Abstract
BACKGROUND
Proton pump inhibitors (PPIs) are widely prescribed, often without clear indications. There are conflicting data on its association with mortality risk and hepatic decompensation in cirrhotic patients. Furthermore, PPI users and PPI exposure in some studies have been poorly defined with many confounding factors.
AIM
To examine if PPI use increases mortality and hepatic decompensation and the impact of cumulative PPI dose exposure.
METHODS
Data from patients with decompensated liver cirrhosis were extracted from a hospital database between 2013 to 2017. PPI users were defined as cumulative defined daily dose (cDDD) ≥ 28 within a landmark period, after hospitalisation for hepatic decompensation. Cox regression analysis for comparison was done after propensity score adjustment. Further risk of hepatic decompensation was analysed by Poisson regression.
RESULTS
Among 295 decompensated cirrhosis patients, 238 were PPI users and 57 were non-users. PPI users had higher mortality compared to non-users [adjusted HR = 2.10, (1.20-3.67); P = 0.009]. Longer PPI use with cDDD > 90 was associated with higher mortality, compared to non-users [aHR = 2.27, (1.10-5.14); P = 0.038]. PPI users had a higher incidence of hospitalization for hepatic decompensation [aRR = 1.61, (1.30-2.11); P < 0.001].
CONCLUSION
PPI use in decompensated cirrhosis is associated with increased risk of mortality and hepatic decompensation. Longer PPI exposure with cDDD > 90 increases the risk of mortality.
Keywords: Proton pump inhibitor, Liver cirrhosis, Mortality, Hospitalisation, Complications, Portal hypertension, Variceal bleeding, Ascites, Spontaneous bacterial peritonitis, Hepatic encephalopathy
Core tip: Most proton pump inhibitor (PPI) studies have issues with poorly defining PPI users and having baseline confounders. Also, studies on PPI use in liver cirrhosis have not been focused on decompensated cirrhosis. Using propensity score analysis, we adjusted for 43 variables including baseline characteristics, comorbidities, PPI indication, and medications (including antiplatelets). Landmark analysis was used to define PPI users to reduce bias. PPI use in patients with decompensated liver cirrhosis was associated with higher mortality and increased risk of hepatic decompensation requiring hospital admissions. Longer PPI exposure with > 90 defined daily doses further increased mortality risk.
INTRODUCTION
Liver cirrhosis is associated with significant morbidity and mortality[1], especially when portal hypertension-related complications or hepatocellular carcinoma (HCC) develop. Several host factors are associated with increased risk of morbidity and mortality in cirrhotic patients including type 2 diabetes[2,3], older age, obesity, and alcohol consumption[4]. Recent studies have shed light on abnormal gut microbiota composition and dysbiosis playing an important role in the pathophysiology of cirrhosis complications such as hepatic encephalopathy (HE), spontaneous bacterial peritonitis (SBP) and acute on chronic liver failure[5,6].
Proton pump inhibitors (PPIs), a frequently prescribed medication worldwide, has been shown to promote alterations in gut microbiota[7,8], leading to dysbiosis and impaired gut barrier function[9]. Its use in cirrhosis patients is associated with increased risk of SBP and HE[9-11]. In addition, Bajaj et al[12] showed that gut microbiota is modulated by PPI and results in increased oral origin microbiota, which can reduce upon PPI withdrawal. They also showed that initiation of PPI was an independent risk factor for hospital readmissions among cirrhotic patients; the 30-d readmission for those discharged with PPI was 50% compared to 32% for those who were not on PPI (P = 0.02).
Despite the increasing concerns of PPI use, it is still widely prescribed in liver cirrhosis patients. One study showed 62.7% of hospitalised cirrhosis patients were prescribed PPIs with unclear indications[13]. It is particularly concerning as PPIs are metabolised in the liver by cytochrome CYP450[11,14], and as a result, their half-life increases by 4-8 h in cirrhotic patients[15]. There have been concerns that PPI use increases the risk of mortality in patients with decompensated liver disease[16], and those with HE[17], but other studies dispute the association of mortality with PPI use in decompensated cirrhosis or cirrhotic patients with SBP[13,18]. Of the published data on PPI use and mortality in cirrhotic patients[13,16,17], “PPI users” are often defined as patients with PPI prescriptions at the study inclusion, and PPI dose duration is not measured. These could potentially lead to guarantee-time bias and exposure classification bias[19,20]. Furthermore, given that PPI is widely used as a gastroprotective agent in patients with cardiovascular disease taking aspirin and antithrombotic agents, these should be adjusted as confounders.
Currently, the evidence supporting PPI exposure and increased mortality in cirrhosis patients is still not clear, with potential biases as PPI user status and dose exposure not well defined. Furthermore, data are lacking on the dose-dependent effect of PPI on mortality risk and further hepatic decompensation among cirrhotic patients, especially when PPI metabolism is affected in this population[15]. Therefore, we assessed if long-term PPI use in decompensated liver cirrhosis patients would increase the risk of mortality after adjusting for potential biases and defining true dosage exposure. The secondary aim was to determine if PPI use increases the risk of hospital admissions for further hepatic decompensation in patients with decompensated liver cirrhosis.
