Abstract
Objectives
We aimed to estimate the prevalence and correlates of QT interval prolongation in rural Uganda.
Methods
We conducted a cross-sectional survey in a sample of adults participating in an ongoing whole-population cohort in Mbarara, Uganda in 2015. Of 1,814 subjects enrolled in the parent whole-population cohort, 856 (47%) participated in the study. Participants completed 12-lead electrocardiography and cardiovascular disease risk factors assessment. We summarized sex-specific, heart rate variation-adjusted QT (QTa) defining prolonged QTa as >460ms in women and >450ms in men. We fit linear and logistic regression models to estimate correlates of (continuous) QTa interval length and (dichotomous) prolonged QTa. Models included inverse probability of sampling weights to generate population-level estimates accounting for study non-participation.
Results
We assessed data from 828 participants with electrocardiograms. The weighted population mean age was 38.4 years (95% CI: 36.3 – 40.4). The weighted population was 50.4% female, 11.5% had elevated blood pressure and 57.6% had a high-sensitivity C-reactive protein >1mg/dL. The population mean QTa was 409.1 ms (95% CI: 405.1 – 413.1), and 10.3% (95% CI: 7.8–13.5) met criteria for prolonged QTa. Women had a higher mean QTa (421.6ms vs. 396.3ms, p<0.001), and a higher proportion of women had a prolonged QTa (14.0% vs. 9.3%, p = 0.122) compared to men. In multivariable-adjusted regression models, female sex and hypertension correlated with higher mean QTa and meeting criteria for prolonged QTa, respectively.
Conclusions
QT interval prolongation is highly prevalent in rural Uganda, and may be more common than in high-income settings. Female sex, age and high blood pressure correlated with QT interval prolongation. Future work should assess whether genetic predisposition or environmental factors in sub-Saharan African populations contribute to prolonged QT and clarify consequences.
Keywords: QT interval prolongation, electrophysiology, population prevalence, Uganda, Sub-Saharan Africa
INTRODUCTION
Cardiovascular diseases (CVD) are a growing cause of morbidity and mortality in sub-Saharan Africa (SSA) [1], yet the precise epidemiology of these conditions is not well characterized, in part due to limitations in diagnostic resources in the region [2]. Resting electrocardiography (ECG) is a low-cost, non-invasive technique that has potential for assessing prevalence and risk of CVD in low-income settings [3]. In the US and Europe, major ECG abnormalities are independently predictive of major cardiovascular adverse events (MACE) [4]. For example, a prolonged QT interval has comparable predictive value for cardiovascular and all-cause mortality as that of many traditional risk factors [5].
However, epidemiologic data on ECG abnormalities, including QT prolongation, and their correlates are based predominantly on studies performed in Western populations [6], [7]. Notably, ethnicity is an effect modifier of the relationship between many ECG abnormalities and CVD outcomes. For example, the association between QT prolongation and MACE is considerably stronger in African Americans than Caucasians in the US [8]. Other determinants of QT interval prolongation include HIV infection, drug exposure, diabetes , hypertension and genetics [9], also differ by region. Thus, establishing regional data on QT intervals to standardize norms and assess for correlations with CVD events in SSA is an important priority. Here, we leverage data from a community-based ECG screening within a population cohort, to estimate the population distribution of prolonged QT intervals in a rural population in Uganda, and describe its relationship with CVD risk factors. In the absence of local data, we use QT interval length norms defined in Western populations.
METHODS
Study design and setting
We conducted a cross sectional survey of adults, 18 years and older, who attended a community health fair held over a 5-day period in Nyakabare Parish, Mbarara District, Uganda in June of 2015. The community health fair was advertised to individuals in the community by means of local radio advertisements, and through community structures (e.g., church and social gatherings). There were no fees for participation in the health fairs. Nyakabare is a predominantly subsistence pastoral-agrarian community, with significant water and food insecurity [10]. Between May 2014 and June 2015, and preceding the health fairs, a parish-wide census was conducted with demographic and health data collection on 98% (1,814/1,851) of all eligible individuals, namely: adults 18 years of age and older (and emancipated minors aged 16-18 years) who considered Nyakabare their primary place of residence. Although pregnant women were not excluded from participation in the health fair, data was not collected on self-reported or biologic tested pregnancy.
Data collection
Participants in the health fair had their height and weight measured. Blood pressure was measured in a seated position using automated sphygmomanometers (Omron HEM 705 LP, Omron Healthcare, Inc., Bannockburn, IL). Venous blood was collected to assess serum lipids, creatinine, C-reactive protein (CRP) and glycated haemoglobin (HbA1c) (Siemens DCA Vantage, Munich, Germany). Human immunodeficiency virus (HIV) infection status was determined by rapid antibody testing according to Ugandan National HIV Testing Guidelines (2010) [11]. We defined diabetes mellitus and pre-diabetes mellitus as HbA1c ≥6.5% and 5.7-6.4%, respectively [12]. A questionnaire based on the International Physical Activity Questionnaire (IPAQ) [13] and the WHO STEPS survey [14] was administered to collect individual participant data on tobacco use and physical activity. Physical activity was measured as metabolic equivalent of task (MET) in minutes per week and categorized as active, minimally active and inactive following IPAQ standard classifications [13]. Lastly, previous medical and family histories of CVD were assessed by self-report. We also documented the use of any medication indicated for common non-communicable diseases, including hypertension, asthma, heart failure, dyslipidemia, and diabetes mellitus.
