Abstract
Myeloperoxidase is a major neutrophil antimicrobial protein, but its role in immunity is often overlooked because individuals deficient in this enzyme are usually in good health. Within neutrophil phagosomes, myeloperoxidase uses superoxide generated by the NADPH oxidase to oxidize chloride to the potent bactericidal oxidant hypochlorous acid (HOCl). In this study, using phagocytosis assays and LC-MS analyses, we monitored GSH oxidation in Pseudomonas aeruginosa to gauge their exposure to HOCl inside phagosomes. Doses of reagent HOCl that killed most of the bacteria oxidized half the cells' GSH, producing mainly glutathione disulfide (GSSG) and other low-molecular-weight disulfides. Glutathione sulfonamide (GSA), a HOCl-specific product, was also formed. When neutrophils phagocytosed P. aeruginosa, half of the bacterial GSH was lost. Bacterial GSA production indicated that HOCl had reacted with the bacterial cells, oxidized their GSH, and was sufficient to be solely responsible for bacterial killing. Inhibition of NADPH oxidase and myeloperoxidase lowered GSA formation in the bacterial cells, but the bacteria were still killed, presumably by compensatory nonoxidative mechanisms. Of note, bacterial GSA formation in neutrophils from patients with cystic fibrosis (CF) was normal during early phagocytosis, but it was diminished at later time points, which was mirrored by a small decrease in bacterial killing. In conclusion, myeloperoxidase generates sufficient HOCl within neutrophil phagosomes to kill ingested bacteria. The unusual kinetics of phagosomal HOCl production in CF neutrophils confirm a role for the cystic fibrosis transmembrane conductance regulator in maintaining HOCl production in neutrophil phagosomes.
Keywords: myeloperoxidase, neutrophil, cystic fibrosis, cystic fibrosis transmembrane conductance regulator (CFTR), Pseudomonas aeruginosa (P. aeruginosa), coenzyme A (CoA), glutathione, glutathione sulfonamide (GSA), hypochlorous acid, immune response
Introduction
Neutrophils, the most abundant white blood cells in circulation, play an indispensable role in host defense (1). Their importance in immunity is underscored by the severe infections that afflict individuals with neutropenia. These granulocytes are attracted to sites of infection where they phagocytize, kill, and digest bacteria and fungi. To kill invading pathogens, neutrophils have an arsenal of antimicrobial strategies, including generation of microbicidal oxidants (2). Oxidative killing requires assembly of the NADPH oxidase on the phagosomal membrane, which uses cytosolic NADPH to reduce molecular oxygen within phagosomes to superoxide (O2˙̄) (3, 4). The neutrophil granule enzyme myeloperoxidase (MPO)2 dismutates superoxide to hydrogen peroxide, which it then uses to oxidize chloride to hypochlorous acid, the most potent antimicrobial oxidant produced in the neutrophil phagosome (3, 5). Hypochlorous acid reacts rapidly with amino acid side chains, particularly cysteine and methionine, lipids, and carbohydrates (6–10). However, the precise contribution hypochlorous acid makes to killing of ingested bacteria is far from resolved. Also, individuals with MPO deficiency generally do not suffer from persistent or severe infections (11). Consequently, MPO's role in host defense is not fully appreciated and has even been challenged (12).
There is, however, a wealth of data that supports a major role for MPO and hypochlorous acid in host defense. First, Rosen and collaborators published two compelling studies (13, 14) that indicate MPO is bactericidal during phagocytosis, but it may play a redundant role in killing depending on the susceptibility of the particular bacteria to other killing mechanisms. They found that although Escherichia coli were killed nonoxidatively, there was sufficient MPO-dependent inactivation of bacterial DNA synthesis that MPO could have killed the bacteria if acting alone in the phagosome (13). In later work, they found that MPO-mediated bacterial methionine oxidation contributes to killing of E. coli by neutrophils and that methionine sulfoxide reductases modulate bacterial susceptibility to hypochlorous acid (14). Although these studies provided strong evidence that MPO contributes to oxidative killing within phagosomes, they did not confirm a role for hypochlorous acid in killing because the targets measured in both cases could have been oxidized by other oxidants.
To directly probe the involvement of hypochlorous acid in the bactericidal action of neutrophils, Hurst and co-workers used fluorescein-coated beads to specifically measure the oxidant inside phagosomes. By measuring fluorescein chlorination, they demonstrated that hypochlorous acid is formed in phagosomes and at sufficient levels to kill ingested bacteria (15). Then hypochlorous acid was shown to chlorinate tyrosine residues in the proteins of E. coli and Staphylococcus aureus ingested by neutrophils (16, 17). This work confirmed that hypochlorous acid is formed in phagosomes and, importantly, reacts with the bacterium. Tyrosine chlorination was monitored in phagocytosed Pseudomonas aeruginosa (PsA) and was found to be low in neutrophils from patients with cystic fibrosis (CF) (18). This result has important clinical ramifications because defective hypochlorous acid production in CF neutrophils may contribute to the severe and persistent lung infections of these young patients (19).
One caveat from our earlier work with S. aureus is that not enough hypochlorous acid appeared to react with ingested bacteria to be responsible for killing. This conclusion was based on our finding that formation of 3-chlorotyrosine in bacterial proteins was low compared with levels obtained with lethal doses of reagent hypochlorous acid. Kinetic modeling of hypochlorous acid's reactivity in the phagosomes also suggested that the majority should react with neutrophil proteins and only a minor quantum with the bacterium (4). This prediction was supported by experimental evidence showing that neutrophil proteins inside phagosomes are chlorinated to a much higher degree than bacterial proteins (20). Furthermore, there was undetectable oxidation of the staphyloxanthin, the golden pigment of S. aureus, when these bacteria were phagocytosed and killed by neutrophils, despite this carotenoid being bleached at lethal doses of the oxidant (21). However, the major limitation of our studies with S. aureus is that chlorination of tyrosine residues and staphyloxanthin is slow compared with other reactions of hypochlorous acid (8, 21). Chlorination should account for a small percentage only of the amount of oxidant that reacted with bacteria. Hence, we may have underestimated how much hypochlorous acid phagocytosed bacteria were exposed to inside neutrophil phagosomes.
To overcome these limitations, we have now chosen to investigate hypochlorous acid–dependent oxidation of bacterial low-molecular-weight thiols during phagocytosis of PsA by neutrophils. Sulfur-containing amino acids, cysteine and methionine, and low-molecular-weight weight (LMW) thiols are the kinetically preferred targets for this oxidant (22). The most abundant LMW thiol in PsA is glutathione (GSH), but it also has coenzyme A (CoA) and cysteine (Cys) (23). Hypochlorous acid oxidizes GSH to a range of products, including glutathione sulfonamide (GSA), which although a minor product is specific to hypochlorous acid (24). Monitoring oxidation of LMW thiols should more accurately reflect the contribution hypochlorous acid makes to the bactericidal capability of neutrophils because it will account for a greater proportion of hypochlorous acid that reacts with the bacteria than the previous targets we or others have measured. We have also used this approach to assess whether hypochlorous acid production is impaired in CF neutrophils as reported earlier (18).
Results
Quantifying low-molecular-weight thiols GSH, CoA, and cysteine in PAO1
Based on the high rate constant reported for the reaction of thiols with hypochlorous acid (25), we hypothesized that microbial LMW thiols are readily oxidized when bacteria are exposed to this oxidant. To assess the effect of hypochlorous acid on LMW thiols in the PsA strain PAO1, the most abundant LMW thiols reported for this bacterial species, i.e. glutathione (GSH), coenzyme A (CoA), and cysteine (23), were quantified by LC with MS (LC-MS). To prevent artifactual oxidation, bacteria suspensions were treated with NEM to alkylate free thiols prior to lysis. GSH–NEM was measured in the lysate using an isotope-dilution LC-MS method previously established in our laboratory (26). CoA–NEM and cysteine–NEM were semi-quantified by specific multiple reaction monitoring (MRM)-based LC-MS methods set up for this study. Representative LC-MS chromatograms for lysates of untreated and hypochlorous acid-treated PAO1 are shown in the supporting Information, along with chromatograms for CoA–NEM and cysteine–NEM standards (Fig. S1).
The content of GSH, CoA, and cysteine in PAO1 lysates was 132 ± 18, 55 ± 9, and 5 ± 2 pmol/108 bacteria corresponding to intracellular concentrations of 0.34 ± 0.05, 0.14 ± 0.02, and 0.01 ± 0.005 mm, respectively (mean ± S.E., n = 6) (Table 1). Intracellular thiol concentrations measured here are in agreement with previously reported concentrations of GSH, CoA, and cysteine measured as thiol-monobimane derivatives by HPLC in PsA strains ATCC 10145 (0.37, 0.03, and 0.23 mm, respectively) and UCSD 24 (2.1, 0.43, and 0.27 mm, respectively) (23, 27). In preliminary work, we found that up to one-third of the total GSH concentration could be measured on the outside of the bacteria, i.e. in the medium, indicating that PAO1 exports GSH.
Table 1.
Thiol content of PsA strain PAO1 (ATCC 47085)
The mean is ±S.E., n = 6.
| Thiol | Thiol content |
Intracellular thiol concentrationb (mm) | |
|---|---|---|---|
| pmol/108 bacteria | μmol/g dry weighta | ||
| GSH | 132 ± 18 | 1.01 ± 0.1 | 0.34 ± 0.05 |
| CoA | 55 ± 9 | 0.42 ± 0.1 | 0.14 ± 0.02 |
| Cysteine | 5 ± 2 | 0.03 ± 0.02 | 0.01 ± 0.005 |
a Data are based on a dry weight of 0.13 mg per 1 × 108 bacteria.
b Data assume a cylindrical shape with a diameter and length of 1 and 5 μm, respectively, per bacterium, i.e. a volume of 3.9 × 10−4 ml per 1 × 108 bacteria.
