Abstract
Wound healing is an important outcome of tissue damage and can be stimulated by adenosine released from cells during events such as tissue injury, ischaemia or tumour growth. The aim of this research was to determine the potency and efficacy of adenosine A1, A2A and A2B receptor agonists on the rate of wound healing and cell proliferation in human EA.hy926 endothelial cells. Real-time PCR data showed that only adenosine A1, A2A and A2B receptor mRNA were expressed in this cell line. All three adenosine receptor agonists, CPA, CGS21680 and NECA, significantly increased the rate of wound healing in human EAhy926 endothelial cells with the following order of potency CGS21680>CPA>NECA and efficacy CPA>NECA>CGS21680. The selective adenosine A1, A2A and A2B receptor antagonists, DPCPX, ZM241385 and MRS1754 (all at 10 nM), reversed the effects of their respective agonists. EAhy926 endothelial cell proliferation was also significantly increased with the adenosine A1 and A2B receptor agonists, CPA and NECA. Western blot analysis demonstrated that adenosine A2A and A1 receptor protein levels were highly expressed compared with the adenosine A2B receptors in the EAhy926 endothelial cell lines. While all three adenosine A1, A2A and A2B receptor subtypes contribute to cell proliferation and wound healing in human EAhy926 endothelial cells, treatments selectively targeting receptor subtypes may further enhance wound healing.
Electronic supplementary material
The online version of this article (10.1007/s11302-019-09668-z) contains supplementary material, which is available to authorized users.
Keywords: Adenosine, Endothelial cells, Wound healing, Cell proliferation
Introduction
Adenosine is a ubiquitous purine nucleoside that is produced following the dephosphorylation of ATP and adenine nucleotides. It protects tissues and cells during tissue damage or hypoxic-ischaemic conditions where its role becomes important to counter metabolic stress and cellular energy imbalance [1, 2]. Adenosine levels range between 20 and 300 nM under physiological conditions and may rise during conditions including intense exercise or in a low-oxygen environment encountered at high altitude [3]. Under severe pathological conditions such as ischaemia, tissue adenosine concentrations can rise to 30 μM [3, 4].
Adenosine is emerging as having an important role in wound healing and remodelling [5] where the repair of damaged tissues is a vital homeostatic mechanism that involves several phases including inflammation, neovascularization, tissue regeneration and tissue reorganisation [6]. Adenosine and its receptors play essential roles in the production of matrix and stimulation of neovascularization, which are both crucial processes for tissue repair and wound healing [2, 6]. Conversely, adenosine receptor stimulation also promotes the development of fibrosis in the skin, lungs and liver [6]. Endothelial cells in the vasculature generate adenosine from adenine nucleotides and respond to adenosine through increased proliferation and migration [2, 7–11].
Cyclopentyladenosine (CPA) has a similar structure to adenosine and is classified as a highly selective agonist for the adenosine A1 receptor [12]. CPA, through binding to the adenosine A1 receptor located on many cell types, can reduce damage associated with ischaemia, attenuate calcium levels in the cytosol or induce coronary vasodilation [12–17]. In addition, CPA through the activation of adenosine A1 receptors in macrophage cell lines mediates both nitric oxide (NO) release and the production of inflammatory cytokines (IL-10 and TNF-α), which are essential for tissue repair [12, 18–21]. In vivo studies using mice have shown that CPA stimulates the proliferation of endothelial cells, keratinocytes and fibroblasts [12, 17]. CPA acts as a mitogen and has strong vasodilator properties, which support cell proliferation and wound healing [12, 17]. CPA induces concentration-dependant increases in human dermal microvascular endothelial cell proliferation [12]. Adenosine A2A and A2B receptor agonists both cooperate to promote angiogenesis and migration of endothelial cells and fibroblasts through cAMP- and PKA (protein kinase A)-dependent pathways [22, 23]. In addition to the promotion of wound healing following activation of adenosine receptors, stimulation of the adenosine A2A receptors may also enhance extracellular matrix production in healing wounds [23]. The adenosine A3 receptor couples to several G proteins such as Gi α2, Gi α3 and Gq α [24–26] and has been reported to induce selective RhoA-dependent activation of phospholipase D (PLD). Adenosine also stimulates angiopoietin-2 secretion through adenosine A3 receptors [27]. To our knowledge, there are no publications regarding the presence of adenosine A3 receptor on human endothelial cells, although they have been reported in mouse endothelium [28, 29]. This project will identify the adenosine receptor subtypes that are involved in mediating wound healing and proliferation of human endothelial cells.
The aims of this study were to measure the effect of adenosine A1, A2A and A2B receptor agonists and antagonists on the rate of wound closure and cell proliferation in human EA.hy926 endothelial cells and to establish the protein expression levels of adenosine A1, A2A and A2B receptor subtypes in EA.hy926 endothelial cells, using Western blot assays.
Materials and methods
Cell culture technique
The EA.hy926 endothelial cell line used for these studies was generously donated to our research group by the Apoptosis Research Group, Griffith University, Gold Coast campus. The EA.hy926 endothelial cells were seeded into a 75 cm2 cell culture flask containing complete media. Complete media comprised high glucose (4500 mg/L, 25 mM) Dulbecco’s Modified Eagle’s Medium (DMEM) (ThermoFisher Scientific, VIC, Australia) supplemented with a mixture of hypoxanthine-aminopterin-thymidine (HAT, Sigma Aldrich, NSW, Australia), 10% foetal bovine serum (FBS) and 1% antibiotic-antimycotic (penicillin/streptomycin/amphotericin B, Invitrogen, VIC, Australia). A high-glucose medium was used to grow the EA.hy926 endothelial cell line as it is the recommended medium for this cell line. These cells do not grow well at lower glucose concentration. Cells were incubated in a humidified incubator at 37 °C and 5% carbon dioxide (CO2). The cells were maintained under these conditions until they adhered to the surface of the flask. They were grown for 4–5 days until they reached the optimal confluency to commence experiments. For these experiments, the cells would have undergone 20 passages for the wound healing experiments and another 20 passages for the cell proliferation assays.
Real-time polymerase chain reaction
The total ribonucleic acid (RNA) from the EA.hy926 endothelial cells was extracted using the RNEasy® Mini Kit (QIAGEN, Netherlands). The Nanodrop® ND-1000 spectrophotometer (Thermo Scientific, USA) was used to determine the yield and purity of RNA which was used in the conversion to cDNA; the samples that remained were stored at −80 °C. The total RNA was converted to cDNA by following the first-strand cDNA synthesis protocol provided in the AffinityScript Multiple Temperature Reverse Transcriptase (Stratagene, USA). The cDNA was stored at − 20 °C until further use.