Electrocardiogram collection and measurement
A 10-second, 12-lead ECG was recorded for each participant in the seated position [15] using a portable ECG machine (CardioCard Digital ECG Box with CardioCard software, Nasiff Associates, New York, USA). ECGs were collected as PDFs and converted to JPEG image format. Those that were visually assessed as being of adequate quality were digitized by conversion into Food and Drug Administration (FDA) standard extensible markup language (aECG FDA HL7 XML) file format using ECG Scan® version 3.3.0 (AMPS LLC, New York, NY). Each digitized XML ECG tracing was then automatically annotated by on-screen digital calipers for QT and RR interval measurement using CalECG® version 3.7.0 (AMPS, New York, NY, USA) [16]. Fully automatic measurements of QT and RR intervals were annotated on limb lead II using the tangent method [17]. The CalECG® on-screen calipers automatically specify the onset of the QT interval as the first deflection of the QRS complex. The end of the T wave is estimated at the point at which a tangent line drawn from the steepest portion of the T wave intersects the isoelectric baseline. All CalECG® measurements were visually verified by a study clinician (IM).
We averaged each of the three QT and RR intervals to obtain the final QT and RR intervals for analysis. We compared agreement between CalECG® and manually derived intervals in a subset of participants for quality assurance purposes. Two reviewers (IM and RM) measured QT and RR intervals from 41 randomly selected ECGs using manual calipers. All measurements were made from limb lead II using the tangent method, as described above, for QT interval measurement. Calculation of the correlation coefficient and the limits of agreement demonstrated that the CalECG® and manual caliper-based methods were similar for the QT interval (Pearson correlation coefficient, r = + 0.96, mean difference ± 2 standard deviations = ± 14.76 ms) and RR interval (r = + 0.96, mean difference ± 2 standard deviations = 25.0 ± 32.05 ms) [18].
Because of recent recommendations that discourage the use of Bazett’s formula for correction of heart-rate-associated variation of the QT interval [19], we used the method described by Sagie et. al. to adjusted for heart rate variability [20]. To do so, we fitted a linear model of QT interval regressed on RR interval and sex, estimating a β-coefficient of the RR interval of 0.187. We then calculated heart-rate adjusted QT (QTa) using the formula: QTa = QT + 0.187*(1 – RR). The application of this formula in our study sample was intended to make the QT interval heart rate-invariant (residual slope of regression, <0.001; 95% confidence interval, −2.88, 2.88; p=0.999).
Statistical analysis
We sought to estimate population-level QTa characteristics and correlates, using inverse probability of sampling weights to account for health fair non-attendance. To do so, we used a propensity score-based technique to correct the likelihood of non-response based on participants’ features from the community census. First, we fit logistic regression models among all census respondents (N=1,1814) with health fair attendance as the outcome of interest and included covariates predicted to correlate with health fair attendance(N=1,1814): food and water insecurity, alcohol use, household asset ownership, sex, age, marital status, village of residence, distance from the health fair, difference between the altitude of the household residence and the altitude of the health fair, educational attainment, self-reported HIV status, self-reported overall health, social network size, and index of social participation (See Supplemental Methods Section). From this, we predicted the propensity to attend a health fair and then produced sampling weights as stabilized inverse probability of treatment weights (IPTW) using methods described previously [21]. Regression models with these weights were then used to make population-representative estimates. We assessed the validity of this method to correlate with population characteristics using variables that were not included in the IPT model, but for which we had values for the whole population from the census (Supplementary Table 1).
We next estimated summary statistics, applying the IPT weights to generate population-representative estimates. We created a graph of mean QTa stratified by age to evaluate their relationship; noting a non-linear relationship between QTa and age, we categorized age as <30 years, 30-50 years, and >50 years for the remainder of our analyses. We next estimated the overall and sex-specific population prevalence of prolonged QTa, defined by a QTa ≥ 460 ms in women and ≥450 ms in men [19]. We repeated our estimates using extreme QTa as the outcome of interest, defined as QTa ≥500 ms for both men and women [19], and using sex-based Bazett-corrected QT (QTcB) (prolonged QTcB ≥470ms in women and ≥450ms in men; extreme QTcB ≥500ms in both women and men), to allow for comparison with prior studies. We then fit multivariable log binomial regression models reporting relative prevalence as suggested by Reichenheim et. al. [22] to estimate correlates of prolonged QTa, including sex, age strata (<30, 30 – 50, and >50 years), elevated Hb1Ac (≤5.7, 5.7-6.5% and ≥6.5%), hypertension (systolic <140 and diastolic <90 versus systolic ≥140 or diastolic ≥90mmHg), eGFR (60 – 89, and ≥90 ml/min/1.73m2), body mass index (<18.5, 18.5 – 24.9, 25.0 – 29.9, and ≥ 30kg/m2), CRP (≤1, 1-3, and ≥3mg/dL), smoking status (current, former and never), serum HDL cholesterol (<40, 40-60 and >60 mg/dL), level of physical activity (inactive, minimal and active), and HIV infection serostatus. We did not include current use of medication as few individuals in the dataset reported use of active medications (<5%). Adjusted models included all factors that achieved a significance level of P<0.25 on univariate analysis. We repeated all analyses considering QTa as a continuous variable, and fitting linear models to estimate correlates. Models were re-estimated with trimmed IPT weights (i.e. removing probability weights >95% and <5%) to assess for robustness to outliers. All statistical analyses were performed using Stata version 14.0 (StataCorp, College Station, TX) with a two-sided P-value <0.05 considered statistically significant.
