Abstract
Dysfunction of the pulmonary endothelium is associated with most lung diseases. Extracellular nucleotides modulate a plethora of endothelial functions in the lung such as vessel integrity, vasodilatation, inflammatory, and thrombotic responses as well as survival and DNA repair, mostly via Ca2+ signaling pathways. However, a comprehensive analysis of the molecular components of the underlying P2 receptor-mediated Ca2+ signaling pathways in the lung has not been conducted so far. Therefore, our aim was to identify the principal P2 receptor Ca2+ signalosome in the human pulmonary endothelium and investigate potential dysregulation in pulmonary vascular disease. Comparative transcriptomics and quantitative immunohistochemistry were performed on publicly available RNA sequencing and protein datasets to identify the specific expression profile of the P2-receptor Ca2+ signalosome in the healthy human pulmonary endothelium and endothelial cells (EC) dysfunctional due to loss of or defective bone morphogenetic protein receptor (BMPR2). Functional expression of signalosome components was tested by single cell Ca2+ imaging. Comparative transcriptome analysis of 11 endothelial cell subtypes revealed a specific P2 receptor Ca2+ signalosome signature for the pulmonary endothelium. Pulmonary endothelial expression of the most abundantly expressed Ca2+ toolkit genes CALM1, CALM2, VDAC1, and GNAS was confirmed by immunohistochemistry (IHC). P2RX1, P2RX4, P2RY6, and P2YR11 showed strong lung endothelial staining by IHC, P2X5, and P2Y1 were found to a much lesser extent. Very weak or no signals were detected for all other P2 receptors. Stimulation of human pulmonary artery (HPA) EC by purine nucleotides ATP, ADP, and AMP led to robust intracellular Ca2+ signals mediated through both P2X and P2Y receptors. Pyrimidine UTP and UDP-mediated Ca2+ signals were generated almost exclusively by activation of P2Y receptors. HPAEC made dysfunctional by siRNA-mediated BMPR2 depletion showed downregulation of 18 and upregulation of 19 P2 receptor Ca2+ signalosome genes including PLCD4, which was found to be upregulated in iPSC-EC from BMPR2-mutant patients with pulmonary arterial hypertension. In conclusion, the human pulmonary endothelium expresses a distinct functional subset of the P2 receptor Ca2+ signalosome. Composition of the P2 receptor Ca2+ toolkit in the pulmonary endothelium is susceptible to genetic disturbances likely contributing to an unfavorable pulmonary disease phenotype found in pulmonary arterial hypertension.
Electronic supplementary material
The online version of this article (10.1007/s11302-019-09674-1) contains supplementary material, which is available to authorized users.
Keywords: ATP, UTP, CD39, Endothelial dysfunction, Purinoceptor, Cardiovascular disease, Lung, Remodeling, Proliferation
Introduction
Endothelial cells (EC) form the contiguous, interconnected luminal interface that completely lines the vasculature of the mammalian circulatory system. The main purpose of the endothelial monolayer in healthy blood vessels is to adaptively maintain vessel integrity by promoting anti-inflammatory and anti-thrombogenic propensities and regulating vascular tone, permeability, structure, and function according to physiological requirements (reviewed in: [1, 2]).
The endothelium of the pulmonary vasculature, a unique high-flow, low-resistance, low-pressure system, is involved in facilitating gas exchange but also maintaining vascular tone; controlling barrier function; regulating inflammatory response and anti-thrombogenic propensities; and preserving vascular-tissue homeostasis [3].
Thus, dysfunction of the pulmonary endothelium is associated with a broad variety of respiratory diseases, ranging from acute (e.g., pulmonary edema or acute respiratory distress syndrome, ARDS) [4] to chronic conditions such as chronic obstructive pulmonary disease (COPD) [5] and pulmonary arterial (PA) hypertension (PAH, [6]).
Extracellular nucleotides such as purines adenosine-5′-triphosphate (ATP), adenosine diphosphate (ADP), and adenosine monophosphate (AMP) as well as pyrimidines uridine-5′-triphosphate (UTP) and uridine-diphosphate (UDP) are released within the pulmonary vascular bed upon stress such as hypoxia or shear stress and mediate vasodilatatory, inflammatory, and thrombotic responses and EC survival [7–11].These nucleotides activate distinct subsets of P2 receptors on the plasma membrane, P2X, and P2Y receptors. P2X receptors (P2RX) are ion channels that increase intracellular Ca2+ concentrations via direct inward gating of extracellular Ca2+. P2Y receptors (P2RY) are G protein-coupled receptors that activate a intracellular phospholipase C (PLC)-IP3 cascade causing Ca2+ release from intracellular stores into the cytosol [12].
Although extracellular nucleotides and P2 receptor-mediated Ca2+ signaling play such pivotal roles in the regulation of the pulmonary ECs, no comprehensive analysis of the underlying Ca2+ signalosome has been conducted in the human pulmonary endothelium.
We, therefore, aimed to identify the principal P2 receptor Ca2+ signalosome in the human pulmonary endothelium and investigate potential dysregulation in pulmonary vascular disease.
In this regard, we performed comparative transcriptomics and quantitative immunohistochemistry on publicly available RNA-seq and protein datasets to identify the specific expression profile of the P2-receptor Ca2+ signalosome in the human pulmonary endothelium. Functional expression of the Ca2+ signalosome components was confirmed using cytosolic Ca2+ imaging upon purine and pyrimidine stimulation.
Recent data suggest that dysregulated nucleotide signaling has been linked to pulmonary arterial (PA) hypertension (PAH) [13, 14], a life-threatening condition, characterized by endothelial dysfunction of the pulmonary vasculature, occlusion of pulmonary arteries, and consecutive right heart failure [15–17].
The most important underlying genetic causes for PAH are mutations of the BMPR2 gene [18]. Dysregulated bone morphogenetic receptor type 2 (BMPR2) signaling is associated with PA EC and pulmonary microvascular (PMV) EC apoptosis and pathological PA remodeling [6] whereas selective activation of BMPR2 reverses PAEC dysfunction and experimental pulmonary hypertension [19].
To test a putative link between endothelial dysfunction and to identify a putative novel therapeutic target structure, we investigated BMPR2-dependency of the P2-receptor Ca2+ signalosome by using datasets of BMPR2-depleted PAEC by siRNA or iPSC-EC from BMPR2-mutant PAH patients.
Material and methods
Comparative transcriptomics
An Encyclopedia of DNA Elements (ENCODE, http://www.encodeproject.org) search was conducted for RNA-Sequencing (RNA-Seq) data of primary human endothelial cells from various organs including pulmonary artery and pulmonary microvasculature to identify P2 receptor Ca2+signalosome signatures in endothelial cells.
Twenty human (H) RNA-Seq datasets performed on a HiSeq 2000 were identified and downloaded using the Gene Expression Omnibus (GEO) website (http://www.ncbi.nlm.nih.gov/gds). mRNA expression values of P2 receptors and Ca2+ signalosome genes (based on KEGG pathway 04020, http://www.kegg.jp, see Suppl. Table 1 for details) were extracted as Transcripts per Million RNA samples (TPM) and used for clustering and correlation analysis. For correlation matrix analysis of endothelial RNA expression signatures, hierarchical clustering was applied after Pearson’s product-moment correlation using Gplot and Corrplot libraries from the Bioconductor repository (http://www.bioconductor.org) in R (version 3.3.3, http://www.r-project.org) and R Studio Desktop. Using Graphpad Prism 7 (Graphpad Inc., San Diego, CA), linear regression was performed for correlation analysis of pulmonary endothelial cells transcript expression. Transcripts with mean TPM > 0 were considered as “expressed.” A detailed list of the RNA-Seq datasets used and links to detailed protocol descriptions per dataset can be found in Suppl. Table 2.