Loss of low-molecular-weight thiols in PAO1 treated with hypochlorous acid
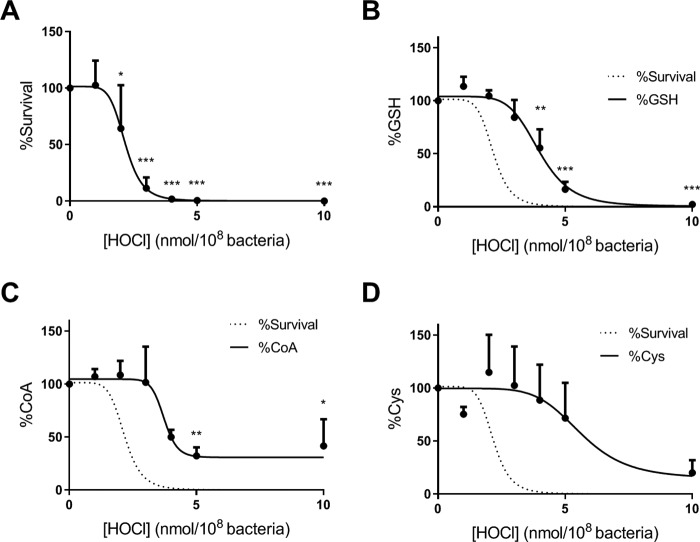
Next we measured GSH, CoA, and cysteine in PAO1 treated with bactericidal concentrations of hypochlorous acid. Fig. 1A shows the loss of bacterial viability with increasing concentrations of hypochlorous acid. The signal for all the reduced LMW thiols decreased over the same concentration range of oxidant in a dose-dependent manner (Fig. 1, B–D). Killing was observed at lower doses of hypochlorous acid than the loss of LMW thiols. At the LD50 (2.3 nmol/108 bacteria), none of the LMW thiols had undergone significant loss. One hundred percent killing was achieved by a dose of hypochlorous acid that caused half of reduced thiols to be depleted (∼4 nmol/108 bacteria).
Figure 1.
Killing of P. aeruginosa by reagent HOCl and loss of low-molecular-weight thiols. Bacteria were treated with increasing concentrations of HOCl in HBSS at 37 °C for 10 min with end–over–end rotation. The reaction was stopped by the addition of 1 mm methionine, and viable bacteria were determined by the CFU plating assay. A, % survival is expressed as (CFUtreated/CFUuntreated) × 100. After exposure to HOCl, NEM (20 mm) and protease inhibitors were added, and the bacteria were lysed by sonication for 5 min. The NEM adducts of reduced glutathione (GSH) (B), cysteine (Cys) (C), and coenzyme A (CoA) (D) were measured by LC-MS/MS and expressed as a percentage of untreated control. Data are presented as mean ± S.E. of at least five independent experiments. Dose-response curves with variable slope (four parameters) were fitted. The line fitted to the survival data in A is also shown as a dotted line in B–D. A significant difference when compared with untreated control was identified by ANOVA with Dunnett's multiple comparison test and is indicated by asterisk (*, p < 0.01; **, p < 0.01; and ***, p < 0.001).
GSH oxidation products in PAO1 treated with hypochlorous acid
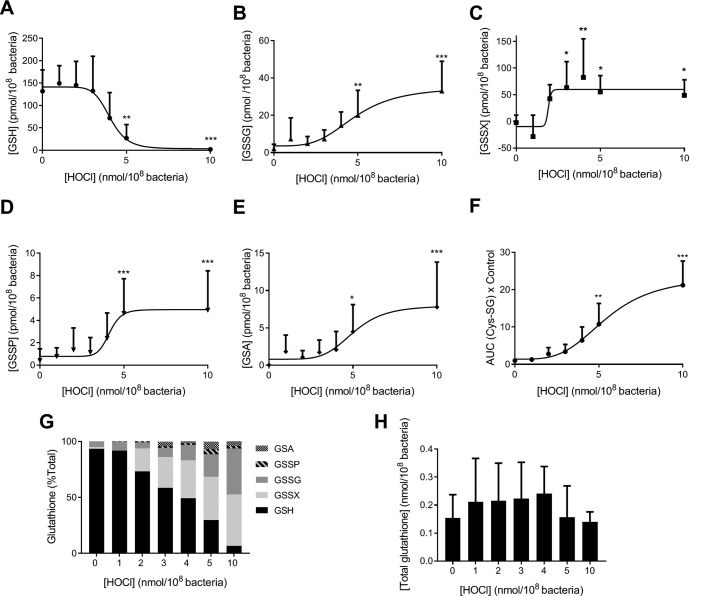
Because GSH is the most abundant LMW thiol in PAO1, we determined GSH oxidation products that could account for the loss of its reduced form observed in hypochlorous acid–treated PAO1 (Fig. 2A). We measured glutathione disulfide (GSSG, Fig. 2B), glutathione present as a mixed disulfide with other LMW thiols (GSSX), or with protein thiols (GSSP) and glutathione sulfonamide (GSA), an oxidation product of GSH specific to hypochlorous acid (24). All of the oxidized glutathione species increased upon hypochlorous acid exposure in a concentration-dependent manner (Fig. 2, B–E).
Figure 2.
GSH oxidation in P. aeruginosa treated with reagent HOCl. Bacteria were grown overnight in LB and treated with increasing concentrations of HOCl in HBSS at 37 °C for 10 min with end–over–end rotation. The reaction was stopped by the addition of 1 mm methionine. NEM (20 mm) and protease inhibitors were added, and the bacteria were lysed by sonication for 5 min. GSH (reduced glutathione) (A), GSSG (glutathione disulfide) (B), GSSX (glutathione present in a mixed disulfide with another low molecular thiol) (C), GSSP (glutathione present in a disulfide with proteins) (D), GSA (glutathione sulfonamide) (E) were quantified by stable isotope dilution LC-MS/MS. F, Cys-SG (glutathione present in a mixed disulfide with cysteine) was detected by LC-MS/MS, and the area under the curve (AUC) was determined and expressed relative to the untreated control. Data are presented as mean ± S.D. of at least four independent experiments, and a dose response with variable slope (four parameters) was fitted. A significant difference when compared with untreated control was identified by ANOVA with Dunnett's multiple comparison test and is indicated by * (p < 0.05), ** (p < 0.01), or *** (p < 0.001). G, GSSG was expressed as GSH equivalents by multiplying by two. Total glutathione was calculated by [GSH] + [GSSX] + 2×[GSSG] + [GSSP] + [GSA]. Individual glutathione species are presented as a percentage of total glutathione. The mean of at least four different experiments is shown. H, total glutathione was calculated as in G, and the mean ± S.D. of at least four independent experiments is shown.
The majority of GSSX is likely to encompass the mixed disulfide with CoA, i.e. CoASSG, because CoA is present at about one-third of the concentration of GSH (55 versus 132 pmol/108 bacteria, respectively). CoASSG could not be measured in PAO1 lysates due to the likely instability of this analyte during sample processing. We could, however, measure the mixed disulfide of GSH with cysteine (CysSSG), and we found its levels to increase with increasing doses of hypochlorous acid (Fig. 2F).
GSSX and GSSG were the major oxidation products of GSH largely accounting for the loss of the reduced form upon treatment with hypochlorous acid, whereas GSSP and GSA were minor products (Fig. 2G). For example, at a dose of 10 nmol of hypochlorous acid/108 bacteria, at which GSH was nearly depleted to only 6% of the total glutathione, GSSX, GSSG, GSSP, and GSA amounted to 47, 39, 2, and 6%, respectively (Fig. 2G). The sum of GSH, 2×GSSG, GSSX, GSSP, and GSA in PAO1 treated with increasing concentrations of hypochlorous acid equaled the GSH concentration in untreated PAO1 indicating that all major GSH oxidation species were accounted for by our measurements (Fig. 2H).
13C labeling of PAO1
To evaluate oxidation of bacterial GSH during phagocytosis of PAO1 by human neutrophils, we had to distinguish bacterial GSH from GSH present in neutrophils. To achieve this, we grew PAO1 in complete medium containing exclusively carbon-13 and adapted our LC-MS assay to measure glutathione species containing the heavy carbon isotope (Fig. S2, A and B). PAO1 grown in 13C-medium produced as much GSH as LB-grown PAO1, and all 10 carbons were replaced with the heavy isotope (Fig. S2, A and B). The hypochlorous acid-induced oxidation pattern of glutathione of PAO1 grown in the 13C-medium (Fig. S2, A–C) and the extent of killing was not significantly different from PAO1 grown in LB medium (Fig. S2D). Methionine (0.5 mm) was able to scavenge hypochlorous acid and prevent the oxidation of bacterial GSH (Fig. S2C).