MATERIALS AND METHODS
Patient selection
Patients with liver cirrhosis using ICD10 coding (Supplemental Table 1) were extracted from January 2013 to June 2017 from the Changi General Hospital electronic database. Patient demographics, medical comorbidities (based on ICD codings forming Charlson’s comorbidity index; Supplementary Table 1 ), biochemical profile, baseline medication use (Supplementary Table 2), and history of prior hepatic decompensation were reviewed and verified by three investigators. Clinical ICD codings of United States Food and Drug Administration (FDA)-approved PPI indications were also extracted such as gastroesophageal reflux disease (GERD), esophagitis, and peptic ulcer disease. Patients over 18 years of age with liver cirrhosis confirmed by histology, imaging or transient elastography and hospital admissions for hepatic decompensation during this period were included. Patients without hepatic decompensation were excluded.
The codings of hospital admission diagnoses were regularly reviewed and audited by the hospital medical record department to maintain data integrity as expected of a restructured public hospital governed by the health ministry. Mortality data were obtained from the Singapore National Registry of Diseases Office, and the date of liver transplant, if any, was obtained from the National Organ Transplant of Singapore.
The study’s protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by our institution's human research committee.
Outcomes
The primary outcome of this study was overall mortality, defined as death or liver transplant, whichever came first. The secondary outcome was the rate of further hepatic decompensation-related hospital admissions after the index admission at baseline. For secondary outcomes, each patient’s hospital admission notes were reviewed by three investigators to verify that coding diagnoses of hepatic decompensation admissions were accurate. Hospital admissions for elective procedures such as radiofrequency ablation or trans-arterial chemoembolisation of HCC and those with incomplete data were excluded from the study.
The hepatic decompensation events were ascites, SBP, HE, variceal bleeding, and hepatorenal syndrome, as defined by current guidelines[21]. Overall survival was calculated from the end of the designated landmark period until the census date of 31st December 2017. Patients who died within the landmark period were excluded from primary analysis to reduce biases.
Definition of PPI user status
In pharmacoepidemiologic studies, there are biases involved in comparing time-to-event data for different groups as classification to “event” or “event-free” groups are dependent on length of follow-up[22]. Therefore, by using the landmark method, a fixed time after the initiation of therapy was selected as a landmark for conducting the survival analysis, which would minimise immortal time, selection, and indication bias. Taking this into consideration, we used a landmark period of 3 mo before to 6 mo after index hepatic decompensation admission (-3 mo to +6 mo), to define PPI user status.
The period of 3 mo before index admission (-3 mo to time 0) was utilised as PPI use in hospitalised cirrhotic patients, as it has been found to increase the risk of 1-mo and 3-mo hospital readmission rates[12]. Exclusion of these patients who were on PPI just prior to liver decompensation would be a bias. Two additional landmark periods were used to validate the primary outcome: -3 mo to +3 mo and -3 mo to +9 mo.
PPI doses were defined using the “defined daily dose (DDD),” which is recommended by the World Health Organization to objectively measure the prescribed amount of a drug[23]. The cumulative defined daily dose (cDDD) ≥ 28 (≥ 1 mo of use) of prescribed medication was chosen, as PPI exposure of 1 mo has been reported to significantly cause adverse outcomes[24]. For the current study, PPI users were defined as those with a cDDD ≥ 28 within the landmark period. Patients with a past history of PPI use more than 3 mo prior to index admission were excluded from the study. Non-users were defined as those with cDDD < 28 within the landmark period, those with no PPI prescribed during the landmark period, or those prescribed with PPI after the landmark period regardless of the cumulative dosage.
Other relevant medication use at baseline, which could influence primary and secondary outcomes were also considered. Long-term use of concurrent medication was defined by more than 3 mo of medication prescribed, and was adjusted for in the analysis (Supplementary Table 2).
Statistical analysis
Categorical data were presented as frequency (percentage). Numeric data were presented as mean [standard deviation (SD)] for parametric distribution and median [interquartile range (IQR)] for non-parametric distribution. The differences in characteristics between PPI users and non-users were examined using the Chi-Square test or Fisher’s Exact test for categorical variables, and two-sample t-test or Mann Whitney U-test for numerical variables, where appropriate.
Propensity score (PS) was first generated using logistic regression to reduce the selection bias of treatment allocation by balancing the characteristics of patients between treatment and control groups. The characteristics of patients such as demographics, aetiology of liver cirrhosis, history of HCC, and previous decompensation (ascites, variceal bleed, SBP, HE, hepatorenal syndrome), medical co-morbidities, baseline MELD score, and baseline medication use (Supplementary Table 2), which could potentially confound the results on mortality and hospitalisation risks were adjusted for. For any significant differences in PS between the two groups, PS was further categorised into four quartiles in the two groups separately for matching.
After PS adjustment for 43 clinically important confounding variables at baseline, which could influence mortality and recurrent hepatic decompensation (Table 1), the effect of PPI use on mortality was assessed using the Cox proportional hazards model. Further variable landmark periods and subgroup analyses were performed to determine subgroups with increased risk of mortality. For secondary outcome of hospital admission for hepatic decompensation, Poisson regression (loglinear) was used with adjustment for PS (similarly as for primary outcome) and overall survival or number of days of follow-up. Relative risk and its 95% confidence interval (CI) were presented. A two-tailed, P value < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS statistical software, version 19.0 (IBM Corp., Armonk, NY, United States). Statistical analysis and review were performed by biomedical statisticians.
Table 1.