In order to identify BMPR2-dependent Ca2+ toolkit genes, an additional GEO search was conducted for human gene array datasets of HPAEC depleted from BMPR2 by siRNA and RNA-Seq of ECs from PAH patients with BMPR2 mutations.
Datasets GSE70456 and GSE79613 were identified. GSE70456 contains RNA expression data from 16 siRNA-transfected HPAEC samples in quadruplicates hybridized to the Affymetrix PrimeView Human Gene Expression Array (GPL15207). PBS-treated replicates transfected with scrambled control siRNA (n = 4) and BMPR2 siRNA (n = 4) were isolated. Normalized gene expression values and ranks were extracted and pre-analyzed using the GEO Dataset Browser data analysis online tools. Differentially expressed genes were quantified locally using Biobase, GEOquery, and limma libraries in R. GSE79613 contains mRNA expression profiles by RNA-Seq from ECs differentiated from induced pluripotentent stem cells (iPSC-ECs) of healthy donors (n = 3) and PAH patients with BMPR2 mutation (n = 5). HTSeq-derived expression values (counts) were extracted and differentially expressed genes were analyzed using DESeq2 in R.
Immunohistochemical analysis
Lung tissue micro array microphotographs of the most abundantly expressed Ca2+ signalosome genes and all known human P2 receptors were located in a publicly available database and downloaded for expression analysis in the pulmonary arterial endothelium in a blinded fashion.
Semi-quantitative immunohistochemistry (IHC) of two to six different TMA cores was performed using a 4-step intensity score (negative = 0, weak = 1+, intermediate = 2+, strong = 3+) as previously described [20]. In order receive reliable protein expression data, based on this 4-step scale, genes with a mean IHC intensity score > = 1 were defined as reliably expressed indicating an intensity score of at least > = 1 for every individual TMA spot. Data for P2RX1 immunohistochemistry was derived from [13] as IHC data for P2RX1 was not available in the Human ProteinAtlas database.
Baseline demographics of donors included and detailed immunohistochemical information regarding antibodies used, Human ProteinAtlas ID, and antigen retrieval method can be found in Suppl. Table 3.
Single-cell Ca2+ imaging
Ca2+ imaging was performed as previously described [21–23]. In brief, 20,000–30,000 healthy donor HPAEC (passage < 10, purchased from PromoCell, Heidelberg, Germany) were seeded onto 0.1% Gelatin-coated glass coverslips and cultured in full EC growth medium (ECGM with 5% FCS, ECGS, PromoCell) for 36 h. Prior to imaging, cells were loaded with 5 μM of the FURA-2/AM Ca2+ dye (Molecular Probes, Leiden, The Netherlands) in Ca2+ containing extracellular solution (ECS). For intracellular Ca2+ depletion, HPAEC were co-treated with thapsigargin, a non-competitive inhibitor of the sarcoplasmatic/endoplasmatic reticulum Ca2+-ATPases (SERCAs), and the phospholipase C inhibitor U73122 at final concentrations of 1 μM and 2 μM, respectively.
For fluorescent Ca2+ imaging, the coverslips were mounted in an imaging/perfusion chamber on a conventional epifluorescence microscope (Olympus, Hamburg, Germany). The imaging chamber was perfused continuously with warm (37 °C) ECS buffer ± 1.8 mM CaCl2. Extracellular nucleotides were added at 100 μM final concentration after 5–10 min of steady baseline recordings. Fluorescence changes upon stimulation were continuously recorded at 3 s intervals and analyzed using MetaFlour Software (Molecular Devices, Downington, PA). Peak fluorescence of 45 to 119 responsive cells from at least three independent experiments per condition was calculated and used for statistical analysis using Prism 7 (GraphPad Inc., San Diego, CA).
Network analysis
Protein-protein interactome networks for siBMPR2-dependent genes were constructed using the NetworkAnalyst software package [24]. Protein-protein interaction network analysis was performed using data from the STRING interactome database [25] with a confidence score cutoff at 900 and experimental evidence required. This approach identified three subnetworks for downregulated genes (#1: 282 nodes and 447 edges, #2: 4 nodes and 3 edges, #3: 3 nodes and 4 edges) and two subnetworks for upregulated genes (#1: 372 nodes and 487 edges, #2: 6 nodes and 5 edges) including a PLCD4 subnetwork. Visualizations were performed using CytoScape 3.5.0 (http://www.cytoscape.org).
Results
Comparative RNA-seq transcriptomics reveal a distinct Ca2+ signalosome signature for the pulmonary endothelium
As the pulmonary endothelium is functionally distinct from endothelia of other organs [3], we aimed to identify P2 receptor Ca2+ signalosome expression signatures specific for the human pulmonary endothelium by comparative transcriptomics using publicly available ENCODE datasets.
Expression signatures of P2-receptor-mediated Ca2+ signalosome toolkit components (derived from KEGG, see Suppl. Table 1) were derived from RNA-Seq data of 11 different human (H) endothelial cell (EC) species. These included pulmonary arterial EC (HPAEC) and pulmonary microvascular EC (HPMVEC) as well as saphenous vein EC (HSaVEC), dermis microvascular EC (HMVEC), mammary microvascular EC (HMMEC), endometrial microvascular EC (HEMEC), dermis lymphatic EC (HDLEC), coronary artery EC (HCAEC), bladder microvascular EC (HBdMEC), thoracic aorta EC (HAoEC), and embryonic glomerular EC (HEGEC) (Fig. 1a). Individual expression values for all investigated Ca2+ signalosome genes are given in Suppl. Table 4.
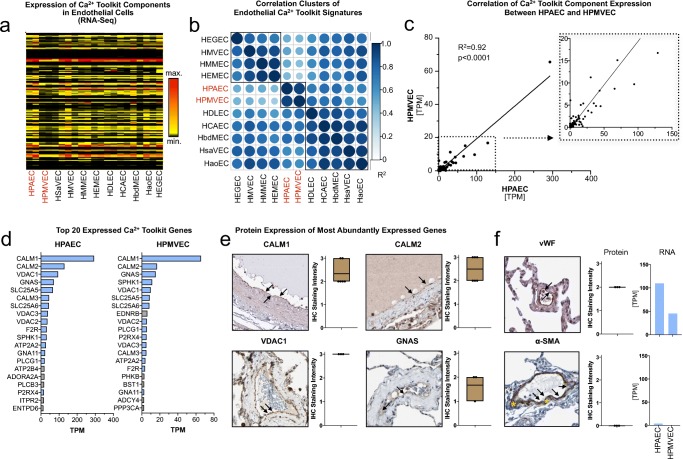
Fig. 1.