For the analysis of adenosine receptor mRNA expression, real-time PCR was used to detect the fluorescence levels and the signal that was proportionate to the amount of double-stranded DNA. The qPCR mix that was used to detect the amplification of cDNA was 5× HOT FIREPol® EvaGreen® qPCR Mix Plus (ROX) (Solis BioDyne, Estonia); the primer sequences that were used for adenosine receptors and the house-keeping genes 18S rRNA and β-actin (ACTB) are as follows: 18S Fw CTTAGAGGGACAAGTGGCG, 18S Rev. GGACATCTAAGGGCATCACA 71BP, hACTB Fw ACCACACCTTCTACAATGAGCT, hACTBRev GAAGGTCTCAAACATGATCTGG 122BP, hADORA1 Fw GGAGTCTGCTTGTCTTAGATG, hADORA1Rev CAACCTCTCTCCTTCCATCC 97 BP, hADORA2AFw GGGTGTCTATTTGCGGATCTTC, hADORA2ARev GTGACTTGGCAGCATGGACC, 122BP, hADORA2BFwGCTGCCTTGTGAAGTGTCTC, hADORA2BRev GCACAGGTAACCAGCACAGG, 255BP, hADORA3Fw GGGCATCACAATCCACTTCT and hADORA3Rev AGGGCCAGCCATATTCTTCT 171BP. cDNA from human blood known to express all 4 adenosine receptor subtypes was used as a positive control. The qPCR reaction was prepared as per manufacturer’s instructions; in brief, 2 μL of cDNA was added to each well with 18 μL of master mix. The master mix consisted of 4 μL of 5× HOT FIREPol® EvaGreen® qPCR Mix Plus, 1 μL of forward and reverse primers with 12 μL of distilled H2O (dH2O).
The qPCR reactions were run on a Bio-Rad IQ iCycler system (Bio-Rad Laboratories, Hercules, USA) that first required initial denaturation for 15 min at 95 °C, which was followed by 40 cycles of 15 s at 95 °C, 20 s at 60 °C and 20 s at 72 °C. The product was loaded onto 2% Agarose gel, to determine real-time PCR products for the specific receptors; this allowed the adenosine receptors specific to the EA.hy926 endothelial cells to be identified under an ultra-violet (UV) fluorescent light.
Western blot technique
The cells were lysed using lysis buffer (1 mM phenylmethanesulfonyl fluoride (PMSF), 10 μM leupeptin, 3 mM benzamidine, 5 μM pepstatin A, 1 mM sodium orthovanadate, 0.1% Triton-X and Kinexus buffer), which was freshly prepared on the day. Kinexus buffer was prepared from a mixture of Mops buffer, 2 mM EGTA, 5 mM EDTA, 30 mM NaF, 40 mM β-glycerophosphate and 20 mM sodium pyrophosphate (NaPP). The cell suspension was centrifuged at 1200 RPM, for 5 min at 4 °C, then the supernatant was removed, and then the cells were washed with ice-cold DPBS. The cells were then re-centrifuged at 1200 RPM, for 5 min at 4 °C. The supernatant was subsequently removed, leaving only the cell pellet. The cells were agitated with the lysis buffer using the pipette tip then transferred into an Eppendorf tube and maintained on ice, with constant agitation for a further 30 min. Afterwards, the cell and lysis buffer mixture was centrifuged at 13200 RPM, for 10 min at 4 °C. The supernatant was then aspirated and preserved in a fresh Eppendorf tube and kept on ice. To determine the protein concentration, the bicinchoninic acid assay (BCA) (Pierce BCA protein assay kit, ThermoFisher Scientific, VIC, Australia) was applied. The BCA protocol was used to measure protein concentrations of cell lysates and compare these to known standard sample concentrations, and absorbance was measured using a plate reader at a wavelength of 562 nm [30].
Protein samples (50 μg) were loaded into the gels and run at 150 V for approximately 50 min until the samples reached the bottom of the gel. The protein samples were then transferred to PVDF membranes using 75 V for 1 h and 30 min. The membranes were treated with blocking buffer for 1 h then they were incubated overnight at 4 °C with rabbit polyclonal primary antibody for adenosine A1 receptor (1:500) (Abcam, Cambridge, UK), adenosine A2A receptor (1:500) (Santa Cruz Biotechnology, CA, USA) or adenosine A2B receptor (1:200) (Merck Millipore, VIC, Australia). Afterwards, the membranes were incubated in horseradish peroxidase–conjugated goat anti-rabbit secondary antibody (1:10000) for adenosine A1 and A2A receptors and (1: 500) for adenosine A2B receptor (Santa Cruz Biotechnology, CA, USA) for 1 h at room temperature. Furthermore, beta (β)-actin antibody was used as a loading control (1:1000) (Thermo Fisher, VIC, Australia) for all three adenosine receptor subtypes and a horseradish peroxidase–conjugated goat anti-rabbit antibody was used as the secondary antibody (1:3000). The expression of the proteins was examined by comparing their size with a protein ladder or molecular weight marker.
Wound healing assay
EA.hy926 endothelial cells (2 × 105 cells) in complete media were seeded into the 12-well plates in triplicate and incubated until the cells reached 80–85% confluence. The cell layer was then scratched with a 200-μL pipette tip and respective adenosine receptor agonists and antagonists were loaded into the wells. For the vehicle control, diluted DMSO (0.03%) was added to the scratch assay. The cell migration was observed using the Nikon ECLIPSE TS100 microscope (Nikon, Tokyo, Japan) with an attached camera. The cells were photographed at 0, 2, 4 and 6 h and the migration distance of the wound was analysed using the TSView 7 software; quantitative data values were obtained in μm/h for each sample.
Adenosine receptor agonists and antagonists utilised in this study
Agonists and antagonists used for these experiments with their published Ki values are listed in Table 1. 5′-N-ethylcarboxamido adenosine (NECA) was used as an agonist for the adenosine A2B receptor; however, NECA is non-selective for the adenosine receptors, so an adenosine A2A receptor antagonist (10 nM ZM241385) was combined with NECA to block the effects of NECA on the adenosine A2A receptor. An adenosine A3 receptor agonist was not used in these experiments as real-time PCR indicated that the adenosine A3 receptor was not present in the EA.hy926 endothelial cells (see Fig. 1). The non-selective adenosine receptor antagonist 8-(p-sulfophenyl)-theophylline hydrate (30 μM) was also used to determine whether endogenous adenosine was basally released by the endothelial cells to affect the wound healing assay. In these experiments, DMSO (0.03%) reduced wound closure rates when compared with control conditions. Analysis of the concentration-response curves to the adenosine receptors agonists was performed using GraphPad Prism software (CA, USA) (version 6.0).
Table 1.
Selective adenosine receptor subtype agonists and antagonists implemented in this study. The Ki values come from the Nomenclature and Classification of Adenosine receptors—an update (Fredholm et al. 2011 [31])
| Adenosine receptor | Agonist | Antagonist |
|---|---|---|
| A1 | N6-cyclopentyladenosine (CPA) Ki value 2.3 nM | 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX)Ki value 3.0 nM |
| A2A | 2-p-(2-carboxyethyl) phenethylamino-5′-N-ethylcarboxamidoadenosine (CGS21680) Ki value 27 nM |
4-(2-[7-Amino-2-(2-furyl)[1,2,4]triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM241385) Ki value 1.6 nM |
| A2B | 5′-N-ethylcarboxamido (NECA) Ki value 140 nM |
N-(4-cyanophenyl)-2-[4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl) phenoxy]-acetamide (MRS1754) Ki value 1.97 nM |
Fig. 1.