Oxidation of GSH in 13C-labeled PAO1 during phagocytic by human neutrophils
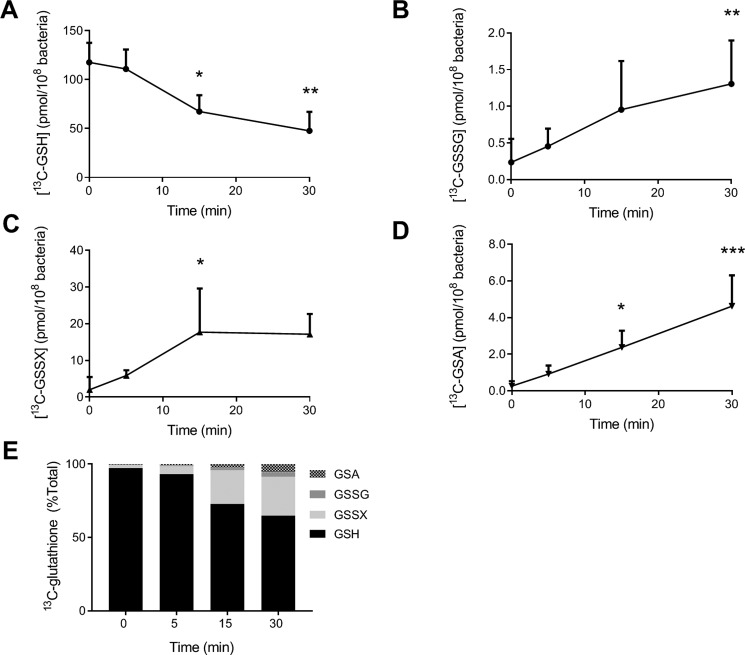
Oxidation of bacterial GSH during phagocytosis by human neutrophils was monitored by measuring 13C-containing glutathione species (Fig. 3). GSH decreased over time with half the amount lost after 30 min (Fig. 3A). GSSG (Fig. 3B) and GSSX (Fig. 3C) increased with time, but did not reach the same levels observed with hypochlorous acid treatment. GSA, in contrast, reached similar levels in the hypochlorous acid and neutrophil systems (Fig. 3D). GSSP was not measured as it was found to be only a minor oxidation product and would be difficult to detect in the presence of excess neutrophil proteins. As with reagent hypochlorous acid, GSSX was the major oxidation product of GSH when PAO1 was phagocytosed by neutrophils (Fig. 3E). The recovery of total glutathione (GSH + 2 × GSSG + GSSX + GSA) was 98, 76, and 63% at 5, 15, and 30 min, respectively, when compared with the control at 0 min (Fig. S3).
Figure 3.
Oxidation of GSH in P. aeruginosa during phagocytosis by human neutrophils. PAO1 (1 × 108/ml) grown on CELTONE Complete 13C-medium was incubated with neutrophils (1 × 107/ml) at 37 °C with end–over–end rotation. At the indicated time points, phagocytosis was stopped by placing the mixtures on melting ice; NEM (20 mm) was added, and neutrophils and bacteria were lysed. [13C]GSH (A), [13C]GSSG (B), [13C]GSSX (C), and [13C]GSA (D) were measured by stable isotope dilution LC-MS/MS. A significant difference when compared with time 0 was identified by ANOVA with Dunnett's multiple comparison test and is indicated by * (p < 0.05), ** (p < 0.01), or *** (p < 0.001). Data were obtained for at least three different donors and presented as mean ± S.D. E, individual glutathione species are presented as a percentage of total glutathione as described in Fig. 2. Data are presented as means from at least three independent experiments.
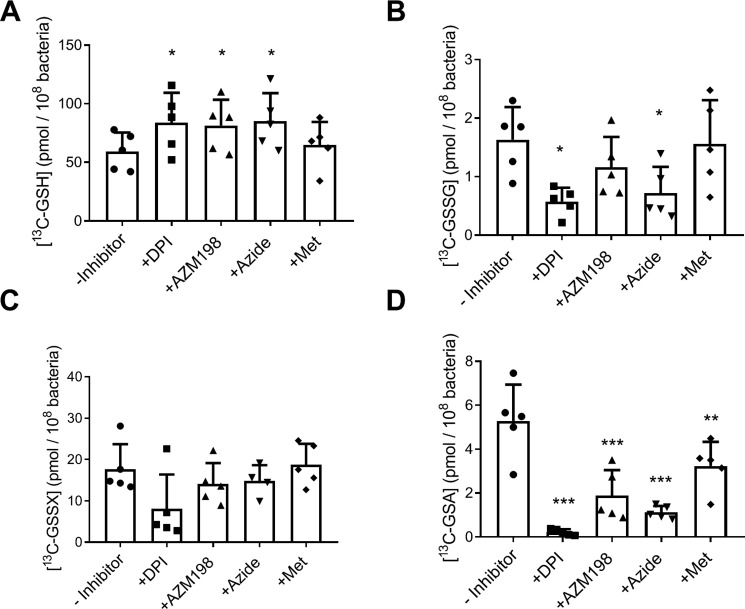
The loss of GSH during phagocytosis by neutrophils could be diminished by pretreatment of neutrophils with inhibitors of the NADPH oxidase (DPI) or MPO (AZM198 and azide) (Fig. 4A). Methionine, a scavenger of extracellular hypochlorous acid (Fig. S2C), did not have this protective effect. Similarly, DPI pretreatment lowered the levels of oxidized glutathione species GSSG and GSSX and prevented formation of GSA (Fig. 4, B–D). The MPO inhibitors AZM198 and azide substantially decreased GSA formation, and azide also lowered GSSG. The decrease in GSA with the MPO inhibitors was not as great as with DPI suggesting an insufficient delivery of these inhibitors to the phagosome or incomplete inhibition of MPO. Methionine also inhibited production of GSA, albeit to a lesser degree than the other inhibitors, indicating that a small proportion of GSA stems from oxidation of extracellular GSH. Collectively, these results show that oxidation of bacterial GSH occurs during neutrophil phagocytosis. The oxidation process is MPO-dependent and predominantly intracellular. Formation of bacterial GSA during phagocytosis can thus be used to assess the hypochlorous acid–production capacity of neutrophils.
Figure 4.
Oxidation of GSH in P. aeruginosa during phagocytosis by human neutrophils and its dependence on active MPO and NADPH oxidase. Neutrophils (1 × 107/ml) were preincubated with inhibitors of the NADPH oxidase (10 μm DPI) and MPO (10 μm AZM198 and 100 μm sodium azide) and a scavenger of extracellular HOCl (0.5 mm methionine) before the addition of 13C-grown PAO1 (1 × 108/ml). After 30 min at 37 °C with end–over–end rotation. [13C]GSH (A), [13C]GSSG (B), [13C]GSSX (C), and [13C]GSA (D) were measured by LC-MS. Bars represent means ± S.D. from five separate experiments using different donors. A significant difference when compared with the untreated control was identified by repeated measures ANOVA with Dunnett's multiple comparison test and is indicated by * (p < 0.05), ** (p < 0.01), or *** (p < 0.001).
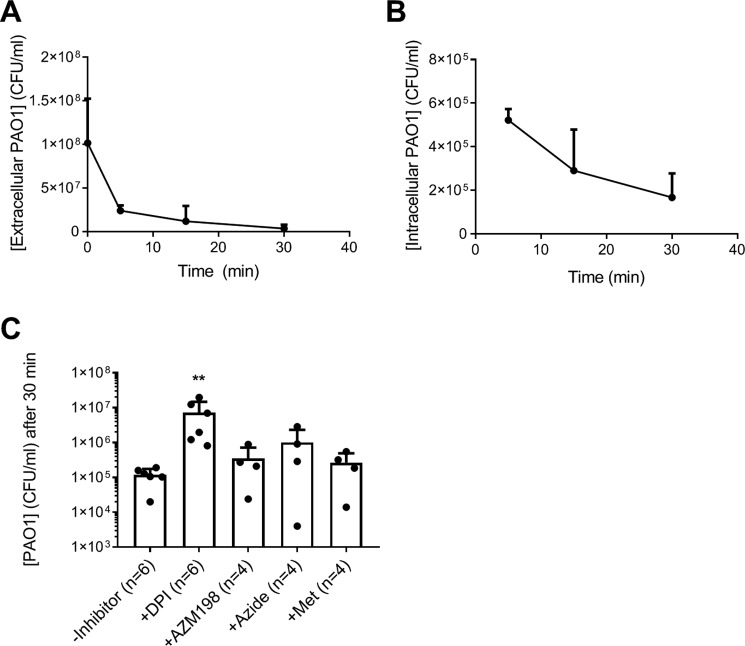
To relate GSH oxidation to phagocytosis and bacterial killing, we performed a two-step killing assay measuring both extracellular and surviving intracellular bacteria. PAO1 was readily taken up by human neutrophils (Fig. 5A). The majority of bacteria (>99%) was killed within 30 min (Fig. 5B), at which point about half of the reduced GSH was depleted (Fig. 3A), and significant amounts of GSA were formed. This mirrored the results from hypochlorous acid treatment, where 50% depletion of reduced GSH and comparable levels of GSA were observed at doses that caused complete loss of bacterial viability (Figs. 1, A and B, and 2E).
Figure 5.
Killing of P. aeruginosa by human neutrophils. PAO1 (1 × 108/ml) was incubated with neutrophils (1 × 107/ml) at 37 °C with end–over–end rotation. At indicated time points, phagocytosis was stopped by placing the mixtures on melting ice. Extracellular (A) and intracellular (B) viable bacteria were determined by CFU plating assay. Means ± S.D. from at least three separate experiments using different donors are shown. C, neutrophils were preincubated with inhibitors of the NADPH oxidase (10 μm DPI) and MPO (10 μm AZM198 and 100 μm sodium azide) and a scavenger of extracellular HOCl (0.5 mm methionine) before the addition of PAO1 and viable intracellular bacteria were determined after 30 min phagocytosis. The dotted line indicates the starting concentration of bacteria. Bars represent means ± S.D. from separate experiments using different donors. A significant difference when compared with the untreated control was identified by ANOVA with Dunnett's multiple comparison test and is indicated by ** (p < 0.01).
When neutrophils were pretreated with the NADPH oxidase inhibitor DPI, significantly more bacteria survived phagocytosis (Fig. 5C). The MPO inhibitor AZM198 had no effect on killing (Fig. 5C). Although pretreatment with the MPO inhibitor azide appeared to increase bacterial survival slightly, this effect was not statistically significant (Fig. 5C).
