Summary
Starch is the most important form of energy storage in cereal crops. Many key enzymes involved in starch biosynthesis have been identified. However, the molecular mechanisms underlying the regulation of starch biosynthesis are largely unknown. In this study, we isolated a novel floury endosperm rice (Oryza sativa) mutant flo16 with defective starch grain (SG) formation. The amylose content and amylopectin structure were both altered in the flo16 mutant. Map‐based cloning and complementation tests demonstrated that FLO16 encodes a NAD‐dependent cytosolic malate dehydrogenase (CMDH). The ATP contents were decreased in the mutant, resulting in significant reductions in the activity of starch synthesis‐related enzymes. Our results indicated that FLO16 plays a critical role in redox homeostasis that is important for compound SG formation and subsequent starch biosynthesis in rice endosperm. Overexpression of FLO16 significantly improved grain weight, suggesting a possible application of FLO16 in rice breeding. These findings provide a novel insight into the regulation of starch synthesis and seed development in rice.
Keywords: floury endosperm mutant, starch synthesis, grain weight, malate, energy supply, redox regulation, rice
Introduction
Starch, large biopolymers of glucose, is the most important form of carbohydrates for most organisms. As the major source of daily carbon intake for humans, starch has a vital role in our diet and health. Cereal endosperm accumulates high levels of starch that provides energy for seed germination and early seedling development. In rice (Oryza sativa), endosperm starch forms insoluble particles referred to as starch grains (SGs) in the amyloplasts. A SG is generally composed of a complex of sharp‐edged polyhedral starch granules (Jane et al., 1994), also named as compound SG (Tateoka, 1962). SGs are easily observed by staining with iodine solution using a light microscope.
Starch synthesis begins with the enzyme ADP‐glucose pyrophosphorylase (AGPase) catalysing the reaction of glucose 1‐phosphate (G1P) and ATP to ADP‐glucose (ADPG), the substrate for starch synthesis (Martin and Smith, 1995). In contrast to most higher plants and leaves of cereal crops (Beckles et al., 2001; Tetlow et al., 2003), the major forms of AGPase in cereal endosperm are located in the cytosol (Tetlow et al., 2003). Synthesized ADPG is then transported from the cytosol to amyloplasts by the ADPG transporter, Brittle 1 (Li et al., 2017; Sullivan et al., 1991). Five starch synthase (SS) isoforms synthesize and elongate glucan chains using ADPG as the substrate. Granule‐bound starch synthase (GBSS) acts in biosynthesis of amylose and extra‐long unit chains of amylopectin in rice (Hanashiro et al., 2008), and other SS isoforms (SSI, SSII, SSIII and SSIV) participate in amylopectin biosynthesis (Nakamura, 2002). Branching enzymes (BEs) and debranching enzymes (DBEs) are required to define amylopectin structure (Ball et al., 1996). In addition to the enzymes mentioned above, additional factors indirectly regulate starch synthesis. For example, Du1 regulates starch synthesis by assisting the splicing of waxy pre‐mRNA (Isshiki et al., 2000). Previous study in wheat revealed that protein phosphorylation regulates activity and integrity of a protein complex formed by BEIIb, BEI and plastidial phosphorylase (PHO1) (Tetlow et al., 2004). Evidence for interaction between BEII, SSI and SSII was also obtained in developing wheat endosperm (Tetlow et al., 2008). However, detailed starch biosynthesis mechanisms, especially their regulation, are far from complete resolution.
Opaque‐kernel mutant phenotypes indicate changes in storage metabolites, varying starch content and structure, aberrant SGs, and other abnormalities. Various rice mutants with opaque endosperm and individual SGs were named floury, including flo(a) (Qiao et al., 2010), flo2 (She et al., 2010), flo3 (Nishio and Iida, 1993), flo4 (Kang et al., 2005), flo5 (Ryoo et al., 2007), flo6 (Peng et al., 2014), flo7 (Zhang et al., 2016) and flo8 (Long et al., 2017). Some mutants with abnormal SGs were named as substandard starch grain (ssg1‐6) (Matsushima et al., 2010, 2014, 2016), dull (du1‐3) (Isshiki et al., 2000, 2008; Zeng et al., 2007) and chalk5 (Li et al., 2014). All previous studies confirmed that defective endosperm mutants are valuable genetic resources for dissecting the mechanisms of amyloplast development and starch biosynthesis.
Malate content contributes to redox homeostasis in the cytosol. Malate participates in the transport of redox equivalents among cell compartments (Kromer and Scheibe, 1996; Scheibe, 2004). In tomato, malate was identified as a potential metabolite regulating starch synthesis. Further analysis suggested that an altered plastidial redox status caused by modified malate metabolism resulted in different AGPase activation states and final starch contents (Centeno et al., 2011). Similar conclusions were made for potato (Tiessen et al., 2002). However, in potato tubers, starch synthesis in plastids was not affected by decreased synthesis of malate in mitochondria, suggesting that redox regulation is tissue dependent (Szecowka et al., 2012). However, the role that malate metabolism plays in starch synthesis in rice endosperm remains unknown.
In this study, a floury mutant, flo16, with a deletion of 4 base pairs in the Cytosolic Malate Dehydrogenase gene was isolated and characterized. Compared to the wild type, decreased starch and increased sucrose contents in the flo16 mutant revealed that the transition from sucrose to starch was partially disrupted during mutant grain filling. Endosperm‐specific overexpression of FLO16 led to increased grain weight. Our results demonstrated that FLO16 is important for starch biosynthesis and seed development in rice.
Results
flo16 endosperm is defective in starch accumulation
As part of our ongoing effort to dissect mechanisms underlying starch biosynthesis and its regulation in rice, we isolated a floury endosperm mutant named flo16 from a 60Co‐irradiated M2 population of indica rice variety N22. The mature grains of flo16 were opaque and slightly shrunken compared with the transparent and fully developed grains of wild type (Figure 1a,b). During endosperm development, the flo16 mutant underwent a much slower grain‐filling rate from 6 days after flowering (DAF), and this difference was maintained until maturation (Figure 1e). The 1000‐grain weight of flo16 was significantly lower than that of wild type (Figure 1f); and the grain thickness of flo16 was smaller than that of the wild type (Figure 1g). Clearly, the flo16 mutation affects starch accumulation during endosperm development. It is worth noting that the plant height of the flo16 mutant was also shorter in height and had less tillers compared to wild type (Figure 1c,d; Table S1). These results suggest that the flo16 mutant is also defective in plant growth and development and that changes in starch metabolism extend beyond the endosperm.
Figure 1.
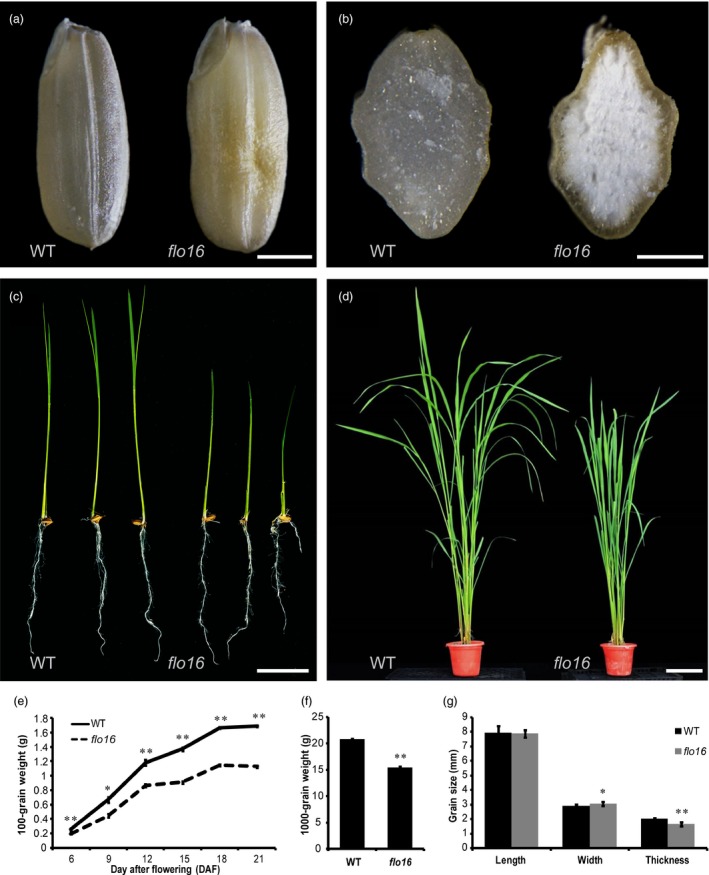
Phenotypes of the flo16 mutant. (a) Images of mature seeds of wild type (left) and flo16 (right). Scale bar, 2 mm. (b) Cross sections of the mature seeds of wild type (left) and flo16 (right). Scale bar, 1 mm. (c) One‐week‐old seedlings of wild type (left) and flo16 (right). Scale bar, 4 cm. (d) Wild type (left) and flo16 (right) plants at the booting stage. Scale bar, 20 cm. (e) Grain weights of wild type and flo16 at various stages post‐fertilization. Grain weight indicates the weight of 100 dehulled dry grains, values are means ± SD, n = 3. (f) 1000‐grain weights of wild type and flo16. Values are means ± SD, n = 5. (g) Grain size comparisons between wild type and flo16. Values are means ± SD, n = 10. Asterisks indicate the statistical significance between the wild type and mutants determined by Student's t‐tests (*P < 0.05; **P < 0.01).
flo16 endosperm has irregular starch grain morphology
Semi‐thin sections of developing endosperm in flo16 at 12 DAF examined after iodine staining had massive unstained spaces apparently caused by delayed filling of amyloplasts (Figure 2c,d). Amyloplasts in wild‐type endosperm were full of densely packed compound SGs consisting of polyhedral granules (Figure 2a,b), whereas in flo16 endosperm they were tiny and disordered (Figure 2c,d). Besides compound SGs, many single granules were scattered in the mutant endosperm (Figure 2d). Scanning electron microscopy (SEM) of transverse sections of mature endosperm revealed that, unlike the regular, compact crystal structure of wild‐type SGs (Figure 2e–g), the endosperm of flo16 consisted of small, spherical and loosely packed SGs with large air spaces (Figure 2h–j). Therefore, formation of compound SGs was severely disrupted in developing flo16 endosperm.