Comparative transcriptomics reveal a distinct Ca2+ signalosome signature for the human pulmonary endothelium. a Expression heatmap of P2 receptor Ca2+ toolkit components in human endothelial cells of various origins based on ENCODE RNA-seq data. b Correlation clusters of endothelial P2 receptor Ca2+ signalosome signatures. Pulmonary endothelial species are labeled in red. c Linear regression analysis of Ca2+ signalosome components comparing expression values (TPM, transcripts per million) of human pulmonary artery endothelial cells (HPAEC) and human pulmonary microvascular endothelial cells (HPMVEC). Dotted area = magnification of lower expression values. d Comparison of most frequently expressed genes in HPAEC and HPMVEC. Common genes between both cell species are shown in blue, different genes in gray. e Microphotographs and quantifications of immunohistochemical (IHC) staining of the most abundantly expressed Ca2+ signalosome genes in the pulmonary endothelium, CALM1 (calmodulin 1), CALM2 (calmodulin 2), VDAC1 (voltage-dependent anion-selective channel 1), and the stimulatory G protein alpha subunit (GNAS). f Endothelial staining (microphotograph + quantification) and RNA expression of von-Willebrand-Factor (vWF) as a positive control. g Lack of endothelial IHC staining and RNA expression for smooth muscle (yellow asterisk) marker gene ACTA2 (smooth muscle actin, a-SMA), used as a negative control. Full names for abbreviated EC species are given in Suppl. Table 1
Correlation clustering of endothelial Ca2+ toolkit component expression revealed a distinct signature for pulmonary ECs (Fig. 1b). mRNA expression of Ca2+ toolkit components was strongly correlated between HPAEC and HPMVEC in a linear fashion (R2 = 0.92, Fig. 1c).
In fact, 15 out of the 20 most abundantly expressed genes were identical between HPAEC and HPMVEC (Fig. 1d, for full list see Suppl. Table 4).
A detailed view of immunohistochemical analysis of the top 4 expressed genes in the pulmonary endothelial showed medium to strong positivity in the pulmonary endothelium, indicating robust expression also on the protein level in tissue microarray samples of healthy donors from publicly available datasets (Fig. 1e).
The well-established endothelial marker von-Willebrand-Factor (vWF [6]) served as a positive control. vWF was robustly expressed in HPAEC and HPMVEC alike based on RNA-Seq data. On the protein level, immunohistochemical staining indicated strong positivity exclusively within the endothelium of pulmonary blood vessels. Smooth muscle marker gene ACTA2 (smooth muscle actin, a-SMA), used as a negative control, showed low mRNA abundance in HPAEC and HPMVEC. On the protein level, a-SMA staining was limited to the media of pulmonary arteries without staining of the of endothelial cell layer (Fig. 1f).
P2 receptor protein expression in the pulmonary endothelium
In order to comprehensively identify the subset of P2 receptors expressed in the human pulmonary arterial and microvascular endothelium, we utilized publicly available immunohistochemistry datasets from The Human Protein Atlas. Using a 4-step intensity score (negative = 0, weak = 1+, intermediate = 2+, strong = 3+, [20]), protein expression was quantified across all available TMA cores from healthy donors for all previously described human ionotropic P2X and metabotropic P2Y receptors [8].
Only P2RX1, P2RX4, P2RY6, and P2RY11 were expressed with a mean score > = 1.0 in the endothelium of pulmonary arteries. P2RX5 and P2RY1 were found to a much lesser extent (mean score > 0.5 and < 1.0). No or very weak signals (mean score < 0.5) were detected for all other P2 receptors (Fig. 2a–c). For P2X2, no interpretable protein data was available.
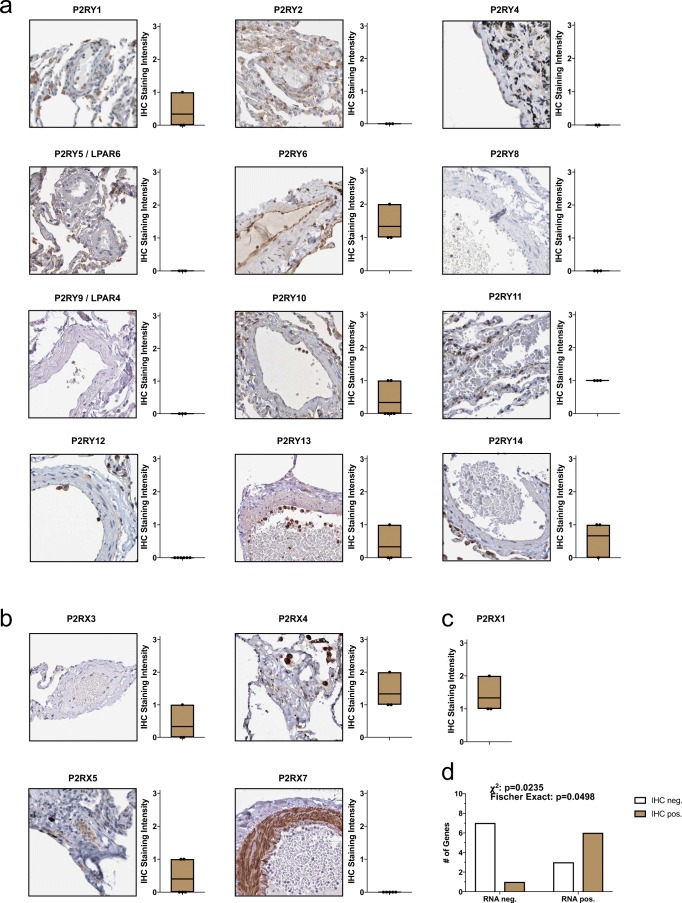
Fig. 2.
P2 receptor protein expression in the pulmonary endothelium. Immunohistochemistry microphotographs and quantifications of metabotropic P2Y receptors (a) and ionotropic P2X receptors (b) in the pulmonary endothelium. P2RX1 data was quantified based on data extracted from [13] as IHC data was unavailable from the Human Protein Atlas database (c). Mean RNA expression values HPAEC and HPMVEC (in TPM) significantly correlate with lung endothelial P2 receptor staining (p < 0.05, chi-square, and Fisher’s exact tests) (d)
In general, protein expression of P2 receptors in the PA endothelium was detectable where mRNA expression for the same genes (based on ENCODE RNA-seq data) were found (Fig. 2d). However, no linear correlation between IHC staining intensity and mRNA transcript abundance was noticed.
Functional expression of the P2 receptor Ca2+ signalosome in human PAEC
Next, we aimed to test the functional significance of the P2 receptor Ca2+ signalosome in the human pulmonary endothelium. Therefore, single-cell fluorescence imaging of intracellular Ca2+ currents was performed in HPAEC after stimulation with agonistic extracellular purine and pyrimidine nucleotides, which, based on previously published ligand affinity data [26], bind to specific subsets of P2Y and P2X receptors as native agonists to induce intracellular Ca2+ increases.
Application of ATP (100 μM) induced a robust increase of the cytosolic Ca2+ concentration [Ca2+] under physiological extracellular ion concentrations (Fig. 3a). Depending on their phosphorylation state purinergic adenosine nucleotides, AMP, ADP, and ATP differentially stimulate P2 receptors at varying degrees [22].
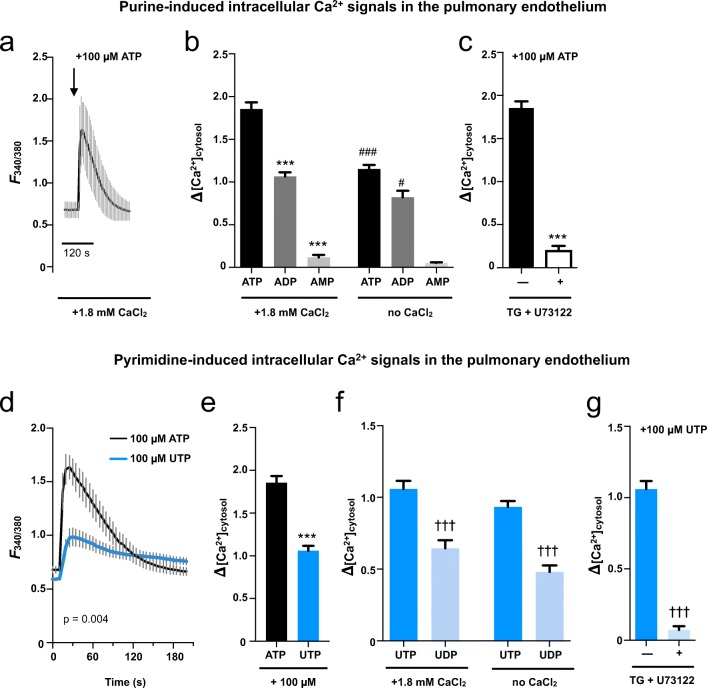
Fig. 3.
