Abstract
Purpose: We conducted this study to determine the relationship between PD-1/PD-L1 inhibitors and the incidence risk of peripheral neuropathy in patients with solid tumors.
Method: The process of the meta-analysis was performed by us according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. Incidence of all-grade and grade 3–5 treatment-related peripheral neuropathy in patients with solid tumors were taken into account.
Results: After screening and eligibility assessment, a total of 17 clinical trials involving 10,500 patients were selected for the final meta-analysis. The incidence risk of peripheral neuropathy for all grade was significantly lower in the PD-1/PD-L1 inhibitor group than that of the control group, either monotherapy (OR = 0.08, 95%CI:[0.03, 0.19]) or chemotherapy (OR = 0.05, 95%CI:[0.03, 0.11]). Similar incidence trend could also be seen for the incidence risk of grade 3–5 peripheral neuropathy. When PD-1/PD-L1 inhibitors were used in combination with chemotherapy, the incidence risk of peripheral neuropathy was higher than in the control chemotherapy group, whether it was all-grade (OR = 1.22, 95%CI:[1.00, 1.49]) or grade 3–5 degree (OR = 1.74, 95%CI:[1.03, 2.92]).
Conclusion: Compared with chemotherapy, incidence risk of peripheral neuropathy related to PD-1/PD-L1 inhibitor was significantly lower than that of the chemotherapy group, while PD-1/PD-L1 inhibitor increased the incidence risk of peripheral neuropathy when it was combined with chemotherapy.
Keywords: incidence risk, peripheral neuropathy, PD-1/PD-L1, solid tumor, meta-analysis
Introduction
Peripheral neuropathy is a syndrome characterized by loss of sensation, muscle weakness and atrophy, loss of tendon reflexes, and/or abnormal vascular motion as a clinical manifestation, either alone or in any combination. Drugs, especially for anti-tumor drugs, are one of the common pathogenic factors for the disease (1–10). During the course of anti-tumor therapy, whether it is chemotherapy or targeted therapy drugs (1–5), peripheral neuropathy is often reported as a drug side effect (1–10). Although reports of death due to peripheral neuropathy were rare, it seriously affected the quality of life for patients with malignant tumors (8–10). Therefore, peripheral neuropathy caused by anti-tumor drugs had increasingly attracted the attention of clinical doctors (11–13).
As a new targeted anti-tumor drug, PD-1/PD-L1 inhibitors have achieved satisfactory clinical efficacy for the treatment of solid tumors, either alone or in combination (14–29). With the increasing clinical applications, more and more drug-related side toxicity effects had been reported, and peripheral neuropathy was one of them (14–29). Because of the low incidence of peripheral neuropathy, we were unable to determine the association between its incidence risk and PD-1/PD-L1 inhibitors. Some chemotherapeutic drugs, such as paclitaxel, might cause delayed peripheral neuropathy (12, 13). It was impossible for us to identify the association between the incidence risk of peripheral neuropathy and PD-1/PD-L1 inhibitors when they were used in combination with other anti-tumor drugs or prescribed as a second-line treatment after chemotherapy (14–30).
For drug-induced peripheral neuropathy, stopping the drug remained to be the primary treatment method (1–10). However, for patients with malignant tumors, when severe drug side effects were encountered (12, 13), careful consideration for stopping the drug should be taken into account. Because of the sudden stop of anti-tumor treatment, it was very likely to cause rapid progression of the tumor. When PD-1/PD-L1 inhibitors were used in combination with chemotherapy, it was particularly important to determine the cause of peripheral neuropathy and then decide which drug to be discontinued (15–18).
To solve the above problems and clarify the association between incidence risk of peripheral neuropathy and PD-1/PD-L1 inhibitors, we designed this meta-analysis.
Methods
The process of the meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (31).
Types of Enrolled Studies
According to the research design, the selected clinical studies must meet the following criteria: (1) Randomized controlled clinical trials would be prioritized, (2) PD-1/PD-L1 inhibitor was prescribed for at least one group of participants, (3)The control group was an anti-tumor drug or PD-1/PD-L1 in combination with an anti-tumor drug rather than a placebo, (4) Participants were diagnosed with solid malignant tumors rather than hematological malignancy, (5) Data on peripheral neuropathy were reported in the study, (6) the enrolled study was published in English.
Search Strategy
Original articles including PD1/PD-L1 inhibitor regimens for solid tumor patients were verified by a systematic search of PubMed. The reported date of the results was limited from Jan 22, 2013 to May 31, 2019. The following subject terms would be used in the literature search process: “cancer,” “tumor,” “PD1/PD-L1,” “nivolumab,” “Opdivo,” “pembrolizumab,” “Keytruda,” “Imfinzi,”,“MK-3475,” “atezolizumab,” “Tecentriq,” “MPDL3280A,” “avelumab,” “Bavencio,” “durvalumab,” “BMS-963558.” Studies limited in human beings, shown in full text, abstract, or poster form, were selected three investigators (Shuisheng Zhang, Yi Zhao, Qingshan Zhu) were appointed to check eligibility and duplicate independently by screening titles and abstracts of relevant studies. If data on peripheral neuropathy had not been reported, we would contact the corresponding author of the article to verify it again, or it would be precluded from the meta-analysis. The basic characteristics information included in the study would be summarized in Table 1.
