Abstract
Background
The aim of this study was to evaluate the effects of ginger supplementation on inflammatory, antioxidant, and periodontal parameters in type 2 diabetes mellitus (T2DM) patients with chronic periodontitis (CP) under non-surgical periodontal therapy (NSPT).
Material and methods
In this double-blind clinical trial study, 46 T2DM patients with CP were randomly allocated to intervention and control groups and received either 4 tablets 500 mg (2 g) ginger or placebo twice a day for 8 weeks. All patients were treated with NSPT during the intervention period. Serum levels of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), hs-C-reactive protein (hs-CRP), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), periodontal indices including clinical attachment loss (CAL), bleeding on probing (BOP), pocket depth (PD), and plaque index were evaluated in all subjects pre- and post-intervention.
Results
Following 8 weeks of ginger treatment with NSPT, significant reductions were observed in the mean levels of IL-6 (p=0.001), hs-CRP (p=0.03), TNF-α (p=0.007), CAL, and PD (p<0.001) in the intervention group. The mean serum levels of SOD and GPx were significantly increased in the intervention group after the intervention (p=0.001 and 0.002, respectively). At the end of the study, the mean changes of GPx were significantly higher in the intervention group compared with the control group (p=0.04). Also, after the administration of the ginger with NSPT, significant decrease occurred in the mean change of IL-6 (p=0.009), hs-CRP (p=0.049), TNF-α (p=0.049), CAL (p=0.003), and PD (p=0.04) compared with the control group.
Conclusion
It is recommended that ginger supplementation along with NSPT may be effective in the improvement of inflammation, oxidative, and periodontal status in T2DM with CP.
Keywords: type 2 diabetes mellitus, periodontal disease, ginger, inflammation, antioxidant enzymes
Introduction
Periodontal disease (a common oral disease all over the world) is an inflammatory condition affects on the supportive tissue of teeth. The prevalence of periodontal disease was reported to be over 50%.1,2 According to previous studies, there was no specific relationship found between the pathogenesis of type 2 diabetes and periodontal disease, evidence recently indicated that these two conditions are broadly in association.3,4 Some factors involved in the pathogenesis of these diseases included smoking, impairment of neutrophil function, age, and polygenic backgrounds.4,5 On the other hand, various studies reported that the prevalence and severity of periodontal disease is greater among diabetic patients. Multivariate risk analysis showed that the prevalence of periodontal disease in diabetic patients is more than threefold of normal subjects.6 The development of periodontal disease may be considered as a complication in diabetes mellitus (DM). Moreover, the progression of periodontal disease is quicker in diabetic patients and interfere glycemic control and may ultimately result in a vicious cycle.7 Moreover, it was found that the macro- and microvascular complications of DM are commonly observed in diabetic patients with periodontal disease.8 Various mechanisms were proposed for the role of DM in the progression of periodontal disease. Because of the infectious nature of periodontal disease, the results of some studies indicated changes in subgingival microbial flora of diabetic patients.9 The other mechanisms involved are changes in the function of immune cells such as neutrophils, monocytes, and macrophages,10 increased levels of inflammatory factors such as tumor necrosis factor-alpha (TNF-α) and altered wound healing.11
Recent studies reported that the treatment of periodontal disease may improve glycemic control and decrease the severity of such faulty cycle.12 Ginger (Zingiber officinale), of the Zingiberaceae, is one of the medicinal plants which is used in many countries.13 The active ingredients in ginger such as β-bisabolene, shogaol, gingerol, and paradol have glycemic control, anti-inflammatory, anti-oxidant, anti-cancer, and anti-obesity effects.14,15 The effectiveness of ginger in treating some diseases such as nausea, rheumatism,16 and lipid profile disorders17 was suggested in some studies. Furthermore, ginger may improve glycemic control18 and reduce inflammatory markers (TNF-α, IL-1β, IL-6)19 in diabetic patients and it can significantly decrease levels of malondialdehyde and increase levels of antioxidant enzymes (superoxide dismutase [SOD], catalase [CAT], glutathione peroxidase [GPx], glutathione reductase [GR]) and glutathione.20 Studies have also indicated that the non-surgical periodontal therapy (NSPT) including oral health education, scaling, and root planning is aimed to reduce the bacterial challenge and inflammation, which may lead to restoration of insulin sensitivity over time and improve metabolic control in diabetic patients and reduce the serum levels of TNF-α and IL-17 in patients with periodontal disease.21,22
To the best of our knowledge, there is no randomized controlled trial assessing the effects of ginger on diabetic patients with periodontal disease and because of insufficient information, this study was designed to evaluate the effects of ginger supplementation on inflammatory, antioxidant, and periodontal parameters in T2DM patients with CP under NSPT.
Materials and methods
Subjects
Forty-six T2DM patients with CP (30–60 years) were enrolled in this randomized double-blind placebo-controlled trial. The subjects were included who had a confirmed diagnosis of T2DM (FBS≥126 mg/dL and HbA1c≥6.5% or 2-hr glucose) 2 hpp (≥200 mg/dL)23 and CP according to the American Academy of Periodontology criteria.24 The inclusion criteria considered in this study were as follows: males and females aged 30–60 years old, body mass index of 18.5–30 kg/m,2 confirmed diagnosis of T2DM for >5 years, individuals with mild and moderate periodontitis (PD≥4 mm and CAL=1–4 mm). Also, the exclusion criteria were as follows: having any systemic diseases, kidney disorder, pregnancy, breastfeeding, smoking, using systemic antibiotic and any dietary supplements or antioxidants, received periodontal treatment in the previous 6 months and using any form of immunosuppressant and anti-inflammatory agents, noticeable change in consumption of medications and treatment of diabetes, the consumption of <90% of ginger tablets, severe periodontitis, and using anticoagulant medications such as warfarin and aspirin.