Ethical Considerations
All study procedures were reviewed and approved by the institutional review boards of Mbarara University of Science and Technology and Partners Healthcare as conforming to the ethical guidelines of the 1975 Declaration of Helsinki. Consistent with national guidelines, we also obtained clearance for the study from the Uganda National Council of Science and Technology and from the Research Secretariat in the Office of the President of Uganda. All study participants gave written informed consent for participation. Participants who could not write were permitted to indicate consent with a thumbprint.
Results
Out of 1,851 eligible adults in the parish, 856 (46.2%) participated in the HF undergoing electrocardiography, and 828 (44.7%) had ECGs deemed of sufficient quality to be included in the final study sample. Health fair attendees were more likely to be female (p<0.001), older (p<0.001), and have less formal educational attainment (p<0.001) than non-attendees. Notably, attendees were twice as likely as non-attendees to report very bad or bad health (1.4% vs. 0.7%, and 26.5% vs. 13.1%, respectively, p <0.001) (Supplementary Table 3). Of those who did attend the health fair, 28 (3.3%) participants had no ECG data. However, those missing ECG data were similar to the rest of the sample in terms of CVD risk factors (Supplementary Table 2).
The weighted mean age for the population was 38.4 years (95% CI: 36.3 – 40.4) (Table 1). The population was 50.4% female, 11.5% had hypertension, 2.0% had diabetes, and 57.6% had a high-sensitivity C-reactive protein >1mg/dL. The majority of the population had never smoked (74.1%) and maintained high levels of physical activity (75.5%). Compared to men, women were older (39.8 vs. 36.1 years; p =0.027), had higher rates of systemic inflammation (CRP >1mg/dL) (69.1 vs. 46.9%; p=0.003), and were more likely to be obese (mean BMI 26.2 vs. 22.4 kg/m2; p <0.001).
Table 1:
Estimated weighted baseline population characteristics
| Characteristics | Weighted estimate# | |||
|---|---|---|---|---|
| Male | Female | P value | Total population | |
| Sex (%) | 49.5 | 50.4 | 0.656 | - |
| Age, mean (years) | 36.1 (33.1 – 39.2) | 39.8 (37.8 – 41.9) | 0.027 | 38.4 (36.3 – 40.4) |
| ≤30 years | 46.4% | 35.2% | 40.7% | |
| 30-50 years | 33.0% | 38.0% | 35.5% | |
| >50 years | 20.6% | 27.0% | 0.105 | 23.8% |
| BMI, mean ( kg/m2) | 22.4 (22.1 – 22.7) | 26.2 (25.6 – 26.8) | <0.001 | 24.3 (23.8 – 24.7) |
| ≤18.5 | 80.0% | 44.9% | 62.2% | |
| 18.5-24.9 | 5.3% | 3.1% | 4.2% | |
| 25-29.9 | 13.2% | 33.1% | 23.2% | |
| ≥30 | 1.7% | 18.9% | <0.001 | 10.3% |
| History of stroke (%) | 4.6 (1.7 – 11.5) | 1.6 (1.0 – 2.9) | 0.065 | 3.1 (1.5 – 6.3) |
| History of heart disease (%) | 1.8 (0.6 – 4.9) | 6.0 (0.4 – 8.4) | 0.013 | 3.9 (2.8 – 5.6) |
| Current hypertension* % | 10.2 (6.9 – 14.9) | 12.8 (9.8 – 16.5) | 0.345 | 11.5 (9.2 – 14.4) |
| Systolic BP (mmHg) | 125.2 (122.0 -128.0) | 121.6 (119.0 - 124.2) | 0.339 | 123.2 (121.3 – 125.0) |
| Diastolic BP (mmHg) | 77.5 (74.8 - 80.2) | 79.6 (78.0 -81.2) | 0.037 | 78.4 (77.0 – 80.0) |
| HbA1c | ||||
| Pre-diabetes | 3.1% | 10.3% | 6.8% | |
| Diabetes mellitus | 1.6% | 2.3% | 0.001 | 2.0% |
| HDL cholesterol (mg/dL) | ||||
| ≥50 | 28.7% | 22.7% | 25.6% | |
| 40-49 | 22.0% | 32.3% | 27.2% | |
| <40 | 49.3% | 45.1% | 0.187 | 47.2% |
| CRP (mg/dL) | ||||
| ≤1 mg/dL | 53.1% | 31.9% | 42.4% | |
| 1-3 mg/dL | 35.7% | 47.0% | 41.4% | |
| >3 mg/dL | 11.2% | 21.1% | 0.006 | 16.2 |
| *eGFR (ml/min/1.73m2) | 150.60 (142.0 - 158.2) | 133.5 (129.8 - 137.1) | <0.001 | 142.0 (137.2 – 146.6) |
| HIV seropositive (%) | 3.5 (1.9 – 6.2) | 6.7 (4.3 – 10.4) | 0.059 | 5.0 (3.5 – 7.1) |
| Physical Activity Category | ||||
| Inactive | 9.0% | 12.1% | 10.5% | |
| Minimal | 16.8% | 11.1% | 14.0% | |
| Active | 74.3% | 76.7% | 0.286 | 75.5% |
| Cigarette smoking | ||||
| Current | 17.9% | 4.5% | 10.6% | |
| Former | 14.7% | 14.9% | 14.5% | |
| Never | 67.4% | 80.6% | <0.001 | 74.1% |
| Use of chronic medication | 3.4 (1.9 – 5.9)% | 5.6 (3.9 – 7.9)% | 0.127 | 4.5 (3.3 -6.1)% |
means reported with 95% confidence intervals
eGFR estimated glomerular filtration according to MDRD study equation.
