Abstract
Immune checkpoint inhibitor (ICI) therapy has greatly improved treatment of various advanced cancers but increasing use of ICI therapy has exposed the risk of ICI-related cardiovascular side effects.
Immune checkpoints are inhibitory regulators of T cell activation and mediate T cell effector functions during physiological responses to shield from autoimmune reactions. ICI therapy for advanced cancers promotes immune activity against tumors and is applied within a broad collective of cancer patients. Widespread use of ICI therapy has revealed the burden of immune related adverse events with various organ manifestations and characteristics. Since immune checkpoints are highly relevant for maintaining myocardial homeostasis as emerging evidence implicates, inhibition of immune checkpoint pathways has been associated with various forms of cardiotoxicity in preclinical models and patients. Although ICI-related cardiotoxicity is rare, it has significant relevance due to high mortality rates.
This review focuses on current knowledge about cardiac ICI-related toxicity. We summarize the most common forms and delineate incidence, presentation, and treatment. Clinical characteristics are correlated to potential underlying pathomechanisms. We outline epidemiology, risk factors, and course of disease. Recommendations for monitoring and critical diagnostic measures are specified within the context of different forms of cardiac involvement. Different therapeutic implications for suspected ICI-related cardiotoxicity and their limitations are critically summarized.
We highlight current gaps of knowledge concerning the underlying pathomechanisms and clinical characteristics of ICI-related cardiotoxicity. Future challenges are depicted for optimum cardio-oncology care of patients receiving ICI therapy.
Keywords: Cardio-oncology, Cardiotoxicity, CTLA4, Immune checkpoint inhibitor, Myocarditis, PD1
Abbreviation: ICI, immune checkpoint inhibitor
1. Immune checkpoints in adaptive immunity
T cell activation in response to antigen presentation is a critical step within the initiation of an adaptive immune response. T cell activation is initiated by antigen recognition of the T cell receptor (TCR). Multiple costimulatory signals are necessary to facilitate this process. T cell activation is counterbalanced by immune checkpoints that prevent exaggerated immune response and account for self-tolerance [1]. Cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and Programmed death 1 (PD1) are the main lymphocyte immune checkpoints [1].
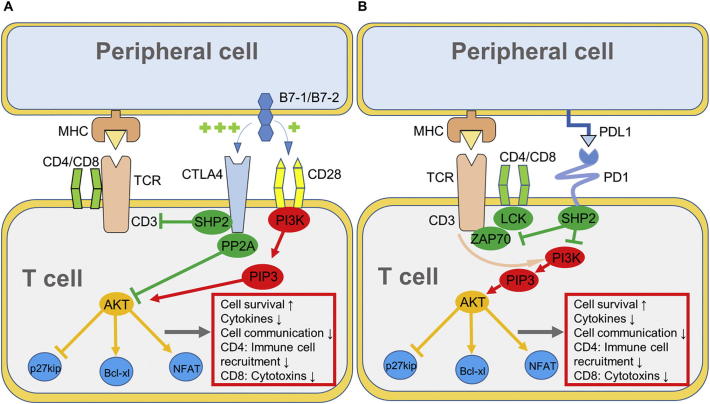
CTLA4 is located intracellular in resting T cells, and translocates to the surface upon activation. CTLA4 then antagonizes the costimulatory receptor cluster of differentiation 28 (CD28) by ligation of CD28 ligands with high affinity to inhibit T cell activation. CTLA4 furthermore inhibits TCR activity to reduce T cell susceptibility to antigen presentation (Fig. 1a) [2,3].
Fig. 1.
Mechanism of A, CTLA4 and B, PD1 in T cell activation upon antigen recognition [1,3]. AKT, protein kinase B; Bcl-xl, B cell lymphoma xl; LCK, lymphocyte-specific protein tyrosine kinase; NFAT, nuclear factor of activated T cells; PI3K, phosphoinositide 3-kinase; PIP3, phosphatidylinositol (3,4,5)-trisphosphate SHP2, Src homology region 2 domain-containing phosphatase-2; ZAP70, zeta-chain-associated protein kinase 70.
PD1 becomes expressed during early antigen-mediated activation of T cells. However, prolonged antigen expression in chronic infections or cancer causes sustained PD1 expression on T cells [1,3]. PD1 is activated upon ligation with its ligands, PDL1 or PDL2 that are expressed on the surface of antigen-presenting cells. In contrast to CTLA4, PD1 inhibits T cell activity solely by the induction of downstream mechanisms. After binding of PDL1 or PDL2, PD1 is activated and initiates further downstream signaling (Fig. 1b) thereby antagonizing TCR and CD28 signaling [1,3].