The ethics approval was obtained from Ahvaz Jundishapur University of Medical Science, Iran (Ref No. IR.AJUMS.REC.1396.925) and was registered in the Iranian Registry of Clinical Trials website (IRCT20170304032874N2). Also, written informed consent was collected from patients.
Study design
This randomized double-blind placebo-controlled trial was performed at endocrinology and metabolism clinics of Imam Khomeini Hospital of Ahvaz Jundishapur University of Medical Science, Iran. In order to diagnose and confirm periodontal disease, all recruited subjects were referred to the Dental Clinic. Finally, 46 subjects were randomly allocated to two groups, including intervention (n=23) or placebo (n=23). The randomization was done through a random permuted block procedure (block design) based on the combined analysis as all recruited subjects were assigned to six groups in four blocks considering two code series of A and B according to the block design (first step; AABB, BBAA, ABAB, BABA, ABBA, BABA and second step; AABB, BBAA, ABAB, BABA, ABBA, BA). The code allocation planning in this study was done by a third blind person (who was not informed of the research) in the health care system. Moreover, the researchers and patients did not inform of the allocation of subjects to either intervention or control groups (double-blinded). To continue the routine treatment considered for DM, daily diet, and lifestyle without any modification during the entire study period were recommended to the patients.
Intervention protocol
The intervention and control groups received 2 g/d (4 tablets of 500 mg as two tablets before lunch and dinner) ginger (containing zingiberene, ar-curcumene, geranial, zingiberol, and Z. officinale) and placebo (containing pea flour), respectively for 8 weeks. The ginger and placebo tablets were produced by Dineh Company, Iran. The placebo tablets were matched with the ginger tablets in terms of shape, color, size, and taste. Any reports regarding with possible side effects of supplements were monitored three times during the study. To evaluate the compliance of subjects the tablet remaining were quantified. Subjects with the consumption of <90% of ginger tablets or self-reported of sensitivity to ginger consumption were excluded from the study.
Measurement of anthropometric indices and assessing dietary intake
Height and body weight were measured while the subjects had light clothing and no shoes. The BMI was calculated by dividing the body weight (in kilograms) by the height (in meters squared). All subjects completed a 3 day 24 hrs dietary recall at baseline and end of the study. The dietary intake was analyzed by Nutritionist IV software specified for Iranian foods. All subjects were advised to maintain their usual diet and physical activity during the study.
Measurement of biochemical parameters
Fasting blood samples (5 mL) were collected from all subjects at baseline and end of the study and centrifuged for 15 mins and stored in − 70°C until biochemical analyses. Serum levels of SOD, CAT, and GPx were measured by reliable spectrophotometric methods using the Zell Bio GmbH kit instructions (Germany). Serum levels of IL-6 and TNF-α and hc-CRP were measured by ELISA kits (hc-CRP ELISA, LDN, Germany) and (Human IL-6 and TNF-α: Elisa kit [IBL, Germany]). All assay procedures were done according to the related kit instructions. (details have not been explained).
Measurement of periodontal indices
The first visit of NSPT (scaling and root surface debridement) was performed for all subjects in intervention and control groups at baseline and the second visit was done with the start of the second month of the intervention. The NSPT involved careful cleaning of the root surfaces to remove plaque and calculus from deep periodontal pockets and surfacing tooth root to remove bacterial toxins. Moreover, some instructions on dental hygiene such as brushing and correct application of dental floss were provided for all subjects. The subjects were asked to avoid using any mouthwash during this study.
Bleeding on probing (BOP) and plaque (the measurement of the status of oral hygiene), PD and CAL were measured as the main periodontal indices in this study by a dentist. The measurement of all periodontal indices was done at six sites of a tooth comprised of mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, and distolingual. The CAL was measured by a full – mouth periodontal examination and determined through measuring the distance between cement – enamel junction and the bottom of the gingival crevice. Periodontitis is considered as severe in individuals with CAL≥5 mm (not on the same tooth) or moderate in individuals with CAL of 3–4 mm (not on the same tooth) or weak in individuals with CAL of 1–2 mm (not on the same tooth). PD (the distance between gingival margin and the base of the gingival sulcus or periodontal pocket) was measured using a UNC-15 (University of North Carolina No. 15) manual periodontal probe.25
Statistical analysis
The results were presented as mean±SD. The P-value of <0.05 was considered statistically as significant in all analyses. The Kolmogorov–Smirnov and Shapiro–Wilk test were used to assess the normality of variables distribution. The Independent sample t-test was done to compare the results between the two groups. The Paired sample t-test was also used to compare the results within groups post-intervention. For analyzing the qualitative data, Chi-square test was used. All the statistical analysis was performed using SPSS (version 19). According to Danwilai et al’s study26 and considering GPx as the main variable, the sample size was calculated with a 95% confidence interval and 90% power of the study. The formula of sample calculation is given in the following:
. The sample size was determined as 19 subjects in each group. However, considering a 20% withdrawal rate, 23 subjects were selected for each group.
