of their localization [19]. Several studies have reported that Prx3 and Prx5 can effectively remove cytosolic and mitochondrial ROS in various cell lines [20,21]. We previously demonstrated a high susceptibility to high-fat diet (HFD)-induced obesity and increased triglyceride level in Prx5-deficient mice. In particular, liver tissues isolated from HFD-fed Prx5 −/− mice had an expanded and greasy appearance. We suggested that Prx5 plays a crucial role in obesity and obesity-associated fatty liver disease [22]. However, the precise role of Prx5 on fatty liver disease remains unclear. Therefore, we hypothesized that Prx5 may improve obesity-induced fatty liver disease by regulating mitochondrial ROS levels in mouse hepatocytes. Also, we confirmed the development of fatty liver disease in a Prx5 knockout mouse model.
In this study, we confirmed that Prx5 ameliorated free fatty acid (FFA)-induced ROS overproduction and lipid accumulation in HepG2 cells. Also, Prx5 overexpression ameliorated hepatic steatosis by regulating lipogenesis and hepatic inflammation. Additionally, when we induced fatty liver disease via HFD feeding, we observed that the expression of lipogenesis-related proteins increased more among Prx5 knockout mice than among wild-type (WT) mice. These findings demonstrate the role of Prx5 in hepatic lipid metabolism and suggest that Prx5 has a potential role in the treatment of obesity-induced NAFLD.
2. Materials and methods
2.1. Materials
N-Acetyl-l-cysteine (NAC), oleic acid, and palmitic acid were obtained from Sigma-Aldrich (MO, USA).
2.2. Cell culture and treatment
We purchased HepG2 cells from the American Type Culture Collection (Manassas, VA, USA). HepG2 cells were cultured in Minimum Essential Medium, Eagle (MEM) (Welgene, Korea), supplemented with 1% penicillin/streptomycin (Welgene) and 10% fetal bovine serum (FBS; Gibco, New Zealand). HepG2 cells were pre-treated with NAC (Sigma-Aldrich) for 30 min after the growth medium was replaced and then treated with 1 mM FFA.
2.3. Fat-overloading induction in HepG2 cells
To induce hepatic steatosis in hepatocytes, HepG2 cells at 75% confluency were cultured with MEM containing 2% FBS overnight, and then we incubated HepG2 cells with 1 mM FFA mixture (oleic acid:palmitic acid = 0.66 mM:0.33 mM) for 24 h. The FFA mixture was prepared in culture medium containing 1% bovine serum albumin (BSA) [23].
2.4. Lentivirus generation and establishment of a stable cell line
Prx5 gene was obtained from Dr. Tae-Hoon Lee (Chonnam National University, Gwangju, Korea). The Prx5 coding sequence was amplified by polymerase chain reaction (PCR) using LA Taq® DNA polymerase (Takara, Shiga, Japan) and cloned into the pCR8/GW/TOPO vector (Invitrogen, CA, USA). The Prx5 gene was then inserted into pLenti6.3/V5-DEST using LR Clonase (Invitrogen). These vectors have 14-amino-acid V5 epitopes in the C-terminal that help with the detection of recombinant proteins during immunoblotting analysis [24]. Construction of the lentivirus was performed as previously described [25]. All the three vectors (pLenti6.3-Prx5 vector, psPAX2 packaging vector, and pMD.2G enveloping vector) were transfected into HEK293FT cells using Lipofectamine 2000 (Invitrogen). After 12 h of transfection, the medium was replaced with fresh medium. After 48–72 h of transfection, lentivirus-containing medium was harvested and purified with a 0.45 μm filter (Sartorius, Gottingen, Germany). The pLenti6.3-Prx5 stable cell lines were generated by infecting HepG2 cells with lentiviral vectors. During the infection, polybrene (Sigma-Aldrich), which increases the transfection efficiency, was added at 8 μg/ml, and the cells were cultured for 72 h. Lentiviral vector-transduced HepG2 cells were selected by growing them in 4 μg/mL of blasticidin (Invitrogen) for several days.
2.5. Analysis of intracellular and mitochondrial ROS
Intracellular and mitochondrial ROS were detected using 2′, 7′-dichlorofluorescein diacetate (CM-H2DCF-DA; Invitrogen) and MitoSOX Red (Molecular Probes, OR, USA), respectively. HepG2 cells were seeded into 6-well plates and following 24 h of incubation, they were treated with or without 1 mM FFA for 24 h. Next, the cells were harvested using 0.25% trypsin-EDTA. The collected cells were washed with phosphate-buffered saline (PBS; pH 7.4) and incubated with 5 μM DCF-DA or MitoSOX containing PBS for 20 min at 37 °C. The cells were then washed with PBS twice and analyzed using flow cytometry (BD Bioscience, CA, USA). ROS levels in liver tissues were assessed using the OxiSelect In Vitro ROS/RNS Assay Kit (Cell Biolabs Inc., CA, USA). This assay kit contains a specific ROS/RNS probe, dichlorodihydrofluorescein DiOxyQ (DCFH-DiOxyQ).
2.6. Analysis of intracellular superoxide
Intracellular superoxide levels were detected via dihydroethidium (DHE) staining. After FFA treatments, the cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) and incubated with 2.5 μM DHE, which was dissolved in phenol red-free MEM, for 10 min. After incubation, a DHE image was obtained by using a DE/DMI 3000B fluorescence microscope (Leica, Wetzlar, Germany).
2.7. Protein extraction and Western blot analysis
Proteins were extracted from the cells using PRO-PREP Protein Extraction Solution (Intron Biotechnology, Seongnam, Korea). The extracted proteins were then separated using 12% SDS-PAGE and transferred onto nitrocellulose membranes (Pall, FL, USA). The membranes were then incubated with primary antibodies against SREBP-1, IκB, NFκB, β-actin (Santa Cruz, CA, USA), AMPK, p-AMPK, ACC, p-ACC, FAS, JNK, p-JNK, Erk, p-Erk, p38, p-p38, p-IκB, COX2, iNOS (Cell Signaling, MA, USA), CPT-1α (Abcam, UK), Prx1, Prx2, Prx3, Prx4, or Prx5 (Abfrontier, Korea). The membranes were further probed with horseradish peroxidase-conjugated anti-mouse IgG or horseradish peroxidase-conjugated anti-rabbit IgG secondary antibodies (Thermo Scientific). The signals were visualized using Clarity Western ECL Substrate (Bio-Rad, CA, USA), and band intensities were analyzed using the Multi Gauge version 3.0 software (Fujifilm, Japan).
2.8. Oil Red O staining
Lipid accumulation was assessed using Oil Red O (Sigma-Aldrich). After the initiation of differentiation and FFA treatment, HepG2 cells were washed with PBS and fixed with 4% paraformaldehyde (Sigma-Aldrich) overnight. The cells were then stained with 0.35% Oil Red O solution for 3 h at 4 °C, followed by three washes with double-distilled water. The red-stained lipid droplets were visualized under a light microscope and photographed. To quantify the lipid production, Oil Red O-stained lipid droplets were extracted with 100% isopropanol, and absorbance was measured at 495 nm.
