Abstract
Background
The Chinese expert consensus on thoracic lymph node (LN) dissection in radical esophagectomy (Chinese Criteria, 2017 edition) was newly promoted. This study examined the prognostic significance and role of thoracic LN metastasis based on the Chinese Criteria for esophageal cancer.
Methods
Data of patients with thoracic esophageal squamous cell carcinoma (ESCC) who underwent curative esophagectomy in the West China Hospital from May 2005 to May 2015 were retrospectively analyzed. Patients’ prognosis and clinicopathological features were compared to determine the role of Chinese Criteria and their relationship with Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) 8th TNM staging.
Results
Overall, 2,285 qualified patients were divided into the no (n=1,148), skip (n=156), local (n=665), and mediastinal (n=316) metastasis groups according to the Chinese Criteria. Significant prognostic differences occurred among the four groups in all the thoracic and lower mediastinal ESCC patients (both P<0.001). The Chinese Criteria grouping was an independent prognostic factor for all thoracic [P<0.001; hazard ratio (HR) =1.261, 95% confidence interval (CI): 1.103–1.441], upper (P<0.001; HR =1.391, 95% CI: 1.264–1.530), lower mediastinal thoracic ESCC patients (P<0.001; HR =1.312, 95% CI: 1.257–1.370) and all thoracic ESCC after adjuvant therapy (P<0.001; HR =1.303, 95% CI: 1.221–1.390). Significant prognostic differences among Chinese Criteria groups occurred with N1 (P=0.014) and N2 (P=0.018) stages only. Significant differences in survival among N stages were found in local (P<0.001) and mediastinal (P=0.009) metastasis groups.
Conclusions
Our study was the first to report the Chinese Criteria in measuring the degree of thoracic LN metastasis. Similar to N-stage, the Chinese Criteria were confirmed as an independent prognostic factor for thoracic ESCC. Further confirmation of our findings is warranted.
Keywords: Esophageal cancer, Chinese Criteria, International Cancer Control/American Joint Committee on Cancer 8th TNM staging (UICC/AJCC 8th TNM staging), prognosis
Introduction
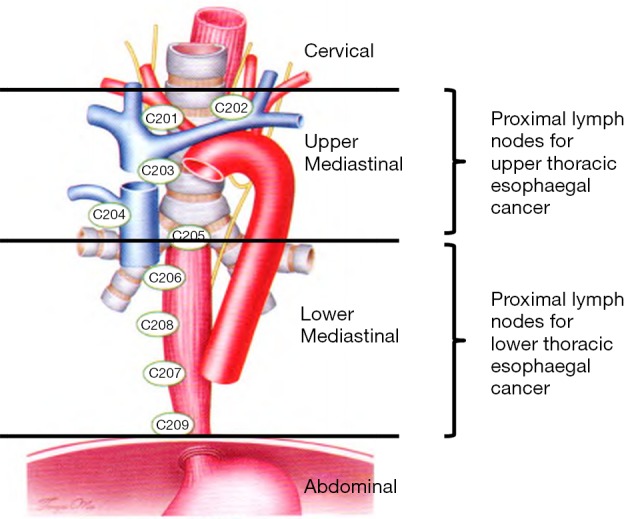
Esophageal cancer is recognized as one of the most malignant tumors worldwide, with postoperative overall survival (OS) rate ranging 10–40% (1,2). In China, from about 270 thousand new cases of esophageal cancer in 2013, about 200 thousand deaths that occurred contributed the fourth highest to mortality due to malignant cancers (3). However, although the primary treatment for esophageal cancer is surgery, the prognosis of localized advanced tumor is unsatisfactory, and the 5-year OS rate in patients with stages IIA–III esophageal squamous cell carcinoma (ESCC) treated by surgical resection alone ranges from 20.6% to 34.0% (4,5). Among the clinical and pathological features of esophageal cancer, lymph node (LN) metastasis is one of the strongest prognostic factors, reported as being related to poor prognosis in patients with esophageal cancer (6-8). Globally, thoracic LN dissection remains controversial; and the two histological forms (squamous cell and adenocarcinoma) of esophageal cancer vary in terms of their geographic prevalence and associated risk factors. Thus, the Chinese expert consensus on thoracic LN dissection for the radical resection of esophageal cancer (Chinese Criteria, 2017 edition) (9) was published by Chinese Society of Esophageal Cancer, China Anti-cancer Association. The consensus is to provide guidance for the standardization of thoracic LN dissection for the radical resection of esophageal cancer. The Chinese Society of Esophageal Cancer laid down the Chinese thoracic LN maps for esophageal cancer with reference to other guidelines. These include the American Joint Committee on Cancer Staging Manual, 8th edition (AJCC 8th TNM), the Union for International Cancer Control protocol (UICC) (10,11), and the Japanese Classification of Esophageal Cancer (JES), 11th Edition: part I (12) (Table 1). These meet the requirements of clinical care of esophageal cancer patients in China to a large degree.
Table 1. Chinese Criteria of thoracic lymph node classification of esophagus cancer and their corresponding relations with UICC/AJCC standards and JES standards.
| Region | Chinese classification and anatomical position description | UICC/AJCC standardsa | JES standardsb |
|---|---|---|---|
| Upper mediastinum | C201: right recurrent laryngeal nerve lymph nodes (initial reentry of right vagus nerves to right terminal subclavian artery, peripheral lymph nodes and adipose tissue of right recurrent laryngeal nerves) | 2R: right upper paratracheal nodes | 106recR: right recurrent laryngeal nerve lymph nodes |
| C202: left recurrent laryngeal nerve lymph nodes (upper left 1/3 of trachea, peripheral lymph nodes and adipose tissue of left recurrent laryngeal nerves of superior border of aortic arch) | 2L: left upper paratracheal nodes | 106recL: left recurrent laryngeal nerve lymph nodes | |
| C203: upper thoracic paraesophageal lymph nodes (lymph nodes from apex pulmonis to inferior border of inferior border of azygos vein) | 8U: upper thoracic paraesophageal lymph nodes | 105: upper thoracic paraesophageal lymph nodes | |
| C204: paratracheal lymph nodes (lymph nodes from right vagus nerves to esophagus, on the right side of tracheae) | 4R: right lower paratracheal nodes | 106: paratracheal lymph nodes (106pre: pre-tracheal lymph nodes; 106tbR: right paratracheal lymph nodes) | |
| – | 4L: left lower paratracheal nodes | 106tbL: left paratracheal lymph nodes | |
| 5: subaortic nodes | 113: lymph nodes of arterial ligament | ||
| 6: anterior mediastinal nodes | 114: anterior mediastinal lymph nodes | ||
| C205: subcarinal lymph nodes (caudal to the carina of the trachea) | 7: subcarinal nodes | 107: subcarinal lymph nodes | |
| Lower mediastinum | C206: middle thoracic paraesophageal lymph nodes (from the tracheal bifurcation to the caudal margin of the inferior pulmonary vein) | 8M: middle thoracic paraesophageal lymph nodes | 108: middle thoracic paraesophageal lymph nodes |
| C207: lower thoracic paraesophageal lymph nodes (paraesophageal lymph nodes from inferior border of inferior pulmonary vein to gastroesophageal junction) | 8Lo: lower thoracic paraesophageal lymph nodes | 110: lower thoracic paraesophageal lymph nodes | |
| C208: inferior pulmonary ligament lymph nodes (lymph nodes that are close to inferior border of right lower inferior pulmonary vein and within inferior pulmonary ligament) | 9L: left inferior pulmonary ligament nodes | 112L: left posterior mediastinal lymph nodes | |
| 9R: right inferior pulmonary ligament nodes | 112R: right posterior mediastinal lymph nodes | ||
| – | 10L: left bronchial paratracheal nodes | 109L: left bronchial paratracheal nodes | |
| 10R: right lower bronchial paratracheal nodes | 109R: right bronchial paratracheal nodes | ||
| C209: diaphragmatic nodes (lymph nodes on the right side of cardiophrenic angle) | 15: diaphragmatic nodes | 111: superior phrenic lymph nodes |
a, is based on literature (10,11); b, is based on literature (12); –, refers to lymph nodes which were not included in Chinese Criteria; “C” in Chinese classification stands for Chinese Criteria, “2” indicates thoracic lymph nodes; AJCC, American Joint Committee on Cancer; UICC, Union for International Cancer Control; JES, Japan Esophagus Society.