Results
Baseline characteristics of the subjects, anthropometric parameters, energy, macro-, and micronutrients
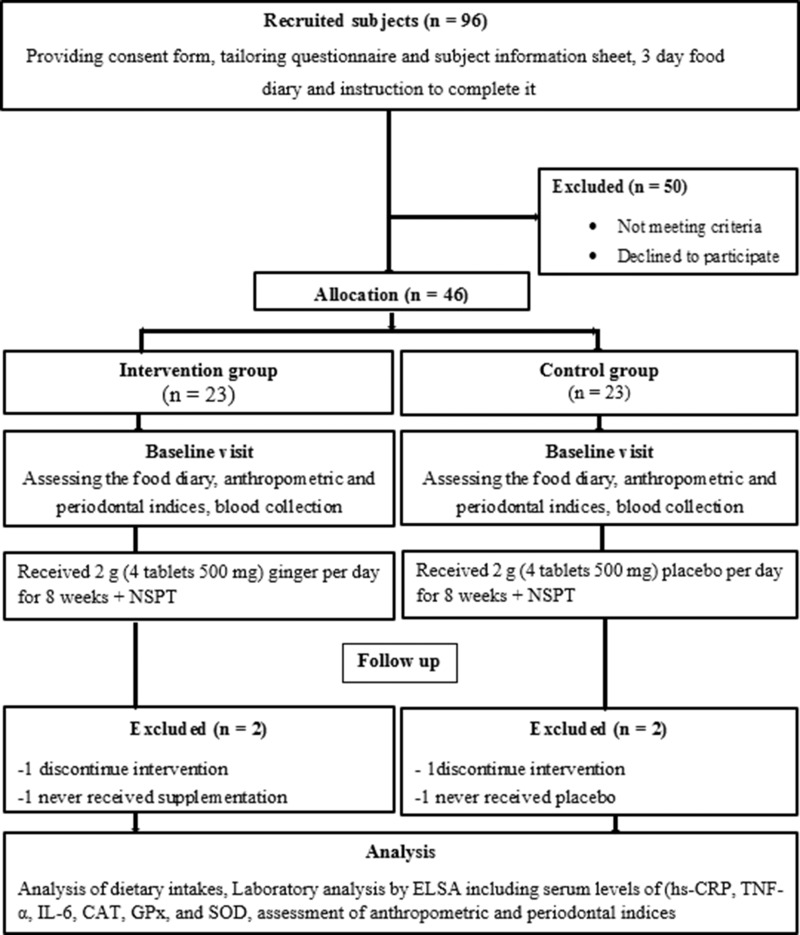
All variables in this study had a normal distribution. Forty-two patients were recruited for the study (control group n=21, and intervention groups n=21). Two patients were excluded from the statistical analysis for each group because they did not use tablets regularly or they did not continue the intervention (Figure 1). The mean age of patients in the intervention and control groups was 52.81±6.44 and 51.62±5.95 years, respectively. No significant differences were seen in demographic characteristics, including age, sex, weight, BMI, hip circumferences, waist circumferences, duration of diabetes, medication uses, and physical activity between two groups at baseline (p≥0.05) (Table 1). No significant differences were also observed between two groups for dietary intake including energy, macronutrients, and micronutrients such as antioxidant vitamins C, E, A, beta-carotene, α-tocopherol, and selenium at baseline and after the intervention (p≥0.05) (Table 2).
Figure 1.
Flow Diagram of the study.
Abbreviations: TNF-α, tumor necrosis factor-alpha; hs-CRP, hs-C-reactive protein; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; NSPT, non-surgical periodontal therapy.
Table 1.
The characteristics of subjects at baseline
| Variable | Control group (n=21) |
Intervention group (n=21) |
*P-value |
|---|---|---|---|
| Age (years) | 51.62±5.95 | 52.81±6.44 | 0.53 |
| Gender | |||
| Female (N) (%) | 12 (57.14) | 11 (52.38) | 0.75** |
| Male (N) (%) | 9 (42.86) | 10 (47.62) | |
| Weight (kg) | 73.28±6.48 | 72.14±10.09 | 0.66 |
| Height (cm) | 164.38±9.58 | 166.33±7.20 | 0.46 |
| BMI (kg/m2) | 27.18±2.15 | 26.06±3.33 | 0.20 |
| WC (cm) | 97.28±4.14 | 95.23±8.96 | 0.35 |
| HC (cm) | 102.76±3.54 | 102.33±8.24 | 0.82 |
| WHR | 0.94±0.02 | 0.93±0.05 | 0.25 |
| Physical activity (met-min/week) | 285.85±162.06 | 311.76±170.99 | 0.61 |
Notes: Values are expressed as means±SD. P<0.05 was considered as significant. *P<0.05 was considered as significant using independent t-test between the two groups at baseline. **P<0.05 was considered as significant using Chi-square test.
Abbreviations: BMI, body mass index; WC, waist circumference; HC, hip circumference; WHR, waist to hip ratio.
Table 2.
Mean±SD of energy, macronutrients, and micronutrients intake at baseline and post-intervention
| Variable | Baseline | Post-intervention | **P-value |
|---|---|---|---|
| Energy (kcal/d) | |||
| Control group | 1910.98±174.28 | 1879.58±143.56 | 0.4 |
| Intervention group | 1898.68±157.76 | 1864.64±151.19 | 0.21 |
| *P-value | 0.81 | 0.74 | |
| Carbohydrate (g/d) | |||
| Control group | 259.67±18.20 | 254.44±17.86 | 0.16 |
| Intervention group | 258.49±21.31 | 252.49±19.47 | 0.2 |
| *P-value | 0.84 | 0.73 | |
| Protein (g/d) | |||
| Control group | 70.77±5.42 | 72.99±6.80 | 0.21 |
| Intervention group | 71.38±6.12 | 72.71±6.54 | 0.42 |
| *P-value | 0.73 | 0.89 | |
| Fat (g/d) | |||
| Control group | 64.17±6.08 | 62.98±5.57 | 0.43 |
| Intervention group | 63.01±5.71 | 61.88±5.12 | 0.21 |
| *P-value | 0.53 | 0.5 | |
| Cholesterol (g/d) | |||
| Control group | 134.68±35.57 | 142.68±38.63 | 0.15 |
| Intervention group | 137.11±34.55 | 142.54±42.61 | 0.47 |
| *P-value | 0.82 | 0.99 | |
| Saturated fat (g/d) | |||
| Control group | 19.43±3.84 | 18.48±2.61 | 0.06 |
| Intervention group | 19.52±4.07 | 18.43±2.54 | 0.34 |
| *P-value | 0.94 | 0.95 | |
| Vitamin A (mcg/d) | |||
| Control group | 363.36±95.97 | 370.42±99.13 | 0.77 |
| Intervention group | 385.53±121.25 | 380.50±125.81 | 0.88 |
| *P-value | 0.51 | 0.77 | |
| Beta-carotene (mcg/d) | |||
| Control group | 4390.40±1559.94 | 4318.85±1420.45 | 0.86 |
| Intervention group | 4445.26±1784.20 | 4018.04±1061.34 | 0.25 |
| *P-value | 0.91 | 0.44 | |
| Selenium (mcg/d) | |||
| Control group | 51.03±13.47 | 54.95±10.49 | 0.13 |
| Intervention group | 55.91±13.35 | 53.90±8.30 | 0.50 |
| *P-value | 0.24 | 0.72 | |
| Vitamin C (mg/d) | |||
| Control group | 100.67±46.21 | 103.26±41.94 | 0.79 |
| Intervention group | 98.35±34.31 | 102.19±36.71 | 0.67 |
| *P-value | 0.85 | 0.93 | |
| α-Tocopherol (mg/d) | |||
| Control group | 7.67±1.78 | 7.72±2.03 | 0.88 |
| Intervention group | 7.39±2.13 | 7.90±2.22 | 0.08 |
| *P-value | 0.64 | 0.77 | |
| Vitamin E (mg/d) | |||
| Control group | 2.29±0.71 | 2.19±0.55 | 0.51 |
| Intervention group | 2.18±0.72 | 2.02±0.61 | 0.25 |
| *P-value | 0.63 | 0.37 |
Notes: Values are expressed as means±SD. *P<0.05 was considered as significant at baseline and post-intervention using independent t-test between two groups. **P<0.05 was considered as significant using Paired t-test.
