Abstract
18α–Glycyrrhetinic acid (18α-GA) is a bioactive triterpenoid that has been shown to activate the nuclear factor (erythroid-derived-2)-like 2 (Nrf2), the main transcription factor that orchestrates the cellular antioxidant response, in both cellular and organismal context. Although various beneficial properties of 18α-GA have been revealed, including its anti-oxidation and anti-aging activity, its possible protective effect against DNA damage has never been addressed. In this study, we investigated the potential beneficial properties of 18α-GA against DNA damage induced by mitomycin C (MMC) treatment. Using human primary fibroblasts exposed to MMC following pre-treatment with 18α-GA, we reveal an Nrf2-mediated protective effect against MMC-induced cell death that depends on extracellular signal–regulated kinase (ERK) signaling. In total, our results reveal an additional beneficial effect of the Nrf2 activator 18α-GA, suggesting that this important phytochemical compound is a potential candidate in preventive and/or therapeutic schemes against conditions (such as aging) or diseases that are characterized by both oxidative stress and DNA damage.
Keywords: 18α–Glycyrrhetinic acid, Nrf2, DNA damage, Mitomycin C, ERK pathway, Phytochemicals
Graphical abstract
Highlights
-
•
18α–glycyrrhetinic acid (18α-GA) protects against MMC-induced cell death.
-
•
The protective effect of 18α-GA against MMC-induced cell death is Nrf2 dependent.
-
•
18α-GA-mediated Nrf2 induction is ERK-dependent.
Abbreviations
- AREs
Antioxidant response elements
- CnC
Cap ‘n’ collar
- DDR
DNA damage response
- DSBs
Double-strand DNA breaks
- EpREs
Electrophile responsive elements
- ERK
Extracellular signal–regulated kinase
- γ-GCS
γ-Glutamyl-cysteine synthetase
- 18α-GA
18α–Glycyrrhetinic acid
- GSTs
Glutathione S transferases
- H2DCFDA
2′, 7′-Dichlorodihydrofluorescein diacetate
- HGPS
Hutchinson–Gilford progeria syndrome
- HO-1
Heme oxygenase 1
- HR
Homologous recombination
- Keap-1
Kelch-like ECH associated protein 1
- Maf
Musculoaponeurotic fibrosarcoma oncogene
- MAPKs
Mitogen-activated protein kinases
- MMC
Mitomycin
- NER
Nucleotide excision repair
- NQO-1
NAD(P)H quinone oxidoreductase-1
- Nrf2
Nuclear factor (erythroid-derived-2)-like 2
- PI3K/AKT
Phosphatidylinositol-3-kinase
- PKC
Protein kinase C
- ROS
Reactive oxygen species
- TXNRD1
Thioredoxin reductase 1
- UPR
Unfolded protein response
1. Introduction
Cells are constantly under various kinds of stress with oxidative, proteotoxic and DNA damage challenges being the most important and frequent ones. Consequently, cells are continuously alert to ensure genome and proteome integrity in the face of challenges posed by exogenous (such as UV irradiation, ionizing radiation or chemicals, among others) and endogenous (such as reactive oxygen species -ROS-, among others) sources of damage. To cope with the various types of stress, cells have developed complex surveillance and repair mechanisms, including the DNA damage response (DDR; [1]), the unfolded protein response (UPR; [2]) or the activation of master transcription factors that regulate the cellular response to other stresses with oxidative stress arguably being the most important [3].
Nuclear factor (erythroid-derived-2)-like 2 (Nrf2) is a master transcription factor, which is induced to orchestrate the cellular antioxidant response upon oxidative/electrophilic stress [4]. Nrf2 belongs to the CnC (Cap ‘n’ collar) family of leucine zipper transcription factors with many well-characterized target genes with antioxidant and detoxifying properties, such as thioredoxins, γ-Glutamyl-cysteine synthetase (γ-GCS), glutathione S transferases (GSTs), NAD(P)H quinone oxidoreductase-1 (NQO-1), heme oxygenase 1 (HO-1) and several proteasome subunits, among others [5]. In the absence of oxidative or electrophilic stress, detectable cytoplasmic Nrf2 levels are low, mainly due to its proteasome-dependent degradation promoted by Kelch-like ECH associated protein 1 (Keap-1; [6]) which is its negative regulator. In contrast, under conditions of cellular stress, key cysteine residues of Keap1 are modified leading to conformational changes that prevent Nrf2 ubiquitination [7] with subsequent stabilization and nuclear translocation of Nrf2. Once in the nucleus, Nrf2 forms heterodimers with small Maf (musculoaponeurotic fibrosarcoma oncogene) proteins and thus recognizing and binding to antioxidant response elements (AREs; [8]) also known as electrophile responsive elements (EpREs; [9]) that are present in the promoter regions of its target genes. As a consequence, the necessary transcriptional complexes are recruited and transcription of the appropriate target genes is upregulated. Several signal transduction pathways have been shown to activate Nrf2 through phosphorylation, including the mitogen-activated protein kinases (MAPKs), phosphatidylinositol-3-kinase (PI3K/AKT), and protein kinase C (PKC) [10].
Mitomycin C (MMC) is a bi-alkylating and DNA crosslinking agent [11] that causes high levels of cytotoxicity through the induction of various types of DNA damage. In addition to its direct effect on DNA topology, MMC's action has also been linked to the generation of ROS and thereby the induction of oxidative stress-mediated DNA damage [12,13]. Thus, a vicious circle ensues, whereby enhanced ROS production leads to a variety of DNA lesions that cause additional DNA damage. DNA repair pathways such as nucleotide excision repair (NER) or homologous recombination (HR) have been shown to be induced following MMC-mediated DNA damage [13].
The term phytochemicals refers to naturally occurring compounds that were shown to possess beneficial properties for human health. In the last decades, research on phytochemicals has flourished, continuously yielding evidence that consumption of foods rich in phytochemicals can provide health gains. Nevertheless, there are still insufficient data to formulate specific recommendations regarding phytochemical intake. 18α-glycyrrhetinic acid (18α-GA) is a bioactive component mainly derived from Glycyrrhiza radix [14]. It is a pentacyclic triterpene glycoside with various attributed pharmacological activities such as anti-oxidative [15,16], anti-inflammatory [17], anti-proliferative (in cancer cells; [18]) but also pro-proliferative (in primary cells; [19]) activities. More recently, we have also shown that it exerts pro-longevity action in human primary fibroblasts [15] as well as in C. elegans [16] through Nrf2/SKN-1-mediated proteasome activation.
In this study, we investigated the potential protective properties of 18α-GA against DNA damage induced by MMC treatment. We identified an Nrf2-mediated protective effect against MMC-induced cell death and we demonstrated that it is ERK-dependent. In total, our results reveal an additional beneficial effect of the Nrf2 activator 18α-GA which further supports its status as a highly promising phytochemical compound.
2. Materials and methods
2.1. 18α-GA treatment
18α-GA (Sigma-Aldrich, Munich, Germany, ≥98% purity) was dissolved as stock solution of 4 mg/ml in DMSO and stored at −20 °C. Cells were exposed to 2 μg/ml 18α-GA or the same amount of DMSO (control) for 24 h before treatment with 20 μg/ml MMC for 1 h. Whenever 24 h recovery was performed, this was done in the presence of 2 μg/ml 18α-GA or DMSO. Since it has been shown that even low DMSO concentrations (>1%) may show antioxidant effects and may influence the experimental results, the final concentration of DMSO in our experiments was 0.05%; no difference was detected between untreated and DMSO-treated cells in agreement with previous work [20,21].