Baseline characteristics between non-users and proton pump inhibitor users for the 6-mo landmark period
| Baseline characteristics | Non-user (n = 57) | PPI user (n = 238) | P value |
| Gender, n (%) | 0.96 | ||
| Male | 39 (68.4) | 162 (68.1) | |
| Female | 18 (31.6) | 76 (31.9) | |
| Age in yr, Mean (± SD) | 60.0 ± 13.3 | 63.3 ± 12.4 | 0.07 |
| Race, n (%) | 0.95 | ||
| Chinese | 33 (57.9) | 132 (55.5) | |
| Malay | 10 (17.5) | 50 (21.0) | |
| Indian | 8 (14.0) | 33 (13.9) | |
| Others | 6 (10.5) | 23 (9.7) | |
| Aetiology of cirrhosis, n (%) | 0.07 | ||
| Hepatitis B | 11 (19.3 | 42 (17.6) | |
| Alcohol | 16 (28.1) | 42 (17.6) | |
| Hepatitis C | 11 (19.3) | 52 (21.8) | |
| NASH | 9 (15.8) | 74 (31.1) | |
| Autoimmune | 4 (7.0) | 4 (1.7) | |
| Others | 6 (10.5) | 24 (10.1) | |
| Index hepatic event, n (%) | |||
| HCC | 6 (10.5) | 20 (8.4) | 0.61 |
| Ascites | 37 (64.9) | 121 (50.8) | 0.06 |
| SBP | 4 (7.0) | 15 (6.3) | 0.77 |
| HE | 9 (15.8) | 59 (24.8) | 0.15 |
| Variceal bleed | 8 (14.0) | 53 (22.3) | 0.17 |
| History of the following, n (%) | |||
| HCC | 0 (0.0) | 9 (3.8) | 0.21 |
| Ascites | 9 (15.8) | 32 (13.4) | 0.65 |
| HE | 1 (1.8) | 10 (4.2) | 0.70 |
| Variceal bleed | 9 (15.8) | 42 (17.6) | 0.74 |
| SBP | 2 (3.5) | 6 (2.5) | 0.65 |
| Biochemical results at baseline; | |||
| Mean (± SD) or median (IQR) | |||
| Albumin in g/L | 27.0 ± 4.7 | 28.1 ± 6.2 | 0.14 |
| INR | 1.12 (1.01-1.26) | 1.13 (1.03-1.28) | 0.73 |
| Creatinine in μmol/L | 79.0 (65.0-124.5) | 86.0 (66.8-117.0) | 0.58 |
| Bilirubin in μmol/L | 29.4 (17.0-56.8) | 25.9 (16.3-74.0) | 0.16 |
| Platelet count as 109/L | 105.5 (67.3-150.3) | 104.0 (71.0-159.0) | 0.82 |
| Haemoglobin in g/dL | 11.4 ± 2.3 | 10.8 ± 2.6 | 0.15 |
| MELD, median (IQR) | 11.0 (8.0-14.5) | 10.5 (8.0-14.3) | 0.56 |
| Medical comorbidities, n (%) | |||
| GERD | 0 (0.0) | 19 (8.0) | 0.03 |
| Esophagitis | 4 (7.0) | 17 (7.1) | 1.00 |
| Peptic ulcer disease | 1 (1.8) | 32 (13.4) | 0.01 |
| Type 2 diabetes1 | 0.16 | ||
| None | 33 (57.9) | 105 (44.1) | |
| Uncomplicated | 14 (24.6) | 70 (29.4) | |
| End-organ damage | 10 (17.5) | 63 (26.5) | |
| Malignancy1 | 0.84 | ||
| None | 47 (82.5) | 199 (83.6) | |
| Leukaemia/lymphoma/localised solid tumour | 8 (14.0) | 33 (13.9) | |
| Metastatic solid tumour | 2 (3.5) | 6 (2.5) | |
| HIV/AIDS1 | 1 (1.8) | 2 (0.8) | |
| Renal impairment1 | 9 (15.8) | 51 (21.4) | |
| Congestive heart failure1 | 4 (7.0) | 21 (8.8) | |
| Myocardial infarct1 | 2 (3.5) | 33 (13.9) | |
| COPD1 | 2 (3.5) | 9 (3.8) | |
| PVD1 | 0 (0.0) | 4 (1.7) | |
| CVA/TIA1 | 2 (3.5) | 24 (10.1) | |
| Dementia1 | 1 (1.8) | 9 (3.8) | |
| Hemiplegia1 | 0 (0.0) | 3 (1.3) | |
| 2 (3.5) | 4 (1.7) | ||
| Connective tissue disease1 | |||
| Baseline medications: | |||
| Antivirals for viral hepatitis: | |||
| Chronic HBV on long-term antivirals | 2/11 (18.2) | 14/42 (33.3) | 0.48 |
| Chronic HCV treated with DAA2 | 0/11 (0.0) | 3/52 (5.8) | 1.00 |
| Use of other concurrent medications, > 3 mo use | |||
| Insulin | 2 (3.5) | 42 (17.6) | 0.01 |
| Sulphonylureas | 8 (14.0) | 51 (21.4) | 0.21 |
| Insulin sensitisers | 2 (3.5) | 24 (10.1) | 0.11 |
| Metformin | 5 (8.8) | 45 (18.9) | 0.07 |
| DPP4 inhibitors | 4 (7.0) | 2 (0.8) | 0.01 |
| Antiplatelet | 5 (8.8) | 45 (18.9) | 0.067 |
| Aspirin | 5 (8.8) | 38 (16.0) | 0.17 |
| Statins | 2 (3.5) | 29 (12.2) | 0.06 |
| ACE-I/ARB | 4 (7.0) | 43 (18.1) | 0.04 |
| Non-selective beta blockers | 8 (14.0) | 81 (34.0) | 0.003 |
| Selective beta blockers | 2 (3.5) | 22 (9.2) | 0.19 |
As defined by Charlson’s comorbidity index;
3 patients given 12 wk of sofosbuvir/daclatasvir/ribavirin for hepatitis C virus cirrhosis; direct acting antiviral only became fully funded in early 2017. NASH: Non-alcoholic steatohepatitis; MELD: Model of end-stage liver disease; GERD: Gastroesophageal reflux disease; HIV/AIDS: Human immunodeficiency virus/acquired immune deficiency syndrome; COPD-Chronic obstructive pulmonary disease; PVD: Peripheral vascular disease; CVA/TIA: Cerebrovascular accident/transient ischemic attack; DPP4: Dipeptidyl peptidase-4; ACE-I/ARB: Angiotensin converting enzyme inhibitor/angiotensin II receptor blocker; DAA: Direct acting antiviral; HE: Hepatic encephalopathy; SBP: Spontaneous bacterial peritonitis; HCC: Hepatocellular carcinoma; IQR: Interquartile range; PPI: Proton pump inhibitor.
