Abstract
The present study reports the role of morphological, physiological and reproductive attributes viz. membrane stability index (MSI), osmolytes accumulations, antioxidants activities and pollen germination for heat stress tolerance in contrasting genotypes. Heat stress increased proline and glycine betaine (GPX) contents, induced superoxide dismutase (SOD), ascorbate peroxidase (APX) and glutathione peroxidase (GPX) activities and resulted in higher MSI in PDL-2 (tolerant) compared to JL-3 (sensitive). In vitro pollen germination of tolerant genotype was higher than sensitive one under heat stress. In vivo stressed pollens of tolerant genotype germinated well on stressed stigma of sensitive genotype, while stressed pollens of sensitive genotype did not germinate on stressed stigma of tolerant genotype. De novo transcriptome analysis of both the genotypes showed that number of contigs ranged from 90,267 to 104,424 for all the samples with N50 ranging from 1,755 to 1,844 bp under heat stress and control conditions. Based on assembled unigenes, 194,178 high-quality Single Nucleotide Polymorphisms (SNPs), 141,050 microsatellites and 7,388 Insertion-deletions (Indels) were detected. Expression of 10 genes was evaluated using quantitative Real Time Polymerase Chain Reaction (RT-qPCR). Comparison of differentially expressed genes (DEGs) under different combinations of heat stress has led to the identification of candidate DEGs and pathways. Changes in expression of physiological and pollen phenotyping related genes were also reaffirmed through transcriptome data. Cell wall and secondary metabolite pathways are found to be majorly affected under heat stress. The findings need further analysis to determine genetic mechanism involved in heat tolerance of lentil.
Subject terms: Agricultural genetics, Heat
Introduction
Lentil (Lens culinaris Medikus) is grown in relatively dry ecology where yield is mainly affected by various abiotic stresses. Increased maximum and minimum growth temperature can result in heat stress in plants. Heat stress can accelerate plant maturity and have detrimental effects on productivity of lentil1. Optimum temperature for growth of lentil ranges between 18–30 °C2. Temperature ≥30 °C can affect plant growth and development adversely and may lead to yield reduction. Kumari et al. showed that seed yield reduced by 38–58 per cent at 33/28 °C3. Heat stress may result in scorching and sunburn of leaves, accelerating leaf senescence; inhibition of root and shoot growth and seedling survival1. It also reduces membrane stability index (MSI), which reflects leaching of ions due to plasma membrane injury under heat stress. Further, heat stress hinders the photochemical activity of photosystem II (PS-II) which results in imbalance between photosynthesis and respiratory processes. Generation of reactive oxygen species (ROS) due to heat stress leads to oxidative damage of plant cells unless it is scavenged by enzymes such as superoxide dismutases (SOD), catalases (CAT) and peroxidases4,5. In addition, osmoprotactant solutes such as proline, glycine betaine and sugars gets accumulated due to activation of various complex regulatory networks6. Most of these traits at seedling stage have been strongly correlated with reproductive stage leading to significant losses in seed yield1,7,8.
Heat stress induces several signal transduction pathways which are inter connected at cellular level9,10. Plant’s survival under heat stress depends on perception, generation and transmission of stress signals along with initiation of required physiological and biochemical responses. Heat tolerance mechanism employs production of compatible solutes for ion transportation, osmo-protection and to maintain cellular structures. At molecular level, heat stress induces expression of genes and production of defence proteins, antioxidants and synthesis of metabolites along with transcriptional controls which are activated to reduce stress alterations11,12. Cytosolic protein misfolding acts as important thermo sensor especially during lenient temperature increase where protein injury is certain. Also, fluidity sensitive Ca2+ channels in plasma membrane are equally responsible. A transient Ca2+ influx was observed in several plants within few minutes of rise in temperature13. Specific CAMs and MAP kinases are associated in downstream events transferring the heat signals from membrane to transcription factors14. Expression of CaM3 and CaM7 under heat stress has been known in Arabidopsis15,16. Members of the family of heat shock transcription factors (HSFs) act as transcriptional regulators that plays a vital role in certain heat signalling and are found to be involved in regulation of thousands of downstream targets17,18. HSFs activated due to heat stress recognize and bind conserved DNA sequences called heat shock elements in HSP promoters19. Activated HSFs displace histones from HSP genes and results in plant acquired thermo-tolerance. Timely expression of HSPs under heat stress is crucial for plants to obtain thermo-tolerance20. Cytoskeleton is also involved in heat stress and cause regulation of HSP expression. Thermo tolerance is also regulated by plant hormones such as salicylic acid and abscisic acid21.
Information on classification of various stress-responsive genes or gene families is insufficient in non-model legumes like lentil, pigeonpea, mungbean etc. under abiotic stress conditions. Apparently, function of candidate genes in response to drought stress has been identified in lentil7. Identification of molecular markers i.e. simple sequence repeats (SSRs) and single nucleotide polymorphisms (SNPs), is a leading target of functional genomic studies. These markers are highly polymorphic, co-dominant, convenient, highly reproducible, stable and authentic. Therefore, identification of such markers in heat stress contrasting genotypes will significantly enrich the recently developed reference genomic pool of lentil i.e. knowpulse.usask.ca.
Illumina platform, a short-read technology has been widely applied to generate large scale genome and transcriptome data in grain legumes such as lentil, chickpea and soybean under various abiotic stress conditions7,22,23. However, no published record is available on transcriptome data in response to heat stress in lentil. Therefore, objectives of this study were to (i) identify differentially expressed genes (DEGs) in heat stressed genotypes, (ii) identify role of candidate genes associated with heat tolerance and (iii) identify SSR, SNP and Insertion-deletion (Indel) markers in heat tolerant and sensitive genotypes. This study will provide a new guide for heat stress tolerance at molecular level which paves the way for improvement of commercial lentil genotypes under heat stress utilizing various breeding and biotechnological strategies. Also, newly identified markers can be utilized in molecular and comparative studies in other legumes as well.
Material and Methods
Material
Two contrasting genotypes viz. PDL-2 and JL-3, (tolerant and sensitive to heat stress) were selected for genome wide differential expression of transcripts in response to heat stress. PDL-2 is a breeding line (ILL-590 × ILL-7663) received from ICARDA- Syria. JL-3 is a landrace and was obtained from Sagar, Madhya Pradesh, India. Both the genotypes were found to be morpho-physiologically and genetically distinct from each other in response to drought and heat stress conditions in earlier studies1,24.
Methods
Evaluation of genotypes for heat tolerance under hydroponic condition
Genotypes were evaluated under heat stress using hydroponic conditions as per protocol of Singh et al. with little modifications1. After surface sterilization using 1% sodium hypochlorite, seeds were germinated on filter paper. Seven days old plants were shifted to nutrient medium having composition as described by Simon et al.25. Growth chamber was maintained for relative humidity (%), temperature of 55.7/68.2% ± 2, 27/16 °C and 50.6/57.2% ± 2, 35/33 °C during day/night and with a photoperiod of 10/14 h to mimic control and heat stress conditions, respectively1. Duration of heat stress was 3 h daily for 3 d, whereas control conditions remained unaltered. Completely randomised design comprising 3 replicates for each genotype and each replicate having 12 seedlings was followed.
Physiological analysis for heat stress tolerance
Leaves from seven days old seedlings were collected from control and stress treated plants for evaluation of physiological parameters viz. membrane stability index (MSI), glycine betaine content, proline content, antioxidants activities [SOD, peroxidase (POD), glutathione peroxidase (GPX) and ascorbate peroxidase (APX)] and lipid peroxidation using methods as described by Singh et al.7.
Analysis of in vitro and in vivo pollen germination for heat stress tolerance
Pollen germination was done following Singh et al.1. Hand pollinations were performed to examine pollen germination and tube growth during in vivo germination. Five flowers were collected after 30 min of incubation and were pollinated to observe germination as well as pollen tube growth on stigma. Each flower was considered as a replication. Each stigma (flower) was pollinated with pollen grains from a particular flower. Flowers were fixed in 80% alcohol for 24 h. Pistils (styles and ovary) were separated, cleared with 6N NaOH for 48 h and gently washed with water. Fluorescent dye aniline blue (0.1%) was used for staining pistils which were observed under fluorescent microscope (Zeiss AXIOSKOP 2)26.
Two cross combinations using stressed stigma (35/20 °C) of sensitive genotype (JL-3) x stressed pollen (35/20 °C) of tolerant genotype (PDL-1); non-stressed stigma of tolerant genotype (PDL-1) x stressed pollen (35/20 °C) of sensitive genotype (JL-3) were made to determine site of sensitivity (pollen or stigma) for heat stress. Five flowers were pollinated in each cross combination and each genotype was exposed to 35/20 °C and 27/16 °C (day/night) conditions.
Ribonucleic acid (RNA) extraction and complementary deoxyribonucleic acid (cDNA) library preparation
Three biological replicates from each of tolerant and sensitive genotypes under control and heat stress conditions were sampled. Each replicate consisted of 12 seedlings which were pooled together (Additional File 1. Fig. S1). Leaf samples were frozen in liquid nitrogen and preserved at −80 °C and were used for RNA extraction for Illumina library construction and quantitative real time polymerase chain reaction (RT-qPCR). TRIZOL reagent (Takara, Japan) was used for extraction of total RNA from control and treated leaf samples followed by RNA integrity test using Bioanalyzer. Library preparation for all the samples was accomplished using TruseqTM RNA sample prep Kit (Illumina, Inc. San Diego, CA, USA). Enriched messenger RNA (mRNA) was sheared into short fragments with the help of magnetic beads containing poly-T molecules. Two hundred base pair (bp) size inserts were selected for post cDNA preparation using Illumina TruSeqTM mRNA Library preparation kit following standard producer’s protocol. Illumina adapters were then ligated to these fragments after their ends were repaired. Clusters were generated and sequencing was done on Illumina HiSeq 2000 platform to generate 2 × 100 bp paired end reads.
De novo assembly of short reads
FastXTool kit was used to clean low quality reads (phred quality below 20). Quality of reads pre and post filtering was analyzed using FASTQC tool. Quality filtered reads were taken for de novo assembly using Trinity denovo assembler. De novo contigs were produced by assembling clean paired end reads. Unigenes i.e. non-redundant transcripts were obtained from those contigs which could not be further extended on either end following redundancy removal process using sequence clustering software. Bowtie software mapped the clean reads back to the assembled transcripts. Flow chart depicting the bioinformatics analysis of this study is represented in Additional File 2. Fig. S2.
Annotation and classification of gene function
Transcripts were annotated using Basic Local Alignment Search Tool (BLAST) program (E value < 1.0E−5) with the help of National Centre for Biotechnology Information (NCBI) non-redundant (nr), Kyoto Encyclopedia of Genes and Genomes (KEGG), Cluster of Orthologous Groups (COG), Swiss-Protand UniProt Reference Clusters (UNIREF) databases. Gene Ontology (GO) terms for characterization of unigene depicting nr annotation results was accomplished by Annocript program followed by construction of GO tree using Web Gene Ontology Annotation Plot (WEGO) tool. Further, functional classification of unigenes was deduced using COG database. KEGG pathway database was used to predict different pathways for all the unigenes. Representation of DEGs involved in tolerant vs sensitive genotypes under heat stress and control conditions was done using various plots like HeatMap, Volcano Plot and Circos. MapMan (v3.51R2) was used for understanding roles of DEGs in different combination sets.
SNP, SSR, Indel filtering and calling
SAMtools mpileup and custom scripts were used to identify SNPs and to call variants on the basis of read depth. Read depth of minimum 10, filtered the heterozygous loci as well as false positive SNPs. Genome Analysis Tool Kit (GATK) was used for SNP calling using haplotype caller command version 3.6–0, where all the parameters were set as default. For SSRs, MIcro SAtellite identification (MISA) software deduced SSRs from high quality filtered reads aligned to the contigs. Primer3 software was assisted to design primers where parameters set for designing the primers were 15 bp for minimum, 21 bp for maximum and 18 bp for optimal primer size. Product sizes ranged between 100 to 300 bp from these SSRs.
Validation of genic SSRs (g-SSRs)
Fifty five g-SSRs were selected and developed to screen 96 genotypes that included cultivars, breeding lines, landraces and wild accessions (Additional File 16. Table S1). These markers were selected on the basis of minimum SSR length of 8mers and GC content < 33.3% (Additional File 17.Table S2). PCR conditions for amplification were similar to Singh et al.1.