2.2. MMC treatment
Mitomycin C (Applichem Panreac, Glenview, IL, USA) was dissolved as stock solution of 0.5 mg/ml in water and stored at 4 °C in the dark. Cells were exposed to 20 μg/ml MMC or water (control) following 24 h pre-treatment with 2 μg/ml 18α-GA or DMSO.
2.3. Antibodies
Antibodies against phospho-ERK (p-ERK; sc-7383), phosho-c-JUN (p-c-JUN; sc-822), GAPDH (sc-25778) and horseradish peroxidase-conjugated secondary antibodies were purchased from Santa Cruz (Heidelberg, Germany). Antibody against phospho-Histone H2AX (Ser139) (#2577) was purchased from Cell signaling (Danvers, MA, USA). The Alexa Fluor 488 secondary antibody used for confocal analysis was purchased from Invitrogen (Carlsbad, CA, USA, Α21206).
2.4. Cell culture
HFL-1 human embryonic fibroblasts were obtained from the European Collection of Cell Cultures and were cultured in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 2 mM glutamine and 1% non-essential amino acids. HFL-1 cells were maintained at 37 °C, 5% CO2 and 95% humidity and they were subcultured when they reached confluency at a split ratio of 1:2. Cell number for each assay was determined in duplicates using a Coulter Z2 counter (Beckman, Brea, CA, USA).
E8.T4 cells, an L929 cells subclone, were cultured in the above mentioned medium supplemented with 200 μg/ml geneticin (G418 sulfate; Invitrogen, Carlsbad, CA, USA). This subclone expresses a mutated, non-functional form of Nrf2 whereas expression of the wild type and functional Nrf2 form is induced following treatment with 1 μg/ml of doxocycline (Sigma-Aldrich, Munich, Germany), a tetracycline analogue.
2.5. RNA isolation and real-time PCR analysis
RNA was extracted using Trizol (Invitrogen, Carlsbad, CA, USA) and converted into cDNA with the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, USA). Real-time PCR reactions were performed in triplicate in a CFX Connect Real-time PCR system (Bio-Rad Laboratories, Hercules, USA). Primers used are summarized in Table 1. Data were analyzed using the comparative 2-ΔΔCt method and are presented as the fold difference in mRNA transcript abundance in 18α-GA-treated cells relative to control (DMSO-treated) cells, normalized to the GAPDH gene, unless otherwise indicated.
Table 1.
Primers used for Real Time PCR analysis.
| Gene | Forward primer (5’→3′) | Reverse primer (5’→3′) |
|---|---|---|
| NRF2 | AAACCAGTGGATCTGCCAAC | GACCGGGAATATCAGGAACA |
| NQO1 | AGTCCCTGCCATTCTGAAAG | AAAGCACTGCCTTCTTACTCC |
| TXNRD1 | GGAACGCTCTCGGAATTGG | TCTGCCCTCCTGATAAGCC |
| HO-1 | GATAGAAGAGGCCAAGACTG | GAATCTTGCACTTTGTTGCT |
| GAPDH | GAAGGTGAAGGTCGGAGT | CATGGGTGGAATCATATTGGAA |
2.6. Immunoblot analysis
Cells were harvested at the indicated time points and lysed in reducing Laemmli buffer. Proteins were then fractionated by SDS-page and transferred to nitrocellulose membranes for probing with appropriate antibodies as previously described [22]. Secondary antibodies used were conjugated with horseradish peroxidase, and blots were developed with chemiluminescence by using the Clarity™ Western ECL substrate (Bio-Rad Laboratories, Hercules, USA). Protein concentration was determined using the Bradford method with bovine serum albumin as standard. Each blot was repeated at least three times. Probing with GAPDH antibody was used to verify equal loading.
2.7. Confocal analysis
HFL-1 cells were grown on coverslips and treated with 18α-GA for 24 h followed by treatment with MMC for 1 h and a 24 h recovery period in the presence of 18α-GA or DMSO, then fixed in 4% parafolmaldehyde followed by cell permeabilization with 0.2% Triton X-100 in phosphate-buffered saline (PBS). Cells were incubated with the appropriate antibody overnight, followed by incubation with Alexa Fluor 488 secondary antibody (Invitrogen, Carlsbad, CA, USA). Nuclei were stained with DAPI (Sigma, Munich, Germany). Slides were mounted using Prolong Gold anti-fade reagent (Life technologies, Carlsbad, CA, USA) and analyzed using a Leica TCS SPE confocal laser scanning microscope (Leica Lasertechnik GmbH, Heidelberg, Germany) equipped with ACS APO 63X/1.30 NA OILCS objective. Fluorescence was excited with a 488 nm line and collected with a 517 nm filter. The LAS AF software (Leica Lasertechnik GmbH, Heidelberg, Germany) was used for image acquisition.
2.8. Survival and cell death detection
Cell death was assessed through scoring of detached cells in the culture supernatant after pre-incubation of the cultures with 18α-GA or DMSO for 24 h, 1 h incubation with MMC and a 24 h recovery period in the presence of 18α-GA or DMSO in triplicate using a Coulter Z2 counter (Beckman, Brea, CA, USA). For MMC optimization, cell viability of HFL-1 cell cultures was assessed through scoring of attached MMC-treated cells after 1 h of incubation with various MMC concentrations in triplicate using a Coulter Z2 counter (Beckman, Brea, CA, USA). E8.T4 cells [23] were continuously supplemented with 1 μg/ml doxocycline (to induce the functional form of Nrf2) or were not supplemented with doxocycline (expression of mutated Nrf2 form) and cell death was assessed in both cultures as described above.
For determination of the survival ratio through crystal violet staining, cells treated as described above were fixed in 4% paraformaldehyde for 20 min and then stained with 0.2% crystal violet in distilled water. Cells were washed with water, air dried and the dye was eluted with 30% acetic acid. Viability was assessed by measuring dye absorbance at 595 nm using the Safire2 Multi-detection Microplate Reader (Tecan, Grödig, Austria).
2.9. Comet assay
Cells were pre-treated with 18α-GA or DMSO followed by treatment with MMC for 1 h and were then left for a 24 h recovery period in the presence of 18α-GA or DMSO. Cells were then harvested and used in comet assay analysis of DNA damage. After the detachment of the cells with trypsin-EDTA, they were washed with PBS, suspended in 1% low melting point agarose (LMP agarose) and maintained at 37 °C. Approximately 10000 cells/sample in 70 μl of PBS were placed on an agarose pre-coated microscope slide and placed at 4 °C for 30 min. The slides were then immersed in cold lysis buffer (2.5 M NaCl, 0.1 M EDTA, 10 mM Tris, 1% Triton-X 100, pH 10) to lyse the cells and to unfold the DNA. The samples were kept for 2 h at 4 °C in the dark and were then placed in a horizontal electrophoresis unit containing electrophoresis buffer (0.3 M NaOH and 1 mM EDTA). Before electrophoresis, cells were maintained in the same buffer for 40 min to allow DNA unwinding and to expose the sites of damage. Electrophoresis was performed for 30 min at 4 °C at 25 V and approximately 255 mA. The samples were then placed in neutralization buffer (0.4 M Tris, pH 7.4) for 10 min, washed with distilled water and air dried. SYBR GOLD was added to each sample for 30 min in the dark and cell were observed using a fluorescence microscope (Zeiss, Oberkochen, Germany).