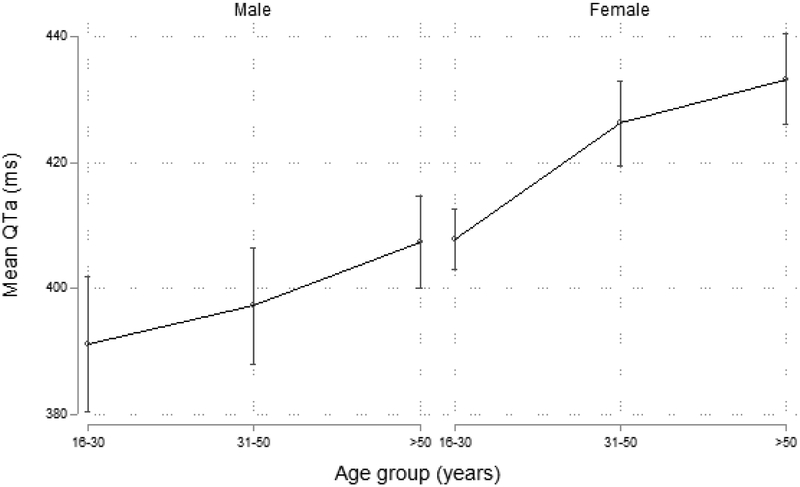
Population-weighted characteristics were as follows: mean QTa, 409.1ms (95% CI: 405.1 – 413.1); prolonged QTa, 11.7% (95% CI: 9.1 – 15.0); extreme QTa prevalence, 2.1% (95% CI: 1.4 – 3.2); prolonged QTcB prevalence, 10.3% (95% CI: 7,8 – 13.5); extreme QTcB prevalence, 4.0% (95% CI: 2.6 – 5.9) (Table 2). Compared with men, women had higher mean QTa (421.6ms vs. 396.3ms, p<0.001) and increased prevalence of extreme QTa (3.3% vs. 0.8%, p = 0.002) (Figure 1). Women also had increased prevalence of prolonged QTa, though the difference was not statistically significant (14.0% vs. 9.3%, p = 0.122). The mean QTcB was 403.5ms in men and 434.2ms in women (p <0.001). QTa increased with age such that those aged 51 years and older had higher mean QTa than their younger counterparts 16-30 years old; however, this association was statistically significant only among women: 433.2ms (95% CI: 426.1 - 440.4) vs. 403.2ms among men (95% CI: 392.3 - 414.2, p <0.001).
Table 2:
Weighted population estimates of ECG characteristics
| ECG features | Weighted estimates* | |||
|---|---|---|---|---|
| Male | Female | p-value | Population | |
| QTa (ms) | 396.3 (390.2 – 402.5) | 421.6 (417.6 – 425.5) | <0.001 | 409.1 (405.1 – 413.1) |
| QTcB (ms) | 403.5 (396.2 – 410.8) | 434.2 (429.5 – 438.8) | <0.001 | 419.0 (414.3 – 423.7) |
| Prolonged QTa (%) | 9.3 (5.7 – 14.6) | 14.0 (10.8 – 18.0) | 0.122 | 11.7 (9.1 – 15.0) |
| Prolonged QTcB (%) | 9.5 (5.9 – 15.1) | 11.1 (8.2 – 15.0) | 0.586 | 10.3 (7.8 – 13.5) |
| Extreme QTa (%) | 0.8 (0.4 – 1.9) | 3.3 (2.1 – 5.3) | 0.002 | 2.1 (1.4 – 3.2) |
| Extreme QTcB (%) | 2.6 (1.0 – 6.8) | 5.3 (3.6 – 7.7) | 0.171 | 4.0 (2.6 – 5.9) |
| Normal ECG (%) | 70.7 (61.0 – 78.8) | 69.7 (64.4 – 74.4) | 0.848 | 70.1 (64.8 – 75.0) |
| aIVCD | 1.3 ((0.04 – 0.4) | 0.4 (0.0 – 1.6) | 0.173 | 0.8 (0.0 – 2.1) |
| bLV hypertrophy | 1.3 (0.6 – 2.9) | 0.9 (0.4 – 2.0) | 0.444 | 0.1 (0.6 – 1.9) |
| cLBBB | 0.3 (0.0 – 2.3) | 0.1 (0.3 – 1.8) | 0.281 | 0.7 (0.0 – 1.8) |
| dRBBB | 1.3 (0.5 – 3.9) | 0.7 (0.3 – 1.6) | 0.298 | 1.0 (0.5 – 2.2) |
| eQ wave MI | 0.8 (0.3 – 1.9) | 0.8 (0.3 – 2.1) | 0.976 | 0.8 (0.4 – 1.5) |
means reported with 95% confidence intervals
IVCD - interventricular conduction delay
LV hypertrophy – left ventricular hypertrophy
LBBB – left bundle branch block
RBBB – right bundle branch block
Q wave MI – pathological Q wave myocardial infarction
Figure 1.
QTa according to age group and sex
In univariable models, female sex, obesity (BMI ≥30kg/mm2), hypertension, CRP, eGFR, HbA1c, and physical activity were correlated with prolonged QTa. However, in multivariable-adjusted logistic regression models, only hypertension (adjusted relative prevalence [ARP], 1.4; 95% CI 1.0 – 3.2; p=0.037) and age (31-50 years: ARP 3.1; 95%CI 1.4 – 6.8; and >50 years: ARP 3.3; 95%CI 1.3 – 7.9) had a statistically significant association with prolonged QTa (Table 2b). In multivariable-adjusted linear regression models, QTa specified as a continuous variable had a statistically significant association with female sex (b=21.2; 95% CI: 14.0 – 28.3, p<0.001) and age >50 years (b=14.0; 95%CI 4.6 – 23.3, p = 0.004) (Table 2a).