Validation using RT-qPCR
RNA isolated from the samples using Trizol reagent was used for RT-qPCR. One Step SYBR PrimeScript RT-PCR Kit II (Takara Biotechnology Co. Ltd, Dalian, Japan) was done for validation of 10 gene specific primers though RT-qPCR following the manufacturer’s instructions. Reaction volume (10 µl) contained 100 ng of total RNA normalized for all the samples using CFX96 Touch RT-PCR detection system (Bio-Rad, Hercules). Details of 10 primers used in RT-PCR is presented in Additional File 18. Table S3. To calculate relative expression, 2−∆∆Ct method for target gene expression was used. Beta-tubulin reference gene was taken as internal expression gene to normalize expressed data.
Results
Phenotypic responses under heat stress
All plants of both the genotypes were found healthy under optimal 27/16 °C (day/night) condition. However, visible differences were found between genotypes at 35/33 °C after continuous exposure for 3 d. Effects of heat stress were initially reflected on the leaves of sensitive genotype after 3 d heat exposure as compared to tolerant one. Level of damage such as leaf dropping, stem lodging and tip burning appeared higher in JL-3 (sensitive) as compared to PDL-2 (tolerant) under heat stress condition (Fig. 1) (Additional File 3. Fig. S3).
Figure 1.
Plant exposed to heat stress (35/33 °C) and control (27/16 °C) in JL-3 (sensitive) and PDL- 2 (tolerant) genotypes at seedling stage in growth chamber under hydroponic conditions.
Physiological and biochemical traits in response to heat stress
Antioxidant enzymes activity
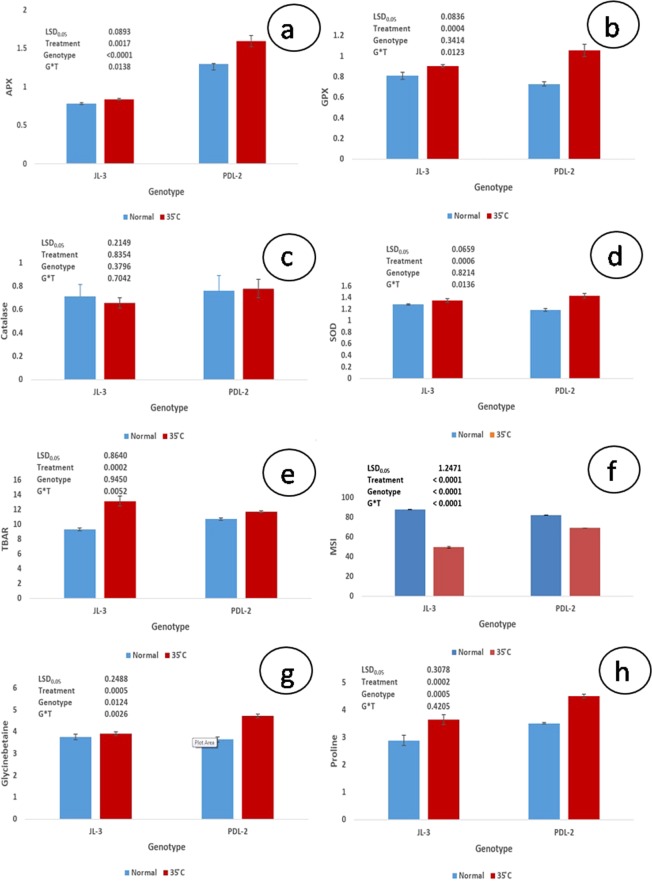
Activities of all the antioxidant enzymes such as APX, GPX and SOD in shoots were significantly higher in response to heat stress conditions than control in both the genotypes (Fig. 2a,b,d). However, CAT activity decreased or was found to be almost unaltered under heat stress in comparison to the control (Fig. 2c).
Figure 2.
Changes in APX (µmol min−1 g−1fr.wt.) (a), GPX (µmol min−1 g−1fr.wt.) (b), CAT (µmol min−1 g−1fr.wt.) (c), SOD (unit min−1 g−1fr.wt.) (d), TBARS (µmol g−1fr.wt.) (lipid peroxidation) (e), MSI (%) (f) Glycine betane content (µmol g−1fr.wt.) (g), Proline content (µmol g−1fr.wt.) (h), of heat tolerant (PDL-2) and sensitive (JL-3) lentil genotypes under control and heat stress.
Lipid peroxidation
Lipid peroxidation level in leaves of both the genotypes, estimated in terms of malondialdehyde (MDA) content, is presented in Fig. 2e. MDA concentration in tolerant and sensitive genotypes was higher than control at 35/33 °C and particularly in sensitive (JL-3) genotype as compared tolerant (PDL-2) genotype exposed to heat stress environment.
Membrane stability index
MSI reduced under heat stress condition for both tolerant and sensitive genotypes. However, tolerant genotype showed higher MSI than sensitive one under heat stress condition (Fig. 2f).
Glycine betaine content
Significant effect of heat stress was observed on glycine betaine content in both the genotypes. In tolerant genotype PDL-2, accumulation of glycine betaine was significantly higher as compared to JL-3 under heat stress (Fig. 2g).
Proline content
Proline accumulated in both the genotypes in response to heat stress (Fig. 2h), where significantly higher content was accumulated in PDL-2 (p < 0.05) than JL-3. Proline concentration increased rapidly as temperature rose from 27/16 °C to 35/33 °C in tolerant genotype.
In vitro and in vivo pollen germination
Pollen germination reduced by 12.1% in PDL-2 (tolerant) at 34.5/23.4 °C. However, JL-3 (sensitive) showed higher reduction (33.2%) at the same level of heat stress (Additional File 4. Fig. S4 and Additional File 5. Fig. S5). In vivo, pollen germination was recorded in heat sensitive (JL-3) as well as heat tolerant (PDL-2) genotypes after 30 min of hand pollination under heat stress. Stressed pollens collected from PDL-2, germinated on stressed stigma of JL-3 (Additional File 6. Fig. S6) but collected stressed pollens from JL-3 did not germinate on stressed stigma of PDL-2 (Additional File 7. Fig. S7).
Transcriptome Assembly
Illumina HiSeq 2000 Raw Fastq files were used for de novo assembly using Trinity de novo assembler software. Minimum number of contigs obtained was 91,926 in Tolerant Treated Rep2 while maximum number of contigs viz. 1,04,424 were obtained in Tolerant Control Rep-3. Smallest contig length filter was applied and only contigs having minimum size of 500 bp were taken. Statistics of assembly and coverage of individual samples as well as details of all the transcripts within different combinations are presented in Table 1, Additional File 19. Table S4 and Additional File 22. Excel sheet. respectively.
Table 1.
Total raw data generated through transcriptome assembly.
| Properties | Total Coverage (Gb) | Number of reads generated | Total contigs | Smallest contig length | Longest contig length | Average contig length | N80 | N50 | N20 |
|---|---|---|---|---|---|---|---|---|---|
| Tolerant- Treated_1 | 6.1 | 22536460 | 93705 | 500 | 16470 | 1437 | 985 | 1770 | 3005 |
| Tolerant- Treated _2 | 5.9 | 24355081 | 91926 | 500 | 16818 | 1426 | 978 | 1766 | 3006 |
| Tolerant- Treated_3 | 5.7 | 23604075 | 90267 | 500 | 15500 | 1423 | 980 | 1760 | 2978 |
| Sensitive-Treated_1 | 6.3 | 26210135 | 101161 | 500 | 16626 | 1465 | 991 | 1796 | 3092 |
| Sensitive-Treated_2 | 6.1 | 22541528 | 99477 | 500 | 16182 | 1463 | 993 | 1797 | 3088 |
| Sensitive-Treated_3 | 5.8 | 23960480 | 97231 | 500 | 16550 | 1444 | 983 | 1781 | 3047 |
| Tolerant-Control_1 | 6.4 | 26685338 | 101480 | 500 | 15597 | 1456 | 1024 | 1844 | 3060 |
| Tolerant-Control_2 | 6.0 | 24935410 | 98301 | 500 | 15365 | 1423 | 1015 | 1822 | 3038 |
| Tolerant-Control_3 | 6.3 | 28521319 | 104424 | 500 | 15858 | 1458 | 1026 | 1832 | 3059 |
| Sensitive-Control_1 | 7.3 | 30210135 | 95978 | 500 | 15764 | 1523 | 990 | 1793 | 3033 |
| Sensitive-Control_2 | 6.8 | 27960145 | 93061 | 500 | 16470 | 1516 | 980 | 1755 | 2965 |
| Sensitive-Control_3 | 7.8 | 32460168 | 94874 | 500 | 15764 | 1524 | 997 | 1795 | 3056 |
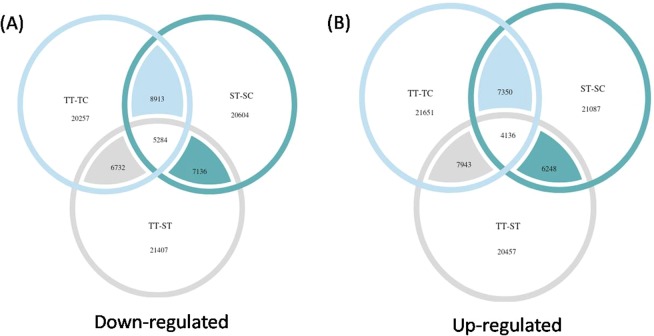
Differential gene expression analysis was performed between three sets of contrasting datasets, viz - tolerant treated vs tolerant control, tolerant treated vs sensitive treated, sensitive treated vs sensitive control using EdgeR software with default parameters. Wherein, TMM normalization was carried out for each set of comparison, total number of up regulated and down regulated genes in all the three comparison groups were quite similar. However, the number of significant DEGs (FDR value < 0.05) were lesser in tolerant treated vs tolerant control than in rest of the groups. Significantly expressed genes and their relative expression levels were compared across all the 3 comparison groups to find out the expression pattern of genes upon heat stress exposure in different genotypes. DEGs for different combinations of tolerant and sensitive genotypes in response to heat stress environment is summarised in Fig. 3. Output of the DEGs were analysed between the samples to assess the correlation using PCA (Additional File 8. Fig. S8).
Figure 3.
Venn diagram showing overall expression of (A) down-regulated (B) up-regulated DEGs in combination tolerant treated vs. tolerant control (TT-TC), sensitive treated vs. sensitive control (ST-SC) and tolerant treated vs. sensitive treated (TT-ST).
Identifications of SSR, SNP and Indel markers
MISA and GATK software were used for identification of SSR and SNP markers, respectively. On an average within 3 replicates of each samples, sensitive control had highest number i.e.14,986 SSRs followed by sensitive-treated (10,960), tolerant-control (10,836) and tolerant-treated (10,233) (Additional File 20. Table 5). P1 (Mono-Nucleotide) type SSRs were abundant in all the samples followed by P2 (Di-Nucleotide), P3 (Tri-Nucleotide), P4 (Tetra-Nucleotide), P5 (Penta-Nucleotide) and P6 (Hexa-Nucleotide). Penta- and hexa-nucleotide types showed similar range of SSRs. Further, average number of SSRs in different samples for tri-nucleotide type ranged from 3,132 to 3,992.
Average SNPs found within the replicates of samples were ranked from tolerant control having highest number of SNPs (17,224) followed by sensitive treated (17,167), tolerant treated (17,110) and sensitive control (13,225) along with number of Indels which ranged from 552 to 683 in all the samples (Additional File 20. Table 5).
Functional annotation of DEGs for plot hierarchical heat map
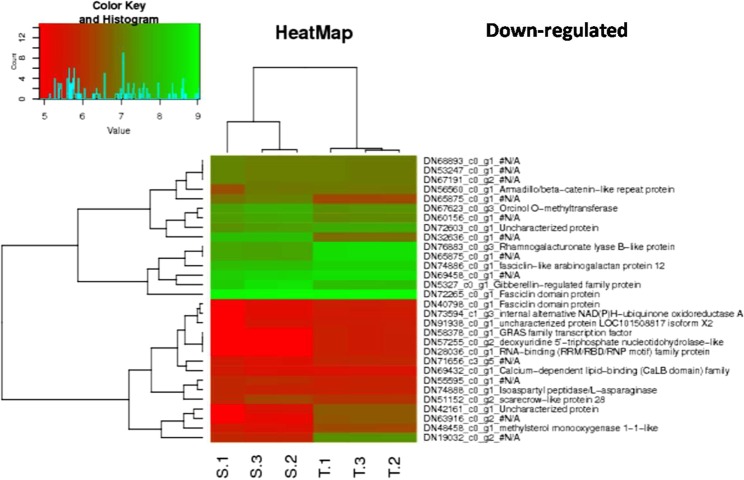
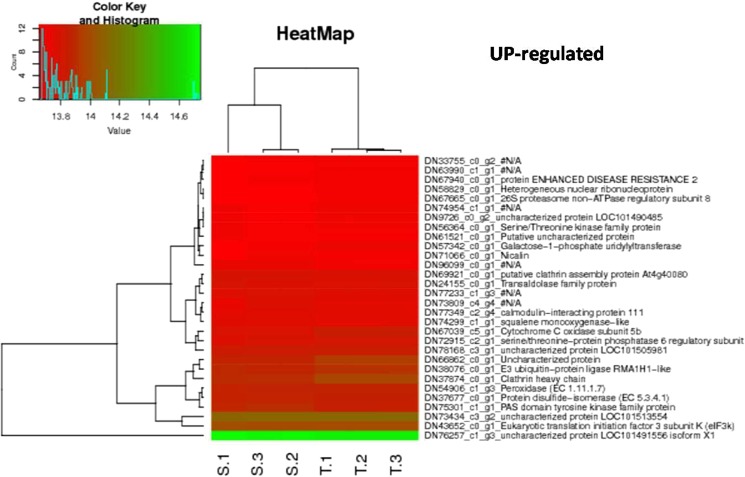
Top 30 up regulated and down regulated heat related DEGs were selected for all the major combinations as represented in Figs 4 and 5, Additional File 9. Fig. S9 and Additional File 10. Fig. S10.