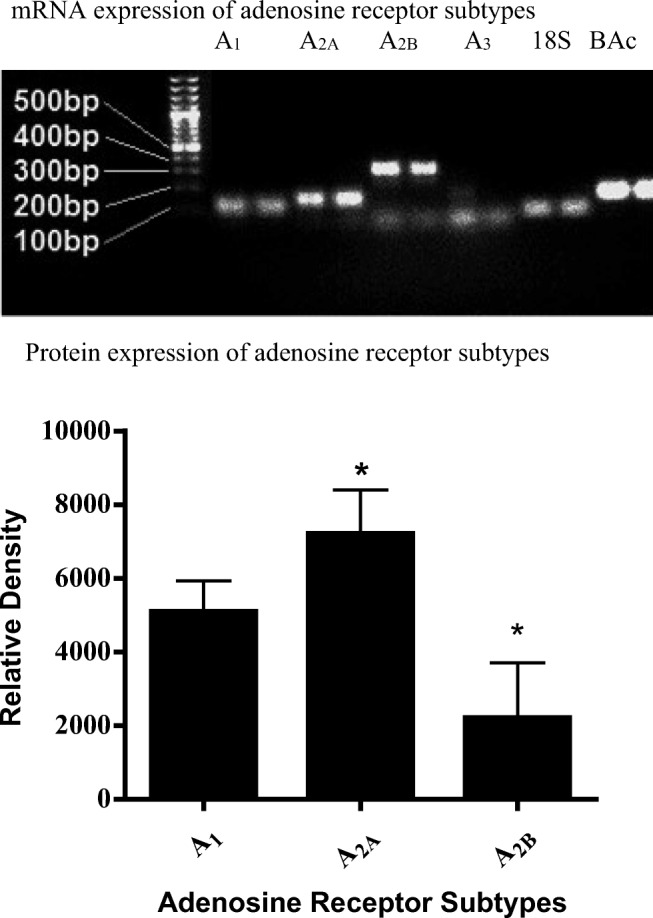
Upper panel: 2% Agarose gel electrophoresis RT-PCR results for adenosine receptors. 18 s ribosomal RNA 71BP, adenosine A1 receptor 97BP, adenosine A2A receptor 122BP, adenosine A2B receptor 255BP, adenosine A3 receptor 171BP and β(B)-actin 122BP. Lower panel: Graph demonstrating the relative expression of adenosine A1, A2A and A2B receptor subtypes on E.Ahy926 endothelial cells. The data was analysed using one-way ANOVA. Data is expressed as mean ± SEM (n = 6), *P < 0.05 versus adenosine A1 receptor
Furthermore, the addition of the non-selective adenosine receptor antagonist 8-sulphylphenyltheophylline (30 μM) did not affect the rate of wound closure in the scratch assay, indicating that residual adenosine (if it is released) in cell culture conditions does not affect cell function in this assay.
Different concentrations of agonists were utilised (1–300 nM) in the initial scratch assay experiments to obtain concentration-response curves, determine the EC50 value and establish an appropriate concentration of agonist required for the rest of the experiments. The Ki values for adenosine receptor antagonists are as follows: DPCPX (adenosine A1 receptor, 3.0 nM), ZM241385 (adenosine A2A receptor, 1.6 nM) and MRS1754 (adenosine A2B receptor, 1.97 nM) [31]. A final concentration of 10 nM was used for all the adenosine receptor antagonists in this study.
Cell proliferation assay
The EA.hy926 endothelial cells were seeded in triplicate at 5000 cells/ well in 96-well plates and also, 4000, 5000, 6000, 7000, 8000 and 9000 cells were seeded in duplicate per well, respectively, to form the standard curve. They were all incubated for 24 h at 37 °C, 5% CO2. The adenosine receptor agonists and antagonists were prepared using high-glucose complete media at 30 nM and 10 nM concentrations, respectively. In addition, for control experiments, cells were treated with normal high-glucose complete media and vehicle controls using DMSO at two different dilutions of 0.004% and 0.03% v/v. Once the cells were seeded, the plate was incubated for 24 h at 37 °C, 5% CO2. CCK-8 was either thawed at room temperature for 30 min or defrosted in the water bath at 37 °C for 5 min. After 24-h incubation, the high-glucose complete media were discarded from each well and subsequently replaced with freshly prepared high-glucose complete media (200 μL) containing 10 μL of respective drugs. A volume of 10 μL of the CCK-8 solution was carefully loaded into each well of the plate to avoid producing any air bubbles (air bubbles in the wells could interfere with the optical density (OD) reading) and the 96-well culture plate was incubated at 37 °C, 5% CO2, for 4 h. The plate was finally placed into the Infinite M200 PRO-TECAN microplate reader (Tecan group, Switzerland) to measure absorbance at 450 nm. The standard curve was used to determine cell numbers and to allow accurate quantification of cell proliferation.
Drug solutions
Stock solutions of the adenosine agonists and antagonists (1 mM) were dissolved in DMSO and stored as aliquots in the − 20 °C freezer until further use. All drugs were freshly prepared on the day of the experiments from the thawed stock solution by diluting with complete media to reach the concentration required for experiments. The reagents used in this experiment are DMSO, CPA, DPCPX, NECA, MRS1754 and CGS21680 which were obtained from Sigma Aldrich (NSW, Australia) and ZM241385 was sourced from Tocris Bioscience (Bristol, UK).
Data analysis
Data is presented as mean ± SEM and the differences were considered statistically significant at P < 0.05. All statistical analysis was performed using GraphPad Prism software (version 6.0) (CA, USA). To examine the rates of wound healing or cell proliferation, a one-way analysis of variance (ANOVA) was used. Statistical analyses for the Western blot implemented an unpaired, two-tailed Student’s t test for a single comparison or a one-way ANOVA for more than two or multiple comparisons.
Results
Adenosine receptor subtype mRNA expression in EA.hy926 endothelial cells using RT-PCR
RT-PCR was conducted on cDNA samples converted from EA.hy926 endothelial cell mRNA. Human blood cDNA sample was used as a positive control as it is known to express all four adenosine receptor subtypes. The PCR product was then loaded onto a 2% agarose electrophoresis gel to determine which receptors subtypes were present. Figure 1 shows adenosine A1, A2A and A2B receptor PCR products in agarose gel. The adenosine A3 receptor was observed in the positive control (human blood cDNA) but not in EA.hy926 endothelial cells. 18 s RNA and β-actin mRNA were also expressed in control and EA.hy926 cells; however, GAPDH PCR product was not observed in either group. From these observations, it was determined that subsequent experiments would only be conducted using adenosine A1, A2A and A2B receptor agonists and antagonists.