According to the Chinese Criteria, the thoracic LNs were divided into two: the upper (including the thoracic LN stations from C201 to C205) and lower (the thoracic LN stations from C206 to C209) mediastinal compartments. However, whether a significant difference exists in the OS of esophageal cancer patients or not using the Chinese Criteria, and the relationship of regional LN metastasis between the Chinese Criteria and AJCC 8th TNM, remain unclear. Therefore, 2,285 patients with surgically resected esophageal cancer were retrospectively investigated. The role that the Chinese Criteria played in the prognostic significance of such patients as well as the relationship between the Chinese Criteria and the AJCC 8th TNM were evaluated.
Methods
Patients
Data of patients with esophageal cancer who underwent radical esophagectomy at the Department of Thoracic Surgery, West China Hospital of Sichuan University from May 2005 to May 2015 were retrospectively reviewed. The patients were excluded using the following exclusion criteria: (I) lost to follow-up; (II) carcinoma located at the cervical esophagus or esophagogastric junction; (III) other pathologically confirmed thoracic esophageal cancer types except ESCC; (IV) palliative surgery and R1 or R2 resection; (V) the total number of LNs removed were <10; (VI) patients who had accepted the preoperative neoadjuvant chemotherapy or radiotherapy; (VII) patients whose status was defined as M1 preoperatively; (VIII) cervical or abdominal LN metastasis. Finally, 2,285 patients were enrolled in our study. The study was approved by the human participants committee of West China Hospital of Sichuan University. Preoperatively, permission for the use of patients resected specimens and written informed consents were obtained.
Surgical procedure and pathology
In this study, the surgical procedures’ selected for each patient depended mainly on the patients’ preoperative computed tomography (CT), magnetic resonance imaging (MRI), specific X-ray, and cervical ultrasonography images. Furthermore, surgeons might evaluate a patient’s general condition to determine the most appropriate surgical procedure for that patient. Generally, the McKeown esophagogastrectomy (right thoracotomy followed by laparotomy and cervical anastomosis) with three-field LN dissection were applied for tumors in the upper, middle, and lower thoracic esophagus; and the Sweet and Ivor-Lewis procedures with two-field LN dissection were applied for middle or lower thoracic ESCC patients. The cervical or thoracic anastomoses, which depended on the resection of the margin of the tumor, were performed in a standardized way with the gastric conduit for reconstruction. The dissected LNs, which were separated from the resected esophagus and the peri-esophagus tissues, were marked and the location according to the guideline of AJCC 8th TNM and UICC protocol was indicated. The mean number of dissected LNs is 15 per patient (range, 10–78). Two experienced pathologists fixed the resected specimens, embedded and stained them with diaminobenzidine chromogen counterstained solution [1:50, EnVisionTM Detection Kit, Gene Tech (Shanghai) Company Limited] and hematoxylin (Zhongshan Golden Bridge Biotechnology Co., Ltd, Beijing, China) subsequently. The routine way of assessing each specimen was adopted histologically, and the pathologists documented the extent and location of metastatic LNs by examining the largest cross section of the dissected LNs.
Tumor location and thoracic LN classification
With reference to the Chinese Criteria, the mediastinum was divided into the upper and lower mediastinum, with the boundary of subcarina. Furthermore, the tumor location and thoracic LNs were also re-classified into two mediastinal compartments, which lie parallel to their location in the mediastinum, according to their classification. The location of the cancer primary site was defined by the cancer epicenter, with the boundary of subcarina; the upper mediastinal thoracic ESCC, which according to the Chinese Criteria was classified as the tumor epicenter was located above the subcarina, including the location of the subcarina. The epicenter of the thoracic ESCC located under the subcarina was regarded as the lower mediastinal thoracic ESCC. Based on the AJCC 8th TNM, the UICC protocol, and the Chinese Criteria, thoracic LN metastasis was not only confined to the number of the metastatic LNs but also focused on the region where the thoracic LNs metastasized to. Typically, according to the longitudinal position of the thoracic LNs relative to the location of the primary tumor, the thoracic LNs should be further classified into proximal and distal nodes. The proximal nodes were defined as those in the nodal group at the same cross section as the primary tumor and its nearest neighborhood groups (13), while the other group of nodes was defined as the distal nodes. Therefore, based on the thoracic tumor location and the thoracic metastatic region, the enrolled patients were divided into four groups (Chinese Criteria grouping) (Figure 1): (I) local metastasis group (defined as the thoracic proximal LN metastasis with the distal LNs free of tumor infiltration, consist of the upper mediastinal esophageal cancer with upper mediastinal LN metastasis only and the lower mediastinal esophageal cancer with lower mediastinal LN metastasis only). (II) Skip metastasis group (regarded as the thoracic metastatic involvement of the distal LNs with the proximal LNs free of tumor infiltration; including the upper mediastinal esophageal cancer with the lower mediastinal LN metastasis only and the lower mediastinal esophageal cancer with the upper mediastinal LN metastasis only). (III) Mediastinal metastasis group (this means that the thoracic metastatic involvement in both proximal and distal LNs, comprised both upper and lower mediastinal esophageal cancer with the entire mediastinal LN metastasis). (IV) No metastasis group.
Figure 1.
Lymph nodes classification referring to Chinese Criteria grouping (cited from (9)).
Adjuvant therapy
After operation, patients in our hospital received the common chemotherapy regimen: cisplatin (DDP) + 5-fluorouracil (5-Fu). The chemotherapy lasted for 4–6 cycles and the concurrent radiotherapy was performed with the clinical target volume dose of 40.0 Gy (36.0–46.0 Gy). Whether the patients received the adjuvant therapy or not depended on the tumor stage, doctor’s opinion, and patients’ status and preference. Generally, the volumes of each regimen were under the control of the oncologist.
Follow up
In the present study, patients were followed-up every 3 months for the first and second year; every 6 months for the third to fifth year after the treatment; and yearly after the fifth year. Routine blood tests, gastroscopy, chest CT, neck and abdominal ultrasound; when necessary, according to the patients’ symptoms and physical examination findings, other examinations such as positron emission tomography-CT, radionuclide bone scanning, and MRI may be included in the patients’ follow-up examinations. The tumor status (including tumor metastasis and recurrence), patients’ status (including survival and death) as well as those lost to follow-up were all documented through outpatient and telephonic/letter follow-up.
Statistical analysis
The clinicopathologic features in the local, skip, mediastinal, and no metastasis groups were analyzed by Fisher’s exact test and the χ2 test. Categorical variables were presented using frequencies and percentages. The logistic regression analysis was performed to determine the independent factors related to each group. The OS of each group was determined from the Kaplan-Meier curves while the log-rank test was used to determine the statistical significance. Multivariate survival analysis was conducted through the Cox proportional hazard regression model. P<0.05 was significant, and all statistical analyses were conducted by IBM® SPSS® Statistics Version 21.0.