2.9. Animal care and experimental design
We used Prx5 −/− mice and WT mice, and both mice are C57BL/6 J. We constructed a targeting vector in which the sequence was replaced with the neo gene. Prx5 −/− mice were produced with Mendelian inheritance, and this was identified by PCR. The primers used were 5′- ATTCTTTGGTGTCTCTCTTTGGG-3′ and 5′-CTTCACTTTCTCCTCCAAATCCC-3′ for the Prx5 gene. All mice were housed at 23 °C and light/dark cycles of 12 h. All animal experiments were performed according to national ethical guidelines and were approved by the Institutional Animal Care and Use Committee at the Kyungpook National University. Mice were fed a standard mouse chow diet (10% calories from fat; D12450B; Research Diets, New Brunswick, NJ, USA) or HFD (60% calories from fat; D12451; Research Diets, NJ, USA). The HFD study with 8-week-old Prx5 −/− mice and WT mice was conducted for 6 weeks.
2.10. Histological analysis
Liver tissues were collected and fixed in 4% (w/v) paraformaldehyde (Sigma-Aldrich). Thick (5 μm) tissue sections were then prepared for histological analysis via paraffin embedding and cryopreservation. Paraffin-embedded sections were stained with hematoxylin and eosin. Oil Red O staining was performed on frozen tissue sections to measure lipid content in the liver.
2.11. Serum analysis
Blood was obtained from the infraorbital vein, followed by centrifugation at 13,000 rpm for 10 min to separate the serum only. Serum was used to measure the level of alanine aminotransferase (ALT)/aspartate aminotransferase (AST) using a commercial kit (ASAN Pharm, Daegu, Korea).
2.12. Immunohistochemistry
Immunohistochemistry was performed on cryo-embedded liver sections. After incubation in blocking solution (10% normal goat serum, Gibco), tissues were incubated with an anti-SREBP-1 antibody (Santa Cruz) at 1:200 dilutions. The tissues were then labeled with Alexa Fluor 594-conjugated secondary antibodies (Molecular Probes, OR, USA) for 1 h. Slices were observed using an LSM-710 confocal microscope (Carl Zeiss, Jena, Germany).
2.13. Statistical analysis
All experiments were repeated as at least three independent experiments. Quantitative data are presented as the mean ± standard deviation of the triple determinations from all experiments. Data were analyzed using analysis of variance (ANOVA) on GraphPad Prism 5.01 (GraphPad Software Inc., La Jolla, CA, USA) to derive the p-values.
3. Results
3.1. FFA treatment induced lipogenesis and lipid accumulation in HepG2 cells
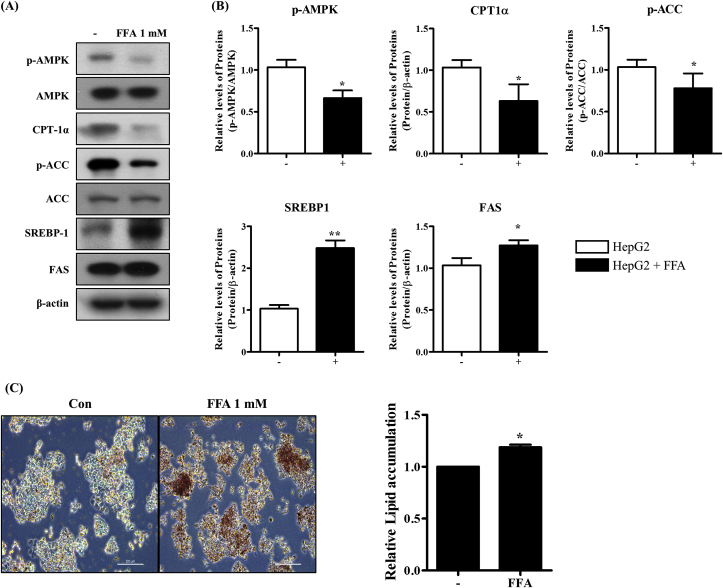
FFA treatment leads to hepatic steatosis and lipid accumulation in hepatocytes [23]. To characterize FFA-induced hepatic steatosis in HepG2 cells, we evaluated the AMPK pathway, which is involved in the regulation of lipid metabolism. To examine the changes in the expression of hepatic steatosis-associated proteins as well as lipid accumulation, we incubated HepG2 cells with a 1 mM FFA mixture for 24 h. As shown in Fig. 1A and B, the FFA altered the AMPK pathway and lipogenesis-associated protein expression. We confirmed that phosphorylated AMPK (p-AMPK)/AMPK, phosphorylated ACC (p-ACC)/ACC (AMPK pathway) and CPT-1α (associated with fatty acid oxidation) exhibited decreased expression levels in FFA-treated HepG2 cells. In contrast, the expression levels of SREBP-1 (a key transcriptional regulator in triglyceride synthesis) and FAS (a lipogenesis enzyme), were remarkably increased by the FFA mixture.
Fig. 1.
Effect of FFA treatment on lipogenesis and lipid accumulation in HepG2 cells. HepG2 cells were treated with 1 mM FFA mixture (oleate/palmitate, 2:1 ratio) for 24 h. (A, B) The levels of protein (AMPK/ACC, CPT-1α, SREBP-1, FAS) were measured by Western blotting. (C) Lipid accumulation was measured using Oil Red O staining. Untreated HepG2 cells were used as a control. Values are presented as mean ± standard deviation (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. control. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The hallmark of hepatic steatosis is abnormal lipid accumulation in hepatocytes. Therefore, we investigated if a mixture of FFAs induced lipid accumulation in HepG2 cells, as demonstrated by Oil Red O staining (Fig. 1C). The FFA mixture indeed promoted the formation of lipid droplets and hepatic lipid accumulation, indicating that FFA mixture treatment induced hepatic steatosis and lipid accumulation via the activation of lipogenesis.
3.2. FFA-induced hepatic steatosis induces ROS production and upregulation of Prx5
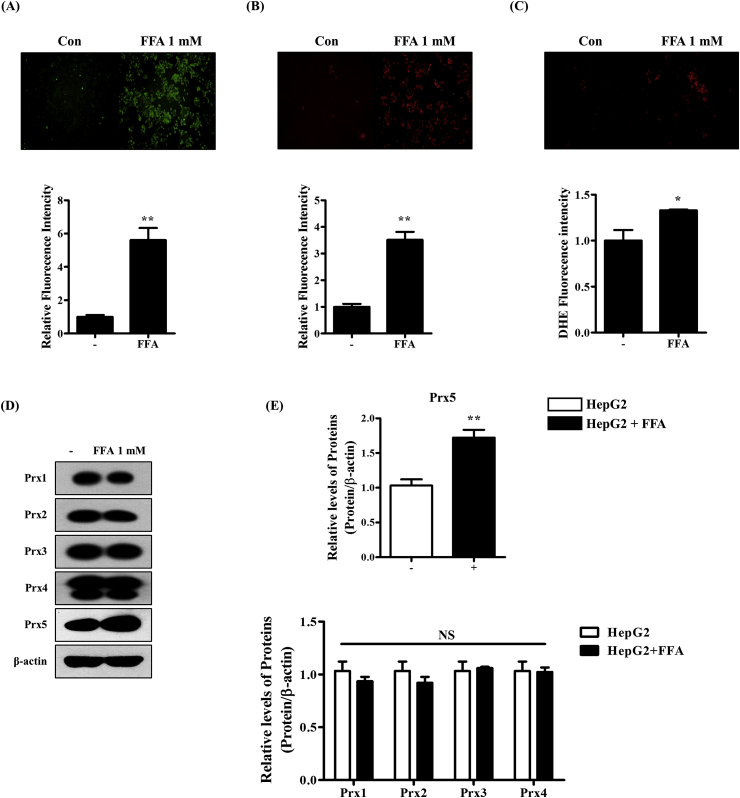
Next, we investigated the effects of FFAs on the intracellular redox system in HepG2 cells. First, we measured the levels of ROS in HepG2 cells treated with FFAs using H2DCF-DA (detects intracellular ROS), MitoSOX (detects mitochondrial ROS), and DHE staining (detects superoxide) (Fig. 2A–C). As a result, FFA treatment increased intracellular and mitochondrial ROS generation and superoxide levels. These results show that the development of FFA-induced hepatic steatosis is closely associated with elevated ROS levels. We then confirmed changes in antioxidant enzyme (Prx) levels. As shown in Fig. 2D and E, there were no changes in the expression levels of Prx1, Prx2, Prx3, Prx4, or Prx6. However, only Prx5 expression increased after FFA treatment. These findings indicated that Prx5 plays a crucial role in the development of FFA-induced hepatic steatosis.