2.10. Measurement of reactive oxygen species (ROS)
Detection of ROS was performed with 2′, 7′-dichlorodihydrofluorescein diacetate H2DCFDA (Molecular Probes, Invitrogen, Carlsbad, CA, USA) as previously described [24]. HFL-1 cells pre-treated with 18α-GA or DMSO for 24 h and then treated with MMC for 1 h, were resuspended in PBS with or without H2DCFDA at a final concentration of 10 μM (loading buffer) and incubated at 37 °C for 30 min. The loading buffer was then removed; cells were resuspended in pre-warmed complete medium and incubated at 37 °C for 5 min. The absorption and the emission of the oxidation product were measured at 493 and 520 nm, respectively using the Safire2 Multi-detection Microplate Reader (Tecan, Grϕdig, Austria). Each sample was measured in triplicate.
2.11. Statistical analysis
Statistical analysis and graphs were produced using Prism (GraphPad Software Inc, La Jolla, CA, USA). Data in all assays (including blots quantification) are depicted as the average of at least 2 independent experiments. Error bars denote ± SEM. Student's t-test and ANOVA were used for comparisons. Asterisks denote p-values as follows: *p < 0.05, **p < 0.01, ***p < 0.001, NS (not significant). Quantification of the adjusted ratio of each protein to GAPDH (using Image Studio Lite Ver 5.2, Li-Cor, Lincoln, NE, USA) expressed as (%) percentage of the DMSO-treated control cells (arbitrarily set to 100%) appears in graphs next to each representative blot.
3. Results
3.1. 18α-GA protects against MMC-induced cell death
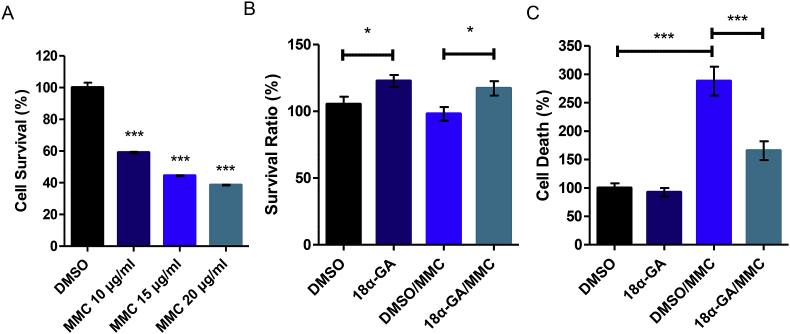
We initially exposed HFL-1 cells to various concentrations of the genotoxic agent MMC (10, 15 and 20 μg/ml MMC). There was a statistically significant difference between treatment groups as determined by one-way ANOVA (F(3,8) = 290.5, p < 0.001). A Tukey post-hoc test revealed that cell survival percentage was significantly lower upon treatment with (a) 10 μg/ml MMC (59.1 ± 0.8, p < 0.001), (b) 15 μg/ml MMC (44.5 ± 0.5, p < 0.001) and (c) 20 μg/ml MMC (38.5 ± 0.5, p < 0.001) as compared to DMSO-treated control group (100 ± 5.5). Cell survival percentage was significantly lower upon treatment with 15 μg/ml MMC (44.5 ± 0.5, p < 0.001) and 20 μg/ml MMC (38.5 ± 0.5, p < 0.001) as compared to treatment with 10 μg/ml MMC (59.1 ± 0.8). There was no significant difference between treatment with 15 μg/ml MMC and 20 μg/ml MMC (p = 0.118). Since 20 μg/ml MMC for 1 h led to death more than 60% of the cellular population (less than 40% survival), this concentration was chosen for all subsequent experiments (Fig. 1A; asterisks display significance levels of each MMC concentration group compared to the DMSO-treated control group). We then pre-treated HFL-1 cells with 2 μg/ml 18α-GA or DMSO for 24 h (a concentration shown to be beneficial in previous work; [15,16]), followed by a 1 h treatment with MMC and a 24 h recovery period in the presence of 18α-GA or DMSO. Survival was assessed by measuring survival ratio through crystal violet staining (Fig. 1B) and by measuring the number of dead cells in the culture supernatant (Fig. 1C); in the presence of 18α-GA, cells were better able to survive from MMC treatment.
Fig. 1.
18α-GA decreases cell death induced by MMC. (A) Optimization of MMC concentration leading to death more than 60% of the cell population as assessed by cell survival (expressed as percentage (%) of live cells). Number of live cells in DMSO-treated cells were set to 100%. Percentage (%) of (B) survival ratio following crystal violet staining, and (C) cell death in HFL-1 cells treated with 18α-GA or DMSO for 24 h and then subjected to genotoxic stress with MMC for 1 h followed by a 24 h recovery in 18α-GA or DMSO. Number of (B) dye absorbance and (C) dead cells in DMSO-treated cells were set to 100%. All values are reported as mean of three independent experiments. Error bars denote ±SEM.*p < 0.05, ***p < 0.001, asterisks in (A) display significance levels of each MMC concentration group compared to the DMSO-treated control group. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
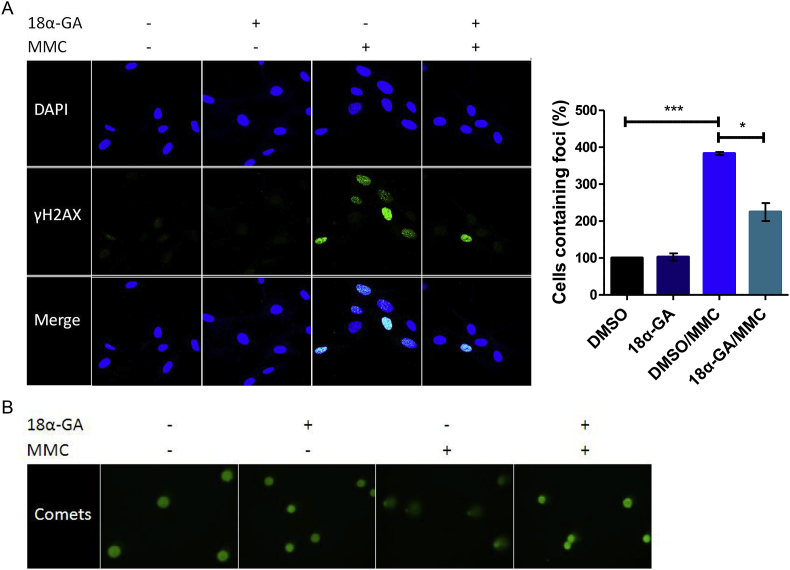
We then investigated whether the increased survival is due to lower DNA damage rates. Phosphorylation of histone H2A variant H2AX at Ser 139 (γH2AX) is abundant, fast, and correlates well with double-strand DNA breaks (DSBs) that are rapidly generated when cells are exposed to DNA-damaging agents such as MMC. Thus, it is a very sensitive marker that can be used to examine the DNA damage produced and the subsequent repair of the DNA lesions [25]. DNA damage foci were increased massively 24 h after MMC treatment in cells treated with MMC as compared to cells pre-treated with 18α-GA and then treated with MMC (Fig. 2A), thus verifying the protective effect of 18α-GA against MMC-mediated DNA damage. To further visualize the protection against DNA damage, comet assay was performed. As shown in fig. 2B, 18α-GA pre-treatment prevented comet formation, thus verifying the protective role of 18α-GA against DNA damage.