Oxidation of PAO1 GSH is dependent on functional CFTR
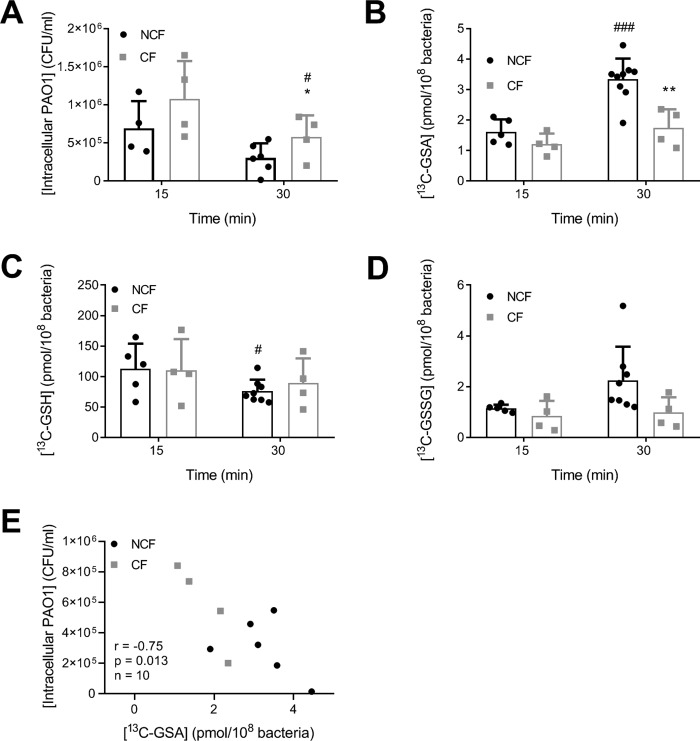
Having demonstrated that the formation of bacterial GSA is a sensitive marker of hypochlorous acid production by neutrophils during phagocytosis of PAO1, we compared formation of GSA and bacterial survival between neutrophils from healthy individuals and those with CF. We used neutrophils from CF patients with the CFTR mutations ΔF508 and G551D. The ΔF508 mutation causes 100% loss of CFTR function as the protein is misfolded and gets degraded before reaching the apical membrane, and the substitution of aspartic acid for glycine at position 551 (G551D) abolishes ATP-dependent gating of the CFTR chloride channel. Methionine was added to prevent extracellular GSH oxidation that could mask differences in the intracellular hypochlorous acid production between the healthy and CF cohorts. The rate of uptake of PAO1 was similar in CF and healthy neutrophils as indicated by a comparable rate of disappearance of viable extracellular bacteria from the medium (data not shown). However, more bacteria survived inside CF neutrophils compared with healthy neutrophils (Fig. 6A). Concomitantly, although GSA levels were not significantly different at 15 min (Fig. 6B), the levels continued to go up in the neutrophils from healthy individuals but plateaued off in neutrophils from CF patients. At each time point, there were no significant differences for bacterial GSH and GSSG between non-CF and CF neutrophils (Fig. 6, C and D). However, there was a significant decrease in GSH over time for non-CF neutrophils, which did not occur in the CF neutrophils (Fig. 6C). Greater production of GSA was associated with decreased survival of PAO1 (Fig. 6E).
Figure 6.
Killing of P. aeruginosa and oxidation of GSH during phagocytosis by human neutrophils depends on functional CFTR. A, neutrophils (1 × 107/ml) from healthy (NCF, black symbols) or cystic fibrosis donors (CF, gray symbols) were incubated with 13C-grown PAO1 (1 × 108/ml) at 37 °C with end–over–end rotation in the presence of 0.5 mm methionine. After 15 or 30 min, phagocytosis was stopped; extracellular bacteria were removed, and intracellular viable bacteria were determined. B, neutrophils and PAO1 were incubated as in A. After 15 or 30 min, NEM (20 mm) was added, and neutrophils and bacteria were lysed, and [13C]GSA (B), [13C]GSH (C) and [13C]GSSG (D) were determined by LC-MS. Bars represent means ± S.D. from at least four separate experiments using different donors. A significant difference when compared with the healthy neutrophils at the same time point was identified by an unpaired t test and is indicated by *, p < 0.05, or **, p < 0.01. A significant difference between 30 and 15 min holding the CF status the same is indicated by #, p < 0.05, or ###, p < 0.001. E, for experiments where matching data from the same donor were available, the number of intracellular viable bacteria was plotted against the level of GSA formed after 30 min of phagocytosis. Pearson r correlation coefficient, p value, and number of individual subjects are shown.
Discussion
In this study, we show that bacterial GSH is oxidized during phagocytosis of PsA by human neutrophils in an MPO-dependent manner. The extent of bacterial GSH loss and GSA formation was analogous to that following treatment with bactericidal doses of HOCl. Our results demonstrate that HOCl is produced inside neutrophil phagosomes at sufficient doses to kill ingested bacteria. Furthermore, by showing that formation of bacterial GSA was diminished in neutrophils from patients with cystic fibrosis, our study supports a role for CFTR in enabling neutrophils to keep generating HOCl in the phagosome.
Previous studies have provided evidence for hypochlorous acid production in the neutrophil phagosome using a variety of methods, including hypochlorous acid–specific fluorescent probes, measurement of 3-chlorotyrosine of phagosomal contents, and bleaching of fluorescently-labeled bacteria (4, 14, 16–18, 20, 28–30). However, these studies used HOCl reporters that are not specific for this oxidant or react only slowly with it and so might overestimate or underestimate the extent of HOCl produced in the phagosome. Conclusions from earlier studies are therefore curbed by their inability to relate oxidative damage of phagocytosed bacteria to that of bacteria treated with bactericidal doses of reagent HOCl. Consequently, it is still debated whether enough hypochlorous acid is produced in neutrophil phagosomes to cause killing of bacteria. Here, by monitoring oxidation of bacterial GSH, we demonstrate that bactericidal amounts of HOCl are formed inside the neutrophil phagosomes. GSH is a major target for HOCl due to its high abundance and reactivity (8, 22), and its oxidation yields the HOCl-specific product GSA (24). Accordingly, our study is more sensitive and specific than previous studies and therefore more conclusive.
Although our data are consistent with a role for HOCl in neutrophil bactericidal activity, blocking oxidant production in neutrophils did not abolish their ability to kill phagocytosed PsA. DPI-treated neutrophils contained significantly more surviving bacteria than control neutrophils, but they were still able to kill 90% of PsA. MPO inhibitors, while inhibiting the formation of GSA, had no significant effect on killing. These results show that PsA can be effectively killed by nonoxidative neutrophil antimicrobial proteins such as neutrophil elastase, lysozyme, defensins, and cathelicidins as reported before (31–35). When inhibitors of oxidant production fail to interfere substantially with neutrophil killing, it is tempting to conclude that oxidants are not involved in bacterial killing. DPI is often used to support or reject a role for oxidative killing. However, inhibiting the NADPH oxidase with DPI will also cause phagosome acidification thus affecting pH-sensitive neutrophil granule enzymes such as neutrophil elastase and cathepsin G (36, 37). The small but significant inhibition of neutrophil killing by DPI observed in this study could have been due to its interference with nonoxidative mechanisms, rather than revealing a small contribution of oxidants to killing. Therefore, inhibitors cannot be used to decisively tease out oxidative and nonoxidative killing mechanisms during neutrophil phagocytosis. Also, the fact that nonoxidative mechanisms can compensate when oxidant production is inhibited, does not deny HOCl a role in bacterial killing when it is being produced. We show that neutrophils produce bactericidal amounts of HOCl even when phagocytosing a microbe that could be killed by their nonoxidative machinery alone. The implication of our finding is that neutrophil killing is a multifactorial process in which redundant antimicrobial tools are deployed to ensure fail-safe killing of bacteria.
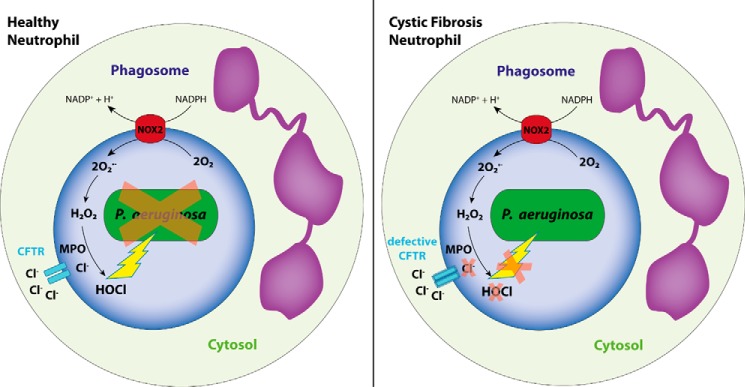
In CF, despite excessive infiltration into the infected lung, neutrophils seem incapable of clearing infections, and thus an intrinsically impaired oxidant production has been suggested to exist in these neutrophils (38–42). So far, evidence for defective HOCl production in particular has come from using nonspecific, indirect, or insensitive markers of oxidant production such as luminol chemiluminescence, R19 fluorescence, and formation of bacterial 3-chlorotyrosine (18, 41, 43). Because the reactivity of tyrosine and R19S with hypochlorous acid is low (8, 22, 28), the observed changes in levels of the respective oxidation products, 3-chlorotyrosine and R19, could have been brought about by insubstantial differences in the amount of oxidant produced by healthy and CF neutrophils. By showing decreased formation of bacterial GSA during phagocytosis of PsA, we confirm that hypochlorous acid production is impaired in CF neutrophils (Fig. 7). This defect is likely caused by an insufficient supply of chloride to the phagosome in the absence of functional CFTR as suggested previously (18, 44). In addition to chloride, CFTR also transports bicarbonate, thereby regulating phagosomal pH (45). Impaired HOCl production with loss of CFTR might thus also result from phagosome acidification, which inhibits both the NADPH oxidase and MPO-chlorinating activity (46, 47).
Figure 7.
Requirement for CFTR in MPO-dependent production of HOCl and killing of P. aeruginosa by human neutrophils.