RESULTS
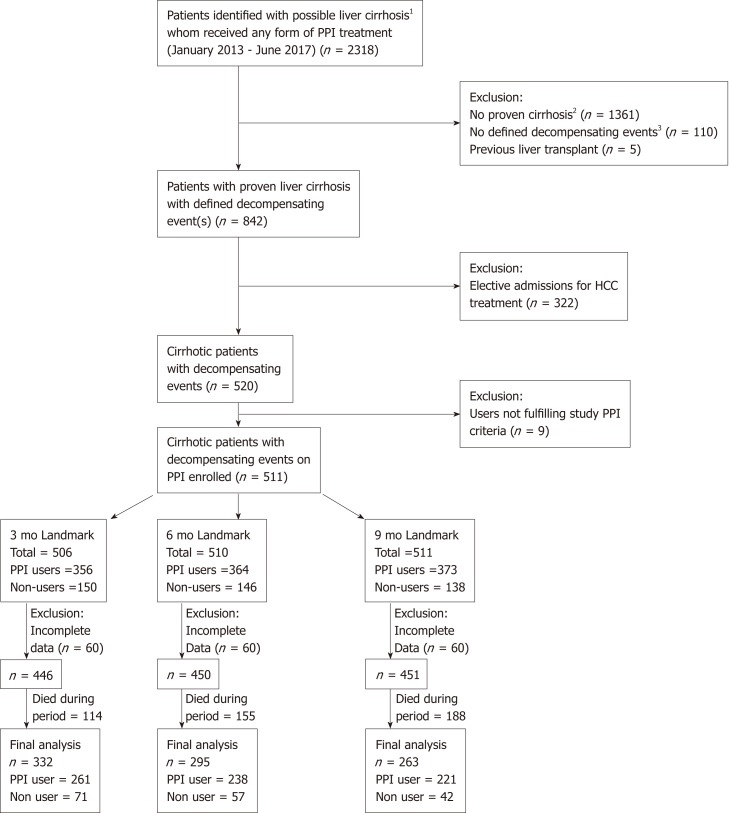
A total of 2318 patients with ICD codings for liver cirrhosis at inpatient admissions were identified. A final cohort of 511 patients was included for landmark analysis (Figure 1), with 295 patients in the chosen landmark period of 6 mo. A total of 238 patients were PPI users and 57 were non-users; their baseline characteristics are described in Table 1. There were no significant differences in history of SBP or HE, between the PPI users and non-users. There was a higher usage of aspirin, anti-platelet drugs, statins, and non-selective beta blockers in the PPI user group compared to non-users. The baseline characteristics described were before propensity adjustment.
Figure 1.
Consort diagram of landmark analysis. PPI: Proton pump inhibitor.
Overall risk of mortality
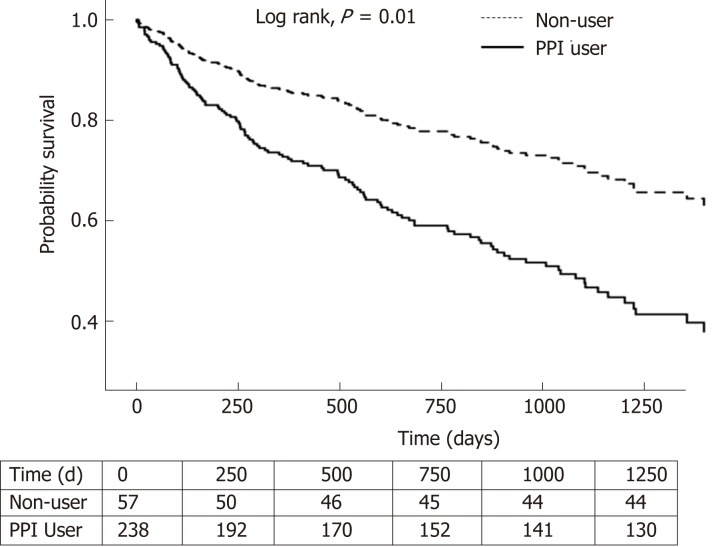
In the 6-mo landmark cohort, 102 of 238 (42.9%) PPI users and 13 of 57 (22.8%) non-users died during the median follow-up period of 551 (IQR: 231-1017) and 584 (289-1152) d, respectively. Seven PPI users and one non-user underwent liver transplant during the follow-up period, before cox regression.
