Abstract
Background
The activation and polarization of macrophages are crucial during the pathogenesis of liver injury induced by the toxin. Human amniotic mesenchymal stromal cells (hAMSCs) are newly identified mesenchymal stem cells and have been shown to have an immunoregulatory ability for multiple autoimmune diseases.
Methods
Mice were intraperitoneally injected with Acetaminophen (APAP) to establish a liver injury model. hAMSCs were injected through the tail vein, and the liver function was observed through a liver function and pathology analysis. To test the regulative ability of hAMSCs in vitro, the supernatant of hAMSCs were collected and co-cultured with Kupffer cells (KCs). Liposome was used to abolish the function of KCs in vivo.
Results
Infusion of hAMSCs reduced the level of liver function injury and inflammation expression in APAP-induced liver injury. hAMSCs markedly promoted M2 polarization of KCs instead of M1 polarization in vitro. Furthermore, the mechanism study also proved that hAMSCs reduced autophagy, as revealed by down-regulated LC3B-II levels. The elimination of KCs in vivo abolished the protective ability of hAMSCs in liver injury, which resulted in a significant increase of liver pathogenesis along with an increase in alanine aminotransaminase (ALT) and aspartate aminotransaminase (AST) levels.
Conclusions
Our results proved that hAMSCs suppressed M1 polarization and promoted M2 polarization of KCs through regulating autophagy in the model of APAP-treated livers. Thus, the injury of the liver was attenuated. This study provides us a new therapeutic strategy for the disease of acute liver injury.
Keywords: Liver injury, Acetaminophen (APAP), human amniotic mesenchymal stromal cells (hAMSCs), Kupffer cell (KC), polarization
Introduction
Mesenchymal stem or stromal (MSC) cells are immunomodulatory cells for numerous immune cell types (1) and protect against the various murine models of autoimmune disease, including colitis (2), autoimmune arthritis, (3) and graft versus host disease (4). Human term placenta has attracted interest as it’s the superior rate in cell yielding and rarely express human leukocyte antigens or co-stimulatory molecules. Human amniotic mesenchymal stromal cells (hAMSCs) derived from term placenta have now been realized as a valuable candidate to be the source of therapeutic MSC generation. Studies have shown that hAMSCs also present a robust protective ability against immune cells.
The most essential function of the Liver is to metabolize the drugs or other toxic compounds, which make itself viable target organ for toxicity. Macrophages in the liver [Kupffer cells, (KCs)] had been identified to play a core role during the pathogenesis of toxin-induced liver injury (5). Ju reported that KCs settled down in the liver and maintained the homeostasis of liver in different liver disease (6). KCs also release cytokines and chemokines to attract other inflammatory cells, which amplificated inflammatory signal (7).
Macrophages can be mainly divided into two cell types, a pro-inflammatory type (M1) and an anti-inflammatory type (M2). M1 macrophage can be controlled by the phosphorylation of STAT1 and IRF5, while the activation of IRF4 and PPARγ regulated the polarization of M2 macrophage (8). Recent studies had defined that autophagy controlled the function and polarization in macrophages (9). The autophagy of macrophage increases liver injury by accelerating M1 polarization (10,11).
Given the findings above, we investigated the function of hAMSCs in regulating liver injury and inflammation as well as the cross-talk between hAMSCs and KC autophagy and polarization.
Methods
Animals
C57BL/6J male mice (8 weeks old) were obtained from the Animal Resources Center, Nanjing Medical University. The mice were housed with standard rodent diet and water provision. Relevant legal and ethical requirements were followed carefully according to the protocol (number NMU08-092, which was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Isolation and enrichment of amnion-derived MSCs
hAMSCs were isolated, as described previously (12). Briefly, the amnion was manually separated, washed, and cut into small pieces. Trypsin and collagenase I was used to digesting the minced amnion. Finally, the harvested cells were cultured under culture medium composed of DMEM medium with heat-inactivated fetal bovine serum (FBS, 10%), with the addition of penicillin (100 U/mL), streptomycin (100 g/mL), and l-glutamine (Gibco, 2 mM) at 37 °C with 5% CO2. The cell passages between passage 3 to passage 7 were used for this study.