Figure 4.
HeatMap depicting 30 down-regulated DEGs with p value < 0.05 in combination tolerant treated vs sensitive treated.
Figure 5.
HeatMap depicting 30 up-regulated DEGs with p value < 0.05 in combination tolerant treated vs sensitive treated.
In combination tolerant treated vs sensitive treated, highest log FC for up and down regulated DEGs were 9.55 and −10.59, respectively. DN68776_c2_g3, DN19596_c0_g1 and DN81013_c1_g8 were top 3 down regulated DEGs for which, first two did not match to any of the known databases and thus can be called as novel transcripts whereas, third one was annotated as Malate dehydrogenase mitochondrial gene. For upregulated DEGs, DN57816_c0_g1, DN41180_c0_g4, DN60393_c0_g4 were top 3 contigs, which were annotated as Lon protease homolog 2 peroxisomal, Probable acyl-activating enzyme 5 peroxisomal and Uncharacterized protein sll0005, respectively (Table 2). Nine hundred twenty DEGs were up regulated above log FC ≥ 3, whereas only 358 DEGs were down regulated in the combination tolerant treated- sensitive treated.
Table 2.
Top 20 up regulated DEGs for combination tolerant treated-sensitive treated using EdgeR.
| ID | LogFC | LogCPM | PValue | FDR | Description |
|---|---|---|---|---|---|
| TRINITY_DN57816_c0_g1 | 9.55 | 1.21 | 1E-59 | 4.4E-58 | Lon protease homolog 2 peroxisomal |
| TRINITY_DN41180_c0_g4 | 9.37 | 1.12 | 4.11E-56 | 1.7E-54 | Probable acyl-activating enzyme 5 peroxisomal |
| TRINITY_DN60393_c0_g4 | 9.26 | 0.94 | 8.2E-38 | 2.3E-36 | Uncharacterized protein sll0005 |
| TRINITY_DN10789_c0_g2 | 8.77 | 0.48 | 4.25E-28 | 9.1E-27 | F-box protein At3g12350 |
| TRINITY_DN81025_c0_g3 | 8.11 | −0.12 | 1.34E-18 | 2.0E-17 | — |
| TRINITY_DN29122_c0_g2 | 7.64 | −0.45 | 2.16E-18 | 3.2E-17 | Carotene epsilon-monooxygenase chloroplastic |
| TRINITY_DN61834_c0_g4 | 7.62 | −0.53 | 7.9E-14 | 9.1E-13 | Cinnamoyl-CoA reductase-like SNL6 |
| TRINITY_DN70907_c0_g1 | 7.47 | −0.63 | 1.78E-15 | 2.2E-14 | Retrovirus-related Pol polyprotein from transposon TNT 1–94 |
| TRINITY_DN62156_c0_g2 | 7.41 | −0.71 | 6.36E-12 | 6.5E-11 | Zinc finger protein JACKDAW |
| TRINITY_DN73377_c1_g1 | 7.37 | 2.08 | 1.1E-109 | 9.3E-108 | Linoleate 13S-lipoxygenase 2-1 chloroplastic |
| TRINITY_DN71700_c1_g1 | 7.34 | 4.29 | 0 | 0.0E + 00 | #N/A |
| TRINITY_DN47566_c0_g1 | 7.10 | −0.92 | 1.82E-12 | 1.9E-11 | — |
| TRINITY_DN36767_c0_g1 | 7.06 | −0.99 | 1.98E-09 | 1.7E-08 | — |
| TRINITY_DN59987_c0_g4 | 6.82 | −1.10 | 6.36E-12 | 6.5E-11 | UDP-glycosyltransferase 73C6 |
| TRINITY_DN73578_c1_g1 | 6.69 | 3.82 | 0 | 0.0E + 00 | Delta-1-pyrroline-5-carboxylate synthase |
| TRINITY_DN33495_c0_g1 | 6.67 | −1.18 | 3.38E-12 | 3.5E-11 | — |
| TRINITY_DN58346_c0_g2 | 6.60 | −1.31 | 7.45E-09 | 6.0E-08 | — |
| TRINITY_DN32466_c0_g2 | 6.60 | 3.62 | 6E-296 | 2.0E-293 | Umecyanin |
| TRINITY_DN55283_c0_g2 | 6.58 | 2.22 | 6.4E-120 | 6.0E-118 | Outer membrane protein Omp38 |
| TRINITY_DN59842_c0_g2 | 6.55 | −1.37 | 4.77E-07 | 3.2E-06 | Phenylethylamine oxidase |
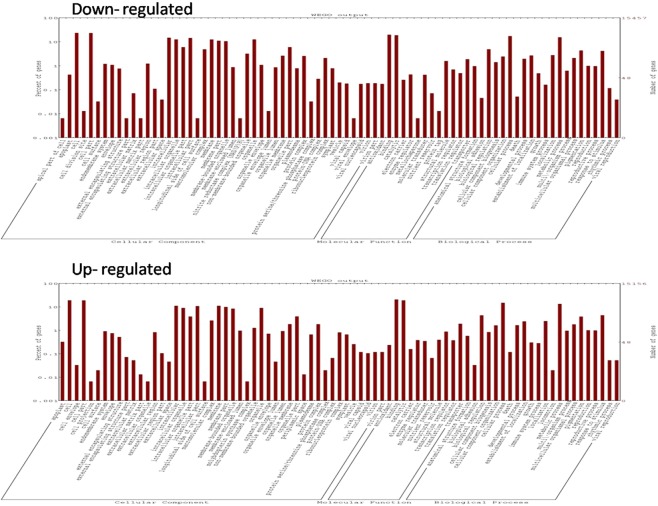
Upregulated and downregulated GO annotations were distributed into three categories viz. biological process, cellular component and molecular functions which were represented by WEGO plots. Upregulated and downregulated GO annotations for the combination tolerant treated vs sensitive treated revealed 15,156 and 15,457 genes, respectively (Fig. 6). Similarly, in tolerant treated vs tolerant control and sensitive treated vs sensitive control comparison groups, a total of 13,508;16,807 and 16,262;15,335 up regulated and down regulated genes were identified, respectively (Additional File 11. Fig. S11 and Additional File 12. Fig. S12). Cellular category had highest components in addition to number of genes per components followed by biological process and molecular functions.
Figure 6.
Wego plot for upregulated and down regulated GO classification in accordance to GO groups: molecular function, biological process and cellular component in combination tolerant treated vs sensitive treated under heat stress.
Among all the GO terms for cellular component, maximum count of genes were associated in apoplast (GO:0048046) and cell division site (GO:0032153) in tolerant treated vs sensitive treated comparison group. These were found to be down regulated in this comparison group, whereas GO terms viz. cell (GO:0005623) and cell parts (GO:0044464) had highest number of genes which were upregulated. For molecular function GO terms, binding (GO:0005488) and catalytic (GO:0003824) which were up regulated as well as down regulated in different combinations, showed highest number of genes and for biological process, cellular process (GO:0009987) and metabolic process (GO:0008152) had highest set of genes for different combinations (Fig. 6).
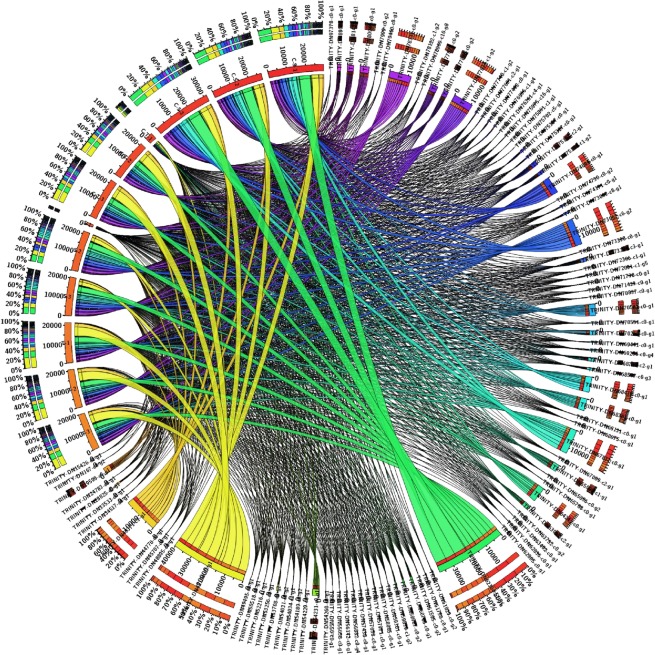
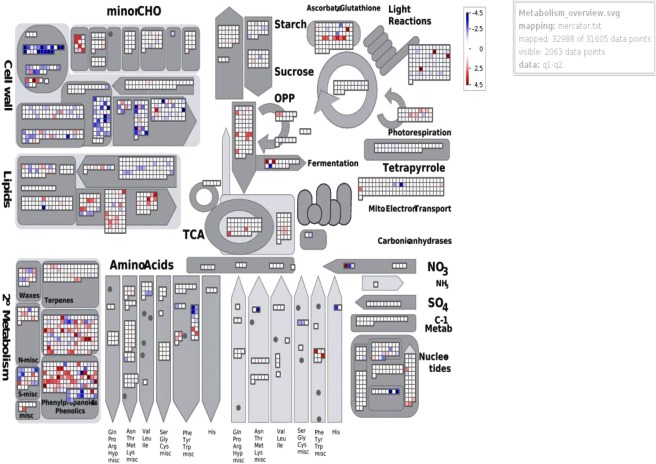
Circos plot was generated showing association of upregulated and downregulated DEGs with tolerant and sensitive genotypes under control and stressed conditions (Fig. 7). MapMan (MapManVersion 3.6.0RC1) represented overall views of metabolic pathways involved under heat stress for different combination (Fig. 8, Additional File 13. Fig. S13 and Additional File 14. Fig. S14). For the combination tolerant treated vs sensitive treated, MapMan showed involvement of nearly all the pathways. Among the major pathways, cell wall and secondary metabolite had highest number of BINs which represented the functional categories to which genes were assigned (Fig. 8).
Figure 7.
Circular plot depicting DEGs involved in tolerant vs sensitive genotypes under heat stress was plotted using Circos version 0.62. Tabular data representing genotypes in columns & DEGs values in rows has been plotted in this circular plot. Rows are represented by circularly arranged segments (outermost ring), whose length is proportional to the total cell values in a row and columns are represented by circularly arranged segments (inner ring) whose length is proportional to the total cell values in a column. Ribbons represent row & column IDs. The 3 outer rings are stacked bar plots that represent relative contribution of a cell to row and column totals. Expression value is expressed in colours with red colour representing lowest value & purple colour representing highest values.
Figure 8.
MapMan display for tolerant vs sensitive under heat stress. Up regulated genes are expressed in increased intense red while down regulated as blue at the amplitude of 4.5 to −4.5 (log2-value).
Validation of g-SSRs
All 55 g-SSRs gave amplification in 96 genotypes. Among which, 18 markers were found to be polymorphic. Primer 10 had highest number of allele, genetic diversity as well as Polymorphism Information Content (PIC) (Additional File 15. Fig. S15). Major allelic frequency, PIC, number of alleles, genetic diversity and heterozygosity were deduced using power marker (Additional File 21. Table S6).