Discussion
Our study is among the first population-based investigations of QT interval length and its correlates in SSA. Population prevalence of prolonged QTa was estimated to be 11.7%, and population prevalence of extreme QTa was estimated to be 2.1%. Both were more prevalent in women versus men. Our findings indicate an unexpectedly high prevalence of QT interval lengthening, which is particularly notable given the relatively young population age (<40 years) and the low burden of recognized QT prolongation risk factors.
In combination with prior data, our findings suggest that QT intervals might be more prolonged in SSA than elsewhere. In the only other population-based study from sub-Saharan Africa, Dewhurst et. al. (2014) [23] reported a mean QTcB of 418 ms (SD 24) in men and 429 ms (SD 24) in women, among a community of adults at least 70 years old in rural Tanzania. Three population-based studies conducted in the US estimated comparatively lower QT intervals. In the Multi-Ethnic Study of Atherosclerosis, which had a mean age of 62 years, and defined regression-corrected prolonged QTa as ≥460 ms in women and ≥450 ms in men [24], prolonged QTa was observed in only 3.5% of individuals, compared to 11.8% in our population. In the REGARDS study [25], mean QTa was estimated to be 409 ms (SD 1.1) among African American men and 417 ms (SD 0.6) among African American women. Interestingly, we found longer QTa among women (421.6ms) in our population, but shorter QTa in men (396.3ms) compared to the African American sample in that study. Lastly, the Jackson Heart Study (JHS) [26] which enrolled an African American population in Mississippi, reported a prevalence of extreme QTcB (≥500ms) in 0.5% of men and 0.4% of women, compared to 2.6% and 5.3% in our Ugandan population. Moreover, our increased estimates of extreme QT prevalence are especially notable given the comparatively younger age and lower prevalence of traditional risk factors (i.e. hypertension 11.5%; current smoking 10.6%; mean age 38.4 years) in our Ugandan cohort (Table 1) compared to the Mississippi cohort (hypertension 57.6%; current smoking 18.0%; mean age 53.2 years) [26].
Our study identified female sex as a correlate of longer QTa and hypertension as a correlate of prolonged QTa. These findings are consistent with a recent systematic review of QT prolongation risk factors in high income settings [9] and including 89,532 adults. That review also identified age, female sex, smoking, hypertension, hypokalemia, use of diuretics and arhythmogenic drugs, and history of myocardial disease as predictive of prolonged QT. The association of QT prolongation with BMI in our study was moderate, while we did not estimate a statistically significant correlation with diabetes, dyslipidemia or renal failure. Although we observed associations between dichotomized and continuous QTa and BMI, CRP, smoking and HbA1c on univariate analysis, these associations did not persist in multivariable-adjusted regression models. Notably, the relationship between CRP and QTa has been previously demonstrated in the setting of chronic inflammatory diseases in high-income settings [27]. This putative link between systemic inflammation and arrhythmic risk, and our observation of highly prevalent (approximately 60%) systemic inflammation (CRP >1mg/dL) in an apparently healthy population, warrant further investigation.
The relevance of our findings for the health of individuals in rural sub-Saharan Africa is unknown. QT prolongation is associated with functional re-entry, torsade de pointes, sudden cardiac death and coronary heart disease [9]. In both individuals with multiple co-morbidities [28], and in the general population, QT interval prolongation is predictive of cardiovascular and all-cause mortality. Such associations between QT prolongation and MACE are comparable in magnitude to the effect of many traditional CVD risk factors [29]. Furthermore, QTc values greater than 500ms are strongly linked with risk of torsade de pointes and sudden cardiac death [30]. However, current clinical guidance recommends against routine ECG screening [31]. Indeed, to date no studies have demonstrated benefit of QT interval screening on patient outcomes in western settings. Thus, an important question for the field is whether ECG screening might have additional value in sub-Saharan Africa where we report significantly higher prevalence of prolonged QTa in a relatively young and healthy population.
Strengths and limitations
The major limitation of our study is its cross-sectional design, which limited our ability to infer causal associations. The cross-sectional design also limited our ability to investigate clinical outcomes (e.g., MACE) over longitudinal follow-up. However, as a population-based study, our results are generalizable to rural Uganda. Moreover, our ability to make population-level inferences is strengthened by the placement of our study within a larger census covering nearly 100% of our study population. Differential participation in the health fairs was accounted for with the use of IPTW-adjusted models to derive population-level estimates. Our study might also be limited by residual confounding from known and unmeasured factors related to QT duration. For example, we did not measure electrolytes such as potassium and magnesium concentrations or current medication use, all of which are known to affect QT intervals. However, we did collect data on chronic medication use, which was rare in this study population (4.4%), so it is unlikely to be a major confounder. In a related study in the same population cohort, no individual reported use of quinolones, macrolides, or neurotropic medications, which are known to affect QT intervals [32].
In summary, this study is among the first population-based studies of QT interval prolongation in rural sub-Saharan Africa. We estimated a high prevalence of prolonged QTa in a relatively young rural sub-Saharan African population, yet failed to identify typical QTa risk factors. Our findings should trigger additional study of the factors contributing to prolonged QT in this setting, and its downstream effects on health. Most importantly, additional data are needed to understand context-specific QT interval norms, their clinical, environmental, and genetic determinants, and if and how they contribute to MACE or death in this setting.