Results
All patients
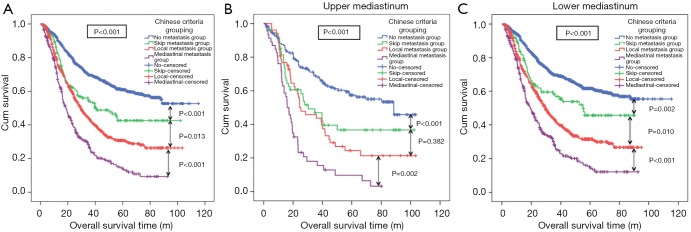
Overall, 2,285 patients with thoracic ESCC were finally enrolled in our study, of which, 1,148 (50.24%) were in the no metastasis group. The median (range) age of the enrolled patients was 59 years (25–88 years) while the median survival time was 27.17 months (1.03–115.4 months). Furthermore, 156 (6.83%), 665 (29.10%), and 316 (13.83%) patients were divided in the skip, local, and mediastinal metastasis groups, respectively. The clinicopathological features of the four groups are presented in Table S1. Sex (P<0.001), tumor differentiation (P<0.001), adjuvant therapy (P<0.001), T-stage (P<0.001), N-stage (P<0.001), and tumor location (P<0.001) showed significant differences between the four groups. The median follow-up time for all patients was 38.27 (1.43–115.4) months, and the median OS was 44.90 [95% confidence interval (CI): 40.39–49.41] months. The 3- and 5-year survival rates were 54.9% and 44.4%, respectively. The Kaplan-Meier curves showed that the OS rate in the thoracic ESCC patients in the mediastinal metastasis group was significantly lower than that in the local metastasis group (P<0.001, Figure 2A). The OS rate of patients in local metastasis group was significantly lower than that in the skip metastasis group (P=0.013, Figure 2A). The survival difference between the skip and no metastasis groups was significant (P<0.001, Figure 2A). The effects of clinicopathologic features including Chinese Criteria grouping on OS evaluated through the Cox proportional hazard regression indicated that T-stage [P<0.001; hazard ratio (HR) =1.407, 95% CI: 1.284–1.540], N-stage (P<0.001; HR =1.442, 95% CI: 1.279–1.626), age (P=0.001; HR =1.261, 95% CI: 1.103–1.441), and Chinese Criteria grouping (P<0.001; HR =1.261, 95% CI: 1.103–1.441) were independent prognostic factors for thoracic ESCC patients (Table S2).
Table S1. Clinicopathological characters of thoracic ESCC patients in different Chinese Criteria groups and the relationship between tumor location and Chinese Criteria grouping.
| Character | No (n=1,148) (%) | Skip (n=156) (%) | Local (n=665) (%) | Mediastinal (n=316) (%) | P | Upper mediastinum (n=320) (%) | Lower mediastinum (n=1,965) (%) | P (univariate analyses) | P (multivariate analyses) |
OR, 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | <0.001 | 0.066 | ||||||||
| Male | 871 (75.9) | 119 (76.3) | 559 (84.1) | 276 (87.3) | 245 (13.4) | 1,580 (86.6) | ||||
| Female | 277 (24.1) | 37 (23.7) | 106 (15.9) | 40 (12.7) | 75 (16.3) | 385 (83.7) | ||||
| Differentiation | <0.001 | 0.023 | 0.030 | 1.196 (1.093–1.440) | ||||||
| High | 144 (12.5) | 14 (9.0) | 59 (8.9) | 17 (5.4) | 46 (19.7) | 188 (80.3) | Ref | Ref | ||
| Moderate | 625 (54.4) | 84 (53.8) | 320 (48.1) | 139 (44.0) | 162 (13.9) | 1,006 (86.1) | 0.017 | 1.571 (1.084–2.276) | ||
| Low | 379 (33.0) | 58 (37.2) | 286 (43.0) | 160 (50.6) | 112 (12.7) | 771 (87.3) | 0.010 | 1.668 (1.128–2.465) | ||
| Adjuvant therapy | <0.001 | 0.400 | ||||||||
| No | 800 (69.7) | 86 (55.1) | 361 (54.3) | 170 (53.8) | 201 (14.2) | 1,216 (85.8) | ||||
| Yes | 348 (30.3) | 70 (44.9) | 304 (45.7) | 146 (46.2) | 119 (13.7) | 749 (86.3) | ||||
| T stage | <0.001 | 0.226 | ||||||||
| T1 | 187 (16.3) | 9 (5.8) | 33 (5.0) | 7 (2.2) | 41 (17.4) | 195 (82.6) | ||||
| T2 | 240 (20.9) | 33 (21.2) | 104 (15.6) | 27 (8.5) | 58 (14.4) | 346 (85.6) | ||||
| T3 | 651 (56.7) | 104 (66.7) | 463 (69.6) | 239 (75.6) | 190 (13.0) | 1,267 (87.0) | ||||
| T4 | 70 (6.1) | 10 (6.4) | 65 (9.8) | 43 (13.6) | 31 (16.5) | 157 (83.5) | ||||
| N stage | <0.001 | 0.305 | ||||||||
| N0 | 1,148 (100.0) | 0 (0) | 0 (0) | 0 (0) | 167 (14.5) | 981 (85.5) | ||||
| N1 | 0 (0) | 126 (80.8) | 435 (65.4) | 62 (19.6) | 94 (15.1) | 529 (84.9) | ||||
| N2 | 0 (0) | 26 (16.7) | 202 (30.4) | 169 (53.5) | 46 (11.6) | 351 (88.4) | ||||
| N3 | 0 (0) | 4 (2.6) | 28 (4.2) | 85 (26.9) | 13 (11.1) | 104 (88.9) | ||||
| Tumor location | <0.001 | - | ||||||||
| Upper mediastinum | 167 (14.5) | 52 (33.3) | 55 (8.3) | 46 (14.6) | – | – | ||||
| Lower mediastinum | 981 (85.5) | 104 (66.7) | 610 (91.7) | 270 (85.4) | – | – | ||||
| Age (y) | 0.062 | 0.012 | 0.050 | 1.344 (1.000–2.306) | ||||||
| <55 | 830 (72.3) | 116 (74.4) | 497 (74.7) | 252 (79.7) | 254 (15.0) | 1,441 (85.0) | Ref | Ref | ||
| ≥55 | 318 (27.7) | 40 (25.6) | 168 (25.3) | 64 (29.3) | 66 (11.2) | 524 (88.8) | 0.050 | 1.344 (1.000–2.306) | ||
| Chinese Criteria grouping | - | <0.001 | <0.001 | 1.070 (1.049–1.868) | ||||||
| No | – | – | – | – | 167 (14.5) | 981 (85.5) | Ref | Ref | ||
| Skip | – | – | – | – | 52 (33.3) | 104 (66.7) | <0.001 | 0.341 (0.235–0.495) | ||
| Local | – | – | – | – | 55 (8.3) | 610 (91.7) | <0.001 | 1.906 (1.382–2.628) | ||
| Mediastinal | – | – | – | – | 46 (14.6) | 270 (85.4) | 0.904 | 1.024 (0.719–1.439) |
ESCC, esophageal squamous cell carcinoma; no, no metastasis group; skip, skip metastasis group; local, local metastasis group; mediastinal, mediastinal metastasis group; OR, odds ratio; 95% CI, 95% confidence interval; Ref, reference.