IL-6, hs-C-reactive protein (hs-CRP), and TNF-α
At baseline, there were no significant differences in the mean serum levels of IL-6, TNF-α, and hs-CRP between two groups (p≥0.05). The mean serum levels of IL-6, hs-CRP, and TNF-α were significantly decreased in the intervention group after the intervention (2.33±0.7 vs 1.72±0.73 pg/mL, respectively; p=0.001), hs-CRP (1482±541 vs 1081±428 ng/mL, respectively; p<0.03) and TNF-α (11.67±3.28 vs 9.6±3.59 pg/mL, respectively; p<0.007). Also, the mean changes of IL-6, hs-CRP, and TNF-α were significantly lower in the intervention group compared with the control group (−0.6±0.71 vs −0.04±0.61 pg/mL, respectively; p=0.009), hs-CRP (−401.35±801.12 vs −9.33±339.40 ng/mL, respectively; p<0.03) and TNF-α (−2.07±3.13 vs −0.19±2.8 pg/mL, respectively; p<0.007) (Table 3).
Table 3.
Inflammatory and antioxidant markers at baseline and post-intervention
| Variables | Intervention group (n=21) | Control group (n=21) | P-value* | P-value** |
|---|---|---|---|---|
| SOD (U/mL) | ||||
| Baseline | 14.90±3.28 | 15.04±2.94 | 0.88 | |
| End | 15.71±3.01 | 15.28±2.53 | 0.278 | |
| P-value | 0.001 | 0.3 | ||
| Difference | 0.8±0.92 | 0.23±1.04 | 0.06 | |
| CAT (U/mL) | ||||
| Baseline | 16.68±3.73 | 16.49±3.16 | 0.85 | |
| End | 16.77±3.35 | 16.30±2.69 | 0.250 | |
| P-value | 0.49 | 0.36 | ||
| Difference | 0.09±0.61 | −0.19±0.94 | 0.25 | |
| GPx (U/mL) | ||||
| Baseline | 131.80±27.73 | 128.47±25.83 | 0.68 | |
| End | 150.23±27.58 | 132.61±24.10 | 0.03 | |
| P-value | 0.002 | 0.39 | ||
| Difference | 18.42±23.56 | 4.14±21.74 | 0.04 | |
| TNF-α (pg/mL) | ||||
| Baseline | 11.67±3.28 | 11.62±3.76 | 0.90 | |
| End | 9.6±3.59 | 11.42±3.88 | 0.12 | |
| P-value | 0.007 | 0.76 | ||
| Difference | −2.07±3.13 | −0.19±2.8 | 0.049 | |
| IL-6 (pg/mL) | ||||
| Baseline | 2.33±0.7 | 2.45±1.04 | 0.66 | |
| End | 1.72±0.73 | 2.41±0.87 | 0.009 | |
| P-value | 0.001 | 0.74 | ||
| Difference | −0.6±0.71 | −0.04±0.61 | 0.009 | |
| hs-CRP (ng/mL) | ||||
| Baseline | 1482±541 | 1326±380 | 0.20 | |
| End | 1081±428 | 1317±409 | 0.643 | |
| P-value | 0.03 | 0.90 | ||
| Difference | −401.35±801.12 | −9.33±339.40 | 0.049* |
Notes: Values are expressed as means±SD. P<0.05 was considered as significant using Paired t-test. *P<0.05 was considered as significant using independent t-test between the two groups at baseline and post-intervention. **P<0.05 was considered as significant changes using independent t-test between the two groups post-intervention. Significant values have been placed in bold.
Abbreviations: TNF-α, tumor necrosis factor-alpha; hs-CRP, hs-C-reactive protein; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase.
SOD, CAT, and GPx
No significant differences were observed in the mean serum levels of SOD, CAT, and GPx between two groups at baseline (p≥0.05). The results of this study showed that the mean serum levels of SOD and GPx were significantly increased in the intervention group after the intervention (14.90±3.28 vs 15.71±3.01 U/mL, respectively; p=0.001) and GPx (131.80±27.73 vs 150.23±27.58 U/mL, respectively; p=0.002). In addition, the mean changes of serum levels of GPx were significantly higher in the intervention group compared with the control group (18.42±23.56 vs 4.14±21. U/mL, respectively; p=0.04) (Table 3).