Table 1.
Basic characteristics of the included studies.
| No. | Study name | Drug name | Drug type | Treatment regimen | Number of evaluable patients | Peripheral neuropathy | Previous therapy | Phase | Randomized controlled trial (RCT) | Tumor type |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cohen et al. (14) | Pembrolizumab | PD-1 | Pembrolizumab vs. (Methotrexate, Docetaxel, or Cetuximab) | 480 | 7 | Platinum-based | III | RCT | Head-and-neck squamous cell carcinoma |
| 2 | Schmid et al. (15) | Atezolizumab | PD-L1 | Atezolizumab + Nab-paclitaxel vs. Placebo + Nab-paclitaxel | 890 | 195 | NO | III | RCT | Advanced Triple-Negative Breast Cancer (BC) |
| 3 | Horn et al. (16) | Atezolizumab | PD-L1 | Atezolizumab + Carboplatin + Etoposide vs. Placebo + Carboplatin + Etoposide | 394 | 6 | NO | III | RCT | SCLC |
| 4 | Socinski et al. (17) | Atezolizumab | PD-L1 | Atezolizumab + BCP vs. BCP | 787 | 274 | NO | III | RCT | Metastatic non squamous NSCLC |
| 5 | Paz-Ares et al. (18) | Pembrolizumab | PD-1 | Pembrolizumab + Carboplatin + Paclitaxel vs. Placebo + Carboplatin + Paclitaxel | 558 | 102 | NO | III | RCT | Metastatic squamous NSCLC |
| 6 | Shitara et al. (19) | Pembrolizumab | PD-1 | Pembrolizumab vs. Paclitaxel | 570 | 41 | YES | III | RCT | Advanced gastric or gastro-oesophageal junction cancer |
| 7 | Powles et al. (20) | Atezolizumab | PD-L1 | Atezolizumab vs. Chemotherapy | 1,128 | 53 | Platinum-based | III | RCT | Locally advanced or metastatic urothelial carcinoma (UC) |
| 8 | Hida et al. (21) | Atezolizumab | PD-L1 | Atezolizumab vs. Docetaxel | 101 | 14 | Platinum-based | III | RCT | Locally advanced/metastatic NSCLC |
| 9 | Bellmunt et al. (22) | Pembrolizumab | PD-1 | Pembrolizumab vs. Chemotherapy | 521 | 28 | Platinum-based | III | RCT | Advanced Urothelial Carcinoma (UC) |
| 10 | Rittmeyer et al. (23) | Atezolizumab | PD-L1 | Atezolizumab vs. Docetaxel | 1187 | 89 | Platinum based | III | RCT | Squamous or non squamous NSCLC |
| 11 | Ferris et al. (24) | Nivolumab | PD-1 | Nivolumab vs. (Methotrexate, Docetaxel, or Cetuximab) | 347 | 8 | Platinum-based | III | RCT | Recurrent Squamous-Cell Carcinoma of the Head and Neck |
| 12 | Antonia et al. (25) | Nivolumab | PD-1 | Nivolumab vs. Nivolumab + Ipilimumab | 213 | 1 | Platinum-based | I/II | N/A | Recurrent SCLC |
| 13 | Fehrenbacher et al. (26) | Atezolizumab | PD-L1 | Atezolizumab vs. Doctaxel | 277 | 16 | Platinum-based | II | RCT | NSCLC |
| 14 | Herbst et al. (27) | Pembrolizumab | PD-1 | Pembrolizumab vs. Docetaxel | 991 | 33 | Platinum-containing | II/III | RCT | Advanced NSCLC |
| 15 | Borghaei et al. (28) | Nivolumab | PD-1 | Nivolumab vs. Docetaxel | 555 | 28 | Platinum-based | III | RCT | Non-squamous NSCLC |
| 16 | Brahmer et al. (29) | Nivolumab | PD-1 | Nivolumab vs. Docetaxel | 260 | 16 | Platinum-based | III | RCT | Squamous NSCLC |
| 17 | Mok et al. (30) | Pembrolizumab | PD-1 | Pembrolizumab vs. Chemotherapy | 1,241 | 51 | NO | III | RCT | NSCLC |
RCT, Randomized controlled trial; BCP, Bevacizumab plus Carboplatin plus Paclitaxel; NSCLC, Non-Small Cell Lung Cancer; PD-1, Programmed Cell Death 1; PD-L1, Programmed Cell Death Ligand 1; SCLC, Small Cell Lung Cancer; CP, Carboplatin plus Paclitaxel; PC, Pemetrexed plus a platinum-based drug; Chemotherapy, Carboplatin plus Pemetrexed, Cisplatin plus Pemetrexed, Carboplatin plus Gemcitabine, Cisplatin plus Gemcitabine, or Carboplatin plus Paclitaxel; N/A, No Available.