APAP-induced acute liver injury
For the establishment of liver injury model, C57BL/6J mice were intraperitoneally injected with Acetaminophen (APAP) (Sigma, Saint Louis, MO, USA, 400 mg/Kg). Mice from the control group received PBS via intraperitoneal injection. After 24 hours, the mice were sacrificed while the tissue, as well as the serum, were collected. In some experiments, mice were pretreated with liposome (Clodronate liposomes, Haarlem, The Netherlands) to eliminate the KCs (200 µL per mouse, i.v.) before APAP administration.
Liver function analysis
Murine serum samples were collected, centrifuged, and analyzed with automated chemical analyzer (Olympus Company, Tokyo, Japan) to test the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Immunohistochemical staining and histopathology
Liver tissue sections obtained from the model (4 µm) were stained with hematoxylin-eosin and observe through light microscopy. Rat anti-mouse F4/80, CD11b, and Ly6G mAb (BD Biosciences, San Jose, CA, USA) were used to label and evaluate the macrophages and neutrophils in the liver. Cells were counted blindly in 10 HPF/section.
KCs isolation and cell culture
KCs were isolated and cultured, as described previously (13). Briefly, Mouse livers were perfused via the portal vein with HBSS and collagenase IV. Then, the perfused livers were dissected and teased, followed by suspension in culture media for 15 min at 37 °C. Then we removed the non-adherent cells and collected the adherent cells for further in vitro experiments.
Western blots and quantitative reverse transcription-polymerase chain reaction
Proteins were extracted from with ice-cold lysis buffer, and then the protein was subjected to 10% SDS-PAGE and transferred to polyvinylidene difluoride nitrocellulose membrane. Abs against LC3B and β-actin (Cell Signaling Technology, MA, USA) were used.
Total RNA was extracted from the frozen liver tissues and cells using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA) and was reverse-transcribed into cDNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Indianapolis, IN, USA). Quantitative real-time PCR was performed using SYBR green (Roche, Indianapolis, IN, USA). The expression levels of target genes and the results were normalized against GAPDH expression.
ELISA
The expression of Cytokines such as TNF-α, IL-6, IL-10, CXCL-10 in the serum was measured by ELISA according to the manufacturer's protocol (eBioscience, San Diego, CA, USA).
Immunofluorescence staining
Rat anti-mouse F4/80, CD11b (BD Biosciences, San Jose, CA, USA), Ly6G, iNOS, and CD206 mAb (Cell Signaling Technology, MA, USA) were applicated to identify the macrophages and neutrophils. Goat anti-mouse Texas Red-conjugated IgG (Sigma, St. Louis, MO, USA) were incubated with VECTASHIELD medium with DAPI (Vector). Positive cells were blindly observed in 10 HPF/section (200×).
Statistical analysis
Results are shown as the mean ± SEM. All analyses were performed using Stata software (version 11.0). P values less than 0.05 (two-tailed) was considered statistically significant.
