Abstract
This study was conducted to investigate the effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors on individual renal outcomes in patients with type 2 diabetes. We searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from inception to September 2017 to identify randomized controlled trials comparing SGLT2 inhibitors with placebo or antidiabetic drugs and reporting any renal outcomes in patients with type 2 diabetes. Additionally, we identified 4 articles which were published after the predefined period to include relevant data. A meta-analysis was performed to calculate weighted mean differences (WMDs) and relative risks (RRs) with 95% confidence intervals (CIs) for each renal outcome. We included 48 studies involving 58,165 patients in the analysis. SGLT2 inhibitors significantly lowered urine albumin-to-creatinine ratio (UACR) (WMD, −14.64 mg/g; 95% CI, −25.15 to −4.12; P = 0.006) compared with controls. The UACR-lowering effects of SGLT2 inhibitors were greater with a higher baseline UACR. Overall changes in estimated glomerular filtration rate (eGFR) were comparable between two groups (WMD, 0.19 mL/min/1.73 m2; 95% CI, −0.44 to 0.82; P = 0.552). However, SGLT2 inhibitors significantly slowed eGFR decline in patients with a higher baseline eGFR and a longer duration of treatment. Compared with controls, SGLT2 inhibitors significantly reduced the risk of microalbuminuria (RR, 0.69; 95% CI, 0.49 to 0.97; P = 0.032), macroalbuminuria (RR, 0.49; 95% CI, 0.33 to 0.73; P < 0.001), and worsening nephropathy (RR, 0.73; 95% CI, 0.58 to 0.93; P = 0.012). In addition, the risk of end-stage renal disease was significantly lower in SGLT2 inhibitors than in controls (RR, 0.70; 95% CI, 0.57 to 0.87; P = 0.001). In conclusion, SGLT2 inhibitors had beneficial renal effects by lowering the risk of albuminuria development or progression and reducing the risk of end-stage renal disease compared with placebo or other antidiabetic drugs.
Subject terms: Diabetes complications, Type 2 diabetes
Introduction
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are a novel class of antidiabetic agents which lower blood glucose levels mainly by reducing glucose reabsorption in the renal proximal tubule, leading to an increase in urinary glucose and sodium excretion1–3. As a result of increased glycosuria and natriuresis, the beneficial effects of SGLT2 inhibitors extend beyond glycemic control to reducing intraglomerular hypertension, promoting plasma volume contraction, lowering blood pressure (BP), reducing body weight, and decreasing uric acid levels4,5. Given their intrarenal and extrarenal effects, SGLT2 inhibitors have been suggested to confer renoprotection in patients with type 2 diabetes.
Several studies have reported beneficial effects of SGLT2 inhibitors on the kidney6–10. In large clinical trials, empagliflozin and canagliflozin similarly reduced the risk of progression of albuminuria and the composite of sustained decrease in renal function, renal replacement therapy (RRT), or renal death compared with placebo6,9. Recently, dapagliflozin also reduced the composite renal outcome of 40% decrease in estimated glomerular filtration rate (eGFR), end-stage renal disease (ESRD), or renal death compared with placebo11. However, these studies were performed in patients with an established cardiovascular disease or high cardiovascular risk9,11,12. Therefore, it is difficult to conclude that the beneficial renal effects of SGLT2 inhibitors extend to overall patients, especially with low cardiovascular risk. Furthermore, individual renal outcomes of these studies have been inconsistent. In the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial, empagliflozin significantly lowered the risk of initiation of RRT but did not affect new-onset microalbuminuria6. In the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program, canagliflozin significantly reduced the risk of new-onset microalbuminuria and 40% decrease in eGFR with no difference in the need for RRT9,13. In both studies6,9, renal outcomes were secondary endpoints and the number of events indicating ESRD was not sufficient to provide conclusive information. Moreover, the Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 (DECLARE-TIMI 58) trial did not reported individual renal outcomes11. Consequently, the renoprotective effects of SGLT2 inhibitors need to be elucidated by more convincing evidence.
Concerns have also been raised over the adverse renal events in patients with SGLT2 inhibitor treatment because these agents are associated with a decrease in intravascular volume and an acute decline in eGFR14–16. Canagliflozin and dapagliflozin have increased the risk of acute kidney injury in patients who have predisposing factors including hypoglycemia, chronic kidney disease (CKD), heart failure, and potentially nephrotoxic drugs14,17,18.
In this regard, we conducted this systematic review and meta-analysis of randomized controlled trials (RCTs) to investigate the effects of SGLT2 inhibitors on individual renal outcomes compared with placebo or other antidiabetic drugs in patients with type 2 diabetes.
Methods
Study design
This systematic review and meta-analysis was performed based on a prespecified protocol developed by the authors (Supplementary Appendix 1), and the results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplementary Appendix 2)19.