Fig. 2.
Reduced DNA damage by MMC in the presence of 18α-GA. Representative images of (A) DNA damage foci containing γH2AX and the quantification of cells containing foci in each condition (at least 80 cells measured/condition, number of foci in DMSO-treated cells set as 100%), and (B) comet fluorescence microscopy visualization in HFL-1 cells pre-treated with 18α-GA or DMSO for 24 h and then subjected to genotoxic stress with MMC for 1 h followed by a 24 h recovery in 18α-GA or DMSO. Error bars denote ±SEM.*p < 0.05, ***p < 0.001.
3.2. The protective effect of 18α-GA against MMC-induced cell death is mediated by Nrf2
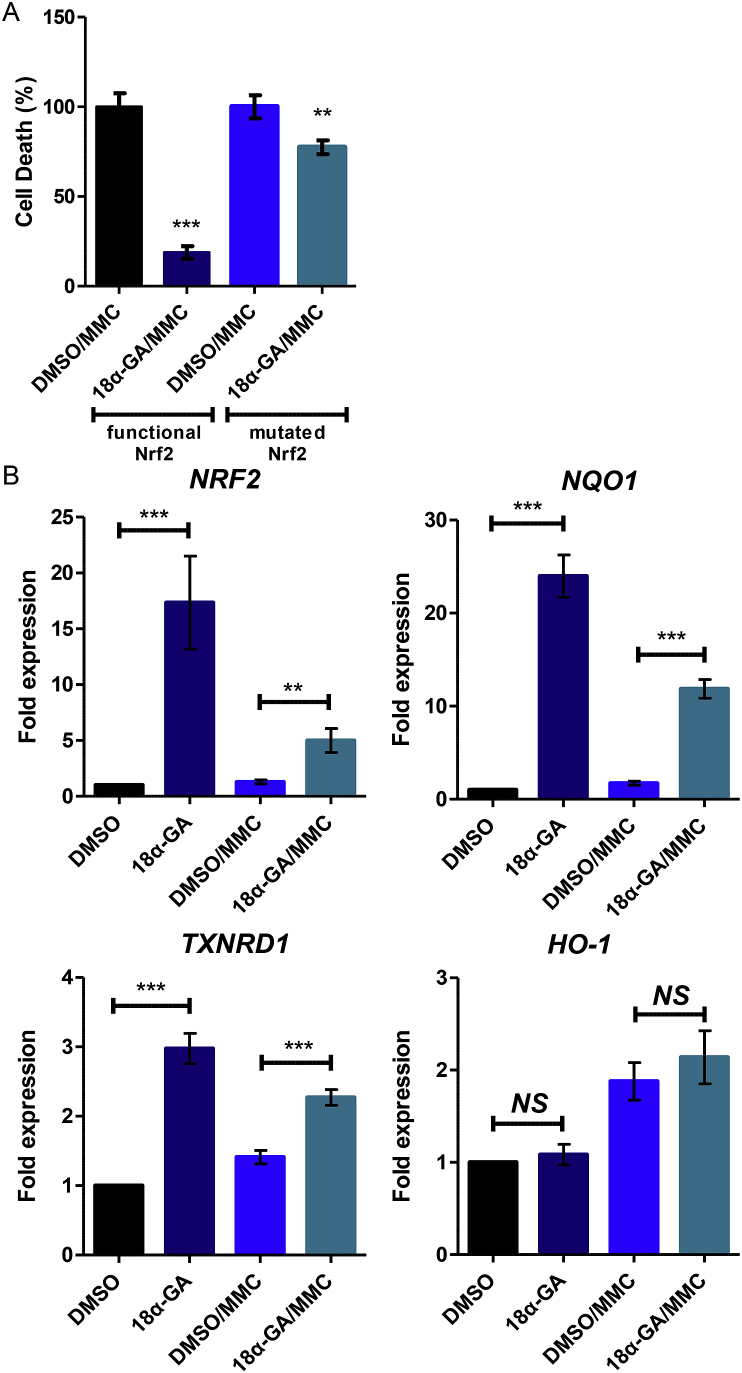
In our previous studies, we have shown that 18α-GA is an Nrf2 activator [15,16]. We therefore sought to investigate whether the observed 18α-GA protection is Nrf2-dependent. We used E8.T4 cells that express the functional form of Nrf2 upon doxocycline supplementation [23]. Cells expressing the functional Nrf2 (in the presence of doxocycline) and cells expressing the mutated Nrf2 (in the absence of doxocycline) were pre-treated with 18α-GA or DMSO for 24 h, followed by 1 h treatment with MMC and a 24 h recovery period in the presence of 18α-GA or DMSO and cell death was assessed. Pre-treatment of cells expressing the functional Nrf2 with 18α-GA led to only ~20% cell death following MMC treatment as compared to the cell death levels in their relative DMSO/MMC control culture (set as 100%; Fig. 3A). The relative levels of cell death in cells expressing the mutated Nrf2 were ~80% as compared to their relative DMSO/MMC control culture (set as 100%; Fig. 3A), thus being significantly higher than the ones in the cultures expressing the functional Nrf2. The observation that 18α-GA treatment also decreased cell death in the cells expressing the mutated Nrf2 by ~20% as compared to the relative DMSO/MMC control culture could suggest an additional Nrf2-independent role of 18α-GA or a potential leakiness of the on/off gene expression system that should be further investigated.
Fig. 3.
Activation of Nrf2 and its target genes by 18α-GA during DNA damage. (A) Percentage (%) of cell death in E8.T4 cells supplemented (expression of functional Nrf2) or non-supplemented (expression of mutated Nrf2) with 1 μg/ml doxocycline, treated with 18α-GA or DMSO for 24 h and then subjected to genotoxic stress with MMC for 1 h followed by a 24 h recovery in 18α-GA or DMSO. Number of dead cells in DMSO/MMC-treated cells were set to 100%. (B) Real-time PCR analysis of NRF2, NQO1, TXNRD1 and HO-1 mRNA levels (expression levels of each gene were arbitrarily set to 1 in DMSO-treated cells, GAPDH mRNA levels were used as normalizer) in cells pre-treated with 18α-GA or DMSO for 24 h and then treated or not with MMC for 1 h. Error bars denote ±SEM.**p < 0.01, ***p < 0.001, NS not significant.
We then sought to further investigate the potential Nrf2 activation in our experimental set up. The mRNA expression levels of NRF2 and its transcriptional target genes, namely NQO1 and TXNRD1 (thioredoxin reductase 1), were found to be significantly induced in the presence of 18α-GA (Fig. 3B). Moreover, the mRNA levels of these genes were significantly higher in 18α-GA/MMC-treated cells as compared to DMSO/MMC-treated cells (Fig. 3B). Not all Nrf2 target genes were induced; HO-1 was not induced under our experimental conditions (Fig. 3B).