Figure 2.
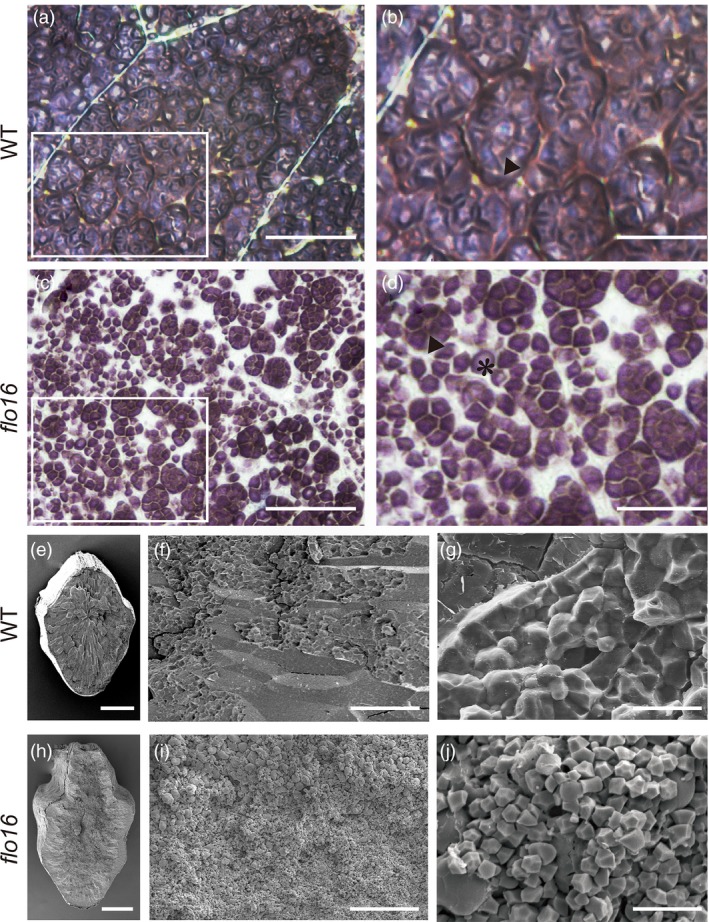
Abnormal starch grain (SG) formation in flo16 endosperm. (a‐d) Semi‐thin sections of the wild type (a, b) and flo16 (c, d) endosperms at 12 days after flowering (DAF). (b) and (d) are enlargements of the boxed areas in (a) and (c), respectively. Small and scattered SGs are displayed in lower left of (b, d). Triangles indicate compound starch grains in (b) and (d); a asterisk indicates a single starch grain in (d). (e–j) SEM of endosperm of wild type (e‐g) and flo16 (h‐j). Scale bars, 40 μm in (a, b), 20 μm in (c, d), 1 mm in (e, h); 200 μm in (f, i); 10 μm in (g, j).
Physicochemical properties of flo16 starch
Total starch and amylose contents in dry weight of mature flo16 seeds were significantly less than in wild‐type seeds (Figure 3a,b), whereas protein and lipid contents were both elevated (Figure 3c,d). Sucrose content in flo16 endosperm was 8 times higher than in the wild type (Figure 3e), suggesting disruption of transition from sucrose to starch. Although amylopectin content taken up in mature flo16 seeds was almost unchanged, the degrees of polymerization (DP) in the ranges 6–10 and 13–14 were significantly increased, whereas those in the ranges of DP 11‐12 and DP 15‐48 were decreased (Figure 3f). There were no visible alterations in the amounts of A‐chains with DP 6‐12 and B‐chains with DP > 36 as classified by Hanashiro et al. (2002). However, elongation of both A‐chains and B‐chains was abnormal in the flo16 mutant (Figure 3f). The solubility of starch in urea solution was measured to test the gelatinization properties (Nishi et al., 2001). Even in 9 M urea powdered flo16 starch was difficult to gelatinize, whereas wild‐type starch began to gelatinize in 5 M urea and was thoroughly gelatinized in 9 M urea (Figure 3g). Analyses of pasting properties revealed that the viscosity of flo16 pasting starch was low after the rise in temperature, but the curve patterns showed no difference between wild type and flo16 (Figure 3h). Collectively, the physicochemical properties of flo16 starch were significantly altered compared with the wild type.
Figure 3.
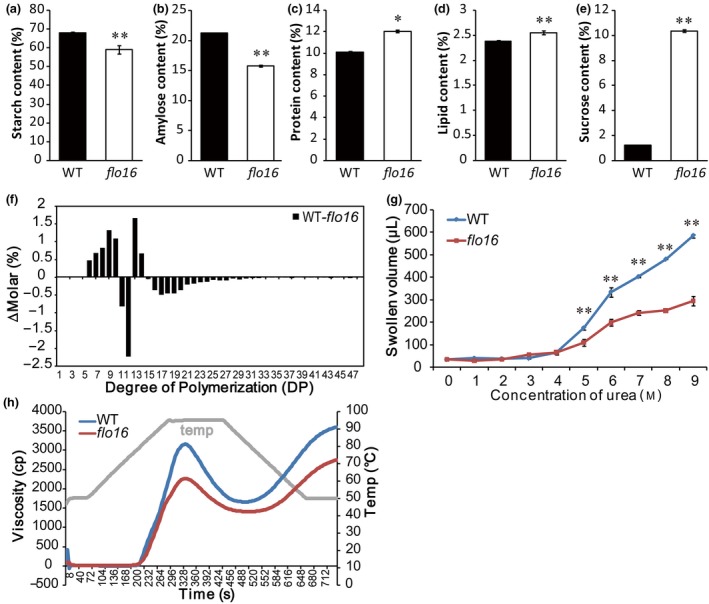
Properties of grains and physicochemical characteristics of starch in the flo16 mutant. (a‐e) The contents of total starch (a), amylose (b), protein (c), lipid (d) and sucrose (e) were proportions of grain dry weight in wild type and flo16. Values are means ± SD, n = 3. (f) Differences in amylopectin chain length distributions between the wild type and the flo16 mutant. (g) Swollen volumes of wild‐type and flo16 starch in urea solutions at various concentrations (0–9 M). Values are means ± SDs, n = 3. (h) Pasting properties of endosperm starch of the wild type and flo16 mutant. Grey line indicates temperature changes during measurements. Asterisks indicate the statistical significance between the wild type and mutant determined by Student's t‐tests (*P < 0.05; **P < 0.01).
The flo16 mutation occurred in gene Os10 g0478200
When flo16 was crossed with wild type, approximately one‐quarter of the F2 seeds had floury endosperm (296 of 1,205, χ2 3:1 = 0.122 < χ2 0.05,1 = 3.84), indicating that the mutant phenotype was inherited as a single recessive allele. We then undertook map‐based cloning to identify the underlying gene. First, 10 individuals with floury endosperm selected from the F2 progeny of cross flo16 (indica) × DJY (japonica) were used for linkage detection. The FLO16 locus was initially mapped between markers I10‐6 and 10‐26 on the long arm of chromosome 10. Then, 125 flo16 individuals were used to position FLO16 between markers H188‐15 and H188‐2. Finally, 1,502 individuals with the recessive phenotype reduced the position of FLO16 to an 88 kb region between markers 188–21 and 188–2 (Figure 4a; Table S3). Six predicted open reading frames (ORFs) in the region were sequenced, and we found a deletion of 4 base pairs in the coding region of Os10 g0478200, leading to a frame shift from amino acid residue 229 (Figure 4b), and a premature termination codon. Os10 g0478200 was predicted to encode a cytosolic malate dehydrogenase (CMDH) composed of 332 amino acids.
Figure 4.
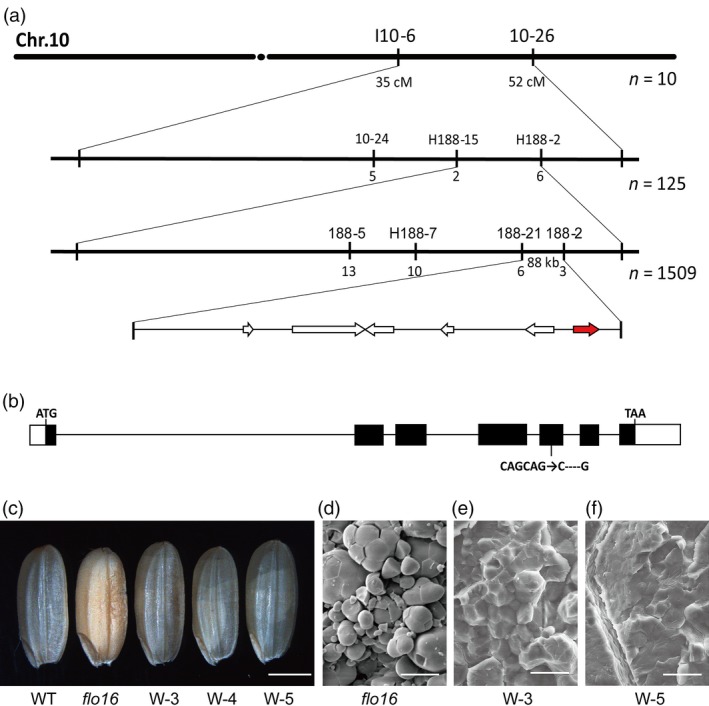
Map‐based cloning of FLO16. (a) Fine mapping of the FLO16 locus. The FLO16 locus was mapped to an 88 kb region by markers 188–21 and 188–2 on chromosome 10 (Chr.10), containing six predicted genes. Numbers of recombinants are indicated below the map. (b) The flo16 mutant has a 4 bp deletion in the fifth exon of Os10 g0478200. White boxes indicate untranslated regions; black boxes indicate exons; lines indicate the introns. (c‐f) Functional complementation lines of flo16 restore normal seed appearance. Complemented seeds became translucent (c), and SGs were restored to normal (d‐f). W‐3, W‐4 and W‐5 are representative positive transgenic lines. Scale bars, 2 mm in (c), 25 μm in (d‐f).