2. Immune checkpoint inhibitors for cancer therapy
Cancer cells are characterized by an evasion of immune response resulting from a lack of recognizable neo-antigens and specific transcriptomic programs to avoid T cell recognition [4,5]. Immune checkpoint inhibitor (ICI) therapy aims to trigger enhanced immune activity to facilitate anti-tumor immune response [5].
The first successful application of CTLA4 ICI therapy in a tumor mouse model was achieved in 1996 by the group of James P. Allison, who shared the 2018 Nobel Prize [6]. The first human CTLA4 antibody (ipilimumab) was initially used in clinical trials in patients with advanced cancers in 2000 [7] and FDA approval was granted in 2011 [5]. Nivolumab was the first PD1 antibody that was used in patients in 2006 and also the first to reach FDA approval for melanoma in 2014 [8]. Superior efficacy of ipilimumab and nivolumab when used as combination therapy for melanoma was demonstrated in studies [9]. Since 2011, six ICIs gained FDA approval for an increasing number of advanced malignant diseases (Table 1). While ICI therapy was initially limited to palliative therapy of advanced cancers, it has recently been approved for therapy after complete resection in an adjuvant, potentially curative setting [8].
Table 1.
Available immune checkpoint inhibitors and indications.
| Drug (brand name) First approval |
Type of cancer |
|---|---|
| Ipilimumab (Yervoy) Mar 2011 |
Unresectable or metastatic melanoma in adult and juvenile (≥12 years) patients; melanoma stage III after complete resection as adjuvant therapy. |
| Nivolumab (Opdivo) Dec 2014 |
Unresectable or metastatic melanoma as monotherapy or in combination with ipilimumab; Melanoma stage III-IV after complete resection as adjuvant therapy; Metastatic NSCLC refractory to platinum-based CTX; Renal cell carcinoma as monotherapy or in combination with ipilimumab; Relapsed classical Hodgkin lymphoma after HSCT and ≥ 3 lines of therapy; Recurrent or metastatic HNSCC with progression after CTX; Microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer with progression after CTX. |
| Pembrolizumab (Keytruda) Sep 2014 |
Unresectable or metastatic melanoma; Metastatic NSCLC with high PDL1 expression or progression after CTX; Recurrent or metastatic HNSCC with progression after CTX; Relapsed classical Hodgkin lymphoma; Advanced or metastatic urothelial carcinoma; Microsatellite instability-high or mismatch repair deficient tumors and no satisfactory alternative; Recurrent local or metastatic gastric cancer with expression of PDL1; Recurrent or metastatic cervical cancer with PDL1 expression and progression after CTX |
| Atezolizumab (Tecentriq) May 2016 |
Advanced or metastatic urothelial carcinoma not not suitable for CTX and/or PDL1 expression; Metastatic NSCLC refractory to platinum-based CTX. |
| Avelumab (Bavencio) Mar 2017 |
Metastatic Merkel cell carcinoma; Advanced or metastatic urothelial carcinoma with progression after CTX. |
| Durvalumab (Imfinzi) May 2017 |
Advanced or metastatic urothelial carcinoma with progression after CTX; NSCLC stage III with stable disease or remission following CTX and radiotherapy. |
The introduction of immune checkpoint inhibitor therapy has greatly improved treatment for many advanced cancers and the number of patients receiving ICI therapy has rapidly increased. However, the augmented use of ICI therapy has led to an increased perception of relevant side effects, the so-called immune-related Adverse Events (irAE).
3. Immune-related adverse events (irAE)
ICI therapy conducts the closely balanced regulation of T cell activation and inhibition towards enhanced T cell activity throughout the whole organism and therefore facilitates autoimmune reactions. In this setting, ICI may exacerbate pre-existing processes, trigger autoimmunity in patients with genetic or acquired predisposing factors, or induce novel autoimmune disease in affected organs [10]. It is hypothesized that genetic variances and microbiome predispose to development of irAEs [9].
irAEs typically occur within the early phase of therapy (≤12 weeks of therapy) and can rarely be observed after 1 year of therapy [11]. Adverse events can show variable presentation, ranging from asymptomatic laboratory findings to fulminant, life-threatening disease [11,12]. irAEs are classified as low-grade (grades 1–2), high-grade (grades 3–4) and lethal (grade 5) according to Common Terminology Criteria for Adverse Events [13]. The incidence of irAEs varies between CTLA4 inhibitors and PD1 inhibitors. Exemplarily, gastrointestinal and skin irAEs are more common with CTLA4 inhibitors, while pulmonary irAEs are rarely seen with CTLA4 inhibitors compared to PD1 inhibitors [14]. High-grade adverse events were tripled in combination therapy compared to anti-PD1 monotherapy [15]. Minor irAEs occur in up to 90% of patients receiving anti-CTLA4 ICI therapy and 70% of patients receiving anti-PD1 or anti-PDL1 therapy. Major irAEs are seen in 10–15% of patients, and lethal irAEs were ranged from 0%–3.2% [11,12,16].