Assessment of Study Quality and Publication Bias
Funnel plot, Egger's test and Newcastle-Ottawa scale, proposed by the Cochrane Collaboration, were taken to evaluate the bias (31–35). Three investigators (Shuisheng Zhang, Yi Zhao, Qingshan Zhu) were appointed to check the quality of all studies. The results, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting, would be summarized in a figure together.
Outcome and Exposure of Interest
The study name, year, phase, tumor type, PD-1 and PD-L1 inhibitor regimen, previous therapy regimen, number of evaluable cases, and number of peripheral neuropathy events were extracted from every enrolled study. Both all-grade and grade 3–5 peripheral neuropathy data were taken into account for the final comprehensive meta-analysis.
Assessment of Heterogeneity and Statistical Analysis
Cochrane's Q statistic and the I2 statistic were taken into account for evaluating the heterogeneity among enrolled studies just as suggested by Moher et al. (31) and Higgins et al. (36). The grade of heterogeneity was calculated by the range of I2 values. Heterogeneity was considered low, moderate or high according to I2 values <25%, 25–50%, and >50%, respectively.
Odds ratio (OR) value was reported to be a much more conservative evaluation parameter and might be more inclined to reveal a safety signal, as the method by which an OR is calculated provided a point estimate farther from unity than that provided by a HR. Odds ratio (OR), and 95% confidence interval (CI) would be calculated by random effect (RE) (37). Risk Ratio (RR) and Risk Difference (RD) were also calculated as secondary reference indicators for a more detailed interpretation of the results. P < 0.05 was considered to be of statistically significance. In order to clarify the correlation between peripheral neuropathy and PD-1/PD-L1 inhibitors, we performed a large number of subgroup analyses based on the type of tumor, the treatment regimen and the specific drug. The software of Review Manager 5.3 was used for data consolidation and analysis. Statistical tests were all two-sided.
Results
Literature Search Results
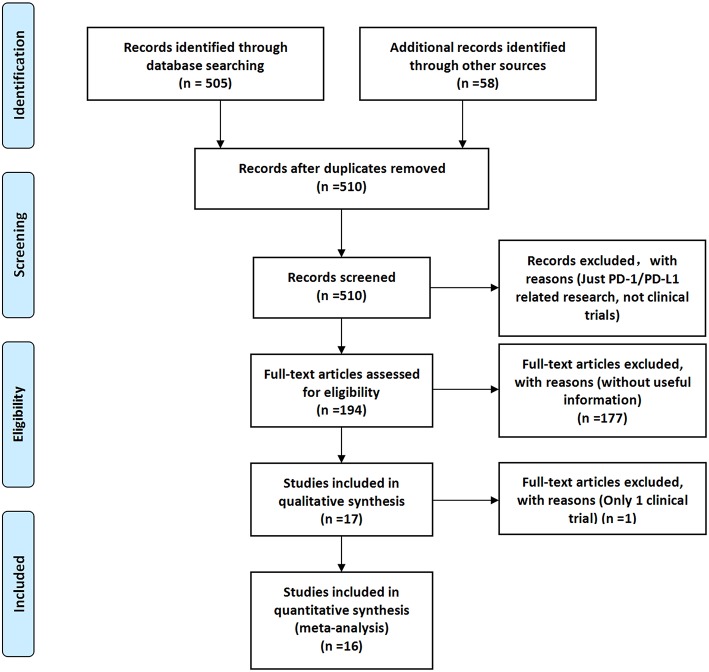
According to the searching principle set by our team, 505 related documents were retrieved on the PubMed website, and 58 related documents were found in other websites or published documents.
After screening and eligibility assessment, a total of 17 clinical trials involving 10,500 patients were selected for the final meta-analysis. The flow diagram of the meta-analysis was shown in Figure 1, while the risk of bias summary was shown in Supplemental Figure 1. All clinical trials enrolled in the meta-analysis included at least one experimental group and one control group (14–30).
Figure 1.
Study flow diagram of inclusion.
Characteristics of Identified Trials
The basic characteristics of all the enrolled clinical trials were summarized in Table 1 (14–30). The involving PD-1/PD-L1 inhibitors were nivolumab (n = 4) (24, 25, 28, 29), pembrolizumab (n = 6) (14, 18, 19, 22, 27, 30), and atezolizumab (n = 7) (15–17, 20, 21, 23, 26). Of all the clinical trials included, 14 were phase III (14–24, 28–30), 1 was phase II (26), 1 was phase II/III (27), and 1 was phase I/II (25). The tumors involved in 17 clinical trials included lung cancer (n = 11) (16–18, 21, 23, 25–30), urothelial cancer (n = 2) (20, 22), breast cancer (n = 1) (15), head and neck carcinoma (n = 2) (14, 24), and advanced gastric or gastro-esophageal junction cancer (n = 1) (19). Of the 11 lung cancer-related clinical trials, nine were limited to non-small cell lung cancer (NSCLC) and two were limited to small cell lung cancer (SCLC) (16, 25). 16 clinical trials were reported to be randomized controlled trial (RCT) (14–24, 26–30), while the information of one clinical trial was unavailable (25). Twelve trials underwent previous platinum-based treatments before PD-1/PD-L1 inhibitors (14, 19–29), while PD-1/PD-L1 inhibitors were prescribed as the first line therapy regimens for the other five trials (15–18, 30). The drugs used in 10 clinical trials were PD-1 inhibitors (14, 18, 19, 22, 24, 25, 28–30), while PD-L1 inhibitors were just given for the other seven clinical trials (15–17, 20, 21, 23, 26).