To verify the identity of flo16, we cloned the genomic sequence of FLO16 starting from 2,000 nucleotides upstream of the putative starting codon (ATG) to the stop codon (TAA) of Os10 g0478200 into a plant expression vector, which was then introduced into flo16. Seed and SG morphologies of positive transgenic plants were similar to wild‐type plants, indicating successful rescue of the defects caused by the flo16 mutation (Figure 4c‐f). Therefore, Os10 g0478200 was confirmed to be the gene responsible. In addition, we generated transgenic lines expressing CMDH driven by Glutelin C promoter in flo16, and the endosperm‐specific expression of CMDH rescued the phenotype of the mutant (Figure S1), indicating that the mutant phenotype in flo16 was mainly due to the defects in seeds rather than in vegetative tissues.
FLO16 is cytosol‐located protein that is ubiquitously expressed in rice
We performed quantitative RT‐PCR (qRT‐PCR) analyses in wild‐type plants to investigate the expression profile of FLO16. Expression levels of FLO16 in vegetative tissues, especially leaf sheaths and panicles, were higher than in the developing endosperm. Expression of FLO16 during endosperm development increased at 6–15 DAF, but reduced at 18 DAF (Figure 5a). Hence, FLO16 expression occurs in the early and mid‐endosperm development stages, corresponding with the time of starch biosynthesis. GUS staining in transgenic lines carrying a β‐glucuronidase (GUS) expression vector driven by the FLO16 promoter confirmed that FLO16 was ubiquitously expressed in rice. FLO16 also showed a relatively high level of expression in anthers, early developing grains and stem nodes, and had a spotty distribution in leaves (Figure 5b‐j). Constitutive expression suggested that functions of FLO16 might not be limited to the endosperm.
Figure 5.
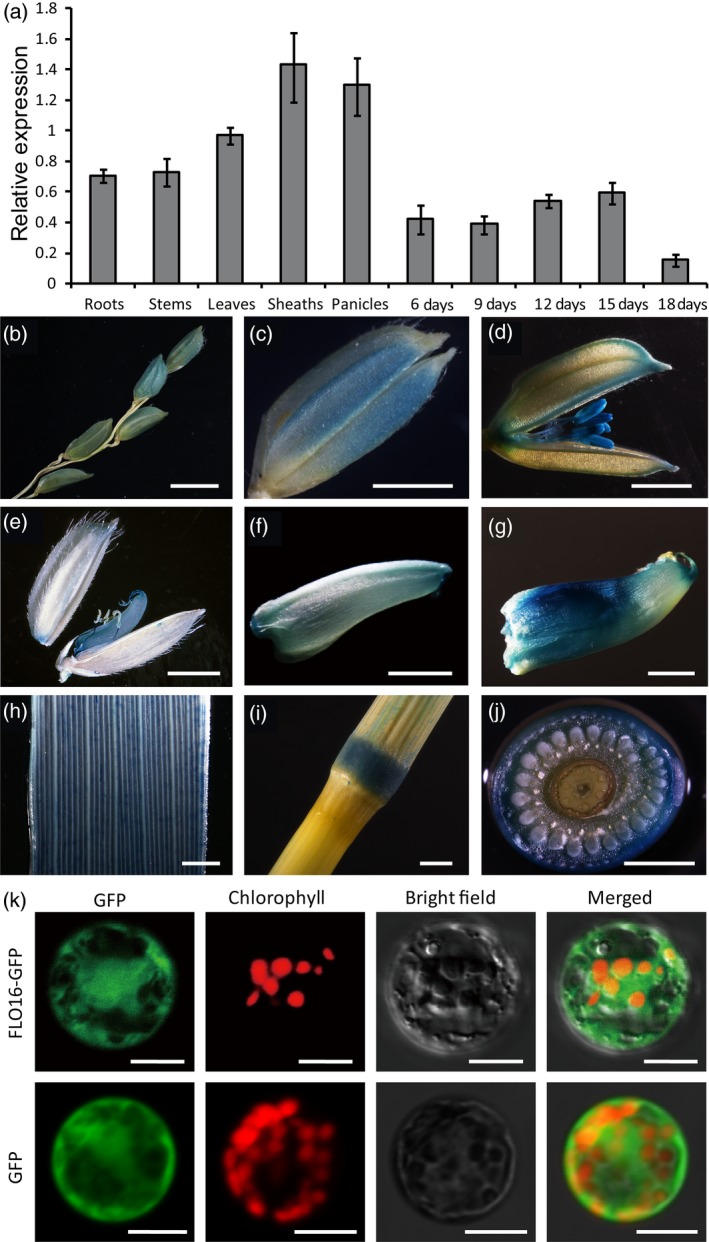
Spatial expression patterns of FLO16. (a) Expression levels of FLO16 in various tissues. Developing seeds were sampled at 6, 9, 12, 15 and 18 days after flowering (DAF), and other tissues were sampled at heading. The value of Actin I mRNA was used as an internal control for data normalization. Values are means ± SD, n = 3. (b‐g) GUS activity in panicles (b), spikelet before heading (c), during heading (d), and at 3 DAF, and grains at 3 (e), 6 DAF (f) and 9 DAF (g), leaf (h), node (i, j). (j) Cross section of the node in (i). Scale bars, 10 mm in (b), 2 mm in (c‐j). (k) Subcellular localization of FLO16. Free GFP served as a control (lower panel). The fusion construct FLO16‐GFP was expressed in the rice protoplasts (upper panel). Green fluorescence signals, red chlorophyll autofluorescence, and bright field and merged images are shown in each panel. Scale bars, 10 μm.
The FLO16 gene was predicted to encode a cytosolic protein. To verify the subcellular location, the coding sequence of FLO16 was C‐terminally fused with GFP driven by the cauliflower mosaic virus 35S (CaMV35S) promoter. Free GFP in the control was diffused evenly in cytoplasm (Figure 5k lower panel). The distribution pattern of the FLO16‐GFP signal (Figure 5k upper panel) was almost the same as that of free GFP, suggesting that FLO16 protein was diffused in the cytosol as predicted.
Disruption of FLO16 causes multiple metabolic changes
MDH isoforms catalyse the reversible conversion of malate to OAA (Gietl, 1992; Heber, 1974; Scheibe, 2004). To determine endogenous MDH activity, crude enzyme was extracted from developing endosperm 6 and 9 DAF for native polyacrylamide gel electrophoresis (PAGE) analysis. As expected, one of the activity bands was not detected in the flo16 mutant, which was recovered in the complementation lines. Thus, flo16 seems to be a knockout mutant of CMDH. Interestingly, other activity bands showed a slight compensatory increase (Figure 6a). The total NAD‐MDH activity in flo16 endosperm at 9 DAF was less than one‐half of that in the wild type (Figure 6b). The lack of CMDH activity might result in alterations in substrates and products of malate metabolism. We evaluated the levels of closely related metabolites. There was an increase in malate levels in young leaves and roots relative to wild type, and malate levels in grains were increased only at 9 DAF (Figures S3A and 6c). We inferred that malate was mainly the substrate of CMDH. Considering that a ‘malate valve’ plays an essential role in redox regulation and energy conversion (Scheibe, 2004), we evaluated the major form of redox power and energy supplying, and identified decreases in the NADPH and ATP content (Figure S2). This indicated that energy metabolism and redox homeostasis were unstable in the flo16 mutant.
Figure 6.
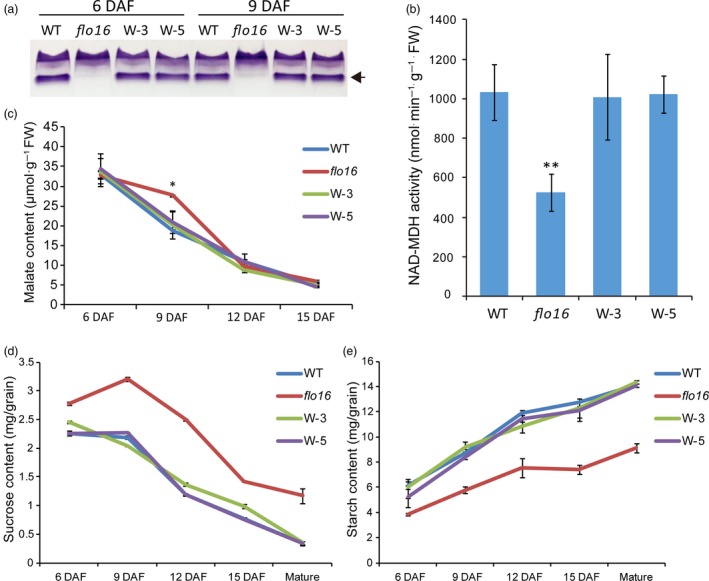
Responses of the metabolic pathways to deletion of FLO16 activity. (a) Native PAGE profiles of malate dehydrogenase (MDH) activities in developing endosperms at 6 and 9 DAF. (b) Malate contents in developing endosperms. (c) NAD‐MDH activity in wild‐type and flo16 developing endosperm. (d) Sucrose contents in developing and mature endosperm. (e) Starch contents in wild‐type and flo16 endosperm. All values are means ± SD, n = 3. Asterisks indicate statistical significance between the wild type and mutant, determined by Student's t‐tests (*P < 0.05; **P < 0.01).