Protein expression of adenosine receptor subtypes in EA.Hy926 cells
The results of Western blotting techniques confirmed the presence of adenosine A1, A2A and A2B receptors of expected molecular size A1 (≈ 37 kDa), A2A (≈ 45 kDa) and A2B (≈ 50–52 kDa) in EA.hy926 endothelial cells. Furthermore, β-actin with a molecular size of ≈ 42 kDa was used as a loading control. Three different blots were utilised for each adenosine A1, A2A and A2B receptor antibody and they were individually analysed against internal controls. Data analysis shows that the protein expression of adenosine A2A receptor was considerably higher in EA.hy926 endothelial cells when compared with adenosine A1 and A2B receptors (P < 0.05). Moreover, the adenosine A1 receptor had a higher expression when compared with adenosine A2B receptor (P < 0.05, see Fig. 1).
The wound healing scratch assay
The data for the wound healing scratch assay was obtained by measuring the width of the scratch at 2 hourly intervals, using Image J software (see Fig. 2). The average speed of wound healing was examined by comparing the effect of different concentrations of adenosine receptor agonists. The results were analysed using a one-way ANOVA. The effective concentration producing 50% maximal effective concentration response (EC50) to agonist (log concentration) was calculated based on curve fitting using GraphPad Prism (see Fig. 3). The adenosine A1 receptor agonist CPA caused a concentration-dependant increase in the speed/rate of scratch closure (which is a surrogate marker for wound healing in this assay) at all concentrations of CPA when compared with the control group (P < 0.05). The EC50 value for CPA in the wound healing scratch assay of EA.hy926 endothelial cell was 7.16 × 10−9 M (confidence intervals 4.75 × 10−9–1.08 × 10−8 M). The data also shows that the efficacy of CPA and its ability to stimulate wound healing, based on its ability to increase the rate of wound closure, were greater than the two other adenosine receptor agonists, CGS21680 and NECA (adenosine A2A receptor and A2B receptor agonists, respectively). However, CPA was not the most potent agonist in stimulating the wound healing scratch assay with the order of potency being CGS21680>CPA>NECA.
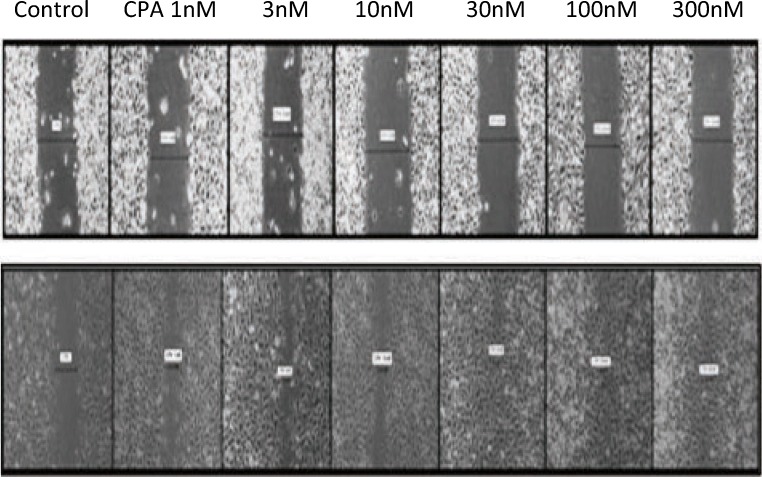
Fig. 2.
Wound healing scratch assay using different concentrations of CPA on EA.hy926 endothelial cells. Upper panel depicts scratch widths at 0 times and lower panel shows scratch widths after 6 h. CPA concentrations from the left to right are control (0 nM), 1 nM, 3 nM, 10 nM, 30 nM, 100 nM and 300 nM CPA. Magnification, × 40
Fig. 3.
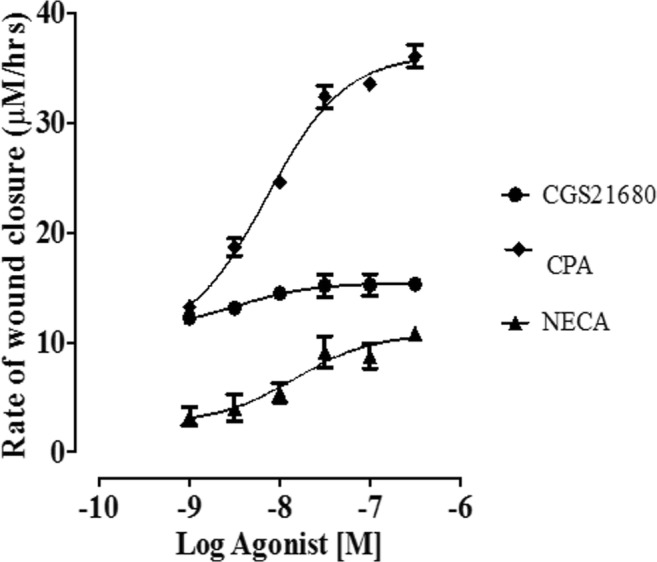
Concentration-response curves (CRC) showing the effect of selective adenosine receptor agonists on EA.hy926 endothelial cells and the rate of wound healing, n = 6 per group. Note that NECA concentration-response curves were done in the presence of 10 nM ZM241385
CGS21680 was used as a selective agonist for the adenosine A2A receptors. The rates of wound healing at all concentrations of CGS21680 were significantly increased when compared with those of the control group (P < 0.05). The EC50 value for CGS21680 in the wound healing scratch assay of EA.hy926 endothelial cells was 3.25 × 10−9 M (confidence intervals 2.83 × 10−10–3.74 × 10−8 M). CGS21680 was more potent than the other two adenosine A1 and A2B receptor agonists (CPA and NECA) used in this assay. While CGS21680 was the most potent of the selective agonists used in this assay, its efficacy was low when compared with the other two agonists with respect to rates of wound healing. NECA was used as an agonist for adenosine A2B receptor in this study; however, as previously explained, NECA is a non-selective agonist for the adenosine A2 receptor. Therefore, NECA was combined with the adenosine A2A receptor antagonist ZM241385 (10 nM) to block the adenosine A2A subtype. The rates of wound healing at all concentrations of NECA were significantly increased when compared with those of the control group (P < 0.05). The EC50 value for NECA in the wound healing scratch assay of EA.hy926 endothelial cells was 1.48 × 10−8 M (confidence intervals 2.83 × 10−10–3.74 × 10−8 M). Of the three agonists tested using the scratch assay, NECA provided the least potent wound healing stimulus in EA.hy926 endothelial cells.