Periodontal parameters (PD, CAL, BOP, and plaque)
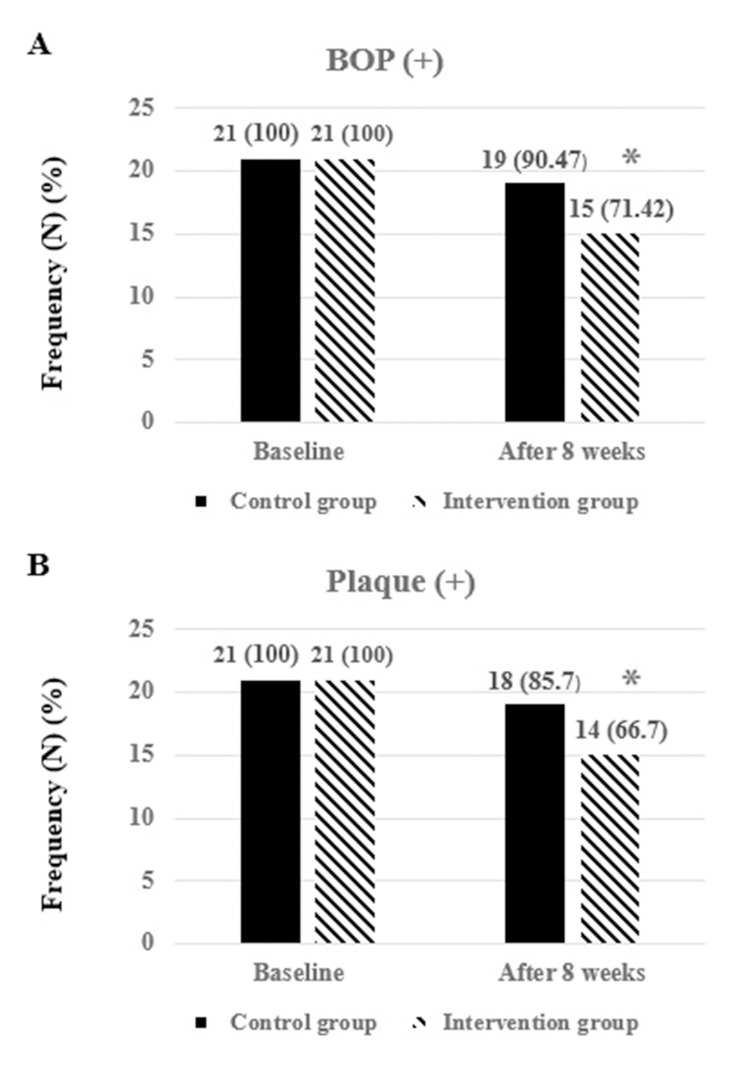
Within-group comparison in the intervention group showed that there were no significant differences in the mean of CAL and PD between two groups (p≥0.05). The mean of PD and CAL was significantly decreased in the intervention group post-intervention (4.95±1.16 vs 4.42±1.39 mm, respectively; p<0.001) and CAL (3.04±0.86 vs 2.47±0.60 mm, respectively; p<0.001). At the end of the study, there was a significant difference in the mean changes of CAL and PD between intervention and control groups (−0.57±0.50 vs −0.14±0.35 mm, respectively; p=0.003) and PD (−0.52±0.51 vs −0.19±0.51 mm, respectively; p=0.04) (Table 4). Although the plaque and BOP were reduced in both intervention and control groups after the intervention, the differences were not significant between two groups post-intervention (p=0.14 vs p=0.11, respectively) (Figure 2).
Table 4.
Periodontal status at baseline and post-intervention
| Variable | Baseline | After 8 weeks | Δ | P-value | P-value* | P-value** | P-value*** |
|---|---|---|---|---|---|---|---|
| PD (mm) | |||||||
| Control group (n=21) |
4.85±1.01 | 4.66±0.91 | −0.19±0.51 | 0.1 | 0.77 | 0.51 | 0.04 |
| Intervention group (n=21) |
4.95±1.16 | 4.42±1.39 | −0.52±0.51 | <0.001 | |||
| CAL (mm) | |||||||
| Control group (n=21) |
3.00±0.77 | 2.85±0.72 | −0.14±0.35 | 0.08 | 0.85 | 0.07 | 0.003 |
| Intervention group (n=21) |
3.04±0.86 | 2.47±0.60 | −0.57±0.50 | <0.001 |
Notes: Values are expressed as means±SD. P<0.05 was considered as significant using Paired t-test. *P<0.05 was considered as significant using independent t-test between the two groups at baseline. **P<0.05 was considered as significant using independent t-test between the two groups post-intervention. ***P<0.05 was considered as significant changes using independent t-test between the two groups post-intervention. Δ: difference between PD (mm) and CAL (mm) at baseline and after 8 weeks.
Abbreviations: CAL, clinical attachment loss; PD, pocket depth.
Figure 2.
The effects of ginger with NSPT on qualitative indices of periodontal (BOP and plaque) at baseline and post-intervention. The numbers are expressed in terms of frequency and percentage. *P<0.05 was considered as significant using Chi-square test.
Abbreviations: BOP, bleeding on probing; NSPT, non-surgical periodontal therapy.
Discussion
According to recent studies, it was indicated that the prevention or delaying the cellular damages mediated by the pathogenesis of free radical is possible due to the oxidative defense capacity of the cell through antioxidant intake. Therefore, much attention has been devoted to health beneficial effect of natural antioxidants in biological systems.27 Ginger is considered as an antioxidant with high antioxidant properties and capability of scavenging several free radicals and protect cell membrane lipids from oxidation.28 To the best of our knowledge, there is no study investigates the effects of ginger supplementation along with NSPT on inflammatory, antioxidant, and periodontal parameters in T2DM patients with CP.