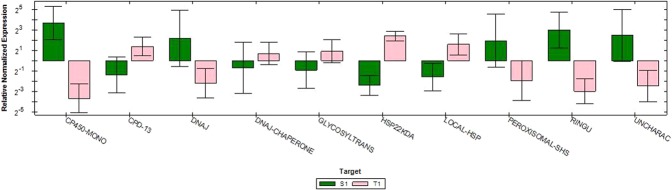
Validation of DEGs through RT-qPCR
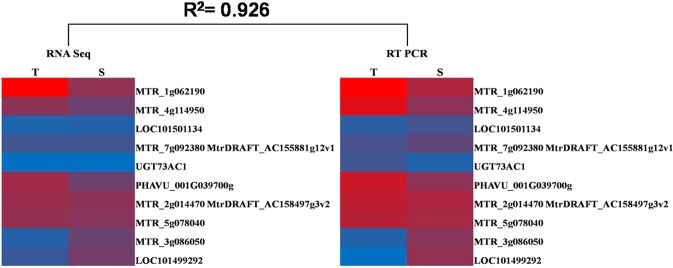
Ten DEGs with log FC ≥1.27 selected from the combination group of tolerant treated vs sensitive treated were validated on two samples (heat treated tolerant and sensitive) with three technical replicates using RT-qPCR. Degree of expression of genes amplified using RT-qPCR is represented in Fig. 9. Log2 fold change was deduced from the raw data and when compared to that of RNA Seq data, it exhibited a close association and concordance with differential expression results of genes under heat stress conditions (Fig. 10).
Figure 9.
Genes relative expression profile using real time PCR. 2-(ΔΔCT) method was used to obtain relative quantification using β-tubulin as reference gene. Data represents average from three biological replicates and error bars indicates standard deviation (±SD).
Figure 10.
Correlation between RNA Seq and real time PCR. Log fold change is depicted through colour intensity as described in heat maps.
Discussion
Heat stress response at seedling stage
Hydroponics is cost effective, easy and empirical method for evaluating genotypes utilizing minimum space and time1. Use of hydroponics diminishes role of water stress during heat stress and confounding plant responses as in soil rhizosphere is also mitigated, therefore abiotic stress is accredited just because of heat stress alone27. Although, hydroponics does not replicate field environment where plants observe different kinds of interaction, but since here our target is to reveal molecular mechanism just because of heat stress alone, eliminating other stress factors is essential which can’t be possible under field conditions. Hence, hydroponics is one of the best approaches to reveal true genes responsible for heat stress. In present study, plants were subjected to heat stress continuous at 35/33 °C for 3 d. As a result, plants of sensitive genotype showed marked wilting of leaves along with lodging of stems when compared to plants of tolerant one (Fig. 1). This indicated that 3 d continuous heat exposure is sufficient to discriminate genotypes as tolerant or sensitive, in addition to the fact that the genotypes has already been selected from a whole spectrum of 119 genotypes by Singh et al.1. Same duration of heat stress did not affect the basic metabolism in tolerant genotype which was affected in case of sensitive genotype. This indicates that visual evaluation of plant wilting and necrosis provides a reliable method for ranking of genotypes in response to long term and/or more severe heat stress conditions. Momonoki et al. elucidated that wilting after thermal stress in plants like cowpea, cucumber and radish is associated with acetylcholine movement and similar to this study, they have used low degree of wilting as a parameter for distinguishing cultivars under heat stress28.
Heat stress response at reproductive stage
Heat stress reduced pollen germination in tolerant and sensitive genotypes and the level of reduction was less in tolerant (PDL-2) genotype at 35/20 °C under controlled conditions. Heat condition damages pollen germination due to decrease in moisture content i.e. desiccation and soluble sugar concentration in pollen29,30. Reduction in pollen germination under heat stress has been reported in many crops such as rice and soybean31,32. Low pollen germination under heat stress could be result of carbohydrate metabolism of anthers and subsequently of their tapetum cells33. These results support the fact that high temperature stress affects pollen germination and consequently results in reduction in seed yield as also reported in several species earlier.
In controlled environment, stressed pollens from tolerant (PDL-2) genotype germinated on stressed stigma of sensitive genotype (JL-3). However, stressed pollens from sensitive (JL-3) genotype did not germinate on stressed stigmas of tolerant (PDL-2) genotype, indicating that pollen was fertile at 35/20 °C (Additional File 6. Fig. 6 and Additional File 7. Fig. S7). This suggests that stigma in lentil is less sensitive to heat than pollen as reported in rice by Yoshida et al.34. Several studies in crops like beans and flax seed have reported that pollen is more sensitive to high temperature than carpel35,36. However, impairment of pollen and stigma tissues depends upon critical temperature of the crop and degree of heat tolerance among genotypes. Every genotype possesses optimum temperature tolerance levels and if level of temperature exceeds, percentage pollen germination and maximum pollen tube length reduces. This optima has been described through bilinear regression model by Kakani et al. and Kakani et al. in groundnut and cotton genotypes, respectively37,38. Overall, in present study also, pollens of lentil were found to be more sensitive to heat stress as compared to stigma when observed under controlled conditions.
Physiological and biochemical responses under heat stress
MSI was affected in both tolerant as well as sensitive genotypes under heat stress. However PDL-2 (tolerant) maintained significantly higher MSI under both control and heat stress conditions (Fig. 2f). This shows that higher MSI elevates the tolerance of the tolerant genotype by enhancing the physio-biochemical processes under heat stress environment. Quantitative trait loci (QTLs) linked with MSI under heat stress have also been mapped in wheat39. Higher MSI was reported to be associated with osmolytes and antioxidant activities in wheat40. Heat can remarkably modify the physical properties of biological membranes41,42. Production of osmoprotactants such as proline and glycine betaine in plants, normalizes the membranes disruption and also retains the protein conformation intact under various abiotic stresses43,44 like drought7,45, salinity46,47, heat48 etc. Present results also showed that heat tolerant genotype accumulated more proline and glycine betaine contents as compared to sensitive ones (Fig. 2g). Higher proline concentration has been reported to reduce photo damage to chloroplast thylakoid membranes through scavenging and/or reducing production of ROS49. Glycine betaine, another compatible solute increases in chloroplast under environmental stresses50. Elevated accumulation of glycine betaine content was observed in PDL-2 compared to JL-3. Similar increase in level of glycine betaine under heat stress at seedling stage has been reported in barley51. Hayashi et al. have observed that transformed Arabidopsis accumulated glycine betaine and exhibited enhanced tolerance to high temperature during seed germination and growth of seedlings52. They proposed that glycine betaine mitigates heat stress by reducing induction of heat shock proteins (Hsp) in those transgenic plants. Rasheed et al., testified involvement of both proline and glycine betaine in heat stress tolerance by giving external pre-treatments in sprouting sugarcane buds. Soaking of buds in proline and glycine betaine solutions markedly lowered hydrogen peroxide production, boosted building up of soluble sugars and shielded developing tissues for heat stress effects53.
MDA has been considered as physiological marker and its concentration varies under abiotic stress conditions54,55. In present study its concentration was found to be higher in both tolerant and sensitive genotypes when compared to their respective control samples, indicating lipid injury in tissues of both the genotypes under heat stress. Lipid peroxidation indicates the prevalence of free radical reactions occurring in tissues which reflects a correlation between heat and oxidative stress56. Degree of lipid peroxidation was lower in shoots of tolerant (PDL-2) genotype (Fig. 2e) as compared to sensitive one. Similar results were found in fababean, wheat and creeping bent grass under heat stress57–59. Induction of activities of antioxidant enzymes and metabolites can play a crucial role in plant’s resistance to heat and other abiotic stresses60,61. Antioxidant enzymes are considered as the first line of defence used by plants to scavenge superoxide radicals into H2O2 and O2. It is reported that production of H2O2 also contributes in transduction of heat signal into expression of HSPs62. Plants also use peroxidases like APX, CAT etc. and antioxidant enzymes to scavenge ROS. Heat stress showed a marked effect on SOD, APX, GPX and CAT activities in both tolerant and sensitive genotypes. Tolerant genotype exhibited a sharp rise in SOD, APX and GPX activities compared to sensitive one (Fig. 2). Less increase in lipid peroxidation level in tolerant genotype could be a result of higher activities of SOD, APX and GPX enzymes (Fig. 2). PDL-2 exhibited no significant effects on CAT activity under heat stress, while its activity decreased significantly in JL-3 suggesting that tolerant genotype had experienced lower level of oxidative stress due to induced antioxidant enzymes activities and detoxified ROS. Similar to our study, increase in SOD activity of temperature tolerant genotypes was reported in wheat, although contrary to our results, activity of CAT was also found to be increased in their study63. Elevated SOD, APX and GPX activities were observed in lily plants under heat stress together with reduction in CAT activity64. Reduction in CAT activity was also reported in tomato plants which were subjected to 35 °C heat stress65.
Transcriptome analysis and stress responsive genes under heat stress
Next Generation Sequencing (NGS) was useful in identification of novel genes correlated with biotic and abiotic stresses66,67. De novo assembly has been used on RNA Seq data for mining out novel genes in several crops like wheat, Vicia faba etc.68,69. Understanding of underlying molecular mechanism of heat tolerance is still unknown in lentil. However, some reports shedding light on molecular mechanisms of other abiotic stresses like drought and cold are available7,70. Transcriptome analysis may provide an overview of novel genes and regulatory networks linked with heat tolerance mechanism in lentil. Previous studies on transcriptome analysis in lentil have provided valuable information and genomic insight for different aspects such as identification of expressed sequenced tags (ESTs) derived SSR and SNP markers7,71,72. Also, heat stress responsive genes have been identified for several crops like Arabidopsis, wheat etc., using transcriptomic analyses73,74.
In the present study, a transcriptome comparison of heat sensitive (JL-3) and heat tolerant (PDL-2) genotypes was attempted to establish potential candidate genes involved in response to heat stress in lentil. cDNA samples of both genotypes were sequenced and in total, 23,605,081 to 32,460,145 clean reads were used for de novo assembly, generating 90,267 to 104,424 contigs for different combinations (Table 1). Number of contigs generated in this study is comparable or similar to other studies7,71,72. Tolerant genotype (PDL-2) showed increased number of DEGs as compared to sensitive genotype at 3 d of continuous heat stress, including both up and downregulated genes. Total range of 13,510 to 16,720 upregulated and 15,141 and 16,817 downregulated genes were found in different combinations (Fig. 3). Results for total DEGs deduced under heat stress, were much higher as compared to that of wheat (6,560), switchgrass (5,365) and Chinese cabbage (625)74–76.