Risk of Bias
Study quality and risk of bias among enrolled studies were checked by Newcastle-Ottawa scale (35). Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias) were assessed by three members of our team independently and summarized in Supplemental Figure 1. Publication bias, evaluated by Harbord's test (31), was displayed by funnel plots (Supplemental Figures 2, 3, 5, 7, 9, 11).
Incidence Risk of All-Grade Peripheral Neuropathy
All included clinical trials were divided into four groups according to different treatment options, and the specific groups were as follows: Group A (PD-1/PD-L1 vs. Mono-therapy) (19, 21, 23, 26–29), Group B (PD-1/PD-L1 vs. Chemotherapy) (14, 20, 22, 24, 30), Group C (PD-1/PD-L1+ Chemotherapy vs. Chemotherapy) (15–18), Group D (PD-1 vs. PD-1+ CTLA-4) (25). Each group was further divided into two subgroups depending on the respective specific drug and tumor type. Meta-analysis was not performed in group D, because only one group of clinical trials was enrolled, and only one patient in both subgroups was reported with peripheral neuropathy (25).
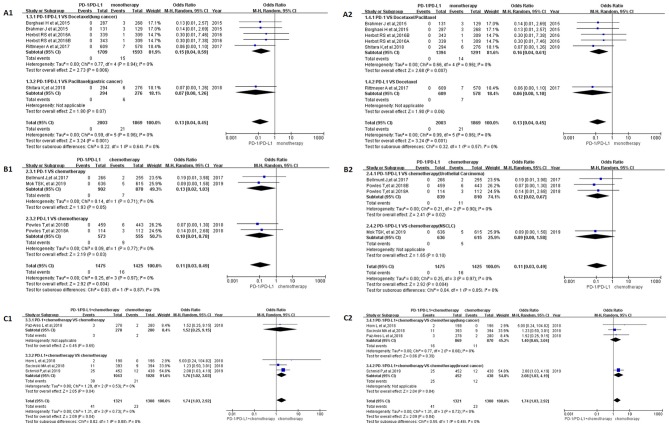
We first performed a meta-analysis on the data of Group A, and the results of the analysis were summarized at the bottom of Figure 2A [OR = 0.08, 95%CI:[0.03, 0.19], I2 = 69%, Z = 5.64 (P < 0.00001)] (19, 21, 23, 26–29). Subgroup analysis was performed according to the different drug types in the control group and the experimental group, and the results were shown in Figures 2A1,A2, respectively. Moderate heterogeneity was found in Group A (I2 = 69%). Subgroup analysis results suggested that the source of heterogeneity was the PD-L1 subgroup [Figure 2A2; (21, 23, 26)]. The funnel plots of OR for Group A could be seen in Supplemental Figures 2A1,A2. Similar to the results of OR, RR and RD of Group A were displayed in Supplemental Figures 4A, 6A.The corresponding funnel plots were gathered in Supplemental Figures 5A, 7A.
Figure 2.
Forest plots for the odds ratio of treatment related peripheral neuropathy for all grade. (A1) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 vs. Docetaxel/Paclitaxel). Subgroup analysis was performed according to the type of chemotherapy drug in the control group. (A2) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 vs. monotherapy). Subgroup analysis was performed based on the drug type (PD-1 or PD-L1) of the experimental group. (B1) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 vs. Chemotherapy). Subgroup analysis was performed based on the drug type (PD-1 or PD-L1) of the experimental group. (B2) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 vs. Chemotherapy). Subgroup analysis was performed based on the specific types of tumors in the experimental and control groups. (C1) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 + Chemotherapy vs. Chemotherapy). Subgroup analysis was performed based on the drug type (PD-1 or PD-L1) of the experimental group. (C2) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 + Chemotherapy vs. Chemotherapy). Subgroup analysis was performed based on the specific types of tumors in the experimental and control groups.