Skin reaction and colitis are the most common irAEs followed by pneumonitis and hepatitis. Skin reactions can be found in 43–45% of patients treated with CTLA4 ICI therapy and 34% in patients treated with PD1 ICI therapy. Interestingly, the occurrence of vitiligo as irAE drepdicts response to PD1 ICI therapy for melanoma [14]. The incidence of high-grade events is below 5%. In contrast to skin irAEs, gastrointestinal irAEs are more severe and represent the most common irAE leading to treatment discontinuation and to treatment-related lethality [14,16]. Enterocolitis can be found in 27–54% of patients treated with anti-CTLA4 ICI therapy. Colon perforation was observed in up to 6.6% of patients, and 1.1% of treated patients died of complications from CTLA4 ICI-related enterocolitis [14]. Cardiovascular immune-related complications are relatively rare, but hold the highest lethality rates [11].
4. ICI-related cardiovascular toxicities in patients
4.1. Myocarditis
4.1.1. Epidemiology and pathomechanism
Since 2016, widespread application of ICI therapy has led to increased reporting of ICI-related myocarditis in several case reports and case series [[17], [18], [19], [20]]. The incidence for clinically manifest ICI-related myocarditis was determined as 0.09% (0.27% for combination ICI therapy) in 2016 according to Bristol-Myers Squibb corporate safety databases [18]. An increasing incidence of 1.14% was reported in a recent multicenter registry [20]. With a fatality rate of 27%–46% [19,21], ICI-related myocarditis is the most lethal form of irAE [11]. ICI-related myocarditis typically develops within the early phase (17–34 days after initiation of ICI therapy) and can show a fulminant course of disease with severely depressed LV function, hemodynamic instability and need for intensive care [20,22].
Despite increasing reports of ICI-related myocarditis and promising experimental models for the role of immune checkpoints in cardiovascular disease, little is known about the underlying pathomechanisms. With regard to detection of troponin I autoantibodies in Pd1−/− mice [23], it was tempting to speculate that patients suffering from ICI-related myocarditis develop specific autoantibodies against cardiac structures but so far, no specific autoantibodies were identified. Emerging data proposes that the tumor itself may play an important role in mediating an immune response against cardiac structures: in a case report using next generation sequencing of TCRs from myocardial T cells and tumor T cells of a patient suffering from ICI-related myocarditis, specific clones present in myocardium and tumor were identified. This finding proposes that shared epitopes between tumor and myocardium may contribute to development of manifest myocarditis, but this has only been demonstrated in a single case so far [18]. The further examination of ICI therapy in preclinical models to precisely characterize ICI-related cardiotoxic effects and their underlying mechanisms is now urgently needed as a basis for the development of targeted, protective measures.
4.1.2. Diagnosis and treatment
Little is known about predisposing factors that promote ICI-related myocarditis in patients undergoing ICI therapy. Myocarditis is more likely in patients receiving combination ICI therapy (ipilimumab and nivolumab) than monotherapy and fatality rates were higher for combination therapy [19,20]. Diabetes mellitus may predispose to ICI-related myocarditis, but no such association has yet been found for other cardiovascular risk factors or presence of coronary artery disease [20]. ICI-related myocarditis is commonly associated with concomitant irAEs like myositis, myasthenia gravis, and hepatitis [19,20]. Lately, several cases of latent, “smoldering” myocarditis with none or minimal symptoms have been reported indicating high variations in clinical presentation of ICI-related myocarditis [24,25]. It may be speculated that the frequency of ICI-related myocarditis is underestimated as many cases may have been missed due to non-specific symptoms, low clinical awareness, and absence of standardized definitions [26,27].
Shortness of breath is the most common primary symptom in ICI-related myocarditis [19,20]. Approximately 50% of patients show an LV ejection fraction (LVEF) <50%. Cardiac troponin appears to be a valid marker with a sensitivity of 94–100% for manifest myocarditis [20,28]. Further signs and symptoms may include angina pectoris, peripheral edema, ECG abnormalities (conductance delay, ventricular arrhythmia), and elevated (N-terminal pro) brain natriuretic peptide (BNP/NT-proBNP) [20,26]. Severe conduction system disease (e.g. complete heart block) and ventricular tachycardia is commonly seen in patients with ICI-related myocarditis [10,26]. The American Society of Clinical Oncology (ASCO) recommends a baseline cardiac workup and extended cardiology workup including echocardiography, chest x-ray, and cardiac biomarkers upon signs or symptoms. A standardized follow-up cardio-oncology visit within the early phase of therapy (week 4) may be beneficial, especially in patients receiving combination ICI therapy [29].
