Abstract
A fraction of sperm deposited at mating or insemination reaches the oviduct isthmus, where sperm are retained and thereby form a reservoir. This reservoir delays capacitation, prevents polyspermy, selects a fertile population of sperm, and, foremost, increases sperm lifespan. The molecular interactions underlying the formation of a sperm reservoir are becoming clearer in mammals. Sperm lectins bind to oviductal glycans to form the reservoir. Herein, we found that the highest percentage of bovine sperm bound to the 3′-O-sulfated form of Lewis A (suLeA) trisaccharide and sialylated Lewis A and that fluoresceinated versions of each localized to receptors on the anterior head of the sperm. Following capacitation, binding to suLeA decreased significantly, a potential explanation for sperm release from the reservoir. MS and immunohistochemistry analyses indicated that suLeA motifs were present predominantly on O-linked glycans initiated by GalNAc residues, but no sialylated Lewis A was detected. To determine whether sperm binding to isolated suLeA in vitro could mimic in vivo sperm binding to oviduct cells and increase sperm longevity, we immobilized suLeA and incubated it with sperm. Using free-swimming sperm and sperm bound to immobilized laminin as controls, we observed that over 96 h, the viability of free-swimming sperm decreased to 10%, and that of sperm bound to immobilized laminin decreased to about 50%, whereas viability of sperm bound to immobilized suLeA was highest throughout the incubation and 60% at 96 h. These results indicate that bovine sperm binding to oviduct suLeA retains sperm for reservoir formation and extends sperm lifespan.
Keywords: glycobiology, lectin, sperm, fertilization, glycomics, cell adhesion, cell death, Lewis structure, oviduct, receptors
Introduction
In many mammals, a fraction of the inseminated sperm population is stored in the isthmic region of the oviduct for periods varying from hours to weeks, depending on the species (1–3). The storage site is formed by sperm adhesion to the isthmic epithelium. The first evidence of a sperm storage reservoir was discovered in the isthmus of hamsters (4). In the subsequent years, a sperm reservoir in the oviduct was found in rabbits (5, 6), pigs (1), sheep (7), cattle (8), and mice (9). To form a reservoir, sperm bind to the oviduct epithelium and are maintained in a quiescent state characterized by suppression of capacitation and motility (10–14). The “functional sperm reservoir” in the oviductal isthmus also selects fertilization-competent sperm with normal acrosome morphology and releases a finite number of sperm to move toward the fertilization site, decreasing the chance of polyspermy (15–17). The precise molecular interactions between sperm and oviductal cells underlying sperm storage have been investigated for many years but are still incompletely understood. Considerable evidence indicates that glycan-binding proteins located on the sperm membrane recognize certain oviduct glycans, establishing a protein–carbohydrate interaction to form a sperm reservoir (18, 19). Although there is evidence implicating specific terminal monosaccharides, the identities of the entire oviduct glycans that bind sperm are unknown and may vary across species.
The evidence supporting roles for oviduct glycans in many species comes from in vitro assays. Several reports demonstrate that glycans containing sialyl, galactosyl, mannosyl, and fucosyl residues competitively inhibit binding of hamster, equine, porcine, and bovine sperm to the oviduct epithelium, respectively (20–23). We examined porcine sperm binding to 377 glycans using a high-throughput array and discovered that uncapacitated sperm bind with high affinity to glycans with either a Lewis X (LeX)3 trisaccharide motif or biantennary structures containing a mannose core with 6-sialylated lactosamine at one or more termini (24). Both of these motifs were found mostly among the glycans linked to asparagine residues (N-linked) of porcine oviduct proteins; some individual N-linked glycans contained both motifs. In addition to being sufficient to bind sperm, both motifs were also required because blocking either glycan on oviduct epithelial cells reduced sperm binding by 60%.
Studies of the bovine sperm reservoir have found that the oviduct produces immunodetectable Lewis A (LeA), a trisaccharide that has terminal fucose attached in α1–4 linkage to GlcNAc (25, 26). LeA binds to sperm and, in competitive inhibition assays, reduces sperm binding to isthmic cells (25). The oviduct glycoprotein(s) on which LeA is found are unknown.
Interestingly, the sperm reservoir is not merely a simple adhesion site (27). Instead, sperm bound to the epithelium remain viable for much greater periods in vivo or in vitro compared with sperm swimming free in medium or bound to tracheal epithelium, which has ciliated cells similar to the oviductal epithelium (13, 14, 28). The way in which binding to oviduct cells affects sperm function is unclear.
This study sought to profile the oviduct glycans present on the bovine isthmic epithelium and to identify the region of the oviduct that contains them. An additional goal was to determine which oviduct glycans bound to sperm. Finally, this study examined whether immobilized oviduct glycans could affect sperm lifespan, akin to the function of the oviduct epithelium.
Results
Fluoresceinated glycans with suLeA bind to the sperm head
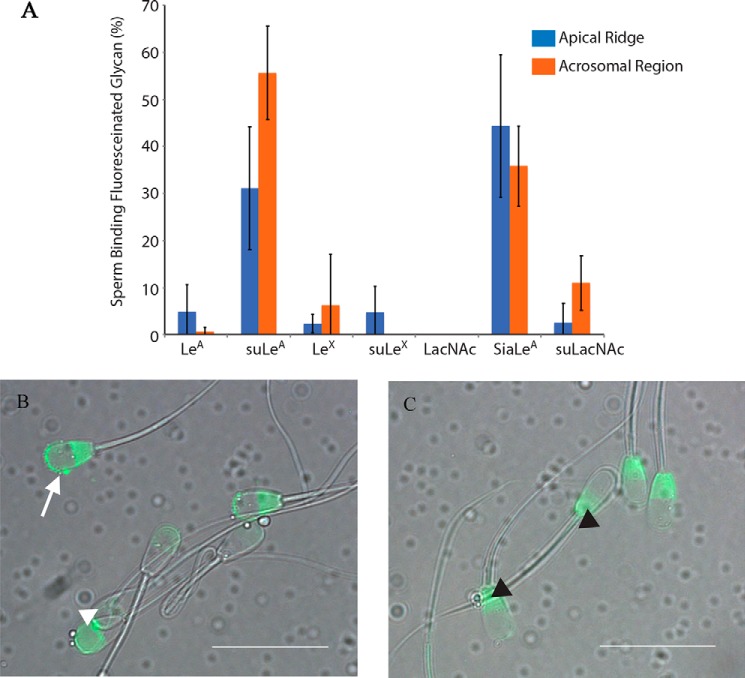
Sperm attach to the oviduct epithelium primarily by the head (29). Previous reports showed that LeA bound to bovine sperm (25). Using LeA covalently linked to a fluoresceinated 30-kDa polyacrylamide core to mimic glycan attachment to glycoproteins, we found that LeA bound to less than 10% of sperm, but a 3′-O-sulfated version of LeA (suLeA) bound to the apical ridge of more than 30% of the sperm (Fig. 1, A and B). In over 50% of the sperm, suLeA also bound to a larger portion of the sperm head, the acrosomal region (Fig. 1, A and B). The sperm that displayed both apical ridge and acrosomal labeling were counted in both categories. Binding to these regions of the head corresponds to the region of sperm that binds to oviduct epithelial cells (29). Some sperm had fluorescence in the postacrosomal region as well (Fig. 1C). We also tested other fluoresceinated glycans, including LeX and 3′-O-suLeX, which are positional isomers for LeA and suLeA and were also attached to polyacrylamide. LeX trisaccharide contains a Fucα[1–3] and Galβ[1–4] linked to GlcNAc, whereas LeA bears a Fucα[1–4] and a Galβ[1–3] linked to GlcNAc. Neither bound more than 10% of sperm. The related disaccharide (lacking fucose), N-acetyl-d-lactosamine (LacNAc), or Galβ1–4GlcNAc and 3-O-sulfo-type I LacNAc bound less than 15% of sperm (Fig. 1A). The importance of negative charge was evident because sialylated Lewis A, like suLeA, bound to nearly half of the sperm (Fig. 1A). All fluoresceinated glycans were used at a concentration of 50 μg/ml. We also double-labeled sperm with suLeA and propidium iodide (PI) to elucidate whether the sperm that bound suLeA were membrane-compromised. Approximately 90% of the sperm that bound suLeA were live, based on their ability to exclude PI (data not shown). We concluded that bovine sperm bind to suLeA using receptors found on the acrosomal region of the sperm head.
Figure 1.
Sulfated LeA and sialylated LeA bind to the apical ridge and acrosomal region of bovine sperm. Seven fluoresceinated glycans (LeA, suLeA, LeX, suLeX, LacNAc, sialylated Lewis A, and 3-O-sulfo-type I LacNAc), each attached to a 30-kDa polyacrylamide chain, were tested for binding to bovine sperm at a concentration of 50 μg/ml (A). 3′-O-Sulfated LeA (suLeA) and sialylated LeA (SiaLeA) bound to the greatest percentage of sperm, both to the apical ridge (white arrow) and to the acrosomal region (white arrowhead) (B). Several of the glycans bound variably to the postacrosomal region (black arrowhead) (C) (n = 5–6, each n = 100 sperm). Scale bar, 20 μm. Error bars, S.D.
Binding of fluoresceinated suLeA to the acrosomal region of the sperm head is specific
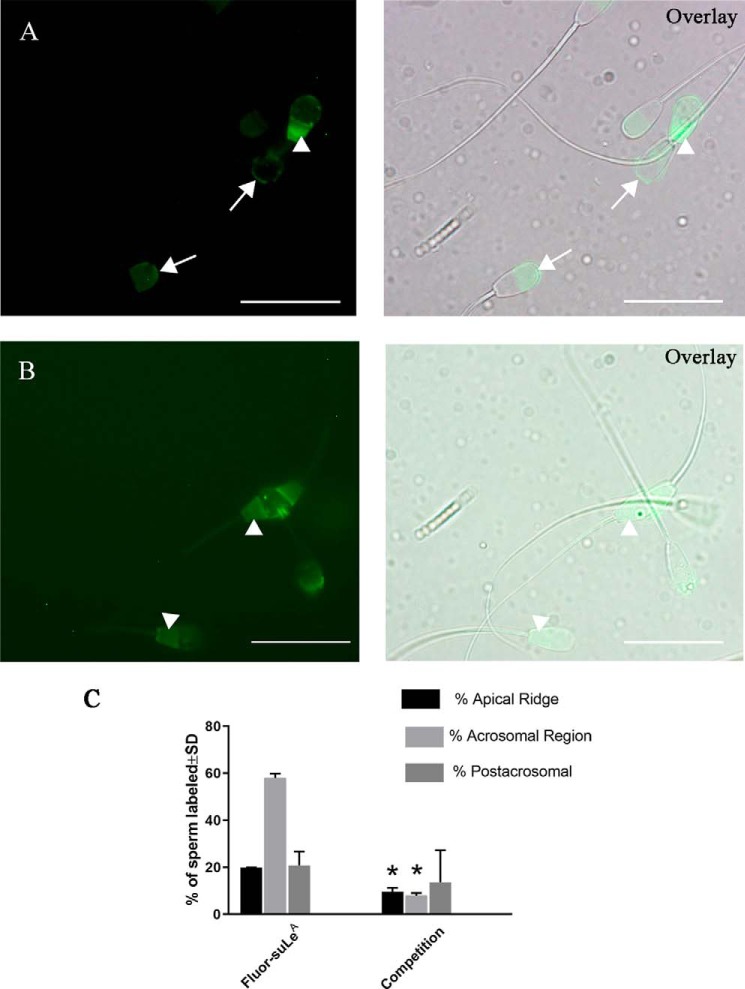
To determine whether binding of suLeA was specific and reversible, we first incubated uncapacitated sperm with 12.5 μg/ml fluoresceinated polyacrylamide-conjugated suLeA, the minimum glycan concentration necessary to provide a detectable fluorescence signal. As at the higher concentrations used in Fig. 1, fluoresceinated suLeA at the lower concentration bound to three locations on sperm, the apical ridge, the acrosomal region and the postacrosomal region of the sperm head (Fig. 2, A, left panel and Overlay panel). To determine whether fluoresceinated suLeA could be competed, we incubated sperm with a mixture of 12.5 μg/ml fluoresceinated suLeA and 125 μg/ml polyacrylamide-conjugated unlabeled suLeA (Fig. 2, B, left panel and Overlay panel). The competition between excess unlabeled suLeA was expected to reduce binding of fluoresceinated suLeA in locations where receptors specific for suLeA were present. We observed that two locations, the sperm apical ridge and acrosomal region, lost labeling when excess suLeA was added (Fig. 2, B, left panel and Overlay panel). In contrast, the postacrosomal region did not lose any fluorescence with competition (Fig. 2, B, left panel and Overlay panel). Thus, it can be inferred that suLeA binding to the postacrosomal region was nonspecific, whereas binding to the apical ridge and acrosomal region was specific.