Functional expression of the P2-receptor Ca2+ signalosome in human pulmonary artery endothelial cells. Purine- (a–c) and pyrimidine-induced (d–g) intracellular Ca2+ signals in HPAEC measured by single cell FURA-2 fluorescence. a Representative fluorescence tracings of ATP treated HPAEC are given (n = 9). b Quantification of peak purine-induced cytosolic Ca2+ signals upon stimulation with 100 μM ATP (black bar), ADP (dark gray bar), and AMP (light gray bar) under physiological extracellular Ca2+ concentrations (left bars) or in a Ca2+ free extracellular buffer (right bars). c Peak fluorescence of HPAEC upon stimulation with 100 μM ATP without (black bar) or after depletion of intracellular Ca2+ stores by combined use of thapsigargin (TG) and PLC inhibitor U73122 (open bar). d Representative tracings of ATP treated (n = 9) vs. UTP-treated HPAEC (n = 12) under physiological extracellular Ca2+ conditions (p = 0.004, Welch’s t test). e Quantification of peak Ca2+ responses to ATP and UTP (100 μM each) under physiological extracellular Ca2+ (ECS + Ca2+). f Quantification of peak pyrimidine-induced cytosolic Ca2+ signals upon stimulation with 100 μM UTP (dark blue bar) and UDP (light blue bar) under physiological extracellular Ca2+ concentrations (left bars) or in a Ca2+-free extracellular buffer (right bars). g Peak fluorescence of HPAEC upon stimulation with 100 μM UTP without (dark blue bar) or after depletion of intracellular Ca2+ stores by combined use of thapsigargin (TG) and PLC inhibitor U73122 (light blue bar). *** = p < 0.0001 vs. ATP; ###,# = p < 0.0001; p < 0.05 vs. corresponding ECS + Ca2+; ††† = p < 0.0001 vs. corresponding UTP; (b) + (f): ANOVA with Bonferroni post-hoc test (c–e) + (g) Welch’s t test
In HPAEC, ATP induced a 190% increase in the cytosolic [Ca2+], followed by ADP (110%) and AMP (12%, 100 μM each, Fig. 3b, left). Purinergic Ca2+ signals were partially dependent on the presence of extracellular Ca2+. Depletion of extracellular Ca2+ from the assay buffer significantly reduced the amplitudes of cytosolic Ca2+ increase for ATP (115.3 vs. 185.5%) and ADP (82.2 vs. 106.5%, Fig. 3b, right). For AMP, a trend towards an attenuated Ca2+ response could be detected which did not reach statistical significance (5.3% vs. 11.7%, Fig. 3b, right). This indicates activation of metabotropic P2Y receptor and ionotropic P2X receptors alike after stimulation of HPAEC. This was further corroborated by the substantially impaired cytosolic Ca2+ response to ATP after pharmacological inhibition of PLC activation by U73122 in combination with depletion of intracellular Ca2+ stores by thapsigargin (TG, 20.3 vs. 185.5%, Fig. 3c) under physiological extracellular [Ca2+].
To investigate differences between the purinergic and the pyrimidinergic Ca2+ response in HPAEC, we performed cytosolic Ca2+ imaging for HPAEC treated with pyrimidines UTP and UDP.
Under physiological extracellular [Ca2+] stimulation, UTP caused a solid yet less pronounced induction of the cytosolic [Ca2+] compared to the purine ATP (Fig. 3d, e). Comparable to the effect of purines on HPAEC, UDP induced a significantly smaller cytosolic Ca2+ increase than UTP (64.6 vs. 105.6%, Fig. 3f, left).
Extracellular Ca2+ did not significantly affect pyrimidine-induced cytosolic Ca2+ signals in HPAEC. Cytosolic Ca2+ amplitudes for UTP and UDP only marginally changed upon withdrawal of extracellular Ca2+ (UTP 93.3 vs. 105.6%, UDP 64.9 vs. 40.0%, p > 0.05; Fig. 3f, left and right). This was in line with a virtually abolished Ca2+ response to UTP after combined U73122/TG pre-treatment (7.3 vs. 105.9%) under physiological extracellular [Ca2+] indicating (almost) exclusive activation of the metabotropic P2YR-phospholipase signaling cascade upon pyrimidine-stimulation of HPAEC.
BMPR2-dependency of the P2-receptor Ca2+ signalosome in human PAEC and BMPR2-mutant iPSC-EC
In order to elucidate a potential role of impaired P2-mediated Ca2+signaling for pulmonary vascular disease, we investigated BMPR2-dependency of the P2R-Ca2+ toolkit components in HPAEC.
Therefore, we used targeted transcriptomics on HPAEC depleted from BMPR2 by siRNA and iPSC-derived ECs from PAH patients with BMPR2 mutations using publicly available RNA microarray and RNA-Seq datasets to identify endothelial signalosome genes that are differentially expressed (DEG) in a BMPR2-dependent manner.
In the first dataset, commercially available HPAEC from healthy donors were successfully depleted from BMPR2 by siRNA (Fig. 4a). Targeted analysis of the P2R-Ca2+ signalosome revealed a subset of DEG which were dependent on BMPR2 (Fig. 4b). A total of 18 signalosome genes were downregulated by loss of BMPR2 (CALM1, ITPKB, PLCB1, ATP2B4, CACNA1A, MYLK, PLCG1, ADCY9, PHKB, ITPR1, GNA14, STIM2, PLCG2, GNAQ, ENTPD4, PLCB4, PRKX, and GNAS), and 19 genes were significantly upregulated by BMPR2 siRNA (ADORA2B, PRKCA, EGFR, ENTPD7, NOS3, CAMK2D, BST1, P2RX4, ENTPD6, ENTPD1, CAMK2G, SPHK1, PLCD4, PRKACB, PLN, ADCY3, EDNRB, ERBB2, and VDAC3) (Fig. 4c).
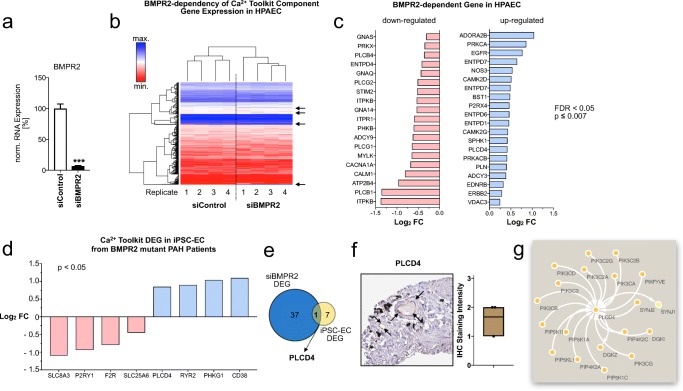
Fig. 4.