When PD-1/PD-L1 drugs were compared with chemotherapy (Group B), the incidence of peripheral neuropathy was significantly lower than that of the control group, and the OR results are summarized in Figure 2B [OR = 0.05, 95%CI:[0.03, 0.11], I2 = 0%, Z = 7.68 (P < 0.00001)] (14, 20, 22, 24, 30). The funnel plots of OR for Group B could be seen in Supplemental Figures 2B1,B2. The subgroup analysis results were also similar to the subgroup analysis results of group A. RR and RD of Group B were displayed in Supplemental Figures 4B, 6B.The corresponding funnel plots were gathered in Supplemental Figures 5B, 7B. No obvious heterogeneity was found among Group B (I2 = 0%).
Different from the met-analysis results of group A and group B, we found that the analysis results of OR were not statistically significant when performing meta-analysis on Group C (Figure 2C) [OR = 1.22, 95%CI:[1.00, 1.49], I2 = 4%, Z = 1.99 (P = 0.05)] (15–18). The same trend could be seen in the results of RD (Supplemental Figure 6C) [RD = 0.03, 95%CI:[0.01, 0.06], I2 = 47%, Z = 1.42(P = 0.16)] (15–18). The corresponding funnel plots of them were gathered in Supplemental Figures 2C, 7C. The RR of Group C showed that the incidence risk of peripheral neuropathy in the PD-1/PD-L1 combined chemotherapy subgroup was significantly higher than that in the chemotherapy subgroup, and the P-value was statistically significant (Supplemental Figure 4C) [RR = 1.16, 95%CI:[1.01, 1.34], I2 = 0%, Z = 2.13(P = 0.03)] (15–18). The corresponding funnel plots of RR were gathered in Supplemental Figure 5C. No obvious heterogeneity was found among Group C (I2 = 0%).
Incidence Risk of Grade 3–5 Peripheral Neuropathy
Twelve clinical trials with the information of grade 3–5 peripheral neuropathy were taken into account for further meta-analysis (15–20, 22, 23, 27–30). The same grouping and subgroup approach as before were taken for dealing with them. In the experimental subgroup of Group A and Group B, using PD-1/PD-L1 inhibitors alone, the incidence rate of peripheral neuropathy was 0% (19, 20, 22, 23, 27–30). In other words, in patients with solid tumors treated with PD-1/PD-L1 alone, the incidence rate of grade 3–5 peripheral neuropathy was 0% (19, 20, 22, 23, 25, 27–30).
In Group A, the incidence risk of PD-1/PD-L1 subgroup was obvious lower than the control group [OR = 0.13, 95%CI:[0.04, 0.45], I2 = 0%, Z = 3.24 (p = 0.001); Figure 3A; (19, 23, 27–29)]. Different grouping methods for subgroup analysis were adopted for dealing with all the data, no statistically significant difference was found among them (Figures 3A1,A2). No heterogeneity was found in Group A (I2 = 0%). Similar to the results of OR, RR, and RD of Group A were displayed in Supplemental Figures 8A, 10A. The corresponding funnel plots were summarized in Supplemental Figures 9A, 11A.
Figure 3.
Forest plots for the odds ratio of treatment related peripheral neuropathy for grade 3–5. (A1) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 vs. Docetaxel/Paclitaxel). Subgroup analysis was performed based on the specific types of tumors in the experimental and control groups. (A2) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 vs. monotherapy). Subgroup analysis was performed based on the drug type (PD-1 or PD-L1) of the experimental group. (B1) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 vs. Chemotherapy). Subgroup analysis was performed based on the drug type (PD-1 or PD-L1) of the experimental group. (B2) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 vs. Chemotherapy). Subgroup analysis was performed based on the specific types of tumors in the experimental and control groups. (C1) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 + Chemotherapy vs. Chemotherapy). Subgroup analysis was performed based on the drug type (PD-1 or PD-L1) of the experimental group. (C2) Forest plots for the odds ratio of treatment related peripheral neuropathy (PD-1/PD-L1 + Chemotherapy vs. Chemotherapy). Subgroup analysis was performed based on the specific types of tumors in the experimental and control groups.
When PD-1/PD-L1 drugs were compared with chemotherapy (Group B), the incidence risk of peripheral neuropathy limited to grade 3–5 was significantly lower than that of the control group, and the OR results are summarized in Figure 3B [OR = 0.11, 95%CI:[0.03, 0.49], I2 = 0%, Z = 2.92 (P = 0.004)] (20, 22, 30). The funnel plots of OR for Group B could be seen in Supplemental Figures 3B1,B2. Similar to the results of OR, RR, and RD of Group B were displayed in Supplemental Figures 8B, 10B.The corresponding funnel plots were gathered in Supplemental Figures 9B, 11B. No heterogeneity was found in Group B (I2 = 0%) (20, 22, 30).