Ginger and inflammatory markers
The results of the present study showed that ginger supplementation with NSPT significantly reduced the serum levels of IL-6, TNF-α, and hs-CRP in the intervention group post-intervention. Similarly, Rehman et al showed that the treatment with zingerone significantly decreased ROS levels, inhibited the nuclear factor-kappa B (NF-kB) activation and reduced the levels of other inflammatory markers including, TNF-a, IL-6, and IL-1b in type 2 diabetic Wistar rat.29 In agreement with the findings of this study, in a double-blinded clinical trial in which diabetic patients received ginger supplementation for 2 months, the results showed that the intervention significantly reduced the levels of TNF-α, IL-6, and hs-CRP in ginger group post-intervention.30 Also, another study reported that administration of ginger supplementation significantly reduced the levels of hs-CRP and PGE2 in patients with T2DM in compare to the control group; however, in this study there was no significant difference in TNF-α between two groups.18 Almost similar results were observed in several studies showing the reducing effects of ginger on inflammatory markers. It is indicated that ginger may inhibit the synthesis of both prostaglandinand leukotriene. Such dual inhibition of cyclooxygenase and lipoxygenase (LOX) causes it a more effective anti‐inflammatory agent.31 Proinflammatory cytokines such as IL1-β, IL-6, and TNF-a are activated through activation of NF-kB because of reactive oxygen species activity in T2DM patients with CP.32 Moreover, ginger is considered as a serotonin blocker and prevents the secretion of substance of P. There are several compounds found in ginger acting as a blocker of serotonin receptors.33 Some laboratory studies have also indicated that the inhibition of serotonin receptors may be correlated with the reduction of TNF-α, IL-1β, IL-6, IL-2, and prostaglandins.34
Ginger and antioxidant status
Several studies have reported that the balance between oxidant and antioxidant system in diabetic patients with periodontal disease disturbed and decreased the production of antioxidant enzymes, such as CAT, SOD, GST, GPx, and GR.35 It was indicated that these antioxidant enzymes may help to protect against the toxic effects of ROS under normal physiological conditions.36 The results of the present study showed that ginger supplementation with NSPT significantly increased mean serum levels of SOD and GPx in intervention group. Similarly, Shanmugam et al indicated that consumption of the ethanol extract of ginger for 30 days significantly increased the activity of GPx, GR, CAT, and SOD in diabetic rats. However, in contrast to this study, in our study increasing CAT was not significant.20 In another study in which the diabetic rats received a powdered ginger along with a normal diet for 30 days, the results showed that 2% concentration increased the levels of antioxidant enzymes (SOD, CAT, GPx, GR) and glutathione.37 Another clinical study showed that receiving ginger extract along with moderate-to-high emetogenic potential adjuvant chemotherapy for 64 days in cancer patients significantly increased activity of antioxidant enzymes, including SOD, CAT, GPx, and GSH/GSSG.26 It is suggested that having a different population, using different dosage of ginger, duration of intervention, size effect of ginger may be considered as possible reasons for different findings. The active components of Z. officinale including 6-shogaol and 6-gingerol are considered as potent antioxidants. A 6-shogaol-rich ginger extract may enhance the antioxidant defense capacity by induction of nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2). Nrf2 activation is suggested to protect against oxidative stress in vitro and in vivo. These findings indicate that Z. officinale may protect against oxidative damages in diabetes patients through decreasing oxidative stress and preventing antioxidant depletion.38 The anti-oxidative effects of ginger are suggested to be due to induction of SOD activity, enhancement of glutathione levels, and decreasing ROS. Moreover, ginger may have an inhibitory effect on xanthine oxidase system which is responsible for the production of reactive oxygen species like superoxide anion.39
Ginger and periodontal parameters
There is no clinical study evaluating the effects of ginger supplementation along with NSPT on periodontal parameters in T2DM. The results of the present study reported that consumption of ginger supplement for 8 weeks along with NSPT significantly reduced the mean of PD and CAL after the intervention. Also, in the control group, NSPT reduced the periodontal parameters, but it was not significant. It is suggested that the main causing factor of developing periodontal disease is the proliferation of pathogenic oral biofilms leading to dental plaque formation.40 The pathogenic biofilm are hard layers of mucilage adhering to solid surfaces and are included several species of bacteria (Gram-negative anaerobic such as Porphyromonas gingivalis, Actinobacillus, Prevotella intermedia, Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, Capnocytophaga spp, and Veillonella spp.) and other microorganisms. This biofilm may result in excessive proliferation of pro-inflammatory mediators, including cytokines, prostanoids, and enzymes.41 According to previous studies, ginger may improve oral health and be beneficial in the management of several oral diseases because of its several properties, such as antioxidant, anti-inflammatory antimicrobial, antibacterial, and antifungal.42 Similar to this study, it was reported that gingerol-related components may prevent the growth of oral bacteria related to CP in the human oral cavity.40 One clinical trial study indicated that NSPT for 3 months could significantly reduce periodontal status in patients with T2DM and CP.43 The different types of treatment and the duration of the intervention may be explained as the reasons for the different findings. Evidence suggests that periodontal therapy may decrease the bacterial community and inflammatory cytokines and increases the levels of anti-inflammatory cytokines and also improves glycemic control in T2DM patients with CP.43 Using only two groups in this study would be a limitation. The inclusion of four study groups in future studies (group 1; diabetes+no periodontal treatment+placebo, group 2; diabetes+no periodontal treatment+ginger, group 3; diabetes+NSPT+placebo, group 4; diabetes+NSPT+ginger) is suggested.
Conclusion
Ginger supplementation along with NSPT decreased oxidative stress and improved the inflammatory and periodontal status and increased serum levels of antioxidant enzymes. Therefore, it is suggested that ginger supplementation along with NSPT may be more effective in the control of systemic inflammation in T2DM patients with CP.
Acknowledgments
The authors express thanks to the Nutrition and Metabolic Disorders Research Center, and Research Center for Diabetes, Endocrinology and Metabolism clinic employees of Imam Khomeini Hospital and Dental Clinic of Ahvaz Jundishapur University of Medical Sciences. This research project was sponsored by the Vice-Chancellor for Research Affairs of Ahvaz Jundishapur University of Medical Sciences (NRC-9631).