BMPR2-dependency of the P2-receptor Ca2+ Signalosome in human PAEC and BMPR2-mutant iPSC-EC. a Effective knockdown of BMPR2 by siRNA in HPAEC dataset GSE70456. b Heatmap of differentially expressed P2 receptor Ca2+ signalosome genes in HPAEC transfected with BMPR2 siRNA (siBMPR2) vs. scrambled control siRNA (siControl). Arrows indicate differentially regulated genes. c Log2 fold changes (Log2 FC) of P2 receptor Ca2+ signalosome genes significantly downregulated (red) or upregulated (blue) by siBMPR2 in HPAEC (vs. siControl, p ≤ 0.007, FDR < 0.05, Benjamini-Hochberg test). d Significantly down- (red) or upregulated (blue) P2 receptor Ca2+ signalosome genes in iPSC-EC derived from PAH patients harboring BMPR2 mutations (p < 0.05, Wald test, GSE79613) e Venn diagram of differentially expressed Ca2+ signalosome genes (DEG) by loss of BMPR2 via knockdown in HPAEC (siBMPR2) or somatic mutation (iPSC-EC). f Microphotograph of PLCD4 immunohistochemistry in the human pulmonary endothelium and quantification. g. PLCD4 subnetwork of protein-protein interaction network analysis
Next, we examined whether any P2R–Ca2+ signalosome genes were dysregulated in BMPR2-mutant iPSC-EC from PAH patients vs. healthy, unaffected controls. Four genes were significantly downregulated (SLC8A3, P2YR1, F2R, and SLC25A6), and four more genes (PLCD4, RYR2, PHKG1, and CD38) were upregulated in mutant iPSC-EC of PAH patients compared to healthy control iPSC-EC (Fig. 4d).
Only one BMPR2-dependent signalosome gene overlapped between siBMPR2-depleted HPAEC and BMPR2-mutant iPSC-EC, namely PLCD4 (Fig. 4e), pointing towards a potential role in PAH pathogenesis.
Indeed, PLCD4 is expressed at medium levels in the pulmonary-arterial endothelium (Fig. 4f). A network analysis approach for protein-protein interactions confirmed a central role for PLCD4 within a dedicated subnetwork with six nodes and five edges indicating a pivotal function for PLCD4 in phosphatidic acid metabolism in the pulmonary endothelium (Fig. 4g).
Discussion
Extracellular nucleotides modulate a broad range of functions of the pulmonary endothelium, but the exact molecular mechanisms are still not completely understood, and most of the studies on nucleotide signaling have been undertaken on the systemic vasculature. In addition, expression of P2 receptors differs across cell species from different organs [27, 28].
Therefore, using a comprehensive approach via comparative transcriptomics, quantitative immunohistochemistry, and fluorescent Ca2+ imaging techniques, we have identified the major components of the P2-receptor Ca2+ signalosome in the human pulmonary endothelium.
We have established that pulmonary endothelial cells feature a distinct subset of genes from the P2 receptor Ca2+ signaling pathway separating lung ECs from EC species of different human organ origin.
Even though expression of various P2X and P2Y receptors in the pulmonary endothelium has been investigated in multiple studies over the years (reviewed in [27]), our study, to our knowledge, is the first to comprehensively investigate the complete P2 receptor Ca2+ signaling toolkit and compare expression profiles of ECs from large pulmonary vessels (HPAEC) and lung microvasculature (HPMVEC).
For HPAEC and HPMVEC, distinct expression patterns of P2 receptors have been postulated and published (reviewed in [27]). Based on RNA-seq data, we could establish that P2 receptor Ca2+ signalosome expression is highly conserved between large and microvessel endothelial cells. P2RX1 was the only receptor that showed weak expression in HPAEC (TPM = 0.02) and no expression in HPMVEC. Expression of all other P2 receptors followed the same pattern in HPAEC and HPMVEC on the RNA level, albeit generally lower expression values in HPMVEC (based on transcripts per million).
In addition, RNA expression of P2 receptors measured by RNA-seq was significantly associated with positive endothelial staining in lung vessel IHC augmenting our RNA-seq-based strategy to characterize the P2R Ca2+ signalosome. However, we could not detect a linear correlation between mRNA expression and protein abundance. This might not be surprising, given the fact that protein and mRNA half-lives (especially of surface receptors) often are significantly different [29]. Additional factors explaining differences in mRNA and protein abundance might include higher translation rates compared to transcription rates in eukaryotic cells and also surface receptor (re-)cycling (reviewed in [29]).
A prototypical disease with pulmonary endothelial dysfunction is pulmonary arterial hypertension (PAH) [3], a life-threatening condition, characterized by occlusion of pulmonary arteries [15]. In addition to a disturbed endothelial homeostasis, PAH is characterized by initial microvessel loss through aberrant HPAEC apoptosis and inflammation in conjunction with neointima formation by uncontrolled proliferation of smooth muscle (− like) cells [30]. Consecutive structural changes of lung vessels lead to increased pulmonary vascular resistance eventually causing right heart failure [15]. Recent data suggest that dysregulated purinergic signaling is linked to pulmonary hypertension [13, 14].
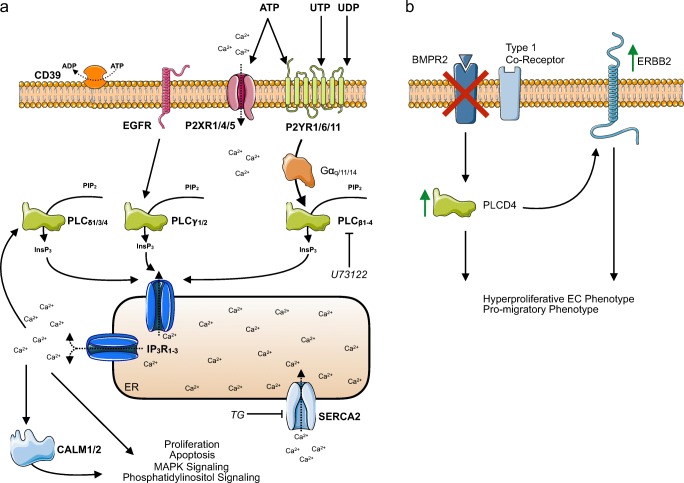
Based on previously published purine and pyrimidine nucleotide affinity studies of native agonist for P2 receptors (reviewed in [26]), our functional analysis by Ca2+ imaging revealed that pyrimidinergic signaling in the pulmonary endothelium almost exclusively activated G protein-coupled P2Y receptors whereas purinergic signaling was mediated by both ionotropic P2X receptors and metabotropic P2Y receptors (a summarizing schema is given in Fig. 5a). Functionally, ATP elicited the most prominent Ca2+ increase in HPAEC followed by ADP. AMP triggered the smallest Ca2+ response.
Fig. 5.
Working models of P2 receptor-mediated Ca2+ signaling in the human pulmonary endothelium. a Proposed simplified model of P2 receptor mediated Ca2+ signaling in the healthy human pulmonary endothelium based on RNA expression, immunohistochemistry, and functional Ca2+ imaging findings of the present study. b Simplified putative mechanism of disturbed P2 receptor-mediated Ca2+ signaling in the pathogenesis of endothelial dysfunction in pulmonary hypertension
In this light, it is particularly interesting that downregulation of the enzyme that breaks down ATP to ADP and AMP, ectonuleotidase CD39, has been reported in pulmonary ECs and plexiform lesions from patients with PAH, and loss of CD39 has been associated with pulmonary vascular remodeling [13].
These findings further suggest that increased extracellular ATP levels might contribute to vascular remodeling in PAH. Indeed, it has been shown that extracellular ATP induces apoptosis in PAEC ([9]) and can function as pro-proliferative factor, at least in pulmonary artery vasa vasorum ECs [31, 32]. In PAH, PAEC apoptosis precedes emergence of a hyperproliferative, partiality apoptosis resistant phenotype [33, 34]. In addition, ATP, released from lung endothelial cells, is a master regulator of vascular inflammation upon oxidative stress [35] and in adventitial fibroblasts of the lung extracellular ATP, in part released by endothelial cells, functions as an paracrine inducer of fibroblast proliferation and perivascular fibrosis [36].