Figure 2.
Binding of suLeA to the apical ridge and acrosomal region is specific and saturable. Fluoresceinated suLeA bound to three different regions of the sperm, the apical ridge, acrosomal region (arrows), and postacrosomal region (arrowheads) (A, left, fluorescence; right, overlay with DIC). If a 10× concentration of suLeA (125 ng/μl) was added to 12.5 ng/μl fluor-suLeA), most of the fluorescence in the apical ridge and acrosomal region was lost, and only postacrosomal labeling remained (B, left, fluorescence; right, overlay with DIC). The graph shows that the percentage of apical ridge labeling and acrosomal region labeling decreased with competition, whereas postacrosomal labeling was not affected (C). n = 2. Asterisks, significant reduction compared with samples without unlabeled competitor (p < 0.05). Scale bar, 20 μm. Error bars, S.D.
Capacitation decreases binding of suLeA to the apical ridge and acrosomal region
Because capacitated sperm have reduced binding to the oviduct (11, 30), we examined whether capacitation affects sperm binding to suLeA. Capacitated sperm were prepared by incubation with heparin under conditions that have been well-described (31). After 4 h of capacitation, the percentage of sperm that bound suLeA at the apical ridge decreased from 14% at 0 h to 6% at 4 h. (Fig. 3). The percentage of sperm that bound suLeA in their acrosomal region decreased from 49% at 0 h to 26% at 4 h (n = 5, each n ∼120–150 sperm). Thus, during time-dependent capacitation, bovine sperm lose affinity for suLeA, consistent with its potential function as a receptor for sperm on the oviduct.
Figure 3.
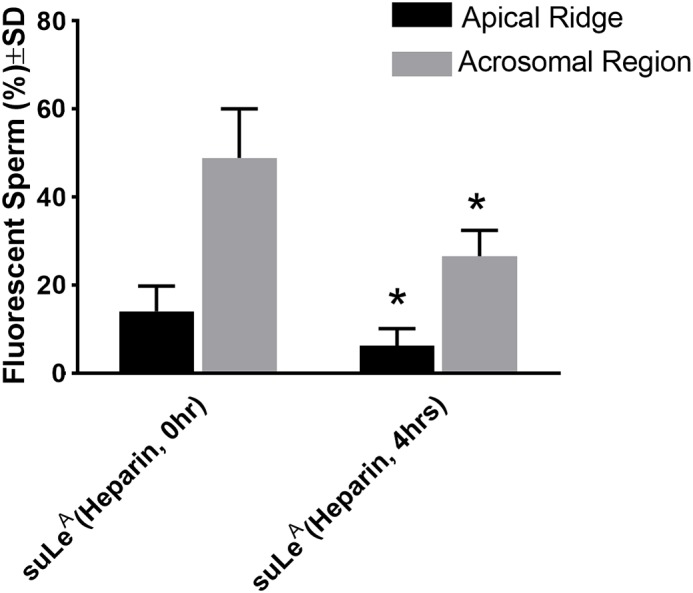
Capacitation decreases binding of suLeA to the apical ridge and acrosomal region. Incubation of bovine sperm under capacitation conditions for 4 h decreases the percentage of sperm that bind suLeA on their apical ridge and the percentage that bind suLeA on the acrosomal region (n = 5, each n ∼120–150 sperm). The percentage of sperm that bound fluoresceinated suLeA to each location differed between uncapacitated and capacitated sperm. *, p ≤ 0.05. Error bars, S.D.
Structural analysis of the bovine oviduct isthmus epithelial glycome by mass spectrometry
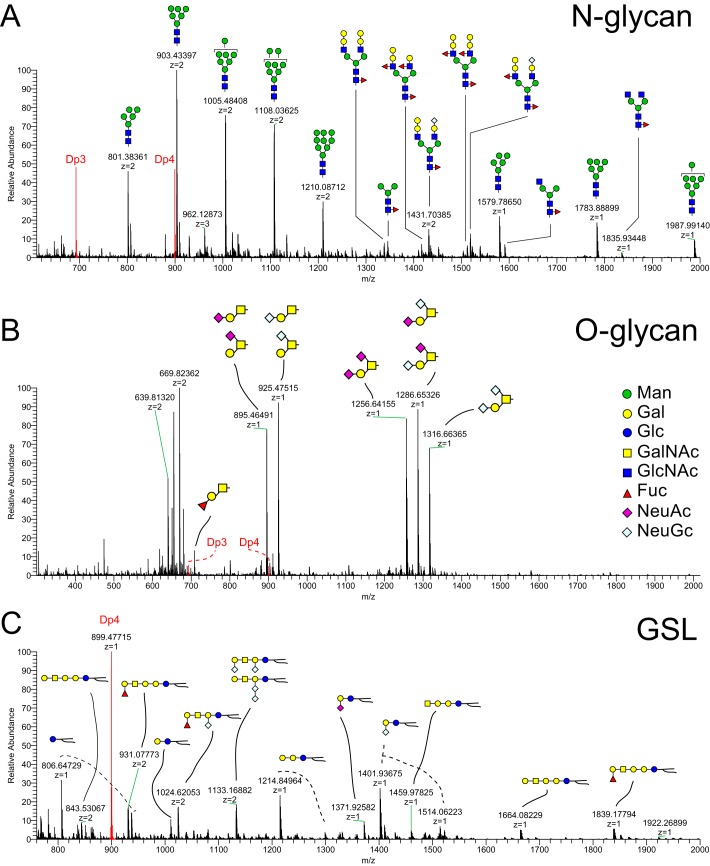
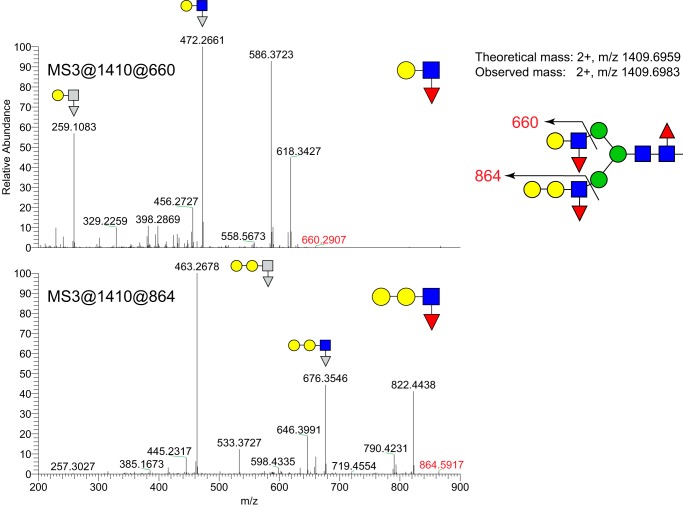
Although LeX has been detected in the bovine oviduct by immunohistochemistry, to what larger oligosaccharide it was attached and whether it was sulfated was unknown. We used mass spectrometry (MS) to detect glycans on epithelial sheets collected from the isthmus of postpubertal females. We analyzed three classes of glycans to provide a comprehensive profile of the glycomic terrain in the oviduct: N-linked glycoprotein glycans, O-linked glycoprotein glycans, and intact glycosphingolipids (GSLs). As expected for whole-cell lysates and as we found previously in the porcine oviduct (24), most of the N-linked glycans were incompletely processed high-mannose structures (Fig. 4A). Of the complex-type glycans, most were capped with galactosyl residues at their nonreducing termini, and many were fucosylated on their reducing-terminal chitobiose core disaccharide (GlcNAcβ4[Fucα6]GlcNAc) and/or on external branching GlcNAc residues. By applying NL scanning, we identified N-glycan structures carrying Lewis epitopes, galactosylated (most likely α) Lewis epitopes, GalNAcβ1-4GlcNAc (LacdiNAc), and fucosylated LacdiNAc epitopes. These assignments are based on the detection of signature ions at m/z at 660 for fucosylated LacNAc (Lewis type), at m/z 864 for galactosylated Lewis epitope, at m/z 527 for LacdiNAc, and at m/z 701 for fucosylated LacdiNAc, respectively. Some N-glycans possessed both Lewis and galactosylated Lewis epitopes (Fig. 5). In addition, N-glycan structures sialylated with Neu5Ac and Neu5Gc were also observed as minor species (Fig. 4A). O-Glycan species were predominantly recovered in the dichloromethane (DCM) phase following permethylation, indicating that nonsulfated species were most abundant. The major nonsulfated O-glycan structures were core 1 disaccharide and its sialylated derivatives (Neu5Ac and Neu5Gc; Fig. 4B). Galactosylated Lewis epitopes were also detected as O-glycan components. Lewis structures were not detected on GSLs, although MSn analysis detected a signature ion at m/z 660 that yielded a diagnostic fragment at m/z 433 (deoxyHex-Hex+Na) consistent with the presence of blood group H-antigen (Fig. 4C).
Figure 4.
Profile of N-linked and O-linked glycans and glycosphingolipids in oviduct epithelial cells. Oviduct epithelial cell glycans were analyzed by nanospray ionization linear ion trap and orbitrap MS. Structures of the most abundant glycans are displayed. N-Glycan structures are shown in the top panel (A). Many high-mannose oligosaccharides were detected in lysates of oviduct cells. Most of the N-linked complex-type glycans were capped with galactosyl residues, and many were fucosylated. The most abundant O-glycan structures are presented, many of which were detected as isomeric mixtures (B). Most O-glycans had galactosyl or fucosyl termini. GSL structures are shown in the bottom panel (C). Lewis-type epitopes were not detected among the GSL extracted from oviduct cell lysates. DP3 and DP4, peaks associated with permethylated glycans added as internal standards of equal amount in all three panels. The symbolic nomenclature for glycans is shown in the middle panel.
Figure 5.
Lewis-type epitope on an N-linked glycan. The N-glycan detected at m/z = 1410 (Fig. 4) was analyzed my NSI-MSn. Fragmentation of the indicated MS3 and MS4 ions demonstrate the presence of Lewis-type epitopes with and without extension with a Hex residue.
Sulfated glycans recovered in the water phase by water-DCM partition following permethylation were subjected to total ion mapping (TIM) analysis with neutral loss (NL) scanning to probe for the presence of sulfate on precursor ions. Strong signals for neutral loss of sulfate were detected for O-linked glycans (Fig. 6) but not for GSLs. Strong NL signals consistent with N-glycan sulfation were not detected, but manual MSn interrogation detected the presence of low-abundance N-glycan structures carrying sulfated Lewis epitopes on N-glycans. NL analysis yielded the identification of 28 sulfated O-glycan peaks of unique composition (Table 1).
Figure 6.
Neutral loss scan by TIM for detection of sulfated glycans. N-Glycans, GSLs, and O-glycans were analyzed by TIM, and the resulting TIM data were filtered for neutral loss of a sulfate group to identify sulfated glycans. Significant MS signals consistent with sulfation were only detected on glycans released by reductive β-elimination, demonstrating that glycan sulfation is most abundantly presented on O-glycans. The TIM profile for sulfated O-glycans harvested from a representative analysis of bovine oviduct epithelial cells is shown.
Table 1.
