Abstract
Surgeons have a steep learning capacity to understand 2-D images provided by conventional cloacagrams. Imaging advances now allow for 3-D reconstruction and 3-D models; but no evaluation of the value of these techniques exists in the literature. Therefore, we sought to determine if advances in 3-D imaging would benefit surgeons, lead to accelerated learning, and improve understanding for operative planning of a cloaca reconstruction. Questionnaires were used to assess the understanding of 2-D and 3-D images by pediatric surgical faculty and trainees. For the same case of a cloacal malformation, a 2D contrast study cloacagram, a 3D model rotatable CT scan reconstruction, a software enhanced 3D video animation (which allowed the observer to manipulate the structure in any orientation), and a printed physical 3D cloaca model that could be held in the observer’s hand were employed. Logistic mixed effect models assessed whether the proportion of questions about the case that were answered correctly differed by imaging modality, and whether the proportion answered correctly differed between trainee and attending surgeons for any particular modality. Twenty-nine pediatric surgery trainees (27 pediatric general surgery and 2 pediatric urology surgery trainees) and 30 pediatric surgery and urology faculty participated. For trainees, the percentage of questions answered correctly was: 2-D 10.5%, 3-D PACS 46.7%, 3-D Enhanced 67.1%, and 3-D Printed 73.8%. For faculty, the total percentage of questions answered correctly was: 2-D 22.2%, 3-D PACS 54.8%, 3D Enhanced 66.2%, and 3-D printed 74.0%. The differences in rates of correctness across all four modalities were significant in both fellows and attendings (p < 0.001), with performance being lowest for the 2-D modality, and with increasing percentage of correct answers with each subsequent modality. The difference between trainees and attendings in correctness rate was significant only for the 2-D modality, with attendings answering correctly more often. The 2-D cloacagram, as the least complex model, was the most difficult to interpret. The more complex the modality, the more correct were the responses obtained from both groups. Trainees and attendings had similar levels of correct answers and understanding of the cloacagram for the more advanced modalities. Mental visualization skills of anatomy and complex 3-D spatial arrangements traditionally have taken years of experience to master. Now with novel surgical education resources of a 3-D cloacagram, a more quickly advancing skill is possible.
Keywords: Cloaca, 3D printing, Surgical education, Anorectal malformation
Introduction
A cloacal malformation occurs when the common urogenital/rectal tract fails to separate, leading to a single opening in the perineum for the urinary, gynecologic, and intestinal systems. This anomaly is rare in females and has variable presentations.1 The anatomy is complex and needs to be fully delineated for parental counseling on prognosis for continence as well as operative planning. As newborns, these patients undergo a diverting colostomy, and occasionally a vaginostomy to divert a hydrocolops if hydronephrosis is present. A vesicostomy is rarely required.2 These are only some of the indications for a vaginostomy. After the newborn period, radiographic imaging is critical for operative planning; a detailed anatomical mapping of the urologic, gynecologic, and colonic structures is vital for proper operative preparation.
Traditionally, diagnostic imaging has been done in a two dimensional (2-D) format, using high-pressure contrast via the distal limb of the colostomy with 2-D fluoroscopy in various projections including frontal, lateral, and oblique orientations in order to map the relationships between the vagina, bladder, and rectum.3 However, overlapping of structures and the inability to clearly define the common channel make proper interpretation of this modality possible only for those with many years of experience. The trainee has a difficult time conceptualizing the structural anatomy for operative planning.
2-D fluoroscopic imaging has progressed to three-dimensional (3-D) computed tomography (CT) reconstructions, which has improved cloacal imaging greatly. 3-D enhanced isotropic soft-tissue volume reconstruction allows the overlapping structures to be rotated for further delineation, facilitating better measurements of the common channel. However, to the trainee, this modality may still have conceptual limitations.