The OR of Group C showed that the incidence risk of peripheral neuropathy in the PD-1/PD-L1 combined chemotherapy subgroup was significantly higher than that in the chemotherapy subgroup, and the P-value was statistically significant (Figure 3C) [OR = 1.74, 95%CI:[1.03, 2.92], I2 = 0%, Z = 2.09 (P = 0.04)] (15–18). The corresponding funnel plots of OR were gathered in Supplemental Figure 3C. No heterogeneity was found in Group C (I2 = 0%). Similar analysis results could also be seen in Supplemental Figure 8C, when the data of Group C was evaluated by RR [RR = 1.71, 95%CI:[1.03, 2.83], I2 = 0%, Z = 2.09 (P = 0.04)]. Different from OR and RR, the meta-analysis result was of no statistical significance (Supplemental Figure 10), when it was calculated by RD [RD = 0.01, 95%CI:[0.00, 0.02], I2 = 10%, Z = 1.82 (P = 0.07)]. The corresponding funnel plots of RD were gathered in Supplemental Figure 11C. Low heterogeneity related to RD was found in Group C (I2 = 10%). Subgroup analysis revealed that the source of heterogeneity might be related to the inclusion of this clinical trial (15).
Discussion
Peripheral neuropathy is a painful condition deriving from many and varied etiologies (38, 39). Certain medications have been implicated in the iatrogenic development of drug induced peripheral neuropathy (DIPN) and include chemotherapeutic agents, antimicrobials, cardiovascular drugs, psychotropic, anticonvulsants, among others (39). Chemotherapy-induced peripheral neuropathy (CIPN), reported in several studies, especially for paclitaxel induced peripheral neuropathy, was common for cancer patients (40, 41). CIPN was a dose limiting toxicity, negatively impacting both quality of life and disease outcomes (42). However, during the process of anti-tumor treatment, combinations of drugs that were unknown to cause CIPN were prescribed for cancer patients, and sequential treatment for recurrence with additional CIPN-inducing drugs would also be suggested (43). Therefore, it would be difficult for us to determine which specific drug was responsible for the occurrence of peripheral neuropathy, especially for some newly marketed targeted anti-tumor drugs without fully understanding of toxicities, such as PD-1/PD-L1 inhibitors and Brentuximab vedotin (3, 14–30). To clarify the association between incidence risk of peripheral neuropathy and PD-1/PD-L1 inhibitors, we designed this meta-analysis.
After screening and eligibility assessment, a total of 17 clinical trials involving 10,500 patients were selected for the final meta-analysis. The flow diagram of the meta-analysis was shown in Figure 1, while the risk of bias summary was shown in Supplemental Figure 1. All clinical trials enrolled in the meta-analysis included at least one experimental group and one control group (14–30). Study quality and risk of bias among enrolled studies were checked by Newcastle-Ottawa scale (35). All clinical trials included were considered to be of higher quality. Therefore, the analytical conclusions based on the data of these clinical trials could represent certain reliability, authenticity, and credibility (14–30). In this study, we tried as many subgroup analysis methods as possible, and conducted a systematic and comprehensive analysis of the results, so the analysis results obtained were much more accurate (Figures 2, 3 and Supplemental Figures 4, 6, 8, 10) than that was analyzed just by one model.
The incidence of peripheral neuropathy for all grade was significantly lower in the PD-1/PD-L1 inhibitor group than that of the control group, either monotherapy (OR = 0.08, 95%CI:[0.03, 0.19], Figure 2A) or chemotherapy (OR = 0.05, 95%CI:[0.03, 0.11], Figure 2B) (14, 19–24, 26–30). Moderate heterogeneity was found in Group A (I2 = 69%) but Group B (I2 = 0%). Subgroup analysis results suggested that the source of heterogeneity was the PD-L1 subgroup (Figure 2A2) (21, 23, 26). The funnel plots of OR for Group A could be seen in Supplemental Figures 2A1,A2. Similar to the results of OR, Forest plots of RR and RD for Group A were displayed in Supplemental Figures 4A, 6A. The corresponding funnel plots were summarized in Supplemental Figures 5A, 7A. We found the existence of asymmetry of the funnel plot of Group A analysis (19, 21, 23, 26–29), so we concluded that there might be publication bias, but we could not rule out the possibility of asymmetry caused by other factors. Similar incidence risk of peripheral neuropathy for grade 3–5 could also be seen in Figure 3A (OR = 0.13, 95%CI:[0.04, 0.45]) (19, 23, 27–29). However, the heterogeneity (I2 = 0%) and the asymmetry of the funnel chart were not found [Supplemental Figure 3A; (19, 23, 27–29)]. Based on the above analysis results, we concluded that the heterogeneity and the asymmetry of the funnel plot were mainly derived from those two clinical trials (21, 26).
When PD-1/PD-L1 inhibitors were used in combination with chemotherapy (Group C), the risk of peripheral neuropathy was higher than in the control chemotherapy group, whether it was all-grade (OR = 1.22, 95%CI:[1.00, 1.49], Figure 2C) or grade 3–5 degree (OR = 1.74, 95%CI:[1.03, 2.92], Figure 3C) (15–18). Similar incidence trend could also be obtained when they were evaluated by RR (Supplemental Figures 4C, 8C). No obviously statistical significant results of RD were only seen in Supplemental Figures 6C, 8C. Obvious heterogeneity and the asymmetry of the funnel chart were not found in Group C (Supplemental Figures 2C, 3C, 5C, 7C, 9C, 11C). It proved that the analytical conclusions we had obtained were credible.