Defective starch biosynthesis in flo16
Sucrose contents in single developing and mature grains of flo16 were much higher than in the wild type (Figure 6d), while starch contents were obviously lower (Figure 6e). Real‐time quantitative RT‐PCR was performed to examine possible effects of FLO16 on starch biosynthesis. Expression levels of many genes participating in starch biosynthesis during endosperm development were decreased, including genes coding for UDPG pyrophosphatase (UGPase), AGPases (AGPL2 and AGPS2b), soluble SSs (SSI), GBSSI, BEI, BEIIb, isoamylases (ISA1 and ISA2) and pullulanase (PUL). In contrast, genes coding for cytosolic phosphorylase (PHO2), disproportionating enzyme (DPE1 and DPE2) and sucrose synthase (Susy3) were increased, whereas FLO2, PPDKB (Pyruvate orthophosphate dikinase B) and RSR1 (Rice starch regulator 1) were only slightly increased (Figure 7a). It seems that genes functioning upstream of AGPase were highly expressed, and those acting downstream of AGPase were significantly reduced.
Figure 7.
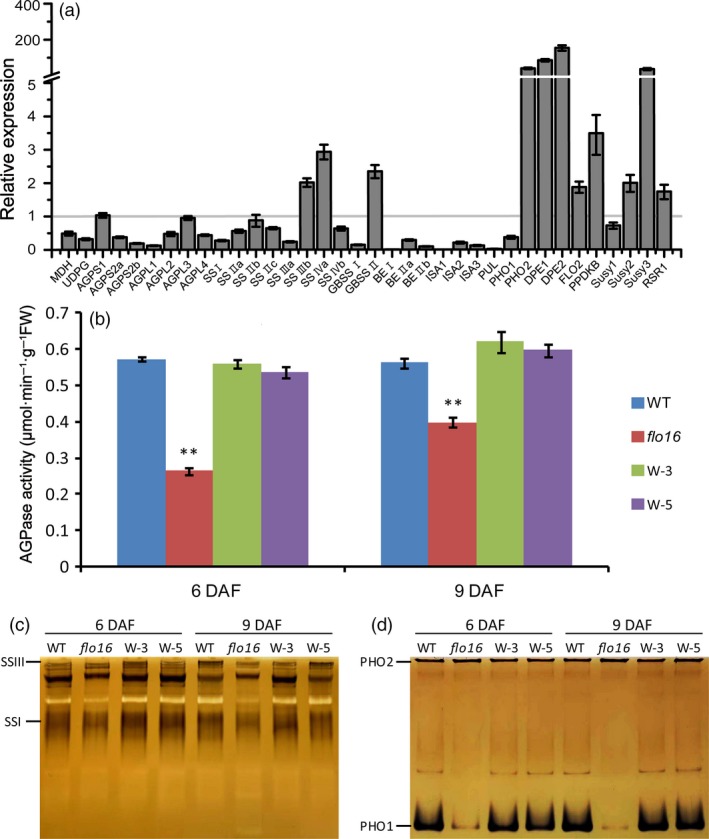
Gene expression and protein activity analyses of starch synthesizing enzymes. (a) Expression levels of starch synthesis‐related enzymes in developing endosperm 10 DAF. Data represent ratios of expression levels in flo16 to that of wild type. Values are means ± SD, n = 3. (b) AGPase activities in developing endosperm of wild type and flo16. Values are means ± SD, n = 3. Asterisks indicate the statistical significance determined by Student's t‐tests (*P < 0.05; **P < 0.01). (c) Activity bands for SSI, and SSIII. (d) Activity bands for PHO1 and PHO2.
The activities of key enzymes in starch synthesis were investigated. We first investigated AGPase activity since it was the rate‐limiting enzyme in starch synthesis. As predicted, AGPase activity in developing flo16 endosperm was lower than in the wild type (Figure 7b). Zymogram analyses performed to detect activities of several essential starch synthetic enzymes showed decreased activities of SSI (Figure 7c) and PHO1 (Figure 7d) in the flo16 mutant relative to wild type, but there was no significant change in SSIII and PHO2 activity. Thus, mutation of the FLO16 gene showed obvious effects on the activities of the key starch synthetic enzymes.
Starch biosynthesis in flo16 has almost no respond to exogenous malate or sucrose
Alterations in malate level caused dramatic effects on transitory starch metabolism in tomato fruit (Centeno et al., 2011). However, no difference in starch content was observed when malate level was decreased in potato (Szecowka et al., 2012). We investigated the influence of malate on starch synthesis in the developing endosperm of rice. Isolated flo16 and wild‐type endosperms at 9–12 DAF were incubated in the presence of 0, 5 or 125 mM malate for 3 h, respectively. Endosperm discs were washed twice, and the activities of starch synthesis‐related enzymes were determined. Catalytic activity of AGPase in the wild type was induced by malate treatment, whereas that in flo16 was significantly lower and barely responded to malate treatment (Figure 8a). Concurrently, expression levels of several key enzymes in starch synthesis were tested for induction in wild‐type endosperm (Figure 8b). Those experiments revealed up‐regulation in starch biosynthetic flux, suggesting that exogenous malate promoted transition from sucrose to starch. The deletion of CMDH at least partly blocked the response of starch synthesis to malate. Similar results were obtained when developing endosperms of flo16 and wild type were treated with sucrose (Figure 8c). Thus, the starch synthesis in flo16 mutant is defective in response to exogenous malate and sucrose.
Figure 8.
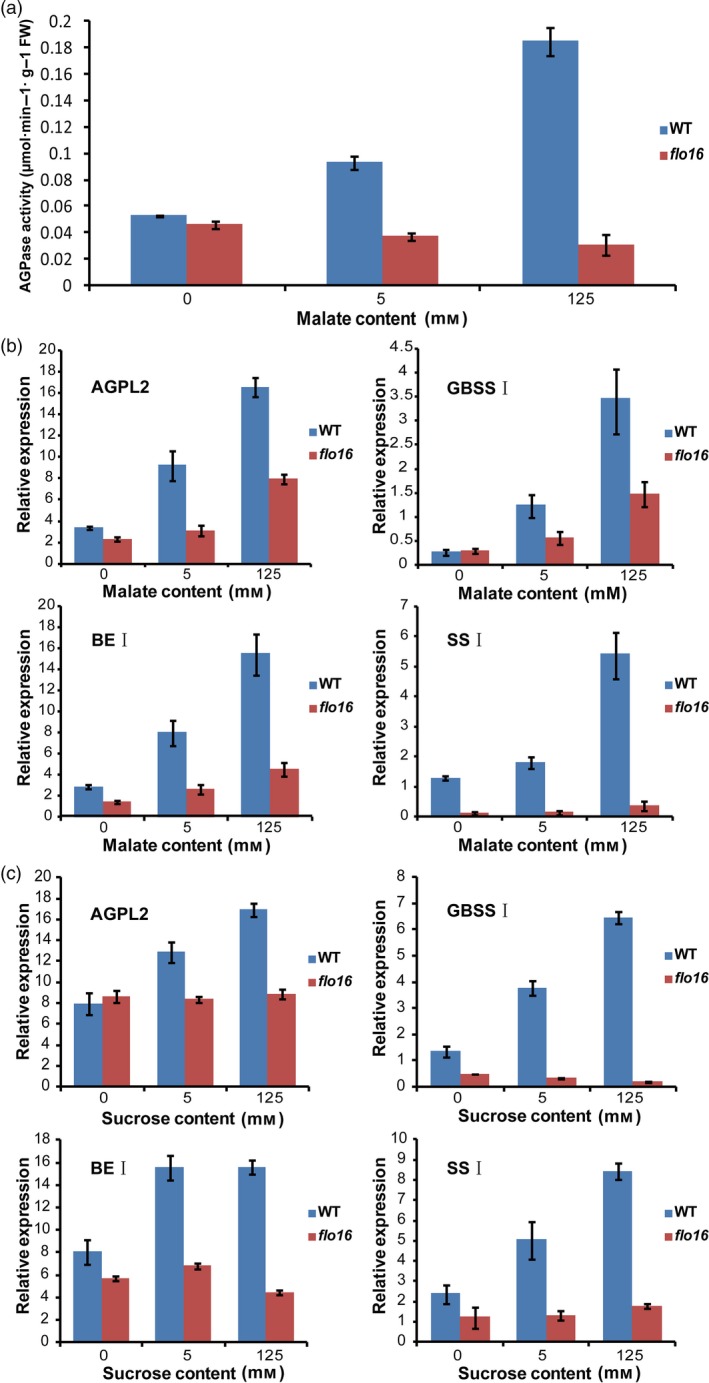
Influence of exogenous malate and sucrose on starch synthesis enzymes in wild‐type and flo16 endosperm. (a) AGPase activities in wild‐type and flo16 developing endosperms at 9–12 DAF treated with malate. (b) Expression levels of starch synthesis enzymes in the developing endosperm at 9–12 DAF incubated in malate. (c) The Expression levels of starch synthesis enzymes in developing endosperm at 9–12 DAF treated with sucrose. Values are means ± SD, n = 3.