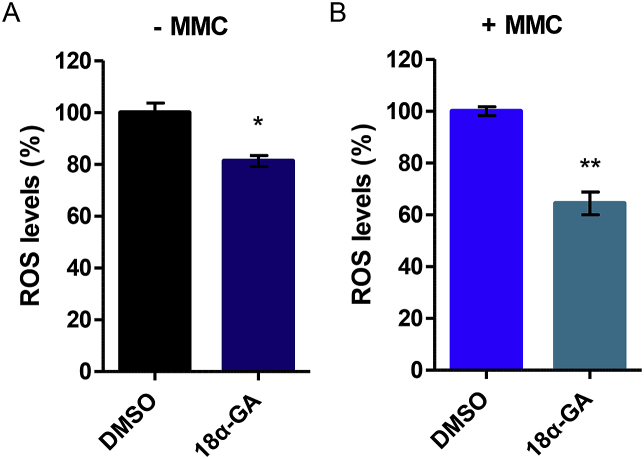
While the canonical mechanism of action of MMC involves alterations in DNA topography that induce DNA damage, MMC has also been shown to effect ROS production; formation of monoadducts and free radical-induced DNA strand breaks have been shown to underlie its toxic effect [12,13]. Since we have previously shown that 18α-GA possesses anti-oxidant properties [15,16], we assessed ROS levels in our experimental setting. Indeed, cells treated with 18α-GA exhibited significantly lower ROS levels as compared to their DMSO-treated counterparts (Fig. 4A). Moreover, cells pre-treated with 18α-GA before the addition of MMC, exhibited even lower ROS levels as compared to their DMSO/MMC-treated counterparts (Fig. 4B); it seems like the addition of the stressor (MMC) is an extra boost for the Nrf2 pathway in accordance with a previous study [26]. These results are consistent with the induction of NQO1 and TXNRD1 genes (Fig. 3B), which are both known to be involved in cellular antioxidant responses [27,28].
Fig. 4.
Cytoprotective effect of 18α-GA against oxidative stress. Percentage (%) of ROS levels in (A) HFL-1 cells treated with 18α-GA or DMSO for 24 h, and in (B) HFL-1 cells treated with 18α-GA or DMSO for 24 h and then treated with MMC for 1 h. Levels of ROS in DMSO-treated cells were arbitrarily set to 1. Error bars denote ±SEM.*p < 0.05, **p < 0.01.
3.3. 18α-GA-mediated Nrf2 induction is ERK-dependent
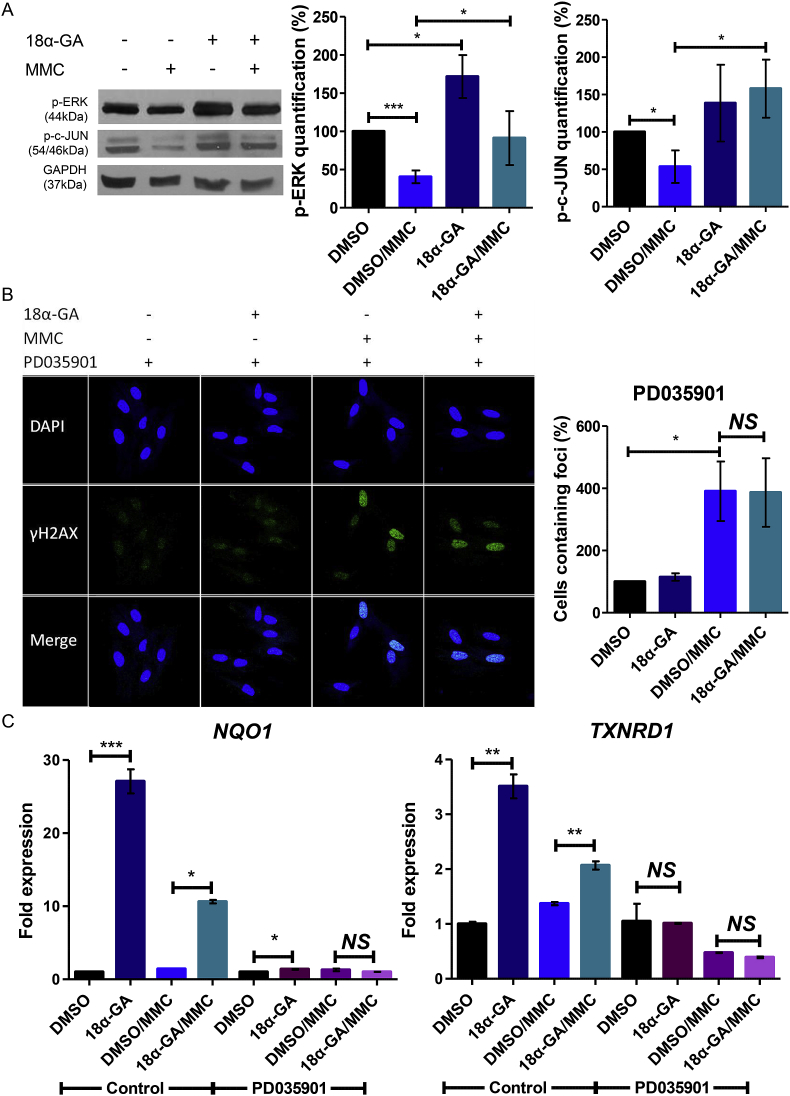
Earlier studies have shown that the cellular defense capacity is dependent on the activation of the ERK/Nrf2 signaling pathway. Moreover, phosphorylation of ERK is negatively influenced by pro-oxidant exposures [29], while p-ERK is induced by several antioxidant compounds like red ginseng [30], morin [31], curcumin [32] and sulforaphane [33], among others. Treatment of cells with 18α-GA led to increased phosphorylation of ERK whereas treatment with MMC negatively influenced ERK phosphorylation (Fig. 5A). Nevertheless, the MMC-mediated decrease in ERK phosphorylation was attenuated by 18α-GA supplementation (Fig. 5A). In accordance with p-ERK levels, phosphorylation of c-JUN was reduced upon MMC treatment. Upon pre-treatment with 18α-GA and MMC challenge, p-c-JUN levels were significantly elevated as compared to the levels found in DMSO/MMC-treated cells (Fig. 5A). The increased levels of p-c-JUN are consistent with the enhanced expression of NQO1 upon 18α-GA supplementation, since it has been shown that Nrf2 cooperates with p-c-JUN to drive NQO1 transcription [34].
Fig. 5.
18α-GA exerts its protective role against DNA damage through induction of ERK/Nrf2 signaling pathway. (Α) Immunoblot analysis and quantification of p-ERK (number of blots = 5) and p-c-JUN (number of blots = 3) in HFL-1 cells pre-treated with 18α-GA or DMSO for 24 h and then subjected to genotoxic stress with MMC for 1 h. Representative gels are shown. (B) DNA damage foci containing γH2AX and the quantification of cells containing foci in each condition (at least 80 cells measured/condition, number of foci in DMSO-treated cells set as 100%), and (C) real time PCR analysis of NQO1 and TXNRD1 mRNA levels (expression levels of each gene were arbitrarily set to 1 in DMSO-treated cells or DMSO/PD035901-treated cells, GAPDH mRNA expression was used as normalizer) in HFL-1 cells pre-treated with 18α-GA or DMSO for 24 h and then subjected to genotoxic stress with MMC for 1 h in the presence or absence of PD035901 inhibitor followed by a 24 h recovery in 18α-GA or DMSO. Error bars denote ±SEM. *p < 0.05, **p < 0.01, ***p < 0.001, NS not significant.