Whereas CFTR loss–of–function and treatment with MPO inhibitors both caused a similar decrease in the amount of bacterial GSA formed during neutrophil phagocytosis, only loss of CFTR was associated with a significant increase in bacterial survival. It is possible that in addition to diminishing HOCl production in neutrophils, defective CFTR also disrupts nonoxidative killing mechanisms. Dysregulation of pH might affect the activity of pH-dependent proteases (36, 37, 48). Also, chloride is needed to activate cathepsin C, which in turn cleaves neutrophil serine proteases such as neutrophil elastase and cathepsin G into their active forms (49, 50). It is also important to consider the different kinetics of HOCl production between CF neutrophils and inhibition of MPO activity in normal neutrophils. In CF neutrophils, HOCl production during the early stages of phagocytosis may interfere with the functioning of nonoxidative mechanisms so that at later time points both oxidative and nonoxidative killing would be compromised. In contrast, when MPO is inhibited in normal neutrophils, nonoxidative mechanisms would not be compromised during phagocytosis.
Although we found a significant decrease in the ability of neutrophils from CF patients to kill PsA as reported previously by Painter et al. (51), the majority of bacteria (>98%) were still killed. About twice the number of bacteria survived inside neutrophils from CF patients compared with those from healthy donors. This small difference in the killing capacity of neutrophils may contribute to the persistent bacterial infections frequently observed in CF lungs (52, 53), particularly as it occurs in combination with other antimicrobial defects in CF such as inadequate mucociliary clearance and decreased activity of antimicrobial proteins in the epithelial lining fluid (54). Consistent with this theory, a previous study showed that MPO knockout mice were more susceptible to infections with PsA than WT mice when inoculation doses were high (55). At low PsA doses, MPO-deficient and WT mice were not different suggesting that MPO antimicrobial activity is particularly important when a greater number of bacteria are required to be killed.
Kinetic data suggest that bacterial thiols will be preferentially oxidized when bacteria are exposed to hypochlorous acid (22, 25), and bacterial LMW thiols have been proposed to protect bacteria against oxidative stress by preventing other crucial cellular targets from becoming oxidized. But little is known about the fate of LMW thiols in bacteria exposed to hypochlorous acid. Loss of reduced GSH was previously observed in E. coli upon treatment with bactericidal doses of reagent hypochlorous acid (56). Also, phagocytosis of E. coli expressing roGFP2-based fusion probes by a neutrophil-like cell line revealed a disruption of bacterial thiol redox balance (57). To our knowledge, this study is the first to comprehensively characterize the fate of all major endogenous LMW thiols in a bacterial species treated with hypochlorous acid and during phagocytosis by human neutrophils. We had postulated that GSH would be a major target for hypochlorous acid in PsA; however, a near 100-fold excess of hypochlorous acid (10 nmol/108 bacteria) was required to achieve complete loss of bacterial GSH (0.13 nmol/108 bacteria) indicating that only about 1% of the oxidant had reacted with GSH. The stoichiometry of hypochlorous acid to GSH was reported to be 4:1 for E. coli (56). The difference can in part be explained by the fact that E. coli has 10–30-fold higher levels of GSH than PsA (27, 56). Our findings suggest that there are other biomolecules in PsA that become preferentially oxidized by hypochlorous acid.
Protein thiols constitute up to 70% of overall cellular thiols (58). They are likely to be the major targets for HOCl within phagocytosed bacteria, but glutathionylation may protect critical thiol residues from becoming irreversibly oxidized (59). However, we showed that GSSP was only a minor oxidation product of GSH in PsA treated with HOCl. This result suggests that at least with PsA other oxidation products of cysteine residues must be favored over glutathionylation. In contrast to our result, Xie et al. (60) recently reported substantial reversible protein thiol oxidation in E. coli phagocytosed by a neutrophil-like cell line. This reversible thiol oxidation may have involved glutathionylation because GSH levels in E. coli are much higher than PsA (27, 56). In future work it will be of interest to quantitatively assess oxidation of protein thiols in different pathogenic bacteria during neutrophil phagocytosis and to determine what oxidation products are formed.
Finally, we need to acknowledge the limitations of this study. We only recovered about 60% of the total bacterial glutathione after 30 min of phagocytosis. This loss was slightly inhibited by DPI indicating that GSH was oxidized to a product that we had not accounted for, e.g. glutathione sulfonic acid or oxidized GSSG. Indeed, a smaller amount of GSSG was measured in PsA exposed to the neutrophil system compared with reagent hypochlorous acid suggesting that the disulfide was perhaps further oxidized (61). Alternatively, some of the GSH and GSSG loss can be explained by the action of γ-glutamyltransferases present in the bacterial periplasm (62, 63). This enzyme could have come into contact with intracellular GSH and GSSG following damage to the bacterial plasma membrane by neutrophil granule enzymes.
Our finding that HOCl production is impaired in neutrophils from CF patients is based on a small number of patients (n = 4). However, it is difficult to recruit CF children free of respiratory pathogens who are old enough and willing to donate the amount of blood needed for these experiments. Therefore, we ended our study when a statistically significant difference was observed between groups, given that our results were consistent with prior reports on defective HOCl production in CF neutrophils (18, 43).
In conclusion, this work demonstrates that neutrophils produce bactericidal amounts of HOCl during phagocytosis of bacteria thereby clarifying the involvement of MPO in neutrophil killing. This study also supports a role for CFTR in neutrophil's ability to produce hypochlorous acid and to ensure efficient killing of phagocytosed bacteria. We suggest that bacterial GSA can be used as a tool for monitoring defects in oxidant production by neutrophils by directly assessing hypochlorous acid-induced damage to microbes.
Experimental procedures
Materials
Coenzyme A (CoA), l-cysteine (Cys), l-methionine (Met), reduced and oxidized glutathione (GSH and GSSG), N-ethylmaleimide (NEM), iodoacetamide (IAM), diphenylene iodonium chloride (DPI), sodium azide, saponin, DNase I type II from bovine pancreas, Hanks' balanced salt solution (HBSS), and phosphate-buffered saline (PBS) for cell culture were purchased from Sigma. Protease inhibitor mixture tablets cOmplete were from Roche Applied Science (Basel, Switzerland), and LB Broth powder (Miller's) was from Thermo Fisher Scientific (Waltham, MA). CELTONE Complete medium (13C, 98%+) and heavy-labeled glutathione (GSH, 13C2 + 15N) were obtained from Cambridge Isotopes Laboratories (Tewksbury, MA). HOCl (ϵ292 = 350 m−1 cm−1 for OCl− at pH 12) (64) was purchased as a commercial chlorine bleach from Household and Body Care (Auckland, New Zealand). AZM198, a specific MPO inhibitor, was provided as a gift by AstraZeneca (Mölndal, Sweden) (65). Ficoll-Paque (GE Healthcare, Sweden) and dextran from Leuconostoc mesenteroides (average Mr 150,000, Sigma) were used for isolation of neutrophils.
PsA culture
PsA strain PAO1 (ATCC 47085) was stored and grown under standard conditions and maintained on Columbia sheep blood agar plates. For experiments, PAO1 was grown overnight in LB or CELTONE Complete 13C-medium at 37 °C. Cells were pelleted by centrifugation at 12,000 × g for 5 min, washed twice with PBS, and then resuspended in HBSS. Biofilms and bacterial aggregates were removed by centrifugation at 100 × g for 5 min, and the concentration of bacteria in the supernatant was determined by absorbance measurement at 550 nm and conversion to colony-forming units (CFU) using A550 = 0.127 for 1 × 108 CFU/ml as determined by a standard curve. For the purpose of this study, it was assumed that one bacterium will form one colony, i.e. CFU was equaled to the number of bacteria.
Treatment of PsA with hypochlorous acid
Five hundred μl of HBSS containing increasing concentrations of hypochlorous acid (0–200 μm) was added to 500 μl of bacteria (1 or 2 × 109 bacteria/ml) while mixing vigorously and incubated for 10 min at 37 °C with end–over–end rotation. The reaction was stopped by the addition of 1 mm methionine. A 50-μl aliquot was removed for viability plating assay; NEM (20 mm final) and protease inhibitors (1× final) were added, and after 20 min of incubation at 20–22 °C, cells were lysed by sonication, and cell debris was removed by centrifugation at 12,000 × g for 5 min. glutathione species were analyzed in the supernatant by LC-MS/MS as described below.
Peripheral blood neutrophils
Blood was obtained from healthy human volunteers and CF patients with informed consent and with ethical approval from the Southern Health and Disability Ethics Committee, New Zealand. Our studies abide by the Declaration of Helsinki principles. There was an even split of male and female donors among the donors, and the ages ranged from 21 to 48 years and 8 to 14 years for healthy volunteers (NCF) and CF patients, respectively. CF patients (n = 4) were clinically stable and had no identified pathologic bacterial infections at the time the blood was obtained as indicated by negative cough swab or bronchoalveolar lavage (BAL) culture. One BAL showed moderate growth of fungal species, and one patient was on treatment with i.v. cefuroxime. Three CF patients were ΔF508 homozygotes, and one was a ΔF508/G551D compound heterozygote.
Human neutrophils were isolated from freshly drawn heparinized blood under sterile conditions by dextran sedimentation followed by Ficoll-Paque centrifugation. Human granulocytes, including neutrophils, were isolated from the Ficoll pellet by erythrocyte lysis in hypotonic buffer (66). Neutrophils were resuspended in HBSS supplemented with 10% autologous serum.