A lot of clinical trials had reported that PD-1/PD-L1 inhibitors had better safety and satisfactory clinical efficacy in the process of anti-tumor therapy (14–30, 44). In the experimental subgroup of Group A and Group B, using PD-1/PD-L1 inhibitors alone, the incidence rate of peripheral neuropathy for grade 3–5 was 0% (19, 20, 22, 23, 27–30). In other words, if we encounter peripheral neuropathy of grade 3–5 in the course of anti-tumor therapy, the possibility caused by the PD-1/PD-L1 inhibitor was firstly excluded. Chemotherapy-induced peripheral neuropathy (CIPN), reported in several studies, especially for paclitaxel induced peripheral neuropathy, was common for cancer patients (40, 41). Stopping the use of related drugs remained to be the primary principle for the treatment of drug-related peripheral neuropathy. However, stopping all anti-tumor treatment for cancer patients, especially for advanced cancer patients, might lead to rapid progression of the tumor, and even endanger the patient's life. Based on the results of our analysis, we found that PD-1/PD-L1 inhibitors often played a secondary role for patients suffering from severe drug-related peripheral neuropathy [Figures 2, 3; (14–30)]. Therefore, when it was necessary to stop anti-tumor therapy to alleviate severe peripheral neuropathy in patients, chemotherapy drugs other than PD-1/PD-L1 would be considered first (Figures 2C, 3C) (15–18). This finding had an important clinical guiding significance in clinical work.
Conclusions
Compared with chemotherapy, incidence risk of peripheral neuropathy related to PD-1/PD-L1 inhibitor was significantly lower than that of the chemotherapy group, while PD-1/PD-L1 inhibitor increased the incidence risk of peripheral neuropathy when it was combined with chemotherapy.
Ethics Statement
This study belongs to the type of data analysis and rearrangement, and does not involve human or animal related ethical issues.
Author Contributions
YT had full access to all data in the study and all authors had final responsibility for the decision to submit for publication. ZS, SZ, XY, ND, and YT had the full data of the paper. MX, QZ, YL, LY, HS, JX, and YM were responsible for the collection of clinical data. ZS helped to gather online data and write the report.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Glossary
Abbreviations
- CI
confidence interval
- CIPN
Chemotherapy-induced peripheral neuropathy
- DIPN
drug induced peripheral neuropathy
- FE
fixed effect
- HR
hazard ratios
- OR
odds ratio
- PD-1
programmed cell death-1
- PD-L1
programmed cell death ligand 1
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RD
risk difference
- RE
random effect
- RR
risk ratio.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2019.00866/full#supplementary-material
References
- 1.Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. (2019) 393:229–40. 10.1016/S0140-6736(18)32984-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prince HM, Kim YH, Horwitz SM, Dummer R, Scarisbrick J, Quaglino P, et al. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. (2017) 390:555–66. 10.1016/S0140-6736(17)31266-7 [DOI] [PubMed] [Google Scholar]
- 3.Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2015) 385:1853–62. 10.1016/S0140-6736(15)60165-9 [DOI] [PubMed] [Google Scholar]
- 4.Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. (2013) 381:1203–10. 10.1016/S0140-6736(12)61763-2 [DOI] [PubMed] [Google Scholar]
- 5.Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. (2013) 309:1359–67. 10.1001/jama.2013.2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. (2019) 380:617–28. 10.1056/NEJMoa1814017 [DOI] [PubMed] [Google Scholar]
- 7.Brown TJ, Sedhom R, Gupta A. Chemotherapy-induced peripheral neuropathy. JAMA Oncol. (2019) 5:750. 10.1001/jamaoncol.2018.6771 [DOI] [PubMed] [Google Scholar]
- 8.Sánchez-Barroso L, Apellaniz-Ruiz M, Gutiérrez-Gutiérrez G, Santos M, Roldán-Romero JM, Curras M, et al. Concomitant medications and risk of chemotherapy-induced peripheral neuropathy. Oncologist. (2018). 24:e784–92. 10.1634/theoncologist.2018-0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hincker A, Frey K, Rao L, Wagner-Johnston N, Ben Abdallah A, Tan B, et al. Somatosensory predictors of response to pregabalin in painful chemotherapy-induced peripheral neuropathy: a randomized, placebo-controlled, crossover study. Pain. (2019) 160:1835–46. 10.1097/j.pain.0000000000001577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yardley DA, Shipley D, Zubkus J, Wright GL, Ward PJ, Mani A, et al. A randomized phase II study of eribulin/cyclophosphamide or docetaxel/cyclophosphamide as neoadjuvant therapy in operable HER2-negative breast cancer. Clin Breast Cancer. (2019) 19:1–9. 10.1016/j.clbc.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Lee KM, Jung D, Hwang H, Son KL, Kim TY, Im SA, et al. Pre-treatment anxiety is associated with persistent chemotherapy-induced peripheral neuropathy in women treated with neoadjuvant chemotherapy for breast cancer. J Psychosom Res. (2018) 108:14–9. 10.1016/j.jpsychores.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 12.Bandos H, Melnikow J, Rivera DR, Swain SM, Sturtz K, Fehrenbacher L, et al. Long-term peripheral neuropathy in breast cancer patients treated with adjuvant chemotherapy: NRG oncology/NSABP B-30. J Natl Cancer Inst. (2018) 110:djx162. 10.1093/jnci/djx162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mustafa Ali M, Moeller M, Rybicki L, Moore HCF. Long-term peripheral neuropathy symptoms in breast cancer survivors. Breast Cancer Res Treat. (2017) 166:519–26. 10.1007/s10549-017-4437-8 [DOI] [PubMed] [Google Scholar]