Data sources and search strategy
We searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials to identify RCTs of SGLT2 inhibitors with full-text articles published from inception to September 2017, regardless of language and publication status. The search terms used for SGLT2 inhibitors were SGLT2 inhibitor or SGLT-2 inhibitor or canagliflozin or dapagliflozin or empagliflozin or ertugliflozin or ipragliflozin or luseogliflozin or remogliflozin or sergliflozin or tofogliflozin (Supplementary Appendix 3). In addition, we identified 4 articles11,20–22 which were published after the predefined period to include all relevant data.
Study selection
The RCTs comparing SGLT2 inhibitors with placebo or other antidiabetic drugs with ≥ 12 weeks of study duration in type 2 diabetes were included. We eliminated duplicate publications of original RCT and screened titles and abstracts. Among them, we selected RCTs that reported at least one of the following renal outcomes: changes in urine albumin-to-creatinine ratio (UACR) or eGFR, and incident microalbuminuria, macroalbuminuria, doubling of serum creatinine, renal failure, ESRD, RRT, dialysis, or kidney transplantation. The selection criteria for renal outcomes have been reported elsewhere23. Pooled analyses or secondary analyses were included when they provided additional information about renal outcomes beyond that found in original RCT articles. We included two publications of canagliflozin trials20,22 which were reported after the prespecified analysis considering the effect size and weight of these studies.
Data extraction
Two authors (J.H.B. and E.P.) independently extracted data according to the prespecified protocol. The procedure of extracting data from publications have been reported elsewhere23. Briefly, the renal outcomes of interests were changes in UACR and eGFR, incident microalbuminuria (UACR >30 mg/g) and macroalbuminuria (UACR >300 mg/g), worsening nephropathy (defined as development of microalbuminuria or macroalbuminuria from normoalbuminuria, or progression from microalbuminuria to macroalbuminuria), and the development of ESRD. We extracted mean changes from baseline and their standard deviations for continuous variables in the intervention (SGLT2 inhibitors) and control (placebo or other antidiabetic drugs) groups and used them as the summary measures. The number of patients reporting each renal outcome was extracted for dichotomous variables. We also collected information about the first author, publication year, number and mean age of randomized participants, study duration, intervention and comparison treatment, background therapy for glycemic control, baseline UACR and eGFR, and the history of cardiovascular disease, heart failure, and CKD (eGFR <60 mL/min/1.73 m2).
Quality assessment
We used the Cochrane Risk of Bias Tool to assess quality and risk of bias for included studies24. Two authors (J.H.B. and E.P.) independently reviewed each RCT and classified the risk of bias as adequate (low risk of bias), unclear (unclear risk of bias), or inadequate (high risk of bias) based on six aspects of trials: sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective reporting, and other sources of bias24. Any disagreements were resolved by consensus among the authors (J.H.B., N.H.K., and S.H.).
Data synthesis and analysis
We calculated weighted mean differences (WMDs) with 95% confidence intervals (CIs) to assess effect size for continuous variables including UACR and eGFR. We also calculated the relative risks (RRs) with 95% CIs to estimate effect size for dichotomous variables including microalbuminuria, macroalbuminuria, worsening nephropathy, and ESRD. In the meta-analysis, we used a random effects model to combine estimators. We also considered a fixed effect model additionally for exploration of the discrepancy in results. The I2 statistic, τ2 statistic, and Cochran’s Q test were used to assess statistical heterogeneity among the studies25. We regarded the I2 statistic of 0% to 40%, 30% to 60%, 50% to 90%, and 75% to 100% as not significant, moderate, substantial, and considerable heterogeneity, respectively26. To detect reporting bias, such as publication bias, asymmetry in the funnel plot was evaluated for renal outcomes27,28. We performed a subgroup analysis of eGFR based on baseline eGFR (<60, 60–90, and >90 mL/min/1.73 m2) and study duration (<26, 26–52, and >52 weeks), and sensitivity analyses of microalbuminuria, macroalbuminuria, worsening nephropathy, and ESRD by considering only canagliflozin, dapagliflozin, and empagliflozin for SGLT2 inhibitors. Additionally, prespecified meta-regression was conducted for changes in UACR according to baseline UACR and for changes in eGFR according to baseline eGFR and study duration, respectively. Some outliers were eliminated by diagnostic measures29. All statistical analyses were performed using R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria). P values of < 0.05 and <0.10 were regarded as statistically significant for treatment effects and test for heterogeneity, respectively.