Phagocytosis of PAO1 by human neutrophils
PAO1 was opsonized at 2 × 108/ml in HBSS containing 10% fresh autologous human serum for 20–40 min at 37 °C with end–over–end rotation. Five hundred μl of opsonized PAO1 (2 × 108/ml) was mixed with 500 μl of neutrophils (2 × 107/ml) pre-warmed to 37 °C and incubated for up to 30 min at 37 °C with end–over–end rotation. At various time points, the mixture was placed on melting ice, and 1 ml of ice-cold PBS was added to stop phagocytosis. Nonphagocytosed bacteria were removed by centrifugation at 100 × g at 4 °C, and the pellet was washed twice with 1 ml of ice-cold PBS. All supernatants were combined, and after further dilution, 100 μl was plated on Columbia sheep blood agar plates to determine viable extracellular bacteria. The pellets were resuspended in pH 11 water containing 0.05% saponin, passed three times through a 25-gauge needle, and treated with DNase (addition of 10 μl of each: 1 m Tris-HCl (pH 7.4), 100 mm CaCl, 50 mm MgCl2, DNase 10,000 units/ml) for 10 min at 37 °C with end–over–end rotation. After further dilution, 100 μl was plated on Columbia sheep blood agar plates to determine viable intracellular bacteria. Neutrophils were preincubated with inhibitors at 37 °C for 10 min prior to phagocytosis at double the final concentration (1 mm methionine, 200 μm sodium azide, 20 μm DPI, 20 μm AZM198).
For determining oxidation of GSH, phagocytosis was stopped by placing the neutrophil/PAO1 mixtures on melting ice at the indicated time points, and 500 μl of ice-cold PBS was added. One hundred μl of 200 mm NEM was added and incubated for 10 min at 20–22 °C. Neutrophils and bacteria were lysed by sonication on ice for 5 min with a 50% pulse (Omni Sonic Ruptor 400 microtip, Omni International, NW Kennesaw, GA) or bead beating using a Precellys Evolution Homogenizer and the VK05 lysing kit (Bertin Instruments, Montigny-le-Bretonneux, France) three times for 30 s at 6800 rpm with 10-s intervals at 4 °C. Cell debris was removed by centrifugation at 12,000 × g for 5 min, and glutathione species in the supernatant were analyzed by LC-MS/MS as described below.
Measurement of glutathione species by LC/MS
GSH, GSSG, GSA, and GSSP were measured using a previously described multiple reaction monitoring (MRM)-based stable isotope dilution LC tandem MS assay (LC-MS/MS) method (26, 67). An Ultimate 3000 RS system (Thermo Fisher Scientific, Waltham, MA) coupled to a 4000 QTrap mass spectrometer (Sciex, Framingham, MA) or an Infinity 1290 LC system (Agilent, Santa Clara, CA) coupled to a 6500 QTrap mass spectrometer (Sciex) was used. Internal standards ([13C2,15N1]GSH–NEM, [13C4,15N2]GSSG, and [13C2,15N1]GSA) were added to a volume of PAO1 lysate containing 107 bacteria; protein was precipitated by the addition of ice-cold ethanol (80% v/v), and the protein pellet was removed by centrifugation. The supernatant was dried by vacuum evaporation, and after resuspension into water containing 0.1% formic acid, GSH (as the GSH–NEM adduct), GSSG, and GSA were quantified by LC-MS/MS in MRM mode using the transitions shown in Table S1.
To measure GSSP, a volume of PAO1 lysate containing 2 × 108 bacteria was mixed with an equal volume of 3.3% metaphosphoric acid; the resulting protein pellet was washed with 1% metaphosphoric acid and reduced with 10 mm DTT. Any free thiol was alkylated with IAM (20 mm); the sample was spiked with [13C2,15N1]carbamidomethyl-GSH as the internal standard; the protein was precipitated by adding ice-cold ethanol (80% v/v) and removed by centrifugation; and carbamidomethyl-GSH was analyzed in the supernatant by LC-MS/MS using the MRM transitions shown in Table S1.
Any mixed disulfides of GSH with other LMW thiols (GSSX) were measured by reducing the PAO1 lysate with 10 mm DTT for 10 min at 20–22 °C followed by the addition of 20 mm NEM and incubation for 20 min. GSH–NEM was measured by LC-MS/MS after precipitation of protein as described above. GSSX was determined using Equation 1.
| (Eq. 1) |
For measuring glutathione species from PAO1 grown on 13C-containing medium, MRM transitions in Table S1 were used.
Preparation of Cys-NEM, CysSSG, CoA-NEM, and CoASSG standards
CoA–NEM and Cys-NEM standards were prepared by incubating 1 mm reduced CoA and Cys with 20 mm NEM for 20 min at 20–22 °C. LC-MS analysis in negative ion mode (described in more detail below) showed the formation of ions with m/z of 891 and 245 consistent with the [M − H]− of CoA–NEM and Cys-NEM, respectively. Conversion to the NEM adducts was nearly complete as indicated by negligible signals for CoA-SH (m/z 776) and Cys-SH (m/z 120). CysSSG was prepared by incubating 0.5 mm cysteine with 5 mm GSSG in water (pH 9), for 2 h at 37 °C. LC-MS analysis in negative ion mode showed the formation of an ion with the m/z of 245 consistent with the [M − H]− of Cys-SSG, respectively. Conversion to CysSSG was nearly complete as indicated by a negligible signal for Cys-SH (m/z 120).
CoASSG was prepared by mixing 0.25 mm CoA, 0.25 mm GSH, and 0.13 mm hypochlorous acid and incubated for 10 min at 20–22 °C. LC-MS analysis in negative ion showed the formation of an ion with the m/z of 425 consistent with the [M − H]− of CysSSG. Conversion to CoASSG was not complete as indicated by the presence of an ion with an m/z consistent with CoA-SH (m/z 776).
Measurement of Cys-NEM, CoA-NEM, CysSSG, and CoASSG by LC-MS
Protein in PAO1 lysate containing 107 bacteria was removed by ethanol precipitation as described above. Analytes in the supernatant were measured by LC-MS/MS using an Infinity 1290 LC system (Agilent, Santa Clara, CA) coupled to a 6500 QTrap mass spectrometer (Sciex, Framingham, MA), and concentrations were calculated using a corresponding calibration curve. Detection of Cys-NEM, CysSSG, CoA–NEM, and CoASSG was by MRM in negative ion mode. Cys-NEM, CysSSG, CoA–NEM, and CoASSG standards prepared above were used to optimize MRM parameters, and settings for the target analytes were m/z 891 → 543.9, 245 → 119.8, 425 → 304, and 535 → 991 (parent ion → fragment ion) for CoA-NEM, Cys-NEM, CysSSG, and CoASSG, respectively.
Cys-NEM and CysSSG were separated on a Hypercarb column (150 × 2.1 mm, 3 μm, Thermo Fisher Scientific, Waltham, MA) operated at 60 °C. Eluent A was water containing 0.25% formic acid (v/v), and eluent B was acetonitrile/propan-2-ol (50:50, v/v) containing 0.25% formic acid (v/v). A linear gradient from 100% eluent A to 50% eluent B was run over 8 min, followed by a 3-min wash with 95% eluent B and re-equilibration to the initial conditions. The flow rate was 0.25 ml/min, and the injection volume was 10 μl. Cys-NEM eluted at 9.1 min and CysSSG at 8.7 min (Fig. S1, A and B). This method is linear over the range of 60 to 0.03 pmol of Cys-NEM and 500 to 0.01 pmol for CysSSG injected onto the column, respectively. Recovery of 2.5 pmol of Cys-NEM and CysSSG standards added to PAO1 lysates after removal of protein by ethanol precipitation was 115 ± 20 and 97 ± 14%, respectively (n = 3, mean ± S.D. using three different PAO1 lysates).
CoA as the CoA–NEM adduct was separated on a Kinetex C18 column (150 × 2.1 mm, 2.6 μm, Phenomenex, Torrance, CA) operated at 40 °C. Eluent A was water containing 10 mm ammonium acetate (pH 6.8), and eluent B was methanol. A linear gradient from 5 to 50% eluent B was run over 8 min, followed by a 3-min wash with 95% eluent B and re-equilibration to the initial conditions. The flow rate was 0.2 ml/min and injection volume was 10 μl. CoA–NEM eluted at 9.5 min (Fig. S1C). This method is linear over the range of 60 to 2 pmol of CoA–NEM injected onto the column. Recovery of 100 pmol of CoA–NEM standards added to PAO1 lysates was 141 ± 11% (n = 2, mean ± S.D. with different PAO1 lysates).
CoASSG was separated on a Kinetex Biphenyl column (100 × 2.1 mm, 2.6 μm, Phenomenex, Torrance, CA) operated at 40 °C in isocratic mode with 10% eluant B. Eluent A was water containing 10 mm ammonium acetate (pH 6.8), and eluent B was methanol. The flow rate was 0.25 ml/min, and injection volume was 10 μl. CoASSG eluted at 0.9 min (Fig. S1D). This method is linear over the range of 125 to 0.25 pmol of CoASSG injected onto the column. CoASSG standards added to PAO1 lysates could not be recovered after removal of protein by ethanol precipitation indicating the instability or loss of this analyte during processing.
Statistics
Graphs were plotted, and statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA). Differences between groups were determined using one-way ANOVA with Dunnett's multiple comparison test. Alternatively, an unpaired t test was used when only two groups were compared. A p value < 0.05 was considered significant.
Author contributions
N. D., P. P., M. B. H., and A. J. K. conceptualization; N. D. data curation; N. D. formal analysis; N. D., M. B. H., and A. J. K. funding acquisition; N. D. investigation; N. D. methodology; N. D. writing-original draft; V. I., P. P., M. B. H., and A. J. K. resources; V. I., P. P., M. B. H., and A. J. K. writing-review and editing; M. B. H. and A. J. K. supervision.
Supplementary Material
Acknowledgments
We thank AstraZeneca (Gothenburg, Sweden) for the gift of AZM198. We also acknowledge Dr. Heather Parker and Dr. Louisa Ashby from the Centre for Free Radical Research for assistance with the neutrophil-killing assays and Dr. Nicholas Magon for assistance with the graphics in Fig. 7. We thank all blood donors and their families for participating in this study.
This work was supported by Health Research Council of New Zealand Grants 15/333 and 15/479. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S3 and Table S1.