Supplementary Material
Table 3a:
Weighted population univariate and multivariate correlates of (continuous) QTa interval
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p-value | Coefficient | 95% CI | p-value | |
| Sex | ||||||
| Male | REF | REF | REF | REF | ||
| Female | 25.6 | 18.0 – 33.3 | <0.001 | 21.2 | 14.0 – 28.3 | <0.001 |
| Age, (years) | ||||||
| ≤29 | REF | REF | REF | REF | ||
| 30 – 50 | 14.3 | 5.4 – 23.2 | 0.002 | 8.2 | −0.4 – 17.9 | 0.062 |
| >50 | 24.2 | 15.5 – 32.9 | <0.001 | 14.0 | 4.6 – 23.3 | 0.004 |
| BMI, mean ( kg/m2) | ||||||
| ≤18.5 | 0.9 | −11.0 – 12.7 | 0.886 | 9.6 | −4.3 – 23.6 | 0.177 |
| >18.5 – 24.9 | REF | REF | REF | REF | ||
| 25.0 – 29.9 | 9.0 | −3.9 – 21.8 | 0.171 | 10.2 | −4.0 – 24.3 | 0.159 |
| ≥30 | 17.8 | 4.3 – 31.4 | 0.010 | 11.3 | −4.1 – 26.7 | 0.151 |
| HDL, (mg/dL) | ||||||
| <40 | REF | REF | REF | REF | ||
| 40 -59 | 6.3 | −1.6 – 14.2 | 0.116 | 1.6 | −7.4 - 10.7 | 0.716 |
| ≥60 | −1.5 | −9.1 – 6.0 | 0.690 | −4.5 | −14.3 – 5.3 | 0.368 |
| Current hypertension | ||||||
| <140/90 mmHg | REF | REF | REF | REF | ||
| ≥140/90 mmHg | 11.9 | 2.8 – 20.9 | 0.010 | 8.7 | −4.7 – 22.1 | 0.202 |
| eGFR, (ml/min/1.73mm2) | ||||||
| ≥90 | REF | REF | REF | REF | ||
| 60 – 89 | 19.3 | 4.7 – 34.0 | 0.010 | −1.7 | −12.2 – 8.8 | 0.879 |
| CRP, (mg/dL) | ||||||
| ≤1 | REF | REF | REF | REF | ||
| 1-3 | 8.2 | 1.2 – 15.1 | 0.022 | 0.7 | −7.8 – 9.1 | 0.878 |
| >3 | 12.1 | 4.1 – 20.0 | 0.003 | 0.7 | −7.9 – 9.2 | 0.879 |
| HbA1c | ||||||
| Normal | REF | REF | REF | REF | ||
| Pre-diabetes | 16.3 | 1.8 – 30.8 | 0.028 | −4.4 | −14.8 – 5.6 | 0.384 |
| Diabetes mellitus | 23.3 | 1.7 – 44.9 | 0.034 | 17.5 | −12.9 – 47.8 | 0.259 |
| Physical Activity Category | ||||||
| Inactive | REF | REF | ||||
| Minimal | −3.5 | −16.3 – 9.3 | 0.595 | |||
| Active | −4.5 | −13.4 – 4.5 | 0.329 | |||
| Cigarette smoking | ||||||
| Never | REF | REF | REF | REF | ||
| Former | 6.9 | −10.2 – 14.0 | 0.056 | 0.3 | −8.3 – 8.9 | 0.950 |
| Current | −4.5 | −15.6 – 6.5 | 0.423 | 0.3 | −13.5 – 14.1 | 0.965 |
Table 3b:
Weighted population univariate and multivariate correlates of prolonged QTa interval
| Characteristic | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Relative Prevalence |
95% CI | p-value | Adjusted Relative Prevalence |
95% CI | p-value | |
| Sex | ||||||
| Male | REF | REF | REF | REF | ||
| Female | 1.5 | 0.9 – 2.6 | 0.136 | 1.2 | 0.7 – 2.0 | 0.450 |
| Age | ||||||
| ≤29 | REF | REF | REF | REF | ||
| 30 – 50 | 3.6 | 1.5 – 8.6 | 0.003 | 3.1 | 1.4 – 6.8 | 0.007 |
| >50 | 4.4 | 1.8 – 10.6 | 0.001 | 3.3 | 1.3 – 7.9 | 0.009 |
| BMI, mean ( kg/m2) | ||||||
| ≤18.5 | 1.2 | 0.5 – 3.1 | 0.716 | 1.7 | 0.6 – 4.9 | 0.361 |
| >18.5 – 24.9 | REF | REF | REF | REF | ||
| 25.0 – 29.9 | 1.3 | −.5 – 3.4 | 0.625 | 1.4 | 0.4 – 4.5 | 0.607 |
| ≥30 | 2.0 | 0.7 – 5.8 | 0.183 | 1.3 | 0.3 – 4.7 | 0.715 |
| HDL cholesterol, (mg/dL) | ||||||
| <40 | REF | REF | ||||
| 40 -59 | 0.9 | 0.4 – 1.8 | 0.706 | |||
| ≥60 | 0.8 | 0.4 – 1.5 | 0.495 | |||
| Current hypertension | ||||||
| <140/90 mmHg | REF | REF | REF | REF | ||
| ≥140/90 mmHg | 2.5 | 1.4 – 4.4 | 0.002 | 1.4 | 1.0 – 3.2 | 0.037 |
| eGFR, ( ml/min/1.73mm2) | ||||||
| ≥90 | REF | REF | REF | REF | ||
| 60 – 89 | 2.1 | 1.0 – 4.6 | 0.056 | 0.8 | 0.4 – 1.7 | 0.622 |
| CRP, (mg/dL) | ||||||
| ≤1 | REF | REF | REF | REF | ||
| 1-3 | 2.1 | 1.1 – 3.9 | 0.026 | 1.7 | 0.9 – 3.1 | 0.081 |
| >3 | 1.8 | 0.9 – 3.9 | 0.118 | 1.2 | 0.5 – 2.5 | 0.707 |
| HbA1c | ||||||
| Normal | REF | REF | REF | REF | ||
| Pre diabetes | 2.2 | 1.1 – 4.4 | 0.019 | 1.0 | 0.5 – 1.9 | 0.952 |
| Diabetes | 1.7 | 0.6 – 4.5 | 0.321 | 2.0 | 0.7 – 5.5 | 0.184 |
| Physical Activity Category | ||||||
| Inactive | REF | REF | REF | REF | ||
| Minimal | 0.8 | 0.3 – 2.3 | 0.738 | 0.7 | 0.3 – 2.1 | 0.560 |
| Active | 0.6 | 0.3 – 1.2 | 0.143 | 0.7 | 0.4 – 1.4 | 0.360 |
| Cigarette smoking | ||||||
| Never | REF | REF | ||||
| Former | 1.2 | 0.7 – 2.0 | 0.438 | |||
| Current | 1.5 | 0.6 – 3.4 | 0.330 | |||
Highlights.