The use of 3-D printed models in medicine has increased dramatically.4 This technology can be used to create custom-made, anatomically precise models. 3-D images are used frequently for operative planning, especially in cases involving difficult anatomy in the fields of cardiothoracic, orthopedics, and otorhinolaryngology.5–7 3-D images can create a powerful visual aid to improve the learning curve of the surgical trainee. Visual anatomical models are already a proven tool in medical education.8–9 Therefore, in this study we sought to examine the surgical understanding of different cloacagram modalities by surgical trainees. We also had surgical faculty answer the same questionnaire to see if trainees could achieve a similar level of understanding as a more experienced surgeon based upon different imaging modalities.
Methods
Pediatric surgery trainees were queried using a questionnaire to assess their understanding of 2-D and 3-D cloacagram images of a single case of a complex cloacal malformation. The questionnaire consisted of 12 questions that a surgeon would need to answer for a pre-operative plan. Participants individually viewed the images in numerical order and were given an unlimited amount of time to complete each station (Tables 1). A total of four models were tested. The first was a 2-D contrast study with fluoroscopy (Fig. 1). The second model was a 3-D isotopic volume auto-rotating reconstruction using the computed tomography (CT) picture archiving and communication system (PACS) (Fig. 2). The third model is a 3D .stl file which allowed users to interact with (manually rotate the image in any orientation) the 3D polygon mesh on a standard computer software (Fig. 3). The fourth imaging model was a 1:1 scale physical 3-D print of the same .stl file in the previous modality that the observer could hold and manipulate in their hands (Fig. 4). Experienced surgical faculty was also asked to participate in the questionnaire at the same four stations as a comparison. The answers to the questionnaire were verified by experts in the field of pediatric colorectal surgery based on the imaging and anatomy of the patient. Logistic mixed effects regression models with surgeon-level random intercepts were used to assess whether the proportion of questions answered correctly differed by imaging modality. Separate statistics were used for trainees and attending surgeons. Additional logistic mixed effects regression models stratified by imaging modality were used to evaluate whether the proportion of questions answered correctly differed between trainee and attending surgeons for any particular modality. SAS version 9.4 was used for the statistical analysis (SAS Institute Inc., Cary, NC). MeshLab_64bit_v1.3.3 was used to display the .stl file for the third model in the study. The fourth model .stl file was then printed on an Ultimaker 2 3-D printer. The model was printed with polylactic acid (PLA) material and took approximately 18 h to print in 0.1 mm layers at 45 mm/s.
Table 1.
Survey questions
| Transcending dimensions, 2D/3D project | |
|---|---|