- MPO
- myeloperoxidase
- CF
- cystic fibrosis
- CFTR
- cystic fibrosis transmembrane regulator
- CFU
- colony-forming unit
- CoA
- coenzyme A
- CoASSG
- mixed disulfide of CoA and glutathione
- CysSSG
- mixed disulfide of cysteine and glutathione
- DPI
- diphenylene iodonium chloride
- GSA
- glutathione sulfonamide
- GSH
- reduced glutathione
- GSSG
- glutathione disulfide
- GSSP
- glutathione bound in a mixed disulfide with protein thiol
- GSSX
- glutathione bound in a mixed disulfide with low molecular weight thiol
- HOCl
- hypochlorous acid
- IAM
- iodoacetamide
- LMW
- low molecular weight
- MRM
- multiple reaction monitoring
- NEM
- N-ethylmaleimide
- PsA
- Pseudomonas aeruginosa
- ANOVA
- analysis of variance
- HBSS
- Hanks' balanced salt solution
- BAL
- bronchoalveolar lavage.
References
- 1. Nathan C. (2006) Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6, 173–182 10.1038/nri1785 [DOI] [PubMed] [Google Scholar]
- 2. Rada B. (2017) Interactions between neutrophils and Pseudomonas aeruginosa in cystic fibrosis. Pathogens 6, E10 10.3390/pathogens6010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hampton M. B., Kettle A. J., and Winterbourn C. C. (1998) Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92, 3007–3017 [PubMed] [Google Scholar]
- 4. Winterbourn C. C., Hampton M. B., Livesey J. H., and Kettle A. J. (2006) Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J. Biol. Chem. 281, 39860–39869 10.1074/jbc.M605898200 [DOI] [PubMed] [Google Scholar]
- 5. Harrison J. E., and Schultz J. (1976) Studies on the chlorinating activity of myeloperoxidase. J. Biol. Chem. 251, 1371–1374 [PubMed] [Google Scholar]
- 6. Hawkins C. L., and Davies M. J. (2002) Hypochlorite-induced damage to DNA, RNA, and polynucleotides: formation of chloramines and nitrogen-centered radicals. Chem. Res. Toxicol. 15, 83–92 10.1021/tx015548d [DOI] [PubMed] [Google Scholar]
- 7. Hawkins C. L., Pattison D. I., and Davies M. J. (2003) Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids 25, 259–274 10.1007/s00726-003-0016-x [DOI] [PubMed] [Google Scholar]
- 8. Pattison D. I., and Davies M. J. (2001) Absolute rate constants for the reaction of hypochlorous acid with protein side chains and peptide bonds. Chem. Res. Toxicol. 14, 1453–1464 10.1021/tx0155451 [DOI] [PubMed] [Google Scholar]
- 9. Pattison D. I., Hawkins C. L., and Davies M. J. (2003) Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: absolute rate constants, product analysis, and computational modeling. Chem. Res. Toxicol. 16, 439–449 10.1021/tx025670s [DOI] [PubMed] [Google Scholar]
- 10. Schiller J., Arnhold J., Gründer W., and Arnold K. (1994) The action of hypochlorous acid on polymeric components of cartilage. Biol. Chem. Hoppe Seyler. 375, 167–172 10.1515/bchm3.1994.375.3.167 [DOI] [PubMed] [Google Scholar]
- 11. Lanza F. (1998) Clinical manifestation of myeloperoxidase deficiency. J. Mol. Med. 76, 676–681 10.1007/s001090050267 [DOI] [PubMed] [Google Scholar]
- 12. Segal A. W. (2005) How neutrophils kill microbes. Annu. Rev. Immunol. 23, 197–223 10.1146/annurev.immunol.23.021704.115653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosen H., and Michel B. R. (1997) Redundant contribution of myeloperoxidase-dependent systems to neutrophil-mediated killing of Escherichia coli. Infect. Immun. 65, 4173–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosen H., Klebanoff S. J., Wang Y., Brot N., Heinecke J. W., and Fu X. (2009) Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc. Natl. Acad. Sci. U.S.A. 106, 18686–18691 10.1073/pnas.0909464106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Q., Griffin D. A., Barofsky D. F., and Hurst J. K. (1997) Intraphagosomal chlorination dynamics and yields determined using unique fluorescent bacterial mimics. Chem. Res. Toxicol. 10, 1080–1089 10.1021/tx9700984 [DOI] [PubMed] [Google Scholar]
- 16. Chapman A. L., Hampton M. B., Senthilmohan R., Winterbourn C. C., and Kettle A. J. (2002) Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J. Biol. Chem. 277, 9757–9762 10.1074/jbc.M106134200 [DOI] [PubMed] [Google Scholar]
- 17. Rosen H., Crowley J. R., and Heinecke J. W. (2002) Human neutrophils use the myeloperoxidase-hydrogen peroxide-chloride system to chlorinate but not nitrate bacterial proteins during phagocytosis. J. Biol. Chem. 277, 30463–30468 10.1074/jbc.M202331200 [DOI] [PubMed] [Google Scholar]
- 18. Painter R. G., Valentine V. G., Lanson N. A. Jr., Leidal K., Zhang Q., Lombard G., Thompson C., Viswanathan A., Nauseef W. M., Wang G., and Wang G. (2006) CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 45, 10260–10269 10.1021/bi060490t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lyczak J. B., Cannon C. L., and Pier G. B. (2002) Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15, 194–222 10.1128/CMR.15.2.194-222.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Green J. N., Kettle A. J., and Winterbourn C. C. (2014) Protein chlorination in neutrophil phagosomes and correlation with bacterial killing. Free Radic. Biol. Med. 77, 49–56 10.1016/j.freeradbiomed.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 21. Coker M. S., Forbes L. V., Plowman-Holmes M., Murdoch D. R., Winterbourn C. C., and Kettle A. J. (2018) Interactions of staphyloxanthin and enterobactin with myeloperoxidase and reactive chlorine species. Arch. Biochem. Biophys. 646, 80–89 10.1016/j.abb.2018.03.039 [DOI] [PubMed] [Google Scholar]
- 22. Storkey C., Davies M. J., and Pattison D. I. (2014) Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach. Free Radic. Biol. Med. 73, 60–66 10.1016/j.freeradbiomed.2014.04.024 [DOI] [PubMed] [Google Scholar]
- 23. Newton G. L., Arnold K., Price M. S., Sherrill C., Delcardayre S. B., Aharonowitz Y., Cohen G., Davies J., Fahey R. C., and Davis C. (1996) Distribution of thiols in microorganisms: mycothiol is a major thiol in most actinomycetes. J. Bacteriol. 178, 1990–1995 10.1128/jb.178.7.1990-1995.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harwood D. T., Kettle A. J., and Winterbourn C. C. (2006) Production of glutathione sulfonamide and dehydroglutathione from GSH by myeloperoxidase-derived oxidants and detection using a novel LC-MS/MS method. Biochem. J. 399, 161–168 10.1042/BJ20060978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davies M. J., Hawkins C. L., Pattison D. I., and Rees M. D. (2008) Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid. Redox Signal. 10, 1199–1234 10.1089/ars.2007.1927 [DOI] [PubMed] [Google Scholar]
- 26. Harwood D. T., Kettle A. J., Brennan S., and Winterbourn C. C. (2009) Simultaneous determination of reduced glutathione, glutathione disulphide and glutathione sulphonamide in cells and physiological fluids by isotope dilution liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877, 3393–3399 10.1016/j.jchromb.2009.04.018 [DOI] [PubMed] [Google Scholar]
- 27. Newton G. L., Rawat M., La Clair J. J., Jothivasan V. K., Budiarto T., Hamilton C. J., Claiborne A., Helmann J. D., and Fahey R. C. (2009) Bacillithiol is an antioxidant thiol produced in bacilli. Nat. Chem. Biol. 5, 625–627 10.1038/nchembio.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Albrett A. M., Ashby L. V., Dickerhof N., Kettle A. J., and Winterbourn C. C. (2018) Heterogeneity of hypochlorous acid production in individual neutrophil phagosomes revealed by a rhodamine-based probe. J. Biol. Chem. 293, 15715–15724 10.1074/jbc.RA118.004789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Palazzolo A. M., Suquet C., Konkel M. E., and Hurst J. K. (2005) Green fluorescent protein-expressing Escherichia coli as a selective probe for HOCl generation within neutrophils. Biochemistry 44, 6910–6919 10.1021/bi047342s [DOI] [PubMed] [Google Scholar]
- 30. Schwartz J., Leidal K. G., Femling J. K., Weiss J. P., and Nauseef W. M. (2009) Neutrophil bleaching of GFP-expressing staphylococci: probing the intraphagosomal fate of individual bacteria. J. Immunol. 183, 2632–2641 10.4049/jimmunol.0804110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bhavsar T., Liu M., Hardej D., Liu X., and Cantor J. (2010) Aerosolized recombinant human lysozyme ameliorates Pseudomonas aeruginosa-induced pneumonia in hamsters. Exp. Lung Res. 36, 94–100 10.3109/01902140903154608 [DOI] [PubMed] [Google Scholar]
- 32. Cole A. M., Thapa D. R., Gabayan V., Liao H. I., Liu L., and Ganz T. (2005) Decreased clearance of Pseudomonas aeruginosa from airways of mice deficient in lysozyme M. J. Leukoc. Biol. 78, 1081–1085 10.1189/jlb.0205073 [DOI] [PubMed] [Google Scholar]
- 33. Hirche T. O., Benabid R., Deslee G., Gangloff S., Achilefu S., Guenounou M., Lebargy F., Hancock R. E., and Belaaouaj A. (2008) Neutrophil elastase mediates innate host protection against Pseudomonas aeruginosa. J. Immunol. 181, 4945–4954 10.4049/jimmunol.181.7.4945 [DOI] [PubMed] [Google Scholar]