It was found that most of DEGs were mainly confined to the cell wall and secondary metabolic components (Fig. 8). BINs which showed significant up and down regulation in the combination tolerant treated vs sensitive treated were also visualized under cell wall and secondary metabolism. Changes in BINs related to cell wall have been reported in barley caryopses under heat stress by Mangelsen et al.77. Modification of cell wall under abiotic stress would prevent plant from severe damage78,79. Genes encoding diverse cell wall enzymes like arabino galactan protein, cellulose synthase, expansin etc. were significantly up regulated following high temperature stress in Brassica rapa L75. In the present investigation, contig DN88332_c0_g1 (Plasmodesmata Callose-Binding Protein 3 (PDCB) up regulated (log FC = −7.67) in tolerant treated vs sensitive treated was localized in the plasmodesmata neck region that gives structural anchor between plasma membrane component of plasmodesmata and cell wall. Increased PDCB1 elevates callose accumulation that enhances correlation between PDCB-mediated callose deposition and plant’s cell-to-cell communication80,81. Callose accumulation has been found to be beneficial for plants in many important aspects such as plasmodesmata regulation and avoiding biotic and abiotic stresses through multifaceted defence response controlled by distinct signalling pathways82. Detectable callose deposition appears within minutes of impairment caused by various stresses including raised temperatures. Callose plugs at plasmodesmata are involved in maintenance of dormancy by isolating meristem from symplastic continuity with neighbouring tissues82. Rinne et al. has proposed a dormancy cycling model to depict subsequent states of cellular transmission in meristem with attributed sensitivities to different abiotic stresses83. Further, it was reported that callose synthase was needed for exine formation during micro gametogenesis and pollen viability in Arabidopsis84. Also, callose is crucial for pollen wall patterning but not pollen tube growth85. Callose deposition (GO: 0052542) and callose localization (GO: 0052545) were found to be involved in heat stress response of sweet maize varieties through transcriptome analysis86. DN70092_c0_g4 (log FC = 1.94) Phosphatidylinositol/phosphatidylcholine transfer protein SFH13 (Sec Fourteen Homolog 13); DN59454_c0_g1(log FC = 2.02) CDP-diacylglycerol–glycerol-3-phosphate 3-phosphatidyltransferase 1 chloroplastic; DN72602_c0_g1(log FC = 1.52) Probable glycerol-3-phosphate acyltransferase 2 (GPAT2); DN73858_c0_g1(1.34) O-acyltransferase WSD; DN92437_c0_g1(−1.21786624590465) Phosphatidylcholine diacylglycerol choline phosphotransferase 1 were noticed to be upregulated in tolerant genotype under heat stress. These were found to be directly or indirectly involved in formation of glycerolipids. These lipid species were found to get induced in response to heat stress conditions in Arabidopsis87. Phosphatidylinositol/phosphatidylcholine transfer protein SFH13 is needed for transport of secretory proteins from golgi complex and that of phosphatidylinositol/phosphatidylcholine between membranes in vitro88. CDP-diacylglycerol–glycerol-3-phosphate 3-phosphatidyltransferase 1 is an integral membrane protein which is involved in glycerophospholipid metabolism. This protein hold high activity with CDP-dipalmitoylglycerol and low activity with CDP-dioleoylglycerol89,90. GPAT2 mediates initial step of glycerolipid biosynthesis which involves esterification of acyl-group from acyl-ACP to sn-1 position of glycerol-3-phosphate91. Various isoforms of GPAT have been classified in yeast animal and plant cells92,93. Zheng et al. has reported pivotal role of Arabidopsis ATGPAT 1 in pollen development91. OsGPAT3 has been reported to be involved in anther development and male fertility in rice94. In present study, since GPAT2 is upregulated in tolerant genotype, at later stages of plant development, it might be involved in anther development in lentil as well. This can be further confirmed through mutation studies in lentil. GPAT enzyme has been partially purified from chilling tolerant plants of spinach and pea95. O-acyltransferase WSD catalyzes the terminal step in triacylglycerol synthesis by using diacylglycerol and fatty acyl CoA as substrates96. Diacylglycerol acetyltransferase WSD1 is required for stem wax ester biosynthesis in Arabidopsis97 whereas, Phosphatidylcholine diacylglycerol cholinephosphotransferase 1 is involved in triacylglycerol synthesis pathway98,99. Triglycerols are widely found as carbon and energy reserve in pollen grains. It is known that these surface lipids are required for male fertility and productive pollen-pistil interactions. A layer of lipophilic material called tryphine is embedded in exine of pollen grains. This tryphine includes small lipid bodies that primarily contains wax monomers and is responsible for reducing water loss from pollen grains. The normal exine coat plays signalling role in pollen-pistil interactions100. Therefore, O-acyltransferase WSD might be upregulated in tolerant genotype to protect this normal exine coat in later reproductive stage of life cycle in response to heat stress. Secondary metabolites play important roles in plant survival under heat stress101. Several pathways like phenyl propanoids, shikimate, mevalonate or erthritol phosphate pathway (MEP) are involved in secondary metabolite production102. Contig DN32591_c0_g2 (Pyruvate phosphate dikinase chloroplastic) down regulated in tolerant-sensitive treated with log FC = −8.04 catalyses the phosphorylation of pyruvate in presence of ATP to produce phospho enolpyruvate (PEP). Both cytosolic and plastidiciso-forms found in higher plants are induced under drought and salt stress103. In case of Nicotiana tabacum L. under drought condition, PPDK increased upto 2.7 folds104. PEP is an essential compound for Shikimate pathway in which it is converted into chorismate, a central branch point metabolite that is converted to secondary metabolites105. Microarray analysis in Arabidopsis has shown that there is a correlation between PPDK and AtRP2 in pollen which has led to an opinion that AtRP2 plays crucial part in pollen development by regulatory inactivation of PPDK106. This suggests that here in lentil under heat stress, down regulation of PPDK might have some correlation to pollen development later at reproductive stages.
In the present study, to identify similarity and differences in molecular mechanisms, transcriptome profiles were compared to publicly available proteome datasets of legume species for similarity percentage. Medicago sativa (~95.8%) showed highest similarity percentage followed by Cajanus canjan (~95.2%), Cicer arietinum (~94.9), Lotus japonicas (~93.8%) and least similarity was observed with Glycine max (~92%). Kaur et al. reported 12,639 unique hits matches with Medicago truncatula and 20,419 unique hits with G. max107. Therefore, number of genes along with its transcript coverage that represent EST collection are important objectives which require completely annotated reference genome sequence107.
Gene ontology analysis showed number of DEGs involved in apoplast were downregulated in tolerant treated vs sensitive treated genotypes whereas cell division, binding & catalytic GO terms were up regulated (Fig. 6). Cytochrome P450 enzymes which are associated with oxidative metabolism of various exogenous and endogenous lipophilic compounds were found to be down regulated in heat tolerant genotype. This was also reported in rice under heat stress108. Similarly GmCYP82A3 which is a soybean cytochrome P450 family gene participates in jasmonic acid and ethylene signalling pathways and thereby have roles in providing tolerance to biotic and abiotic stresses109. Hsps play paramount role in protecting plants against stresses by reconstructing normal protein conformation and ultimately cellular homeostasis110. Data from genome wide expression profile of Arabidopsis plants exposed to heat stress has appreciably expanded our information on Hsps111. Extreme heat stress may cause protein denaturation in vivo and accumulation of misfolded proteins activates HSFs by inducing the disengagement of HSF and HSP70 and/or HSP90 complexes112. HSP70 and/or HSP90 are called up to repair protein damage. Transcriptional profiling of Arabidopsis Hsps and Hsfs has revealed extensive cross talk between heat and non-heat stress response pathways113. In present investigation, many Hsps were induced during heat stress in PDL-2 (tolerant) genotype, confirming role of upregulated Hsps in providing tolerance to PDL-2. Overall GO enrichment analysis showed 15,156 up and 15,457 down regulated GO terms in the combination tolerant-sensitive which were much higher when compared to that of chinese cabbage and switch grass in response to heat stress75,114. Biological membranes circumscribe sensory aids competent of perceiving specific signals and transducing them into apt gene expressions115. In present study, plasma membrane and cell wall were affected under heat stress which might have triggered interaction of signal molecules at one or more specific sites resulting in changes in anabolic and catabolic processes. These results were similar to that of heat exposed chinese cabbage and switch grass75,114.
Contigs DN57816_c0_g1 and DN41180_c0_g4 with log FC of 9.54, 9.36 and gene descriptions as Lon protease homolog 2 peroxisomal and Probable acyl-activating enzyme 5 peroxisomal, respectively were top two up regulated in the combination tolerant treated vs sensitive treated (Table 2). These were confined to sustained matrix protein import into peroxisomes; transporter for β-oxidation during germination and establishment; jasmonic acid biosynthesis and conversion of indole butyric acid to indole acetic acid. Lon protease homolog 2 peroxisomal is serine protease and helps in selective break down of polypeptides in the peroxisomal matrix. Deficiency of Lon 2 in Arabidopsis has resulted in enhanced peroxisomal degeneration by autophagy and there by peroxisomal proteins was accumulated in the cytosol116. Probable acyl-activating enzyme 5 peroxisomal may act as an acid-thiol ligase that stimulates carboxylic acids by forming acyl-CoAs. OPCL1 peroxisomal acyl-activating enzyme was found to be involved in jasmonic acid biosynthesis in Arabidopsis117. An acyl activating enzyme is also reported to be involved in formation of precursor for cannabinoid biosynthesis in Cannabis sativa trichomes118. Further, top two down regulated contigs DN68776_c2_g3 and DN19596_c0_g1 with log FC −10.58 and −9.45, respectively had no gene descriptions. Though, first one had protein description of Protein detoxification (Multidrug and toxic compound extrusion protein (MATE), AT3G26590.1) but later has no annotation. MATE transporters are conserved transporter families in nature which are involved in the maintenance of homostasis by excretion of waste products and xenobiotics119. Wang et al. have demonstrated that MATE gene family has expanded via tandem and segmental duplication in rice and Arabidopsis120.
Contigs annotated as peroxidase 15, 42, (DN56326, DN54906) viz. Glyoxylate/succinic semialdehyde reductase 2 chloroplastic and Cytochrome P450 (DN54719) were upregulated in tolerant genotype against sensitive genotype under heat stress conditions with Log FC above 5. Peroxidases are involved in scavenging superoxide radical, toxic reductant’s oxidation, lignin metabolism, catabolism of auxin and oxidative stress121,122. Glyoxylate/succinic semialdehyde reductase 2 is involved in detoxification of reactive aldehydes, glyoxylate and succinic semialdehyde into glycolate and γ hydroxyl butyrate, respectively and ultimately functions in maintenance of redox homeostasis123,124. Cytochrome P450 is involved in removal of ROS and synthesis of secondary metabolites such as stilbenoid, diarylhepatanoid and gingerol109. During physiological analysis of genotypes under heat stress also, tolerant genotype has exhibited a sharp rise in peroxidases like SOD, APX and GPX activities compared to sensitive one. Detection of increase in antioxidant activity in tolerant genotype due to heat stress in the present study reaffirms the transcriptomics evaluation. Similarly, expressions of several other physiological genes were altered under heat stress in tolerant genotype as compared to sensitive one. In this class of genes, those which were highly upregulated were Delta-1-pyrroline-5-carboxylate synthase (P5CS), 36.4 kDa proline-rich protein, Respiratory burst oxidase homolog protein C and D, Proline transporter 1 and Ornithine amino transferase mitochondrial. Delta-1-pyrroline-5-carboxylate synthase is the rate-limiting enzyme for proline biosynthesis and undergo feedback inhibition by proline which is lost in plants due to stress conditions125. In the present study, up-regulation of this enzyme under heat stress reflects tolerant genotype strategy to avoid feedback inhibition by proline, so that more proline can be synthesized under heat stress, which is true as per physiological analysis. Similarly, Pyrroline-5-carboxylate (P5C) is a common intermediate in the synthesis and metabolism of proline, which is produced by ornithine aminotransferase (OAT). This enzyme functions in an alternative proline metabolic pathway of mitochondria in response to stress conditions126. Up regulation of OAT represents operation of alternative proline metabolic pathway under heat stress in mitochondria of tolerant genotype. DEGs which were downregulated included Purple acid phosphatase 17, Cationic peroxidase 1, Proline-tRNA ligasechloroplastic/mitochondrial, Proline-rich receptor-like protein kinase PERK1 and Hydroxyproline O-arabinosyltransferase 1.
When expression of transcription factors between heat tolerant and sensitive genotypes for heat stress were compared, expression was found higher in PDL-2 compared to JL-3. ABA responsive protein (DN22374_c0_g1), WRKY transcription factor WRKY24 (DN10173_c0_g1), DnaJ homolog subfamily B member 13 (DN73300_c5_g2), 17.1 kDa class II heat shock protein (DN96073_c0_g1) were some of the well-known stress responsive genes which were common in both the genotypes with Log FC of 3.12, 2.81, 2.90, 3.90, respectively. Similar findings were reported in barley caryopses, Chinese cabbage and switch grass under heat stress conditions75,77,114. Signalling by stress phytohormone ABA is involved in acquired thermo tolerance127,128. Wang et al., revealed that HSFA2 is associated in ABA mediated heat stress tolerance in tall fescue and Arabidopsis129. Liu et al., reported that ABA can regulate the expression of several small Hsps (sHsps) in maize leaves when imposed to combined drought and heat stresses130. In the present study, NAC and WRKY transcription factors were found over-expressed in PDL-2 compared to JL-3. NAC has already been reported to be involved in heat stress in Arabidopsis, rice and wheat131–133. Whereas implication of WRKY25 in thermo tolerance in Arabidopsis was demonstrated by Li et al.134.
Validation of g-SSRs
Out of 55 g-SSRs, 18 were found polymorphic (32.72%) while genotyping against 96 genotypes. All the markers showed amplification indicating the presence of marker locus within the lentil genome. These markers can be used for QTL identification and molecular assortment of species and can be further utilized for varied applications in lentil breeding. Similar to this study Hou et al. has also used transcriptomic analysis for identification of gSSR markers in proso millet. They also validated identified gSSRs by detecting polymorphism in 56 accessions135.