| With regard to the cloacal malformation depicted in 2D, 3D PACS, 3D reconstruction, and 3D printed, please answer the following questions | |
| General questions | |
| 1. Is the patient likely to require a posterior sagittal approach or an abdominal approach to reach the rectum? | |
| a) Posterior sagittal approach alone | |
| b) Abdominal approach in addition to the posterior sagittal approach | |
| c) I cannot tell from this image | |
| 2. Is the common channel: | |
| a) Shorter than 3 cm | |
| b) Equal to or Longer than 3 cm | |
| c) I cannot tell from this image | |
| 3. If approached via a posterior sagittal approach what is the first structure encountered? | |
| a) Rectum | |
| b) Vagina(s) | |
| c) Urinary tract | |
| d) I cannot tell from this image | |
| 4. How long do you predict the operation will take? | |
| a) Less than 4 h | |
| b) 4 to 6 h | |
| c) Greater than 6 h | |
| d) I cannot tell from this image | |
| Gynecologic aspects | |
| 5. How many vaginas are there? | |
| a) One | |
| b) Two | |
| c) I cannot tell from this image | |
| 6. If you believe there are two vaginas, are these vaginas symmetric? | |
| a) They are the same size | |
| b) The left hemivagina is larger than the right | |
| c) The right hemivagina is larger than the left | |
| d) There is only one vagina | |
| e) I cannot tell from this image | |
| 7. Is a vaginal replacement likely to be required? | |
| a) Yes | |
| b) No | |
| c) I cannot tell from this image | |
| Rectal aspects | |
| 8. Where does the rectum insert? | |
| a) Vestibule | |
| b) In the lower part of the septum between the 2 hemivaginas | |
| c) In the middle of the septum between the 2 hemivaginas | |
| d) High in the dome between the 2 hemivaginas | |
| e) I cannot tell from this image | |
| 9. The length of the distal bowel from the mucous fistula is: | |
| a) Plenty of length for the distal rectum to reach the perineum | |
| b) The distal rectum will not reach the perineum without taking down the mucous fistula | |
| c) I cannot tell from this image | |
| Urologic aspects | |
| 10. A total urogenital mobilization is _____ to bring down urethra and vagina | |
| a) Likely | |
| b) Unlikely | |
| c) I cannot tell from this image | |
| 11. Do the kidneys demonstrate any abnormality? | |
| a) No, normal kidneys | |
| b) Reflux | |
| c) I cannot tell from this image | |
| 12. What is the length of the urethra, from the urethral take off from the common channel to the bladder neck? | |
| a) 1 cm | |
| b) > 1.5 cm | |
| c) 3 cm | |
| d) I cannot tell from this image | |
| Urologic aspects | |
| 10. A total urogenital mobilization is _____ to bring down urethra and vagina | |
| a) Likely | |
| b) Unlikely | |
| c) I cannot tell from this image | |
| 11. Do the kidneys demonstrate any abnormality? | |
| a) No, normal kidneys | |
| b) Reflux | |
| c) I cannot tell from this image | |
| 12. What is the length of the urethra, from the urethral take off from the common channel to the bladder neck? | |
| a) 1 cm | |
| b) > 1.5 cm | |
| c) 3 cm | |
| d) I cannot tell from this image |
Fig. 1.
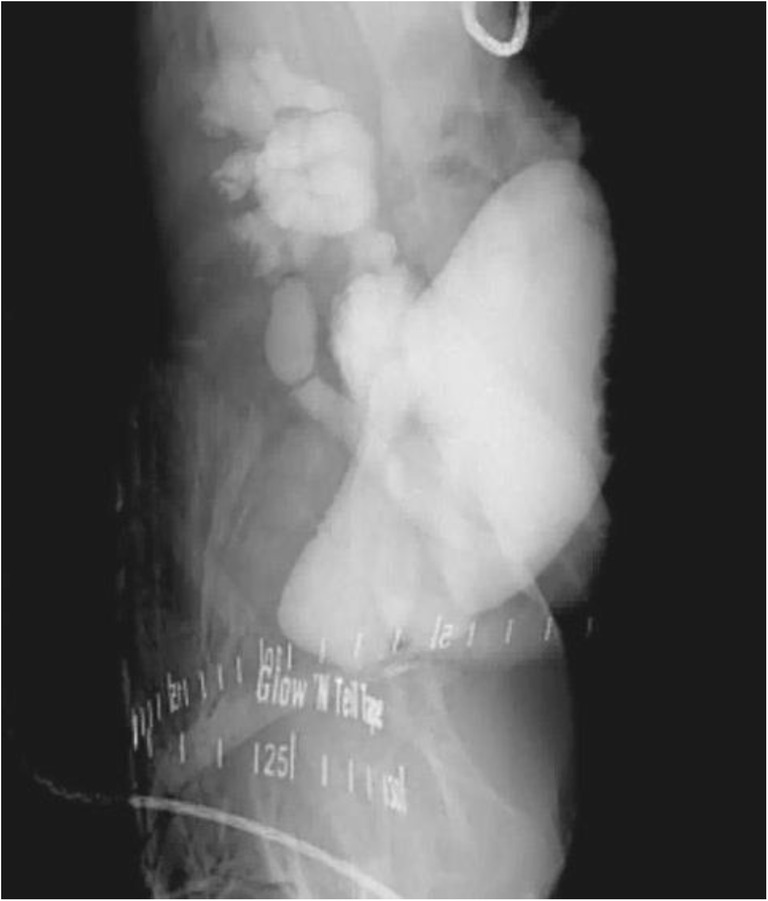
2-D contrast study with fluoroscopy.