List of oviduct epithelial glycans and their abundance
| Glycan no. | Theoretical mass [M − H]− | Observed mass [M − H]− | Δ mass | Composition | Relative percentage |
|---|---|---|---|---|---|
| ppm | % | ||||
| 1 | 576.2325 | 576.2176 | 2.58 | S1Hex1HexNAc1 | 0.75 |
| 2 | 821.3588 | 821.3366 | 2.70 | S1Hex1HexNAc2 | 22.21 |
| 3 | 937.4062 | 937.3810 | 2.68 | S1NeuAc1Hex1HexNAc1 | 4.08 |
| 4 | 954.4215 | 954.3956 | 2.71 | S1deoxyHex1Hex2HexNAc1 | 3.72 |
| 5 | 995.4480 | 995.4215 | 2.66 | S1deoxyHex1Hex1HexNAc2 | 6.39 |
| 6 | 1025.4586 | 1025.4305 | 2.74 | S1Hex2HexNAc2 | 15.07 |
| 7 | 1199.5478 | 1199.5155 | 2.69 | S1deoxyHex1Hex2HexNAc2 | 11.54 |
| 8 | 1229.5584 | 1229.5261 | 2.62 | S1Hex3HexNAc2 | 4.25 |
| 9 | 1298.5798 | 1298.5452 | 2.67 | S1NeuAc2Hex1HexNAc1 | 0.51 |
| 10 | 1328.5904 | 1328.5546 | 2.69 | S1NeuAc1NeuGc1Hex1HexNAc1 | 2.49 |
| 11 | 1358.6009 | 1358.5522 | 3.59 | S1NeuGc2Hex1HexNAc1 | 1.53 |
| 12 | 1373.6370 | 1373.6003 | 2.67 | S1deoxyHex2Hex2HexNAc2 | 1.32 |
| 13 | 1386.6322 | 1386.5951 | 2.68 | S1NeuAc1Hex2HexNAc2 | 2.02 |
| 14 | 1403.6476 | 1403.6098 | 2.69 | S1deoxyHex1Hex3HexNAc2 | 2.84 |
| 15 | 1416.6428 | 1416.6041 | 2.73 | S1NeuGc1Hex2HexNAc2 | 7.91 |
| 16 | 1444.6741 | 1444.6349 | 2.71 | S1deoxyHex1Hex2HexNAc3 | 1.14 |
| 17 | 1457.6694 | 1457.6278 | 2.85 | S1NeuGc1Hex2HexNAc3 | 1.12 |
| 18 | 1474.6847 | 1474.6459 | 2.63 | S1Hex3HexNAc3 | 1.03 |
| 19 | 1560.7215 | 1560.6787 | 2.74 | S1NeuAc1deoxyHex1Hex2HexNAc2 | 1.05 |
| 20 | 1590.7320 | 1590.6893 | 2.69 | S1NeuGc1deoxyHex1Hex2HexNAc2 | 3.85 |
| 21 | 1620.7426 | 1620.7000 | 2.63 | S1NeuGc1Hex3HexNAc2 | 1.14 |
| 22 | 1648.7739 | 1648.7312 | 2.59 | S1deoxyHex1Hex3HexNAc3 | 0.69 |
| 23 | 1661.7691 | 1661.7257 | 2.61 | S1Neu5Gc1Hex2HexNAc3 | 0.79 |
| 24 | 1678.7845 | 1678.7398 | 2.66 | S1Hex4HexNAc3 | 0.68 |
| 25 | 1807.8270 | 1807.7799 | 2.61 | S1Neu5Gc2Hex2HexNAc2 | 0.71 |
| 26 | 1822.8631 | 1822.8119 | 2.81 | S1deoxyHex2Hex3HexNAc3 | 0.67 |
| 27 | 1835.8583 | 1835.8109 | 2.58 | S1NeuGcdeoxyHex1Hex2HexNAc3 | 0.34 |
| 28 | 1865.8689 | 1865.8270 | 2.25 | S1NeuGc12Hex3HexNAc3 | 0.16 |
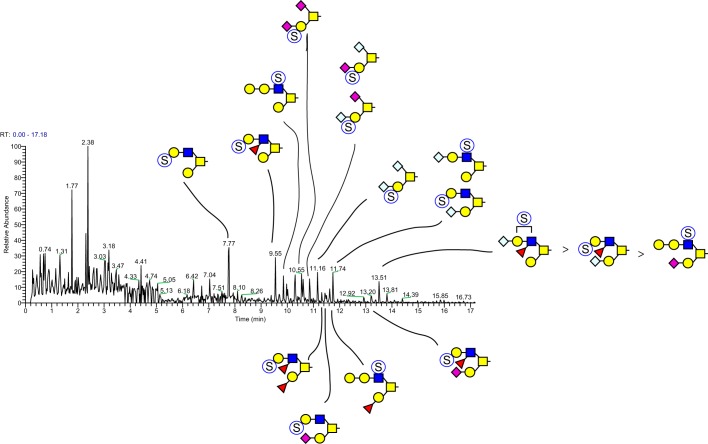
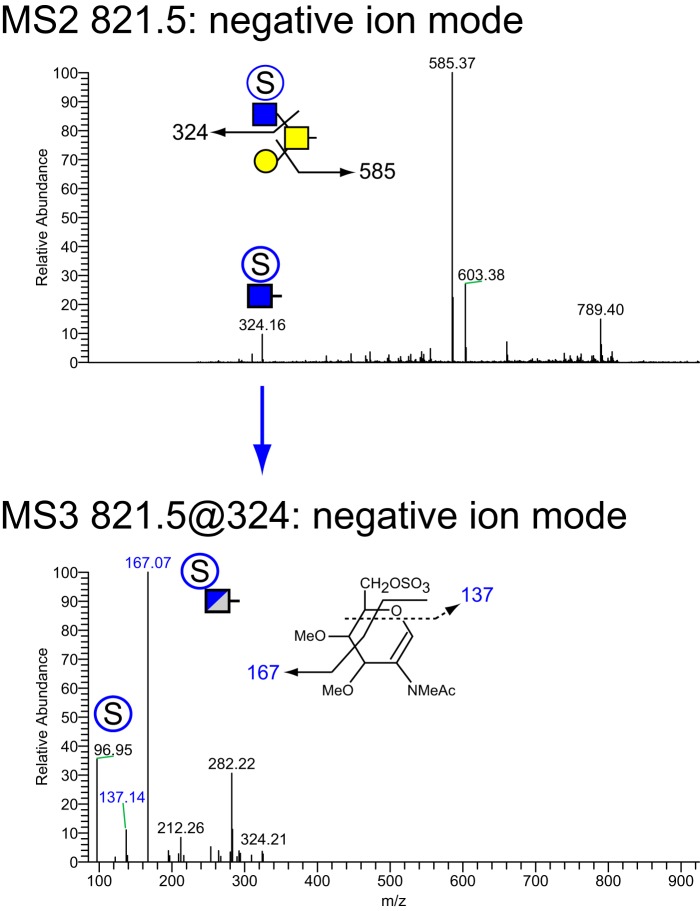
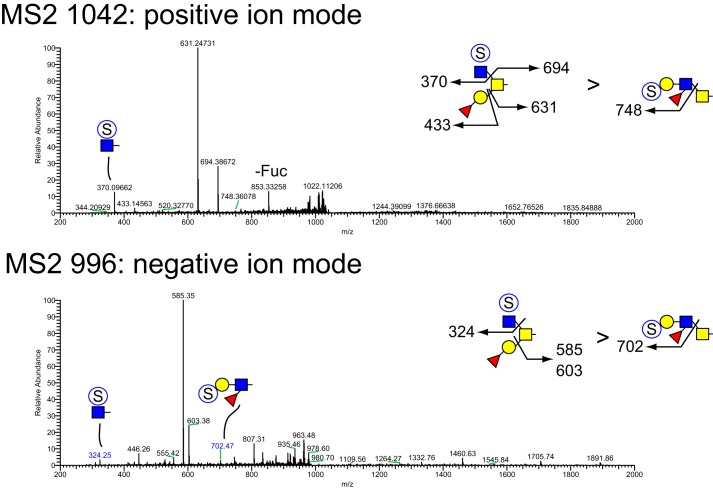
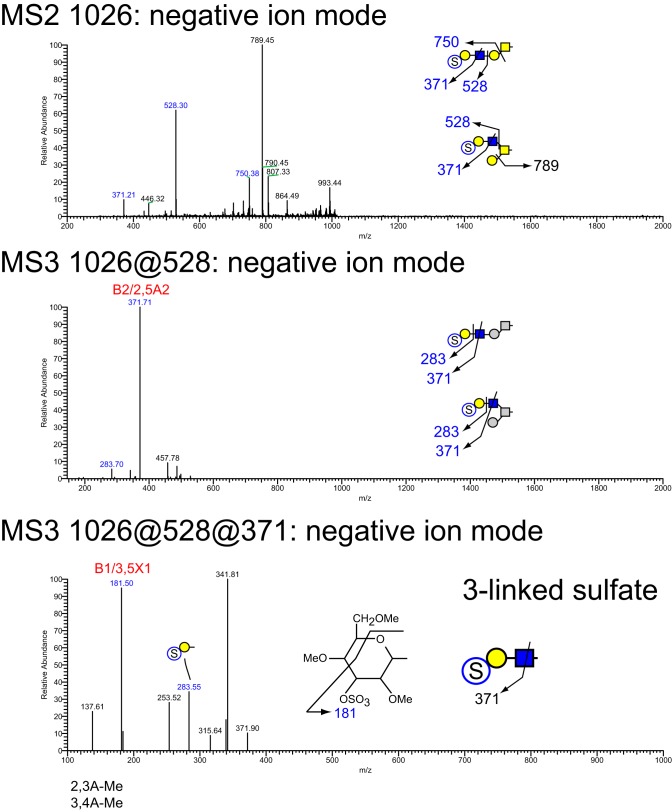
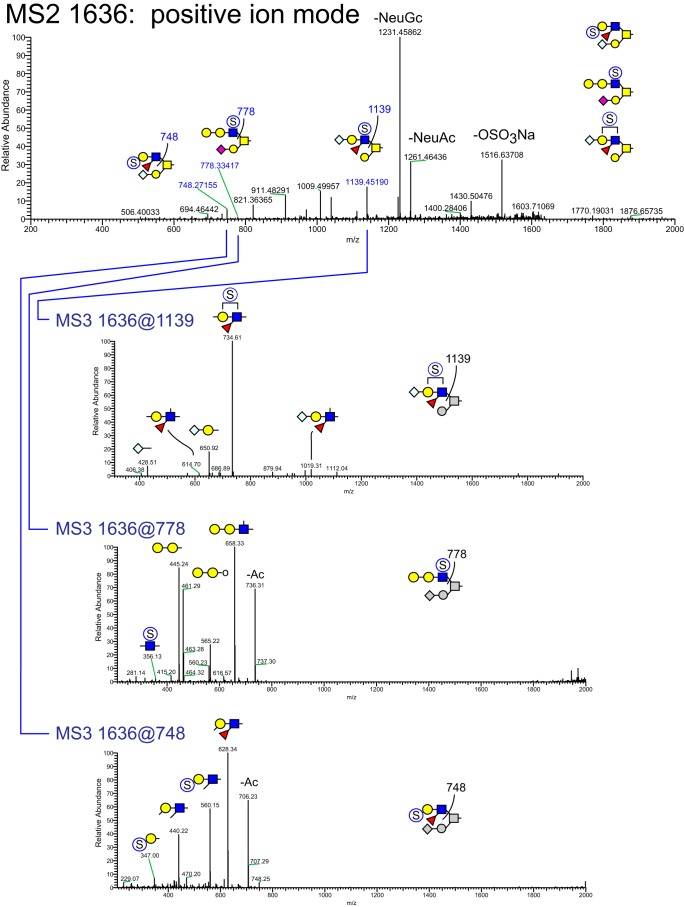
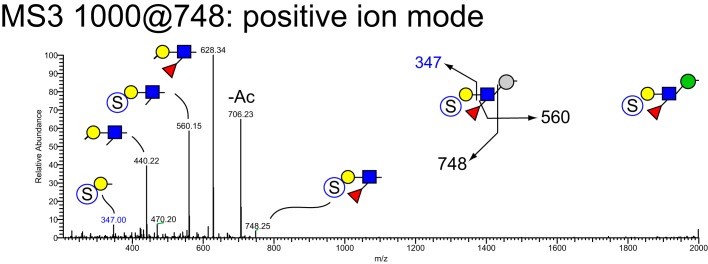
MSn analysis in positive and negative mode identified 41 isomers among these 28 compositions whose sulfation positions and isomeric topologies were assigned by MSn analysis (Fig. 7). For example, the most abundant sulfated O-glycan (structure 2, Table 1) was shown to be sulfated at the 6-position of its core 2 type branching HexNAc residue by the detection of two informative cross-ring fragments generated by MS2 at m/z 324, namely the 3,5A fragment at m/z 167 and the 0,4X fragment at m/z 137 (Fig. 8). A monofucosylated form of structure 2 was detected at m/z 1042 in positive mode and m/z 996 in negative mode along with an isomeric core 3 type glycan carrying a suLe (sulfo-Gal) motif (Fig. 9). At m/z 1026 in negative ion mode, two isomeric glycans were detected, one being a core 1 type with a sulfo-LacNAc extension and the other a core 2 type capped with a sulfated Gal (Fig. 10). The ion observed at m/z 750 in MS2 indicates the presence of the liner core 1 type O-glycan, and the ion at m/z 789 indicates branched core 2 type. Both O-glycans carry the same terminal epitope, sulfated LacNAc with a 3-sulfated Gal residue, indicated by the signature MS3 ion at m/z 371 and the MS4 cross-ring 3,5X fragment at m/z 181, which distinguishes between 3- and 6-sulfation. O-Glycans possessing sialic acid and sulfate were also detected. The two most abundant of these glycans were detected as a sulfo-sialyl-core 1 disaccharide (structure 3, Table 1) and a more complex mixture of extended and branched core 2 glycans (structure 20, Table 1). MSn analysis of the more complex sulfo-sialylated glycans at m/z 1636 in positive mode revealed the presence of suLe motifs as well as Gal-Gal and NeuGc constituents (Fig. 11). Although these sialylated glycans were detected, no sialylated LeA structures were observed. In addition to sulfated glycans initiated by GalNAc linked to Ser/Thr, a single sulfated O-Man glycan was detected (structure 4, Table 1). Signature fragments at MS3 demonstrated that the terminal Gal residue carries the sulfate modification on the O-Man glycans (Fig. 12).