Fig. 2.
Effect of FFA on the generation of reactive oxygen species (ROS) and the expression of peroxiredoxins. HepG2 cells were treated with 1 mM FFA mixture (oleate/palmitate, 2:1 ratio) for 24 h. (A) Intracellular ROS levels were assessed with 2, 7- dichlorofluorescein diacetate (CM-H2DCF-DA) assays. (B) Mitochondrial ROS levels were measured with MitoSOX. (C) ROS levels were measured through DHE staining. (D, E) Western blotting was used to measure expression levels of peroxiredoxins. Untreated HepG2 cells were used as controls. Values are presented as mean ± standard deviation (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. control.
3.3. Prx5 regulated FFA-induced intracellular and mitochondrial ROS generation
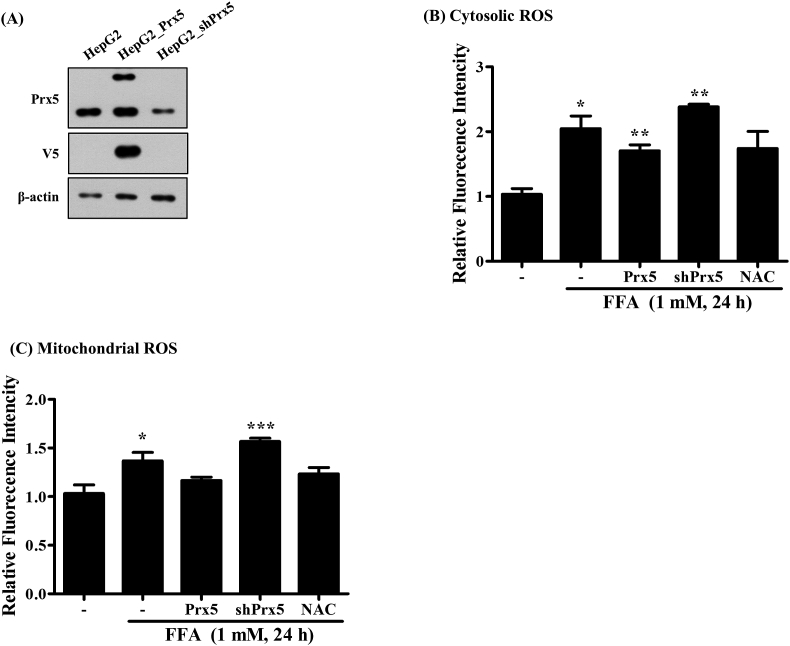
To investigate if Prx5 regulates FFA-induced ROS generation in HepG2 cells, we induced Prx5 (HepG2_Prx5) and mutated Prx5 (HepG2_shPrx5) expression in stably expressing HepG2 cells using lentivirus. The HepG2_shPrx5 was an artificial structure that could silence the target gene expression. First, we identified the exogenous expression of Prx5 and the suppression of endogenous Prx5 by Western blotting using Prx5 and V5-tag antibodies (Fig. 3A). The lentivirus-mediated Prx5 gene was overexpressed successfully. Also, endogenous Prx5 expression was almost entirely suppressed in HepG2_shPrx5 cells. We observed that FFAs induced the generation of intracellular and mitochondrial ROS, but Prx5 overexpression inhibited this activity. On the other hand, silencing endogenous Prx5 (shPrx5) promoted FFA-induced intracellular and mitochondrial ROS generation, relative to HepG2_Prx5 cells (3B and C). When we treated HepG2 cells with FFA and10mM NAC, which is an effective antioxidant, intracellular and mitochondrial ROS levels decreased. These findings suggested that Prx5 might ameliorate liver steatosis through regulating the generation of intracellular and mitochondrial ROS.
Fig. 3.
Effect of Prx5 on FFA-induced intracellular and mitochondrial ROS generation. Before FFA treatment, HepG2 cells were incubated with 10 mM N-acetyl-L cysteine (NAC). HepG2 cells treated with NAC were used as positive controls. (A) Stable expression of Prx5 or knockdown of Prx5 (shPrx5) were detected in HepG2 cells through Western blotting with Prx5 and V5-tag antibodies. (B) Flow cytometry analysis of intracellular and mitochondrial ROS in HepG2, HepG2_Prx5, and HepG2_shPrx5 cells treated with FFA mixture. ROS levels were measured with CM-H2DCFDA and MitoSOX. Untreated HepG2 cells were used as controls. Values are presented as mean ± standard deviation (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. control.
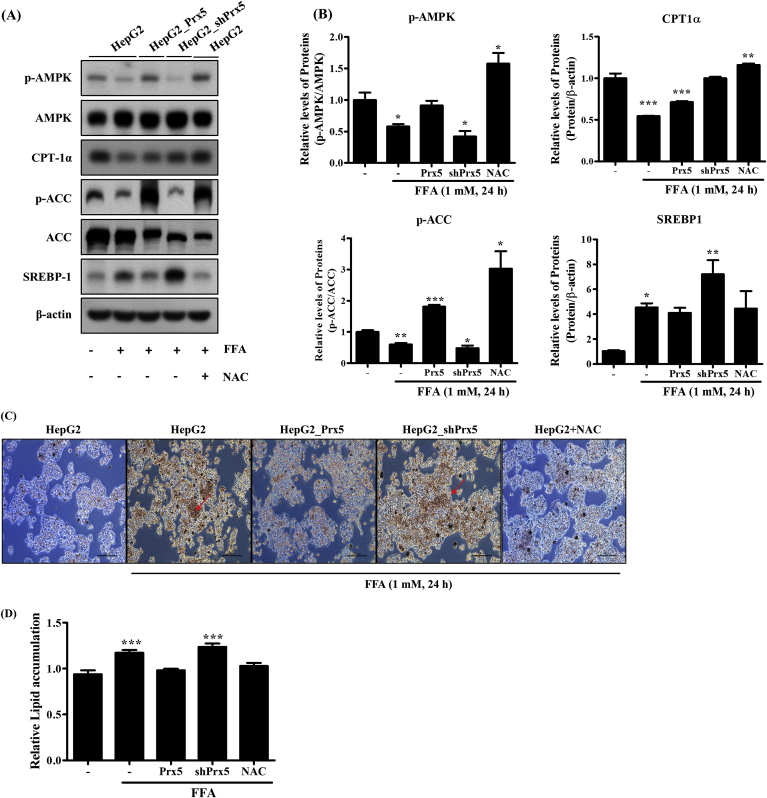
3.4. Prx5 suppressed FFA-induced hepatic steatosis and lipid accumulation
To further investigate the precise molecular mechanism underlying the hepatic steatosis-mitigating effects of Prx5, we evaluated the expression of hepatic steatosis-associated proteins. As shown in Fig. 4A and B, Prx5 overexpression induced the phosphorylation of AMPK and ACC and inhibited the expression of lipogenesis-associated proteins, including SREBP-1 and FAS. Prx5 overexpression also upregulated CPT-1α expression. These findings suggested that Prx5 inhibited FFA-induced lipogenesis and facilitated fatty acid oxidation. Prx5 knockdown (shPrx5) had the opposite effects, thereby complementing our Oil Red O staining results. Subsequently, we performed Oil Red O staining to assess the production of lipid droplets in HepG2 cells. Oil Red O staining demonstrated that Prx5 overexpression decreased the production of lipid droplets. Conversely, more Oil Red O-stained lipid droplets were observed in HepG2_shPrx5 cells than control HepG2 cells (Fig. 4C and D). These results confirmed that Prx5 knockdown promoted lipid accumulation and hepatic steatosis, which was in line with the hypothesis that Prx5 is a key regulator in FFA-induced hepatic steatosis.