Prolongation of QT interval is known to be associated with incident major adverse cardiovascular events and death in populations in high income settings.
QT interval prolongation is highly prevalent in rural Uganda, and may be more common than in high-income settings.
In rural Uganda, female sex, age and high blood pressure are correlated with QT interval prolongation.
Additional data are needed to understand context-specific QT interval norms, and how, if, they contribute to major adverse cardiovascular events or death in this setting.
Acknowledgments
Conflict of Interest: The study was funded by Friends of a Healthy Uganda. The authors report no relationships that could be construed as a conflict of interest. The funding sources had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Dr. Magodoro receives additional support from the NIH Fogarty International Center (D43 TW010543), and the AIDS Health Foundation, and a scholarship from Harvard Medical School. Dr. Siedner receives support from the National Institute of Health (K23 MH099916) and the Harvard Center for AIDS Research (P30 AI060354). Dr. Tsai also acknowledges salary support from NIH R01MH113494-01. Dr. Muthalaly is supported by a Doctors-in-Training scholarship from Avant Mutual and a scholarship from Monash Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mensah G, Roth G, Sampson U, Moran A, Feigin V, Forouzanfar M, et al. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013: cardiovascular topic. Cardiovasc J Afr 2015;26:S6–10. doi: 10.5830/CVJA-2015-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Moran A, Forouzanfar M, Sampson U, Chugh S, Feigin V, Mensah G. The epidemiology of cardiovascular diseases in sub-saharan Africa: The global burden of diseases, injuries and risk factors 2010 study. Prog Cardiovasc Dis 2013. doi: 10.1016/j.pcad.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Now W, Screening W. Annals of Internal Medicine Editorial What Now With Screening Electrocardiography ? 2018. [Google Scholar]
- [4].Ashley EA, Raxwal VK, Froelicher VF. The prevalence and prognostic significance of electrocardiographic abnormalities. Curr Probl Cardiol 2000;25:1–72. doi: 10.1016/S0146-2806(00)70020-X. [DOI] [PubMed] [Google Scholar]
- [5].Montanez A, Ruskin JN, Hebert PR, Lamas GA, Hennekens CH. Prolonged QTc interval and risks of total and cardiovascular mortality and sudden death in the general population: a review and qualitative overview of the prospective cohort studies. Arch Intern Med 2004;164:943–8. doi: 10.1001/archinte.164.9.943 [doi]\r164/9/943 [pii]. [DOI] [PubMed] [Google Scholar]
- [6].Beinart R, Zhang Y, Lima JAC, Bluemke DA, Soliman EZ, Heckbert SR, et al. The QT interval is associated with incident cardiovascular events: the MESA study. J Am Coll Cardiol 2014;64:2111–9. doi: 10.1016/j.jacc.2014.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Straus SMJM, Kors JA, De Bruin ML, Van Der Hooft CS, Hofman A, Heeringa J, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 2006;47:362–7. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- [8].Williams ES, Thomas KL, Broderick S, Shaw LK, Velazquez EJ, Al-Khatib SM, et al. Race and gender variation in the QT interval and its association with mortality in patients with coronary artery disease: Results from the Duke Databank for Cardiovascular Disease (DDCD). Am Heart J 2012;164:434–41. doi: 10.1016/j.ahj.2012.05.024. [DOI] [PubMed] [Google Scholar]
- [9].Vandael E, Vandenberk B, Vandenberghe J, Willems R, Foulon V. Risk factors for QTc-prolongation: systematic review of the evidence. Int J Clin Pharm 2017;39:16–25. doi: 10.1007/s11096-016-0414-2. [DOI] [PubMed] [Google Scholar]
- [10].Tsai AC, Kakuhikire B, Mushavi R, Vořechovská D, Perkins JM, McDonough AQ, et al. Population-based study of intra-household gender differences in water insecurity: Reliability and validity of a survey instrument for use in rural Uganda. J Water Health 2016;14:280–92. doi: 10.2166/wh.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Uganda Ministry of Health. Uganda National Policy Guidelines for HIV Counselling and Testing THE REPUBLIC OF UGANDA 2003:1–41. [Google Scholar]
- [12].Statements P Standards of medical care in diabetes - 2012. Diabetes Care 2012;35. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].The IPAQ Group. International Physical Activity Questionnaire. IPAQ Website 2015. doi: 10.2165/11531930-000000000. [DOI] [Google Scholar]