Figure 7.
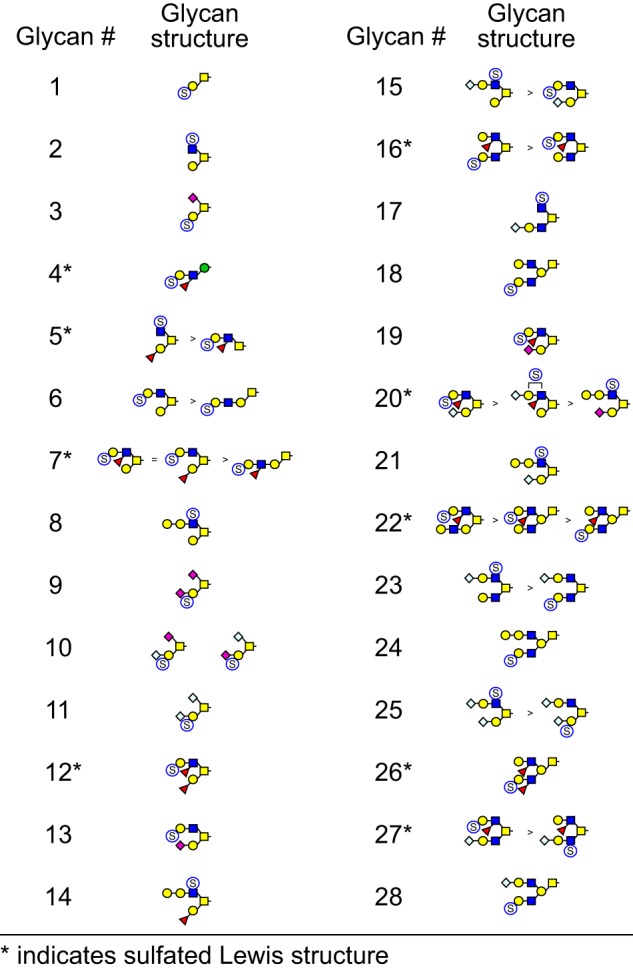
Sulfated O-glycans of bovine oviduct epithelial cells. TIM analysis identified 28 m/z values that exhibited MS/MS fragmentation consistent with neutral loss of sulfate. Subsequent NSI-MSn analysis revealed the isomeric complexity associated with these m/z values. A total of 42 isomeric structures were detected, nine of which yielded fragmentation consistent with the presence of suLeA epitopes.
Figure 8.
NSI-MSn analysis of sulfated O-glycan structure 2. The glycan composition detected at m/z = 821.5 in negative mode (Table 1 and Fig. 7) was fragmented to determine the site and position of sulfation. Informative cross-ring fragments placed the sulfate at the 6-position of the branching HexNAc residue of this core 2 glycan.
Figure 9.
NSI-MSn analysis of sulfated O-glycan structure 5. The glycan composition detected at m/z = 995.4 in negative mode (Table 1 and Fig. 7) or m/z = 1042 in positive mode was fragmented to detect the site and position of sulfation and the presence of Lewis-type structural features. Two isomeric species were detected, one bearing sulfate on a branching HexNAc, similar to structure 2, the other bearing sulfate on a Lewis-type structure.
Figure 10.
NSI-MSn analysis of sulfated O-glycan structure 6. The glycan composition detected at m/z = 1025.4 in negative mode (Table 1 and Fig. 7) was fragmented to determine the site and position of sulfation. Two isomeric species were detected, one bearing sulfate on an extended core 1 type glycan and the other bearing sulfate on a core 2 type glycan. In both cases, cross-ring fragments detected at MS3 were consistent with sulfation at the 3-position on the terminal Hex of a LacNAc moiety.
Figure 11.
NSI-MSn analysis of sulfated O-glycan structure 20. The glycan composition detected at m/z = 1636 in positive mode, corresponding to 1590.7 in negative mode (Table 1 and Fig. 7), was fragmented to detect the site and position of sulfation and the presence of Lewis-type structural features. Three isomeric core 2 structures were detected at this m/z ratio, two of which contain Lewis-type epitopes. One of these is sulfated on a terminal Hex residue (see MS3 at m/z = 748), and the other is a sialylated Lewis glycan (see MS3 and 1139) whose sulfation position was not determinable from the fragmentation achieved (Hex or HexNAc). The third glycan (see MS3 at m/z = 778) is extended by a Hex residue and is sulfated on its HexNAc.
Figure 12.
NSI-MSn analysis of sulfated O-glycan structure 4. The glycan composition detected at m/z = 1000 in positive mode, corresponding to 955.4 in negative mode (Table 1 and Fig. 7), was fragmented to detect the site and position of sulfation and the presence of Lewis-type structural features. The glycan composition and fragmentation pattern are consistent with an O-Man–initiated structure with a Lewis-type nonreducing terminus bearing a sulfated Hex residue. The sulfate group was detected at the 3-position of the terminal hexose through informative cross-ring fragments generated in negative mode.
suLeA is present in the luminal epithelium of the isthmus and ampulla
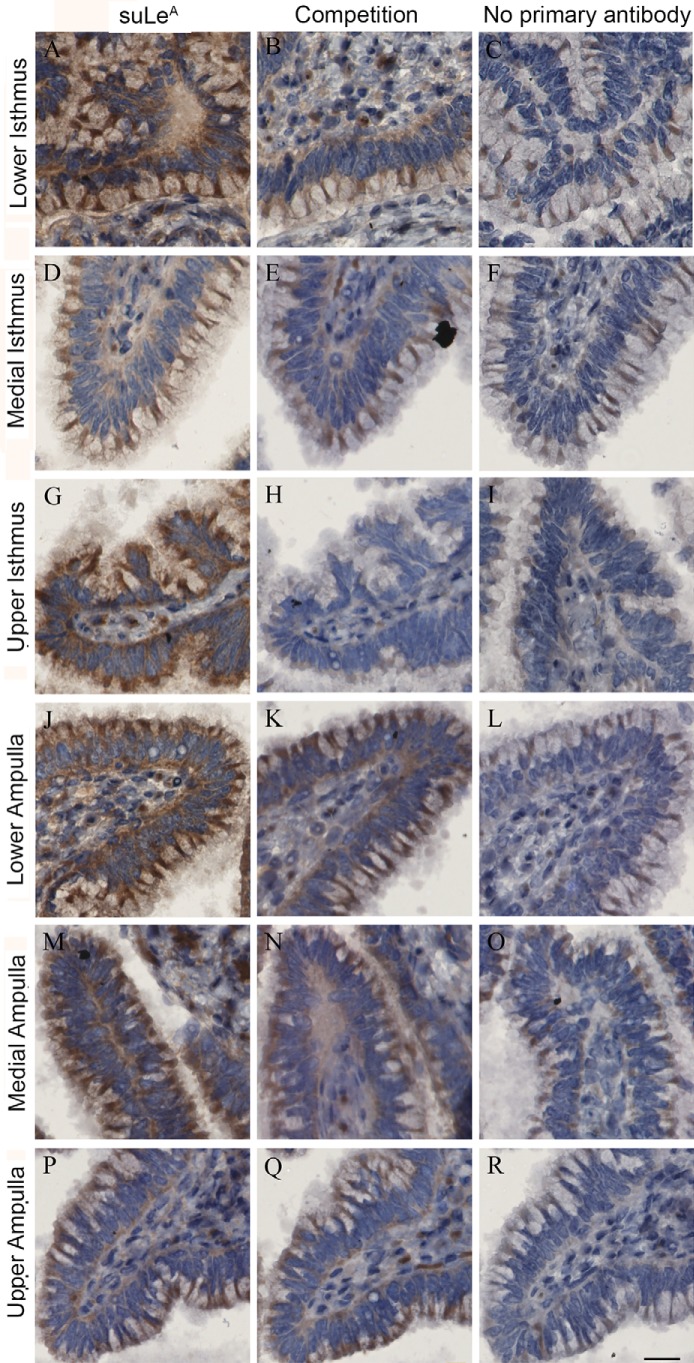
Because MS distinguishes structures based on mass/charge ratio, and anomericity and linkage positions are not always identified, structural analysis did not differentiate between the positional isomers, LeA and LeX, in the oviduct. To confirm whether the Lewis structures identified by MS included suLeA and to determine the precise location of suLeA, we used immunohistochemistry. Bovine oviducts were collected from animals in proestrus/estrus and in diestrus, and serial sections were made from the upper, medial, and lower regions of the isthmus and ampulla. The sections were incubated with a LeA antibody (Clone ID MA17) (32). The specificity of this antibody was initially confirmed using specific biotinylated glycans immobilized to Sepharose beads; results show that MA17 recognized both unsulfated and sulfated LeA (Figs. S1 and S2). Immunostaining was localized to the luminal surface of isthmic and ampullary epithelial cells of all regions analyzed. Glycoproteins containing LeA/suLeA were present on the isthmic and ampullary epithelium in a punctate pattern on both ciliated and nonciliated cells (Fig. 13, A, D, G, J, M, and P). For further confirmation of the specificity of MA17 for suLeA, the antibody was preincubated with 25 μg/ml unlabeled suLeA (2 times the lowest concentration of fluoresceinated suLeA that yielded fluorescence when incubated with sperm) before incubating with sections. We observed that preabsorbing the antibody reduced staining on all sections (Fig. 13, B, E, H, K, N, and Q). We further verified this step by preincubating the MA17 antibody with 25 μg/ml unlabeled suLeX (Figs. S1 and S2). We found that preincubation of MA17 antibody with suLeX did not block the activity of the antibody, and it labeled the sections the same as MA17 antibody used alone (Fig. S1 and S2). Another specificity control was performed in which incubation with the primary antibody (MA17) was not done. In that control, we observed very little staining (Fig. 13, C, F, I, L, O, and R). Thus, at least some of the sulfated Lewis trisaccharides detected by MS were suLeA.