Fig. 2.
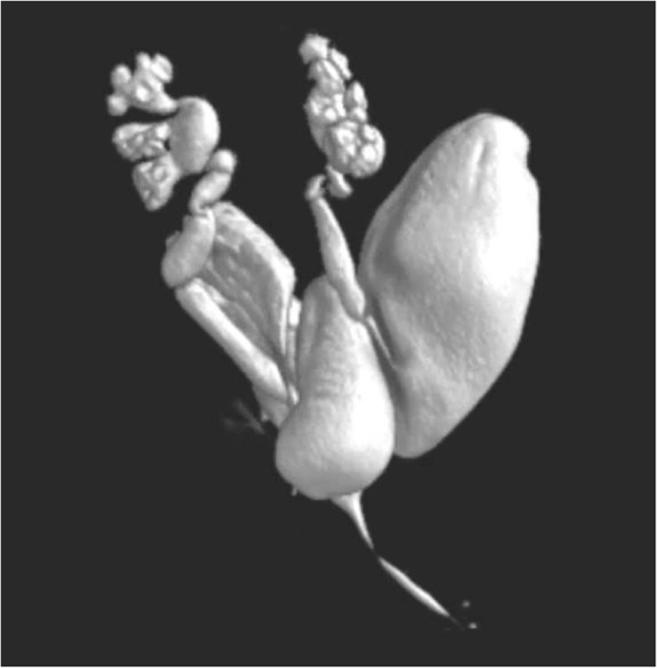
3-D isotopic volume auto-rotating reconstruction using picture archiving and communication system (PACS).
Fig. 3.
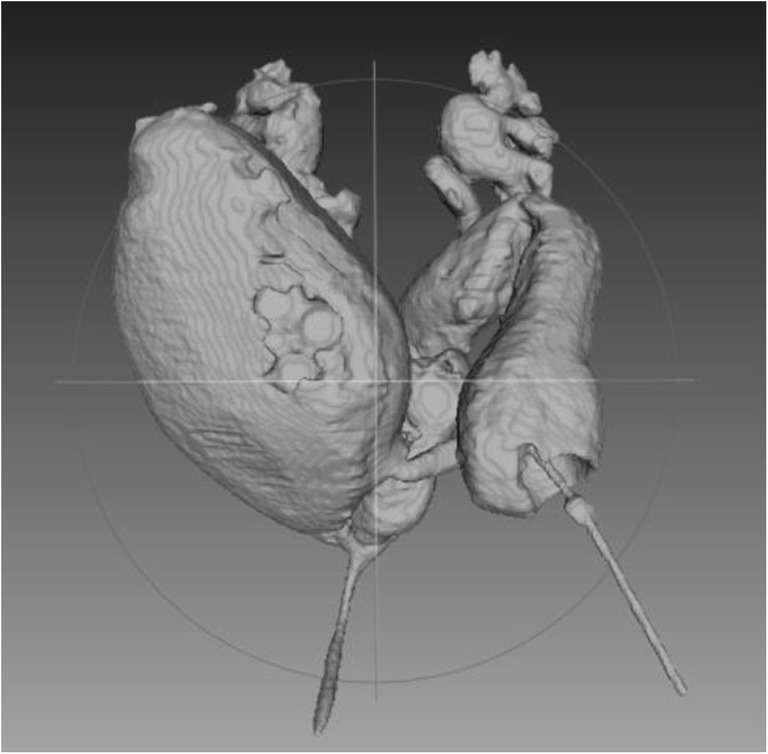
Software enhanced 3-D video animation that allows participant to manually rotate image in any orientation
Fig. 4.