Results
Characteristics of included studies
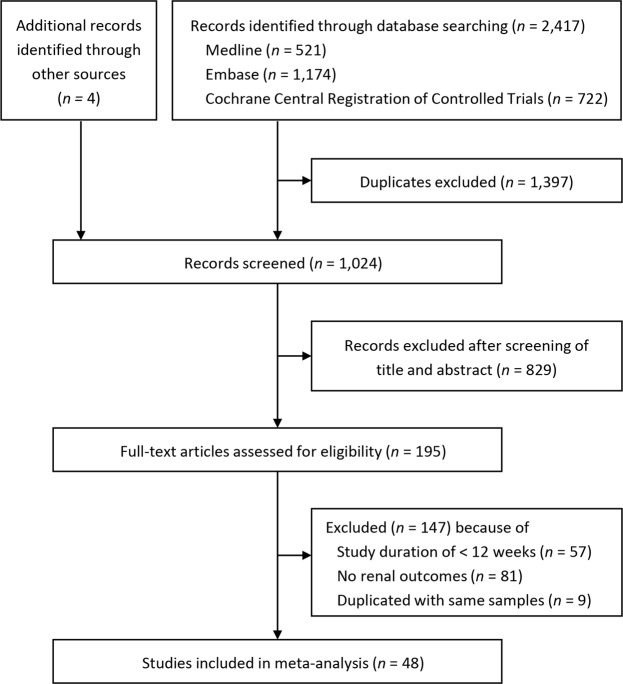
The study screening and selection process is shown in Fig. 1. Of 2,421 records retrieved through the database search, 48 studies were included in the systematic review and meta-analysis. Two of the 48 studies7,30 were pooled analyses of five RCTs31–35 and another five RCTs15,36–39. The characteristics of the included studies and their participants are described in Supplementary Table 1. The total number of participants was 58,165 (34,661 in the SGLT2 inhibitor group and 23,504 in the control group). The number of participants in each study ranged from 114 to 10,142. Three studies had a duration of 187 to 296 weeks6,9,22, whereas the remaining studies had a duration ranging from 12 to 104 weeks. The baseline eGFR of the participants was ≥55 (or 60) mL/min/1.73 m2 in 24 studies4,8,38–59, ≥30 mL/min/1.73 m2 in 14 studies6,7,9,15,20,22,30,32–34,60–63, ≥20 mL/min/1.73 m2 and in 1 study21. In one study, 74 of 741 participants had an eGFR of ≥15 and <30 mL/min/1.73 m2 at baseline35.
Figure 1.
Study screening and selection process.
Assessment of study quality and risk of bias
The risk of bias assessment is summarized in Supplementary Fig. 1. Forty-four of the 48 studies reported adequate random sequence generation and adequate allocation concealment. Three studies did not describe the method of sequence generation and allocation concealment15,63,64. All 48 studies described adequate blinding of participants and personnel. Moreover, 14 studies reported incomplete outcome data due to losses to follow-up15,30,42,46,48–51,58,60–62,64,65. One study had the possibility of selective reporting in the dapagliflozin 5 mg group40.
Changes in UACR and eGFR
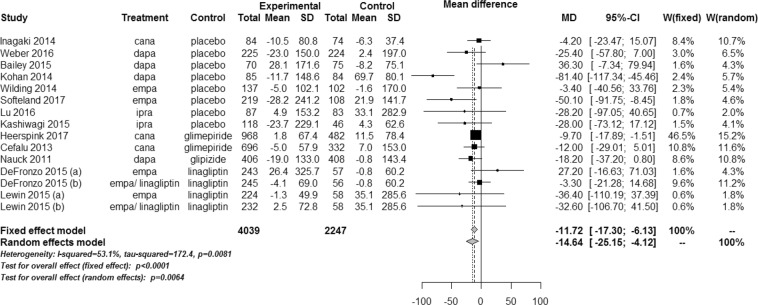
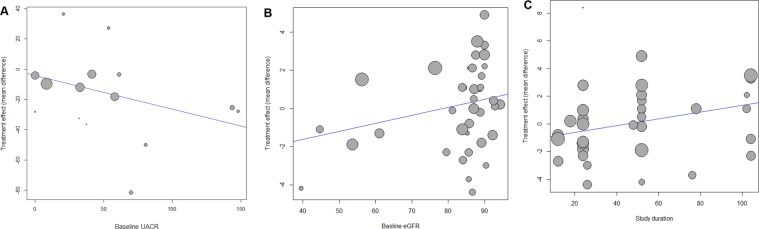
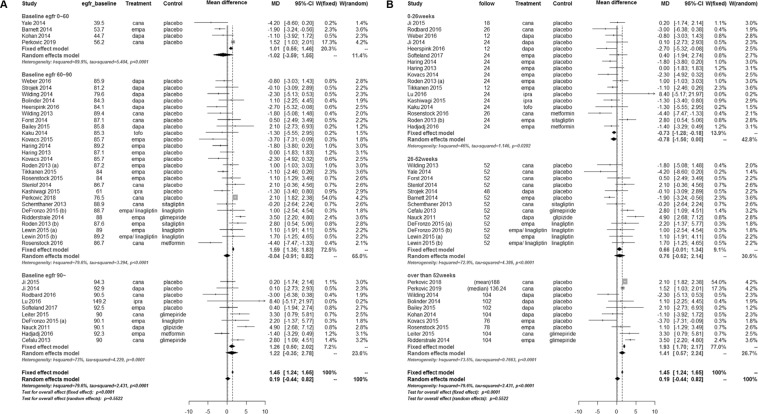
SGLT2 inhibitors significantly lowered the UACR compared with controls (WMD, −14.64 mg/g; 95% CI, −25.15 to −4.12; P = 0.006) (Fig. 2). The test for heterogeneity showed moderate heterogeneity across the studies (I2 = 53.1%; P = 0.008). In the meta-regression, the UACR-lowering effects of SGLT2 inhibitors tended to be greater with higher levels of baseline UACR (P = 0.081) (Fig. 3A). The changes in eGFR were not significantly different between SGLT2 inhibitors and controls (WMD, 0.19 mL/min/1.73 m2; 95% CI, −0.44 to 0.82; P = 0.552) (Fig. 4A,B). The test for heterogeneity for this showed substantial heterogeneity across the studies (I2 = 79.6%; P < 0.001). There was a large discrepancy noted in estimated treatment effects between fixed effect and random effects models, depending on weights given to two large trials20,22. However, SGLT2 inhibitors significantly slowed the decline in eGFR in patients with >52 weeks of treatment duration compared with controls (Fig. 4B). In the meta-regression, the decline in eGFR were slower in patients with a higher baseline eGFR (P = 0.116) (Fig. 3B) and a longer duration of follow-up (P = 0.038) (Fig. 3C).