Fig. 4.
Effect of Prx5 on FFA-induced lipogenesis and lipid accumulation in HepG2 cells. Before FFA treatment, HepG2 cells were incubated with 10 mM N-acetyl-L cysteine (NAC). HepG2 cells treated with NAC were used as positive controls. (A, B) The levels of protein (AMPK/ACC, CPT-1α, SREBP-1, FAS) were measured by Western blotting. (C, D) Lipid accumulation was measured using Oil Red O staining. Untreated HepG2 cells were used as controls. Values are presented as mean ± standard deviation (n = 3). *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. control. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
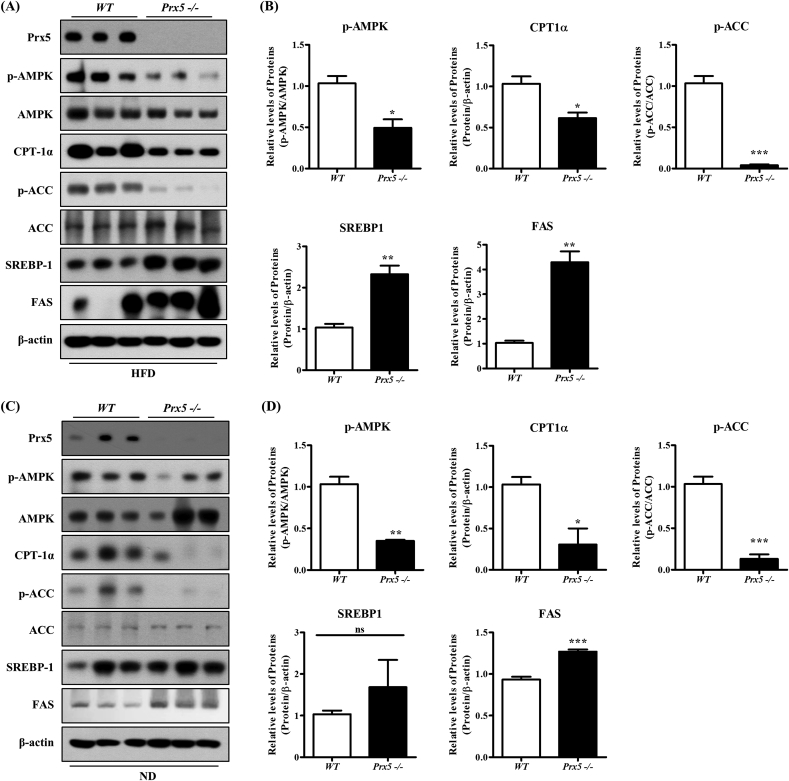
3.5. Deletion of Prx5 accelerated hepatic lipogenesis in HFD-induced obese mice
Previously, we demonstrated that Prx5 −/− mice exhibited adipocyte hypertrophy and increased expression of adipogenic proteins compared with WT mice. This difference resulted in the high susceptibility to HFD-induced obesity and obesity-related complications [22]. Therefore, we harvested the liver tissues from WT and Prx5 −/− mice to confirm the physiological role of Prx5 in obesity-induced hepatic steatosis. First, to elucidate the molecular mechanisms of metabolic changes in the liver, we evaluated the expression levels of hepatic steatosis-associated proteins. In accordance with in vitro results (Fig. 4), we observed increased expression of lipogenesis proteins, such as SREBP-1 and FAS, following the dephosphorylation of AMPK and ACC in Prx5 −/− mice. However, deletion of Prx5 decreased CPT-1α expression. These results were found regardless of the type of feeding diet (normal or HFD) (Fig. 5A–D). Our findings confirmed that Prx5 is involved in hepatic lipid metabolism even in vivo systems, and it might be a suppressor for obesity-induced fatty liver diseases.
Fig. 5.
Effects of Prx5 deficiency on in vivo hepatic lipogenesis. (A–D) The expression of the hepatic steatosis-associated proteins in liver tissues of WT and Prx5 −/− mice was measured by Western blotting (n = 3). WT mice were used as controls. Values are presented as mean ± standard deviation. *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. control.
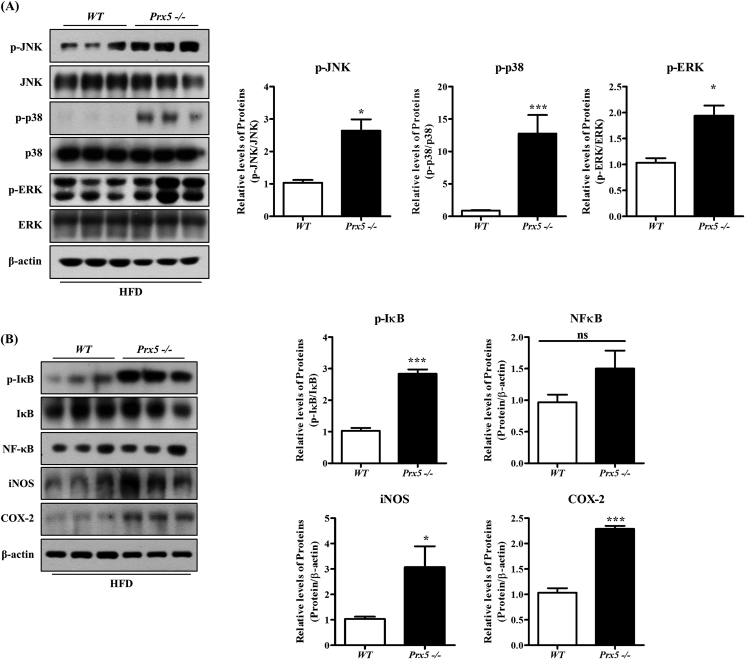
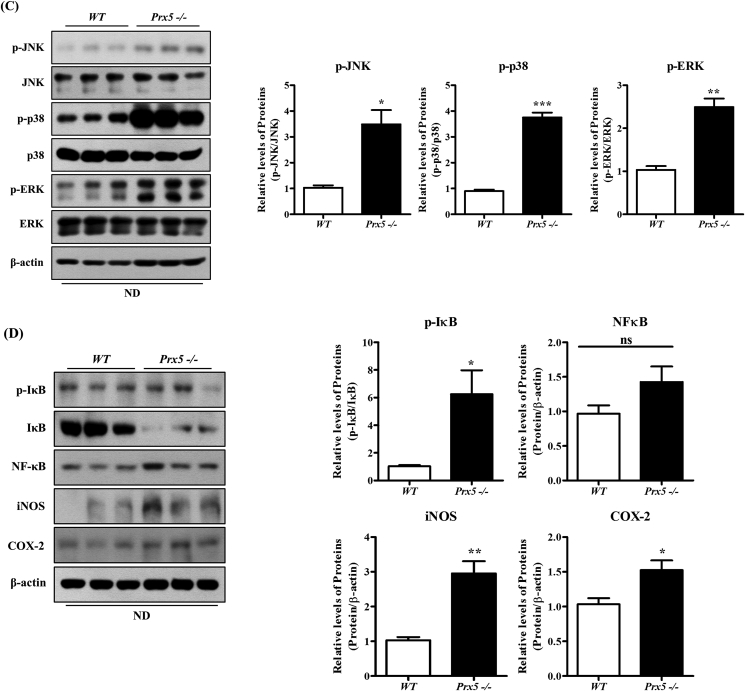
3.6. Prx5 −/− mice exhibited increased hepatic inflammation
Recent studies suggested that the activation of mitogen-activated protein kinase (MAPK) signaling (JNK, ERK, and p38) is closely associated with insulin resistance and hepatic inflammation, which could lead to NAFLD. In particular, hepatic inflammation is a key factor for the progression of hepatic steatosis [26,27]. Thus, we performed Western blotting to confirm the inflammation. First, we evaluated the expression of p-JNK, p-ERK, and p-p38. Deletion of Prx5 increased phosphorylation of p-JNK, p-ERK, and p-p38, compared to WT mice (Fig. 6A and C). We also measured the effect of Prx5 knockout on the proinflammatory response, including iNOS and COX-2 expression. As shown in Fig. 6B and D, iNOS and COX-2 expression markedly increased in Prx5 −/− mice.