3-D printed model that observer can hold and manipulate in their hands.
Results
Twenty-nine trainees and 30 attendings participated. The total percentage of questions answered correctly for each group is shown in Table 2 for all four imaging models. For trainees, the percentage of questions answered correctly for each type of model was: 2-D 10.5%, 3-D PACS 46.7%, 3-D enhanced 67.1%, and 3-D printed 73.8%. For attendings, the percentage of questions answered correctly for each model was: 2-D 22.2%, 3-D PACS 54.8%, 3-D enhanced 66.2%, and 3-D printed 74%. Across each model, there was a significant difference in rates of correctness for both trainees and attendings (p < 0.001). Performance was lowest for the 2-D imaging and improved as the complexity of the imaging increased and this was true for both attendings and trainees. When trainees’ and attending surgeons’ performances were compared, they differed significantly only for the 2-D imaging model, with the attendings answering correctly more often (p = 0.003).
Table 2.
Total percentage of questions answered correctly
| Trainees (%) | Attendings (%) | P value for difference in rates of correctness across modalities in fellows* | P value for difference in rates of correctness across modalities in attendings* | P value for difference in rates of correctness for this modality between fellows and attendings* | |
|---|---|---|---|---|---|
| 2-D | 10.5** | 22.2** | < .001 | < .001 | 0.003 |
| 3D PACS | 46.7** | 54.8** | 0.10 | ||
| 3D enhanced | 67.1 | 66.2 | 0.82 | ||
| 3D printed | 73.8 | 74.0 | 0.97 |
*All p values are from logistic mixed effects models for answer correctness. These models included random physician effects to account for the clustering of answers within physicians, and each question was treated as a distinct observation
**P < .05 as compared to modality 4 in this group of physicians (evaluated in the same logistic mixed effects models described above) (all 4 of these P values were < .001)
Discussion
In our study we have shown that improved imaging can increase trainee and faculty understanding of the complex anatomy of a cloaca. Similar studies have shown that trainees can be advanced to a level of anatomic understanding that otherwise may take years of practice to achieve using a 3-D model compared to a more rudimentary 2-D model that lacks the anatomical detail to develop and form spatial conceptualization mapping. Using a 3-D aortic aneurysm model, for example, trainees were able to more effectively advance their level of expertise for surgical planning for endovascular aortic aneurysm repair.10 Similarly, 3-D models are used in understanding complex congenital heart defects for preoperative planning, communication with the patient, and education of trainees (Fig. 5).11
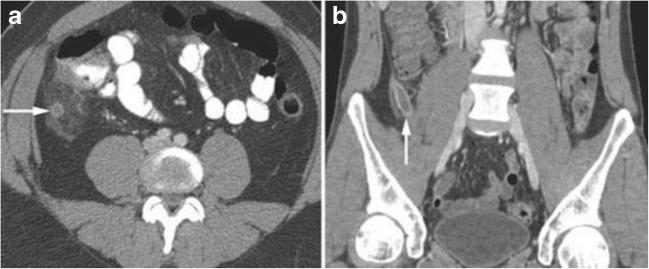
Fig. 5.
CT scan cross-sectional imaging a axial and b coronal for comparison to coronal 3-D CT reconstruction. This advancement in CT technology improved the ability for less experienced observers to understand the anatomic findings; in this case of acute appendicitis. (White arrows pointing to the dilated appendix)
In our study, the more complex the imaging model, the more correct the responses were to the questions about the cloaca anatomy. This was true for both the trainees and the attendings. This was not surprising as the 2-D imaging had only 10.5% correct from the trainees and only 22.2% correct responses from the attendings. The 2-D cloacagram was the least complex and most difficult for the trainees and the attendings to correctly answer pertinent questions that aid in operative planning. This is in stark contrast to the 3-D printed model that enabled where 73.8% of the trainees and 74% of both trainees and attendings answered the questions correctly. Therefore, a 3-D printed cloaca model was able to aid the trainees in achieving a level of understanding similar to that of a more experienced surgeon. However, even the 3-D reconstruction available on any PACS system made an enormous difference in anatomic understanding.