Figure 2.
Prognosis of Chinese Criteria grouping in all thoracic ESCC patients, upper and lower mediastinal ESCC patients. (A) The OS rate in the thoracic ESCC patients in the mediastinal metastasis group was significantly lower than that in the local metastasis group (P<0.001). The OS rate of patients in local metastasis group was significantly lower than that in the skip metastasis group (P=0.013). The survival difference between the skip and no metastasis groups was significant (P<0.001). (B) In upper mediastinum, OS rate in the mediastinal metastasis group was significantly lower than in the local metastasis group (P=0.002). The OS rate in the local metastasis group tended to be lower than that in the skip metastasis group, the difference was not significant (P=0.382). There was a significant difference in OS rate between the skip and the no metastasis groups (P<0.001). (C) In lower mediastinum, the OS rate in the local metastasis group was significantly lower than in the skip metastasis group (P=0.010). ESCC, esophageal squamous cell carcinoma; OS, overall survival.
Table S2. Univariate and multivariate Cox regression analyses of thoracic ESCC patients and the relationship between tumor location and Chinese Criteria grouping.
| Character | All patients | Upper mediastinum | Lower mediastinum | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analyses | Multivariate analyses | Univariate analyses | Multivariate analyses | Univariate analyses | Multivariate analyses | ||||||||||||
| P | HR, 95% CI | P | HR, 95% CI | P | HR, 95% CI | P | HR, 95% CI | P | HR, 95% CI | P | HR, 95% CI | ||||||
| Gender | 0.002 | 0.785 (0.672–0.917) | 0.303 | 0.918 (0.785–1.075) | 0.510 | 0.890 (0.630–1.258) | – | – | 0.002 | 0.757 (0.636–0.901) | 0.145 | 0.879 (0.737–1.049) | |||||
| Male | Ref | Ref | Ref | Ref | Ref | Ref | – | – | Ref | Ref | Ref | Ref | |||||
| Female | 0.002 | 0.785 (0.672–0.917) | 0.303 | 0.918 (0.785–1.075) | 0.510 | 0.890 (0.630–1.258) | – | – | 0.002 | 0.757 (0.636–0.901) | 0.145 | 0.879 (0.737–1.049) | |||||
| Differentiation | <0.001 | 1.214 (1.104–1.334) | 0.112 | 1.099 (0.898–1.211) | 0.183 | 1.203 (0.963–1.502) | – | – | 0.001 | 1.225 (1.103–1.360) | 0.084 | 1.263 (0.811–1.610) | |||||
| High | Ref | Ref | Ref | Ref | Ref | Ref | – | – | Ref | Ref | Ref | Ref | |||||
| Moderate | 0.111 | 1.192 (0.960–1.478) | 0.869 | 1.018 (0.820–1.266) | 0.225 | 1.028 (0.663–1.595) | – | – | 0.069 | 1.260 (0.982–1.617) | 0.113 | 1.062 (0.826–1.366) | |||||
| Low | 0.001 | 1.458 (1.171–1.814) | 0.196 | 1.159 (0.927–1.448) | 0.066 | 1.359 (0.862–2.142) | – | – | 0.001 | 1.526 (1.186–1.964) | 0.029 | 1.211 (1.045–1.580) | |||||
| Adjuvant therapy | 0.494 | 1.044 (0.923–1.182) | – | – | 0.856 | 0.972 (0.714–1.323) | – | – | 0.413 | 1.058 (0.924–1.211) | – | – | |||||
| No | Ref | Ref | – | – | Ref | Ref | – | – | Ref | Ref | – | – | |||||
| Yes | 0.494 | 1.044 (0.923–1.182) | – | – | 0.856 | 0.972 (0.714–1.323) | – | – | 0.413 | 1.058 (0.924–1.211) | – | – | |||||
| T stage | <0.001 | 1.620 (1.484–1.769) | <0.001 | 1.407 (1.284–1.540) | <0.001 | 1.560 (1.273–1.912) | <0.001 | 1.526 (1.235–1.886) | <0.001 | 1.638 (1.485–1.805) | <0.001 | 1.400 (1.266–1.548) | |||||
| T1 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |||||
| T2 | <0.001 | 1.580 (1.162–2.147) | 0.084 | 1.313 (0.965–1.788) | 0.224 | 1.492 (0.783–2.846) | 0.886 | 1.050 (0.543–2.027) | 0.006 | 1.631 1.150–2.313) | 0.059 | 1.402 (0.987–1.992) | |||||
| T3 | <0.001 | 2.721 (2.078–3.562) | <0.001 | 1.989 (1.513–2.616) | 0.006 | 2.195 (1.260–3.826) | 0.039 | 1.808 (1.030–3.176) | <0.001 | 2.905 (2.133–3.956) | <0.001 | 2.106 (1.539–2.883) | |||||
| T4 | <0.001 | 4.065 (2.970–5.562) | <0.001 | 2.610 (1.894–3.579) | <0.001 | 3.980 (2.043–7.755) | 0.001 | 3.154 (1.595–6.239) | <0.001 | 4.172 (2.920–5.961) | <0.001 | 2.635 (1.830–3.794) | |||||
| N stage | <0.001 | 1.755 (1.651–1.864) | <0.001 | 1.442 (1.279–1.626) | <0.001 | 1.755 (1.495–2.060) | <0.001 | 1.685 (1.434–1.980) | <0.001 | 1.759 (1.647–1.878) | <0.001 | 1.654 (1.543–1.772) | |||||
| N0 | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |||||
| N1 | <0.001 | 2.001 (1.727–2.319) | <0.001 | 1.849 (1.593–2.146) | <0.001 | 2.180 (1.545–3.076) | <0.001 | 2.140 (1.511–3.031) | <0.001 | 1.961 (1.666–2.307) | <0.001 | 1.781 (1.510–2.101) | |||||
| N2 | <0.001 | 3.264 (2.791–3.818) | <0.001 | 2.935 (2.500–3.445) | <0.001 | 2.812 (1.862–4.271) | <0.001 | 2.750 (1.806–4.188) | <0.001 | 3.354 (2.830–3.976) | <0.001 | 2.969 (2.494–3.535) | |||||
| N3 | <0.001 | 5.073 (4.032–6.383) | <0.001 | 4.518 (3.579–5.702) | <0.001 | 6.165 (3.289–11.544) | <0.001 | 4.986 (2.606–9.358) | <0.001 | 4.983 (3.891–6.380) | <0.001 | 4.417 (3.439–5.674) | |||||
| Tumor location | 0.171 | 0.894 (0.761–1.050) | – | – | – | – | – | – | – | – | – | – | |||||
| Upper mediastinum | Ref | Ref | – | – | – | – | – | – | – | – | – | – | |||||
| Lower mediastinum | 0.171 | 0.894 (0.761–1.050) | – | – | – | – | – | – | – | – | – | – | |||||
| Age (y) | 0.011 | 1.188 (0.761–1.050) | 0.001 | 1.261 (1.103–1.441) | 0.344 | 1.186 (0.833–1.686) | – | – | 0.016 | 1.194 (1.034–1.379) | 0.003 | 1.242 (1.075–1.434) | |||||
| <55 | Ref | Ref | Ref | Ref | Ref | Ref | – | – | Ref | Ref | Ref | Ref | |||||
| ≥55 | 0.011 | 1.188 (0.761–1.050) | 0.001 | 1.261 (1.103–1.441) | 0.344 | 1.186 (0.833–1.686) | – | – | 0.016 | 1.194 (1.034–1.379) | 0.003 | 1.242 (1.075–1.434) | |||||
| Chinese Criteria grouping | <0.001 | 1.370 (1.319–1.423) | <0.001 | 1.261 (1.103–1.441) | <0.001 | 1.381 (1.257–1.518) | <0.001 | 1.391 (1.264–1.530) | <0.001 | 1.371 (1.315–1.429) | <0.001 | 1.312 (1.257–1.370) | |||||
| No | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | |||||
| Skip | <0.001 | 1.892 (1.494–2.397) | <0.001 | 1.614 (1.285–2.310) | 0.007 | 1.806 (1.178–2.768) | 0.018 | 1.690 (1.096–2.607) | 0.003 | 1.603 (1.176–2.184) | 0.010 | 1.503 (1.101–2.050) | |||||
| Local | <0.001 | 2.320 (2.012–2.676) | <0.001 | 2.123 (1.837–2.454) | <0.001 | 2.234 (1.498–3.329) | <0.001 | 2.300 (1.537–3.441) | <0.001 | 2.403 (2.063–2.801) | <0.001 | 2.179 (1.865–2.545) | |||||
| Mediastinal | <0.001 | 3.857 (3.271–4.547) | <0.001 | 3.367 (2.843–3.987) | <0.001 | 4.436 (2.987–6.588) | <0.001 | 4.341 (2.894–6.514) | <0.001 | 3.756 (3.134–4.503) | <0.001 | 3.225 (2.675–3.888) | |||||
ESCC, esophageal squamous cell carcinoma; no, no metastasis group; skip, skip metastasis group; local, local metastasis group; mediastinal, mediastinal metastasis group; HR, hazard ratio; 95% CI, 95% confidence interval; Ref, reference.