Figure 2.
Weighted mean differences in changes in urine albumin-to-creatinine ratio from baseline (mg/g) for sodium-glucose cotransporter 2 inhibitors versus placebo or other antidiabetic drugs. CI, confidence interval; MD, mean difference; SD, standard deviation; W, weight.
Figure 3.
Meta-regression of changes in urine albumin-to-creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) for sodium-glucose cotransporter 2 inhibitors versus placebo or other antidiabetic drugs. (A) Changes in UACR according to baseline UACR. (B) Changes in eGFR according to baseline eGFR. (C) Changes in eGFR according to treatment duration.
Figure 4.
Weighted mean differences in estimated glomerular filtration rate from baseline (mL/min/1.73 m2) for sodium-glucose cotransporter 2 inhibitors versus placebo or other antidiabetic drugs. (A) According to baseline estimated glomerular filtration rate. (B) According to treatment duration. CI, confidence interval; MD, mean difference; SD, standard deviation; W, weight.
Development or progression of albuminuria
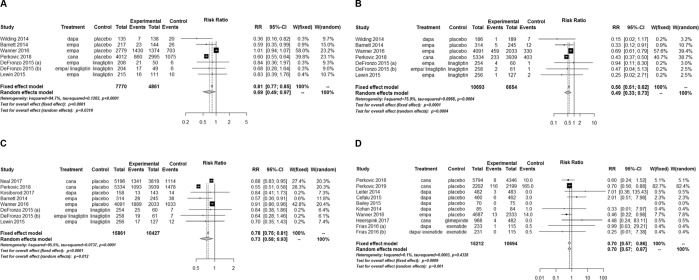
SGLT2 inhibitors significantly lower the risk of developing microalbuminuria compared with controls (RR, 0.69; 95% CI, 0.49 to 0.97; P = 0.032) (Fig. 5A). The beneficial effects on microalbuminuria were primarily driven by one large trial with canagliflozin22. The test for heterogeneity showed considerable heterogeneity across the studies (I2 = 94.7%; P < 0.001). SGLT2 inhibitors significantly lowered the risk of developing macroalbuminuria compared with controls (RR, 0.49; 95% CI, 0.33 to 0.73; P < 0.001) (Fig. 5B). There was substantial heterogeneity across the studies for this (I2 = 75.9%; P < 0.001). In addition, SGLT2 inhibitors significantly lowered the risk of worsening nephropathy compared with controls (RR, 0.73; 95% CI, 0.58 to 0.93; P = 0.012) (Fig. 5C). There was considerable heterogeneity across the studies (I2 = 95.5%; P < 0.001). In subgroup analyses, canagliflozin reduced both the risk of microalbuminuria and macroalbuminuria. On the other hand, dapagliflozin and empagliflozin reduce the risk of microalbuminuria and macroalbuminuria, respectively (Supplementary Fig. 2A–C).
Figure 5.
Relative risks of microalbuminuria, macroalbuminuria, worsening nephropathy, and end-stage renal disease for sodium-glucose cotransporter 2 inhibitors versus placebo or other antidiabetic drugs. (A) Microalbuminuria. (B) Macroalbuminuria. (C) Worsening nephropathy. (D) End-stage renal disease. CI, confidence interval; RR, relative risk; W, weight.
Development of ESRD
SGLT2 inhibitors significantly reduced the risk of ESRD compared with controls (RR, 0.70; 95% CI, 0.57 to 0.87; P = 0.001) (Fig. 5D). The number of events was 151 of 15,212 and 194 of 10,694 participants in the SGLT2 inhibitor and control groups, respectively. Heterogeneity was regarded as not significant across the studies (I2 = 0.1%; P = 0.433).
Assessment of funnel plot asymmetry
In the changes in UACR and eGFR, the funnel plots did not show any notable asymmetry apart from a few outlying values (Supplementary Fig. 3A,B). Although it was hard to determine asymmetry of the plots for in incident microalbuminuria, incident macroalbuminuria, and worsening nephropathy (Supplementary Fig. 4A–C) due to the small number of studies, the funnel plot still appeared to be quite symmetric in the development of ESRD (Supplementary Fig. 4D).