Compliance with ethical standards
This study was done according to the guidelines of the Helsinki Declaration and all procedures involving human patients were approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Ethical Code. IR.AJUMS.REC.1396.925). Written informed consent was obtained from all patients before initiating the study.
Data sharing statement
The datasets are not publicly available because of lack of agreement for disclosing individual raw data in public but are available from the corresponding author on reasonable request.
Abbreviations
DM, diabetes mellitus; CP, chronic periodontitis; TNF-α, tumor necrosis factor-alpha; hs-CRP, hs-C-reactive protein; SOD, superoxide dismutase; CAT, catalase; GPx, glutathione peroxidase; GST, glutathione S-transferase; GPx, glutathione peroxidase; GR, glutathione reductase; CAL, clinical attachment loss; BOP, bleeding on probing; PD, pocket depth; NSPT, non-surgical periodontal therapy; WC, waist circumference; HC, hip circumference; WHR, waist to hip ratio.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dahiya P, Kamal R, Gupta R, Bhardwaj R, Chaudhary K, Kaur S. Reactive oxygen species in periodontitis. J Indian Soc Periodontol. 2013;17(4):411. doi: 10.4103/0972-124X.118306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babaei H, Forouzandeh F, Maghsoumi-Norouzabad L, Yousefimanesh HA, Ravanbakhsh M, Zare Javid A. Effects of chicory leaf extract on serum oxidative stress markers, lipid profile and periodontal status in patients with chronic periodontitis. J Am Coll Nutr. 2018;37(6):479-86. [DOI] [PubMed] [Google Scholar]
- 3.Covani U, Marconcini S, Derchi G, Barone A, Giacomelli L. Relationship between human periodontitis and type 2 diabetes at a genomic level: a data-mining study. J Periodontol. 2009;80(8):1265–1273. [DOI] [PubMed] [Google Scholar]
- 4.Gursoy UK, Marakoglu I, Oztop AY. Relationship between neutrophil functions and severity of periodontitis in obese and/or type 2 diabetic chronic periodontitis patients. Quintessence Int (Berl). 2008;39:6. [PubMed] [Google Scholar]
- 5.Xiao L, Yan Y, Xie C, et al. Association among interleukin‐6 gene polymorphism, diabetes and periodontitis in a Chinese population. Oral Dis. 2009;15(8):547–553. [DOI] [PubMed] [Google Scholar]
- 6.Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc. 1990;121(4):532–536. [DOI] [PubMed] [Google Scholar]
- 7.Ide R, Hoshuyama T, Wilson D, Takahashi K, Higashi T. Periodontal disease and incident diabetes: a seven-year study. J Dent Res. 2011;90(1):41–46. [DOI] [PubMed] [Google Scholar]
- 8.Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28(1):27–32. [DOI] [PubMed] [Google Scholar]
- 9.Mealey BL. Periodontal disease and diabetes: A two-way street. J Am Dent Assoc. 2006;137:S26–S31. [DOI] [PubMed] [Google Scholar]
- 10.Manouchehr-Pour M, Spagnuolo P, Rodman H, Bissada N. Comparison of neutrophil chemotactic response in diabetic patients with mild and severe periodontal disease. J Periodontol. 1981;52(8):410–415. doi: 10.1902/jop.1981.52.8.410 [DOI] [PubMed] [Google Scholar]
- 11.Zare Javid A, Maghsoumi-Norouzabad L, Ashrafzadeh E, et al. Impact of cranberry juice enriched with omega-3 fatty acids adjunct with nonsurgical periodontal treatment on metabolic control and periodontal status in type 2 patients with diabetes with periodontal disease. J Am Coll Nutr. 2018;37(1):71–79. doi: 10.1080/07315724.2017.1357509 [DOI] [PubMed] [Google Scholar]
- 12.Bissett S, Pumerantz A, Preshaw P. Periodontal disease and diabetes. J Diabetes Nurs. 2015;19:134–140. [Google Scholar]
- 13.Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medicinal properties of food: a review. J Food Sci Technol. 2015;52(5):2522–2529. doi: 10.1007/s13197-014-1396-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shidfar F, Rajab A, Rahideh T, Khandouzi N, Hosseini S, Shidfar S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J Evid Based Complementary Altern Med. 2015;12(2):165–170. doi: 10.1515/jcim-2014-0021 [DOI] [PubMed] [Google Scholar]
- 15.Srinivasan KJP. Ginger rhizomes (Zingiber officinale): A spice with multiple health beneficial potentials. PharmaNutrition. 2017;5(1):18–28. [Google Scholar]
- 16.Amorndoljai P, Taneepanichskul S, Niempoog S, Nimmannit U. A comparative of ginger extract in Nanostructure Lipid Carrier (NLC) and 1% diclofenac gel for treatment of knee osteoarthritis (OA). J Med Assoc Thai. 2017;100(4):447–456. [PubMed] [Google Scholar]
- 17.Pourmasoumi M, Hadi A, Rafie N, Najafgholizadeh A, Mohammadi H, Rouhani MH. The effect of ginger supplementation on lipid profile: A systematic review and meta-analysis of clinical trials. Phytomedicine. 2018;43:28–36. doi: 10.1016/j.phymed.2018.03.043 [DOI] [PubMed] [Google Scholar]
- 18.Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr. 2014;65(4):515–520. doi: 10.3109/09637486.2014.880671 [DOI] [PubMed] [Google Scholar]
- 19.Mazidi M, Gao HK, Rezaie P, Ferns GA. The effect of ginger supplementation on serum C-reactive protein, lipid profile and glycaemia: a systematic review and meta-analysis. Food Nutr Res. 2016;60:32613. doi: 10.3402/fnr.v60.32613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanmugam KR, Mallikarjuna K, Nishanth K, Kuo CH, Reddy KS. Protective effect of dietary ginger on antioxidant enzymes and oxidative damage in experimental diabetic rat tissues. Food Chem. 2011;124(4):1436–1442. doi: 10.1016/j.foodchem.2010.07.104 [DOI] [Google Scholar]