Selective adenosine receptor antagonists were used in the EA.hy926 endothelial cell wound healing scratch assay. Using submaximal concentrations for the concentration-response curves from the wound healing data (see Fig. 3), single concentrations of the adenosine receptor agonists (30 nM) were used in the wound healing scratch assay and tested against selective adenosine receptor antagonists at 10 nM. The rates of wound healing with all the adenosine receptor agonists increased as follows: adenosine A1 receptor agonist (CPA, 30 nM) increased by 42.29%, adenosine A2A receptor agonist (CGS21680, 30 nM) rose by 49% and adenosine A2B receptor agonist (NECA, 30 nM + ZM241385, 10 nM) was elevated by 20.57% when compared with their respective control group (P < 0.05) (Fig. 4). The selective adenosine receptor antagonists alone did not alter the rates of wound healing in comparison with the control group (P > 0.05, see Fig. 4). However, the adenosine receptor antagonists inhibited adenosine receptor agonist-induced increases in the rates of wound healing indicating that all three adenosine receptor subtypes are involved in stimulating wound healing (P < 0.05).
Fig. 4.
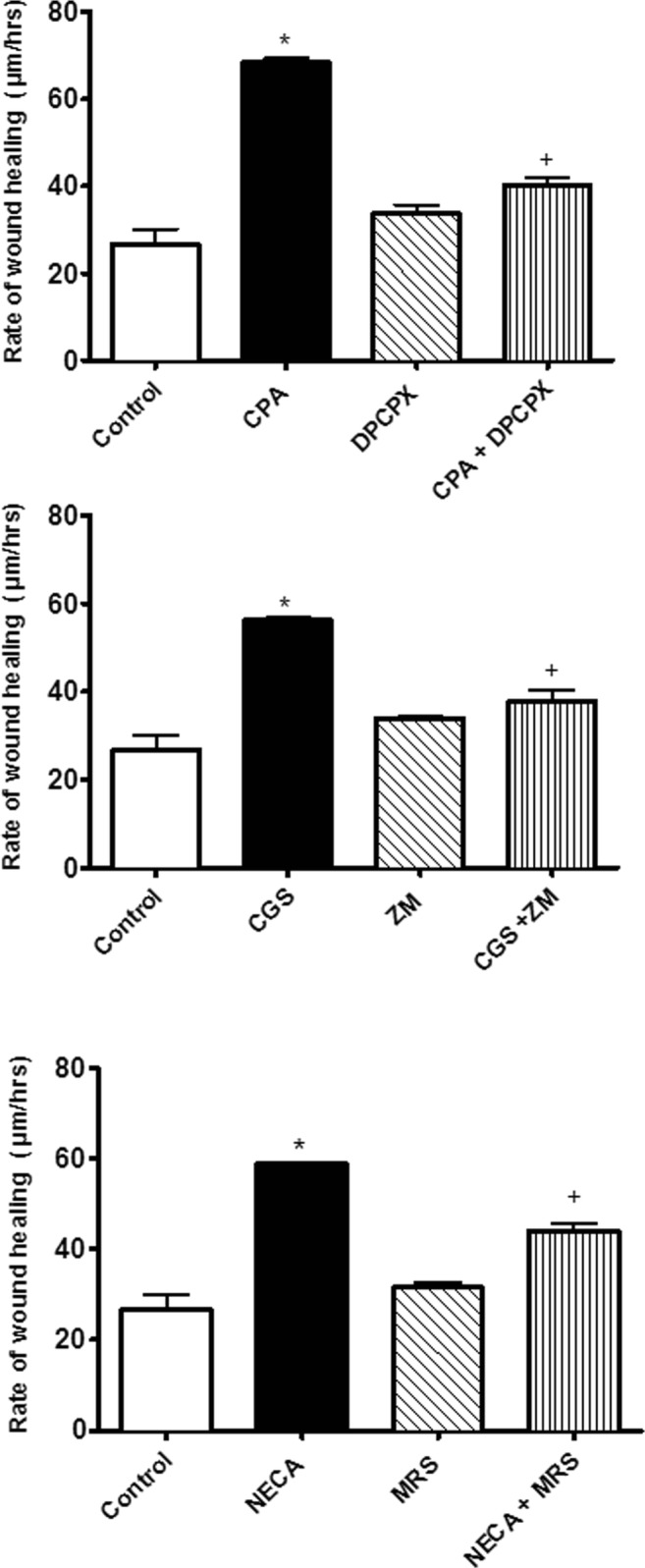
The effect of adenosine receptor agonist, antagonist and their combination on the rate of wound healing. Note that responses to NECA were done in the presence of 10 nM ZM241385. One-way ANOVA was applied to analyse the data. Data is expressed as mean ± SEM, n = 6, *P < 0.05 versus the control, +P < 0.05 versus selective agonist. CGS21680 (CGS), ZM241385 (ZM) and MRS1754 (MRS)
Cell proliferation assay
The results show that cell proliferation was significantly decreased by the vehicle control treatment (0.004% and 0.03% DMSO) when compared with the control group (data not shown, P < 0.05). Also, increased cell proliferation was observed in cells treated with the adenosine A1 and A2B receptor agonists (CPA, 30 nM and NECA, 30 nM + ZM241385, 10 nM) when compared with the control groups (P < 0.05). However, in comparison with the control groups, the treatment with the selective antagonists alone or combined agonist and antagonist groups did not show any changes (P > 0.05). Figure 5 shows that the adenosine receptor subtype agonists (CPA and NECA, 30 nM + ZM241385, 10 nM) increased cell proliferation but they did not have any significant differences when compared with each other (P > 0.05). Moreover, the results showed that agonist-induced cell proliferation was reversed by selective antagonist treatment.
Fig. 5.
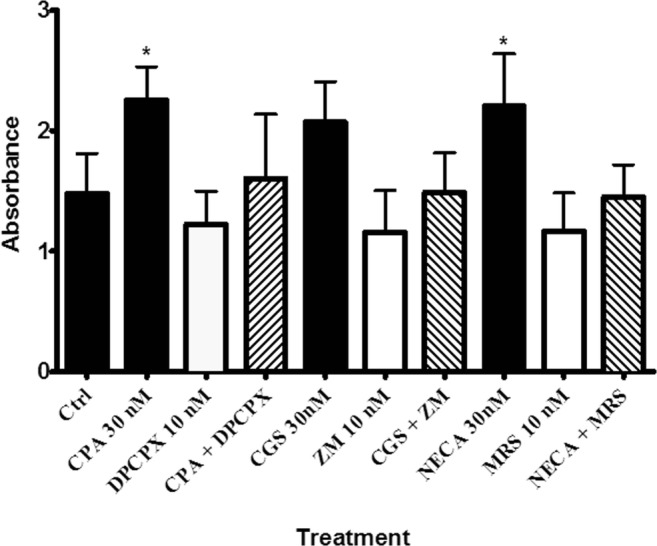
The effect of adenosine receptor agonist, antagonist and their combination on proliferation rates. Note that responses to NECA were done in the presence of 10 nM ZM241385. One-way ANOVA was applied to analyse the data. Data is expressed as mean ± SEM, n = 6, *P < 0.05 versus the control. CGS21680 (CGS), ZM241385 (ZM) and MRS1754 (MRS)
Discussion
This study measured the effect of selective adenosine A1, A2A and A2B receptor agonists and antagonists on the rate of wound closure in EA.hy926 endothelial cells. This project also determined the effect of adenosine receptor agonists on cell proliferation and determined the adenosine A1, A2A and A2B receptor expression levels in EA.hy926 endothelial cells.