Cardiac magnetic resonance imaging (CMR) serves as the preferable imaging tool for ICI-related myocarditis but due to moderate sensitivity according to recent reports, CMR should be evaluated together with further diagnostic measures [30]. According to a recent report on a cohort of 77 patients with confirmed ICI-related myocarditis, late gadolinium enhancement in CMR was found in 52% of cases and should be evaluated together with cardiac edema, necrosis, and fibrosis [31,32]. Sensitivity of CMR was increased in patients with reduced LVEF but decreased when LV function was preserved (66% versus 43%) [31]. CMR has additionally been suggested for follow-up monitoring, but systematic evidence is not yet available [33].
Endomyocardial biopsy should be evaluated for suspected ICI-related myocarditis [30]. Particularly, evidence for myocarditis in tissue pathology has been proposed as central diagnostic criterion with high specificity for definitive myocarditis together with CMR and echocardiography in a recent proposal of case definition [30]. The availability of sufficient expertise for biopsy procedure and pathological interpretation is critical for optimum diagnostic quality [30].
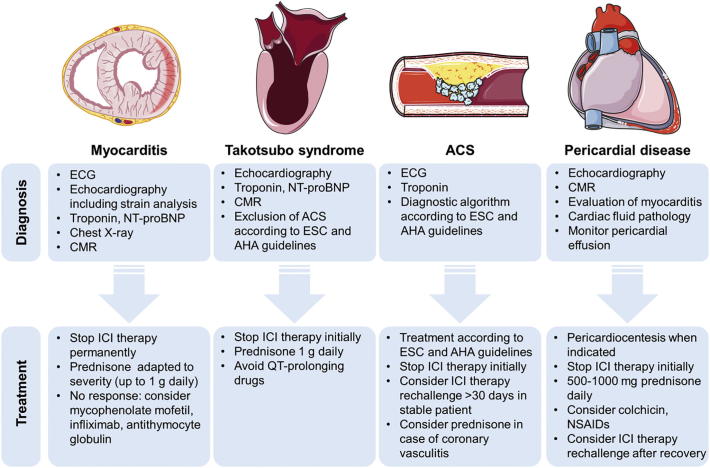
Recommendations on the treatment of ICI-related myocarditis are available from clinical practice guidelines by the ASCO and have been proposed in recent reviews (Fig. 2) [26,29,34]. However, no prospective data is yet available and all recommendations are based on small case series and anecdotal evidence. At any sign for cardiotoxicity, it is recommended to stop ICI therapy initially. Cardiotoxic effects can however be expected even after termination of ICI therapy since autoreactive T cells that recognize cardiac antigens can sustain beyond the period of ICI therapy [9,26]. Interdisciplinary management of suspected ICI-related cardiotoxicity between oncologists and a specialized cardio-oncologist is required and intensive care management of major cardiovascular complications like severe heart failure or arrhythmia may be necessary and should be conducted in accordance to current European Society of Cardiology (ESC) and American Heart Association (AHA) guidelines [35,36].
Fig. 2.
Common presentations of ICI-related cardiotoxicity. Diagnosis and treatment of ICI therapy-related myocarditis, takotsubo syndrome, acute coronary syndrome, and pericardial disease are summarized. ACS, acute coronary syndrome, AHA, American Heart Association; CMR, cardiac magnet resonance tomography; ECG, electrocardiography; ESC, European Society of Cardiology; ICI, immune checkpoint inhibitor; NSAID, nonsteroidal anti-inflammatory drug; NT-proBNP, N-terminal pro brain natriuretic peptide.
Immunosuppressive therapy with corticosteroids is recommended as initial therapy. Corticosteroid dose should be individually determined according to severity of disease. In patients suffering from myocarditis with hemodynamic impairment, 1 g per day intravenous prednisone is reasonable as first line therapy [33]. In patients without immediate response to corticosteroid treatment, addition of mycophenolate mofetil or tacrolimus should be considered [26,33,34,37]. In a recent case report, antithymocyte globulin was effective for the treatment of corticoid-refractory ICI-related myocarditis [38]. As promising new targeted approaches, the CTLA4 agonist abatacept and the anti-CD52 antibody alemtuzumab were successfully applied in cases of steroid-refractory myocarditis [39,40]. Particularly abatacept, a soluble IgG-CTLA4, may serve to improve treatment of steroid-refractory, ICI-related myocarditis by specifically targeting immune checkpoint pathways with less off-target effects [39].
No systematic data is yet available on the use of conventional cardiac medication in ICI-related myocarditis. β blockers and angiotensin converting enzyme (ACE) inhibitors are indicated in patients with reduced LVEF according to heart failure guidelines, but a cardioprotective role to prevent or mitigate ICI-related myocarditis has not yet been shown [26,35,36]. A recent review recommends the use of ACE inhibitors in patients with confirmed ICI-related myocarditis and left ventricular ejection fraction <50% in the absence of contraindications [26].