Figure 13.
suLeA is present in the luminal epithelium of isthmus and ampulla. Isthmus sections collected from the lower (near the utero-tubal junction, A–C), upper (near the ampullar-isthmic junction, G–I), and medial regions (between the upper and lower junctions, D–F) were labeled with a LeA/suLeA antibody (MA17). Ampulla sections were collected from three different regions, the lower (near the ampullary-isthmus junction, J–L), medial (M–O), and upper ampulla (near the infundibulum, P–R). These sections were similarly stained with MA17 antibody. Sections on the left were labeled with MA17 (A, D, G, J, M, and P), those in the middle (competition) were labeled with MA17 preincubated with 25 μg/ml suLeA (B, E, H, K, N, and Q), and those on the right (control) were prepared without primary antibody (C, F, I, L, O, and R). Scale bar, 20 μm. All of the sections are from a single proestrus/estrus animal and are representative of three animals.
Binding of sperm to suLeA-coated beads prolongs sperm lifespan
To determine whether sperm binding to immobilized suLeA under in vitro conditions could mimic effects of in vivo sperm binding to oviduct epithelial cells by prolonging sperm lifespan, we coupled biotinylated suLeA to streptavidin-Sepharose beads and incubated these beads with sperm for 96 h (Fig. 14). Immobilization of glycans by coupling to beads was designed to mimic the insolubility of cell-bound glycans and has the advantage of the ease with which glycan-bound sperm can be distinguished. At 0.5, 8, 24, 48, 72, and 96 h of incubation, aliquots of suLeA-coated beads with bound sperm were removed and stained with SYBR14 and PI to label live and dead sperm, respectively (Fig. 14B) (33). Free sperm at each time were also stained with SYBR14 and PI and assessed for sperm viability (Fig. 14D). As a control to assess any general effects of sperm tethering to beads, sperm were incubated with laminin-coated beads (Fig. 14C) (34). The percentage of sperm bound to immobilized suLeA or laminin that were alive at 0.5 h was 75–80% (Fig. 15A), not different from the percentage of free sperm that were alive. Over the next 96 h, the percentage of free-swimming sperm that were alive decreased to about 10%, but about 60% of the sperm bound to immobilized suLeA were alive. Over the 96-h period, more sperm that bound to immobilized suLeA were alive than those bound to immobilized laminin or those that were free-swimming (Fig. 15A). Furthermore, a higher percentage of sperm bound to immobilized laminin were alive over the 96-h period than in droplets of free-swimming sperm.
Figure 14.
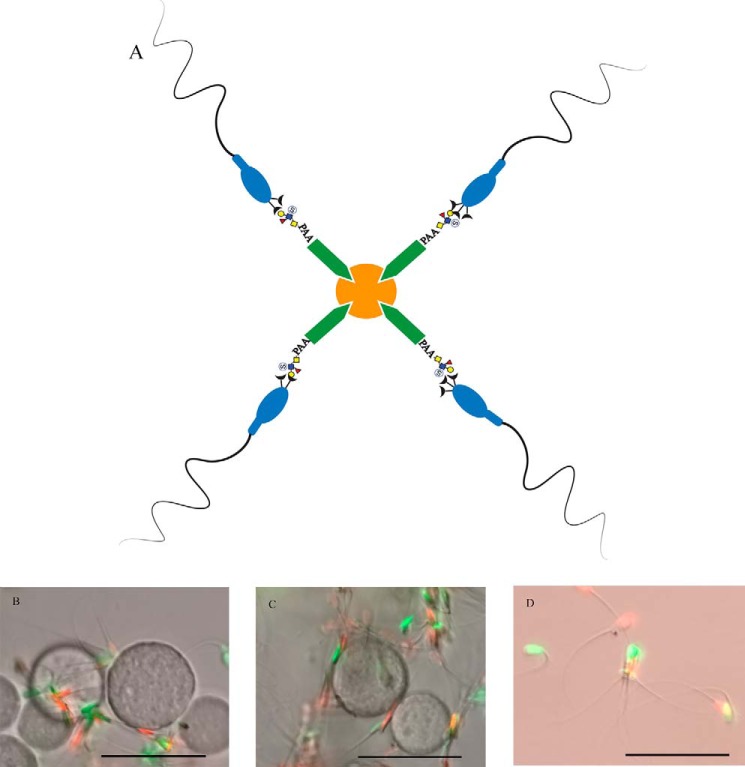
Sperm bind to suLeA-beads. A schematic shows how biotinylated suLeA or laminin were attached to streptavidin-agarose beads (A). Sperm that bound to suLeA-beads (B) or laminin-beads (C), or were free-swimming (D) were incubated for 8 h and labeled with SYBR14 (green, live) and PI (red, dead). Scale bar, 50 μm.
Figure 15.
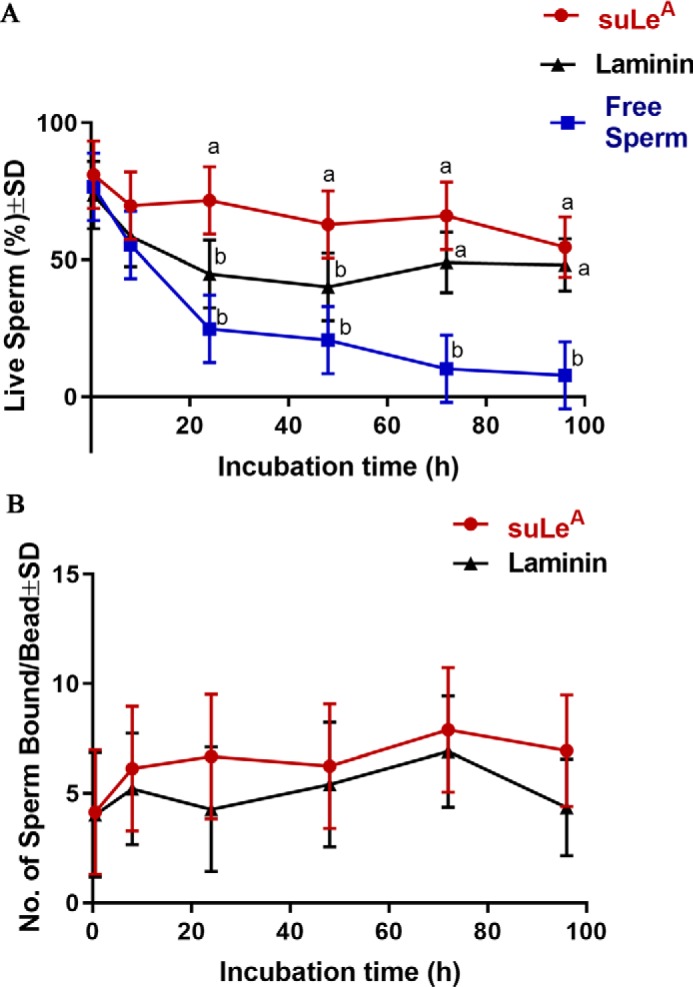
Binding of sperm with suLeA-coated beads prolongs sperm lifespan. Sperm were incubated with suLeA- and laminin-coated beads for 96 h. Aliquots were stained with SYBR14 (live) and PI (dead) sperm at various times. Free sperm at these times were also collected and stained with SYBR14 and PI (A). The total number of sperm bound to each bead was calculated at each time point and expressed as total sperm bound/bead (B). The experiments were replicated five times, and data were plotted as mean ± S.D. (error bars). Within each specific incubation time point, the means labeled with different letters (a or b) were significantly different (p < 0.05).
The possibility that the difference in viability could be accounted for by dead sperm detachment from the beads was considered. If dead sperm detached, one would expect to observe a loss in the number of sperm bound to beads. Therefore, the total number of sperm bound to each suLeA and laminin-covered bead at each of the time points was enumerated (Fig. 15B). There was no decrease in the number of sperm bound to beads, and in fact, the number of sperm bound to suLeA at 72 h was significantly higher compared with the number of sperm bound at 0.5 h. Thus, binding to suLeA immobilized on beads extended the lifespan of sperm; there was no bias due to release of dead sperm.
Discussion
There are several salient findings in this report. First, the head of bovine sperm binds to both soluble and immobilized suLeA trisaccharide. Binding is specific because, although sialylated Lewis A also binds sperm, unsulfated LeA or the structurally related suLeX binds far fewer sperm and because an excess of unlabeled suLeA can competitively reduce binding of the fluoresceinated suLeA. After capacitation, far fewer sperm bind suLeA. suLeA epitopes were found on O-linked glycans attached to oviduct epithelial cell glycoproteins. Finally, binding to immobilized suLeA simulates some effects of the oviduct epithelium because it can lengthen the lifespan of sperm.
Results herein show that the sulfated trisaccharide, suLeA, binds to the head of bovine sperm. Most glycan-binding proteins have relatively low affinity for their glycan ligands. But there is compelling evidence that many cell surface glycoproteins and glycolipids function as multimers to form higher-order aggregates that increase overall avidity (35). Our experiments were designed to mimic the multivalent interaction. In all experiments in this report, multiple copies of suLeA were attached to a 30-kDa polyacrylamide core to provide the multivalent interactions that this trisaccharide would have with sperm if there were multiple copies of suLeA on a large oligosaccharide or a glycoprotein.
Sperm binding to suLeA was highly specific. Of the glycans tested, a much higher percentage of bovine sperm bound to fluoresceinated suLeA trisaccharide than to its nonsulfated version (LeA). The binding to sialylated Lewis A as well as suLeA suggests that negative charge is important. Binding to fluoresceinated suLeA could be reduced by the addition of an excess of unlabeled suLeA, providing further indication of the specific nature of this interaction. The phenomenon where the addition of a sulfate group to a glycan can regulate its affinity for a lectin has also been observed in other systems, such as lymphocyte homing (36). Bovine sperm had little affinity for suLeX, the positional isomer of suLeA or LeX. Previous studies reported that LeA trisaccharide bound to bovine sperm, but these studies did not examine suLeA (25). Interestingly, porcine sperm do not bind to LeA or suLeA but, instead, bind to suLeX (37). This is consistent with many reports suggesting that sperm from different species have affinity for glycans with distinct terminal monosaccharides (20–23). The observation that bovine and porcine sperm have affinity for different Lewis trisaccharides is intriguing and demonstrates the remarkable specificity of sperm interaction with glycans. Other systems that involve carbohydrate recognition also require a high degree of lectin-glycan specificity, including human sperm binding to glycans in the zona pellucida (38, 39), host–pathogen cell interactions (40), and cancer cell proliferation and survival (41).
In addition to the specificity, the affinity of the interaction between suLeA and sperm must be relatively high because of the affinity necessary to tether a motile sperm (42). The affinity was reduced after capacitation, as we observed a reduction in the percentage after capacitation that still bound suLeA. It is uncertain whether this is due to changes in the function of the plasma membrane and resident lectins or to proteolysis by released proteasomes (43), but this loss in affinity for suLeA during capacitation may contribute to the release of sperm from the reservoir so that they can fertilize oocytes.
Even prior to capacitation, almost 50% of the sperm did not bind fluoresceinated suLeA. The reason for this is unclear and could be due to sperm heterogeneity or to unknown technical reasons. There may be a second glycan-based interaction or perhaps a protein-based interaction that allows these sperm to bind to oviduct cells, or perhaps these sperm are simply incapable of binding to oviduct cells.