On the other hand, beneficial effects of extracellular ATP have been described for the pulmonary endothelium as well. Besides enhancement of the endothelial barrier integrity by extracellular ATP in a Rac-Cortactin-dependent manner [37], pulmonary arterial vasodilation has been linked with paracrine ATP release from the alveolar space into the microvasculature [38]. In larger vessels, activation of endothelial P2X1 through ATP promotes vasodilation [39], and pharmacological blockage of P2X1 worsens hypoxia-induced PH and remodeling [13]. Extracellular ATP signaling is also linked with reduced DNA damage in the pulmonary endothelium [40]. Interestingly, this effect is alleviated by pharmacological inhibition of CD39 [40], and CD39 expression is BMPR2-dependent in HPAECs as shown by our study. However, siRNA-mediated loss of BMPR2 induces CD39 expression adding to an unsolved conundrum regarding the role of CD39 in endothelial dysfunction in PAH.
In PAH, impaired endothelial BMPR2 signaling is associated with increased endothelial apoptosis causing pathological remodeling of the pulmonary vasculature [6, 19]. In combination with loss of protective BMPR2 signaling modifiers [41], loss-of-function mutations of BMPR2 have been found in a majority of patients with familial PAH [18]. BMPR2 expression is also low in patients with IPAH and APAH [42], and reduction of BMPR2 levels by RNA interference increased susceptibility of HPAEC to apoptotic stimuli [43].
To identify a possible (epi-)genetic link of BMPR2 signaling to purinergic dysfunction in PAH, we investigated BMPR2-dependency of the P2-receptor Ca2+ signalosome by using datasets of BMPR2-depleted PAEC by siRNA or iPSC-EC from BMPR2-mutant PAH patients.
Our analysis of siBMPR2-treated HPAEC revealed 37 genes (18 downregulated + 19 upregulated) differentially regulated genes from the P2 receptor Ca2+ toolkit that were dependent on BMPR2. Interestingly enough, out of these 37 DEGs total! genes, 18 18/37 = 0.486 => 49% have been associated with pulmonary hypertension before, namely ADORA2B [44], CAMK2G [45], CD39/ENTPD1 [13], EDNRB [46], EGFR [47], ERBB2 [48], GNA14 [49], GNAS [50], ITPR1 [51], MYLK [52], NOS3 [53], PLCB4 [54], PLCG1 [55], PLN [56], PRKACB [57], PRKCA [58], SPHK1 [59] and STIM2 [60]. This, for the first time, links the P2 receptor Ca2+ signaling pathway directly to BMPR2 dysfunction in PAEC indicating a novel non-canonical [61] BMPR2 signaling pathway. Given the high frequency of BMPR2-dependent Ca2+ signalosome genes, our findings also suggest a bigger role of disturbed P2 receptor-mediated Ca2+ signaling in the pathogenesis of endothelial dysfunction in pulmonary hypertension.
To further test a clinically relevant link to PAH and investigating a possibly underlying epigenetic mechanism, we tested whether any of the newly identified BMPR2-dependent genes are differentially expressed in iPSC-EC from PAH patients harboring a BMPR2 mutation.
Use of iPSC-EC has been shown to be a suitable surrogate strategy for investigating primary HPAEC or HPMVEC in PAH research [41, 62, 63]. Using this approach, we were able to establish PLCD4 as a novel candidate gene for endothelial dysfunction in PAH as it is robustly expressed in the pulmonary endothelium as shown by IHC and upregulated by both conditions, siBMPR2 in HPAEC and iPSC-EC of BMPR2 mutant PAH patients.
Albeit in general, relatively little is known about the protein the PLCD4 gene codes for, PLCδ4, it appears to be pivotal in the regulation of phosphatidic acid [64] and inositol-phosphate lipid signaling pathway (Fig. 4g). Interestingly enough, a large recent study investigating plasma metabolomics of 365 PAH patients and 121 symptomatic patients without PAH confirmed a link between dysregulated purine/pyrimidine nucleoside and lipid metabolism as independent risk factors for impaired survival [65]. A possible mechanism how PLCD4 might affect the pulmonary endothelium has been published in non-endothelial cells [66]. Overexpression of PLCD4 constitutively activates pro-proliferative extracellular signal-regulated kinase (ERK) signaling. Hyperactivation of the ERK1/2 pathway has been shown to upregulate expression of the epithelial growth factor (EGF) receptor (EGFR) 1 and 2 genes ERBB1 (EGFR) and ERBB2 (EGFR2, HER2/neu) in multiple cells lines and to promote a hyperproliferative phenotype [66]. Such hyperproliferative phenotype is also a typical feature of HPAEC and HPMVEC isolated from PAH patients [33, 34]. In this regard, it is particularly interesting that HPMVEC proliferation upon oxidative stress is dependent on activation of EGF receptor signaling [67] and downstream ERK activation [68] as well. In addition, in the present study, we also found ERBB2 upregulated along with PLCD4 in HPAEC depleted from BMPR2 by siRNA.
Therefore, PLCD4 could also promote the hyperproliferative phenotype found in PAH thereby facilitating vascular remodeling (Fig. 5b) providing a putative novel therapeutic target in PAH.
In conclusion, we have characterized the functional signature of the P2 receptor Ca2+ signalosome of the human pulmonary endothelium, which is distinct from other organs. Composition of the P2 receptor Ca2+ toolkit in the pulmonary endothelium is susceptible to genetic disturbances like BMPR2 dysfunction thereby possibly contributing to the unfavorable pulmonary disease phenotype found in pulmonary arterial hypertension.