Whether to resume ICI therapy after ICI-related cardiovascular events remains an important question since it may represent the best possible treatment for many advanced cancers. Rechallenge of ICI therapy after resolved irAEs has been extensively discussed [26,29,41,42]. For manifest ICI-related myocarditis, the ASCO recommends permanent discontinuation for all grades (Table 2). In addition, ICI rechallenge is not recommended in patients with advanced conduction disease or critical ventricular arrhythmia [26]. In the context of low-grade, or asymptomatic forms, e.g. an isolated elevation of cardiac troponin without clinically manifest myocarditis or other forms of myocardial injury, it remains questionable if ICI rechallenge is reasonable for selected cases considering the potential effectiveness of ICI within advanced disease. Here, it is particularly important that nivolumab and ipilimumab have recently been approved for the treatment of melanoma patients in an adjuvant setting after complete resection augmenting a two-year disease-free survival of 50% to even higher rates [[43], [44], [45]]. Management of cardiac adverse effects of ICI therapy is therefore particularly important in these patients that are likely to be long-term cancer survivors. Currently, patients receiving adjuvant therapy are not exposed to the risk of ICI rechallenge after experiencing cardiac toxicities in clinical routine, but studies are still needed to support this practice.
Table 2.
ASCO classification for ICI-related myocarditis.
| Grade | Presentation |
|---|---|
| 1 | Elevated cardiac troponin, BNP/NT-proBNP, or abnormal ECG |
| 2 | Elevated cardiac troponin, BNP/NT-proBNP, abnormal ECG, abnormal echocardiography, abnormal chest X-ray + mild symptoms |
| 3 | Moderately abnormal testing or symptoms with mild activity |
| 4 |
Decompensation, intravenous medication, intervention required, life-threatening |
Adapted and modified from Brahmer et al. J Clin Oncol 2018 [34]. ASCO, American Society of Clinical Oncology; BNP, brain natriuretic peptide; NT-proBNP, N-terminal pro brain natriuretic peptide; ECG, electrocardiography.
4.2. Takotsubo syndrome
Several reports have identified takotsubo syndrome-like cardiac adverse events during ICI therapy [21,46]. The incidence of takotsubo syndrome is elevated in patients with cancer, and treatment with different forms of cytotoxic chemotherapy and targeted cancer therapy has been associated with the development of takotsubo syndrome [46]. In a retrospective study, 14% of patients with unselected ICI-related cardiotoxicity showed a takotsubo syndrome-like appearance [21]. In contrast to ICI-related myocarditis, the underlying pathomechanism of ICI-related takotsubo syndrome is expected to be non-inflammatory. It is unknown whether takotsubo syndrome is a direct ICI-related effect or indirectly induced by adrenergic stress during early ICI therapy [26]. Evaluation of takotsubo syndrome should include clinical examination, ECG, echocardiography, and cardiac biomarkers. Acute coronary syndrome should be excluded as underlying as underlying cause. CMR may help to evaluate presence of myocarditis [46].
Interruption of ICI therapy is recommended in case of ICI-related takotsubo-syndrome [26]. No generalized recommendations on immunosuppression are available, but high-dose corticosteroid therapy (1 g methylprednisone intravenously) was proven to be effective in two reported cases [46]. QT-prolonging drugs should be avoided. Few data is available on the feasibility of ICI rechallenge following takotsubo syndrome. Therefore, ICI rechallenge should be evaluated individually after recovery of LV function under intensified cardiac monitoring [26,47].
4.3. Acute coronary syndrome (ACS)
4.3.1. Epidemiology and pathomechanism
Emerging data indicates a potential association of ICI therapy to other forms of cardiovascular disease. Particularly, a recent review highlights an increased incidence of acute myocardial infarction in ICI trials [26]. The authors hypothesize that ICI therapy may enhance inflammation of atherosclerotic plaques, destabilize pre-existing plaques, and promote plaque rupture. However, diverging evidence on the role of immune checkpoint signaling and its inhibition in atherosclerosis is available [26].
CTLA4 and PD1 immune checkpoint signaling during formation of atherosclerosis has been evaluated in different preclinical and clinical settings. In an atherosclerosis mouse model using apolipoprotein E knockout (Apoe−/−) mice, overexpression of CTLA4 ameliorated formation of atherosclerotic lesions by downregulation of CD4+ T cell activity and inhibition of macrophage migration into atherosclerotic plaques [48]. Similar results were obtained from another group after treatment of atherosclerotic mice with soluble CTLA4-IgG (abatacept) [49]. Here, the CTLA4 agonist abatacept reduced formation of atherosclerosis in a model of homocysteine-accelerated atherosclerosis in Apoe−/− mice [49].