Discussion
This systematic review and meta-analysis found that SGLT2 inhibitors were associated with a significantly lower risk of development or progression of albuminuria compared with placebo or other antidiabetic drugs in patients with type 2 diabetes. The UACR-lowering effects of SGLT2 inhibitors were associated with a higher baseline UACR. The overall changes in eGFR were not different between two groups. However, SGLT2 inhibitors slowed the decline in eGFR in patients with a higher baseline eGFR and a longer duration of treatment. In addition, SGLT2 inhibitor significantly reduced the risk of ESRD compared with controls.
Considering the direct action of SGLT2 inhibitors on the renal tubules and their favorable effects on BP, body weight, and heart failure, these agents have been suggested theoretically to improve renal outcomes in patients with type 2 diabetes5,66. The large clinical trials already showed improvement in the composite renal outcomes with SGLT2 inhibitors6,9,11. However, these studies were conducted in patients with an average age of 60 years, a long diabetes duration of about 10 years, and an established cardiovascular disease or high cardiovascular risk. In the present study, we demonstrated that SGLT2 inhibitors had the renoprotective effects by reducing the risk of albuminuria and ESRD in patients with a wide range of cardiovascular risk.
SGLT2 inhibitors may reduce albuminuria by several mechanisms including a decrease in glomerular hyperfiltration67, improvement in tubulointerstitial fibrosis68, systemic BP reduction69, changes in plasma volume expansion70, and a decrease in uric acid levels71. In patients with type 2 diabetes and either microalbuminuria or macroalbuminuria, empagliflozin reduced the UACR independent of changes in hemoglobin A1c (HbA1c), BP, and body weight7. Dapagliflozin also reduced the UACR for over 2 years of treatment in patients with type 2 diabetes and stage 3 CKD regardless of changes in HbA1c, BP, eGFR, and uric acid72. These findings suggest that SGLT2 inhibitors reduce albuminuria through their direct effects on the kidney. In diabetic mice, SGLT2 inhibitors reduced albuminuria by ameliorating intraglomerular hypertension and tubulointerstitial fibrosis73,74, which are the two key contributors to renal damage in diabetic kidney disease (DKD). In line with these findings, our meta-analysis showed that albuminuria-lowering effects of SGLT2 inhibitors were higher on macroalbuminuria than on microalbuminuria. It could be partly explained by the greater UACR reduction in patients with a higher baseline UACR after SGLT2 inhibitor treatment. Therefore, SGLT2 inhibitors may have beneficial effects on albuminuria in the later stage rather than the early stage of DKD, which needs to be evaluated in further studies.
The overall changes in eGFR showed no difference between SGLT2 inhibitors and controls. In the subgroup-analysis and meta-regression, we found that changes in renal function were affected by baseline eGFR and duration of treatment. The changes in renal function after SGLT2 inhibitor treatment were characterized by a rapid decline in eGFR within the first 4–5 weeks, followed by progressive recovery over time15,35,50. In addition, the decrease in eGFR was reversible within 2 weeks after drug discontinuation35. These findings indicate that the changes in eGFR with SGLT2 inhibitor treatment were a consequence of the drug’s hemodynamic effects. In patients with type 1 diabetes, empagliflozin attenuated renal hyperfiltration accompanied by a decrease in eGFR through affecting tubuloglomerular feedback67. Reduction in renal hyperfiltration may be beneficial against progressive decline in renal function because intraglomerular hyperfiltration increases the risk of development and progression of DKD75–77. Besides intrarenal effects, SGLT2 inhibitors may affect eGFR by reducing BP and body weight. In two recent trials, dapagliflozin and empagliflozin similarly maintained BP and weight reduction despite a decline in renal function, whereas HbA1c reduction was decreased78,79, suggesting that they may slow the progressive decline in renal function independent of their glucose-lowering effects8.
Finally, SGLT2 inhibitors significantly reduced the risk of ESRD compared with placebo or other antidiabetic drugs. The direction of treatment effects of dapagliflozin was different from those of canagliflozin and empagliflozin but it did not change the overall treatment effect. Considering their beneficial effects on albuminuria, progressive eGFR decline, and glomerular hyperfiltration4, SGLT2 inhibitors have been expected to improve hard renal outcomes. In the Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial, which evaluated the primary composite renal outcome, canagliflozin showed 30% risk reduction of the composite of ESRD, doubling of serum creatinine, or renal or cardiovascular death, and 32% of risk reduction of ESRD compared with placebo in patients with type 2 diabetes and albuminuric CKD22. All the patients in this study were receiving angiotensin-converting-enzyme inhibitor or angiotensin-receptor blocker22. Interestingly, the magnitude of renal benefit of SGLT2 inhibitors was greater in patients with less severe kidney disease at baseline in a meta-analysis of cardiovascular outcome trials80. Therefore, further investigation is required to determine whether the risk reduction of ESRD is a class effect of SGLT2 inhibitors in patients with or without CKD.