Overexpression of FLO16 increases grain weight
We created transgenic lines overexpressing FLO16 driven by the endosperm‐specific Glutelin C promoter to evaluate the effect of elevated FLO16 expression level in rice endosperm. qRT‐PCR analysis showed that the expression levels of FLO16 in positive transgenic lines were much higher than those in the endosperm of the recipient cultivar Zhonghua 11 (Figure 9c). The major agronomic traits of these transgenic plants showed almost no difference with the recipient (Table S2). Notably, the 1000‐grain weight of these lines were significantly higher than Zhonghua 11 (Figure 9g), indicating that endosperm‐specific overexpression of CMDH will significantly promote starch biosynthesis in rice. Further analysis showed the elevated grain weight are mainly to the enlarged grain size as the grain length of transgenic lines were much larger than Zhonghua 11 (Figure 9a,d). This trend was even more obvious when dehulled grains were compared (Figure 9b). It is noteworthy that constitutive overexpression of CMDH could also result in increased grain weight (Figure S5).
Figure 9.
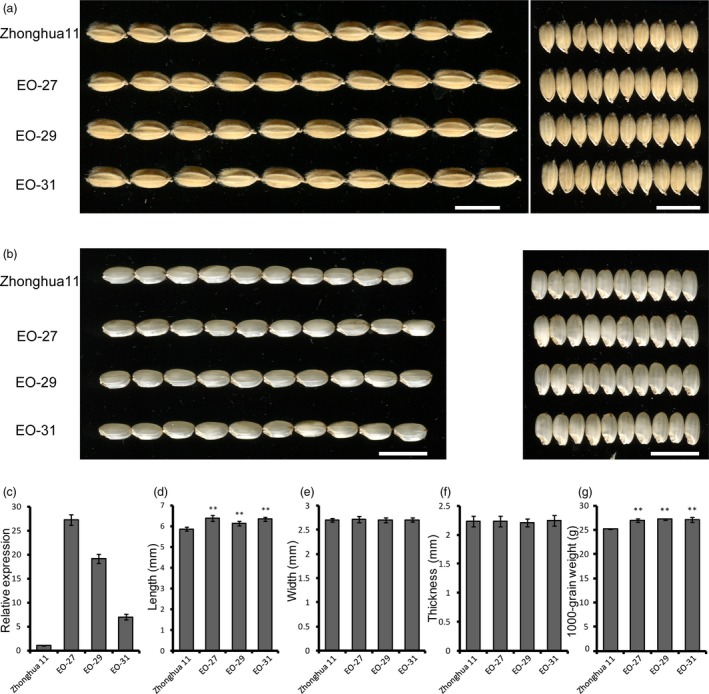
Effects of endosperm‐specific FLO16 overexpression on grains. (a, b) Images of hulled (a) and dehulled (b) seeds of the recipient and transgenic lines. EO‐27, EO‐29 and EO‐31 are independent transgenic lines. Scale bars, 1 cm. (c) Enhanced expression levels of FLO16 in overexpression endosperms. Expression level in Zhonghua 11 was considered as sample control. Values are means ± SDs, n = 3. (d–f) Size comparisons between the wild‐type and transgenic seeds. Values are means ± SDs, n = 10. (g) 1000‐grain weights of overexpression lines. Values are means ± SDs, n = 3. Asterisks indicate the statistical significance between the wild type and the mutant, determined by Student's t‐tests (**P < 0.01).
Discussion
CMDH has strong effects on starch biosynthesis during seed development
Previous studies characterized several mutants that affected starch synthesis in rice. Many of them have opaque endosperm, such as flo5 (ssIIIa), waxy (gbssI) and amylose‐extender (ae, beIIb) (Nishi et al., 2001). Endosperm‐defective mutants are valuable resources for elucidating molecular mechanisms underlying seed development and/or starch biosynthesis. In this study, we isolated mutant flo16 with floury endosperm and retarded growth. Its opaque endosperm appearance seemed to be caused by defective SG formation (Figure 2). Further analyses revealed that reduction in starch synthesis in flo16 endosperm led to reduced grain weight (Figure 1f), and changes in amylose content and amylopectin structure (Figure 3a,b).
The FLO16 locus was located within an 88 kb region in chromosome 10S. Sequence analysis and subsequent complementation tests determined that the underlying gene was Os10 g0478200. A 4 bp deletion caused premature termination of FLO16, indicating that flo16 is most probably a knockout mutant of that gene (Figure 4). MDH activity assays confirmed this result (Figure 6a). Os10 g0478200 encodes a cytosolic malate dehydrogenase (CMDH) that is expressed ubiquitously. Expression of CMDH driven by endosperm‐specific Glutelin C and Ubiquitin promoters both rescued the opaque phenotype of mutant seeds, indicating that the grain phenotype is due to lack of CMDH in the endosperm. Therefore, CMDH is important for starch biosynthesis during seed development.
CMDH functions in supplying reducing power and energy for starch biosynthesis in rice endosperm
Plant MDHs function in equilibrating reducing equivalents between organelles in the cell, and catalyse reversible conversion of malate and NAD(P) to OAA and NAD(P)H. In the cytosol, malate is oxidized to oxaloacetate by the cytosolic MDHs, generating reducing equivalent in the form of NADH. Oxaloacetate is then transported to the plastids, where the plastidial MDH converts it back to malate. This so‐called ‘malate valve’ is vital in equilibrating reducing equivalents in the cytosol and between organelles as well as regulating malate for further metabolic synthesis (Scheibe, 2004; Taniguchi and Miyake, 2012). An Arabidopsis double mutant lacking both the mitochondrial MDH isoforms was defective in seed maturation and post‐germination growth (Sew et al., 2016; Tomaz et al., 2010), while the mutant deficient in peroxisomal isoforms requires exogenous sugars for seedling (Pracharoenwattana et al., 2010). Plastidial NADP‐MDH can be redox‐regulated and activated dependent on light (Scheibe, 1987), while plastidial NAD‐MDH is not redox sensitive and acts in dark and non‐green parts (Berkemeyer et al., 1998). Plant growth of Arabidopsis nadp‐mdh mutants was either not affected (Hebbelmann et al., 2012) or slight weakened (Heyno et al., 2014) compared to the wild type, while pdnad‐mdh knockout mutant has an embryo–lethal phenotype (Beeler et al., 2014). Although it is well established that MDHs play important roles in central metabolism in plants, no mdh mutants were reported in rice. Recently, Heng et al. (2018) revealed that the defect in malate transport results in apical abortion panicles, suggesting an important role of malate metabolism in rice. However, the importance of CMDH in rice remains unclear.
In this study, CMDH activity was not detected in the flo16 mutant, resulting in overaccumulated malate and decreased NADPH and ATP contents (Figures 6c and S2). This result suggested that rice CMDH mainly functions in converting malate and NAD(P) to OAA and NAD(P)H. Further exogenous malate treatment indicated that high levels of malate had no detrimental effect on starch biosynthesis (Figure 8a,b). Sucrose flux is the main form of substrate supply for starch synthesis in the endosperm. Exogenous sucrose in sweet potato increased the transcript level of GBSSI (Wang et al., 2001). In our study, expression levels of GBSSI and several key enzymes in the wild type were induced by sucrose and malate, whereas no significant differences were observed in flo16 (Figure 8), indicating that starch synthesis in response to malate and sucrose is blocked in the flo16 mutant. Starch synthesis requires a high rate of turnover of reducing equivalents and a mass of energy supply. Previous study in tomato showed that alterations in mitochondrial malate metabolism have strong effects on starch biosynthesis in the amyloplast due to an altered cellular redox status (Centeno et al., 2011). In flo16, significantly decreased NADPH content indicated a lack of reduction potential required for starch synthesis. Moreover, ATP is referred to as the energy currency of the cell. Lower level of ATP in flo16 is expected to result in a shortage of energy, which in turn greatly affects starch biosynthesis. Therefore, it is likely that alterations in cytosolic malate‐related metabolism have strong effects on starch biosynthesis during seed development. Notably, mutation of CMDH led to defective plant growth, increased malate and reduced ATP content in flo16 seedling, indicating that the malate‐related metabolism also existed in other part of rice plant (Figures 1c,d and S3). However, the endosperm‐specific expression of CMDH almost completely rescued these deficiencies. Considering the significant role of photosynthesis in regulating redox state, we thus speculated that CMDH is more important in sink organs than source organs in rice.
Previous study in potato declared that the redox state affects starch synthesis by post‐translational regulation of AGPase (Tiessen et al., 2002). Moreover, the redox control of AGPase activity was also reported in rice endosperm (Tuncel et al., 2014). However, our study clearly indicates that AGPase activity is independent of redox control as the decrease ratio was constant (Figure S4). Whether and how reducing equivalents alter the redox state of other starch synthesis‐related enzymes remains to be further addressed.
Overexpression of FLO16 increases grain weight in rice
Grain weight is the major component of crop yield and an important agronomic trait in the breeding of cereal crops. Grain weight is positively associated with grain size. Several genes affecting grain weight have been identified in rice, such as GS3 (Fan et al., 2006; Mao et al., 2010), GW2 (Song et al., 2007), qSW5/GW5 (Liu et al., 2017; Shomura et al., 2008; Weng et al., 2008), GS5 (Li et al., 2011), qGL3/qGL3.1 (Qi et al., 2012; Zhang et al., 2012), GW8 (Wang et al., 2012), TGW6 (Ishimaru et al., 2013),GL7/GW7 (Wang et al., 2015a,b), Gn1a (Ashikari et al., 2005), DEP1 (Huang et al., 2009) and OsSPL14 (Jiao et al., 2010), but the mechanisms that control grain weight are rarely reported.
Our endosperm‐specific overexpressing of CMDH in Zhonghua 11 significantly increased grain weight which might due to the elevated grain length, demonstrated that elevated CMDH activity in the endosperm significantly promotes starch biosynthesis. Therefore, FLO16 has the potential for improving grain weight and grain yield in rice.
Experimental procedures
Plant materials and growing conditions
The flo16 mutant was generated by 60Co treatment of indica cv. N22 (Nagina22, an Indian traditional variety). An F2 population from cross flo16 mutant × japonica cv. DJY (Dianjingyou 1, from Yunnan Academy of Agricultural Sciences, Japonica Rice Breeding Center) was developed for fine mapping. Rice plants were grown in experimental fields at Nanjing or Beijing. Developing seeds of the wild type (N22) and flo16 at 6–21 days after flowering (DAF) were harvested and stored at −80°C if not used immediately.