The role of PD1 in atherosclerosis formation is unclear. Since PD1-deficient low-density lipoprotein receptor knockout (Ldlr−/−) mice showed enhanced formation of atherosclerotic lesions with increased numbers of infiltrating macrophages and T cells, it was expected that PD1 ICI therapy accelerated development of atherosclerosis [50]. Additionally, it has been hypothesized that ICI therapy could induce coronary vasculitis that leads to acute myocardial infarction in the absence of atherosclerosis, but this mechanism has not yet been demonstrated [26]. However, nivolumab treatment was associated with improvement of atherosclerotic plaques in a retrospective study [51]. The authors hypothesize that strong PDL1 expression on dendritic cells within complicated plaques may govern so far unknown, beneficial mechanisms [51]. Taken together, despite preclinical evidence indicating deleterious effects of immune checkpoint deficiency on atherosclerosis, the role of immune checkpoints for atherosclerosis development in humans is not yet understood.
4.3.2. Diagnosis and treatment
In patients with suspected ICI-related ACS, diagnostic measures should be taken according to ESC and AHA myocardial infarction guidelines [[52], [53], [54], [55]]. Particularly, elevated troponin should prompt further ACS diagnostics including coronary angiography. Potential differential diagnoses include ICI-triggered coronary vasculitis and focal myocarditis. However, evaluation of ICI-related myocarditis as a potential cause for chest pain and troponin elevation should follow after rule-out of ACS [29].
General treatment should be conducted according to appropriate ESC and AHA guidelines [[52], [53], [54], [55]]. Coronary angiography and percutaneous coronary intervention upon identification of a culprit lesion with subsequent dual antiplatelet therapy and supportive therapy (e.g. statins) are recommended. ICI therapy in patients with suspected ICI-related myocardial infarction should be interrupted [26]. Upon successful therapy, an ICI therapy rechallenge after >30 days in clinically stable patients under intensified cardiac monitoring may be considered [26,29]. So far, there is no evidence for the use of immunosuppressive therapy in this collective.
In patients with myocardial infarction under ICI therapy and without evidence for manifest coronary atherosclerosis during coronary angiography, ICI-triggered coronary vasculitis should be considered as differential diagnosis. Although there is no evidence on immunosuppressive therapy for coronary vasculitis, intravenous prednisone can be considered as an additional measure [26].
4.4. Pericardial disease
Several cases of pericarditis have been reported in association with ICI therapy. Among cardiac adverse drug reactions, pericardial disease was the second most commonly reported event with 13.6% of all cases in an analysis of adverse drug reactions using the VigiBase database (World Health Organization) [56]. In a second retrospective study of patients with ICI-related cardiotoxicity of any kind, pericardial effusion was found in 7% [21]. Pericarditis can occur in isolation or together with ICI-related myocarditis (perimyocarditis). Retrospective data indicates a mortality rate of 21% [57]. Post-mortem and post-operative tissue pathology from three patients with ICI-related pericarditis revealed moderate to severe lymphocytic infiltration and fibrinous exudate [58].
Symptoms of pericardial disease are often unspecific and diagnosis is challenging. Shortness of breath is the most common symptom of ICI-related pericardial disease [56]. Further symptoms may include pericardial pain in the absence of pericardial effusion or upper venous congestion and cardiogenic shock in case of pericardial effusion [59]. In case of clinical signs or symptoms, pericardial disease is evaluated by physical examination, ECG, X-ray, and cardiac biomarkers [26,56]. Concomitant presence of ICI-related myocarditis should be evaluated in the event of pericardial disease. CMR may serve as an additional imaging modality in selected cases and for the assessment of myocardial involvement. Cancer-associated pericardial effusion represents an important differential diagnosis. Here, examination of pericardial fluid may serve to differentiate the underlying pathology [59].
Interruption of ICI therapy is recommended for all forms of pericarditis. Immunosuppressive therapy with 500–1000 mg prednisone daily followed by oral prednisone with slow weaning can be given as initial therapy. Colchicine and nonsteroidal anti-inflammatory drugs may be beneficial as additional treatment [26]. In case of steroid-refractory pericarditis, mycophenolate mofetil, infliximab, or anti-thymocyte globulin may serve as second line therapy but no systematic evidence is yet available [26,56]. Management of complications including pericardiocentesis in the event of cardiac tamponade is recommended according to guidelines [59]. In a recent case series, a pericardial window procedure was described for cardiac tamponade during ICI-related pericarditis. ICI rechallenge can be considered after pericarditis is completely resolved under intensified cardiac monitoring [26,56].