The metastasis groups
The median (range) follow-up time for the no, skip, local, and mediastinal metastasis groups were 43.28 (95% CI: 1.47–115.4), 40.17 (95% CI: 2.27–104.97), 33.53 (95% CI: 1.43–103.73), and 29.98 (95% CI: 1.63–93.83) months, respectively. The median survival time for these four groups were 35.25 (95% CI: 1.03–115.4), 39.47 (95% CI: 25.23–53.71), 29.63 (95% CI: 26.44–32.83), and 18.69 months (95% CI: 16.14–21.24) months, respectively. The 3- and 5-year survival rates for these four groups were 69.6% and 60.9%; 52.9% and 37.3%; 43.6% and 32.0%; and 26.3% and 13.6%, respectively.
From the Cox proportional hazard regression analysis, while T-stage (P=0.002; HR =2.318, 95% CI: 1.375–3.908) and age (P=0.002; HR =2.065, 95% CI: 1.296–3.290) were independent prognostic factors of thoracic ESCC in local metastasis group; adjuvant therapy (P=0.039; HR =0.802, 95% CI: 0.651–0.989), T-stage (P=0.005; HR =1.339, 95% CI: 1.133–1.582), N-stage (P<0.001; HR =1.655, 95% CI: 1.400–1.956), and age (P=0.003; HR =1.407, 95% CI: 1.125–1.758) were such factors in the local metastasis group. In the mediastinal metastasis group, independent prognostic factors of thoracic ESCC were adjuvant therapy (P=0.003; HR =0.674, 95% CI: 0.520–0.873) and N-stage (P=0.006; HR =1.346, 95% CI: 1.105–1.639).
The relationship between tumor location and the Chinese Criteria grouping
According to the Chinese Criteria, the relationship between the thoracic LN metastasis and the newly defined tumor location is shown in Table S1. Differentiation (P=0.023), age (P=0.012), and Chinese Criteria grouping (P<0.001) were significantly associated with the location of thoracic ESCC. However, no significant differences were found with any other clinicopathologic features. Logistic regression showed that differentiation [P=0.030; odds ratio (OR) =1.196, 95% CI: 1.093–1.440], age (P=0.050; OR =1.344, 95% CI: 1.000–2.306), and Chinese Criteria grouping (P<0.001; OR =1.070, 95% CI: 1.049–1.868) were independent factors of the location of thoracic ESCC.
The upper and lower mediastinal ESCC
A total of 320 and 1,965 patients were found with the upper and lower mediastinal ESCC, respectively. The median (range) follow-up time for the upper and lower ESCC were 41.98 (1.90–105.00) and 37.77 (1.43–115.40) months, respectively. The median OS for the two groups were 39.72 (95% CI: 31.20–48.24) and 46.27 (95% CI: 40.02–52.52) months, respectively. The 3- and 5-year survival rates for the upper (53.10% and 41.00%) and lower (55.2% and 45.0%) mediastinal ESCC are shown, respectively.
In the upper mediastinal ESCC, the Kaplan-Meier survival curves showed that OS rate in the mediastinal metastasis group was significantly lower than in the local metastasis group (P=0.002, Figure 2B). Whereas, although the OS rate in the local metastasis group tended to be lower than that in the skip metastasis group, the difference was not significant (P=0.382, Figure 2B). There was a significant difference in OS rate between the skip and the no metastasis groups (P<0.001, Figure 2B). Furthermore, T-stage (P<0.001; HR =1.526, 95% CI: 1.235–1.886), N-stage (P<0.001; HR =1.685, 95% CI: 1.434–1.980), and Chinese Criteria grouping (P<0.001; HR =1.391, 95% CI: 1.264–1.530) were independent prognostic factors of upper mediastinal ESCC.
The lower ESCC, mediastinal metastasis group had the worst prognosis while the no metastasis group had the best. The OS rate in the local metastasis group was significantly lower than in the skip metastasis group (P=0.010, Figure 2C). Furthermore, T-stage (P<0.001; HR =1.400, 95% CI: 1.266–1.548), N-stage (P<0.001; HR =1.654, 95% CI: 1.543–1.772), age (P=0.003; HR =1.242, 95% CI: 1.075–1.434), and Chinese Criteria grouping (P<0.001; HR =1.312, 95% CI: 1.257–1.370) were independent prognostic factors of the lower mediastinal ESCC.
The relationship between N-stage and the Chinese Criteria grouping
To investigate the similarity or otherwise between Chinese Criteria grouping and N-stage, we compared the prognosis in thoracic ESCC patients using either the N-stage or the Chinese Criteria grouping.
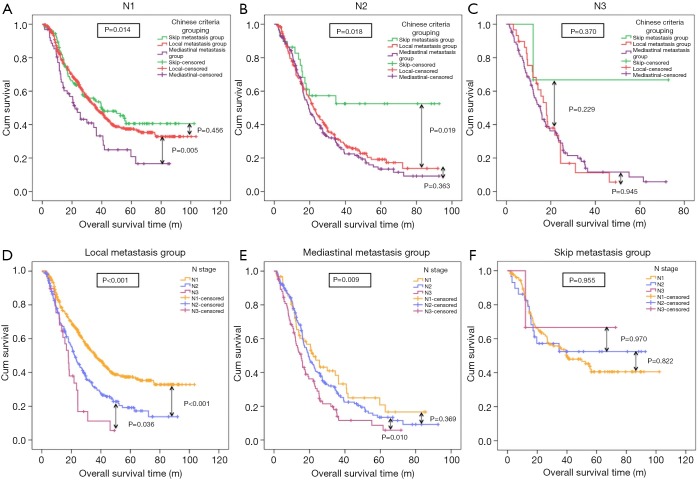
In N-stage
Using the Kaplan-Meier survival curves, in N1 stage, the OS rate in the mediastinal metastasis group was significantly lower than in the local metastasis group (P=0.005, Figure 3A). Although the OS rate in the local metastasis group tended to be lower than that in the skip metastasis group, the difference was not significant (P=0.456, Figure 3A). Furthermore, in N2 stage, the OS rates in the mediastinal metastasis group and the local metastasis group were not significantly different (P=0.363, Figure 3B), while that in the local metastasis group was significantly lower than that in the skip metastasis group (P=0.019, Figure 3B). Nevertheless, no significant difference in OS rate in the Chinese Criteria groups was found in N3 stage (Figure 3C).