Starch grain observation
Transverse sections (~1 mm in thickness) of developing endosperm were fixed in 2% (W/V) paraformaldehyde, 2% (V/V) glutaraldehyde and 250 mm sucrose buffered with 50 mm PIPES‐KOH (pH 7.2). The fixed endosperm was then dehydrated in an ethanol series and embedded in LR White resin (London Resin, Berkshire, UK). After sectioning with an ultramicrotome (UC7, Leica, Solms, Germany), specimens (1 μm) were stained with I2‐KI and observed under an optical microscope (Nikon, Tokyo, Japan).
Naturally fractured sections of mature seeds were observed with a scanning electron microscope (S‐3400N, Hitachi, Tokyo, Japan) as described (Kang et al., 2005).
Physicochemical properties of starch in endosperm
Starch content in the endosperm was measured using a starch assay kit (Megazyme, Ireland). Amylose, lipid and protein contents were measured as described previously (Kang et al., 2005; Liu et al., 2009). Chain length distribution of amylopectin was measured using DSA‐FACE (Han et al., 2012). Pasting properties were determined with a rapid visco analyzer (RVA‐Tec Master, Perten, Sweden). Gelatinization and swelling coefficient of starch were measured by mixing flour with different concentrations of urea (0–9 m) and incubating at 25°C for 24 h (Nishi et al., 2001).
Mapping and cloning of FLO16
A total of 1502 F2 individuals with recessive phenotype were used for map‐based cloning. Molecular markers were developed according to nucleotide polymorphisms between N22 and DJY (Table S3). Six open reading frames (ORFs) in the fine‐mapped region were predicted by the Rice Annotation Project Database (http://rapdb.dna.affrc.go.jp/). The mutation was confirmed by sequencing PCR products for these genes.
Vector construction and plant transformation
For functional complementation of flo16, the wild‐type FLO16 sequence under control of its native promoter (2000 bp upstream of ATG) was inserted into vector pCUbi1390. This plasmid was introduced into flo16 callus by Agrobacterium‐mediated transformation (Hiei and Komari, 2008; Hiei et al., 1994). For endosperm‐specific functional complementation of flo16 and overexpression of FLO16, the wild‐type FLO16 sequence was inserted into vector pCUbi1390, in which FLO16 was driven by the Glutelin C promoter. This plasmid was introduced into flo16 and Zhonghua 11 callus, respectively. For constitutive overexpression of FLO16, the wild‐type FLO16 sequence was inserted into vector pCUbi1390, in which FLO16 was driven by the maize Ubiquitin promoter, and this plasmid was introduced into Zhonghua 11 callus. For promoter analysis, the FLO16 promoter was inserted into a β‐glucuronidase (GUS) expression vector and introduced into Nipponbare callus as described above. All primers used were listed in Table S4.
GUS staining
Transgenic plants obtained as mentioned above were stained as described (Jefferson et al., 1987). Images were captured with a stereoscope (Leica Application Suite 3.3, Germany)
Subcellular localization
The coding sequence of FLO16 was cloned into the pAN580‐GFP vector to express FLO16‐GFP under the control of a double 35S promoter. The control (GFP alone) and FLO16‐GFP were each expressed in rice protoplasts (Chen et al., 2006). Images of GFP fluorescence were captured using a confocal laser scanning microscope (LSM710, Zeiss, Germany).
RNA extraction and qRT‐PCR analysis
Total RNA was extracted using an RNA Prep Pure Plant kit (TIANGEN Biotech, Beijing). First‐strand cDNA was synthesized from 2 μg of total RNA by priming with oligo (dT) in 20 μL reaction volumes, using a PrimeScript Reverse Transcriptase Kit (TaKaRa, Tokyo, Japan). qRT‐PCR was performed using the SYBR Premix Ex Taq (TaKaRa) in an ABI7500 Real‐time PCR system. Gene‐specific primers used in this analysis are listed in Table S3 or the previous study (She et al., 2010). ActinI was used as an internal control.
Determination of metabolite levels
Levels of sucrose were quantified by UPLC as described previously (Fernie et al., 2001). Malate contents were measured by enzymatic analysis (Nunes‐Nesi et al., 2007). ATP contents were detected using an ATP assay kit (Beyotime, Shanghai, China) according to the manufacturer's instructions. Developing grains and other tissues of rice were captured into liquid N2 and stored in −80°C if not extracted immediately, and all extractions were performed on ice as soon as possible to reduce loss of substance. Furthermore, the extractions for ATP and malate measurement were filtered across the 10 kDa ultrafiltration centrifugal tube (Millipore, Billerica, MA) to decrease degradation of the metabolites.
Enzyme activity and zymogram analysis
Crude enzymes from developing endosperm were extracted for enzyme activity assays (Peng et al., 2014). MDH isozymes were separated in 10% native polyacrylamide gels stained according to a previous study (Brown et al., 1978). Total MDH activity assays were performed in reaction mixtures containing 10 μL crude enzyme, 3 mm malate, 1 mm NAD buffered with 50 mm glycine‐NaOH (pH 8.5) at 25°C and determined by monitoring altered NADH content at 340 nm (Zheng et al., 2005). AGPase was extracted in 50 mm Hepes‐KOH (pH 7.8) buffer with 5 mm MgCl2 and analysed in 50 mm Hepes‐KOH (pH 7.8), 5 mm MgCl2, 0.6 mm NAD, 2.5 mm Na‐PPi, 1 unit/mL phosphoglucomutase (Sigma‐Aldrich, Saint Louis, MO), 2.5 units/mL Glc‐6‐P dehydrogenase (Sigma‐Aldrich) and 1 mm ADP‐Glc with or without 5 mm DTT (Tiessen et al., 2002). Activities measured with or without DTT were termed Vred or Vsel, respectively. Activities of starch synthases (SSI and SSIII) were determined according to Nishi et al. (2001). Phosphorylase (PHO1 and PHO2) activities were tested in a polyacrylamide gel containing 0.8% (w/v) oyster glycogen (Sigma‐Aldrich) (Satoh et al., 2008). All electrophoreses were conducted at 4°C.
Incubation of endosperm discs with exogenous malate or sucrose
Developing seeds at 9–12 DAF were harvested and then sliced into 1‐mm‐thickness discs, washed three times in fresh incubation medium (50 mM HEPES‐KOH, pH7.4), and incubated (~200 mg) in 5 mL of incubation medium containing Na‐malate or sucrose in various concentration for 3 h. All incubations were performed at 30°C with 90 rpm shaking. After incubation, samples were washed three times in incubation medium and blotted with filter papers. Further assays were performed as mentioned above.
Supporting information
Figure S1 Endosperm‐specific functional complementation lines of flo16 restore normal appearance.
Figure S2 NADP+/NADPH and ATP contents in developing endosperm.
Figure S3 Metabolic differences between wild type and flo16 in young seedling.
Figure S4 Redox activation state of AGPase.
Figure S5 Effects of FLO16 over‐expression on grains.
Table S1 Comparison of agronomic traits between the wild type and flo16 mutant.
Table S2 Agronomic traits of overexpression lines.
Table S3 Oligonucleotide primers used in map‐based cloning.
Table S4 Gene‐specific primers used in this study.
Acknowledgements
This work was supported by the National Transgenic Science and Technology Program (2016ZX08009003‐004), the National Natural Science Foundation of China (Grant 31330054), the Jiangsu Science and Technology Development Program (BE2017368), the Agricultural Science and Technology Innovation Fund project of Jiangsu Province (CX(16)1029) and the Fundamental Research Funds for the Central Universities (KYTZ201601). This work was also supported by the Key Laboratory of Biology, Genetics and Breeding of Japonica Rice in Mid‐lower Yangtze River, Ministry of Agriculture, P.R. China, and the Jiangsu Collaborative Innovation Center for Modern Crop Production.
Contributor Information
Yihua Wang, Email: yihuawang@njau.edu.cn.
Jianmin Wan, Email: wanjm@njau.edu.cn, Email: wanjianmin@caas.cn.