5. Conclusion and future perspective
Considering the increasing use of ICI therapy within palliative and curative therapeutic settings, there is an imminent medical need for understanding ICI-related cardiovascular side effects, their underlying pathomechanisms and best possible monitoring and treatment of patients suffering from ICI-related cardiotoxicity. For this, the availability of a specialized cardio-oncology unit is highly important to ensure optimum diagnosis and management of ICI-related cardiac adverse events. Additionally, prospective studies aiming to record and characterize cardiac function during ICI therapy are necessary. Systematic registration of patients suffering from ICI-related cardiotoxicity may help to develop standardized treatment recommendations for this collective.
At last, translational research including preclinical models to reproduce ICI-related cardiotoxicity in context of presence of a malignant tumor are necessary and may help to understand the underlying mechanisms and to identify new cardioprotective measures that will help to improve treatment of ICI-related cardiotoxicity.
Declaration of competing interest
None.
Acknowledgments
Acknowledgements
The figures were created using SMART Servier Medical Art templates (https://smart.servier.com/).
Funding sources
Lars Michel acknowledges the following funding source: IFORES research grant from the Medical Faculty, University Duisburg-Essen, Germany. This work was supported by the IFORES research grant from the Medical Faculty, University Duisburg-Essen, Hufelandstraße 55, 45147 Essen, Germany (LM).
References
- 1.Sharpe A.H., Pauken K.E. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder E.I., Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seidel J.A., Otsuka A., Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Ribas A., Wolchok J.D. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 7.Hodi F.S., Mihm M.C., Soiffer R.J. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4712. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schadendorf D., van Akkooi A.C.J., Berking C. Melanoma. Lancet. 2018;392:971–984. doi: 10.1016/S0140-6736(18)31559-9. [DOI] [PubMed] [Google Scholar]
- 9.Postow M.A., Chesney J., Pavlick A.C. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson D.B., Chandra S., Sosman J.A. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320:1702–1703. doi: 10.1001/jama.2018.13995. [DOI] [PubMed] [Google Scholar]
- 11.Wang D.Y., Salem J.E., Cohen J.V. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michot J.M., Bigenwald C., Champiat S. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur. J. Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Department of Health and Human Servicesn National Cancer Institute Common terminology criteria for adverse events (CTCAE) version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50
- 14.Haanen J., Carbonnel F., Robert C. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:iv264–iv266. doi: 10.1093/annonc/mdy162. [DOI] [PubMed] [Google Scholar]
- 15.Wolchok J.D., Chiarion-Sileni V., Gonzalez R. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Y., Zhang N., Pang H., Gao X., Zhang H. Risk and incidence of fatal adverse events associated with immune checkpoint inhibitors: a systematic review and meta-analysis. Ther. Clin. Risk Manag. 2019;15:293–302. doi: 10.2147/TCRM.S191022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzerling L., Ott P.A., Hodi F.S. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. doi: 10.1186/s40425-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escudier M., Cautela J., Malissen N. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 22.Michel L., Rassaf T. Cardio-oncology: need for novel structures. Eur. J. Med. Res. 2019;24(1) doi: 10.1186/s40001-018-0359-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okazaki T., Tanaka Y., Nishio R. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 24.Norwood T.G., Westbrook B.C., Johnson D.B. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. 2017;5:91. doi: 10.1186/s40425-017-0296-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thibault C., Vano Y., Soulat G., Mirabel M. Immune checkpoint inhibitors myocarditis: not all cases are clinically patent. Eur. Heart J. 2018;39:3553. doi: 10.1093/eurheartj/ehy485. [DOI] [PubMed] [Google Scholar]
- 26.Lyon A.R., Yousaf N., Battisti N.M.L., Moslehi J., Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 27.Neilan T.G., Rothenberg M.L., Amiri-Kordestani L. Myocarditis associated with immune checkpoint inhibitors: an expert consensus on data gaps and a call to action. Oncologist. 2018;23:874–878. doi: 10.1634/theoncologist.2018-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel L., Rassaf T., Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. Journal of Thoracic Disease. 2018;10(Suppl. 35):S4282–S4295. doi: 10.21037/jtd.2018.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Totzeck M., Schuler M., Stuschke M., Heusch G., Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int. J. Cardiol. 2019;280:163–175. doi: 10.1016/j.ijcard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 30.Bonaca Marc P., Olenchock Benjamin A., Salem J.-E. Myocarditis in the setting of cancer therapeutics. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L., Awadalla M., Mahmood S.S. Late gadolinum enhancement in patients with myocarditis from immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2019;73:675. [Google Scholar]