Results
hAMSCs attenuate acute liver injury induced by APAP
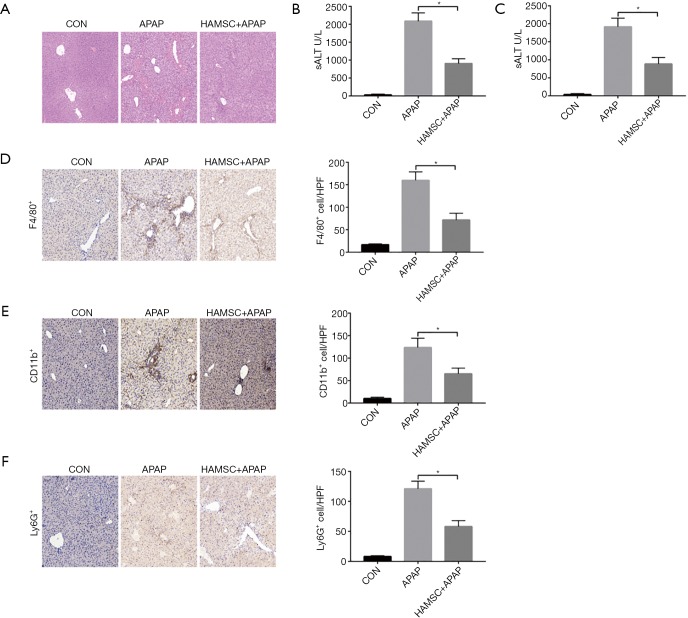
Firstly, we evaluated the level of liver injury induced by APAP with or without hAMSCs. 5×106 hAMSCs was transferred on the same day while APAP (500 mg/kg) were injected intraperitoneally into B6 mice. Adoptive infusion with hAMSCs attenuated APAP-induced acute liver injury significantly comparing with APAP group (Figure 1A), in the meanwhile, lower serum ALT and AST levels were detected in the hAMSCs group (Figure 1B,C). Thus, hAMSC treatment ameliorates APAP-induced acute liver injury.
Figure 1.
hAMSC pretreatment attenuates APAP-induced acute liver injury and reduces innate immune cell activation in APAP-treated livers. Liver injury was evaluated in terms of liver histopathology (A), serum ALT (B), and AST (C). F4/80+ macrophage (D), CD11b+ macrophage (E), and Ly6G+ neutrophil (F) infiltration in the liver was detected by immunohistochemical staining. *, P<0.05, n=6 for each group. hAMSC, human amniotic mesenchymal stromal cell; APAP, Acetaminophen; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Innate immune responses were reduced by hAMSCs in APAP-exposed livers
Next, we evaluated the regulative function of hAMSCs in macrophage and neutrophil infiltration in vivo. We found that hAMSCs reduced intrahepatic macrophages (F4/80+) in the APAP + hAMSCs group comparing with control group or APAP group (Figure 1D). Besides, hAMSCs also significantly downregulated CD11b+ infiltrating macrophages or Ly6G+ neutrophils in APAP + hAMSCs treated livers (Figure 1E,F).
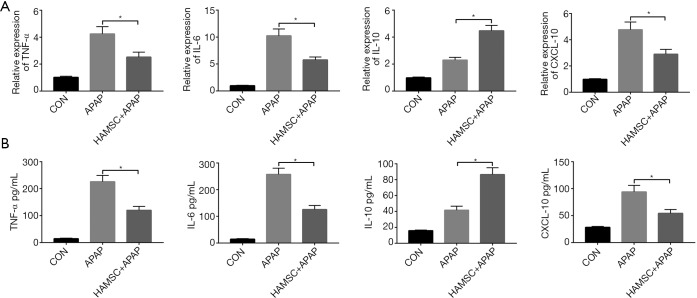
Previous data had already proved that the activation of innate immune responses participated in the pathogenesis of APAP-induced acute liver injury (14). We examined the gene induction of cytokine or chemokine for different groups by qRT-PCR. As shown in Figure 2A, APAP treatment significantly increased TNF-α, IL-6, IL-10, and CXCL-10 compared with the control group. Meanwhile, co-infusion of hAMSCs presented significantly lower TNF-α, IL-6, and CXCL-10, but much higher IL-10 gene induction comparing with APAP group. Furthermore, we evaluated the expression of TNF-α, IL-6, and CXCL-10 in the serum, which is parallel to the qRT-PCR result (Figure 2B).
Figure 2.