PPI users had a higher risk of overall mortality, compared to non-users with [adjusted HR (aHR) of 2.10, 95%CI (1.20-3.670); P = 0.009] (Table 2 and Figure 2). This was also observed in the 9-mo landmark cohort with aHR 3.44, (1.50-7.85); P = 0.003. In the 3-mo landmark cohort, the aHR was 1.36, but this was not statistically significant (P = 0.143). Longer PPI exposure with cDDD 91-180 was associated with higher mortality [aHR 2.27, (1.10-5.14); P = 0.038] compared to non-users in the 6-mo landmark cohort (Table 2). Long-term PPI exposure with cDDD > 180 was also associated with higher mortality in the 6-mo landmark cohort [aHR 2.08, (1.17-3.61); P = 0.011] (Table 2) and the 9-mo landmark cohort [aHR 3.52, (1.53-8.09); P = 0.003].
Table 2.
Mortality risk of proton pump inhibitor users by landmark periods and cumulative dose exposure
| Periods | Number of patients | Adjusted HR (95%CI) | P value |
| 6-mo landmark: (-3 to +6 mo) | Non-user = 57 PPI user = 238 | Ref 2.10 (1.20-3.67) | 0.009 |
| 3-mo landmark: (-3 to +3 mo) | Non-user = 71 PPI user = 261 | Ref 1.36 (0.90-2.06) | 0.143 |
| 9-mo landmark: (-3 to +9 mo) | Non-user = 42 PPI user = 221 | Ref 3.44 (1.50-7.85) | 0.003 |
| Variable Dose Exposure | Number of patients | Adjusted HR (95%CI) | P value |
| 6-mo landmark: (-3 to +6 mo) | |||
| Non-user | 57 | Ref | |
| cDDD 28-90 | 18 | 1.34 (0.48-3.73) | 0.579 |
| cDDD 91-180 | 27 | 2.27 (1.10-5.14) | 0.038 |
| cDDD > 180 | 193 | 2.08 (1.17-3.61) | 0.011 |
| 3-mo landmark: (-3 to +3 mo) | |||
| Non-user | 71 | Ref | |
| cDDD 28-90 | 24 | 1.49 (0.74-3.03) | 0.266 |
| cDDD 91-180 | 34 | 2.04 (1.13-3.07) | 0.019 |
| cDDD > 180 | 203 | 1.33 (0.87 – 2.03) | 0.188 |
| 9-mo landmark : (-3 to + 9 mo) | |||
| Non-user | 42 | Ref | |
| cDDD 28-90 | 20 | 4.02 (1.33-12.12) | 0.013 |
| cDDD 91-180 | 22 | 3.38 (1.17 – 9.82) | 0.025 |
| cDDD > 180 | 179 | 3.52 (1.53 – 8.09) | 0.003 |
HR: Hazard ratio (with propensity score adjustment); CI: Confidence interval; cDDD: Cumulative defined daily dose; PPI: Proton pump inhibitor.
Figure 2.
Survival analysis of PPI users and non-users for the 6-mo landmark period. PPI: Proton pump inhibitor.
Subgroup and sensitivity analyses for mortality
In the subgroup analyses, PPI users with MELD15 was associated with increased mortality risk compared to non-users [aHR = 10.30, (1.41-75.58); P = 0.022] (Supplementary Table 3). There was a trend towards significance among patients with viral hepatitis aetiology [aHR 3.23, (0.99-10.52); P = 0.052], ascites [aHR 1.91, (0.96-3.78); P = 0.063], and those without prior decompensation at baseline [aHR 1.99, (0.98-4.00); P = 0.057] (Supplementary Table 3).
Risk of hospitalisation for hepatic decompensation
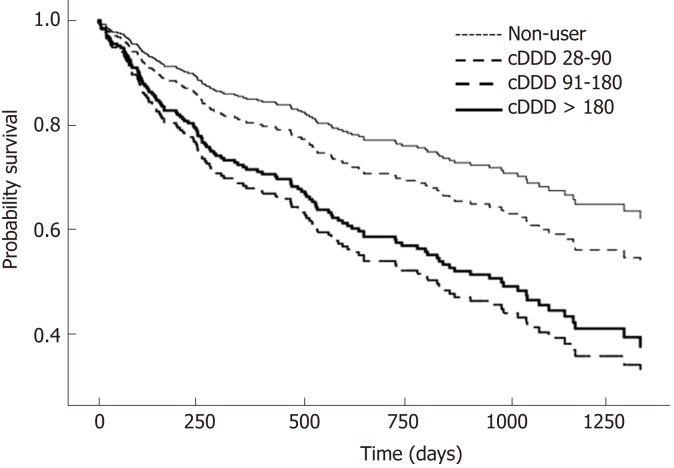
The clinical characteristics of 335 PPI users and 116 non-users, for secondary outcome analysis, are described in Supplementary Table 4. There were 835 and 231 hospital admissions for PPI users and non-users respectively, for hepatic decompensation during the follow-up period. PPI users had a higher incidence of hospital admissions for hepatic decompensation with adjusted relative risk (aRR) of 1.61 [95%CI: 1.30-2.11, P < 0.001] (Table 3). Similar to the survival analysis for primary outcome, there was a dose-dependent effect of PPI on increased risk of hospitalisations for hepatic decompensation. Those with cDDD > 180 were more likely to have admissions for hepatic decompensation [aRR 1.91, (1.49-2.45); P < 0.001], compared to non-users (Figure 3).