Bovine oviduct epithelial cell glycans were analyzed by nanospray ionization linear ion trap (NSI)-MSn to assess the diversity and relative abundance of sulfated Lewis-type glycans. Sulfated Lewis structures were found predominantly on O-linked glycans initiated by GalNAc residues, consistent with their existence on mucin proteins, but direct confirmation of the proteins that carry sulfated Lewis epitopes remains to be determined. Sialylated Lewis A was not detected. Whereas MSn provides a very sensitive means to detect glycan structural compositions and topologies, the assignment of linkage anomericity and linkage position is more challenging. Therefore, we used immunohistochemistry to confirm the identity of Lewis trisaccharides and determine their cellular expression patterns. LeA was found on both ciliated and nonciliated cells of the oviductal epithelium, and the pattern of expression was similar at pro-estrus/estrus and diestrus. The abundance throughout the estrous cycle is consistent with previous observations that the stage of the estrous cycle does not affect sperm binding to the oviduct in cattle (44). The staining pattern of suLeA was uniform on the apical ridge of the entire bovine oviduct epithelium, which is similar to the uniform apical staining pattern of 6′-sialylated biantennary glycans that we observed previously in the sow oviduct (24). In contrast, the immunoreactivity for suLeX in the sow oviducts was present more in a punctuate fashion (37) and limited to the isthmus. That LeA is found throughout the oviduct provides support for the hypothesis that the reason the sperm reservoir is formed in the isthmus rather than the ampulla is because sperm first encounter suLeA in the isthmus and are retained there to form the sperm reservoir. Recent studies using mice revealed that sperm could also remain attached to the ampullar epithelium once they have passed through the isthmus (45, 46). It appears that sperm continue to adhere intermittently to the oviductal epithelium as they move through the oviduct.
Potential receptors for suLeA found on bovine sperm are unknown; however, a candidate receptor for LeA, a 16.5-kDa sperm protein known as Binder of Sperm1 (BSP1) has been identified by affinity chromatography using immobilized LeA (27). BSP1 is synthesized and secreted by seminal vesicles (47) and is adsorbed onto sperm during contact with seminal plasma secretions at ejaculation (26). Bull epididymal sperm lacking BSP1 on their membranes show reduced binding to oviductal epithelium under in vitro conditions (27), although in vivo fertility of cauda epididymal sperm appears normal (48). Two other BSP proteins, BSP3 and BSP5, produced by the seminal vesicles act in conjunction with BSP1 to promote sperm binding to the oviduct (49). All three BSPs contain a unique N-terminal domain followed by two FN2 domains with heparin- and phospholipid-binding sites (50). Although they bind heparin, fibronectin, and phospholipids, it is uncertain whether these proteins have a function beyond sperm adhesion to the oviduct.
Binding to oviduct cells affects sperm function. Sperm bound to oviduct cells have decreased intracellular free Ca2+, which could suppress capacitation and maintain sperm viability (12, 30, 51). Such effects on viability are also seen in vitro when sperm are incubated with oviductal apical plasma membrane proteins rather than live oviduct cells (52, 53). When we attached suLeA to beads to immobilize them, the percentage of live sperm was increased dramatically, compared with free-swimming sperm. The effect of binding to an oviduct glycan was greater than simply providing an anchor to sperm because sperm bound to laminin-coated beads had reduced viability compared with sperm bound to suLeA-coated beads. But we also observed that providing an anchor to sperm using laminin-coated beads increased their lifespan compared with free-swimming sperm. The underlying basis for the prolonged lifespan in laminin and suLeA-anchored sperm is intriguing.
In summary, we show that (i) suLeA binds to the head of bull sperm and binding is decreased following capacitation, (ii) suLeA motifs are attached primarily to serine/threonine residues of proteins in the bovine oviductal epithelium, and (iii) co-incubation of sperm with suLeA-coated beads under in vitro conditions promotes sperm longevity. The interaction between sperm and oviduct glycans not only retains sperm in a reservoir but also can affect sperm function to lengthen their fertile life in situations where mating and ovulation are not well-synchronized.
Experimental procedures
Collection and processing of sperm
All experiments were performed using freshly collected bovine semen pooled from different groups of at least five bulls sampled from >25 bulls at Genex Cooperative, Inc. (Tiffin, OH) and diluted in extender. The extended semen was cooled to 17 °C, shipped to the laboratory, and processed within 48 h after collection. Pooled extended semen (1 ml) was washed through a Percoll gradient containing a mixture of 4 ml of noncapacitating medium (NC-TALP; 2.1 mm CaCl2, 3.1 mm KCl, 1.5 mm MgCl2, 100 mm NaCl, 0.29 mm KH2PO4, 0.36% lactic acid, 0.6% polyvinyl alcohol, 1 mm pyruvic acid, 35 mm HEPES (pH 7.3), filter-sterilized), 0.6 ml of 10× HBS (1.3 m NaCl, 40 mm KCl, 10 mm CaCl2, 5 mm MgCl2, 140 mm fructose, 5% Fraction V BSA, sterile-filtered), and 5.4 ml of Percoll for 10 min at 800 × g. The resulting pellet was resuspended in 5 ml of NC-TALP and centrifuged for 3 min at 600 × g. The final sperm concentration was estimated by a hemocytometer. Only those samples ≥80% motility were used for the experiments.
Localization of glycan binding to sperm
Labeling of noncapacitated and capacitated sperm with fluoresceinated Lewis structures was performed to identify the sites of glycan binding. Sperm concentration was adjusted to 2 × 107 cells/ml. The noncapacitated sperm group was incubated for 4 h at 39 °C under 5% CO2 in NC-TALP. Another sperm group were capacitated by incubating sperm with C-TALP (2.1 mm CaCl2, 3.1 mm KCl, 1.5 mm MgCl2, 100 mm NaCl, 0.29 mm KH2PO4, 0.36% lactic acid, 26 mm NaHCO3, 0.6% BSA, 1 mm pyruvic acid, 20 mm HEPES, pH 7.3, 10 units/ml penicillin, 10 μg/ml streptomycin) containing 10 μg/ml heparin for 4 h at 39 °C. Sperm were immobilized with 0.01% formaldehyde, a concentration that does not permeabilize sperm, and incubated with various glycans coupled to a 30-kDa fluoresceinated polyacrylamide chain. From 0.8 to 0.9 μmol of each glycan was coupled to 1 mg of polyacrylamide (54). Each fluorescein-conjugated glycan was added to the sperm suspension at 50 μg/ml (55) final concentration and incubated for 30 min at 39 °C. After the incubation, 10 μl of the sperm were transferred onto a microscope slide and covered. The samples were observed under fluorescence microscopy and DIC optics using a Zeiss Axioskop and Plan-Neofluar ×63/1.25 oil objective. Images were captured with an AxioCam Hc camera and analyzed using Axiovision version 4.5 software. A minimum of 100 sperm were observed for each group in each experiment.
Delipidation and protein enrichment of bovine oviduct epithelial cells
Cells from 70 freshly collected bovine oviducts (supplied by Applied Reproductive Technology, Monona, WI) were stripped from the oviducts and centrifuged at 10,000 rpm for 10 min at 4 °C. The supernatant was aspirated, and the pellet was suspended in a total volume of 1 ml of C-TALP. Using a 1000-μl pipette, the cells were pipetted 20 times. The cells were brought to 3–5 ml and further disaggregated by pipetting with a 22-gauge needle and a 1-ml serological syringe (20 times). The disaggregated cells were counted using a hemocytometer (average number of cells was 1.3 × 108). To enrich for glycoproteins, the oviduct isthmic epithelial cells were delipidated as described previously (24, 56, 57). Briefly, epithelial cells were disrupted on ice by Dounce homogenization (24, 56, 57) to extract lipids with a solvent mixture of chloroform/methanol/water to 4:8:3 by volume. The insoluble proteinaceous material was collected by centrifugation for 10 min, and the clarified supernatant was set aside. The extracted lipids were combined and dried under a nitrogen stream. The precipitated protein fractions were washed with aqueous acetone (20% water). The final insoluble pellets were dried under a nitrogen stream, providing protein powder for protein-linked glycan analysis.
N-Glycan release from bovine oviduct epithelial cells
Bovine oviduct N-glycans were prepared as described previously (24, 56, 57). Briefly, 4 mg of oviduct protein powder was digested with a mixture of trypsin and chymotrypsin (25 μg each). Using peptide-N-glycosidase F, N-glycans were released from glycopeptides that were enriched by C18 Sep-Pak chromatography. Released N-glycans were permethylated for MS analysis. Permethylated N-glycans were further separated into nonsulfated glycan and sulfated glycan populations by water-DCM partitioning (58, 59).
O-Glycan release from bovine oviduct epithelial cells
Bovine oviduct O-glycans were prepared as described previously (60). Briefly, 5 mg of oviduct protein powder was subjected to O-glycan release by reductive β-elimination. O-Glycans were purified by desalting on a cation-exchange ion chromatography column. The released O-glycans were lyophilized, purified on Sep-Pak C18 cartridge columns, and permethylated for MS analysis. Sulfated O-glycans were enriched by water-DCM partition following permethylation (58, 59).
Mass spectrometry for glycan analysis
N-glycans, O-glycans, and glycosphingolipids were analyzed by NSI-MS (LTQ-Orbitrap, Thermo Fisher Scientific) as described previously (24, 56–59, 61). Briefly, nonsulfated glycans recovered in the DCM phase following permethylation were analyzed in positive ion mode, and sulfated glycans recovered in water phase were analyzed in both positive and negative ion mode. The TIM functionality of the Excalibur control software was used to screen for sulfated glycan species by filtering for the NL of sulfate groups in positive ion mode. MSn analyses were carried out in both positive and negative ion modes to characterize glycan structures. To calculate mass accuracy for the detected glycan species, theoretical m/z values were obtained using GlycoWorkbench (62). Graphical representations of monosaccharide residues are shown in the middle panel of Fig. 4 and are consistent with the Symbol Nomenclature for Glycans (SNFG), which has been broadly adopted by the glycomics community (63). Most permethylated glycans were identified as singly or doubly charged, sodiated species [M + Na] in positive mode or as singly charged [M − H] species in negative mode. All spectra were manually interpreted and annotated. The explicit identity of individual monosaccharide residues was assigned based on the known biosynthetic pathways for N-linked, O-linked, and glycosphingolipid glycans in vertebrates. The MS-based glycomics data generated in these analyses and the associated annotations are presented in accordance with the MIRAGE standards and the Athens Guidelines (64, 65).
Immunohistochemical localization of LeA trisaccharide
Bovine oviducts were collected and sorted by reproductive stage. The stages included were proestrus/estrus (small follicles in the range of 1–3 mm in diameter in both ovaries, dominant follicle at 4–6 mm in diameter, no corpus luteum), diestrus (corpus luteum in one or two of the ovaries in the range of 0.5–2 cm), and anestrus (no follicles >1-mm diameter and no corpus luteum) (66–68). Each isthmus was equally divided into three regions. The lower region was located 10 mm from the uterine-isthmic junction, the upper region was at the ampullary-isthmic junction, and the medial region was halfway between the lower and upper regions. Each isthmic region was ∼20 mm long. Immunohistochemical procedures were carried out on oviductal tissues fixed in 10% neutral-buffered formalin overnight at room temperature. The tissues were dehydrated in 70% ethanol and embedded in paraffin in an automated Leica ASP300 S tissue processor (Leica Microsystems Inc.). The paraffin blocks were sectioned at 5-μm thickness and mounted on glass slides for staining with antibody. The samples were deparaffinized and rehydrated (69). For antigen retrieval, the sections were boiled in a pressure cooker for 10 min (time calculated after steaming) in 1× Dako Target Retrieval Solution, pH 9 (Agilent Technologies). The pressure cooker was allowed to cool to room temperature for 2 h. The sections were then treated with Bloxall endogenous peroxidase and alkaline phosphatase blocking solution (Vector Laboratories) for 10 min for inactivation of endogenous peroxidase and alkaline phosphatase. The samples were then incubated with mouse anti-LeA antibody (Clone ID MA17), (ETS-Bretagne, France) (32) at 1:50 dilution for 24 h at room temperature. All immunoreactions were performed using a Vectastain Elite ABC horseradish peroxidase kit (Vector Laboratories). The sections were incubated with a biotinylated anti-mouse secondary antibody and an avidin/biotin-based peroxidase system. Peroxidase activity was detected on the basis of oxidation of 3,3′-diaminobenzidine tetrahydrochloride producing a brown color at the sites of antibody binding. The sections were counterstained with hematoxylin to detect the nuclei (purple). Dehydration, clearing, and mounting of sections followed standard hematoxylin and eosin protocols (69). Images of sections were documented using the Nanozoomer slide scanning system (Hamamatsu).