Lastly, we measured DNA damage foci 24 h after MMC treatment in cells pre-treated with 18α-GA or DMSO in the presence or absence of the specific MEK inhibitor, PD035901 that effectively prevents ERK phosphorylation. Although there were significantly fewer DNA damage foci 24 h after MMC treatment in 18α-GA/MMC-treated cells as compared to DMSO/MMC-treated cells (~2-fold less foci; Fig. 2A), the beneficial effect of 18α-GA was abolished in the presence of PD035901 (Fig. 5B), suggesting that this protective action is ERK-dependent. To further link Nrf2 induction to the ERK pathway, we measured the mRNA expression levels of the Nrf2 target genes NQO1 and TXRDN1 in cells treated with 18α-GA or DMSO in the presence of PD035901. Indeed, while those genes were upregulated upon treatment with 18α-GA and upon combined treatment with 18α-GA and MMC, the induction was abolished (either totally or near totally) when ERK phosphorylation was inhibited by PD035901 (Fig. 5C). Taken together, these data indicate that 18α-GA exerts a protective role against MMC genotoxicity through the activation of the ERK/Nrf2 pathway. Fig. 6 summarizes our results and outlines potential mechanisms that drive the protective effects of 18α-GA against MMC.
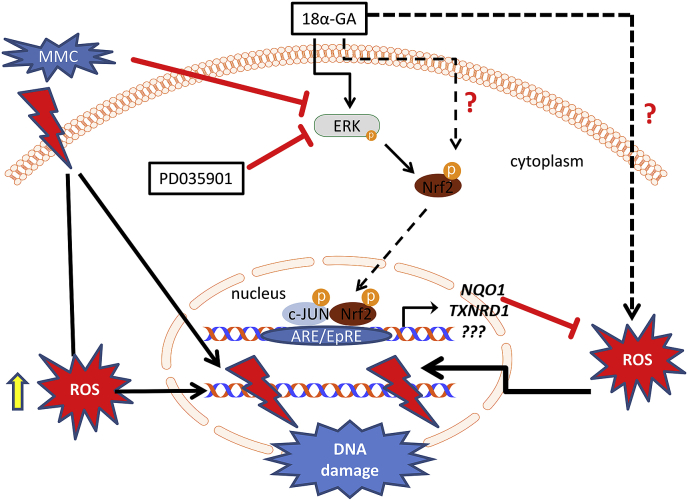
Fig. 6.
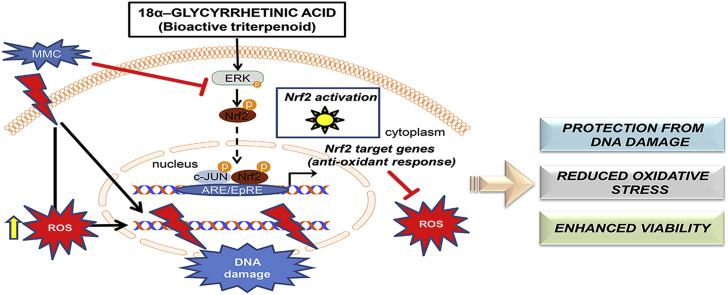
Proposed model for the cytoprotective effect of 18α-GA against MMC cytotoxicity. Apart from the canonical mechanism of action of MMC on DNA topology, MMC alters the cellular redox status, promoting ROS production and contributing to oxidative stress-mediated DNA damage. Moreover, MMC leads to decreased p-ERK levels, thereby limiting Nrf2 activation and its antioxidant effect and further contributing to oxidative stress-mediated DNA damage. 18α-GA induces ERK phosphorylation that promotes Nrf2 phosphorylation and nuclear translocation. Nuclear Nrf2 heterodimerizes with p-c-JUN (among other possible partners) upregulating the transcription of several target genes like NQO1 and -TXNRD1 (among others). The proteins encoded by those genes are part of the antioxidant cellular mechanisms leading to reduced ROS levels that normally promote DNA damage. This cascade may be inhibited through treatment with the specific MEK inhibitor PD035901 that effectively prevents ERK phosphorylation thus abolishing the protective effect of 18α-GA against MMC cytotoxicity. 18α-GA may also activate Nrf2 through other pathways as well (e.g. through the redox regulation of Keap1) while it may impact cellular ROS levels through additional direct or indirect mechanisms.
4. Discussion
Constantly growing evidence indicates that dietary compounds exert beneficial effects against various types of damage (mainly oxidative stress-mediated damage), aging and age-related diseases through the modulation of the Nrf2 signaling pathway. Intracellular signaling pathways such as the MAP kinase cascades are crucial in this activation [35]. In this study, we demonstrate that 18α-GA, which has previously been shown to activate Nrf2, protects cells against MMC-induced cytotoxicity through activation of the ERK/Nrf2 signaling pathway. This is an additional beneficial effect of this dietary compound against DNA damage, which is a stress that all organisms have to cope with continuously.
Nrf2 is one of the pivotal transcription factors regulating the cellular antioxidant response through the directed upregulation of several antioxidant and detoxifying genes [5]. MMC has been linked to ROS generation [12,13] and ROS levels are known to be the main mediators of Nrf2 induction [3]. It is therefore not surprising that MMC led to Nrf2 activation and to increased expression of antioxidant genes such as NQO1 and TXNRD1. The observed reduced ROS levels in cells that were pre-treated with 18α-GA even following MMC addition are in accordance with the previously established antioxidant properties of the compound [15,16]. Moreover, our results are consistent with data revealing higher sensitivity of Nrf2-deficient human colon cancer cells to MMC exposure; in the absence of Nrf2 and its downstream effects on antioxidant genes, MMC is much more potent as a genotoxic agent [36]. Consequently, the Nrf2 activation that occurs in our experimental set up is expected to limit MMC's genotoxicity.
Our data suggest that Nrf2 activation by 18α-GA is at least partially regulated by the MAPK (ERK1/2) pathway, as MEK-ERK pathway inhibition by the pharmacological inhibitor PD035901 abolished the protective effects of 18α-GA and reduced the expression of Nrf2 target genes following MMC-induced DNA damage. Many phytochemical components with antioxidant properties have been shown to exert their (usually antioxidant) protective effects through the ERK/Nrf2 pathway; red ginseng [30], morin [31], curcumin [32], sulforaphane [33], genistein [26] and quercetin [37] are only few of them. We have previously demonstrated that in C. elegans 18α-GA promotes the nuclear translocation of SKN-1 (the ortholog of Nrf2 in C. elegans; [38]) through activation and phosphorylation by PMK-1 (a MAPK family member, ortholog of human MAPK12, 13 and 14) [16]. Moreover, it has been shown that the ERK-MAPK pathway regulates longevity through SKN-1 and insulin-like signaling in C. elegans [39], and that phytochemicals like resveratrol exert their pro-longevity effects through the MPK-1/ERK/SKN-1 pathway [40]. Thus, our findings are in agreement with other studies implicating MAPK cascade in Nrf2 activation at both the cellular and organismal level.