We found that only adenosine A1, A2A and A2B receptor mRNA were expressed in this cell line and that all three adenosine A1, A2A and A2B receptor agonists, CPA, CGS21680 and NECA + ZM241385, significantly increased the rate of wound healing in human EAhy926 endothelial cells with the following order of potency CGS21680>CPA>NECA and efficacy CPA>NECA>CGS21680. The selective adenosine A1, A2A and A2B receptor antagonists, DPCPX, ZM241385 and MRS1754 (all at 10 nM), reversed the effects of their respective agonists and that EAhy926 endothelial cell proliferation was also significantly increased with the adenosine A1 and A2B receptor agonists, CPA and NECA + ZM241385.
Several studies have used cultured endothelial cells to mimic different physiological and pathological conditions, particularly in the study of angiogenesis [32]. Primary endothelial cells have a restricted lifespan and they may display variable characteristics due to their multi-donor origin [32]. Immortalised cell lines have been reported to be generally better characterised and more constant in their endothelial properties than primary endothelial cells that were given an extended life span [32]. This study reported that the best characterised human macrovascular endothelial cell lines and human microvascular endothelial cell lines are EA.hy926 and HMEC-1, respectively [32]. The permanent human endothelial hybrid cell line (EA.hy926) was generated by hybridisation of primary human umbilical vein endothelial cells (HUVEC) and the human lung carcinoma cell line (A549/8) [33, 34].
The results of this study show that the rates of wound healing in EAhy926 endothelial cells were significantly increased by the adenosine A1, A2A and A2B receptor agonists (CPA, CGS21680 and NECA + ZM241385 respectively) when compared with their control group indicating that all three adenosine receptor subtypes stimulated wound healing in this assay. Also, the concentration-response curves to the adenosine A1, A2A and A2B receptor agonists show that the rate of wound healing was concentration-dependent. Adenosine exerts its effects via G protein–coupled cell surface receptors that can be divided into four subtypes: adenosine A1 and A3 receptors that inhibit adenylyl cyclase and adenosine A2A and A2B receptors that stimulate adenylyl cyclase [12]. Another research has determined that stimulation of adenosine receptors increased endothelial cell migration via cAMP- and PKA-dependent pathways [23]. Also, studies using human endothelial cells confirmed that adenosine is a mitogen for endothelial cells [12, 35, 36]. Another study observed that the topical application of the selective adenosine A2A receptor agonist (CGS21680) in mice significantly increased the wound closure rates when compared with mice treated with a vehicle 1.5% methylcellulose (control group) [23].
The efficacy and potency of the three adenosine receptor agonists (CPA, CGS21680 and NECA) on the rate of wound healing which varies in the EAhy926 endothelial cell line were assessed. The adenosine A2A receptor agonist (CGS21680) was more potent than CPA and NECA + ZM241385 (adenosine A1 and A2B receptor agonists, respectively) on wound closure. On the other hand, the efficacy of CPA on the rate of wound healing in EAhy926 endothelial cells was greater than that in CGS21680 and NECA + ZM241385. Corroborating previous findings, the adenosine A2B receptor agonist (NECA + ZM241385) was the least potent and had reduced efficacy in stimulating wound healing when compared with the other two adenosine receptor agonists (CPA and CGS21680). The adenosine A2B receptor has the lowest affinity for the agonists used in this study; that is, the agonists for the other receptor subtypes have potencies in the low nanomolar range while the most potent of adenosine A2B receptor agonists have affinities around the 1-μM range [37]. Similarly, a study using human umbilical vein endothelial cells reported that the selective adenosine A2A receptor agonist CGS 21680 was more potent in activating adenylyl cyclase production than the non-selective adenosine A2B receptor agonist (NECA). Although NECA was less potent than CGS21680, it exhibited higher efficacy in the activation of adenylyl cyclase [38]. In this study, the cell proliferating effects of NECA were inhibited by MRS1754, a selective adenosine A2B receptor antagonist which has low affinity for the adenosine A1 receptor with a Ki value of 403 nM [39]. Also, while there are limited options for selective agonists at the adenosine A2B receptor, the compound BAY 60-6583 may offer more insight into the potential role of this receptor in stimulating endothelial cell proliferation [40].
Moreover, an in vivo study using mice showed that the topical application of the selective adenosine A2 receptor antagonist DMPX (3,7-dimethyl-1-propargyl xanthine) did not affect the rate of wound healing itself; however, it reversed the effect of the selective adenosine A2A receptor agonist (CGS21680) on the rate of wound closure [23]. It should be noted that DMPX and ZM 241385 are highly selective adenosine A2A receptor antagonists. Thus, both are able to reverse the effect of the selective adenosine A2A receptor agonist (CGS-21680) [41].
This study also showed that the adenosine A1 and A2B receptor agonists stimulated the proliferation of EAhy926 endothelial cells, an effect that was inhibited by their respective adenosine receptor antagonists. The adenosine receptor subtypes have been reported to stimulate cell proliferation and wound healing in other in vitro studies, as well as in vivo studies. The adenosine A1 and A2B receptors have been reported to regulate proliferation and cell differentiation in vascular smooth muscle cells and to promote cell proliferation and migration of endothelial cells in wound healing studies using mice [37, 42, 43]. Moreover, the selective adenosine A1 receptor agonist (CPA) has been shown to significantly increase the proliferation of keratinocytes, fibroblasts and endothelial cells using bromodeoxyuridine-labelled cells in an in vivo wound healing study in mice [12]. The non-selective adenosine A2B receptor agonist (NECA) stimulates retinal endothelial cell angiogenesis, cell proliferation and cell migration as well as stimulating cAMP response element-binding protein (CREB) and extracellular signal-regulated kinase (ERK) signalling pathways associated with cell survival and proliferation [44]. This same study also reported that selective adenosine A2B receptor antagonists blocked the effect of NECA and attenuated the proliferation of human retinal endothelial cells, leading to abnormal angiogenesis as observed in diabetic retinopathy [44].