- 32.Müller O.J., Spehlmann M.E., Frey N. Cardio-toxicity of checkpoint inhibitors. Journal of Thoracic Disease. 2018;10(Suppl. 35):S4400–S4404. doi: 10.21037/jtd.2018.12.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ganatra S., Neilan T.G. Immune checkpoint inhibitor-associated myocarditis. Oncologist. 2018;23:879–886. doi: 10.1634/theoncologist.2018-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponikowski P., Voors A.A., Anker S.D. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016;37(2016):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 36.Yancy C.W., Jessup M., Bozkurt B. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2017;136:e137–e161. doi: 10.1161/CIR.0000000000000509. [DOI] [PubMed] [Google Scholar]
- 37.Arangalage D., Delyon J., Lermuzeaux M. Survival after fulminant myocarditis induced by immune-checkpoint inhibitors. Ann. Intern. Med. 2017;167:683–684. doi: 10.7326/L17-0396. [DOI] [PubMed] [Google Scholar]
- 38.Jain V., Mohebtash M., Rodrigo M.E., Ruiz G., Atkins M.B., Barac A. Autoimmune myocarditis caused by immune checkpoint inhibitors treated with antithymocyte globulin. J. Immunother. 2018;41:332–335. doi: 10.1097/CJI.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 39.Salem J.-E., Allenbach Y., Vozy A. Abatacept for severe immune checkpoint inhibitor–associated myocarditis. N. Engl. J. Med. 2019;380:2377–2379. doi: 10.1056/NEJMc1901677. [DOI] [PubMed] [Google Scholar]
- 40.Esfahani K., Buhlaiga N., Thébault P., Lapointe R., Johnson N.A., Miller W.H. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N. Engl. J. Med. 2019;380:2375–2376. doi: 10.1056/NEJMc1903064. [DOI] [PubMed] [Google Scholar]
- 41.Puzanov I., Diab A., Abdallah K. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5:95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Som A., Mandaliya R., Alsaadi D. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J Clin Cases. 2019;7:405–418. doi: 10.12998/wjcc.v7.i4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber J., Mandala M., Del Vecchio M. Adjuvant Nivolumab versus Ipilimumab in resected stage III or IV melanoma. N. Engl. J. Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 44.FDA Highlights of prescribing information. https://www.fda.gov/
- 45.Eggermont A.M.M., Blank C.U., Mandala M. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N. Engl. J. Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 46.Ederhy S., Cautela J., Ancedy Y., Escudier M., Thuny F., Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. JACC Cardiovasc. Imaging. 2018;11:1187–1190. doi: 10.1016/j.jcmg.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 47.Ederhy S., Dolladille C., Thuny F., Alexandre J., Cohen A. Takotsubo syndrome in patients with cancer treated with immune checkpoint inhibitors: a new adverse cardiac complication. Eur. J. Heart Fail. 2019;21:945–947. doi: 10.1002/ejhf.1497. [DOI] [PubMed] [Google Scholar]
- 48.Matsumoto T., Sasaki N., Yamashita T. Overexpression of cytotoxic T-lymphocyte-associated antigen-4 prevents atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2016;36:1141–1151. doi: 10.1161/ATVBAHA.115.306848. [DOI] [PubMed] [Google Scholar]
- 49.Ma K., Lv S., Liu B. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE−/− mice. Cardiovasc. Res. 2012;97:349–359. doi: 10.1093/cvr/cvs330. [DOI] [PubMed] [Google Scholar]
- 50.D-x Bu, Tarrio M., Maganto-Garcia E. Impairment of the programmed cell death-1 pathway increases atherosclerotic lesion development and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:1100–1107. doi: 10.1161/ATVBAHA.111.224709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gelsomino F., Fiorentino M., Zompatori M. Programmed death-1 inhibition and atherosclerosis: can nivolumab vanish complicated atheromatous plaques? Ann. Oncol. 2017;29:284–286. doi: 10.1093/annonc/mdx718. [DOI] [PubMed] [Google Scholar]
- 52.Roffi M., Patrono C., Collet J.P. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2016;37:267–315. doi: 10.1093/eurheartj/ehv320. [DOI] [PubMed] [Google Scholar]
- 53.Amsterdam E.A., Wenger N.K., Brindis R.G. AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;64(2014):e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 54.Ibanez B., James S., Agewall S. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 55.O'Gara P.T., Kushner F.G., Ascheim D.D. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 56.Upadhrasta S., Elias H., Patel K., Zheng L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med. 2019;5:6–14. doi: 10.1016/j.cdtm.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Salem J.E., Manouchehri A., Moey M. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Altan M., Toki M.I., Gettinger S.N. Immune checkpoint inhibitor-associated pericarditis. J. Thorac. Oncol. 2019;14:1102–1108. doi: 10.1016/j.jtho.2019.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adler Y., Charron P., Imazio M. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2015;36:2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]