Viability of sperm bound to glycans coupled to beads
Glycans coupled to a biotinylated polyacrylamide core were attached to Streptavidin-Sepharose High Performance beads (GE Healthcare). The average diameter of the agarose beads was 34 μm. Each 30-kDa molecule of polyacrylamide had 20% glycan and 5% biotin by molar ratio. Approximately 60 μg of biotinylated glycan conjugate were incubated with 20 μl of streptavidin-Sepharose beads for 90 min at 22 °C in PBS, and then beads were washed twice. Once the glycan-coupled beads were ready for use, 2 μl of glycan-coupled beads were incubated with 100-μl droplets containing 2 × 105 sperm/ml. As a control for sperm binding to something other than defined glycans, biotinylated laminin (Cytoskeleton, Inc.) at a concentration of 1 mg/ml was used to coat the streptavidin-coated beads. 60 μg of biotinylated laminin was incubated with 20 μl of streptavidin-Sepharose beads, and the beads were prepared by the above method.
The droplets were covered with mineral oil and incubated in 5% CO2 and 95% air. Sperm and beads were incubated for 0.5, 8, 24, 36, 72, and 96 h at 39 °C and then stained with 100 nm SYBR14 (labels live) and 12 μm PI (labels dead) and observed by fluorescence microscopy. Nonspecific binding was determined by incubating sperm with 3′-O-sulfated LeX (suLeX), a positional isomer of suLeA, coupled to beads.
Statistical analysis
Results are presented as mean ± S.D., and differences were considered to be significant if p was ≤0.05 (Figs. 1–3). For Figs. 2 and 3, unpaired t tests (Mann–Whitney U test) were performed. To analyze the change in the percentage of live sperm over a period of 96 h, the percentages of live sperm under each condition at each time point were compared by two-way analysis of variance followed by Dunnett's multiple-comparison test using GraphPad Prism version 7 (GraphPad Software, Inc.). For Fig. 15, data were analyzed by two-way analysis of variance. Tukey's multiple-comparison test was used to identify differences between means. Data were plotted as mean ± pooled S.D.
Author contributions
S. D., K. A., M. T., N. B., and D. J. M. designed the experiments; S. D., K. A., and K. D. performed the experiments; S. D., K. A., K. D., M. T., and D. J. M. analyzed the results; and S. D., K. A., M. T., and D. J. M. wrote the paper.
Supplementary Material
Acknowledgments
The LeA antibody, Clone ID MA17 (ETS-Bretagne, France), was a generous gift from Stephen Henry (Auckland University of Technology). We also acknowledge Drs. Kingsley Boateng and Mayandi Sivaguru (Carl R. Woese Institute for Genomic Biology, University of Illinois) for technical guidance and assistance with immunohistochemistry.
This project was supported by Agriculture and Food Research Initiative Competitive Grant 2015-67015-23228 from the United States Department of Agriculture National Institute of Food and Agriculture and National Science Foundation Grant 1450227 (to D. J. M.); National Institutes of Health (NIH) Common Fund Grant R21AI129873 (to K. A.); NIGMS, NIH, Grant P41GM103490 (to M. T.); and Russian Science Foundation Grant 14-50-00131 (to N. B.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1 and S2.
- LeX
- Lewis X
- LeA
- Lewis A
- MSn
- sequential MS
- PI
- propidium iodide
- GSL
- glycosphingolipid
- LacdiNAc
- GalNAcβ1-4GlcNAc
- TIM
- total ion mapping
- NL
- neutral loss
- DIC
- differential interference contrast
- NSI
- nanospray ionization
- Man
- mannose
- Lac
- lactose
- Hex
- hexose
- LacNAc
- N-acetyl-d-lactosamine
- HexNAc
- N-acetylhexosamine.
References
- 1. Hunter R. H. (1981) Sperm transport and reservoirs in the pig oviduct in relation to the time of ovulation. J. Reprod. Fertil. 63, 109–117 10.1530/jrf.0.0630109 [DOI] [PubMed] [Google Scholar]
- 2. Holt W. V., and Fazeli A. (2016) Sperm storage in the female reproductive tract. Annu. Rev. Anim. Biosci. 4, 291–310 10.1146/annurev-animal-021815-111350 [DOI] [PubMed] [Google Scholar]
- 3. Birkhead T. R., and Moller A. P. (1993) Sexual selection and the temporal separation of reproductive events: sperm storage data from reptiles, birds and mammals. Biol. J. Linnean Soc. 50, 295–311 10.1111/j.1095-8312.1993.tb00933.x [DOI] [Google Scholar]
- 4. Yanagimachi R., and Chang M. C. (1963) Sperm ascent through the oviduct of the hamster and rabbit in relation to the time of ovulation. J. Reprod. Fertil. 6, 413–420 10.1530/jrf.0.0060413 [DOI] [PubMed] [Google Scholar]
- 5. Harper M. J. (1973) Relationship between sperm transport and penetration of eggs in the rabbit oviduct. Biol. Reprod. 8, 441–450 10.1093/biolreprod/8.4.441 [DOI] [PubMed] [Google Scholar]
- 6. Overstreet J. W., and Cooper G. W. (1978) Sperm transport in the reproductive tract of the female rabbit: II. The sustained phase of transport. Biol. Reprod. 19, 115–132 10.1095/biolreprod19.1.115 [DOI] [PubMed] [Google Scholar]
- 7. Hunter R. H., and Nichol R. (1983) Transport of spermatozoa in the sheep oviduct: preovulatory sequestering of cells in the caudal isthmus. J. Exp. Zool. 228, 121–128 10.1002/jez.1402280113 [DOI] [PubMed] [Google Scholar]
- 8. Hunter R. H., and Wilmut I. (1984) Sperm transport in the cow: peri-ovulatory redistribution of viable cells within the oviduct. Reprod. Nutr. Dev. 24, 597–608 10.1051/rnd:19840508 [DOI] [PubMed] [Google Scholar]
- 9. Suarez S. S., Vincenti L., and Ceglia M. W. (1987) Hyperactivated motility induced in mouse sperm by calcium ionophore A23187 is reversible. J. Exp. Zool. 244, 331–336 10.1002/jez.1402440218 [DOI] [PubMed] [Google Scholar]
- 10. Rodríguez-Martínez H., Saravia F., Wallgren M., Tienthai P., Johannisson A., Vázquez J. M., Martínez E., Roca J., Sanz L., and Calvete J. J. (2005) Boar spermatozoa in the oviduct. Theriogenology 63, 514–535 10.1016/j.theriogenology.2004.09.028 [DOI] [PubMed] [Google Scholar]
- 11. Suarez S. S. (2008) Regulation of sperm storage and movement in the mammalian oviduct. Int. J. Dev. Biol. 52, 455–462 10.1387/ijdb.072527ss [DOI] [PubMed] [Google Scholar]
- 12. Suarez S. S., and Pacey A. A. (2006) Sperm transport in the female reproductive tract. Hum. Reprod. Update 12, 23–37 10.1093/humupd/dmi047 [DOI] [PubMed] [Google Scholar]
- 13. Kawakami E., Kashiwagi C., Hori T., and Tsutsui T. (2001) Effects of canine oviduct epithelial cells on movement and capacitation of homologous spermatozoa in vitro. Anim. Reprod. Sci. 68, 121–131 10.1016/S0378-4320(01)00135-X [DOI] [PubMed] [Google Scholar]
- 14. Pollard J. W., Plante C., King W. A., Hansen P. J., Betteridge K. J., and Suarez S. S. (1991) Fertilizing capacity of bovine sperm may be maintained by binding of oviductal epithelial cells. Biol. Reprod. 44, 102–107 10.1095/biolreprod44.1.102 [DOI] [PubMed] [Google Scholar]
- 15. Hunter R. H., and Léglise P. C. (1971) Polyspermic fertilization following tubal surgery in pigs, with particular reference to the role of the isthmus. J. Reprod. Fertil. 24, 233–246 10.1530/jrf.0.0240233 [DOI] [PubMed] [Google Scholar]
- 16. Teijeiro J. M., Dapino D. G., and Marini P. E. (2011) Porcine oviduct sperm binding glycoprotein and its deleterious effect on sperm: a mechanism for negative selection of sperm? Biol. Res. 44, 329–337 10.4067/S0716-97602011000400003 [DOI] [PubMed] [Google Scholar]
- 17. Teijeiro J. M., and Marini P. E. (2012) The effect of oviductal deleted in malignant brain tumor 1 over porcine sperm is mediated by a signal transduction pathway that involves pro-AKAP4 phosphorylation. Reproduction 143, 773–785 10.1530/REP-11-0314 [DOI] [PubMed] [Google Scholar]
- 18. Suarez S. S. (2001) Carbohydrate-mediated formation of the oviductal sperm reservoir in mammals. Cells Tissues Organs 168, 105–112 10.1159/000016811 [DOI] [PubMed] [Google Scholar]
- 19. Töpfer-Petersen E., Ekhlasi-Hundrieser M., and Tsolova M. (2008) Glycobiology of fertilization in the pig. Int. J. Dev. Biol. 52, 717–736 10.1387/ijdb.072536et [DOI] [PubMed] [Google Scholar]
- 20. DeMott R. P., Lefebvre R., and Suarez S. S. (1995) Carbohydrates mediate the adherence of hamster sperm to oviductal epithelium. Biol. Reprod. 52, 1395–1403 10.1095/biolreprod52.6.1395 [DOI] [PubMed] [Google Scholar]
- 21. Lefebvre R., Lo M. C., and Suarez S. S. (1997) Bovine sperm binding to oviductal epithelium involves fucose recognition. Biol. Reprod. 56, 1198–1204 10.1095/biolreprod56.5.1198 [DOI] [PubMed] [Google Scholar]
- 22. Wagner A., Ekhlasi-Hundrieser M., Hettel C., Petrunkina A., Waberski D., Nimtz M., and Töpfer-Petersen E. (2002) Carbohydrate-based interactions of oviductal sperm reservoir formation-studies in the pig. Mol. Reprod. Dev. 61, 249–257 10.1002/mrd.1154 [DOI] [PubMed] [Google Scholar]
- 23. Sabeur K., and Ball B. A. (2007) Characterization of galactose-binding proteins in equine testis and spermatozoa. Anim. Reprod. Sci. 101, 74–84 10.1016/j.anireprosci.2006.08.028 [DOI] [PubMed] [Google Scholar]
- 24. Kadirvel G., Machado S. A., Korneli C., Collins E., Miller P., Bess K. N., Aoki K., Tiemeyer M., Bovin N., and Miller D. J. (2012) Porcine sperm bind to specific 6-sialylated biantennary glycans to form the oviduct reservoir. Biol. Reprod. 87, 147 10.1093/biolreprod/87.s1.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suarez S. S., Revah I., Lo M., and Kölle S. (1998) Bull sperm binding to oviductal epithelium is mediated by a Ca2+-dependent lectin on sperm that recognizes Lewis-a trisaccharide. Biol. Reprod. 59, 39–44 10.1095/biolreprod59.1.39 [DOI] [PubMed] [Google Scholar]
- 26. Ignotz G. G., Lo M. C., Perez C. L., Gwathmey T. M., and Suarez S. S. (2001) Characterization of a fucose-binding protein from bull sperm and seminal plasma that may be responsible for formation of the oviductal sperm reservoir. Biol. Reprod. 64, 1806–1811 10.1095/biolreprod64.6.1806 [DOI] [PubMed] [Google Scholar]
- 27. Suarez S. S. (2016) Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 363, 185–194 10.1007/s00441-015-2244-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ellington J. E., Ignotz G. G., Varner D. D., Marcucio R. S., Mathison P., and Ball B. A. (1993) In vitro interaction between oviduct epithelial and equine sperm. Arch. Androl. 31, 79–86 10.3109/01485019308988384 [DOI] [PubMed] [Google Scholar]
- 29. Suarez S., Redfern K., Raynor P., Martin F., and Phillips D. M. (1991) Attachment of boar sperm to mucosal explants of oviduct in vitro: possible role in formation of a sperm reservoir. Biol. Reprod. 44, 998–1004 10.1095/biolreprod44.6.998 [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez-Martinez H. (2007) Role of the oviduct in sperm capacitation. Theriogenology 68, S138–S146 10.1016/j.theriogenology.2007.03.018 [DOI] [PubMed] [Google Scholar]