Various compounds characterized as Nrf2 activators have been shown to exert protective effects against DNA damage. In most cases, this has been proposed to encompass oxidative stress-mediated DNA damage since most of the challenges (treatment with chemical compounds or irradiation) modulate ROS production in addition to their canonical mechanism of action. For example, ferulic acid, which is a naturally occurring plant flavonoid, protects cells from γ-irradiation-mediated oxidative stress through Nrf2 activation and ROS scavenging [41]. Similarly, Nrf2-dependent protective action against oxidative stress-induced DNA damage has been shown for morin [42], fish oil omega-3 fatty acids [43] and puerarin [44], among others. Likewise, red raspberry extract was shown to protect against UVB-induced skin photo-damage by activating Nrf2 and its target genes and consequently through the ROS scavenger properties and through protection against inflammatory responses [45]. Mangiferin has been shown to upregulate NQO1 expression through Nrf2 activation, thereby significantly reducing etoposide-induced DNA damage [46]. Nevertheless, the redox status of the cells upon etoposide treatment was not investigated to rule out the possibility of oxidative stress-induced DNA damage and consequent Nrf2 activation. Interestingly, despite the vast majority of studies referring to oxidative stress-mediated DNA damage there are also studies reporting a protective effect of Nrf2 against DNA damage independently of ROS. For example, BLAST analysis on upstream regions of DNA repair genes revealed the presence of antioxidant response elements (AREs) and found that many repair genes that are involved in the HR pathway may be regulated by Nrf2 [47]. Likewise, transcription of 53BP1 during DSBs repair has been shown to be partially dependent on Nrf2 [48], while BRCA1 has been shown to promote Nrf2 expression through enhanced transcription and reduced degradation [49]. Additionally, more efficient DNA damage repair was documented in Hutchinson–Gilford progeria syndrome (HGPS) fibroblasts in the presence of the Nrf2 activator sulforaphane [50]. Our results indicate oxidative stress-mediated DNA damage protection through 18α-GA, without however ruling out the possibility of a ROS-independent action of the compound contributing to the observed Nrf2 activation.
Over the last few years, functional foods have been increasingly attracting attention due to their potential health preserving properties. Such foods are thoroughly investigated to reveal properties that can be exploited to prevent disease, to delay its manifestation and/or to facilitate its treatment. Consumption of natural products with health maintenance claims possesses the advantage that such products may be delivered through a normal diet at any phase of human life, even from a young age. Most importantly, they can exert their positive actions even during preclinical stages of disease initiation when current methods of clinical screening are unable to make a diagnosis. For example, DNA damage occurs daily in cells, yet carcinogenesis requires certain cumulative doses. Therefore, enhancement of cellular resistance or repair capacity against DNA damage through diet that prevents or delays the accumulation of insults is a highly desirable strategy for disease prevention, especially if we refer to natural compounds with limited side effects that we would consume anyway.
5. Conclusions
Our study demonstrates the protective effect of 18α-GA, a pentacyclic triterpenoid already shown to activate the Nrf2/SKN-1 pathway [15,16] against DNA damage. Our study suggests that this triterpenoid could be used in a prophylactic (i.e. chemopreventive) or therapeutic strategy against conditions (such as aging) or diseases that are characterized by both oxidative stress and DNA damage.
Declaration of interest
None.
Acknowledgements
We would like to thank Ms M. Bekyrou for technical assistance with comet assay. This work was funded by two Research Funding Programs: Thales “GenAge” [QΑLΗS ΑP:10479/3.7.12 MIS380228] (to N.C.) and “MAESTRO” (to N.C.) co-financed by the European Union and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) and an Empirikion Foundation Scientific Project to N.C. Research from N.C. lab is currently co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH – CREATE – INNOVATE (project code: T1EDK-00353 and T1EDK-01610) as well by the project “STHENOS-b'' (MIS 5002398), which is funded by the Operational Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014–2020) and co-financed by Greece and the EU (European Regional Development Fund). N.P. receives a PhD fellowship from Empirikion Foundation. J.A.T. is funded by CIBEROBN (Physiopathology of Obesity and Nutrition) CB12/03/30038 (Instituto de Salud Carlos III, Spain and the European Regional Development Fund. G.P.S. is funded by the Swiss National Science Fund [IZCOZ0-177070 and 31003A_182105] and by a Leenaards Foundation 2016 Fellowship for Academic Promotion in Clinical Medicine. This article/publication is based upon work from COST Action NutRedOx-CA16112 supported by COST (European Cooperation in Science and Technology) (to J.A.T., G.P.S. and N.C.).
Contributor Information
Maria Lefaki, Email: lefakimaria@yahoo.gr.
Nikoletta Papaevgeniou, Email: npapaevgeniou@eie.gr.
Josep A. Tur, Email: pep.tur@uib.es.
Constantinos E. Vorgias, Email: cvorgias@biol.uoa.gr.
Gerasimos P. Sykiotis, Email: gerasimos.sykiotis@chuv.ch.
Niki Chondrogianni, Email: nikichon@eie.gr.
References
- 1.Ciccia A., Elledge S.J. The DNA damage response: making it safe to play with knives. Mol. Cell. 2010;40(2):179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frakes A.E., Dillin A. The UPR(ER): sensor and coordinator of organismal homeostasis. Mol. Cell. 2017;66(6):761–771. doi: 10.1016/j.molcel.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niture S.K., Khatri R., Jaiswal A.K. Regulation of Nrf2-an update. Free Radic. Biol. Med. 2014;66:36–44. doi: 10.1016/j.freeradbiomed.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lefaki M., Papaevgeniou N., Chondrogianni N. Redox regulation of proteasome function. Redox. Biol. 2017;13:452–458. doi: 10.1016/j.redox.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao S. p97 Negatively regulates NRF2 by extracting ubiquitylated NRF2 from the KEAP1-CUL3 E3 complex. Mol. Cell. Biol. 2017;37(8) doi: 10.1128/MCB.00660-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang D.D., Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003;23(22):8137–8151. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itoh K. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 9.Itoh K., Tong K.I., Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004;36(10):1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 10.Huang Y. The complexity of the Nrf2 pathway: beyond the antioxidant response. J. Nutr. Biochem. 2015;26(12):1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomasz M., Palom Y. The mitomycin bioreductive antitumor agents: cross-linking and alkylation of DNA as the molecular basis of their activity. Pharmacol. Ther. 1997;76(1–3):73–87. doi: 10.1016/s0163-7258(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 12.Sartorelli A.C. Mitomycin C: a prototype bioreductive agent. Oncol. Res. 1994;6(10–11):501–508. [PubMed] [Google Scholar]
- 13.Shi K. Endoplasmic reticulum stress signaling is involved in mitomycin C (MMC)-induced apoptosis in human fibroblasts via PERK pathway. PLoS One. 2013;8(3) doi: 10.1371/journal.pone.0059330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zong L. 18alpha-glycyrrhetinic acid extracted from Glycyrrhiza radix inhibits proliferation and promotes apoptosis of the hepatic stellate cell line. J. Dig. Dis. 2013;14(6):328–336. doi: 10.1111/1751-2980.12041. [DOI] [PubMed] [Google Scholar]