Our study has some limitations. First, most of the studies included in our meta-analysis were originally designed to investigate glucose-lowering effects and safety of SGLT2 inhibitors. Therefore, the analysis of renal outcomes should be interpreted cautiously. Second, 14 of 48 studies reported incomplete outcome data due to losses to follow-up, suggesting the possibility of attrition bias. Third, we could not evaluate the renal effects of SGLT2 inhibitors stratified by cardiovascular risk of included studies because they provided missing or inconsistent data. Recently, another meta-analysis showed that SGLT2 inhibitors reduced the risk of the composite of worsening of renal function, ESRD, or renal death similarly in patients with or without atherosclerotic cardiovascular disease80.
In conclusion, our meta-analysis demonstrated that SGLT2 inhibitors had beneficial effects on the kidney by lowering the risk of albuminuria development or progression and reducing the risk of ESRD compared with placebo or other antidiabetic drugs in patients with type 2 diabetes. In addition, the renoprotective effects of SGLT2 inhibitors were greater in patients with a higher UACR and GFR, and a long duration of treatment.
Supplementary information
Acknowledgements
This work was supported by the Korean Endocrine Society of EnM Research Award 2017.
Author Contributions
All authors contributed to conception and design of the study. J.H.B. and E.P. contributed to acquisition and analysis of data. J.H.B., E.P., S.K., N.H.K. and S.H. contributed to interpretation of data. J.H.B., E.P. and N.H.K. contributed to drafting of the manuscript. J.H.B., E.P., S.H. and N.H.K. contributed to critical revision of the manuscript. All authors contributed to approval of the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jae Hyun Bae and Eun-Gee Park contributed equally.
Contributor Information
Seokyung Hahn, Email: hahns@snu.ac.kr.
Nam Hoon Kim, Email: pourlife@korea.ac.kr.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-49525-y.
References
- 1.Ferrannini E, Solini A. SGLT2 inhibition in diabetes mellitus: rationale and clinical prospects. Nat Rev Endocrinol. 2012;8:495–502. doi: 10.1038/nrendo.2011.243. [DOI] [PubMed] [Google Scholar]
- 2.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 3.Jung CH, Jang JE, Park JY. A Novel Therapeutic Agent for Type 2 Diabetes Mellitus: SGLT2 Inhibitor. Diabetes Metab J. 2014;38:261–273. doi: 10.4093/dmj.2014.38.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 6.Wanner C, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med. 2016;375:323–334. doi: 10.1056/NEJMoa1515920. [DOI] [PubMed] [Google Scholar]
- 7.Cherney D, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59:1860–1870. doi: 10.1007/s00125-016-4008-2. [DOI] [PubMed] [Google Scholar]
- 8.Heerspink HJ, et al. Canagliflozin Slows Progression of Renal Function Decline Independently of Glycemic Effects. J Am Soc Nephrol. 2017;28:368–375. doi: 10.1681/ASN.2016030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, Matthews DR. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:2099. doi: 10.1056/NEJMc1712572. [DOI] [PubMed] [Google Scholar]
- 10.Petrykiv SI, Laverman GD, de Zeeuw D, Heerspink HJL. The albuminuria-lowering response to dapagliflozin is variable and reproducible among individual patients. Diabetes Obes Metab. 2017;19:1363–1370. doi: 10.1111/dom.12936. [DOI] [PubMed] [Google Scholar]
- 11.Wiviott SD, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 12.Zinman B, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 13.Mahaffey KW, et al. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study) Circulation. 2018;137:323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasilakou D, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 15.Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perlman A, et al. Acute renal failure with sodium-glucose-cotransporter-2 inhibitors: Analysis of the FDA adverse event report system database. Nutr Metab Cardiovasc Dis. 2017;27:1108–1113. doi: 10.1016/j.numecd.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Tang H, et al. Sodium-glucose co-transporter-2 inhibitors and risk of adverse renal outcomes among patients with type 2 diabetes: A network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2017;19:1106–1115. doi: 10.1111/dom.12917. [DOI] [PubMed] [Google Scholar]
- 18.U. S. Food and Drug Administration. FDA Drug Safety Communication: FDA strengthens kidney warnings for diabetes medicines canagliflozin (Invokana, Invokamet) and dapagliflozin (Farxiga, Xigduo XR), https://www.fda.gov/Drugs/DrugSafety/ucm505860.htm (2016).