Validation of DEGs
To confirm credibility of DEGs obtained in this study, 10 DEGs belonging to different gene families, (including 5 upregulated and 5 downregulated in tolerant-sensitive combination) were selected to examine their expression patterns via RT-qPCR (Additional File 17. Table S3). RT-qPCR results showed that expression of all these DEGs were similar to those obtained from Illumina sequencing analysis (Figs 9 and 10). Besides, the fold changes obtained by DEGs were generally higher than those obtained by RT-qPCR, which was a comprehensive phenomenon in some studies. These findings showed that the method used to ascertain DEGs in this study was valid. Up regulated DEGs validated through RT-qPCR analysis were MTR_1g062190 (Cytochrome P450 monooxygenase), MTR_4g114950 (ABC transporter domain protein), PHAVU_001G039700g (Uncharacterized protein), MTR_2g014470 [DnaJ homolog subfamily B member 4 (DNAJB4)] and MTR_5g078040 (peroxisomal small heat shock protein). Cytochrome P450-dependent monooxygenases represents a large group of heme-containing enzymes, where classical forms catalyzes NADPH- and O2-dependent hydroxylation reactions. Non-classical forms are monogeneses that either do not employ flavoproteins for dioxygen activation or fail to integrate molecular oxygen in their concluding product. Cytochrome P450-dependent mono oxygenases are involved in biosynthetic or detoxification pathways136,137. Plant ABC transporter proteins play paramount role in plant growth and development processes. These are involved in detoxification, chlorophyll biosynthesis, arrangement of Fe/S clusters, stomatal movement and ion fluxes138. STAR1 and STAR2 are bacterial type ABC transporters found to be involved in aluminium stress tolerance in rice139. OsABCG15, an ABC transporter gene was found to play crucial role in anther cuticle and pollen exine development in rice140. DNAJB4 belongs to heat shock protein (Hsp 40) family. sHsps are widespread molecular chaperons. Plants employ these Hsps in peroxisomal matrix to avoid undetailed gathering of partially denatured proteins under both physiological and stress conditions141. Similar to this study, expression of AtHsp15.7, which is a peroxisomal located sHsp is reported to be induced under heat and oxidative stress in Arabidopsis141. Earlier studies have shown that considerable tolerance to drought and high light can be attained by over-expressing cytosolic or plastidic heat-shock proteins142,143. Validated DEGs which were downregulated in RT-PCR analysis includes LOC101501134 [Cytochrome P450 superfamily protein (CPD 13)], MTR_7g092380 (DNAJ chaperone), UGT73AC1 (β glucosyltransferase), MTR_3g086050 (localized small heat shock protein) and LOC101499292 (HSP 22 KDa). Glycosyl transferases constitute indispensable multigene family present in all organisms. In plants they have role in glycosylation of plant products which can help them in survival under adverse conditions144. Expression of UGTs was reported to be localized in parts of expeditiously dividing cells145. High expressions of UGTs are involved in acute cell division which indicates involvement in cell cycle regulation145.
Supplementary information
Acknowledgements
Authors express their gratitude to the Head, Division of Genetics and Incharge, National Phytotron Facility, ICAR-Indian Agricultural Research Institute, New Delhi, for supporting research activities. Authors thank Ashwani Kumar Mishra, DNA Xperts Pvt Ltd, Delhi for support in Bioinformatics data analysis.
Author Contributions
D.S., M.P. and K.G. formulated the experiments. D.S., C.K.S., J.T., V.J. executed molecular and statistical data analysis. D.S., C.K.S. accomplished experiments of physiological and biochemical parameters. M.P. analysed physiological data. D.S., C.K.S., J.T. and M.P. drafted the revised manuscript. All the authors have read and approved the final manuscript.
Data Availability
The dataset for two genotypes supporting the conclusions of this article is deposited in Sequence Read Archive repository (SRA) with SRA submission ID – SUB3390924. [http://www.ncbi.nlm.nih.gov/sra].
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dharmendra Singh, Email: dharmendrapbg@rediffmail.com.
Madan Pal, Email: madanpal@yahoo.com.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49496-0.
References
- 1.Singh D, et al. Exploring genetic diversity for heat tolerance among lentil (Lens culinaris Medik.) genotypes of variant habitats by simple sequence repeat markers. Plant Breed. 2016;135:215–223. doi: 10.1111/pbr.12341. [DOI] [Google Scholar]
- 2.Roy CD, Tarafdar S, Das M, Kundagrami S. Screening lentil (Lens culinaris Medik.) germplasms for heat tolerance. Trends Biosci. 2012;5:143–146. doi: 10.3389/fpls.2017.00744. [DOI] [Google Scholar]
- 3.Sita Kumari, Sehgal Akanksha, Bhandari Kalpna, Kumar Jitendra, Kumar Shiv, Singh Sarvjeet, Siddique Kadambot HM, Nayyar Harsh. Impact of heat stress during seed filling on seed quality and seed yield in lentil (Lens culinaris Medikus) genotypes. Journal of the Science of Food and Agriculture. 2018;98(13):5134–5141. doi: 10.1002/jsfa.9054. [DOI] [PubMed] [Google Scholar]
- 4.Allakhverdiev SI, et al. Heat stress: an overview of molecular responses in photosynthesis. Photosynth Res. 2008;98:541–550. doi: 10.1007/s11120-008-9331-0. [DOI] [PubMed] [Google Scholar]
- 5.Mittler R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006;11:15–19. doi: 10.1016/j.tplants.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Livingston DP, Hincha DK, Heyer AG. Fructan and its relationship to abiotic stress tolerance in plants. Cell Mol. Life Sci. 2009;66:2007–2023. doi: 10.1007/s00018-009-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van den Ende W, Valluru R. Sucrose, sucrosyl oligosaccharides, and oxidative stress: scavenging and salvaging? J. Exp. Bot. 2009;60:9–18. doi: 10.1093/jxb/ern297. [DOI] [PubMed] [Google Scholar]
- 8.Singh D, et al. Transcriptome analysis of lentil (Lens culinarisMedikus) in response to seedling drought stress. BMC Genomics. 2017;18:206. doi: 10.1186/s12864-017-3596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyko A, et al. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. Plos One. 2010;5(3):e9514. doi: 10.1371/journal.pone.0009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkindale J, Hall JD, Knight MR, Vierling E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005;138:882–897. doi: 10.1104/pp.105.062257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkindale J, Vierling E. Core genome responses involved in acclimation to high temperature. Plant Physiol. 2008;146:748–761. doi: 10.1104/pp.107.112060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, et al. Molecular and genetic evidence for the key role of AtCaM3 in heat-shock signal transduction in Arabidopsis. Plant Physiol. 2009;149:1773–1784. doi: 10.1104/pp.108.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu HC, Jinn TL. Ethylesterase activity and cytosolic Ca2+ oscillation are crucial for plant thermotolerance. Plant Signal. & Behav. 2010;5:1252–1256. doi: 10.4161/psb.5.10.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saidi Y, Finka A, Goloubinoff P. Heat perception and signalling in plants: a tortuous path to thermotolerance. New Phytologist. 2011;190(3):556–565. doi: 10.1111/j.1469-8137.2010.03571.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu HT, Un DY, Zhou RG. Ca2+ and AtCaM3 are involved in the expression of heat shock protein gene in Arabidopsis. Plant, Cell &Environment. 2005;28:1276–1284. doi: 10.1111/j.1365-3040.2005.01365.x. [DOI] [Google Scholar]
- 16.Zhang W, et al. Molecular and genetic evidence for the key role of AtCaM3 inheat-shock signal transduction in Arabidopsis. Plant Physiol. 2009;149:1773–1784. doi: 10.1104/pp.108.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo JK, et al. Genome-wide analysis of heat shock transcription factor families in rice and Arabidopsis. J. Genet Genomics. 2008;35:105–118. doi: 10.1016/S1673-8527(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 18.Mittal D, Chakrabarti S, Sarkar A, Singh A, Grover A. Heat shock factor gene family in rice: genomic organization and transcript expression profiling in response to high temperature, low temperature and oxidative stresses. Plant Physiol. Biochem. 2009;47:785–795. doi: 10.1016/j.plaphy.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Von Koskull-Doring P, Scharf KD, Nover L. The diversity of plant heat stress transcription factors. Trends Plant Sci, 2007;12:452–457. doi: 10.1016/j.tplants.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 20.Hua J. From freezing to scorching, transcriptional responses to temperature variations in plants. Curr Opin Plant Biol. 2009;12:568–573. doi: 10.1016/j.pbi.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Iba K. Acclimative response to temperature stress in higher plants: approaches of gene engineering for temperature tolerance. Annu Rev Plant Biol. 2002;53:225–245. doi: 10.1146/annurev.arplant.53.100201.160729. [DOI] [PubMed] [Google Scholar]
- 22.Kudapa H, et al. Comprehensive transcriptome assembly of chickpea (Cicer arietinum L.) using Sanger and next generation sequencing platforms: Development and Applications. Plos One. 2014;9(1):e86039. doi: 10.1371/journal.pone.0086039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libault M, et al. Complete transcriptome of the soybean root hair cell, a single-cell model, and its alteration in response to Bradyrhizobiumjaponicum infection. Plant Physiol. 2010;152:541–552. doi: 10.1104/pp.109.148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh Dharmendra, Singh Chandan Kumar, Tomar Ram Sewak Singh, Taunk Jyoti, Singh Ranjeet, Maurya Sadhana, Chaturvedi Ashish Kumar, Pal Madan, Singh Rajendra, Dubey Sarawan Kumar. Molecular Assortment of Lens Species with Different Adaptations to Drought Conditions Using SSR Markers. PLOS ONE. 2016;11(1):e0147213. doi: 10.1371/journal.pone.0147213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon L, Smalley TJ, Jr., Benton J, Lasseigne FT. Aluminum toxicity in tomato: part I. Growth and mineral nutrition. J Plant Nutr. 1994;17:293–306. doi: 10.1080/01904169409364728. [DOI] [Google Scholar]
- 26.Devasirvatham V, et al. Effect of high temperature on the reproductive development of chickpea genotypes under controlled environments. Funct. Plant Biol. 2012;39:1009–1018. doi: 10.1071/FP12033. [DOI] [PubMed] [Google Scholar]
- 27.Giri A, Heckathorn S, Mishra S, Krause C. Heat stress decreases levels of nutrient-uptake andassimilation proteins in tomato roots. Plants. 2017;6(1):6. doi: 10.3390/plants6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Momonoki YS, Momonoki T. Changes in acetylcholine levels following leaf wilting and leaf recovery by heat stress in plant cultivars. Jpn J Crop Sci. 1991;60(2):283–290. doi: 10.1626/jcs.60.283. [DOI] [Google Scholar]
- 29.Naveed S, et al. M.Physiology of high temperature stress tolerance at reproductive stage in maize. J of Ani & Plant Sci. 2014;24(4):1141–1145. [Google Scholar]
- 30.Pressman E, Peet MM, Pharr DM. The effect of heat stress on tomato pollen characteristics is associated with changes in carbohydrate concentration in the developing anthers. Ann Bot. 2002;90(5):631–636. doi: 10.1093/aob/mcf240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prasad PVV, Boote KJ, Allen LHJR, Sheehy JE, Thomas JMG. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res. 2006;95:398–411. doi: 10.1016/j.fcr.2005.04.008. [DOI] [Google Scholar]
- 32.Koti S, Reddy KR, Kakani VG, Zhao D, Reddy VR. Interactive effects of carbon dioxide, temperature and ultraviolet-B radiation on flower and pollen morphology, quantity and quality of pollen in soybean (Glycine max L.) genotypes. J. Exp. Bot. 2005;56:725–736. doi: 10.1093/jxb/eri044. [DOI] [PubMed] [Google Scholar]
- 33.Jain M, Prasad PVV, Boote KJ, Hartwell AL, Chourey PS. Effects of season-long high temperature growth conditions on sugar-to-starch metabolism in developing microspores of grain sorghum (Sorghum bicolor L. Moench) Planta. 2007;227:67–7. doi: 10.1007/s00425-007-0595-y. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida, S. Fundamentals of rice crop science. IRRI, Los Banos, Philippines, 269 (1981).