- 21.Aryal S, Pradhan A, Shrestha SM, et al. Does improved periodontal health affect metabolic and inflammatory markers in patients with diabetes mellitus? A comparative study. J Nep Soc Perio Oral Implantol. 2017;1:(2):6. [Google Scholar]
- 22.Duarte PM, Da Rocha M, Sampaio E, et al. Serum levels of cytokines in subjects with generalized chronic and aggressive periodontitis before and after non-surgical periodontal therapy: a pilot study. J Periodontol. 2010;81(7):1056–1063. [DOI] [PubMed] [Google Scholar]
- 23.Alberti KGMM, Zimmet P. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Med. 1998;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 24.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4(1):1–6. [DOI] [PubMed] [Google Scholar]
- 25.Zare Javid A, Seal C, Heasman P, Moynihan P. Impact of a customised dietary intervention on antioxidant status, dietary intakes and periodontal indices in patients with adult periodontitis. J Hum Nutr Diet. 2014;27(6):523–532. [DOI] [PubMed] [Google Scholar]
- 26.Danwilai K, Konmun J, Sripanidkulchai B-O, Subongkot S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: a pilot study. Cancer Manag Res. 2017;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nwozo SO, Osunmadewa DA, Oyinloye BE. Anti-fatty liver effects of oils from Zingiber officinale and Curcuma longa on ethanol-induced fatty liver in rats. J Integr Med. 2014;12(1):59–65. [DOI] [PubMed] [Google Scholar]
- 28.Deol PK, Khare P, Bishnoi M, Kondepudi KK, Kaur IP. Coadministration of ginger extract–Lactobacillus acidophilus (cobiotic) reduces gut inflammation and oxidative stress via downregulation of COX‐2, i‐NOS, and c‐Myc. Phytother Res. 2018;32(10):1950–1956. [DOI] [PubMed] [Google Scholar]
- 29.Rehman MU, Rashid SM, Rasool S, et al. Zingerone (4-(4-hydroxy-3-methylphenyl) butan-2-one) ameliorates renal function via controlling oxidative burst and inflammation in experimental diabetic nephropathy. Arch Physiol Biochem.2019;125(3):201–209. [DOI] [PubMed] [Google Scholar]
- 30.Mahluji S, Ostadrahimi A, Mobasseri M, Attari VE, Payahoo L. Anti-inflammatory effects of Zingiber officinale in type 2 diabetic patients. Adv Pharm Bull. 2013;3(2):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang F, Ma N, Gao YF, Sun LL, Zhang JG. Therapeutic effects of 6‐gingerol, 8‐gingerol, and 10‐gingerol on dextran sulfate sodium‐induced acute ulcerative colitis in rats. Phytother Res. 2017;31(9):1427–1432. [DOI] [PubMed] [Google Scholar]
- 32.Torumtay G, Kırzıoğlu F, Öztürk Tonguç M, Kale B, Calapoğlu M, Orhan H. Effects of periodontal treatment on inflammation and oxidative stress markers in patients with metabolic syndrome. J Periodontal Res. 2016;51(4):489–498. [DOI] [PubMed] [Google Scholar]
- 33.Marella S, Reddy KS. Ginger extract defies changes in brain serotonin levels and enzymes of monoamine metabolism during withdrawal following chronic ethanol ingestion. Global J Biotechnol Biochem. 2012;7(4):115–124. [Google Scholar]
- 34.Muller W, Fiebich BL, Stratz T. New treatment options using 5-HT3 receptor antagonists in rheumatic diseases. Curr Top Med Chem. 2006;6(18):2035–2042. [DOI] [PubMed] [Google Scholar]
- 35.Pendyala G, Thomas B, Joshi SR. Evaluation of total antioxidant capacity of saliva in type 2 diabetic patients with and without periodontal disease: A case-control study. N Am J Med Sci. 2013;5(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abolaji AO, Kamdem JP, Lugokenski TH, et al. Ovotoxicants 4-vinylcyclohexene 1, 2-monoepoxide and 4-vinylcyclohexene diepoxide disrupt redox status and modify different electrophile sensitive target enzymes and genes in Drosophila melanogaster. Redox Biol. 2015;5:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shanmugam KR, Mallikarjuna K, Kesireddy N, Reddy KS. Neuroprotective effect of ginger on anti-oxidant enzymes in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49(4):893–897. [DOI] [PubMed] [Google Scholar]
- 38.Al Hroob AM, Abukhalil MH, Alghonmeen RD, Mahmoud AM. Ginger alleviates hyperglycemia-induced oxidative stress, inflammation and apoptosis and protects rats against diabetic nephropathy. Biomed Pharmacother. 2018;106:381–389. [DOI] [PubMed] [Google Scholar]
- 39.Aryaeian N, Tavakkoli H. Ginger and its effects on inflammatory diseases. Adv Food Technol Nutr Sci Open J. 2015;1(4):97–101. [Google Scholar]
- 40.Park M, Bae J, Lee DS. Antibacterial activity of [10]‐gingerol and [12]‐gingerol isolated from ginger rhizome against periodontal bacteria. Phytother Res. 2008;22(11):1446–1449. [DOI] [PubMed] [Google Scholar]
- 41.Milovanova-Palmer J, Pendry B. Is there a role for herbal medicine in the treatment and management of periodontal disease? J Herb Med. 2018;12:33–48. [Google Scholar]
- 42.Rashmi K, Tiwari R. Pharmacotherapeutic properties of ginger and its use in diseases of the oral cavity: A narrative review. J Adv Oral Res. 2016;7:2. [Google Scholar]
- 43.Cosgarea R, Heumann C, Juncar R, et al. One year results of a randomized controlled clinical study evaluating the effects of non-surgical periodontal therapy of chronic periodontitis in conjunction with three or seven days systemic administration of amoxicillin/metronidazole. PLoS One. 2017;12(6):e0179592. [DOI] [PMC free article] [PubMed] [Google Scholar]