- 14.Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. (2019) 393:156–67. 10.1016/S0140-6736(18)31999-8 [DOI] [PubMed] [Google Scholar]
- 15.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. (2018) 379:2108–21. 10.1056/NEJMoa1809615 [DOI] [PubMed] [Google Scholar]
- 16.Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. (2018) 379:2220–9. 10.1056/NEJMoa1809064 [DOI] [PubMed] [Google Scholar]
- 17.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. (2018) 378:2288–301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 18.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. (2018) 379:2040–51. 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 19.Shitara K, Özgüroglu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. (2018) 392:123–33. 10.1093/annonc/mdy208.004 [DOI] [PubMed] [Google Scholar]
- 20.Powles T, Durán I, van der Heijden MS, Loriot Y, Vogelzang NJ, De Giorgi U, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. (2018) 391:748–57. 10.1016/S0140-6736(17)33297-X [DOI] [PubMed] [Google Scholar]
- 21.Hida T, Kaji R, Satouchi M, Ikeda N, Horiike A, Nokihara H, et al. Atezolizumab in Japanese patients with previously treated advanced non-small-cell lung cancer: a subgroup analysis of the phase 3 OAK study. Clin Lung Cancer. (2018) 19:e405–15. 10.1016/j.cllc.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 22.Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. (2017) 376:1015–26. 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. (2017) 389:255–65. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. (2016) 375:1856–67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonia SJ, López-Martin JA, Bendell J, Ott PA, Taylor M, Eder JP, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. (2016) 17:883–95. 10.1016/S1470-2045(16)30098-5 [DOI] [PubMed] [Google Scholar]
- 26.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. (2016) 387:1837–46. 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 27.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. (2016) 387:1540–50. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 28.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. (2015) 373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. (2015) 373:123–35. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. (2019) 393:1819230. 10.1016/S0140-6736(18)32409-7 [DOI] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane bias methods group; cochrane statistical methods group. the cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. (2009). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed July 6, 2012).
- 36.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 38.Dabelea D, Stafford JM, Mayer-Davis E-J, D'Agostino R, Dolan J, Jr, Imperatore G, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. (2017) 317:825–35. 10.1001/jama.2017.0686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones MR, Urits I, Wolf J, Corrigan D, Colburn L, Peterson E, et al. Drug-induced peripheral neuropathy, a narrative review. Curr Clin Pharmacol. (2019). 10.2174/1574884714666190121154813. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gianni L, Mansutti M, Anton A, Calvo L, Bisagni G, Bermejo B, et al. Comparing neoadjuvant nab-paclitaxel vs paclitaxel both followed by anthracycline regimens in women with ERBB2/HER2-negative breast cancer-the evaluating treatment with neoadjuvant abraxane (ETNA) trial: a randomized phase 3 clinical trial. JAMA Oncol. (2018) 4:302–8. 10.1001/jamaoncol.2017.4612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Untch M, Jackisch C, Schneeweiss A, Schmatloch S, Aktas B, Denkert C, et al. NAB-paclitaxel improves disease-free survival in early breast cancer: GBG 69-GeparSepto. J Clin Oncol. (2019) 37:2226–34. 10.1200/JCO.18.01842 [DOI] [PubMed] [Google Scholar]
- 42.Hu S, Huang KM, Adams EJ, Loprinzi CL, Lustberg MB. Recent developments of novel pharmacologic therapeutics for prevention of chemotherapy-induced peripheral neuropathy. Clin Cancer Res. (2019). 10.1158/1078-0432.CCR-18-2152. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wozniak KM, Vornov JJ, Wu Y, Liu Y, Carozzi VA, Rodriguez-Menendez V, et al. Peripheral neuropathy induced by microtubule-targeted chemotherapies: insights into acute injury and long-term recovery. Cancer Res. (2018) 78:817–29. 10.1158/0008-5472.CAN-17-1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortese I, Muranski P, Enose-Akahata Y, Ha SK, Smith B, Monaco M, et al. Pembrolizumab treatment for progressive multifocal leukoencephalopathy. N Engl J Med. (2019) 380:1597–605. 10.1056/NEJMoa1815039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.