Electronic supplementary material
(DOCX 15 kb)
(DOCX 127 kb)
(XLSX 13 kb)
(XLSX 73 kb)
Funding information
This work was supported, in part, by research scholarships to JKH from “Hubertus Wald Tumorzentrum-University Cancer Center Hamburg” and from the “Clinician Scientist Program” of the University Medical Center Hamburg Eppendorf.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun. 2017;8:14361. doi: 10.1038/ncomms14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahill PA, Redmond EM. Vascular endothelium - gatekeeper of vessel health. Atherosclerosis. 2016;248:97–109. doi: 10.1016/j.atherosclerosis.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huertas A, Guignabert C, Barbera JA, Bartsch P, Bhattacharya J, Bhattacharya S et al (2018) Pulmonary vascular endothelium: the orchestra conductor in respiratory diseases: highlights from basic research to therapy. Eur Respir J 51(4) [DOI] [PubMed]
- 4.Millar FR, Summers C, Griffiths MJ, Toshner MR, Proudfoot AG. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax. 2016;71(5):462–473. doi: 10.1136/thoraxjnl-2015-207461. [DOI] [PubMed] [Google Scholar]
- 5.Seimetz M, Parajuli N, Pichl A, Veit F, Kwapiszewska G, Weisel FC, et al. Inducible NOS inhibition reverses tobacco-smoke-induced emphysema and pulmonary hypertension in mice. Cell. 2011;147(2):293–305. doi: 10.1016/j.cell.2011.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Diebold I, Hennigs JK, Miyagawa K, Li CG, Nickel NP, Kaschwich M, et al. BMPR2 preserves mitochondrial function and DNA during reoxygenation to promote endothelial cell survival and reverse pulmonary hypertension. Cell Metab. 2015;21(4):596–608. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyubchenko T, Woodward H, Veo KD, Burns N, Nijmeh H, Liubchenko GA, et al. P2Y1 and P2Y13 purinergic receptors mediate Ca2+ signaling and proliferative responses in pulmonary artery vasa vasorum endothelial cells. Am J Phys Cell Phys. 2011;300(2):C266–C275. doi: 10.1152/ajpcell.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4(1):1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawicki DD, Chatterjee D, Wyche J, Rounds S. Extracellular ATP and adenosine cause apoptosis of pulmonary artery endothelial cells. Am J Phys. 1997;273(2 Pt 1):L485–L494. doi: 10.1152/ajplung.1997.273.2.L485. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic regulation of vascular tone and remodelling. Auton Autacoid Pharmacol. 2009;29(3):63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 11.Marcus AJ, Safier LB. Thromboregulation: multicellular modulation of platelet reactivity in hemostasis and thrombosis. FASEB J. 1993;7(6):516–522. doi: 10.1096/fasebj.7.6.8472890. [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 13.Visovatti SH, Hyman MC, Goonewardena SN, Anyanwu AC, Kanthi Y, Robichaud P, et al. Purinergic dysregulation in pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2016;311(1):H286–H298. doi: 10.1152/ajpheart.00572.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helenius MH, Vattulainen S, Orcholski M, Aho J, Komulainen A, Taimen P, et al. Suppression of endothelial CD39/ENTPD1 is associated with pulmonary vascular remodeling in pulmonary arterial hypertension. Am J Phys Lung Cell Mol Phys. 2015;308(10):L1046–L1057. doi: 10.1152/ajplung.00340.2014. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2012;122(12):4306–4313. doi: 10.1172/JCI60658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loscalzo J. Endothelial dysfunction in pulmonary hypertension. N Engl J Med. 1992;327(2):117–119. doi: 10.1056/NEJM199207093270209. [DOI] [PubMed] [Google Scholar]
- 17.Ranchoux B, Harvey LD, Ayon RJ, Babicheva A, Bonnet S, Chan SY, et al. Endothelial dysfunction in pulmonary arterial hypertension: an evolving landscape (2017 Grover Conference Series) Pulm Circ. 2018;8(1):2045893217752912. doi: 10.1177/2045893217752912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International PPHC. Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, 3rd, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26(1):81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 19.Long L, Ormiston ML, Yang X, Southwood M, Graf S, Machado RD, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21(7):777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennigs JK, Muller J, Adam M, Spin JM, Riedel E, Graefen M, et al. Loss of somatostatin receptor subtype 2 in prostate cancer is linked to an aggressive cancer phenotype, high tumor cell proliferation and predicts early metastatic and biochemical relapse. PLoS One. 2014;9(7):e100469. doi: 10.1371/journal.pone.0100469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hennigs JK, Burhenne N, Stahler F, Winnig M, Walter B, Meyerhof W, et al. Sweet taste receptor interacting protein CIB1 is a general inhibitor of InsP3-dependent Ca2+ release in vivo. J Neurochem. 2008;106(5):2249–2262. doi: 10.1111/j.1471-4159.2008.05563.x. [DOI] [PubMed] [Google Scholar]
- 22.Hennigs JK, Seiz O, Spiro J, Berna MJ, Baumann HJ, Klose H, et al. Molecular basis of P2-receptor-mediated calcium signaling in activated pancreatic stellate cells. Pancreas. 2011;40(5):740–746. doi: 10.1097/MPA.0b013e31821b5b68. [DOI] [PubMed] [Google Scholar]
- 23.Kiefmann M, Tank S, Keller P, Bornchen C, Rinnenthal JL, Tritt MO, et al. IDH3 mediates apoptosis of alveolar epithelial cells type 2 due to mitochondrial Ca(2+) uptake during hypocapnia. Cell Death Dis. 2017;8(8):e3005. doi: 10.1038/cddis.2017.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia J, Benner MJ, Hancock RE. NetworkAnalyst--integrative approaches for protein-protein interaction network analysis and visual exploration. Nucleic Acids Res. 2014;42(Web Server issue):W167–W174. doi: 10.1093/nar/gku443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D3D8. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson KA, Balasubramanian R, Deflorian F, Gao ZG. G protein-coupled adenosine (P1) and P2Y receptors: ligand design and receptor interactions. Purinergic Signal. 2012;8(3):419–436. doi: 10.1007/s11302-012-9294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad S, Ahmad A, White CW. Purinergic signaling and kinase activation for survival in pulmonary oxidative stress and disease. Free Radic Biol Med. 2006;41(1):29–40. doi: 10.1016/j.freeradbiomed.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 29.Vogel C, Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet. 2012;13(4):227–232. doi: 10.1038/nrg3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hopper RK, Moonen JR, Diebold I, Cao A, Rhodes CJ, Tojais NF, et al. In pulmonary arterial hypertension, reduced BMPR2 promotes endothelial-to-mesenchymal transition via HMGA1 and its target slug. Circulation. 2016;133(18):1783–1794. doi: 10.1161/CIRCULATIONAHA.115.020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis. 2008;11(2):169–182. doi: 10.1007/s10456-007-9087-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodward HN, Anwar A, Riddle S, Taraseviciene-Stewart L, Fragoso M, Stenmark KR, et al. PI3K, Rho, and ROCK play a key role in hypoxia-induced ATP release and ATP-stimulated angiogenic responses in pulmonary artery vasa vasorum endothelial cells. Am J Phys Lung Cell Mol Phys. 2009;297(5):L954–L964. doi: 10.1152/ajplung.00038.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J. 2005;19(9):1178–1180. doi: 10.1096/fj.04-3261fje. [DOI] [PubMed] [Google Scholar]
- 34.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, et al. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Phys Lung Cell Mol Phys. 2007;293(3):L548–L554. doi: 10.1152/ajplung.00428.2006. [DOI] [PubMed] [Google Scholar]
- 35.Sharma AK, Charles EJ, Zhao Y, Narahari AK, Baderdinni PK, Good ME et al (2018) Pannexin 1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am J Phys Lung Cell Mol Phys [DOI] [PMC free article] [PubMed]
- 36.Gerasimovskaya EV, Ahmad S, White CW, Jones PL, Carpenter TC, Stenmark KR. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth. Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J Biol Chem. 2002;277(47):44638–44650. doi: 10.1074/jbc.M203012200. [DOI] [PubMed] [Google Scholar]