Innate immune responses were reduced by hAMSCs in APAP-Exposed Livers. Inflammatory gene (A) and cytokine (B) expression were measured from liver tissue or serum by quantitative RT-PCR and ELISA. *, P<0.05. Data are mean ± SEM from 5 independent experiments. *, P<0.05, n=6 for each group. hAMSC, human amniotic mesenchymal stromal cell; APAP, Acetaminophen; RT-PCR, reverse transcription-polymerase chain reaction.
hAMSCs regulate KCs M1/M2 polarization in response to APAP treatment through autophagy
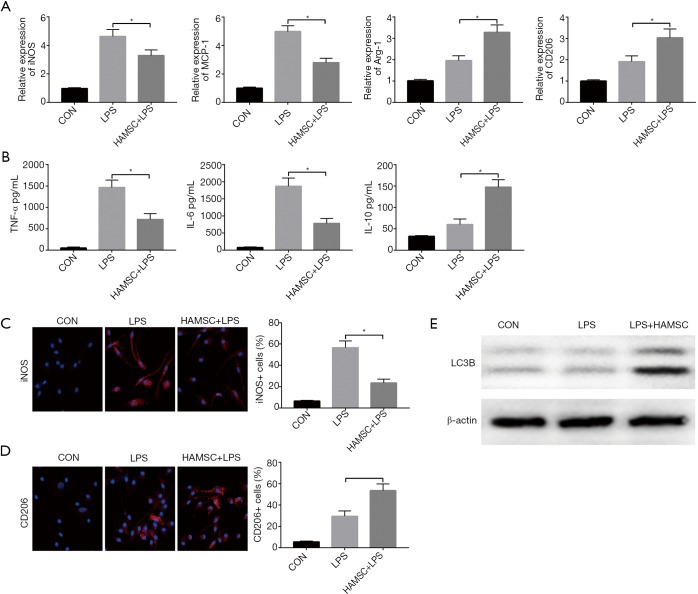
As Macrophages can be classified into M1 (classical) and M2 (alternative) subtypes (15), therefore, we identified the regulative effect of hAMSCs for M1/M2 polarization. KCs isolated from B6 mice were plated and cultured in vitro with the absence of LPS. Indeed, after 6 h, KCs stimulated with LPS exhibited a higher induction of M1 markers (iNOS and MCP-1) but a lower level of M2 markers (Arg-1 and CD206) compared with the KCs-alone group. Interestingly, pretreatment with the supernatant of hAMSCs suppressed the expression of iNOS and MCP-1 while improving the expression of Arg-1 and CD206 (Figure 3A). We next analyzed levels of TNF-α, IL-6, and IL-10 protein during KC culturing. KCs were treated with supernatant for 6 h; then the cells were washed and cultured in standard culture medium for 24 h, while the expression of multiple cytokines was evaluated through ELISA. As shown in Figure 3B, hAMSCs reduced the expression of pro-inflammatory cytokines such as TNF-α and IL-6, while increasing that of IL-10. We also tested the polarization of KCs through immunofluorescence staining, which also proved that hAMSCs reduced the iNOS+ M1 cells and enriched CD206+ M2 cells. These data indicate that hAMSCs preferentially polarized into the M2-like phenotype (Figure 3C,D).
Figure 3.
hAMSCs regulate KC M1/M2 polarization in response to APAP treatment through autophagy. (A) KCs were treated with hAMSCs supernatant for 6 hours, the level of gene induction of iNOS, MCP-1, Arg-1, and CD206 were analyzed by qRT-PCR; (B) the cytokine expression of the KCs’ supernatant was measured by ELISA. Representative figures of immunofluorescence staining of iNOS (C) and CD206 (D) in KCs; (E) representative figure of the expression of LC3B in different groups as analyzed by Western Blot. Data are mean ± SEM from 5 independent experiments, and each experiment was repeated four times. *, P<0.05, n=6 for each group. hAMSC, human amniotic mesenchymal stromal cell; APAP, Acetaminophen; KC, Kupffer cell; qRT-PCR, quantitative reverse transcription-polymerase chain reaction.