Western blot results of this study determined that the protein expression of adenosine A1, A2A and A2B receptor subtypes varies in the EAhy926 endothelial cells. This study showed that the adenosine A2A receptors are predominantly expressed in EAhy926 endothelial cells when compared with the adenosine A1 and A2B receptors. The expression of adenosine receptor subtypes has been reported to vary in different cell lines such as isolated cerebral arterial muscle cells (CAMCs), dermal fibroblast and human umbilical vein endothelial cells (HUVECs), human microvascular endothelial cell-1 (HMEC-1), and human and porcine coronary artery endothelial cells [23]. The presence of the adenosine A2A, A2B, and A3 receptor subtypes was observed in cultured dermal fibroblasts and human umbilical vein endothelial cells using the Western blot technique and reverse transcriptase PCR which were expressed in both cell lines [23]. In contrast, the adenosine A1 receptor was only expressed in human umbilical vein endothelial cells (HUVECs) but not in dermal fibroblasts [23]. Previous studies investigating the expression of adenosine A2A and A2B receptors reported that the adenosine A2A receptors were the predominant subtype and highly expressed in HUVECs and human and porcine coronary artery endothelial cells [38, 45]. This confirms our work as the EAhy926 endothelial cells are derived from HUVEC lines. In contrast, the expression of adenosine A2B receptors was higher in human retinal microvascular endothelial cells and in HMEC-1 [38, 46, 47]. Feoktistov et al. [38] suggested that the adenosine receptor populations might depend on the location in the vasculature, with the adenosine A2A receptor subtypes predominantly expressed in endothelial cells lining large conduit vessels while the adenosine A2B receptor subtypes were mainly expressed in endothelial cells lining capillaries [38].
Conclusion
Adenosine A1, A2A and A2B receptor agonists (CPA, CGS21680 and NECA + ZM241385) significantly increased the rate of the wound healing and cell proliferation in EAhy926 endothelial cells. This work shows that all three adenosine receptors can individually stimulate cell proliferation and wound healing in the EA.hy926 endothelial cells and probably all contribute to wound healing when endogenous adenosine is released. However, adenosine A1 receptor agonists or allosteric modulators may be the most efficacious compounds to promote wound healing.
Electronic supplementary material
(DOCX 198 kb)
Acknowledgements
The EA.hy926 endothelial cell line used for these studies was generously donated to our research group by the Apoptosis Research Group, Griffith University, Gold Coast campus.
Compliance with ethical standards
Conflicts of interest
Zeinab Bonyanian declares that he has no conflict of interest.
Matthew Walker declares that he has no conflict of interest.
Eugene Du Toit declares that he has no conflict of interest.
Roselyn B. Rose’Meyer declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardiovasc Res. 2001;52(1):25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- 2.Cronstein BN. Adenosine receptors and fibrosis: a translational review. F1000 Biol Rep. 2011;3:21. doi: 10.3410/B3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredholm BB. Adenosine receptors as drug targets. Exp Cell Res. 2010;316(8):1284–1288. doi: 10.1016/j.yexcr.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andine P, Rudolphi KA, Fredholm BB, Hagberg H. Effect of propentofylline (HWA 285) on extracellular purines and excitatory amino acids in CA1 of rat hippocampus during transient ischaemia. Br J Pharmacol. 1990;100(4):814–818. doi: 10.1111/j.1476-5381.1990.tb14097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borea PA, Gassi S, Merighi S, Varani K. Adenosine as a multi-signalling guardian angel in human diseases: when, where and how does it exert protective effects? Trends Pharmacol Sci. 2016;37(6):419–434. doi: 10.1016/j.tips.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Feoktistov I, Biaggioni I, Cronstein BN. Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb Exp Pharmacol. 2009;193:383–397. doi: 10.1007/978-3-540-89615-9_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berne RM, Belardinelli L. Effects of hypoxia and ischaemia on coronary vascular resistance, A-V node conduction and S-A node excitation. Acta Medica Scand Suppl. 1985;694:9–19. doi: 10.1111/j.0954-6820.1985.tb08795.x. [DOI] [PubMed] [Google Scholar]
- 8.Gawlowski DM, Duran WN. Dose-related effects of adenosine and bradykinin on microvascular permselectivity to macromolecules in the hamster-cheek pouch. Circ Res. 1986;58(3):348–355. doi: 10.1161/01.res.58.3.348. [DOI] [PubMed] [Google Scholar]
- 9.Des Rosiers C, Nees S. Functional evidence for the presence of adenosine A2-receptors in cultured coronary endothelial cells. Naunyn Schmiedeberg's Arch Pharmacol. 1987;336(1):94–98. doi: 10.1007/BF00177757. [DOI] [PubMed] [Google Scholar]
- 10.Bache RJ, Dai XZ, Schwartz JS, Homans DC. Role of adenosine in coronary vasodilation during exercise. Circ Res. 1988;62(4):846–853. doi: 10.1161/01.res.62.4.846. [DOI] [PubMed] [Google Scholar]
- 11.Meininger CJ, Schelling ME, Granger HJ. Adenosine and hypoxia stimulate proliferation and migration of endothelial-cells. Am J Phys. 1988;255(3):H554–H562. doi: 10.1152/ajpheart.1988.255.3.H554. [DOI] [PubMed] [Google Scholar]
- 12.Sun LL, Xu LL, Nielsen TB, Rhee P, Burris D. Cyclopentyladenosine improves cell proliferation, wound healing, and hair growth. J Surg Res. 1999;87(1):14–24. doi: 10.1006/jsre.1999.5716. [DOI] [PubMed] [Google Scholar]
- 13.Dana A, Baxter GF, Walker JM, Yellon DM. Prolonging the delayed phase of myocardial protection: repetitive adenosine A1 receptor activation maintains rabbit myocardium in a preconditioned state. J Am Coll Cardiol. 1998;31(5):1142–1149. doi: 10.1016/s0735-1097(98)00054-0. [DOI] [PubMed] [Google Scholar]
- 14.Carr CS, Hill RJ, Masamune H, Kennedy SP, Knight DR, Tracey WR, Yellon DM. Evidence for a role for both the adenosine A1 and A3 receptors in protection of isolated human atrial muscle against simulated ischaemia. Cardiovasc Res. 1997;36(1):52–59. doi: 10.1016/s0008-6363(97)00160-0. [DOI] [PubMed] [Google Scholar]
- 15.Stambaugh K, Jacobson KA, Jiang JL, Liang BT. A novel cardioprotective function of adenosine A1 and A3 receptors during prolonged simulated ischemia. Am J Phys. 1997;273(1 Pt 2):H501–H505. doi: 10.1152/ajpheart.1997.273.1.H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casati C, Forlani A, Lozza G, Monopoli A. Hemodynamic changes do not mediate the cardioprotection induced by the A1, adenosine receptor agonist CCPA in the rabbit. Pharmacol Res. 1997;35(1):51–55. doi: 10.1006/phrs.1996.0114. [DOI] [PubMed] [Google Scholar]