- [14].Riley L, Guthold R, Cowan M, Savin S, Bhatti L, Armstrong T, et al. The world health organization STEPwise approach to noncommunicable disease risk-factor surveillance: Methods, challenges, and opportunities. Am J Public Health 2016;106:74–8. doi: 10.2105/AJPH.2015.302962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Madias JE. Comparability of the standing and supine standard electrocardiograms and standing sitting and supine stress electrocardiograms. J Electrocardiol 2006;39:142–9. doi: 10.1016/j.jelectrocard.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [16].Badilini F, Sarapa N. Implications of methodological differences in digital electrocardiogram interval measurement. J Electrocardiol 2006;39:152–6. doi: 10.1016/j.jelectrocard.2006.05.030. [DOI] [PubMed] [Google Scholar]
- [17].Salvi V, Karnad DR, Panicker GK, Natekar M, Hingorani P, Kerkar V, et al. Comparison of 5 methods of QT interval measurements on electrocardiograms from a thorough QT/QTc study: Effect on assay sensitivity and categorical outliers. J Electrocardiol 2011;44:96–104. doi: 10.1016/j.jelectrocard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- [18].Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud 2010;47:931–6. doi: 10.1016/j.ijnurstu.2009.10.001. [DOI] [PubMed] [Google Scholar]
- [19].Rautaharju PM, Surawicz B, Gettes LS. AHA/ACCF/HRS Recommendations for the Standardization and Interpretation of the Electrocardiogram. Part IV: The ST Segment, T and U Waves, and the QT Interval A Scientific Statement From the American Heart Association Electrocardiography and Arrhythmias Co. J Am Coll Cardiol 2009;53:982–91. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- [20].Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol 1992. [DOI] [PubMed] [Google Scholar]
- [21].Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health 2006;60:578–86. doi: 10.1136/jech.2004.029496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reichenheim ME, Coutinho ESF. Measures and models for causal inference in cross-sectional studies: arguments for the appropriateness of the prevalence odds ratio and related logistic regression 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dewhurst MJ, Di Marco LY, Dewhurst F, Adams PC, Murray A, Orega GP, et al. Electrocardiographic reference values for a population of older adults in sub-Saharan Africa. Ann Noninvasive Electrocardiol 2014;19:34–42. doi: 10.1111/anec.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].O’Neal WT, Efird JT, Kamel H, Nazarian S, Alonso A, Heckbert SR, et al. The association of the QT interval with atrial fibrillation and stroke: the Multi-Ethnic Study of Atherosclerosis. Clin Res Cardiol 2015;104:743–50. doi: 10.1007/s00392-015-0838-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soliman EZ, Howard G, Cushman M, Kissela B, Kleindorfer D, Le A, et al. Prolongation of QTc and risk of stroke: The regards (REasons for Geographic and Racial Differences in Stroke) study. J Am Coll Cardiol 2012;59:1460–7. doi: 10.1016/j.jacc.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Newton-Cheh C, Akylbekova EL, Crow RS, Johnson WD, Buxbaum SG, Njemanze S, et al. Clinical correlates and heritability of qt interval duration in blacks: The jackson heart study. Circ Arrhythmia Electrophysiol 2009;2:427–32. doi: 10.1161/CIRCEP.109.858894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lazzerini PE, Acampa M, Capecchi PL, Hammoud M, Maffei S, Bisogno S, et al. Association between high sensitivity C-reactive protein, heart rate variability and corrected QT interval in patients with chronic inflammatory arthritis. Eur J Intern Med 2013;24:368–74. doi: 10.1016/j.ejim.2013.02.009. [DOI] [PubMed] [Google Scholar]
- [28].Poncet A, Gencer B, Blondon M, Gex-Fabry M, Combescure C, Shah D, et al. Electrocardiographic screening for prolonged QT interval to reduce sudden cardiac death in psychiatric patients: A cost-effectiveness analysis. PLoS One 2015;10:1–14. doi: 10.1371/journal.pone.0127213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhang Y, Post WS, Blasco-Colmenares E, Dalal D, Tomaselli GF, Guallara E. Electrocardiographic QT interval and mortality: A meta-analysis. Epidemiology 2011;22:660–70. doi: 10.1097/EDE.0b013e318225768b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Van Noord C, Eijgelsheim M, Stricker BHC. Drug- and non-drug-associated QT interval prolongation. Br J Clin Pharmacol 2010;70:16–23. doi: 10.1111/j.1365-2125.2010.03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chou R, Arora B, Dana T, Fu R, Walker M HL. Annals of Internal Medicine Review Screening Asymptomatic Adults With Resting or Exercise Electrocardiography : A Review of the Evidence for the. Ann Intern Med 2011; 155:375–86. [DOI] [PubMed] [Google Scholar]
- [32].Feinstein MJ, Kim J-H, Bibangambah P, Sentongo R, Martin JN, Tsai AC, et al. Ideal Cardiovascular Health and Carotid Atherosclerosis in a Mixed Cohort of HIV-Infected and Uninfected Ugandans. AIDS Res Hum Retroviruses 2017;33:49–56. doi: 10.1089/aid.2016.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.