Figure 3.
Comparison on prognosis of the Chinese Criteria grouping and N stage. (A) In N1 stage, the OS rate in the mediastinal metastasis group was significantly lower than in the local metastasis group (P=0.005). The OS rate in the local metastasis group tended to be lower than that in the skip metastasis group but the difference was not significant (P=0.456). (B) In N2 stage, the OS rates in the mediastinal metastasis group and the local metastasis group were not significantly different (P=0.363). The OS in the local metastasis group was significantly lower than that in the skip metastasis group (P=0.019). (C) In N3 stage, no significant difference in OS rate in the Chinese Criteria groups was found in N3 stage. (D) In local metastasis group, the OS rates in each N-stage was significantly lower in the local metastasis group, with N3 stage being significantly lower than in N2 stage (P=0.036), and N2 stage was significantly lower than in N1 stage (P<0.001). (E) In mediastinal metastasis group, significant differences in OS rates between the N3 and N2 stages were found (P=0.010). The OS rate in N2 stage tended to be lower than that in N1 stage; but the difference was not significant (P=0.369). (F) In skip metastasis group, No significant differences in OS rate in all N-stages were found in the skip metastasis group. OS, overall survival.
In Chinese Criteria grouping
The OS rates in each N-stage was significantly lower in the local metastasis group, with N3 stage being significantly lower than in N2 stage (P=0.036, Figure 3D), and N2 stage was significantly lower than in N1 stage (P<0.001, Figure 3D). Equally, in the mediastinal metastasis group, significant differences in OS rates between the N3 and N2 stages were found (P=0.010, Figure 3E). The OS rate in N2 stage tended to be lower than that in N1 stage; but the difference was not significant (P=0.369, Figure 3E). No significant differences in OS rate in all N-stages were found in the skip metastasis group (Figure 3F).
The relationship between Chinese Criteria grouping and adjuvant therapy
Among all the patients, there are 868 patients received the adjuvant therapy after operation, and the median (range) follow-up time for them were 38.30 (95% CI: 1.43–115.40) months, respectively. The median OS for them was 44.77 (95% CI: 38.52–51.02) months, respectively. The 3- and 5-year survival rates for all patients after adjuvant therapy were 55.40% and 42.4%, respectively.
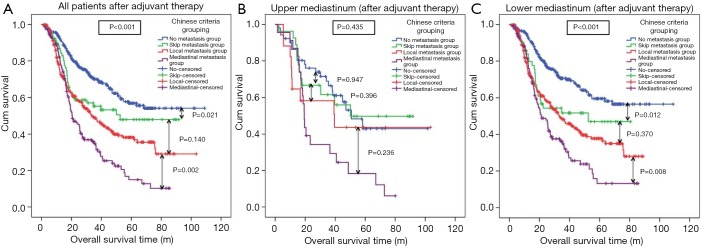
From the Kaplan-Meier curves, in all patients (after adjuvant therapy), the OS rate in the mediastinal metastasis group was significantly lower than that in the local metastasis group (P=0.002). The OS rate in the local metastasis group tended to be lower than that in the skip metastasis group, the difference was not significant (P=0.140). The survival difference between the skip and no metastasis groups was significant (P=0.021) (Figure 4A). In upper mediastinum (after adjuvant therapy), no significant prognostic differences were found among four groups (Figure 4B). In lower mediastinum (after adjuvant therapy), the OS rate in the mediastinal metastasis group was significantly lower than in the local metastasis group (P=0.008), meanwhile, the survival difference between the skip and no metastasis groups was significant (P=0.012). However, no significant difference of prognosis was found between skip and local metastasis group (P=0.370) (Figure 4C). Furthermore, the Chinese Criteria grouping (P<0.001; HR =1.303, 95% CI: 1.221–1.390) was demonstrated as the independent prognostic factors for all thoracic ESCC patients after adjuvant therapy.
Figure 4.
Prognosis of Chinese Criteria grouping in all thoracic ESCC patients, upper and lower mediastinal ESCC patients after adjuvant therapy. (A) In all patients (after adjuvant therapy), the OS rate in the mediastinal metastasis group was significantly lower than that in the local metastasis group (P=0.002). The OS rate in the local metastasis group tended to be lower than that in the skip metastasis group, the difference was not significant (P=0.140). The survival difference between the skip and no metastasis groups was significant (P=0.021). (B) In upper mediastinum (after adjuvant therapy), no significant prognostic differences were found among four groups. (C) In lower mediastinum (after adjuvant therapy), the OS rate in the mediastinal metastasis group was significantly lower than in the local metastasis group (P=0.008), meanwhile, the survival difference between the skip and no metastasis groups was significant (P=0.012). However, no significant difference of prognosis was found between skip and local metastasis group (P=0.370). ESCC, esophageal squamous cell carcinoma; OS, overall survival.
Discussion
The indications, approaches, the number, and the extent of thoracic LN metastasis dissection in radical esophagectomy are still being debated. Some scholars hold the opinion that the radical lymphadenectomy performs well in restricting tumor recurrence and eliminating the micro-metastatic involvement in the LNs, and is also good at prolonging the OS time of esophageal cancer patients (14-16); however, some differ, because they believe that radical lymphadenectomy contributes to a high morbidity postoperatively (17-19). The current grouping criteria for thoracic LN in esophageal cancer are currently documented in the UICC/AJCC 8th TNM manual (10,11), wherein the N-stage is based on the number of metastatic LNs, and the JES criteria (12), where both the number and the extent of metastatic LNs need to be taken into consideration in the N-stage. The UICC/AJCC 8th TNM manual is easily incorporated into practice and was confirmed with the association of OS (8,20). However, the JES criteria are rarely used in China because of the preference for the relatively sophisticated sets of N-staging. In fact, radical lymphadenectomy for esophageal cancer patients in China has not been unified regardless of the regions of China and the grade of the hospital. In considering the fundamental situation of China, combining the UICC/AJCC 8th TNM manual and the JES criteria, the Chinese Society of Esophageal Cancer first proposed the Chinese grouping criteria for thoracic LN in esophageal cancer. On the one hand, the Chinese grouping criteria simplified the grouping of thoracic LNs, for example, we removed groups of 4L, 5, 6, 10L, and 10R, and amalgamated groups of 9L and 9R into one single LN group (C208). However, we divided the thoracic LNs into two mediastinal compartments: the upper and the lower mediastinum with the boundary of subcarina; and either the thoracic LNs or the location of thoracic esophageal cancer, which were mutually parallel with the classification, were partitioned. In these regard, the Chinese grouping criteria differ from the other two criteria from which the new pattern of thoracic LN metastasis are consequently derived. Thus, the prognostic significance and role of the new thoracic LN metastasis pattern in esophageal cancer became our focus.
According to the Chinese Criteria grouping, the new patterns of thoracic LN metastasis of esophageal cancer were classified into four groups: no, skip, local, and mediastinal metastasis groups.