- 17.Olanrewaju HA, Mustafa SJ. Effects of adenosine analogues on tension and cytosolic Ca2+ in porcine coronary artery. Am J Phys. 1996;270(1 Pt 2):H134–H141. doi: 10.1152/ajpheart.1996.270.1.H134. [DOI] [PubMed] [Google Scholar]
- 18.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157(10):4634–4640. [PubMed] [Google Scholar]
- 19.Schaffer MR, Fuchs N, Proksch B, Bongartz M, Beiter T, Becker HD. Tacrolimus impairs wound healing: a possible role of decreased nitric oxide synthesis. Transplantation. 1998;65(6):813–818. doi: 10.1097/00007890-199803270-00008. [DOI] [PubMed] [Google Scholar]
- 20.Wallace HJ, Stacey MC. Levels of tumor necrosis factor-α (TNF-α) and soluble TNF receptors in chronic venous leg ulcers–correlations to healing status. J Investig Dermatol. 1998;110(3):292–296. doi: 10.1046/j.1523-1747.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 21.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160(1):419–425. [PubMed] [Google Scholar]
- 22.Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol. 2002;160(6):2009–2018. doi: 10.1016/S0002-9440(10)61151-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186(9):1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer TM, Gettys TW, Stiles GL. Differential interaction with and regulation of multiple G-proteins by the rat A3 adenosine receptor. J Biol Chem. 1995;270(28):16895–16902. doi: 10.1074/jbc.270.28.16895. [DOI] [PubMed] [Google Scholar]
- 25.Klinger M, Freissmuth M, Nanoff C. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell Signal. 2002;14(2):99–108. doi: 10.1016/s0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- 26.Headrick JP, Peart J. A3 adenosine receptor-mediated protection of the ischemic heart. Vasc Pharmacol. 2005;42(5–6):271–279. doi: 10.1016/j.vph.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ Res. 2003;92(5):485–492. doi: 10.1161/01.RES.0000061572.10929.2D. [DOI] [PubMed] [Google Scholar]
- 28.Ansari HR, Nadeem A, Tilley SL, Mustafa SJ. Involvement of COX-1 in A3 adenosine receptor-mediated contraction through endothelium in mice aorta. Am J Physiol Heart Circ Physiol. 2007;293:H3338–H3455. doi: 10.1152/ajpheart.00764.2007. [DOI] [PubMed] [Google Scholar]
- 29.Zhao Z, Makaritsis K, Francis CE, Gavras H, Ravid K. A role for the A3 adenosine receptor in determining tissue levels of cAMP and blood pressure: studies in knock-out mice. Biochim Biophys Acta. 2000;1500(3):280–290. doi: 10.1016/s0925-4439(99)00111-8. [DOI] [PubMed] [Google Scholar]
- 30.Scientific T (2012) How to use a protein assay standard curve
- 31.Fredholm BB, AP IJ, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev. 2011;63(1):1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouis D, Hospers GA, Meijer C, Molema G, Mulder NH. Endothelium in vitro: a review of human vascular endothelial cell lines for blood vessel-related research. Angiogenesis. 2001;4(2):91–102. doi: 10.1023/a:1012259529167. [DOI] [PubMed] [Google Scholar]
- 33.Edgell CJ, Haizlip JE, Bagnell CR, Packenham JP, Harrison P, Wilbourn B, Madden VJ. Endothelium specific Weibel-Palade bodies in a continuous human cell line, EA.hy926. In Vitro Cell Dev Biol. 1990;26(12):1167–1172. doi: 10.1007/BF02623694. [DOI] [PubMed] [Google Scholar]
- 34.Edgell CJ, McDonald CC, Graham JB. Permanent cell line expressing human factor VIII-related antigen established by hybridization. Proc Natl Acad Sci U S A. 1983;80(12):3734–3737. doi: 10.1073/pnas.80.12.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ethier MF, Chander V, Dobson JG., Jr Adenosine stimulates proliferation of human endothelial cells in culture. Am J Phys. 1993;265(1 Pt 2):H131–H138. doi: 10.1152/ajpheart.1993.265.1.H131. [DOI] [PubMed] [Google Scholar]
- 36.Ethier MF, Dobson JG., Jr Adenosine stimulation of DNA synthesis in human endothelial cells. Am J Phys. 1997;272(3 Pt 2):H1470–H1479. doi: 10.1152/ajpheart.1997.272.3.H1470. [DOI] [PubMed] [Google Scholar]
- 37.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 38.Feoktistov I, Goldstein AE, Ryzhov S, Zeng D, Belardinelli L, Voyno-Yasenetskaya T, Biaggioni I. Differential expression of adenosine receptors in human endothelial cells: role of A2B receptors in angiogenic factor regulation. Circ Res. 2002;90(5):531–538. doi: 10.1161/01.res.0000012203.21416.14. [DOI] [PubMed] [Google Scholar]
- 39.Kim Y-C, Ji X-D, Melman N, Linden J, Jacobson KA. Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A2B adenosine receptors. J Med Chem. 2000;43:1165–1172. doi: 10.1021/jm990421v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baraldi PG, Tabrizi MA, Fruttarolo F, Romagnoli R, Preti D. Recent improvements in the development of A2B adenosine receptor agonists. Purinergic Signal. 2009;5:3–19. doi: 10.1007/s11302-009-9140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richter A, Hamann M. Effects of adenosine receptor agonists and antagonists in a genetic animal model of primary paroxysmal dystonia. Br J Pharmacol. 2001;134(2):343–352. doi: 10.1038/sj.bjp.0704268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jonzon B, Nilsson J, Fredholm BB. Adenosine receptor-mediated changes in cyclic AMP production and DNA synthesis in cultured arterial smooth muscle cells. J Cell Physiol. 1985;124(3):451–456. doi: 10.1002/jcp.1041240314. [DOI] [PubMed] [Google Scholar]
- 43.Shaikh G, Cronstein B. Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal. 2016;12(2):191–197. doi: 10.1007/s11302-016-9498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant MB, Davis MI, Caballero S, Feoktistov I, Biaggioni I, Belardinelli L. Proliferation, migration, and ERK activation in human retinal endothelial cells through A(2B) adenosine receptor stimulation. Invest Ophthalmol Vis Sci. 2001;42(9):2068–2073. [PubMed] [Google Scholar]
- 45.Olanrewaju HA, Qin W, Feoktistov I, Scemama JL, Mustafa SJ. Adenosine A(2A) and A(2B) receptors in cultured human and porcine coronary artery endothelial cells. Am J Phys Heart Circ Phys. 2000;279(2):H650–H656. doi: 10.1152/ajpheart.2000.279.2.H650. [DOI] [PubMed] [Google Scholar]
- 46.Fischer S, Sharma HS, Karliczek GF, Schaper W. Expression of vascular permeability factor/vascular endothelial growth factor in pig cerebral microvascular endothelial cells and its upregulation by adenosine. Brain Res Mol Brain Res. 1995;28(1):141–148. doi: 10.1016/0169-328x(94)00193-i. [DOI] [PubMed] [Google Scholar]
- 47.Grant MB, Tarnuzzer RW, Caballero S, Ozeck MJ, Davis MI, Spoerri PE, Feoktistov I, Biaggioni I, Shryock JC, Belardinelli L. Adenosine receptor activation induces vascular endothelial growth factor in human retinal endothelial cells. Circ Res. 1999;85(8):699–706. doi: 10.1161/01.res.85.8.699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 198 kb)