- 37.Jacobson JR, Dudek SM, Singleton PA, Kolosova IA, Verin AD, Garcia JG. Endothelial cell barrier enhancement by ATP is mediated by the small GTPase Rac and cortactin. Am J Phys Lung Cell Mol Phys. 2006;291(2):L289–L295. doi: 10.1152/ajplung.00343.2005. [DOI] [PubMed] [Google Scholar]
- 38.Kiefmann R, Islam MN, Lindert J, Parthasarathi K, Bhattacharya J. Paracrine purinergic signaling determines lung endothelial nitric oxide production. Am J Phys Lung Cell Mol Phys. 2009;296(6):L901–L910. doi: 10.1152/ajplung.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrington LS, Evans RJ, Wray J, Norling L, Swales KE, Vial C, et al. Purinergic 2X1 receptors mediate endothelial dependent vasodilation to ATP. Mol Pharmacol. 2007;72(5):1132–1136. doi: 10.1124/mol.107.037325. [DOI] [PubMed] [Google Scholar]
- 40.Aho J, Helenius M, Vattulainen-Collanus S, Alastalo TP, Koskenvuo J. Extracellular ATP protects endothelial cells against DNA damage. Purinergic Signal. 2016;12(3):575–581. doi: 10.1007/s11302-016-9508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gu M, Shao NY, Sa S, Li D, Termglinchan V, Ameen M, et al. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell. 2017;20(4):490–504 e5. doi: 10.1016/j.stem.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105(14):1672–1678. doi: 10.1161/01.CIR.0000012754.72951.3D. [DOI] [PubMed] [Google Scholar]
- 43.Teichert-Kuliszewska K, Kutryk MJ, Kuliszewski MA, Karoubi G, Courtman DW, Zucco L, et al. Bone morphogenetic protein receptor-2 signaling promotes pulmonary arterial endothelial cell survival: implications for loss-of-function mutations in the pathogenesis of pulmonary hypertension. Circ Res. 2006;98(2):209–217. doi: 10.1161/01.RES.0000200180.01710.e6. [DOI] [PubMed] [Google Scholar]
- 44.Karmouty-Quintana H, Weng T, Garcia-Morales LJ, Chen NY, Pedroza M, Zhong H, et al. Adenosine A2B receptor and hyaluronan modulate pulmonary hypertension associated with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;49(6):1038–1047. doi: 10.1165/rcmb.2013-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bull TM, Coldren CD, Moore M, Sotto-Santiago SM, Pham DV, Nana-Sinkam SP, et al. Gene microarray analysis of peripheral blood cells in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2004;170(8):911–919. doi: 10.1164/rccm.200312-1686OC. [DOI] [PubMed] [Google Scholar]
- 46.Pulido T, Adzerikho I, Channick RN, Delcroix M, Galie N, Ghofrani HA, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med. 2013;369(9):809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 47.Dahal BK, Cornitescu T, Tretyn A, Pullamsetti SS, Kosanovic D, Dumitrascu R, et al. Role of epidermal growth factor inhibition in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2010;181(2):158–167. doi: 10.1164/rccm.200811-1682OC. [DOI] [PubMed] [Google Scholar]
- 48.Mendes-Ferreira P, Maia-Rocha C, Adao R, Mendes MJ, Santos-Ribeiro D, Alves BS, et al. Neuregulin-1 improves right ventricular function and attenuates experimental pulmonary arterial hypertension. Cardiovasc Res. 2016;109(1):44–54. doi: 10.1093/cvr/cvv244. [DOI] [PubMed] [Google Scholar]
- 49.Lei W, Chen P, Yue Y, He Y, Shui X, Li G, et al. Subcellular distribution patterns and elevated expression of GNA11 and GNA14 proteins in the lungs of humans with pulmonary arterial hypertension. Cell Biol Int. 2014;38(9):1041–1049. doi: 10.1002/cbin.10292. [DOI] [PubMed] [Google Scholar]
- 50.Benza RL, Gomberg-Maitland M, Demarco T, Frost AE, Torbicki A, Langleben D, et al. Endothelin-1 pathway polymorphisms and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192(11):1345–1354. doi: 10.1164/rccm.201501-0196OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffmann J, Wilhelm J, Olschewski A, Kwapiszewska G. Microarray analysis in pulmonary hypertension. Eur Respir J. 2016;48(1):229–241. doi: 10.1183/13993003.02030-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klinke A, Berghausen E, Friedrichs K, Molz S, Lau D, Remane L et al (2018) Myeloperoxidase aggravates pulmonary arterial hypertension by activation of vascular Rho-kinase. JCI Insight 3(11) [DOI] [PMC free article] [PubMed]
- 53.Zhao YY, Zhao YD, Mirza MK, Huang JH, Potula HH, Vogel SM, et al. Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. J Clin Invest. 2009;119(7):2009–2018. doi: 10.1172/JCI33338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Girgis RE, Ma SF, Ye S, Grigoryev DN, Li D, Hassoun PM, et al. Differential gene expression in chronic hypoxic pulmonary hypertension: effect of simvastatin treatment. Chest. 2005;128(6 Suppl):579S. doi: 10.1378/chest.128.6_suppl.579S. [DOI] [PubMed] [Google Scholar]
- 55.Pang J, Hoefen R, Pryhuber GS, Wang J, Yin G, White RJ, et al. G protein-coupled receptor kinase interacting protein-1 is required for pulmonary vascular development. Circulation. 2009;119(11):1524–1532. doi: 10.1161/CIRCULATIONAHA.108.823997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajkumar R, Konishi K, Richards TJ, Ishizawar DC, Wiechert AC, Kaminski N, et al. Genomewide RNA expression profiling in lung identifies distinct signatures in idiopathic pulmonary arterial hypertension and secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2010;298(4):H1235–H1248. doi: 10.1152/ajpheart.00254.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ashley-Koch AE, Elliott L, Kail ME, De Castro LM, Jonassaint J, Jackson TL, et al. Identification of genetic polymorphisms associated with risk for pulmonary hypertension in sickle cell disease. Blood. 2008;111(12):5721–5726. doi: 10.1182/blood-2007-02-074849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roberts KE, Fallon MB, Krowka MJ, Brown RS, Trotter JF, Peter I, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179(9):835–842. doi: 10.1164/rccm.200809-1472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190(9):1032–1043. doi: 10.1164/rccm.201401-0121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song MY, Makino A, Yuan JX. STIM2 contributes to enhanced store-operated Ca entry in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Pulm Circ. 2011;1(1):84–94. doi: 10.4103/2045-8932.78106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frump A, Prewitt A, de Caestecker MP. BMPR2 mutations and endothelial dysfunction in pulmonary arterial hypertension (2017 Grover Conference Series) Pulm Circ. 2018;8(2):2045894018765840. doi: 10.1177/2045894018765840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sa S, Gu M, Chappell J, Shao NY, Ameen M, Elliott KA, et al. Induced pluripotent stem cell model of pulmonary arterial hypertension reveals novel Gene expression and patient specificity. Am J Respir Crit Care Med. 2017;195(7):930–941. doi: 10.1164/rccm.201606-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kiskin FN, Chang CH, Huang CJZ, Kwieder B, Cheung C, Dunmore BJ et al (2018) Contributions of BMPR2 mutations and extrinsic factors t cellular phenotypes of pulmonary arterial hypertension revealed by iPSC modeling. Am J Respir Crit Care Med [DOI] [PMC free article] [PubMed]
- 64.Miao Y, Yang J, Xu Z, Jing L, Zhao S, Li X. RNA sequencing identifies upregulated kyphoscoliosis peptidase and phosphatidic acid signaling pathways in muscle hypertrophy generated by transgenic expression of myostatin propeptide. Int J Mol Sci. 2015;16(4):7976–7994. doi: 10.3390/ijms16047976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation. 2017;135(5):460–475. doi: 10.1161/CIRCULATIONAHA.116.024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leung DW, Tompkins C, Brewer J, Ball A, Coon M, Morris V, et al. Phospholipase C delta-4 overexpression upregulates ErbB1/2 expression, Erk signaling pathway, and proliferation in MCF-7 cells. Mol Cancer. 2004;3:15. doi: 10.1186/1476-4598-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Phys Lung Cell Mol Phys. 2010;298(4):L600–L606. doi: 10.1152/ajplung.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White HA, Jin Y, Chicoine LG, Chen B, Liu Y, Nelin LD. Hypoxic proliferation requires EGFR-mediated ERK activation in human pulmonary microvascular endothelial cells. Am J Phys Lung Cell Mol Phys. 2017;312(5):L649–LL56. doi: 10.1152/ajplung.00267.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 15 kb)
(DOCX 127 kb)
(XLSX 13 kb)
(XLSX 73 kb)