In our study, the 2,285 thoracic ESCC patients without cervical or abdominal LN metastasis were classified into these four groups, and more patients were males, and had advanced T stages and lower thoracic esophageal cancer. Except for no metastasis group, earlier N stage was seen in skip and local metastasis groups while the mediastinal metastasis group didn’t show that. Most of the results for the four groups were similar to that reported in other studies (21,22); however, the T stage result in the skip metastasis group was different. By virtue of the abundant submucosal lymphatic vessel communication in esophagus, the cancer cells spread from the mucosal lymphatic ducts to drain into a rich submucosal plexus, and then spread longitudinally through this dense lymphatic network (23). Therefore, this unique pattern of LN metastasis contributes to the metastatic spread to neighboring LNs from the esophageal cancer to any segments of the esophagus. The skip metastasis therefore occurred not only in thoracic esophageal cancer, but also in the cervical and abdominal esophageal cancers. Secondly, owing to the study on thoracic LN metastasis, the definition of skip metastasis in our study did not include the LN metastasis to the cervical and abdominal lymphatic stations, but was confined to the skip metastasis in the thorax. Furthermore, the earlier part of the esophagus is equipped with the bilateral vascular supply and lymphatic drainage system; however, the separation at the level of tracheal bifurcation delimits the joining of the two separate lymphatic drainage system during the embryonic and fetal development (24). Therefore, to this end, this results in the diversity of the patterns of LN metastasis in different segments of the esophagus despite their intensive lymphatic vessel communication. Therefore, the chances of thoracic LN metastasis to either upper or lower thoracic esophageal cancer are similar. This finally results in the different T stages in our study when compared with other studies.
The present study demonstrated that the thoracic LN metastasis according to the grouping of Chinese Criteria could be used to stratify the patients based on different prognosis. For example, the four thoracic LN metastasis groups referring to the Chinese Criteria could be regarded as the classification of the number of fields with LN metastasis. Different numbers of fields with LN metastasis, as reported in many studies, is a convenient and better index in evaluating the prognosis of patients with esophageal cancer (21,25,26). The fields included the neck, chest, and abdomen; in our study, the skip and local metastasis groups could be considered to be the field with LN metastasis in the thoracic ESCC while the mediastinal metastasis could be regarded as the two fields with LN metastasis in the thoracic ESCC. Obviously, significant differences occur in prognosis between the one field and the two fields, as confirmed in previous studies (20-22). Moreover, the prognostic difference in skip metastasis has been assessed in several studies in which the positive impact on survival was well observed, when compared with the local and mediastinal LN metastasis (23,27), which confirmed the result in our study. Meanwhile, the Chinese Criteria grouping as well as the T-stage, N-stage, and age were identified as independent prognostic factors of thoracic ESCC patients in our present study, which indicates that the Chinese Criteria grouping play the analogous function as N-stage does in thoracic ESCC patients.
According to Skandalakis et al. (24), the joining of two separate lymphatic drainage systems was delimited by the tracheal bifurcation during the embryonic and fetal developments, as well as in adulthood. The classification of thoracic LNs with the boundary of subcarina according to the Chinese Criteria suits the two separated lymphatic drainage systems anatomically. Furthermore, the tumor location parallel to the newly classified thoracic LNs should be defined once more. In our study, at the subcarina level, the thoracic esophagus was divided into two parts: upper and lower thoracic esophagus. Based on the logistic regression, none of the other clinicopathological characteristics in the thoracic ESCC patients was associated with the tumor location except for tumor differentiation, age, and Chinese Criteria grouping. Meanwhile, the difference in prognosis in thoracic ESCC patients in these different groups was obviously distinguished both in the upper and lower thoracic esophagus. Moreover, the Chinese Criteria grouping, and the T and N stages were identified as the independent prognostic factors in upper and lower esophagus. Therefore, the role of Chinese Criteria grouping in directing the prognosis of the thoracic ESCC patients was confirmed once again as with the N stage in UICC/AJCC 8th TNM (10,11) and in JES criteria (12).
As shown above, the N-stage and the Chinese Criteria grouping are two criteria reflecting the thoracic LN status and evaluating the esophageal cancer patients’ prognosis. Moreover, the relationship between the two criteria was examined in the present study. On the one hand, with the increase in the number of metastatic thoracic LNs, the prognostic difference in the thoracic ESCC patients were always stratified into the local and mediastinal metastasis groups, except with the skip metastasis group. The explanation of this result might be interpreted as follows: first, with the small amount of thoracic ESCC patients in the skip metastasis group, the prognostic difference could not be easily stratified among N1, N2, and N3 stages when compared with other groups. Secondly, the different LN classification systems used in various studies led to the diverse results as well. On the other hand, with the N stage, the most favorable and the worst prognosis of the thoracic ESCC patients were always found in the skip and mediastinal metastasis groups, respectively, whereas the prognostic difference in each group in the different N stages were not statistically significant. Several studies have reported that the number and the ratio of LN were independent prognostic factors in patients with esophageal cancer (28-30). In the present study, the LN ratio in the skip metastasis group was lower in N1, N2, and N3 stages. Long et al. (31) once described that the development of the skip metastasis might attribute to the proliferative potential of metastatic tumor cells mediated by adhesion molecules and growth factors. Therefore, as several studies have reported (23,32) that the lower ratio of the metastatic LNs in the skip metastasis group contributes to the positive influence on survival when compared to the continuous LN metastasis, which, based on our speculation, leads to a better prognosis among the thoracic ESCC patients in the skip metastasis group in N1, N2, and N3 stages, consequently. Similar studies on esophageal cancer were not available, thus far.
There are also some limitations to our study. First, because the newly issued Chinese Criteria were mainly focused on the thoracic LN dissection in the radical resection of esophageal cancer, the present study excluded patients with the cervical and abdominal esophageal cancer; however, the skip metastasis group in this study was restricted to the thoracic ESCC; thus, selection bias was inevitable. Secondly, some studies that reported LN micrometastases drew our attention (27,33). Hosch et al. (27) demonstrated that lymphatic micrometastases, detected by immunohistochemistry, were an independent prognostic factor of esophageal cancer, with higher detection ratio, higher precision, and positive prognosis when compared with those detected through histopathology. Nevertheless, we have hardly developed an assessment of LN micrometastases in clinic work, and the feasibility of this approach required evaluation in our hospital. Thirdly, it is common in China for the Sweet esophagectomy to be widely conducted in areas with high incidence of esophageal cancer because of its lower morbidity and shorter operation time (3). In the present study, some patients also accepted the Sweet approach; however, the disadvantage of this was the incomplete lymphadenectomy, especially for the C02 node in the Chinese Criteria (34). Obviously, for those reasons, we could not negate the current N-stage based on the UICC/AJCC 8th TNM; however, the Chinese Criteria grouping was demonstrated as another schema to measure the degree of LN metastasis, which played almost the same role as N-stage in our study. A multicenter randomized controlled trial is required to confirm our results.
Conclusions
In conclusion, the present results demonstrated that when compared with the N-stage based on the UICC/AJCC 8th TNM, the Chinese Criteria grouping played almost the same function in measuring the degree of LN metastasis in EC as N-stage did. The Chinese Criteria grouping significantly stratified the patients with different prognosis, and it was confirmed as an independent prognostic factor of thoracic ESCC in patients with esophageal cancer. Further confirmation is warranted.
Acknowledgments
The authors thank Department of Pathology of West China Hospital, Sichuan University, China for the substantial work in diagnosing and examining the dissected tissues and lymph nodes pathologically.