- 19.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 20.Perkovic V, et al. Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials. Lancet Diabetes Endocrinol. 2018;6:691–704. doi: 10.1016/S2213-8587(18)30141-4. [DOI] [PubMed] [Google Scholar]
- 21.Pollock Carol, Stefánsson Bergur, Reyner Daniel, Rossing Peter, Sjöström C David, Wheeler David C, Langkilde Anna Maria, Heerspink Hiddo J L. Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial. The Lancet Diabetes & Endocrinology. 2019;7(6):429–441. doi: 10.1016/S2213-8587(19)30086-5. [DOI] [PubMed] [Google Scholar]
- 22.Perkovic Vlado, Jardine Meg J., Neal Bruce, Bompoint Severine, Heerspink Hiddo J.L., Charytan David M., Edwards Robert, Agarwal Rajiv, Bakris George, Bull Scott, Cannon Christopher P., Capuano George, Chu Pei-Ling, de Zeeuw Dick, Greene Tom, Levin Adeera, Pollock Carol, Wheeler David C., Yavin Yshai, Zhang Hong, Zinman Bernard, Meininger Gary, Brenner Barry M., Mahaffey Kenneth W. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. New England Journal of Medicine. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 23.Bae JH, et al. Effects of Dipeptidyl Peptidase-4 Inhibitors on Renal Outcomes in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Endocrinol Metab (Seoul) 2019;34:80–92. doi: 10.3803/EnM.2019.34.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins, J. P. & Altman, D. G. In Cochrane Handbook for Systematic Reviews of Interventions (eds Higgins, J. P. T. & Green, S.) 187–242 (John Wiley & Sons Ltd, 2008).
- 25.Deeks, J. J., Higgins, J. P. & Altman, D. G. In Cochrane Handbook for Systematic Reviews of Interventions (eds Higgins, J. P. T. & Green, S.) 243–296 (John Wiley & Sons Ltd, 2008).
- 26.Higgins, J. P. & Green, S. In Cochrane handbook for systematic reviews of interventions (eds Higgins, J. P. T. & Green, S.) 243–296 (John Wiley & Sons Ltd, 2008).
- 27.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 29.Viechtbauer W, Cheung MW. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1:112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 30.Kosiborod M, Gause-Nilsson I, Xu J, Sonesson C, Johnsson E. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and concomitant heart failure. J Diabetes Complications. 2017;31:1215–1221. doi: 10.1016/j.jdiacomp.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Roden M, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 32.Häring HU, et al. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–3404. doi: 10.2337/dc12-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Häring HU, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–1659. doi: 10.2337/dc13-2105. [DOI] [PubMed] [Google Scholar]
- 34.Kovacs CS, et al. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–158. doi: 10.1111/dom.12188. [DOI] [PubMed] [Google Scholar]
- 35.Barnett AH, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384. doi: 10.1016/S2213-8587(13)70208-0. [DOI] [PubMed] [Google Scholar]
- 36.Strojek K, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011;13:928–938. doi: 10.1111/j.1463-1326.2011.01434.x. [DOI] [PubMed] [Google Scholar]
- 37.Wilding JP, et al. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012;156:405–415. doi: 10.7326/0003-4819-156-6-201203200-00003. [DOI] [PubMed] [Google Scholar]
- 38.Leiter LA, et al. Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: a 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc. 2014;62:1252–1262. doi: 10.1111/jgs.12881. [DOI] [PubMed] [Google Scholar]
- 39.Cefalu WT, et al. Dapagliflozin’s Effects on Glycemia and Cardiovascular Risk Factors in High-Risk Patients With Type 2 Diabetes: A 24-Week, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study With a 28-Week Extension. Diabetes Care. 2015;38:1218–1227. doi: 10.2337/dc14-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber MA, et al. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211–220. doi: 10.1016/S2213-8587(15)00417-9. [DOI] [PubMed] [Google Scholar]
- 41.Leiter LA, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355–364. doi: 10.2337/dc13-2762. [DOI] [PubMed] [Google Scholar]
- 42.Schernthaner G, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013;36:2508–2515. doi: 10.2337/dc12-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji L, et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17:23–31. doi: 10.1111/dom.12385. [DOI] [PubMed] [Google Scholar]
- 44.DeFronzo RA, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38:384–393. doi: 10.2337/dc14-2364. [DOI] [PubMed] [Google Scholar]
- 45.Ridderstråle M, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700. doi: 10.1016/S2213-8587(14)70120-2. [DOI] [PubMed] [Google Scholar]
- 46.Bolinder J, et al. Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab. 2014;16:159–169. doi: 10.1111/dom.12189. [DOI] [PubMed] [Google Scholar]
- 47.Nauck MA, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011;34:2015–2022. doi: 10.2337/dc11-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilding JP, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267–1282. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forst T, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467–477. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cefalu WT, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382:941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 51.Rodbard HW, et al. Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin. Diabetes Obes Metab. 2016;18:812–819. doi: 10.1111/dom.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu CH, et al. Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: Results of a phase 3 randomized, placebo-controlled, double-blind, multicenter trial. J Diabetes Investig. 2016;7:366–373. doi: 10.1111/jdi.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Søfteland E, et al. Empagliflozin as Add-on Therapy in Patients With Type 2 Diabetes Inadequately Controlled With Linagliptin and Metformin: A 24-Week Randomized, Double-Blind, Parallel-Group Trial. Diabetes Care. 2017;40:201–209. doi: 10.2337/dc16-1347. [DOI] [PubMed] [Google Scholar]