Fig. 6.
Effects of Prx5 deficiency on hepatic inflammation. (A–D) The expression of the JNK/ERK/p38 pathway and inflammation-associated proteins in liver tissues of WT and Prx5−/− mice was measured by Western blotting (n = 3). WT mice were used as controls. Values are presented as mean ± standard deviation. *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. control.
NF-κB is involved in the regulation of inflammatory responses, and translocation of NF-κB to the nucleus activates phosphorylation of IκB [28]. To confirm whether Prx5 regulates the NF-κB pathway, we assessed NF-κB activation by measuring the phosphorylation of IκB. Prx5 −/− mice showed a higher level of phosphorylated IκB than WT mice. Altogether, these results demonstrated that the deletion of Prx5 enhanced inflammatory responses, aggravating hepatic steatosis.
3.7. Prx5 −/− mice exhibited severe hepatic steatosis
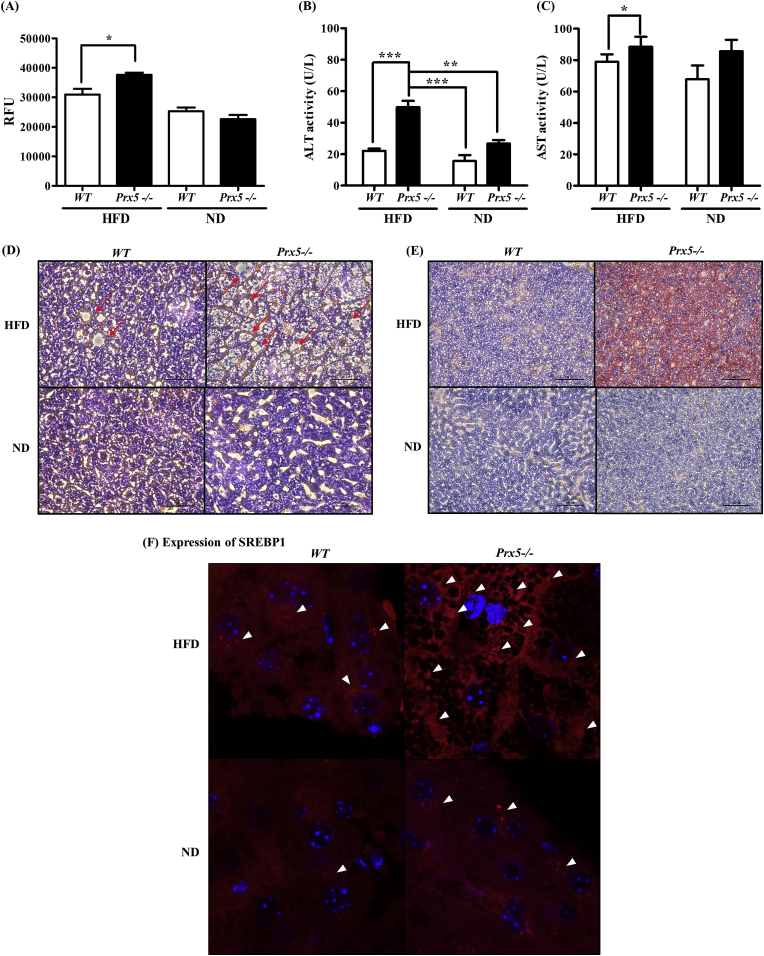
ROS generation and lipid accumulation are associated with excessive energy intake, and this leads to obesity and obesity-related complications. We previously observed that the liver tissue of Prx5 −/− mice appeared a greasier and larger appearance than that of WT mice. Additionally, Prx5 −/− mice showed the high level of triglyceride and insulin resistance. These results indicated that deletion of Prx5 predisposed mice to obesity and obesity-induced NAFLD [22]. In the present study, we confirmed the progression of NAFLD in liver tissues and serum. First, we measured ROS levels in liver tissues using the Oxiselect In vitro ROS/RNS Assay Kit (Cell Biolabs Inc.). As shown in Fig. 7A, deletion of Prx5 increased ROS levels in HFD-fed mice. Serum ALT and AST are markers of hepatocellular damage. Thus, ALT and AST levels are used in NAFLD diagnosis [29]. As expected, we observed higher levels of ALT and AST— reflecting hepatic lipotoxicity—in Prx5 −/− mice than WT mice (Fig. 7B and C). Therefore, our serum analysis implied that the excessive lipid accumulation associated with hepatic steatosis occurs in association with liver tissue damage.
Fig. 7.
Effects of Prx5 deficiency on hepatic steatosis. (A) ROS level assessed by OxiSelect In Vitro ROS/RNS Kit in liver tissues. (B, C) Activities of serum alanine aminotranferase (ALT) and aspartate aminotranferase (AST) were measured by commercial kits. (D) Histological sections of the liver were stained with hematoxylin and eosin. (E) Lipid accumulation in mouse livers was assessed using Oil Red O staining. (F) Immunohistochemistry staining of SREBP-1, involved in lipogenesis in the liver tissues of WT and Prx5 −/− mice. WT mice were used as controls. Values are presented as mean ± standard deviation (n = 3). *, p < 0.05; ***, p < 0.001 vs. control. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Next, we found many small vacuoles and severe hepatic steatosis in the livers of the HFD-fed Prx5 −/− mice, but not in HFD-fed WT mice (Fig. 7D). Subsequently, we performed Oil Red O staining to assess hepatic lipid accumulation in mice. In Oil Red O-stained liver sections (Fig. 7E), lipid droplets were not visible in the normal diet-fed groups and HFD-fed WT groups. However, Oil Red O-stained lipid droplets were more prominent in sections taken from the HFD-fed Prx5 −/− mice. Immunohistochemistry analysis of mouse liver sections revealed severe microvesicular steatosis and higher immunostaining for SREBP-1 protein among HFD fed Prx5 −/− mice, while the HFD-fed WT mice had relatively healthy liver tissue and weak expression of SREBP-1 (Fig. 7F). Taken together, our results suggested that Prx5 could protect against abnormal lipid accumulation and histological hepatic damage, which were exacerbated by obesity-induced hepatic steatosis.