Funding: This study was supported by the Key Innovation Team of Shanxi 1331 Project approved by Shanxi Education Department and Shanxi Finance Department (2107 #12).
Ethical Statement: The study was approved by the human participants committee of West China Hospital of Sichuan University. Preoperatively, permission for the use of patients resected specimens and written informed consents were obtained. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Nakagawa S, Kanda T, Kosugi S, et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004;198:205-11. 10.1016/j.jamcollsurg.2003.10.005 [DOI] [PubMed] [Google Scholar]
- 2.Daly JM, Fry WA, Little AG, et al. Esophageal cancer: results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg 2000;190:562-72. 10.1016/S1072-7515(00)00238-6 [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zhang Z, et al. Analysis of the incidence and mortality of malignant tumors in China in 2012. Chinese Cancer 2016;25:1-8. [Google Scholar]
- 4.Pingyu M, Zhang Y, Du X, et al. Proceedings of the First International Conference on Esophageal Cancer and the Seventh National Conference on Esophageal Cancer 2005;2005:167-71. [Google Scholar]
- 5.Shao L, Gao Z, Xu J, et al. Surgical treatment of esophageal and cardiac cancer: a summary of 15707 cases with an overview of prevention and treatment of esophageal cancer in Henan Province. Proceedings of the First International Conference on Esophageal Cancer and the Seventh National Conference on Esophageal Cancer 2005;2005:40-5. [Google Scholar]
- 6.Kato H, Fukuchi M, Miyazaki T, et al. Surgical treatment for esophageal cancer. Current issues. Dig Surg 2007;24:88-95. 10.1159/000101894 [DOI] [PubMed] [Google Scholar]
- 7.Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72. 10.1097/00000658-199409000-00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991;48:411-20. 10.1159/000226971 [DOI] [PubMed] [Google Scholar]
- 9.Li H, Fang W, Yu Z, et al. Chinese expert consensus on mediastinal lymph node dissection in esophagectomy for esophageal cancer (2017 edition). J Thorac Dis 2018;10:2481-9. 10.21037/jtd.2018.03.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amin MB, Edge S, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer, 2017:185-202. [Google Scholar]
- 11.Amin MB. The 2009 version of the cancer protocols of the college of American pathologists. Arch Pathol Lab Med 2010;134:326-30. [DOI] [PubMed] [Google Scholar]
- 12.Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu Z, Yu W, Li H, et al. Nodal skip metastasis is not a predictor of survival in thoracic esophageal squamous cell carcinoma. Ann Surg Oncol 2013;20:3052-8. 10.1245/s10434-013-2987-5 [DOI] [PubMed] [Google Scholar]
- 14.Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. 10.1007/s10388-015-0515-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;347:1662-9. 10.1056/NEJMoa022343 [DOI] [PubMed] [Google Scholar]
- 16.Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007;246:992-1000; discussion 1000-1. 10.1097/SLA.0b013e31815c4037 [DOI] [PubMed] [Google Scholar]
- 17.Davies AR, Sandhu H, Pillai A, et al. Surgical resection strategy and the influence of radicality on outcomes in oesophageal cancer. Br J Surg 2014;101:511-7. 10.1002/bjs.9456 [DOI] [PubMed] [Google Scholar]
- 18.Khullar OV, Jiang R, Force SD, et al. Transthoracic versus transhiatal resection for esophageal adenocarcinoma of the lower esophagus: A value-based comparison. J Surg Oncol 2015;112:517-23. 10.1002/jso.24024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ovrebo KK, Lie SA, Laerum OD, et al. Long-term survival from adenocarcinoma of the esophagus after transthoracic and transhiatal esophagectomy. World J Surg Oncol 2012;10:130. 10.1186/1477-7819-10-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Udagawa H, Ueno M, Shinohara H, et al. The importance of grouping of lymph node stations and rationale of three-field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol 2012;106:742-7. 10.1002/jso.23122 [DOI] [PubMed] [Google Scholar]
- 21.Xu QR, Zhuge XP, Zhang HL, et al. The N-classification for esophageal cancer staging: should it be based on number, distance, or extent of the lymph node metastasis? World J Surg 2011;35:1303-10. 10.1007/s00268-011-1015-9 [DOI] [PubMed] [Google Scholar]
- 22.Fujita H, Kakegawa T, Yamana H, et al. Lymph node metastasis and recurrence in patients with a carcinoma of the thoracic esophagus who underwent three-field dissection. World J Surg 1994;18:266-72. 10.1007/BF00294412 [DOI] [PubMed] [Google Scholar]
- 23.Prenzel K.L., Bollschweiler E, Schröder W, et al. Prognostic relevance of skip metastases in esophageal cancer. Ann Thorac Surg 2010;90:1662-7. 10.1016/j.athoracsur.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 24.Skandalakis JE, Ellis H. Embryologic and anatomic basis of esophageal surgery. Surg Clin North Am 2000;80:85-155. 10.1016/S0039-6109(05)70399-6 [DOI] [PubMed] [Google Scholar]
- 25.An FS, Huang JQ, Chen SH. Analysis of lymph node metastases of 217 cases of thoracic esophageal carcinoma and its impact on prognosis. Ai Zheng 2003;22:974-7. [PubMed] [Google Scholar]
- 26.Shimada H, Okazumi SI, Matsubara H, et al. Impact of the number and extent of positive lymph nodes in 200 patients with thoracic esophageal squamous cell carcinoma after three-field lymph node dissection. World J Surg 2006;30:1441-9. 10.1007/s00268-005-0462-6 [DOI] [PubMed] [Google Scholar]
- 27.Hosch SB, Stoecklein NH, Pichlmeier U, et al. Esophageal cancer: the mode of lymphatic tumor cell spread and its prognostic significance. J Clin Oncol 2001;19:1970-5. 10.1200/JCO.2001.19.7.1970 [DOI] [PubMed] [Google Scholar]
- 28.Wilson M, Rosato EL, Chojnacki KA, et al. Prognostic significance of lymph node metastases and ratio in esophageal cancer. J Surg Res 2008;146:11-5. 10.1016/j.jss.2007.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. 10.1097/SLA.0b013e31815aaadf [DOI] [PubMed] [Google Scholar]
- 30.Gu Y, Swisher SG, Ajani JA, et al. The number of lymph nodes with metastasis predicts survival in patients with esophageal or esophagogastric junction adenocarcinoma who receive preoperative chemoradiation. Cancer 2006;106:1017-25. 10.1002/cncr.21693 [DOI] [PubMed] [Google Scholar]
- 31.Long L, Rubin R, Brodt P. Enhanced invasion and liver colonization by lung carcinoma cells overexpressing the type 1 insulin-like growth factor receptor. Exp Cell Res 1998;238:116-21. 10.1006/excr.1997.3814 [DOI] [PubMed] [Google Scholar]
- 32.Riquet M, Assouad J, Bagan P, et al. Skip mediastinal lymph node metastasis and lung cancer: a particular N2 subgroup with a better prognosis. Ann Thorac Surg 2005;79:225-33. 10.1016/j.athoracsur.2004.06.081 [DOI] [PubMed] [Google Scholar]
- 33.Izbicki JR, Hosch SB, Pichlmeier U, et al. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med 1997;337:1188-94. 10.1056/NEJM199710233371702 [DOI] [PubMed] [Google Scholar]
- 34.Adachi W, Koike S, Nimura Y, et al. Clinicopathologic characteristics and postoperative outcome in Japanese and Chinese patients with thoracic esophageal cancer. World J Surg 1996;20:332-6. 10.1007/s002689900053 [DOI] [PubMed] [Google Scholar]