- 54.Tikkanen I, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38:420–428. doi: 10.2337/dc14-1096. [DOI] [PubMed] [Google Scholar]
- 55.Frías JP, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:1004–1016. doi: 10.1016/S2213-8587(16)30267-4. [DOI] [PubMed] [Google Scholar]
- 56.Lewin A, et al. Initial combination of empagliflozin and linagliptin in subjects with type 2 diabetes. Diabetes Care. 2015;38:394–402. doi: 10.2337/dc14-2365. [DOI] [PubMed] [Google Scholar]
- 57.Hadjadj S, Rosenstock J, Meinicke T, Woerle HJ, Broedl UC. Initial Combination of Empagliflozin and Metformin in Patients With Type 2 Diabetes. Diabetes Care. 2016;39:1718–1728. doi: 10.2337/dc16-0522. [DOI] [PubMed] [Google Scholar]
- 58.Rosenstock J, et al. Initial Combination Therapy With Canagliflozin Plus Metformin Versus Each Component as Monotherapy for Drug-Naive Type 2 Diabetes. Diabetes Care. 2016;39:353–362. doi: 10.2337/dc15-1736. [DOI] [PubMed] [Google Scholar]
- 59.Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18:590–597. doi: 10.1111/dom.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yale JF, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–1027. doi: 10.1111/dom.12348. [DOI] [PubMed] [Google Scholar]
- 61.Kovacs CS, et al. Empagliflozin as Add-on Therapy to Pioglitazone With or Without Metformin in Patients With Type 2 Diabetes Mellitus. Clin Ther. 2015;37:1773–1788 e1771. doi: 10.1016/j.clinthera.2015.05.511. [DOI] [PubMed] [Google Scholar]
- 62.Rosenstock J, et al. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–948. doi: 10.1111/dom.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kashiwagi A, et al. A randomized, double-blind, placebo-controlled study on long-term efficacy and safety of ipragliflozin treatment in patients with type 2 diabetes mellitus and renal impairment: results of the long-term ASP1941 safety evaluation in patients with type 2 diabetes with renal impairment (LANTERN) study. Diabetes Obes Metab. 2015;17:152–160. doi: 10.1111/dom.12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stenlöf K, et al. Long-term efficacy and safety of canagliflozin monotherapy in patients with type 2 diabetes inadequately controlled with diet and exercise: findings from the 52-week CANTATA-M study. Curr Med Res Opin. 2014;30:163–175. doi: 10.1185/03007995.2013.850066. [DOI] [PubMed] [Google Scholar]
- 65.Wilding JP, et al. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2014;16:124–136. doi: 10.1111/dom.12187. [DOI] [PubMed] [Google Scholar]
- 66.Ferrannini E, Veltkamp SA, Smulders RA, Kadokura T. Renal glucose handling: impact of chronic kidney disease and sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care. 2013;36:1260–1265. doi: 10.2337/dc12-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cherney DZ, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. doi: 10.1161/CIRCULATIONAHA.113.005081. [DOI] [PubMed] [Google Scholar]
- 68.Panchapakesan U, et al. Effects of SGLT2 inhibition in human kidney proximal tubular cells–renoprotection in diabetic nephropathy? PLoS One. 2013;8:e54442. doi: 10.1371/journal.pone.0054442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baker WL, et al. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262–275 e269. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab. 2013;15:853–862. doi: 10.1111/dom.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lytvyn Y, et al. Glycosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am J Physiol Renal Physiol. 2015;308:F77–83. doi: 10.1152/ajprenal.00555.2014. [DOI] [PubMed] [Google Scholar]
- 72.Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjostrom CD. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59:2036–2039. doi: 10.1007/s00125-016-4017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gembardt F, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol. 2014;307:F317–325. doi: 10.1152/ajprenal.00145.2014. [DOI] [PubMed] [Google Scholar]
- 74.Terami N, et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS One. 2014;9:e100777. doi: 10.1371/journal.pone.0100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magee GM, et al. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52:691–697. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 76.Ruggenenti P, et al. Glomerular hyperfiltration and renal disease progression in type 2 diabetes. Diabetes Care. 2012;35:2061–2068. doi: 10.2337/dc11-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim SS, Kim JH, Kim IJ. Current Challenges in Diabetic Nephropathy: Early Diagnosis and Ways to Improve Outcomes. Endocrinol Metab (Seoul) 2016;31:245–253. doi: 10.3803/EnM.2016.31.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Petrykiv S, et al. Differential Effects of Dapagliflozin on Cardiovascular Risk Factors at Varying Degrees of Renal Function. Clin J Am Soc Nephrol. 2017;12:751–759. doi: 10.2215/CJN.10180916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cherney DZI, et al. Pooled analysis of Phase III trials indicate contrasting influences of renal function on blood pressure, body weight, and HbA1c reductions with empagliflozin. Kidney Int. 2018;93:231–244. doi: 10.1016/j.kint.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 80.Zelniker TA, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.