- 34. Tai K. P., Kamdar K., Yamaki J., Le V. V., Tran D., Tran P., Selsted M. E., Ouellette A. J., and Wong-Beringer A. (2015) Microbicidal effects of α- and θ-defensins against antibiotic-resistant Staphylococcus aureus and Pseudomonas aeruginosa. Innate Immun. 21, 17–29 10.1177/1753425913514784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Beaumont P. E., McHugh B., Gwyer Findlay E., Mackellar A., Mackenzie K. J., Gallo R. L., Govan J. R., Simpson A. J., and Davidson D. J. (2014) Cathelicidin host defence peptide augments clearance of pulmonary Pseudomonas aeruginosa infection by its influence on neutrophil function in vivo. PLoS ONE 9, e99029 10.1371/journal.pone.0099029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Foote J. R, Levine A. P., Behe P., Duchen M. R., and Segal A. W. (2017) Imaging the neutrophil phagosome and cytoplasm using a ratiometric pH indicator. J. Vis. Exp. 2017, 10.3791/55107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Levine A. P., Duchen M. R., de Villiers S., Rich P. R., and Segal A. W. (2015) Alkalinity of neutrophil phagocytic vacuoles is modulated by HVCN1 and has consequences for myeloperoxidase activity. PLoS ONE 10, e0125906 10.1371/journal.pone.0125906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Frühwirth M., Ruedl C., Ellemunter H., Böck G., and Wolf H. (1998) Flow-cytometric evaluation of oxidative burst in phagocytic cells of children with cystic fibrosis. Int. Arch. Allergy Immunol. 117, 270–275 10.1159/000024022 [DOI] [PubMed] [Google Scholar]
- 39. Houston N., Stewart N., Smith D. S., Bell S. C., Champion A. C., and Reid D. W. (2013) Sputum neutrophils in cystic fibrosis patients display a reduced respiratory burst. J. Cyst. Fibros. 12, 352–362 10.1016/j.jcf.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 40. McKeon D. J., Cadwallader K. A., Idris S., Cowburn A. S., Pasteur M. C., Barker H., Haworth C. S., Bilton D., Chilvers E. R., and Condliffe A. M. (2010) Cystic fibrosis neutrophils have normal intrinsic reactive oxygen species generation. Eur. Respir. J. 35, 1264–1272 10.1183/09031936.00089709 [DOI] [PubMed] [Google Scholar]
- 41. Robledo-Avila F. H., Ruiz-Rosado J. D., Brockman K. L., Kopp B. T., Amer A. O., McCoy K., Bakaletz L. O., and Partida-Sanchez S. (2018) Dysregulated calcium homeostasis in cystic fibrosis neutrophils leads to deficient antimicrobial responses. J. Immunol. 201, 2016–2027 10.4049/jimmunol.1800076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Witko-Sarsat V., Allen R. C., Paulais M., Nguyen A. T., Bessou G., Lenoir G., and Descamps-Latscha B. (1996) Disturbed myeloperoxidase-dependent activity of neutrophils in cystic fibrosis homozygotes and heterozygotes, and its correction by amiloride. J. Immunol. 157, 2728–2735 [PubMed] [Google Scholar]
- 43. Ng H. P., Valentine V. G., and Wang G. (2016) CFTR targeting during activation of human neutrophils. J. Leukoc. Biol. 100, 1413–1424 10.1189/jlb.4A0316-130RR [DOI] [PubMed] [Google Scholar]
- 44. Painter R. G., Marrero L., Lombard G. A., Valentine V. G., Nauseef W. M., and Wang G. (2010) CFTR-mediated halide transport in phagosomes of human neutrophils. J. Leukoc. Biol. 87, 933–942 10.1189/jlb.1009655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen J. H., Stoltz D. A., Karp P. H., Ernst S. E., Pezzulo A. A., Moninger T. O., Rector M. V., Reznikov L. R., Launspach J. L., Chaloner K., Zabner J., and Welsh M. J. (2010) Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell 143, 911–923 10.1016/j.cell.2010.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kettle A. J., and Winterbourn C. C. (1989) Influence of superoxide on myeloperoxidase kinetics measured with a hydrogen peroxide electrode. Biochem. J. 263, 823–828 10.1042/bj2630823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gabig T. G., Bearman S. I., and Babior B. M. (1979) Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood 53, 1133–1139 [PubMed] [Google Scholar]
- 48. Wang G. (2016) Chloride flux in phagocytes. Immunol. Rev. 273, 219–231 10.1111/imr.12438 [DOI] [PubMed] [Google Scholar]
- 49. Gorter J., and Gruber M. (1970) Cathepsin C: an allosteric enzyme. Biochim. Biophys. Acta 198, 546–555 10.1016/0005-2744(70)90132-4 [DOI] [PubMed] [Google Scholar]
- 50. Korkmaz B., Lesner A., Letast S., Mahdi Y. K., Jourdan M. L., Dallet-Choisy S., Marchand-Adam S., Kellenberger C., Viaud-Massuard M. C., Jenne D. E., and Gauthier F. (2013) Neutrophil proteinase 3 and dipeptidyl peptidase I (cathepsin C) as pharmacological targets in granulomatosis with polyangiitis (Wegener granulomatosis). Semin. Immunopathol. 35, 411–421 10.1007/s00281-013-0362-z [DOI] [PubMed] [Google Scholar]
- 51. Painter R. G., Bonvillain R. W., Valentine V. G., Lombard G. A., LaPlace S. G., Nauseef W. M., and Wang G. (2008) The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J. Leukoc. Biol. 83, 1345–1353 10.1189/jlb.0907658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cohen T. S., and Prince A. (2012) Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat. Med. 18, 509–519 10.1038/nm.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miao X. Y., Ji X. B., Lu H. W., Yang J. W., and Xu J. F. (2015) Distribution of major pathogens from sputum and bronchoalveolar lavage fluid in patients with noncystic fibrosis bronchiectasis: a systematic review. Chin. Med. J. 128, 2792–2797 10.4103/0366-6999.167360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berkebile A. R., and McCray P. B. Jr. (2014) Effects of airway surface liquid pH on host defense in cystic fibrosis. Int. J. Biochem. Cell Biol. 52, 124–129 10.1016/j.biocel.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aratani Y., Kura F., Watanabe H., Akagawa H., Takano Y., Suzuki K., Maeda N., and Koyama H. (2000) Differential host susceptibility to pulmonary infections with bacteria and fungi in mice deficient in myeloperoxidase. J. Infect. Dis. 182, 1276–1279 10.1086/315843 [DOI] [PubMed] [Google Scholar]
- 56. Chesney J. A., Eaton J. W., and Mahoney J. R. Jr. (1996) Bacterial glutathione: a sacrificial defense against chlorine compounds. J. Bacteriol. 178, 2131–2135 10.1128/jb.178.7.2131-2135.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Degrossoli A., Müller A., Xie K., Schneider J. F., Bader V., Winklhofer K. F., Meyer A. J., and Leichert L. I. (2018) Neutrophil-generated HOCl leads to non-specific thiol oxidation in phagocytized bacteria. Elife 7, e32288 10.7554/eLife.32288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hansen R. E., Roth D., and Winther J. R. (2009) Quantifying the global cellular thiol-disulfide status. Proc. Natl. Acad. Sci. U.S.A. 106, 422–427 10.1073/pnas.0812149106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dalle-Donne I., Rossi R., Colombo G., Giustarini D., and Milzani A. (2009) Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem. Sci. 34, 85–96 10.1016/j.tibs.2008.11.002 [DOI] [PubMed] [Google Scholar]
- 60. Xie K., Bunse C., Marcus K., and Leichert L. I. (2019) Quantifying changes in the bacterial thiol redox proteome during host–pathogen interaction. Redox. Biol. 21, 101087 10.1016/j.redox.2018.101087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Horvath A. K., and Nagypal I. (2006) Kinetics and mechanism of the oxidation of sulfite by chlorine dioxide in a slightly acidic medium. J. Phys. Chem. A 110, 4753–4758 10.1021/jp060246b [DOI] [PubMed] [Google Scholar]
- 62. Ishiye M., Yamashita M., and Niwa M. (1993) Molecular cloning of the γ-glutamyltranspeptidase gene from a Pseudomonas strain. Biotechnol. Prog. 9, 323–331 10.1021/bp00021a012 [DOI] [PubMed] [Google Scholar]
- 63. Suzuki H., Kumagai H., and Tochikura T. (1986) γ-Glutamyltranspeptidase from Escherichia coli K-12: formation and localization. J. Bacteriol. 168, 1332–1335 10.1128/jb.168.3.1332-1335.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Morris J. C. (1966) Acid ionization constant of HOCl from 5 to 35 degrees. J. Phys. Chem. 70, 3798–3805 10.1021/j100884a007 [DOI] [Google Scholar]
- 65. Tidén A. K., Sjögren T., Svensson M., Bernlind A., Senthilmohan R., Auchère F., Norman H., Markgren P. O., Gustavsson S., Schmidt S., Lundquist S., Forbes L. V., Magon N. J., Paton L. N., Jameson G. N., Eriksson H., and Kettle A. J. (2011) 2-Thioxanthines are mechanism-based inactivators of myeloperoxidase that block oxidative stress during inflammation. J. Biol. Chem. 286, 37578–37589 10.1074/jbc.M111.266981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Segal A. W., Dorling J., and Coade S. (1980) Kinetics of fusion of the cytoplasmic granules with phagocytic vacuoles in human polymorphonuclear leukocytes. Biochemical and morphological studies. J. Cell Biol. 85, 42–59 10.1083/jcb.85.1.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dickerhof N., Pearson J. F., Hoskin T. S., Berry L. J., Turner R., Sly P. D., Kettle A. J., and AREST, C. F. (2017) Oxidative stress in early cystic fibrosis lung disease is exacerbated by airway glutathione deficiency. Free Radic. Biol. Med. 113, 236–243 10.1016/j.freeradbiomed.2017.09.028 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.