- 15.Kapeta S., Chondrogianni N., Gonos E.S. Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. J. Biol. Chem. 2010;285(11):8171–8184. doi: 10.1074/jbc.M109.031575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papaevgeniou N. 18alpha-Glycyrrhetinic acid proteasome activator decelerates aging and alzheimer's disease progression in Caenorhabditis elegans and neuronal cultures. Antioxidants Redox Signal. 2016;25(16):855–869. doi: 10.1089/ars.2015.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsui S. Glycyrrhizin and related compounds down-regulate production of inflammatory chemokines IL-8 and eotaxin 1 in a human lung fibroblast cell line. Int. Immunopharmacol. 2004;4(13):1633–1644. doi: 10.1016/j.intimp.2004.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi T. Selectivity of action of glycyrrhizin derivatives on the growth of MCF-7 and HEP-2 cells. Anticancer Res. 2003;23(5A):3813–3818. [PubMed] [Google Scholar]
- 19.Kimura M. Glycyrrhizin and some analogues induce growth of primary cultured adult rat hepatocytes via epidermal growth factor receptors. Eur. J. Pharmacol. 2001;431(2):151–161. doi: 10.1016/s0014-2999(01)01424-8. [DOI] [PubMed] [Google Scholar]
- 20.Sanmartin-Suarez C. Antioxidant properties of dimethyl sulfoxide and its viability as a solvent in the evaluation of neuroprotective antioxidants. J. Pharmacol. Toxicol. Methods. 2011;63(2):209–215. doi: 10.1016/j.vascn.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Tuncer S. Low dose dimethyl sulfoxide driven gross molecular changes have the potential to interfere with various cellular processes. Sci. Rep. 2018;8(1):14828. doi: 10.1038/s41598-018-33234-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chondrogianni N. Partial proteasome inhibition in human fibroblasts triggers accelerated M1 senescence or M2 crisis depending on p53 and Rb status. Aging Cell. 2008;7(5):717–732. doi: 10.1111/j.1474-9726.2008.00425.x. [DOI] [PubMed] [Google Scholar]
- 23.Alam J. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274(37):26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 24.Katsiki M. The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts. Rejuvenation Res. 2007;10(2):157–172. doi: 10.1089/rej.2006.0513. [DOI] [PubMed] [Google Scholar]
- 25.Rogakou E.P. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998;273(10):5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 26.Zhai X. Dietary flavonoid genistein induces Nrf2 and phase II detoxification gene expression via ERKs and PKC pathways and protects against oxidative stress in Caco-2 cells. Mol. Nutr. Food Res. 2013;57(2):249–259. doi: 10.1002/mnfr.201200536. [DOI] [PubMed] [Google Scholar]
- 27.Ross D., Siegel D. NQO1 in protection against oxidative stress. Curr. Opin. Toxicol. 2018;7:67–72. [Google Scholar]
- 28.Sykiotis G.P., Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Sci. Signal. 2010;3(112):re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampey B.P. 4-Hydroxy-2-nonenal adduction of extracellular signal-regulated kinase (Erk) and the inhibition of hepatocyte Erk-Est-like protein-1-activating protein-1 signal transduction. Mol. Pharmacol. 2007;71(3):871–883. doi: 10.1124/mol.106.029686. [DOI] [PubMed] [Google Scholar]
- 30.Bak M.J. Induction of Nrf2/ARE-mediated cytoprotective genes by red ginseng oil through ASK1-MKK4/7-JNK and p38 MAPK signaling pathways in HepG2 cells. J. Ginseng. Res. 2016;40(4):423–430. doi: 10.1016/j.jgr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rizvi F. Suppression in PHLPP2 induction by morin promotes Nrf2-regulated cellular defenses against oxidative injury to primary rat hepatocytes. Redox. Biol. 2015;6:587–598. doi: 10.1016/j.redox.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J. Curcumin inhibits heat-induced oxidative stress by activating the MAPK-Nrf2/ARE signaling pathway in chicken fibroblasts cells. J. Therm. Biol. 2019;79:112–119. doi: 10.1016/j.jtherbio.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Vauzour D. Sulforaphane protects cortical neurons against 5-S-cysteinyl-dopamine-induced toxicity through the activation of ERK1/2, Nrf-2 and the upregulation of detoxification enzymes. Mol. Nutr. Food Res. 2010;54(4):532–542. doi: 10.1002/mnfr.200900197. [DOI] [PubMed] [Google Scholar]
- 34.Venugopal R., Jaiswal A.K. Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene. 1998;17(24):3145–3156. doi: 10.1038/sj.onc.1202237. [DOI] [PubMed] [Google Scholar]
- 35.Owuor E.D., Kong A.N. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharmacol. 2002;64(5–6):765–770. doi: 10.1016/s0006-2952(02)01137-1. [DOI] [PubMed] [Google Scholar]
- 36.Park J.Y., Seo Y.R. Enhancement of mitomycin C-induced apoptosis in Nrf2-deficient human colon cancer cells. Mol. Cell. Toxicol. 2010;6(1):51–56. [Google Scholar]
- 37.Yao P. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007;47(2):253–261. doi: 10.1016/j.jhep.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Walker A.K. A conserved transcription motif suggesting functional parallels between Caenorhabditis elegans SKN-1 and Cap'n'Collar-related basic leucine zipper proteins. J. Biol. Chem. 2000;275(29):22166–22171. doi: 10.1074/jbc.M001746200. [DOI] [PubMed] [Google Scholar]
- 39.Okuyama T. The ERK-MAPK pathway regulates longevity through SKN-1 and insulin-like signaling in Caenorhabditis elegans. J. Biol. Chem. 2010;285(39):30274–30281. doi: 10.1074/jbc.M110.146274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon D.S. MPK-1/ERK is required for the full activity of resveratrol in extended lifespan and reproduction. Aging Cell. 2019;18(1) doi: 10.1111/acel.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Das U. Ferulic acid (FA) abrogates gamma-radiation induced oxidative stress and DNA damage by up-regulating nuclear translocation of Nrf2 and activation of NHEJ pathway. Free Radic. Res. 2017;51(1):47–63. doi: 10.1080/10715762.2016.1267345. [DOI] [PubMed] [Google Scholar]
- 42.Vanitha P. Morin activates the Nrf2-ARE pathway and reduces oxidative stress-induced DNA damage in pancreatic beta cells. Eur. J. Pharmacol. 2017;801:9–18. doi: 10.1016/j.ejphar.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Sakai C. Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0187934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma J.Q. Puerarin ameliorates carbon tetrachloride-induced oxidative DNA damage and inflammation in mouse kidney through ERK/Nrf2/ARE pathway. Food Chem. Toxicol. 2014;71:264–271. doi: 10.1016/j.fct.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 45.Wang P.W. Red raspberry extract protects the skin against UVB-induced damage with antioxidative and anti-inflammatory properties. Oxid. Med. Cell. Longev. 2019;2019:9529676. doi: 10.1155/2019/9529676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B. Mangiferin activates the Nrf2-ARE pathway and reduces etoposide-induced DNA damage in human umbilical cord mononuclear blood cells. Pharm. Biol. 2015;53(4):503–511. doi: 10.3109/13880209.2014.927890. [DOI] [PubMed] [Google Scholar]
- 47.Jayakumar S., Pal D., Sandur S.K. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat. Res. 2015;779:33–45. doi: 10.1016/j.mrfmmm.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Kim S.B. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc. Natl. Acad. Sci. U. S. A. 2012;109(43):E2949–E2955. doi: 10.1073/pnas.1207718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorrini C. BRCA1 interacts with Nrf2 to regulate antioxidant signaling and cell survival. J. Exp. Med. 2013;210(8):1529–1544. doi: 10.1084/jem.20121337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabriel D. Sulforaphane enhances progerin clearance in Hutchinson-Gilford progeria fibroblasts. Aging Cell. 2015;14(1):78–91. doi: 10.1111/acel.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]