4. Discussion
To investigate the role of Prx5 in NAFLD with in vitro and in vivo experiments, we established Prx5 overexpressing and knockdown HepG2 cells and generated a novel mouse model with Prx5 deletion. Following HFD feeding, Prx5 −/− mice developed obesity and hepatic steatosis. We previously used this model to explain the relationship between Prx5 and HFD-induced obesity [22]. Here, we uncover a precise role of Prx5 as a regulator that controls hepatic lipid metabolism and thereby mitigates the development of NAFLD. Prx5 deletion caused the activation of hepatocellular fatty acid synthesis (lipogenesis) genes, and this was dependent on the dephosphorylation of AMPK (Figs. 4 and 5). As a result of AMPK dephosphorylation, Prx5 −/− mice displayed aggravated hepatic steatosis upon HFD feeding, due to increased fatty acid synthesis and decreased fatty acid oxidation (Fig. 6).
Prxs are a family of thiol peroxidases that have a pivotal antioxidative function. The Prx family can effectively scavenge peroxides in mammalian cells. The Prx family has six subtypes (Prx1-6), categorized according to subcellular localization. Prx1, Prx2, and Prx6 are mainly expressed in the cytoplasm; Prx4 is found in the endoplasmic reticulum; while Prx3 and Prx5 are observed in mitochondria [30]. The association of Prx enzymes with metabolic disease has emerged from several studies [[31], [32], [33], [34], [35], [36]]. Within the Prx family, due to its subcellular localization, Prx5 has been reported to inhibit obesity through regulating cytosol and mitochondrial ROS levels. We previously addressed the function of Prx5 in obesity and proposed a positive effect of Prx5 on obesity-associated fatty liver disease [22]. Nevertheless, little is known of how Prx5 function links to NAFLD and mitochondrial ROS. In this study, the expression of Prx1-4 did not change even with FFA treatment. However, Prx5 expression was increased by FFA treatment in HepG2 cells (Fig. 2). Based on these results, we believe that Prx5 may ameliorate NAFLD. We, therefore, examined whether Prx5 affects NAFLD development using HepG2 cells and a Prx5 deficiency mouse model.
The liver is a crucial organ that regulates whole-body energy metabolism and is involved in the pathology of endocrine diseases, including obesity, diabetes, and fatty liver disease [37]. The progression of liver disease can be summarized by 4 stages: healthy liver, fatty liver, liver fibrosis, and cirrhosis. Fatty liver disease can be classified into two types, alcoholic liver disease and NAFLD. NAFLD—a representative metabolic disease—is associated with several factors, such as obesity, dyslipidemia, diabetes, parenteral nutrition, and drugs [38,39]. The pathogenesis of NAFLD involves disrupted uptake, synthesis, and oxidation of fatty acids [40,41], and the presence of an abnormal lipid accumulation in the liver (hepatic lipid accumulation of >5% liver weight) is defined as NAFLD [42].
There are a number of causes of chronic liver diseases, including ROS, which are closely associated with the development of NAFLD and aggravate its symptoms [43]. In healthy hepatocytes, there are appropriate antioxidant systems containing antioxidant enzymes, such as catalase, superoxide dismutase, glutathione peroxidase, and Prxs, which can eliminate excessive ROS to maintain the redox balance. However, redox imbalance, resulting from breakdown of antioxidant systems, is responsible for inflammation and abnormal lipid metabolism in hepatocytes. Hepatic proteins, lipids, and DNA are primarily damaged by ROS, resulting in structural changes and hepatic dysfunction [44]. Several studies have emphasized that the deletion of antioxidant enzymes leads to high level of ROS and the development of fatty liver disease. For example, the deletion of CuZn-SOD has been shown to accelerate ROS generation and consequently hepatic lipid accumulation [45]. Our study also demonstrated that cytosolic and mitochondrial ROS levels were increased in hepatic steatosis-induced HepG2 cells by FFA treatment (Fig. 2). Moreover, NAC, an intensive antioxidant, inhibited FFA-induced ROS generation (Fig. 3). We showed that the overexpression of Prx5 effectively prevented FFA-induced cytosolic and mitochondrial ROS generation. In contrast, knockdown of Prx5 (shPrx5) produced the opposite effects (Fig. 3). Consistent with the in vitro results, the liver tissue of HFD-fed Prx5 −/− mice also showed high ROS levels compared with WT mice. These results indicate that hepatocellular lipid accumulation is associated with ROS generation and that antioxidant enzymes play an essential role in maintaining hepatocellular homeostasis.
A growing number of studies have highlighted the relationship between mitochondrial ROS, mitochondrial function, and NAFLD progression [14,41]. The hepatic mitochondrion is the main organelle involved in de novo lipogenesis and lipolysis. ROS derived from mitochondria have been reported to alter hepatic lipid metabolism, with such alteration affecting the development and progression of liver disease [46]. In hepatocyte mitochondria, AMPK signaling is involved in substrate supply for ATP production, leading to the regulation of energy storage and disposal [47]. In obese patients, mitochondria-derived ROS induce a reduction of AMPK activity, which contributes to whole-body insulin resistance and results in liver lipid accumulation. These effects depend on changes of lipogenesis and fatty acid oxidation. The dephosphorylation of AMPK sequentially causes a dephosphorylation of ACC that leads to increased malonyl-CoA and inhibits CPT-1α-dependent fatty acid oxidation. SREBP-1 and FAS, key regulators of lipogenic processes, activate transcriptional factors involved in fatty acid and triglyceride synthesis, resulting in increased hepatic triglyceride deposition [48]. We observed that the phosphorylation of AMPK/ACC and the expression of CPT-1α were decreased. Also, SREBP-1 and FAS were upregulated, and excessive lipid quantities accumulated in the steatosis cell model, induced by FFA treatment (Fig. 1).
In the current study, we investigated the ability of Prx5 to prevent FFA-induced steatosis using a Prx5 overexpression or knockdown (shPrx5) HepG2 cell model. Interestingly, we found that Prx5 overexpression enhanced the phosphorylation of AMPK and ACC, upregulated CPT-1α gene expression, and downregulated the gene expression of SREBP-1 and FAS in FFA-treated HepG2 cells. Also, Prx5 reduced abnormal lipid accumulation despite FFA treatment. However, the suppression of Prx5 (shPrx5) triggered lipogenic gene expression and lipid deposition (Fig. 4). In summary, our study provides evidence to support the hypothesis that Prx5 may improve hepatic steatosis by regulating AMPK-related lipogenesis and ROS generation. Based on the in vitro results, we further verified the role of Prx5 and its association with hepatic steatosis in vivo using a Prx5 knockout mouse model.
It is well established that there is a correlation between hepatic steatosis and MAPK signaling. The MAPK signaling pathway is associated with the regulation of inflammation and fatty acid metabolism [49]. We found that deletion of Prx5 significantly induced the phosphorylation of JNK/p38/ERK, but the expression of total JNK/p38/ERK remained unchanged regardless of Prx5 expression. Prx5 −/− mice exhibited high levels of iNOS and Cox-2 expression and increased phosphorylation of IκB, indicating that the deletion of Prx5 activated the inflammatory response (Fig. 6). The activation of MAPK signaling increases hepatic inflammation and hepatic damage, leading to an increase of de novo lipogenesis and exacerbating fatty liver disease [50]. We also confirmed lipid metabolism levels in the liver tissue of HFD-Prx5 −/− mice. Moreover, Prx5 deletion increased the expression of lipogenic genes (SREBP-1, FAS) and decreased CPT-1α expression through dephosphorylation of AMPK/ACC (Fig. 5). Immunohistochemistry revealed prominent expression of SREBP-1 in liver sections isolated from Prx5 −/− mice (Fig. 7E). Consistent with these results, the liver sections from the HFD-fed Prx5 −/− mice displayed several small fat vacuoles, structural damage, and Oil Red O-stained lipid droplets, unlike HFD-fed WT mice (Fig. 7C and D).