Table 3.
Hospital admissions for hepatic decompensation for proton pump inhibitor users and non-users with decompensated liver cirrhosis
| Number of patients |
Hospital admissions for liver decompensation |
||
| Adjusted RR (95%CI) | P value | ||
| Entire cohort | PPI user = 335 Non-user = 116 | 1.61 (1.30-2.11) | < 0.001 |
| Dose exposure | |||
| Non-user | 116 | Ref | |
| cDDD 28-90 | 49 | 0.65 (0.39-1.08) | 0.10 |
| cDDD 91-180 | 61 | 1.08 (0.74-1.59) | 0.69 |
| cDDD > 180 | 225 | 1.91 (1.49-2.45) | < 0.001 |
cDDD: Cumulative defined daily dose; RR: Relative risk; CI: Confidence interval; PPI: Proton pump inhibitor.
Figure 3.
Survival analysis of PPI users and non-users with decompensated liver cirrhosis by cumulative dose exposure in the 6-mo landmark cohort. PPI: Proton pump inhibitor.
DISCUSSION
In our study of patients with decompensated liver cirrhosis, PPI users had twice the risk of mortality [aHR 2.10, (1.20-3.67); P = 0.009] compared to non-users after adjusting for potential biases and confounders using landmark analysis, PS adjustment, and defined daily doses. We also found that PPI users were 61% more likely to have hospitalisation for hepatic decompensation than non-users [aRR = 1.61, (1.30-2.15); P < 0.001]. Longer exposure to PPI with cDDD 91-180 increased mortality risk [aHR = 2.27, (1.10-5.14); P = 0.038] and long-term PPI use with cDDD > 180 had a higher risk of admission for hepatic decompensation compared to non-users (P < 0.001).
Previous studies have suggested that PPI use may be associated with a higher risk of mortality. Dultz et al[16] reported PPI use to be an independent predictor of mortality in patients with compensated and decompensated liver cirrhosis [HR = 2.33, (1.26–4.29); P = 0.007], but another study performed on hospitalised cirrhotic patients did not show a difference in survival between PPI users and non-users[13]. Hung et al[17] studied the effect of inpatient PPI use on survival in cirrhotic patients admitted with HE and reported a higher 30-d mortality in the PPI group (HR = 1.360, (1.208-1.532); P < 0.001], but not in their separate study of patients with SBP[18]. These studies have not shown consistent results on the association of PPI use and mortality, which could potentially be related to issues with defining the duration of PPI exposure and the classification of PPI user status, leading to potential biases. As PPI use is prevalent particularly in patients with history of stroke or myocardial infarction, the mortality analysis in this population should be adjusted for underlying cardiovascular disease and the use of relevant medications. Our study showed that after correcting for these different potential biases and 43 relevant confounders for mortality, decompensated cirrhotic patients with PPI use, particularly with prolonged duration, have an increased risk of mortality.
The use of PPI has been shown to induce gut dysbiosis[7,8,25], which could increase the risk of hepatic decompensation with HE and SBP[9,10]. Our study found that PPI users with decompensated cirrhosis had a higher risk of portal hypertension-related decompensations requiring hospital admission. Our study findings support the evidence from a recent study showing increased all-cause, 1-mo, and 3-mo hospital readmissions among cirrhotic patients[12].
There are several reasons that could explain higher mortality and increased occurrence of hepatic events with PPI use in patients with decompensated liver cirrhosis. First, pathological bacterial translocation increases with the severity of liver disease[26]. In decompensated cirrhosis, the secretion of antimicrobial peptides diminishes, intestinal permeability increases, and small intestinal bacterial overgrowth accelerates including enhanced transcellular epithelial crossing of viable bacteria[26], all of which lead to an increased risk of pathologic bacterial translocation. Second, gastric hydrochloric acid is bactericidal and is a defence mechanism from ingested microorganisms[27]. However, PPIs are strong gastric acid suppressants, thus limiting this defence[28]. Furthermore, in liver cirrhosis, there is reduced hepatic clearance of PPI[15], which thus increases the overall PPI exposure. Last and perhaps most importantly, PPIs also affect the gut microenvironment by modifying pH in the stomach and small intestine and is proven to cause gut dysbiosis. Dysbiosis in particular, can drive inflammasome-deficiency-associated changes through microbiome derived metabolites, which worsens hepatic inflammation and produces endotoxins that exacerbate intestinal permeability and inflammation[29,30]. These potentially explain why PPI use is a known risk factor for bacterial infections, HE, and SBP. Hence, PPI use, which diminishes the body’s natural defence from microorganisms and causes dysbiosis, in combination with increased pathological bacterial translocation in decompensated cirrhosis could increase hepatic decompensation, infection risk, and ultimately mortality in patients with advanced liver cirrhosis. In our subgroup analysis, PPI users with MELD ≥ 15 were associated with a higher mortality risk compared to non-users. This suggests that patients with advanced cirrhosis are more prone to effects from dysbiosis, infections, and hepatic decompensation. Further studies are required to see if active cessation of PPI in advanced cirrhotic patients would improve survival.