References
- Ashikari, M. , Sakakibara, H. , Lin, S.Y. , Yamamoto, T. , Takashi, T. , Nishimura, A. , Angeles, E.R. et al (2005) Cytokinin oxidase regulates rice grain production. Science, 309, 741–745. [DOI] [PubMed] [Google Scholar]
- Ball, S. , Guan, H.P. , James, M. , Myers, A. , Keeling, P. , Mouille, G. , Buleon, A. et al (1996) From glycogen to amylopectin: a model for the biogenesis of the plant starch granule. Cell, 86, 349–352. [DOI] [PubMed] [Google Scholar]
- Beckles, D.M. , Craig, J. and Smith, A.M. (2001) ADP‐glucose pyrophosphorylase is located in the plastid in developing tomato fruit. Plant Physiol. 126, 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler, S. , Liu, H.C. , Stadler, M. , Schreier, T. , Eicke, S. , Lue, W.L. , Truernit, E. et al (2014) Plastidial NAD‐dependent malate dehydrogenase is critical for embryo development and heterotrophic metabolism in Arabidopsis. Plant Physiol. 164, 1175–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkemeyer, M. , Scheibe, R. and Ocheretina, O. (1998) A novel, non‐redox‐regulated NAD‐dependent malate dehydrogenase from chloroplasts of Arabidopsis thaliana L. J. Biol. Chem. 273, 27927–27933. [DOI] [PubMed] [Google Scholar]
- Brown, A.H.D. , Nevo, E. , Zohary, D. and Dagan, O. (1978) Genetic variation in natural populations of wild barley (Hordeum spontaneum). Genetica, 49, 97–108. [Google Scholar]
- Centeno, D.C. , Osorio, S. , Nunes‐Nesi, A. , Bertolo, A.L. , Carneiro, R.T. , Araujo, W.L. , Steinhauser, M.C. et al (2011) Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell, 23, 162–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S.B. , Tao, L.Z. , Zeng, L.R. , Vega‐Sanchez, M.E. , Umemura, K. and Wang, G.L. (2006) A highly efficient transient protoplast system for analyzing defence gene expression and protein‐protein interactions in rice. Mol. Plant Pathol. 7, 417–427. [DOI] [PubMed] [Google Scholar]
- Fan, C.H. , Xing, Y.Z. , Mao, H.L. , Lu, T.T. , Han, B. , Xu, C.G. , Li, X.H. et al (2006) GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 112, 1164–1171. [DOI] [PubMed] [Google Scholar]
- Fernie, A.R. , Roscher, A. , Ratcliffe, R.G. and Kruger, N.J. (2001) Fructose 2,6‐bisphosphate activates pyrophosphate: fructose‐6‐phosphate 1‐phosphotransferase and increases triose phosphate to hexose phosphate cycling in heterotrophic cells. Planta, 212, 250–263. [DOI] [PubMed] [Google Scholar]
- Gietl, C. (1992) Malate dehydrogenase isoenzymes: cellular locations and role in the flow of metabolites between the cytoplasm and cell organelles. Biochem. Biophys. Acta. 1100, 217–234. [DOI] [PubMed] [Google Scholar]
- Han, X.H. , Wang, Y.H. , Liu, X. , Jiang, L. , Ren, Y.L. , Liu, F. , Peng, C. et al (2012) The failure to express a protein disulphide isomerase like protein results in a floury endosperm and an endoplasmic reticulum stress response in rice. J. Exp. Bot. 63, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanashiro, I. , Tagawa, M. , Shibahara, S. , Iwata, K. and Takeda, Y. (2002) Examination of molar‐based distribution of A, B and C chains of amylopectin by fluorescent labeling The failure to express a protein disulphide isomerase‐like protein results in a floury endosperm and an endoplasmic reticulum stress response in rice with 2‐aminopyridine. Carbohydr. Res. 337, 1211–1215. [DOI] [PubMed] [Google Scholar]
- Hanashiro, I. , Itoh, K. , Kuratomi, Y. , Yamazaki, M. , Igarashi, T. , Matsugasako, J.I. and Takeda, Y. (2008) Granule‐bound starch synthase i is responsible for biosynthesis of extra‐long unit chains of amylopectin in rice. Plant Cell Physiol. 49, 925–933. [DOI] [PubMed] [Google Scholar]
- Hebbelmann, I. , Selinski, J. , Wehmeyer, C. , Goss, T. , Voss, I. , Mulo, P. , Kangasjärvi, S. et al (2012) Multiple strategies to prevent oxidative stress in Arabidopsis plants lacking the malate valve enzyme NADP‐malate dehydrogenase. J. Exp. Bot. 63, 1445–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber, U. (1974) Metabolite exchange between chloroplasts and cytoplasm. Annu. Rev. Plant Physiol. 25, 393–421. [Google Scholar]
- Heng, Y.Q. , Wu, C.Y. , Long, Y. , Luo, S. , Ma, J. , Chen, J. , Liu, J.F. et al (2018) OsALMT7 maintains panicle size and grain yield in rice by mediating malate transport. Plant Cell, 30, 889–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyno, E. , Innocenti, G. , Lemaire, S.D. , Issakidis‐Bourguet, E. and Krieger‐Liszkay, A. (2014) Putative role of the malate valve enzyme NADP‐malate dehydrogenase in H2O2 signalling in Arabidopsis. Philos. Trans. R. Soc. B Biol. Sci. 369, 20130228–20130228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei, Y. and Komari, T. (2008) Agrobacterium‐mediated transformation of rice using immature embryos or calli induced from mature seed. Nat. Protoc. 3, 824–834. [DOI] [PubMed] [Google Scholar]
- Hiei, Y. , Ohta, S. , Komari, T. and Kumashiro, T. (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T‐DNA. Plant J. 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Huang, X.Z. , Qian, Q. , Liu, Z.B. , Sun, H.Y. , He, S.Y. , Luo, D. , Xia, G.M. et al (2009) Natural variation at the DEP1 locus enhances grain yield in rice. Nat. Genet. 41, 494–497. [DOI] [PubMed] [Google Scholar]
- Ishimaru, K. , Hirotsu, N. , Madoka, Y. , Murakami, N. , Hara, N. , Onodera, H. , Kashiwagi, T. et al (2013) Loss of function of the IAA‐glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat. Genet. 45, 707–711. [DOI] [PubMed] [Google Scholar]
- Isshiki, M. , Nakajima, M. , Satoh, H. and Shimamoto, K. (2000) dull: rice mutants with tissue‐specific effects on the splicing of the waxy pre‐mRNA. Plant J. 23, 451–460. [DOI] [PubMed] [Google Scholar]
- Isshiki, M. , Matsuda, Y. , Takasaki, A. , Wong, H.L. , Satoh, H. and Shimamoto, K. (2008) Du3, a mRNA cap‐binding protein gene, regulates amylose content in Japonica rice seeds. Plant Biotechnol. 25, 483–487. [Google Scholar]
- Jane, J.L. , Kasemsuwan, T. , Leas, S. , Zobel, H. and Robyt, J.F. (1994) Anthology of starch granule morphology by scanning electron microscopy. Starch, 46, 121–129. [Google Scholar]
- Jefferson, R.A. , Kavanagh, T.A. and Bevan, M.W. (1987) GUS fusions: beta‐glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao, Y.Q. , Wang, Y.H. , Xue, D.W. , Wang, J. , Yan, M.X. , Liu, G.F. , Dong, G.J. et al (2010) Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat. Genet. 42, 541–544. [DOI] [PubMed] [Google Scholar]
- Kang, H.G. , Park, S. , Matsuoka, M. and An, G. (2005) White‐core endosperm floury endosperm‐4 in rice is generated by knockout mutations in the C‐type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J. 42, 901–911. [DOI] [PubMed] [Google Scholar]
- Kromer, S. and Scheibe, R. (1996) Function of the chloroplastic malate valve for respiration during photosynthesis. Biochem. Soc. Trans. 24, 761–766. [DOI] [PubMed] [Google Scholar]
- Li, Y.B. , Fan, C.C. , Xing, Y.Z. , Jiang, Y.H. , Luo, L.J. , Sun, L. , Shao, D. et al (2011) Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 43, 1266–1269. [DOI] [PubMed] [Google Scholar]
- Li, Y.B. , Fan, C.C. , Xing, Y.Z. , Yun, P. , Luo, L.J. , Yan, B. , Peng, B. et al (2014) Chalk5 encodes a vacuolar H + ‐translocating pyrophosphatase influencing grain chalkiness in rice. Nat. Genet. 46, 657–657. [DOI] [PubMed] [Google Scholar]
- Li, S.F. , Wei, X.J. , Ren, Y.L. , Qiu, J.H. , Jiao, G.A. , Guo, X.P. , Tang, S.Q. et al (2017) OsBT1 encodes an ADP‐glucose transporter involved in starch synthesis and compound granule formation in rice endosperm. Sci. Rep. 7, 40124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Ma, X. , Liu, S. , Zhu, C. , Jiang, L. , Wang, Y. , Shen, Y. et al (2009) Identification and characterization of a novel Waxy allele from a Yunnan rice landrace. Plant Mol. Biol. 71, 609–626. [DOI] [PubMed] [Google Scholar]
- Liu, J.F. , Chen, J. , Zheng, X.M. , Wu, F.Q. , Lin, Q.B. , Heng, Y.Q. , Tian, P. et al (2017) GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plant 3, 17043. [DOI] [PubMed] [Google Scholar]
- Long, W.H. , Dong, B.N. , Wang, Y.H. , Pan, P.Y. , Wang, Y.L. , Liu, L.L. , Chen, X.L. et al (2017) FLOURY ENDOSPERM8, encoding the UDP‐glucose pyrophosphorylase 1, affects the synthesis and structure of starch in rice endosperm. J. Plant Biol. 60, 513–522. [Google Scholar]
- Mao, H.L. , Sun, S.Y. , Yao, J.L. , Wang, C.R. , Yu, S.B. , Xu, C.G. , Li, X.H. et al (2010) Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc. Natl Acad. Sci. USA 107, 19579–19584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, C. and Smith, A.M. (1995) Starch biosynthesis. Plant Cell, 7, 971–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima, R. , Maekawa, M. , Fujita, N. and Sakamoto, W. (2010) A rapid, direct observation method to isolate mutants with defects in starch grain morphology in rice. Plant Cell Physiol. 51, 728–741. [DOI] [PubMed] [Google Scholar]