We have uncovered a novel function of Prx5 in regulating hepatic energy metabolism and clarified the relationship between Prx5, ROS, and fatty liver disease. In conclusion, Prx5 overexpression conferred resistance to hepatic steatosis caused by FFA treatment in HepG2 cells. Furthermore, Prx5 alleviated abnormal lipid deposition. Prx5 knockout mice (Prx5 −/− mouse model) were susceptible to HFD-induced fatty liver disease. This effect might be mediated by the function of Prx5 as an AMPK activator, which further downregulated SREBP-1 expression. Moreover, our results suggest that Prx5 has potential for mitigating liver impairment via the attenuation of intracellular and mitochondrial ROS levels. Based on these results, we propose that Prx5 protects against hepatic steatosis induced by excessive energy intake. Collectively, we therefore suggest that the fundamental therapeutic effect of Prx5 on hepatic steatosis is due to its powerful antioxidant activity, maintaining intracellular redox balance. In conclusion, our present study emphasizes that Prx5, as a pivotal modulator in fatty liver disease, may be an attractive target for the prevention and treatment of NAFLD.
Conflicts of interest
The authors have declared that there are no conflicts of interest.
Funding
This research was supported by the National Research Foundation of Korea (NRF), through a grant funded by the Korean government [NRF-2015R1A4A1042271, NRF-2017R1A2B4008176, and MSIT, NRF-2017R1A5A2015391], as well as a grant from the KRIBB Research Initiative Program [KGM4621922].
Acknowledgments
Mi Hye Kim and Dong-Seok Lee performed the experiment and wrote the paper. Jung Bae Seong, Jae-Won Huh, Yong Chul Bae, Hyun-Shik Lee designed the study and experiments.
References
- 1.Tilg H., Hotamisligil G.S. Nonalcoholic fatty liver disease: cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131(3):934–945. doi: 10.1053/j.gastro.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 2.Kirpich I.A., McClain C.J. Probiotics in the treatment of the liver diseases. J. Am. Coll. Nutr. 2012;31(1):14–23. doi: 10.1080/07315724.2012.10720004. [DOI] [PubMed] [Google Scholar]
- 3.Van De Wier B., Koek G.H., Bast A., Haenen G.R. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit. Rev. Food Sci. Nutr. 2017;57(4):834–855. doi: 10.1080/10408398.2014.952399. [DOI] [PubMed] [Google Scholar]
- 4.Musso G., Gambino R., Cassader M. Non-alcoholic fatty liver disease from pathogenesis to management: an update. Obes. Rev.: Off. J. Int. Assoc. Study Obes. 2010;11(6):430–445. doi: 10.1111/j.1467-789X.2009.00657.x. [DOI] [PubMed] [Google Scholar]
- 5.Koek G.H., Liedorp P.R., Bast A. The role of oxidative stress in non-alcoholic steatohepatitis, Clinica chimica acta. Int. J. Clin. Chem. 2011;412(15–16):1297–1305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Day C.P., James O.F. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27(6):1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 7.Cheung P.C., Salt I.P., Davies S.P., Hardie D.G., Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem. J. 2000;346 Pt 3:659–669. [PMC free article] [PubMed] [Google Scholar]
- 8.Hardie D.G. The AMP-activated protein kinase pathway--new players upstream and downstream. J. Cell Sci. 2004;117(Pt 23):5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein J.L., Brown M.S. From fatty streak to fatty liver: 33 years of joint publications in the JCI. J. Clin. Investig. 2008;118(4):1220–1222. doi: 10.1172/JCI34973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang Y.L., Chen H., Wang C.L., Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: from “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018;24(27):2974–2983. doi: 10.3748/wjg.v24.i27.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonucci L., Porcu C., Iannucci G., Balsano C., Barbaro B. Non-alcoholic fatty liver disease and nutritional implications: special focus on copper. Nutrients. 2017;9(10) doi: 10.3390/nu9101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nomura K., Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J. Nutr. Biochem. 2012;23(3):203–208. doi: 10.1016/j.jnutbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Balaban R.S., Nemoto S., Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120(4):483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Simoes I.C.M., Fontes A., Pinton P., Zischka H., Wieckowski M.R. Mitochondria in non-alcoholic fatty liver disease. Int. J. Biochem. Cell Biol. 2018;95:93–99. doi: 10.1016/j.biocel.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6(1):1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Jeong H.S., Kim K.H., Lee I.S., Park J.Y., Kim Y., Kim K.S., Jang H.J. Ginkgolide A ameliorates non-alcoholic fatty liver diseases on high fat diet mice. Biomed. Pharmacother. 2017;88:625–634. doi: 10.1016/j.biopha.2017.01.114. [DOI] [PubMed] [Google Scholar]
- 17.Geng C., Xu H., Zhang Y., Gao Y., Li M., Liu X., Gao M., Wang X., Liu X., Fang F., Chang Y. Retinoic acid ameliorates high-fat diet-induced liver steatosis through sirt1, Science China. Life Sci. 2017;60(11):1234–1241. doi: 10.1007/s11427-016-9027-6. [DOI] [PubMed] [Google Scholar]
- 18.Wood Z.A., Schroder E., Robin Harris J., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003;28(1):32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- 19.Poynton R.A., Hampton M.B. Peroxiredoxins as biomarkers of oxidative stress. Biochim. Biophys. Acta. 2014;1840(2):906–912. doi: 10.1016/j.bbagen.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Huh J.Y., Kim Y., Jeong J., Park J., Kim I., Huh K.H., Kim Y.S., Woo H.A., Rhee S.G., Lee K.J., Ha H. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Antioxidants Redox Signal. 2012;16(3):229–243. doi: 10.1089/ars.2010.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park J., Choi H., Kim B., Chae U., Lee D.G., Lee S.R., Lee S., Lee H.S., Lee D.S. Peroxiredoxin 5 (Prx5) decreases LPS-induced microglial activation through regulation of Ca(2+)/calcineurin-Drp1-dependent mitochondrial fission. Free Radical Biol. Med. 2016;99:392–404. doi: 10.1016/j.freeradbiomed.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Kim M.H., Park S.J., Kim J.H., Seong J.B., Kim K.M., Woo H.A., Lee D.S. Peroxiredoxin 5 regulates adipogenesis-attenuating oxidative stress in obese mouse models induced by a high-fat diet. Free Radical Biol. Med. 2018;123:27–38. doi: 10.1016/j.freeradbiomed.2018.05.061. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Lechon M.J., Donato M.T., Martinez-Romero A., Jimenez N., Castell J.V., O'Connor J.E. A human hepatocellular in vitro model to investigate steatosis. Chem. Biol. Interact. 2007;165(2):106–116. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Southern J.A., Young D.F., Heaney F., Baumgartner W.K., Randall R.E. Identification of an epitope on the P and V proteins of simian virus 5 that distinguishes between two isolates with different biological characteristics. J. Gen. Virol. 1991;72(Pt 7):1551–1557. doi: 10.1099/0022-1317-72-7-1551. [DOI] [PubMed] [Google Scholar]