Growing evidence illustrates that the polarization of KCs can be regulated through the autophagy pathway (16,17). Based on these findings, we examined whether hAMSCs affect KC autophagy. KCs were pretreated with the supernatant of hAMSCs, and autophagy marker was analyzed by western blot. As shown in Figure 3E, the LC3B-II level was increased in the LPS-treated group but decreased in the LPS + hAMSC KCs, indicating that hAMSCs regulated the polarization of KCs through autophagy-related pathways.
hAMSCs protect APAP-induced acute liver injury through regulating KCs in vivo
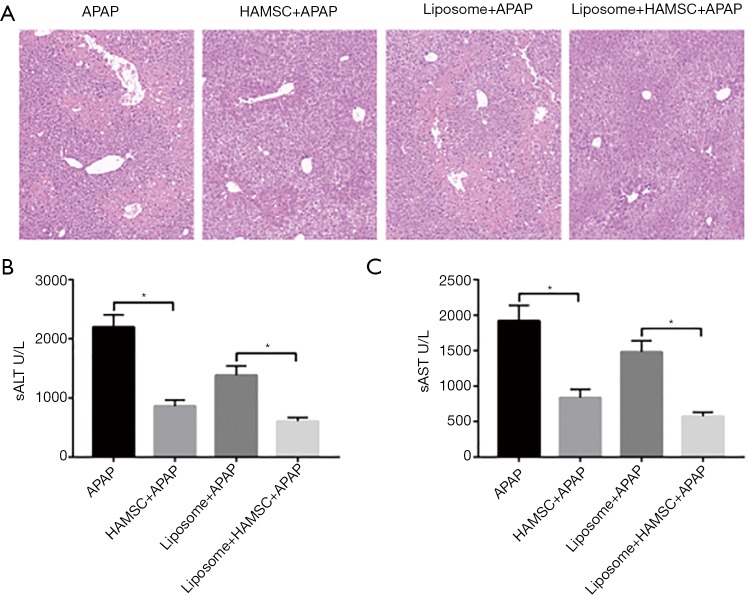
To evaluate whether the effects of hAMSCs on the protective effect of APAP-induced acute liver injury is dependent on controlling KCs, liposome was used to eliminate the function of KCs (18). Liposome was transferred one day before hAMSC transfer and liver injury model establishment. Compared with the APAP group, pretreatment with hAMSCs significantly attenuated APAP-induced acute liver injury (Figure 4A), as demonstrated by lower serum ALT and AST (Figure 4B). However, injection of liposome partly abolished the protective effect of hAMSCs, while the level expression of ALT and AST increased with the addition of liposome.
Figure 4.
hAMSCs protect against APAP-induced acute liver injury through regulating KCs in vivo. Mice were administrated with hAMSC and APAP administration as described previously. Liposome was added through intraperitoneal injection. Liver injury was evaluated in terms of liver histopathology (A), serum ALT, and AST (B). Data are shown as mean ± SEM from 3 independent experiments. *, P<0.05, n=6 for each group. hAMSC, human amniotic mesenchymal stromal cell; APAP, Acetaminophen; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
As studies had proved that the activity and function of MSCs declined with increasing age of the sources (19), researchers had begun to investigate the potential therapeutic efficacy of the hAMSCs which was isolated from the term human amnion due to their MSC-like characteristics. The reported advantages of hAMSCs include higher cell harvest at the beginning, low donor site morbidity, more “youthful” phenotype, compatibility for use in allogeneic transplants, and powerful immunomodulatory properties compared with hBMSCs and hADSCs (20-22). Research has proven that hAMSCs protect multiple disease models, including chronic kidney disease (23), T1 diabetes (24), and smoke-induced lung injury (25). However, no study has yet revealed the function of hAMSCs in APAP-induced liver injury.