- 35.Weaver ML, Timm H. Influence of temperature and plant water status on pollen viability in beans. J. Am. Soc.Hortic.Sci. 1988;113:31–35. [Google Scholar]
- 36.Cross RH, McKay SAB, G McHughen A, Bonham‐Smith PC. Heat‐stress effects on reproduction and seed set in Linumusitatissimum L.(flax) Plant Cell Environ. 2003;26(7):1013–1020. doi: 10.1046/j.1365-3040.2003.01006.x. [DOI] [Google Scholar]
- 37.Kakani VG, Prasad PV, Craufurd PQ, Wheeler TR. Response of in vitro pollen germination and pollen tube growth of groundnut (Arachishypogaea L.) genotypes to temperature. Plant Cell Environ. 2002;25(12):1651–1661. doi: 10.1046/j.1365-3040.2002.00943.x. [DOI] [Google Scholar]
- 38.Kakani VG, et al. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann. Bot. 2005;96(1):59–67. doi: 10.1093/aob/mci149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Talukder SK, et al. Mapping QTL for the traits associated with heat tolerance in wheat (Triticumaestivum L.) BMC Genetics. 2014;15(1):97. doi: 10.1186/s12863-014-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan SU, Jalal U-D, Gurmani AR, Qayyum A, Khan H. Heat tolerance evaluation of wheat (Triticum aestivum L.) genotypes based on some potential heat tolerance indicators. J. Chem. Soc. Pak. 2013;35:3. [Google Scholar]
- 41.Falcone DL, Ogas JP, Somerville CR. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biology. 2004;4:17. doi: 10.1186/1471-2229-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matos AR, et al. Alternative oxidase involvement in cold stress response of Arabidopsis thaliana fad2 and FAD3+ cell suspension saltered in membrane lipid composition. Plant Cell Physiol. 2007;48:856–865. doi: 10.1093/pcp/pcm061. [DOI] [PubMed] [Google Scholar]
- 43.Ashraf M, Foolad M. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007;59(2):206–216. doi: 10.1016/j.envexpbot.2005.12.006. [DOI] [Google Scholar]
- 44.Chen TH, Murata N. (2008). Glycinebetaine: an effective protectant against abiotic stress in plants. Trends Plant Sci. 2007;13(9):499–505. doi: 10.1016/j.tplants.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Wang GP, Zhang XY, Li F, Luo Y, Wang W. Over-accumulation of glycine betaine enhances tolerance to drought and heat stress in wheat leaves in the protection of photosynthesis. Photosynthetica. 2010;48(1):117–126. doi: 10.1007/s11099-010-0016-5. [DOI] [Google Scholar]
- 46.Meloni DA, Gulotta MR, Martínez CA, Oliva MA. The effects of salt stress on growth, nitrate reduction and proline and glycinebetaine accumulation in Prosopisalba. Braz. J. Plant Physiol. 2004;16(1):39–46. doi: 10.1590/S1677-04202004000100006. [DOI] [Google Scholar]
- 47.Yang X, Lu C. Photosynthesis is improved by exogenous glycinebetaine in salt‐stressed maize plants. Physiol. Plant. 2005;124(3):343–352. doi: 10.1111/j.1399-3054.2005.00518.x. [DOI] [Google Scholar]
- 48.Paleg LG, Douglas TJ, Van Daal A, Keech DB. (1981). Proline, betaine and other organic solutes protect enzymes against heat inactivation. Funct. Plant Biol. 1981;8(1):107–114. doi: 10.1071/PP9810107. [DOI] [Google Scholar]
- 49.Hayat S, et al. Role of proline under changing environments. Plant Signal Behav. 2012;7(11):1456–1466. doi: 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kurepin Leonid V., Ivanov Alexander G., Zaman Mohammad, Pharis Richard P., Hurry Vaughan, Hüner Norman P. A. Photosynthesis: Structures, Mechanisms, and Applications. Cham: Springer International Publishing; 2017. Interaction of Glycine Betaine and Plant Hormones: Protection of the Photosynthetic Apparatus During Abiotic Stress; pp. 185–202. [Google Scholar]
- 51.Wahid A, Shabbir A. Induction of heat stress tolerance in barley seedlings by pre-sowing seed treatment with glycine betaine. Plant Growth Regul. 2005;46:133–141. doi: 10.1007/s10725-005-8379-5. [DOI] [Google Scholar]
- 52.Hayashi H, Sakamoto A, Murata N. Enhancement of the tolerance of Arabidopsis to high temperatures by genetic engineering of the synthesis of glycine betaine. Plant J. 1998;16(2):155–161. doi: 10.1046/j.1365-313x.1998.00284.x. [DOI] [PubMed] [Google Scholar]
- 53.Rasheed R, Wahid A, Farooq M, Hussain I, Basra SM. Role of proline and glycine betaine pre-treatments in improving heat tolerance of sprouting sugarcane (Saccharumsp.) buds. Plant Growth Regul. 2011;65(1):35–45. doi: 10.1007/s10725-011-9572-3. [DOI] [Google Scholar]
- 54.Davey MW, Stals E, Panis B, Keulemans J, Swennen RL. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005;15(347(2)):201–7. doi: 10.1016/j.ab.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 55.Moller IM, Jensen PE, Hansson A. Review oxidative modifications to cellular components in plants. Ann. Rev. Plant Biol. 2007;58:459–81. doi: 10.1146/annurev.arplant.58.032806.103946. [DOI] [PubMed] [Google Scholar]
- 56.Rahal Anu, Kumar Amit, Singh Vivek, Yadav Brijesh, Tiwari Ruchi, Chakraborty Sandip, Dhama Kuldeep. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Research International. 2014;2014:1–19. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siddiqui MH, et al. Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi J. Biol. Sci. 2015;22(5):656–663. doi: 10.1016/j.sjbs.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Savicka, M. &Skute, N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.) Ekologija. 56, 1–2, 26–33 (2010).
- 59.Liu X, Huang B. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 2000;40(2):503–510. doi: 10.2135/cropsci2000.402503x. [DOI] [Google Scholar]
- 60.Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006;171(3):382–388. doi: 10.1016/j.plantsci.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Wang WB, et al. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol.Biochem. 2009;47(7):570–577. doi: 10.1016/j.plaphy.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Konigshofer H, Tromballa HW, Loppert HG. Early events in signalling high-temperature stress in tobacco BY2 cells involve alterations in membrane fluidity and enhanced hydrogen peroxide production. Plant, Cell & Environ. 2008;31:1771–1780. doi: 10.1111/j.1365-3040.2008.01880.x. [DOI] [PubMed] [Google Scholar]
- 63.Sairam RK, Srivastava GC, Saxena DC. Increased antioxidant activity under elevated temperatures: a mechanism of heat stress tolerance in wheat genotypes. Biologia Plantarum. 2000;43(2):245–251. doi: 10.1023/A:1002756311146. [DOI] [Google Scholar]
- 64.Yin H, Chen Q, Yi M. (2008). Effects of short-term heat stress on oxidative damage and responses of antioxidant system in Lilium longiflorum. Plant Growth Regul. 2008;54(1):45–54. doi: 10.1007/s10725-007-9227-6. [DOI] [Google Scholar]
- 65.Rivero RM, Ruiz JM, Romero L. Oxidative metabolism in tomato plants subjected to heat stress. J.Hortic.Sci. Biotechnol. 2004;79(4):560–564. doi: 10.1080/14620316.2004.11511805. [DOI] [Google Scholar]
- 66.Varshney RK, Nayak SN, May GD, Jackson SA. Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol. 2009;27(9):522–530. doi: 10.1016/j.tibtech.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 67.Barrera-Figueroa BE, et al. High throughput sequencing reveals novel and abiotic stress-regulated microRNAs in the inflorescences of rice. BMC Plant Bio. 2012;12(1):132. doi: 10.1186/1471-2229-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fox SE, et al. De Novo transcriptome assembly and analyses of gene expression during Photomorphogenesis in diploid wheat (Triticum monococcum) Plos One. 2014;9(5):e96855. doi: 10.1371/journal.pone.0096855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arun-Chinnappa KS, McCurdy DW. De novo assembly of a genome-wide transcriptome map of Vicia faba (L.) for transfer cell research. Front. Plant Sci. 2015;6:217. doi: 10.3389/fpls.2015.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barrios A, et al. Deep Super-SAGE transcriptomic analysis of cold acclimation in lentil (Lens culinarisMedik.) BMC Plant Bio. 2017;17(1):111. doi: 10.1186/s12870-017-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Temel HY, Gol D, Kahriman A, Tanyolac MB. Single nucleotide polymorphism discovery through Illumina- based transcriptome sequencing and mapping in lentil. Turk. J. Agric. 2014;38:1–19. doi: 10.3906/tar-1409-70. [DOI] [Google Scholar]
- 72.Sudheesh, S., Verma, P., Forster, J. W., Cogan, N. O. I. & Kaur, S. Generation and characterisation of a reference transcriptome for lentil (Lens culinaris Medik.). Int. J. Mol. Sci. 17(11), 1887, 10.3390/ijms17111887 (2016). [DOI] [PMC free article] [PubMed]
- 73.Kotak S, et al. Complexity of the heat stress response in plants. Curr Opin Plant Biol. 2007;10(3):310–316. doi: 10.1016/j.pbi.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 74.Qin D, et al. Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat (Triticum aestivum L.) by using Wheat Genome Array. BMC Genomics. 2008;9(1):432. doi: 10.1186/1471-2164-9-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li YF, Wang Y, Tang Y, Kakani VG, Mahalingam R. Transcriptome analysis of heat stress response in switchgrass (Panicum virgatum L.) BMC Plant Bio. 2013;13(1):153. doi: 10.1186/1471-2229-13-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang KA, et al. Identification of cell wall genes modified by a permissive high temperature in Chinese cabbage. Plant Sci. 2006;171(1):175–182. doi: 10.1016/j.plantsci.2006.03.013. [DOI] [Google Scholar]
- 77.Mangelsen E, et al. Transcriptome analysis of high-temperature stress in developing barley caryopses: early stress responses and effects on storage compound biosynthesis. Mol. Plant. 2011;4:97–115. doi: 10.1093/mp/ssq058. [DOI] [PubMed] [Google Scholar]
- 78.Le Gall H, et al. Cell wall metabolism in response to abiotic stress. Plants. 2015;4:112–166. doi: 10.3390/plants4010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lima RB, et al. Heat stress causes alterations in the cell-wall polymers and anatomy of coffee leaves (Coffea arabica L.) Carbohydr. Polym. 2013;93:135–143. doi: 10.1016/j.carbpol.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 80.Simpson C, Thomas C, Findlay K, Bayer E, Maule AJ. An Arabidopsis GPI-anchor plasmodesmal neck protein with callose binding activity and potential to regulate cell-to-cell trafficking. Plant Cell. 2009;21(2):581–594. doi: 10.1105/tpc.108.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fitzgibbon, J. Structure and development of complex plasmodesmata. Ph.D. thesis, University of Edinburgh (2012).
- 82.Chen XY, Kim JY. Callose synthesis in higher plants. Plant Signal Behav. 2009;4(6):489–492. doi: 10.4161/psb.4.6.8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rinne PL, Van der Schoot C. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development. 1998;125(8):1477–1485. doi: 10.1242/dev.125.8.1477. [DOI] [PubMed] [Google Scholar]
- 84.Dong X, Hong Z, Sivaramakrishnan M, Mahfouz M, Verma DP. Callose synthase (CalS5) is required for exine formation during microgametogenesis and for pollen viability in Arabidopsis. Plant J. 2005;42(3):315–328. doi: 10.1111/j.1365-313X.2005.02379.x. [DOI] [PubMed] [Google Scholar]
- 85.Nishikawa S, Zinkl GM, Swanson RJ, Maruyama D, Preuss D. Callose (beta-1, 3-glucan) is essential for Arabidopsis pollen wall patterning, but not tube growth. BMC Plant Biol. 2005;5:22. doi: 10.1186/1471-2229-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi J, Yan B, Lou X, Ma H, Ruan S. Comparative transcriptome analysis reveals the transcriptional alterations in heat-resistant and heat-sensitive sweet maize (Zea mays L.) varieties under heat stress. BMC Plant Biol. 2017;17:26–36. doi: 10.1186/s12870-017-0973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yasuhiro H, Yozo O, Fumiyoshi M, Kazuo S, Kazuki S. Landscape of the lipidome and transcriptome under heat stress in Arabidopsis thaliana. Sci. Rep. 2015;5:10533. doi: 10.1038/srep10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liscovitch M, Cantley LC. Signal transduction and membrane traffic: the PITP/phosphoinositide connection. Cell. 1995;81(5):659–662. doi: 10.1016/0092-8674(95)90525-1. [DOI] [PubMed] [Google Scholar]
- 89.Müller F, Frentzen M. Phosphatidylglycerophosphate synthases from Arabidopsis thaliana. FEBS Letters. 2001;509(2):298–302. doi: 10.1016/S0014-5793(01)03163-5. [DOI] [PubMed] [Google Scholar]
- 90.Xu C, et al. The pgp1 mutant locus of Arabidopsis encodes a phosphatidylglycerolphosphate synthase with impaired activity. Plant Physiol. 2002;129(2):594–604. doi: 10.1104/pp.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng Z, et al. Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. The Plant Cell. 2003;15(8):1872–1887. doi: 10.1105/tpc.012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao Q, Shang Y, Huang W, Wang C. Glycerol-3-phosphate acyltransferase contributes to triacylglycerol biosynthesis, lipid droplet formation, and host invasion in Metarhiziumrobertsii. Appl. Environ. Microbiol. 2013;79(24):7646–7653. doi: 10.1128/AEM.02905-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ishizaki O, Nishida I, Agata K, Eguchi G, Murata N. Cloning and nucleotide sequence of cDNA for the plastid glycerol‐3‐phosphate acyltransferase from squash. FEBS Letters. 1988;238(2):424–430. doi: 10.1016/0014-5793(88)80525-8. [DOI] [PubMed] [Google Scholar]
- 94.Men X, et al. Glycerol-3-Phosphate Acyltransferase 3 (OsGPAT3) is required for anther development and male fertility in rice. J. Exp. Bot. 2017;68(3):513–526. doi: 10.1093/jxb/erw445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bertrams M, Heinz E. Positional specificity and fatty acid selectivity of purified sn-glycerol 3-phosphate acyltransferases from chloroplasts. Plant Physiol. 1981;68(3):653–657. doi: 10.1104/pp.68.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalscheuer R, Steinbüchel A. A novel bifunctional wax ester synthase/acyl-CoA: diacylglycerol acyltransferase mediates wax ester and triacylglycerol biosynthesis in Acinetobacter calcoaceticus ADP1. J. Biol. Chem. 2003;278(10):8075–8082. doi: 10.1074/jbc.M210533200. [DOI] [PubMed] [Google Scholar]
- 97.Li F, et al. Identification of the wax ester synthase/acyl-coenzyme A: diacylglycerol acyltransferase WSD1 required for stem wax ester biosynthesis in Arabidopsis. PlantPhysiol. 2008;148(1):97–107. doi: 10.1104/pp.108.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hu Z, Ren Z, Lu C. The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy fatty acid accumulation in transgenic Arabidopsis. Plant Physiol. 2012;158(4):1944–1954. doi: 10.1104/pp.111.192153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bates PD, Fatihi A, Snapp AR, Carlsson AS, Lu C. Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol. 2012;160(3):1530–1539. doi: 10.1104/pp.112.204438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Buchanan, B. B., Gruissem, W. & Jones, R. L. eds, 2015. Biochemistry and molecular biology of plants. John Wiley & Sons.