- 31. Parrish J. J., Susko-Parrish J., Winer M. A., and First N. L. (1988) Capacitation of bovine sperm by heparin. Biol. Reprod. 38, 1171–1180 10.1095/biolreprod38.5.1171 [DOI] [PubMed] [Google Scholar]
- 32. Williams E., Korchagina E., Frame T., Ryzhov I., Bovin N., and Henry S. (2016) Glycomapping the fine specificity of monoclonal and polyclonal Lewis antibodies with type-specific Lewis kodecytes and function-spacer-lipid constructs printed on paper. Transfusion 56, 325–333 10.1111/trf.13384 [DOI] [PubMed] [Google Scholar]
- 33. Garner D. L., and Johnson L. A. (1995) Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod. 53, 276–284 10.1095/biolreprod53.2.276 [DOI] [PubMed] [Google Scholar]
- 34. Timpl R., Rohde H., Robey P. G., Rennard S. I., Foidart J. M., and Martin G. R. (1979) Laminin–a glycoprotein from basement membranes. J. Biol. Chem. 254, 9933–9937 [PubMed] [Google Scholar]
- 35. Belardi B., O'Donoghue G. P., Smith A. W., Groves J. T., and Bertozzi C. R. (2012) Investigating cell surface galectin-mediated cross-linking on glycoengineered cells. J. Am. Chem. Soc. 134, 9549–9552 10.1021/ja301694s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Patnode M. L., Yu S. Y., Cheng C. W., Ho M. Y., Tegesjö L., Sakuma K., Uchimura K., Khoo K. H., Kannagi R., and Rosen S. D. (2013) KSGal6ST generates galactose-6-O-sulfate in high endothelial venules but does not contribute to L-selectin-dependent lymphocyte homing. Glycobiology 23, 381–394 10.1093/glycob/cws166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Machado S. A., Kadirvel G., Daigneault B. W., Korneli C., Miller P., Bovin N., and Miller D. J. (2014) LewisX-containing glycans on the porcine oviductal epithelium contribute to formation of the sperm reservoir. Biol. Reprod. 91, 140 10.1095/biolreprod.114.119503 [DOI] [PubMed] [Google Scholar]
- 38. Clark G. F., and Dell A. (2006) Molecular models for murine sperm-egg binding. J. Biol. Chem. 281, 13853–13856 10.1074/jbc.R600001200 [DOI] [PubMed] [Google Scholar]
- 39. Pang P. C., Chiu P. C., Lee C. L., Chang L. Y., Panico M., Morris H. R., Haslam S. M., Khoo K. H., Clark G. F., Yeung W. S., and Dell A. (2011) Human sperm binding is mediated by the sialyl-Lewis (x) oligosaccharide on the zona pellucida. Science 333, 1761–1764 10.1126/science.1207438 [DOI] [PubMed] [Google Scholar]
- 40. Bao R., Nair M. K., Tang W. K., Esser L., Sadhukhan A., Holland R. L., Xia D., and Schifferli D. M. (2013) Structural basis for the specific recognition of dual receptors by the homopolymeric pH 6 antigen (Psa) fimbriae of Yersinia pestis. Proc. Natl. Acad. Sci. U.S.A. 110, 1065–1070 10.1073/pnas.1212431110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Astorgues-Xerri L., Riveiro M. E., Tijeras-Raballand A., Serova M., Neuzillet C., Albert S., Raymond E., and Faivre S. (2014) Unraveling galectin-1 as a novel therapeutic target for cancer. Cancer Treat. Rev. 40, 307–319 10.1016/j.ctrv.2013.07.007 [DOI] [PubMed] [Google Scholar]
- 42. Curtis M. P., Kirkman-Brown J. C., Connolly T. J., and Gaffney E. A. (2012) Modelling a tethered mammalian sperm cell undergoing hyperactivation. J. Theor. Biol. 309, 1–10 10.1016/j.jtbi.2012.05.035 [DOI] [PubMed] [Google Scholar]
- 43. Miles E. L., O'Gorman C., Zhao J., Samuel M., Walters E., Yi Y. J., Sutovsky M., Prather R. S., Wells K. D., and Sutovsky P. (2013) Transgenic pig carrying green fluorescent proteasomes. Proc. Natl. Acad. Sci. U.S.A. 110, 6334–6339 10.1073/pnas.1220910110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lefebvre R., Chenoweth P. J., Drost M., LeClear C. T., MacCubbin M., Dutton J. T., and Suarez S. S. (1995) Characterization of the oviductal sperm reservoir in cattle. Biol. Reprod. 53, 1066–1074 10.1095/biolreprod53.5.1066 [DOI] [PubMed] [Google Scholar]
- 45. Nakanishi T., Ikawa M., Yamada S., Parvinen M., Baba T., Nishimune Y., and Okabe M. (1999) Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 449, 277–283 10.1016/S0014-5793(99)00433-0 [DOI] [PubMed] [Google Scholar]
- 46. Chang H., and Suarez S. S. (2012) Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biol. Reprod. 86, 140, 1–8 10.1095/biolreprod.111.096578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manjunath P., Sairam M. R., and Uma J. (1987) Purification of four gelatin-binding proteins from bovine seminal plasma by affinity chromatography. Biosci. Rep. 7, 231–238 10.1007/BF01124794 [DOI] [PubMed] [Google Scholar]
- 48. Amann R. P., and Griel L. C. Jr. (1974) Fertility of bovine spermatozoa from rete testis, cauda epididymidis, and ejaculated semen. J. Dairy Sci. 57, 212–219 10.3168/jds.S0022-0302(74)84862-9 [DOI] [PubMed] [Google Scholar]
- 49. Gwathmey T. M., Ignotz G. G., Mueller J. L., Manjunath P., and Suarez S. S. (2006) Bovine seminal plasma proteins PDC-109, BSP-A3, and BSP-30-kDa share functional roles in storing sperm in the oviduct. Biol. Reprod. 75, 501–507 10.1095/biolreprod.106.053306 [DOI] [PubMed] [Google Scholar]
- 50. Fan J., Lefebvre J., and Manjunath P. (2006) Bovine seminal plasma proteins and their relatives: a new expanding superfamily in mammals. Gene 375, 63–74 10.1016/j.gene.2006.02.025 [DOI] [PubMed] [Google Scholar]
- 51. Waberski D., Magnus F., Ardón F., Petrunkina A. M., Weitze K. F., and Töpfer-Petersen E. (2006) Binding of boar spermatozoa to oviductal epithelium in vitro in relation to sperm morphology and storage time. Reproduction 131, 311–318 10.1530/rep.1.00814 [DOI] [PubMed] [Google Scholar]
- 52. Elliott R. M., Lloyd R. E., Fazeli A., Sostaric E., Georgiou A. S., Satake N., Watson P. F., and Holt W. V. (2009) Effects of HSPA8, an evolutionarily conserved oviductal protein, on boar and bull spermatozoa. Reproduction 137, 191–203 10.1530/REP-08-0298 [DOI] [PubMed] [Google Scholar]
- 53. Holt W. V., Elliott R. M., Fazeli A., Satake N., and Watson P. F. (2005) Validation of an experimental strategy for studying surface-exposed proteins involved in porcine sperm-oviduct contact interactions. Reprod. Fertil. Dev. 17, 683–692 10.1071/RD05070 [DOI] [PubMed] [Google Scholar]
- 54. Galanina O., Feofanov A., Tuzikov A. B., Rapoport E., Crocker P. R., Grichine A., Egret-Charlier M., Vigny P., Le Pendu J., and Bovin N. V. (2001) Fluorescent carbohydrate probes for cell lectins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 57, 2285–2296 10.1016/S1386-1425(01)00478-4 [DOI] [PubMed] [Google Scholar]
- 55. Galanina O. E., Tuzikov A. B., Rapoport E., Le Pendu J., and Bovin N. V. (1998) Carbohydrate-based probes for detection of cellular lectins. Anal. Biochem. 265, 282–289 10.1006/abio.1998.2859 [DOI] [PubMed] [Google Scholar]
- 56. Seppo A., Moreland M., Schweingruber H., and Tiemeyer M. (2000) Zwitterionic and acidic glycosphingolipids of the Drosophila melanogaster embryo. Eur. J. Biochem. 267, 3549–3558 10.1046/j.1432-1327.2000.01383.x [DOI] [PubMed] [Google Scholar]
- 57. Aoki K., Perlman M., Lim J. M., Cantu R., Wells L., and Tiemeyer M. (2007) Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J. Biol. Chem. 282, 9127–9142 10.1074/jbc.M606711200 [DOI] [PubMed] [Google Scholar]
- 58. Kumagai T., Katoh T., Nix D. B., Tiemeyer M., and Aoki K. (2013) In-gel β-elimination and aqueous-organic partition for improved O- and sulfoglycomics. Anal. Chem. 85, 8692–8699 10.1021/ac4015935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nix D. B., Kumagai T., Katoh T., Tiemeyer M., and Aoki K. (2014) Improved in-gel reductive β-elimination for comprehensive O-linked and sulfo-glycomics by mass spectrometry. J. Vis. Exp. e51840 10.3791/51840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aoki K., Porterfield M., Lee S. S., Dong B., Nguyen K., McGlamry K. H., and Tiemeyer M. (2008) The diversity of O-linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J. Biol. Chem. 283, 30385–30400 10.1074/jbc.M804925200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boccuto L., Aoki K., Flanagan-Steet H., Chen C. F., Fan X., Bartel F., Petukh M., Pittman A., Saul R., Chaubey A., Alexov E., Tiemeyer M., Steet R., and Schwartz C. E. (2014) A mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt & pepper syndrome, a neurocutaneous disorder with altered glycolipid and glycoprotein glycosylation. Hum. Mol. Genet. 23, 418–433 10.1093/hmg/ddt434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Damerell D., Ceroni A., Maass K., Ranzinger R., Dell A., and Haslam S. M. (2015) Annotation of glycomics MS and MS/MS spectra using the GlycoWorkbench software tool. Methods Mol. Biol. 1273, 3–15 10.1007/978-1-4939-2343-4_1 [DOI] [PubMed] [Google Scholar]
- 63. Varki A., Cummings R. D., Aebi M., Packer N. H., Seeberger P. H., Esko J. D., Stanley P., Hart G., Darvill A., Kinoshita T., Prestegard J. J., Schnaar R. L., Freeze H. H., Marth J. D., Bertozzi C. R., et al. (2015) Symbol nomenclature for graphical representations of glycans. Glycobiology 25, 1323–1324 10.1093/glycob/cwv091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. York W. S., Agravat S., Aoki-Kinoshita K. F., McBride R., Campbell M. P., Costello C. E., Dell A., Feizi T., Haslam S. M., Karlsson N., Khoo K. H., Kolarich D., Liu Y., Novotny M., Packer N. H., et al. (2014) MIRAGE: the minimum information required for a glycomics experiment. Glycobiology 24, 402–406 10.1093/glycob/cwu018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wells L., Hart G. W., Athens Guidelines for the Publication of Glycomics Data (2013) Glycomics: building upon proteomics to advance glycosciences. Mol. Cell. Proteomics 12, 833–835 10.1074/mcp.E113.027904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jaiswal R. S., Singh J., and Adams G. P. (2004) Developmental pattern of small antral follicles in the bovine ovary. Biol. Reprod. 71, 1244–1251 10.1095/biolreprod.104.030726 [DOI] [PubMed] [Google Scholar]
- 67. Ireland J. J., Murphee R. L., and Coulson P. B. (1980) Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J. Dairy Sci. 63, 155–160 10.3168/jds.S0022-0302(80)82901-8 [DOI] [PubMed] [Google Scholar]
- 68. Ireland J. J., Coulson P. B., and Murphree R. L. (1979) Follicular development during four stages of the estrous cycle of beef cattle. J. Anim. Sci. 49, 1261–1269 10.2527/jas1979.4951261x [DOI] [PubMed] [Google Scholar]
- 69. Fischer A. H., Jacobson K. A., Rose J., and Zeller R. (2008) Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, pdb.prot4986 10.1101/pdb.prot4986 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.