In our study, we calculated PPI exposure using cumulative defined daily doses and used fixed landmark periods to define users, past users and non-users. This method reduces biases in selecting “users”. In the landmark analysis, PPI use 3 mo prior to index admission was accounted for because PPI users with cirrhosis had increased 3-mo hospital readmission rates compared to non-users[12]. Exclusion of the group already exposed to PPI prior to decompensation would be a confounder and reduces the true effect of PPI on hepatic decompensation and mortality. Furthermore, there are significant baseline clinical characteristics, comorbidities, and concurrent medications that would be associated with hepatic decompensation, cardiovascular events, and ultimately overall mortality. Therefore, we considered PS adjustment for these variables (Supplementary Tables 1 and 2).
Our study had several limitations. First, PPI use was measured using physician prescriptions available in our electronic system. We do not have data on patient adherence to the PPI prescribed or data from private practitioners. However, only patients on follow-up at our hospital were included. Prescriptions from and admission to private hospitals were very minimal. To mitigate indication bias of PPI use, we included baseline comorbidities such as GERD, esophagitis, peptic ulcer disease, and those on anti-platelet agents such as aspirin and clopidogrel. We could not adjust for PPI use in functional dyspepsia, but this should not require long-term PPI use. There are several residual confounders that could have impacted mortality and hepatic decompensation in our study such as obesity[4], sarcopenia[31,32], and smoking[33]. We adjusted for antibiotic use but did not include rifaximin, a non-aminoglycoside semi-synthetic antibacterial, as it was only publicly funded in Singapore towards the end of our study period and hence is not yet widely available. Our study did not analyse hospital admission for other reasons without hepatic decompensations such as pneumonia, C. difficile and enteric infections, which are known associations with PPI use[14]. However, most episodes of hepatic decompensation would be triggered as a result of infections and would hence be captured in our study. We used all-cause mortality as an objective measure of primary outcome. The exact cause of death was difficult to ascertain in this retrospective study. For example, when a decompensated patient was admitted for HE and passed on after developing aspiration pneumonia and SBP, it was unclear if the cause of death was pneumonia or a liver-related death. Analysing dichotomised outcomes for liver and non-liver related deaths would then introduce ambiguity and bias. Finally, our study only analysed episodes of decompensation severe enough for hospitalisation, but not those with mild decompensated cirrhosis managed as an outpatient.
In conclusion, PPI use in patients with decompensated liver cirrhosis is associated with higher mortality and severe hepatic decompensations requiring hospital admission. Further prospective studies are required to confirm these findings and determine causality. A cumulative defined daily dose > 90 has a higher risk of mortality and PPI should be limited to a shorter duration and dosage if needed, or stopped if there is no indication.
ARTICLE HIGHLIGHTS
Research background
Proton pump inhibitor (PPI) use is associated with an increased risk of mortality but is not well studied in patients with decompensated liver cirrhosis. The impact and definition of significant dose exposure are also not known. Although previous studies have looked into this relationship, there are several unaddressed issues such as PPI users not being well defined, the presence of many confounding factors, and indications for PPIs not being adjusted for. Also, this particular patient population of decompensated cirrhotic patients has not been well studied. Our study investigated if PPI use is independently associated with increased mortality risk in decompensated liver cirrhosis after adjustment for indications, medications, baseline variables and co-morbidities, and established the impact of dose exposure on mortality.
Research motivation
PPIs are prescribed widely and for long durations even in patients with liver cirrhosis. If a convincing relationship with increased mortality risk and dose exposure is established, stopping or shortening the duration of PPIs when possible should be strongly advocated.
Research objectives
This study confirms our main objective, that PPI usage in decompensated liver cirrhosis patients is an independent factor associated with an increased risk of mortality. In addition, a longer dose exposure of more than 90 cumulative defined daily doses was found to significantly increase this risk. We hence advocate reviewing PPI use in patients with liver cirrhosis with a view to shorten or deprescribe when possible.
Research methods
This is a retrospective cohort study using a hospital database. PPI users were defined as those with more than 28 defined daily doses used within a study landmark period. Users and non-users were compared after adjusting for 43 variables including baseline characteristics, comorbidities, PPI indications, and medications.
Research results
A total of 295 patients were included for analysis in the study. PPI users had a higher mortality compared to non-users and longer PPI use with more than 90 cumulative defined daily doses was associated with higher mortality. PPI users also had a higher incidence of hospitalisation for hepatic decompensation.
Research conclusions
The impact of varying PPI dose exposure in decompensated cirrhotics has not been previously described. This study showed that a cumulative defined daily dose > 90 is associated with higher mortality in patients with decompensated liver cirrhosis. Patients with decompensated liver cirrhosis have increased intestinal permeability and decreased hepatic clearance of PPIs, which predispose to gut dysbiosis and increases the risk of severe hepatic decompensation and ultimately mortality. Higher dose exposure to PPI worsens this. PPIs can be harmful when given for long durations in patients with decompensated liver cirrhosis by increasing the risk of further decompensation and death. Longer PPI dose exposure, in particular more than 90 cumulative defined daily doses can be harmful in patients with decompensated liver cirrhosis. PPIs inhibit the bactericidal effect of gastric hydrochloric acid and predispose to gut dysbiosis. When used in patients with decompensated liver cirrhosis who have decreased hepatic clearance of PPI, there is increased dose exposure that can potentially cause more harm. PPI users were well defined in this study by using defined daily doses and a cumulative dose ≥ 28 within a landmark period. Also, users and non-users were compared after important adjustments such as indication for PPI use and medication use such as antiplatelets, which were not accounted for in prior studies. PPI use should be reviewed regularly especially in patients with liver cirrhosis. It should be stopped when there are no indications. If PPIs are indicated, dosage should be reduced to the lowest possible dose.