This study is limited in that as each individual went through the stations sequentially from the 2-D model and finally the 3-D printed model, it is possible that a certain amount of knowledge from the previous anatomy could be cumulative aiding the final understanding. Therefore the questions may be more likely to be answered correctly in later stations. Additionally, we acknowledge that 3-D reconstruction technology is not available at all institutions throughout the world and may be limited to tertiary referral centers in developed countries.
Conclusions
Complex surgical anatomy requires a great deal of conceptualization in surgical planning that may take years to develop, especially with a 2-D imaging model. However, 3-D reconstruction and printed models enable the surgeon to make significant strides in comprehension of intricate cloacal anatomy and achieve a higher level of preparedness for surgery.
References
- 1.Warne S, Chitty LS, Wilcox DT. Prenatal diagnosis of cloacal anomalies. BJU Int. 2002;89(1):78–81. doi: 10.1046/j.1464-410X.2002.02556.x. [DOI] [PubMed] [Google Scholar]
- 2.Levitt MA, Pena A. Pitfalls in the management of newborn cloacas. Pediatr Surg Int. 2005;21(4):264–9. doi: 10.1007/s00383-005-1380-2. [DOI] [PubMed] [Google Scholar]
- 3.Jaramillo D, Lebowitz RL, Hendren WH. The cloacal malformation: radiologic findings and imaging recommendations. Radiology. 1990;177(2):441–8. doi: 10.1148/radiology.177.2.2217782. [DOI] [PubMed] [Google Scholar]
- 4.Ventola CL. Medical Applications for 3D Printing: Current and Projected Uses. PT. 2014;39(10):704–711. [PMC free article] [PubMed] [Google Scholar]
- 5.Hermsen JL, Burke TM, Seslar SP et al. Scan, plan, print, practice, perform: Development and use of a patient specific 3-dimensional printed model in adult cardiac surgery. J Thorac Cardiovasc Surg. 2016 Aug 20. [DOI] [PubMed]
- 6.Kaye R, Goldstein T, Zeltsman D, et al. Three dimensional printing: A review on the utility within medicine and otolaryngology. In J Pediatr Otorhinolaryngol. 2016;89:145–8. doi: 10.1016/j.ijporl.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Fujita T, Saito N, Minakata K et al. Transfemoral transcatheter aortic valve implantation in the presence of a mechanical mitral valve prosthesis using a dedicated TAVI guidewire: utility of a patient-specific threedimensional heart model. Cardiovasc Interv Ther. 2016 Aug 27. [DOI] [PubMed]
- 8.Valdecasas A, Correas A, Guerrero C, et al. Understanding complex systems: lessons from Auzoux’s and von Hagens’s anatomical models. J Biosci. 2009;34(6):835–843. doi: 10.1007/s12038-009-0097-0. [DOI] [PubMed] [Google Scholar]
- 9.Al-Ramahi J, Luo H, Fang R et al. Development of an Innovative 3D Printed Rigid Bronchoscopy Training Model. Ann Otol Rhinol Laryngol. 2016 Sept 7. [DOI] [PubMed]
- 10.Wilasrusmee C, Suvikrom J, Suthakorn J, et al. Three-dimensional aortic aneurysm model and endovascular repair: an educational tool for surgical trainees. Wilasrusmee C, Suvikrom J, Lertsithichai P et al. Int J Angiol. 2008;17(3):129–133. doi: 10.1055/s-0031-1278295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deferm S, Meyns B, Vlasselaers D, et al. 3-D printing in congenital cardiology: from flatland to spaceland. J Clin Imaging Sci. 2016;6:8. doi: 10.4103/2156-7514.179408. [DOI] [PMC free article] [PubMed] [Google Scholar]