- Matsushima, R. , Maekawa, M. , Kusano, M. , Kondo, H. , Fujita, N. , Kawagoe, Y. and Sakamoto, W. (2014) Amyloplast‐localized SUBSTANDARD STARCH GRAIN4 protein influences the size of starch grains in rice endosperm. Plant Physiol. 164, 623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima, R. , Maekawa, M. , Kusano, M. , Tomita, K. , Kondo, H. , Nishimura, H. , Crofts, N. et al (2016) Amyloplast membrane protein SUBSTANDARD STARCH GRAIN6 controls starch grain size in rice endosperm. Plant Physiol. 170, 1445–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, Y. (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol. 43, 718–725. [DOI] [PubMed] [Google Scholar]
- Nishi, A. , Nakamura, Y. , Tanaka, N. and Satoh, H. (2001) Biochemical and genetic analysis of the effects of amylose‐extender mutation in rice endosperm. Plant Physiol. 127, 459–472. [PMC free article] [PubMed] [Google Scholar]
- Nishio, T. and Iida, S. (1993) Mutants having a low content of 16‐kDa allergenic protein in rice (Oryza sativa L.). Theor. Appl. Genet. 86, 317–321. [DOI] [PubMed] [Google Scholar]
- Nunes‐Nesi, A. , Carrari, F. , Gibon, Y. , Sulpice, R. , Lytovchenko, A. , Fisahn, J. , Graham, J. et al (2007) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J. 50, 1093–1106. [DOI] [PubMed] [Google Scholar]
- Peng, C. , Wang, Y.H. , Liu, F. , Ren, Y.L. , Zhou, K.N. , Lv, J. , Zheng, M. et al (2014) FLOURY ENDOSPERM6 encodes a CBM48 domain‐containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J. 77, 917–930. [DOI] [PubMed] [Google Scholar]
- Pracharoenwattana, I. , Zhou, W. and Smith, S.M. (2010) Fatty acid beta‐oxidation in germinating Arabidopsis seeds is supported by peroxisomal hydroxypyruvate reductase when malate dehydrogenase is absent. Plant Mol. Biol. 72, 101–109. [DOI] [PubMed] [Google Scholar]
- Qi, P. , Lin, Y.S. , Song, X.J. , Shen, J.B. , Huang, W. , Shan, J.X. , Zhu, M.Z. et al (2012) The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin‐T1;3. Cell Res. 22, 1666–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao, Y. , Lee, S.I. , Piao, R. , Jiang, W. , Ham, T.H. , Chin, J.H. , Piao, Z. et al (2010) Fine mapping and candidate gene analysis of the floury endosperm gene, FLO(a), in rice. Mol. Cell 29, 167–174. [DOI] [PubMed] [Google Scholar]
- Ryoo, N. , Yu, C. , Park, C.S. , Baik, M.Y. , Park, I.M. , Cho, M.H. , Bhoo, S.H. et al (2007) Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white‐core floury endosperm in rice (Oryza sativa L.). Plant Cell Rep. 26, 1083–1095. [DOI] [PubMed] [Google Scholar]
- Satoh, H. , Shibahara, K. , Tokunaga, T. , Nishi, A. , Tasaki, M. , Hwang, S.K. , Okita, T.W. et al (2008) Mutation of the plastidial alpha‐glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell 20, 1833–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe, R. (1987) NADP+‐malate dehydrogenase in C3‐plants: regulation and role of a light‐activated enzyme. Physiol. Plant. 71, 393–400. [Google Scholar]
- Scheibe, R. (2004) Malate valves to balance cellular energy supply. Physiol. Plant. 120, 21–26. [DOI] [PubMed] [Google Scholar]
- Sew, Y.S. , Stroher, E. , Fenske, R. and Millar, A.H. (2016) Loss of mitochondrial malate dehydrogenase activity alters seed metabolism impairing seed maturation and post‐germination growth in Arabidopsis. Plant Physiol. 171, 849–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She, K.C. , Kusano, H. , Koizumi, K. , Yamakawa, H. , Hakata, M. , Imamura, T. , Fukuda, M. et al (2010) A novel factor FLOURY ENDOSPERM2 is involved in regulation of rice grain size and starch quality. Plant Cell 22, 3280–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomura, A. , Izawa, T. , Ebana, K. , Ebitani, T. , Kanegae, H. , Konishi, S. and Yano, M. (2008) Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 40, 1023–1028. [DOI] [PubMed] [Google Scholar]
- Song, X.J. , Huang, W. , Shi, M. , Zhu, M.Z. and Lin, H.X. (2007) A QTL for rice grain width and weight encodes a previously unknown RING‐type E3 ubiquitin ligase. Nat. Genet. 39, 623–630. [DOI] [PubMed] [Google Scholar]
- Sullivan, T.D. , Strelow, L.I. , Illingworth, C.A. , Phillips, R.L. and Nelson, O.E. Jr . (1991) Analysis of maize brittle‐1 alleles and a defective suppressor‐mutator‐induced mutable allele. Plant Cell 3, 1337–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szecowka, M. , Osorio, S. , Obata, T. , Araujo, W.L. , Rohrmann, J. , Nunes‐Nesi, A. and Fernie, A.R. (2012) Decreasing the mitochondrial synthesis of malate in potato tubers does not affect plastidial starch synthesis, suggesting that the physiological regulation of ADPglucose pyrophosphorylase is context dependent. Plant Physiol. 160, 2227–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi, M. and Miyake, H. (2012) Redox‐shuttling between chloroplast and cytosol: Integration of intra‐chloroplast and extrachloroplastmetabolism. Curr. Opin. Plant Biol. 15, 252–260. [DOI] [PubMed] [Google Scholar]
- Tateoka, T. (1962) Starch grains of endosperm in grass systematics. Bot. Soc. Japan 75, 377–383. [Google Scholar]
- Tetlow, I.J. , Davies, E.J. , Vardy, K.A. , Bowsher, C.G. , Burrell, M.M. and Emes, M.J. (2003) Subcellular localization of ADPglucose pyrophosphorylase in developing wheat endosperm and analysis of the properties of a plastidial isoform. J. Exp. Bot. 54, 715–725. [DOI] [PubMed] [Google Scholar]
- Tetlow, I.J. , Wait, R. , Lu, Z. , Akkasaeng, R. , Bowsher, C.G. , Esposito, S. , Kosar‐Hashemi, B. et al (2004) Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein‐protein interactions. Plant Cell 16, 694–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow, I.J. , Beisel, K.G. , Cameron, S. , Makhmoudova, A. , Liu, F. , Bresolin, N.S. , Wait, R. et al (2008) Analysis of protein complexes in wheat amyloplasts reveals functional interactions among starch biosynthetic enzymes. Plant Physiol. 146, 1878–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen, A. , Hendriks, J.H. , Stitt, M. , Branscheid, A. , Gibon, Y. , Farre, E.M. and Geigenberger, P. (2002) Starch synthesis in potato tubers is regulated by post‐translational redox modification of ADP‐glucose pyrophosphorylase: a novel regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell, 14, 2191–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaz, T. , Bagard, M. , Pracharoenwattana, I. , Linden, P. , Lee, C.P. , Carroll, A.J. , Stroher, E. et al (2010) Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis. Plant Physiol. 154, 1143–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuncel, A. , Cakir, B. , Hwang, S.K. and Okita, T.W. (2014) The role of the large subunit in redox regulation of the rice endosperm ADP‐glucose pyrophosphorylase. FEBS J. 281, 4951–4962. [DOI] [PubMed] [Google Scholar]
- Wang, S.J. , Yeh, K.W. and Tsai, C.Y. (2001) Regulation of starch granule‐bound starch synthase I gene expression by circadian clock and sucrose in the source tissue of sweet potato. Plant Sci. 161, 635–644. [Google Scholar]
- Wang, S.K. , Wu, K. , Yuan, Q.B. , Liu, X.Y. , Liu, Z.B. , Lin, X.Y. , Zeng, R.Z. et al (2012) Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 44, 950–954. [DOI] [PubMed] [Google Scholar]
- Wang, S.K. , Li, S. , Liu, Q. , Wu, K. , Zhang, J.Q. , Wang, S.S. , Wang, Y. et al (2015a) The OsSPL16‐GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 47, 949–954. [DOI] [PubMed] [Google Scholar]
- Wang, Y.X. , Xiong, G.S. , Hu, J. , Jiang, L. , Yu, H. , Xu, J. , Fang, Y.X. et al (2015b) Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 47, 944–948. [DOI] [PubMed] [Google Scholar]
- Weng, J.F. , Gu, S.H. , Wan, X.Y. , Gao, H. , Guo, T. , Su, N. , Lei, C.L. et al (2008) Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 18, 1199–1209. [DOI] [PubMed] [Google Scholar]
- Zeng, D. , Yan, M.X. , Wang, Y.H. , Liu, X.F. , Qian, Q. and Li, J.Y. (2007) Du1, encoding a novel Prp1 protein, regulates starch biosynthesis through affecting the splicing of Wx(b) supercript stop pre‐mRNAs in rice (Oryza sativa L.). Plant Mol. Biol. 65, 501–509. [DOI] [PubMed] [Google Scholar]
- Zhang, X.J. , Wang, J.F. , Huang, J. , Lan, H.X. , Wang, C.L. , Yin, C.F. , Wu, Y.Y. et al (2012) Rare allele of OsPPKL1 associated with grain length causes extra‐large grain and a significant yield increase in rice. Proc. Natl Acad. Sci. USA 109, 21534–21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Ren, Y.L. , Lu, B.Y. , Yang, C.Y. , Feng, Z.M. , Liu, Z. , Chen, J. et al (2016) FLOURY ENDOSPERM7 encodes a regulator of starch synthesis and amyloplast development essential for peripheral endosperm development in rice. J. Exp. Bot. 67, 633–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, N. , Xu, J. , Wu, Z. , Chen, J. , Hu, X. , Song, L. , Yang, G. et al (2005) Clonorchis sinensis: molecular cloning and functional expression of novel cytosolic malate dehydrogenase. Exp. Parasitol. 109, 220–227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Endosperm‐specific functional complementation lines of flo16 restore normal appearance.
Figure S2 NADP+/NADPH and ATP contents in developing endosperm.
Figure S3 Metabolic differences between wild type and flo16 in young seedling.
Figure S4 Redox activation state of AGPase.
Figure S5 Effects of FLO16 over‐expression on grains.
Table S1 Comparison of agronomic traits between the wild type and flo16 mutant.
Table S2 Agronomic traits of overexpression lines.
Table S3 Oligonucleotide primers used in map‐based cloning.
Table S4 Gene‐specific primers used in this study.