- 101.Bita EC, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013;4:273. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wahid A, Ghazanfar A. Possible involvement of some secondary metabolites in salt tolerance of sugarcane. J. Plant Physiol. 2006;163:723–730. doi: 10.1016/j.jplph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 103.Trejo-Tellez LI, Stenzel R, Gomez-Merino FC, Schmitt JM. Transgenic tobacco plants over-expressing pyruvate phosphate dikinase increase exudation of organic acids and decrease accumulation of aluminum in the roots. Plant Soil. 2010;326:187–198. doi: 10.1007/s11104-009-9994-0. [DOI] [Google Scholar]
- 104.Hyskova VD, Miedzinska L, Dobra J, Vankova R, Ryslava H. Phosphoenolpyruvate carboxylase, NADP-malic enzyme, and pyruvate, phosphate dikinase are involved in the acclimation of Nicotiana tabacum L. to drought stress. J Plant Physiol. 2014;171:19–25. doi: 10.1016/j.jplph.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 105.Vered T, Gad G. The biosynthetic pathways for shikimate and aromatic amino acids in Arabidopsis thaliana. Arabidopsis Book. 2010;8:e0132. doi: 10.1199/tab.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chastain CJ, et al. The pyruvate, orthophosphate dikinase regulatory proteins of Arabidopsis possess a novel, unprecedented Ser/Thr protein kinase primary structure. Plant J. 2008;53(5):854–863. doi: 10.1111/j.1365-313X.2007.03366.x. [DOI] [PubMed] [Google Scholar]
- 107.Kaur S, et al. Transcriptome sequencing of lentil based on second-generation technology permits large-scale unigene assembly and SSR marker discovery. BMC Genomics. 2011;12:265–275. doi: 10.1186/1471-2164-12-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wu C, et al. Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in rice. Front Plant Sci. 2017;8:37110.3389/fpls.2017.00371. doi: 10.3389/fpls.2017.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yan Q, et al. GmCYP82A3, a soybean cytochrome P450 family gene involved in the jasmonic acid and ethylene signaling pathway, enhances plant resistance to biotic and abiotic stresses. Plos One. 2016;11(9):e0162253. doi: 10.1371/journal.pone.0162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang W, Vinocur B, Shoseyov O, Altman A. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci. 2004;9(5):244–252. doi: 10.1016/j.tplants.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 111.Larkindale J, Mishkind M, Vierling E. Plant responses to high temperature. Plant Abiotic Stress. 2005;100:144. [Google Scholar]
- 112.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 113.Swindell WR, Huebner M, Weber AP. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics. 2007;8(1):125. doi: 10.1186/1471-2164-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang AH, et al. Comparative transcriptome analysis reveals heat-responsive genes in chinese cabbage (Brassica rapa ssp. chinensis) Front. Plant Sci. 2016;7:939. doi: 10.3389/fpls.2016.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Los DA, Murata N. Membrane fluidity and its roles in the perception of environmental signals. Biochimicaet Biophysica Acta. 2004;1666:142–157. doi: 10.1016/j.bbamem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 116.Goto-Yamada S, et al. Chaperone and protease functions of LON protease 2 modulate the peroxisomal transition and degradation with autophagy. Plant Cell Physiol. 2014;55(3):482–496. doi: 10.1093/pcp/pcu017. [DOI] [PubMed] [Google Scholar]
- 117.Koo AJ, Chung HS, Kobayashi Y, Howe GA. Identification of a peroxisomal acyl-activating enzyme involved in the biosynthesis of jasmonic acid in Arabidopsis. J. Biol. Chem. 2006;281(44):33511–33520. doi: 10.1074/jbc.M607854200. [DOI] [PubMed] [Google Scholar]
- 118.Stout JM, Boubakir Z, Ambrose SJ, Purves RW, Page JE. The hexanoyl‐CoA precursor for cannabinoid biosynthesis is formed by an acyl‐activating enzyme in Cannabis sativa trichomes. Plant J. 2012;71(3):353–365. doi: 10.1111/j.1365-313X.2012.04949.x. [DOI] [PubMed] [Google Scholar]
- 119.Moriyama Y, Hiasa M, Matsumoto T, Omote H. Multidrug and toxic compound extrusion (MATE)-type proteins as anchor transporters for the excretion of metabolic waste products and xenobiotics. Xenobiotica. 2008;38(7-8):1107–1118. doi: 10.1080/00498250701883753. [DOI] [PubMed] [Google Scholar]
- 120.Wang L, et al. The similar and different evolutionary trends of MAT E family occurred between rice and Arabidopsis thaliana. BMC Plant Biol. 2016;16(1):207. doi: 10.1186/s12870-016-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sato Y, et al. Isolation and characterization of a novel peroxidase gene ZPO-C whose expression and function are closely associated with lignification during tracheary element differentiation. Plant Cell Physiol. 2006;47(4):493–503. doi: 10.1093/pcp/pcj016. [DOI] [PubMed] [Google Scholar]
- 122.Welinder KG, et al. Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. FEBS J. 2002;269(24):6063–6081. doi: 10.1046/j.1432-1033.2002.03311.x. [DOI] [PubMed] [Google Scholar]
- 123.Brikis, C. Characterization of glyoxylate/succinic semialdehyde reductases in plants and impact of elevated CO2 on γ-aminobutyrate metabolism in ‘Empire’ apple fruit stored under controlled atmosphere conditions, http://hdl.handle.net/10214/9105 (2015).
- 124.Simpson JP, et al. Identification and characterization of a plastid-localized Arabidopsis glyoxylate reductase isoform: comparison with a cytosolic isoform and implications for cellular redox homeostasis and aldehyde detoxification. J. Exp.Bot. 2008;59(9):2545–2554. doi: 10.1093/jxb/ern123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hong Z, Lakkineni K, Zhang Z, Verma DPS. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. P. Physiol. 2000;122(4):1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anwar A, She M, Wang K, Riaz B, Ye X. Biological roles of ornithine aminotransferase (OAT) in plant stress tolerance: Present progress and future perspectives. Int. J Mol. Sci. 2018;19(11):3681. doi: 10.3390/ijms19113681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zandalinas SI, et al. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 2016;67(18):5381–5390. doi: 10.1093/jxb/erw299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang YC, Niu CY, Yang CR, Jinn TL. The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol. 2016;172(2):1182–1199. doi: 10.1104/pp.16.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang X, Zhuang L, Shi Y, Huang B. Up-regulation of HSFA2c and HSPs by ABA contributing to improved heat tolerance in tall fescue and Arabidopsis. Int. J. Mol.Sci. 2017;18(9):1981. doi: 10.3390/ijms18091981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu T, et al. Identification of proteins regulated by ABA in response to combined drought and heat stress in maize roots. ActaPhysiol.Plant. 2013;35(2):501–513. [Google Scholar]
- 131.Morishita T, et al. Arabidopsis NAC transcription factor, ANAC078, regulates flavonoid biosynthesis under high-light. Plant Cell Physiol. 2009;50:2210–2222. doi: 10.1093/pcp/pcp159. [DOI] [PubMed] [Google Scholar]
- 132.Hu H, et al. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol. Biol. 2008;67,:169–181. doi: 10.1007/s11103-008-9309-5. [DOI] [PubMed] [Google Scholar]
- 133.Guo W, et al. The wheat NAC transcription factor TaNAC2L is regulated at the transcriptional and post-translational levels and promotes heat stress tolerance in transgenic Arabidopsis. Plos One. 2015;10(8):e0135667. doi: 10.1371/journal.pone.0135667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li S, Fu Q, Huang W, Yu D. Functional analysis of an Arabidopsis transcription factor WRKY25 in heat stress. Plant Cell Rep. 2009;28(4):683–693. doi: 10.1007/s00299-008-0666-y. [DOI] [PubMed] [Google Scholar]
- 135.Hou, S. et al. Transcriptomic analysis, genic SSR development, and genetic diversity of proso millet (Panicummiliaceum; Poaceae). Appl. Plant Sci. 5(7) (2017). [DOI] [PMC free article] [PubMed]
- 136.Chapple C. Molecular-genetic analysis of plant cytochrome P450-dependent monooxygenases. Annu. Rev. Plant Biol. 1998;49(1):311–343. doi: 10.1146/annurev.arplant.49.1.311. [DOI] [PubMed] [Google Scholar]
- 137.Schuler MA. Plant cytochrome P450 monooxygenases. Cri.Rev.Plant Sci. 1996;15(3):235–284. doi: 10.1080/07352689609701942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martinoia E, et al. Multifunctionality of plant ABC transporters–more than just detoxifiers. Planta. 2002;214(3):345–355. doi: 10.1007/s004250100661. [DOI] [PubMed] [Google Scholar]
- 139.Huang CF, et al. A bacterial-type ABC transporter is involved in aluminum tolerance in rice. The Plant Cell. 2009;21(2):655–667. doi: 10.1105/tpc.108.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu L, et al. OsABCG15 encodes a membrane protein that plays an important role in anther cuticle and pollen exine formation in rice. P. Cell Rep. 2014;33(11):1881–1899. doi: 10.1007/s00299-014-1666-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ma C, Haslbeck M, Babujee L, Jahn O, Reumann S. Identification and characterization of a stress-inducible and a constitutive small heat-shock protein targeted to the matrix of plant peroxisomes. Plant Physiol. 2006;141(1):47–60. doi: 10.1104/pp.105.073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sato Y, Yokoya S. Enhanced tolerance to drought stress in transgenic rice plants over-expressing a small heat-shock protein, sHSP17.7. Plant Cell Rep. 2008;27(2):329–334. doi: 10.1007/s00299-007-0470-0. [DOI] [PubMed] [Google Scholar]
- 143.Zou J, Liu C, Liu A, Zou D, Chen X. Over-expression of OsHsp17.0 and OsHsp23.7 enhances drought and salt tolerance in rice. J. Plant Physiol. 2012;169(6):628–635. doi: 10.1016/j.jplph.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 144.Sharma, R. Study of glycosyl transferases and other stress-related proteins encoded in chickpea genome and analysis of their structures and functions using molecular modeling and docking, http://ncl.csircentral.net/1635/1/Thesis.pdf (2014).
- 145.Woo HH, Jeong BR, Hirsch AM, Hawes MC. Characterization of Arabidopsis AtUGT85A and AtGUS gene families and their expression in rapidly dividing tissues. Genomics. 2007;90(1):143–153. doi: 10.1016/j.ygeno.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset for two genotypes supporting the conclusions of this article is deposited in Sequence Read Archive repository (SRA) with SRA submission ID – SUB3390924. [http://www.ncbi.nlm.nih.gov/sra].