The results of the present study revealed that pretreatment with hAMSCs attenuates acute liver injury through adjusting KCs related innate immune inflammation. Although the immune regulative ability of hAMSCs in inflammatory diseases has been preliminarily revealed, its role in acute liver injury remains to be elucidated. Our study showed that hAMSCs inhibited M1 polarization but promoted M2 polarization, thereby attenuated intrahepatic inflammation and alleviated hepatocellular injury in APAP-treated livers. The mechanism study also revealed that hAMSCs regulate KC autophagy. Additionally, we eliminated the macrophage using liposome and tested the role of KCs in vivo, which showed that hAMSCs protected APAP-induced acute liver injury partly via regulating KC cells. This work is the first study to demonstrate hAMSCs regulate KCs polarization via induction of autophagy in the acute liver injury model.
Kupffer cells are liver-resident macrophages which reside in the hepatic sinusoid and serve as important defender against various liver diseases and injuries. M1/M2 polarization of KCs regulated the pathogenesis of liver disease or injuries, Rao proved that hyperglycemia aggravates hepatic ischemia and reperfusion injury by inducing M2 polarization (26). Autophagy is a conserved catabolic process in which cellular components are finally degraded in lysosomes (9). Evidence had shown that autophagy regulated of M1/M2 polarization, but the effect remains not clear. In this study, we proved that hAMSC induced M2 but not M1 polarization of KCs in APAP-treated groups. We first measured the protein expression related to autophagy of LC3B in KCs by western blot, which proved that hAMSCs significantly induces KC autophagy in APAP-treated groups compared with other groups.
In conclusion, the present result proved that hAMSCs markedly protected acute liver injury induced by APAP. Mechanism study indicated hAMSC-regulated autophagy of KCs and promoted M2 polarization. Our findings demonstrated that hAMSCs would be considered a potent candidate for acute liver injury treatment.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animals received humane care, and all animal procedures met the relevant legal and ethical requirements according to a protocol (number NMU08-092) approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Sivanathan KN, Coates PT. Bone Marrow-Derived Progenitor Cells Mediate Immune Cell Regulation. Methods Mol Biol 2019;2029:215-34. 10.1007/978-1-4939-9631-5_17 [DOI] [PubMed] [Google Scholar]
- 2.Kawata Y, Tsuchiya A, Seino S, et al. Early injection of human adipose tissue-derived mesenchymal stem cell after inflammation ameliorates dextran sulfate sodium-induced colitis in mice through the induction of M2 macrophages and regulatory T cells. Cell Tissue Res 2019;376:257-71. 10.1007/s00441-018-02981-w [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Li Q, Zhu J, et al. Comparison of therapeutic effects of different mesenchymal stem cells on rheumatoid arthritis in mice. PeerJ 2019;7:e7023. 10.7717/peerj.7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krasowska-Kwiecien A, Gozdzik J, Jarocha D, et al. Mesenchymal Stem Cells as a Salvage Treatment for Severe Refractory Graft-vs-Host Disease in Children After Bone Marrow Transplantation. Transplant Proc 2019;51:880-9. 10.1016/j.transproceed.2019.01.023 [DOI] [PubMed] [Google Scholar]
- 5.Zhao E, Ilyas G, Cingolani F, et al. Pentamidine blocks hepatotoxic injury in mice. Hepatology 2017;66:922-35. 10.1002/hep.29244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ju C, Tacke F. Hepatic macrophages in homeostasis and liver diseases: from pathogenesis to novel therapeutic strategies. Cell Mol Immunol 2016;13:316-27. 10.1038/cmi.2015.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald B, Kubes P. Innate Immune Cell Trafficking and Function During Sterile Inflammation of the Liver. Gastroenterology 2016;151:1087-95. 10.1053/j.gastro.2016.09.048 [DOI] [PubMed] [Google Scholar]
- 8.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 2011;11:750-61. 10.1038/nri3088 [DOI] [PubMed] [Google Scholar]