- 25.Kim T.S., Choi H.S., Ryu B.Y., Gang G.T., Kim S.U., Koo D.B., Kim J.M., Han J.H., Park C.K., Her S., Lee D.S. Real-time in vivo bioluminescence imaging of lentiviral vector-mediated gene transfer in mouse testis. Theriogenology. 2010;73(1):129–138. doi: 10.1016/j.theriogenology.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 26.Kodama Y., Brenner D.A. c-Jun N-terminal kinase signaling in the pathogenesis of nonalcoholic fatty liver disease: multiple roles in multiple steps. Hepatology. 2009;49(1):6–8. doi: 10.1002/hep.22710. [DOI] [PubMed] [Google Scholar]
- 27.Zeng L., Tang W.J., Yin J.J., Zhou B.J. Signal transductions and nonalcoholic fatty liver: a mini-review. Int. J. Clin. Exp. Med. 2014;7(7):1624–1631. [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatt D., Ghosh S. Regulation of the NF-kappaB-Mediated transcription of inflammatory genes. Front. Immunol. 2014;5:71. doi: 10.3389/fimmu.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang Y., Ryu S., Sung E., Jang Y. Higher concentrations of alanine aminotransferase within the reference interval predict nonalcoholic fatty liver disease. Clin. Chem. 2007;53(4):686–692. doi: 10.1373/clinchem.2006.081257. [DOI] [PubMed] [Google Scholar]
- 30.Hanschmann E.M., Godoy J.R., Berndt C., Hudemann C., Lillig C.H. Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxidants Redox Signal. 2013;19(13):1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito R., Takahashi M., Ihara H., Tsukamoto H., Fujii J., Ikeda Y. Measurement of peroxiredoxin-4 serum levels in rat tissue and its use as a potential marker for hepatic disease. Mol. Med. Rep. 2012;6(2):379–384. doi: 10.3892/mmr.2012.935. [DOI] [PubMed] [Google Scholar]
- 32.Nabeshima A., Yamada S., Guo X., Tanimoto A., Wang K.Y., Shimajiri S., Kimura S., Tasaki T., Noguchi H., Kitada S., Watanabe T., Fujii J., Kohno K., Sasaguri Y. Peroxiredoxin 4 protects against nonalcoholic steatohepatitis and type 2 diabetes in a nongenetic mouse model. Antioxidants Redox Signal. 2013;19(17):1983–1998. doi: 10.1089/ars.2012.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nawata A., Noguchi H., Mazaki Y., Kurahashi T., Izumi H., Wang K.Y., Guo X., Uramoto H., Kohno K., Taniguchi H., Tanaka Y., Fujii J., Sasaguri Y., Tanimoto A., Nakayama T., Yamada S. Overexpression of peroxiredoxin 4 affects intestinal function in a dietary mouse model of nonalcoholic fatty liver disease. PLoS One. 2016;11(4) doi: 10.1371/journal.pone.0152549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacifici F., Arriga R., Sorice G.P., Capuani B., Scioli M.G., Pastore D., Donadel G., Bellia A., Caratelli S., Coppola A., Ferrelli F., Federici M., Sconocchia G., Tesauro M., Sbraccia P., Della-Morte D., Giaccari A., Orlandi A., Lauro D. Peroxiredoxin 6, a novel player in the pathogenesis of diabetes. Diabetes. 2014;63(10):3210–3220. doi: 10.2337/db14-0144. [DOI] [PubMed] [Google Scholar]
- 35.Tang Z., Xia N., Yuan X., Zhu X., Xu G., Cui S., Zhang T., Zhang W., Zhao Y., Wang S., Shi B. PRDX1 is involved in palmitate induced insulin resistance via regulating the activity of p38MAPK in HepG2 cells. Biochem. Biophys. Res. Commun. 2015;465(4):670–677. doi: 10.1016/j.bbrc.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Yamada S., Guo X. Peroxiredoxin 4 (PRDX4): its critical in vivo roles in animal models of metabolic syndrome ranging from atherosclerosis to nonalcoholic fatty liver disease. Pathol. Int. 2018;68(2):91–101. doi: 10.1111/pin.12634. [DOI] [PubMed] [Google Scholar]
- 37.Rui L. Energy metabolism in the liver. Compr. Physiol. 2014;4(1):177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases. Am. Coll. Gastroenterol. Am. Gastroenterol. Assoc. Hepatol. 2012;55(6):2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 39.Lazo M., Hernaez R., Eberhardt M.S., Bonekamp S., Kamel I., Guallar E., Koteish A., Brancati F.L., Clark J.M. Prevalence of nonalcoholic fatty liver disease in the United States: the third national health and nutrition examination survey, 1988-1994. Am. J. Epidemiol. 2013;178(1):38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podrini C., Borghesan M., Greco A., Pazienza V., Mazzoccoli G., Vinciguerra M. Redox homeostasis and epigenetics in non-alcoholic fatty liver disease (NAFLD) Curr. Pharmaceut. Des. 2013;19(15):2737–2746. doi: 10.2174/1381612811319150009. [DOI] [PubMed] [Google Scholar]
- 41.Ucar F., Sezer S., Erdogan S., Akyol S., Armutcu F., Akyol O. The relationship between oxidative stress and nonalcoholic fatty liver disease: its effects on the development of nonalcoholic steatohepatitis. Redox Rep.: Commun. Free Radic. Res. 2013;18(4):127–133. doi: 10.1179/1351000213Y.0000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Angulo P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 2002;346(16):1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 43.Leung T.M., Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013;58(2):395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Ha H.L., Yu D.Y. HBx-induced reactive oxygen species activates hepatocellular carcinogenesis via dysregulation of PTEN/Akt pathway. World J. Gastroenterol. 2010;16(39):4932–4937. doi: 10.3748/wjg.v16.i39.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchiyama S., Shimizu T., Shirasawa T. CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice. J. Biol. Chem. 2006;281(42):31713–31719. doi: 10.1074/jbc.M603422200. [DOI] [PubMed] [Google Scholar]
- 46.Mansouri A., Gattolliat C.H., Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155(3):629–647. doi: 10.1053/j.gastro.2018.06.083. [DOI] [PubMed] [Google Scholar]
- 47.Li Y., Xu S., Mihaylova M.M., Zheng B., Hou X., Jiang B., Park O., Luo Z., Lefai E., Shyy J.Y., Gao B., Wierzbicki M., Verbeuren T.J., Shaw R.J., Cohen R.A., Zang M. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metabol. 2011;13(4):376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith B.K., Marcinko K., Desjardins E.M., Lally J.S., Ford R.J., Steinberg G.R. Treatment of nonalcoholic fatty liver disease: role of AMPK, American journal of physiology. Endocrinol. Metabol. 2016;311(4):E730–E740. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- 49.Lawan A., Zhang L., Gatzke F., Min K., Jurczak M.J., Al-Mutairi M., Richter P., Camporez J.P., Couvillon A., Pesta D., Roth Flach R.J., Shulman G.I., Bennett A.M. Hepatic mitogen-activated protein kinase phosphatase 1 selectively regulates glucose metabolism and energy homeostasis. Mol. Cell. Biol. 2015;35(1):26–40. doi: 10.1128/MCB.00503-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawan A., Bennett A.M. Mitogen-activated protein kinase regulation in hepatic metabolism. Trends Endocrinol. Metab.: TEM (Trends Endocrinol. Metab.) 2017;28(12):868–878. doi: 10.1016/j.tem.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]