- 9.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science 2000;290:1717-21. 10.1126/science.290.5497.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu K, Zhao E, Ilyas G, et al. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 2015;11:271-84. 10.1080/15548627.2015.1009787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakahira K, Haspel JA, Rathinam VA, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol 2011;12:222-30. 10.1038/ni.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D, Jiang M, Miao D. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One 2011;6:e16789. 10.1371/journal.pone.0016789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou S, Gu J, Liu R, et al. Spermine Alleviates Acute Liver Injury by Inhibiting Liver-Resident Macrophage Pro-Inflammatory Response Through ATG5-Dependent Autophagy. Front Immunol 2018;9:948. 10.3389/fimmu.2018.00948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amanzada A, Moriconi F, Mansuroglu T, et al. Induction of chemokines and cytokines before neutrophils and macrophage recruitment in different regions of rat liver after TAA administration. Lab Invest 2014;94:235-47. 10.1038/labinvest.2013.134 [DOI] [PubMed] [Google Scholar]
- 15.Byles V, Covarrubias AJ, Ben-Sahra I, et al. The TSC-mTOR pathway regulates macrophage polarization. Nat Commun 2013;4:2834. 10.1038/ncomms3834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hai Y, Shinsky SA, Porter NJ, et al. Histone deacetylase 10 structure and molecular function as a polyamine deacetylase. Nat Commun 2017;8:15368. 10.1038/ncomms15368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prosser AC, Kallies A, Lucas M. Tissue-resident Lymphocytes in Solid Organ Transplantation: Innocent Passengers or the key to Organ Transplant Survival? Transplantation 2018;102:378-86. [DOI] [PubMed] [Google Scholar]
- 18.Wu LL, Peng WH, Wu HL, et al. Lymphocyte Antigen 6 Complex, Locus C+ Monocytes and Kupffer Cells Orchestrate Liver Immune Responses Against Hepatitis B Virus in Mice. Hepatology 2019;69:2364-80. [DOI] [PubMed] [Google Scholar]
- 19.Nejadnik H, Hui JH, Feng Choong EP, et al. Autologous bone marrow-derived mesenchymal stem cells versus autologous chondrocyte implantation: an observational cohort study. Am J Sports Med 2010;38:1110-6. 10.1177/0363546509359067 [DOI] [PubMed] [Google Scholar]
- 20.Keeley R, Topoluk N, Mercuri J. Tissues reborn: fetal membrane-derived matrices and stem cells in orthopedic regenerative medicine. Crit Rev Biomed Eng 2014;42:249-70. 10.1615/CritRevBiomedEng.2014011591 [DOI] [PubMed] [Google Scholar]
- 21.Miki T. Amnion-derived stem cells: in quest of clinical applications. Stem Cell Res Ther 2011;2:25. 10.1186/scrt66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parolini O, Alviano F, Bagnara GP, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008;26:300-11. 10.1634/stemcells.2007-0594 [DOI] [PubMed] [Google Scholar]
- 23.Cetinkaya B, Unek G, Kipmen-Korgun D, et al. Effects of Human Placental Amnion Derived Mesenchymal Stem Cells on Proliferation and Apoptosis Mechanisms in Chronic Kidney Disease in the Rat. Int J Stem Cells 2019;12:151-61. 10.15283/ijsc18067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R, Zhang D, Zhang T, et al. The differentiation of human MSCs derived from adipose and amniotic tissues into insulin-producing cells, induced by PEI@Fe3O4 nanoparticles-mediated NRSF and SHH silencing. Int J Mol Med 2018;42:2831-8. [DOI] [PubMed] [Google Scholar]
- 25.Cui P, Xin H, Yao Y, et al. Human amnion-derived mesenchymal stem cells alleviate lung injury induced by white smoke inhalation in rats. Stem Cell Res Ther 2018;9:101. 10.1186/s13287-018-0856-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao Z, Sun J, Pan X, et al. Hyperglycemia Aggravates Hepatic Ischemia and Reperfusion Injury by Inhibiting Liver-Resident Macrophage M2 Polarization via C/EBP Homologous Protein-Mediated Endoplasmic Reticulum Stress. Front Immunol 2017;8:1299. 10.3389/fimmu.2017.01299 [DOI] [PMC free article] [PubMed] [Google Scholar]