Research perspectives
There were potential confounding factors that could have affected the results. However, this represents real world data and the current difficulties faced. The differences were also minimised using statistical methods such as propensity adjustment or matching. Future research should be conducted to prove the mechanisms on how PPIs modulate gut microbiota causing dysbiosis and hepatic decompensations and also to determine if PPI withdrawal can reverse mortality risk. Larger cohort, prospective studies should be performed with a view on proving causality.
ACKNOWLEDGEMENTS
We would like to thank Dr. Prem Harichander Thurairajah for critically reviewing and proofreading the manuscript, and Dr. Zhenwei Teo for technical and language editing and proofreading the manuscript.
Footnotes
Institutional review board statement: This study was conducted after receiving approval from the Singhealth Centralized Institutional Review Board with waiver of informed consent.
Informed consent statement: This study was approved by the Singhealth IRB for waiver of informed consent.
Conflict-of-interest statement: All authors declare there are no conflicts of interest. None of the authors received financial support or grants for this study.
Data sharing statement: Statistical coding and dataset is available from the corresponding author at jchsiang@gmail.com.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items
Peer-review started: March 25, 2019
First decision: May 24, 2019
Article in press: July 19, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cholongitas E, Spahr L S-Editor: Ma RY L-Editor: Filipodia E-Editor: Ma YJ
Contributor Information
Marianne Anastasia De Roza, Department of Gastroenterology and Hepatology, Changi General Hospital, Singhealth 529889, Singapore.
Lim Kai, Department of Gastroenterology and Hepatology, Changi General Hospital, Singhealth 529889, Singapore.
Jia Wen Kam, Clinical Trials and Research Unit, Changi General Hospital, Singhealth 529889, Singapore.
Yiong Huak Chan, Biostatistics Unit, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 160608, Singapore.
Andrew Kwek, Department of Gastroenterology and Hepatology, Changi General Hospital, Singhealth 529889, Singapore.
Tiing Leong Ang, Department of Gastroenterology and Hepatology, Changi General Hospital, Singhealth 529889, Singapore.
John Chen Hsiang, Department of Gastroenterology and Hepatology, Changi General Hospital, Singhealth 529889, Singapore. jchsiang@gmail.com.
References
- 1.Maggio M, Corsonello A, Ceda GP, Cattabiani C, Lauretani F, Buttò V, Ferrucci L, Bandinelli S, Abbatecola AM, Spazzafumo L, Lattanzio F. Proton pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173:518–523. doi: 10.1001/jamainternmed.2013.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsiang JC, Gane EJ, Bai WW, Gerred SJ. Type 2 diabetes: a risk factor for liver mortality and complications in hepatitis B cirrhosis patients. J Gastroenterol Hepatol. 2015;30:591–599. doi: 10.1111/jgh.12790. [DOI] [PubMed] [Google Scholar]
- 3.Pang Y, Kartsonaki C, Turnbull I, Guo Y, Clarke R, Chen Y, Bragg F, Yang L, Bian Z, Millwood IY, Hao J, Han X, Zang Y, Chen J, Li L, Holmes MV, Chen Z. Diabetes, Plasma Glucose, and Incidence of Fatty Liver, Cirrhosis, and Liver Cancer: A Prospective Study of 0.5 Million People. Hepatology. 2018;68:1308–1318. doi: 10.1002/hep.30083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart CL, Morrison DS, Batty GD, Mitchell RJ, Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acharya C, Bajaj JS. Gut Microbiota and Complications of Liver Disease. Gastroenterol Clin North Am. 2017;46:155–169. doi: 10.1016/j.gtc.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj JS, Heuman DM, Hylemon PB, Sanyal AJ, White MB, Monteith P, Noble NA, Unser AB, Daita K, Fisher AR, Sikaroodi M, Gillevet PM. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imhann F, Bonder MJ, Vich Vila A, Fu J, Mujagic Z, Vork L, Tigchelaar EF, Jankipersadsing SA, Cenit MC, Harmsen HJ, Dijkstra G, Franke L, Xavier RJ, Jonkers D, Wijmenga C, Weersma RK, Zhernakova A. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedberg DE, Toussaint NC, Chen SP, Ratner AJ, Whittier S, Wang TC, Wang HH, Abrams JA. Proton Pump Inhibitors Alter Specific Taxa in the Human Gastrointestinal Microbiome: A Crossover Trial. Gastroenterology. 2015;149:883–885.e9. doi: 10.1053/j.gastro.2015.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandl K, Schnabl B. Is intestinal inflammation linking dysbiosis to gut barrier dysfunction during liver disease? Expert Rev Gastroenterol Hepatol. 2015;9:1069–1076. doi: 10.1586/17474124.2015.1057122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dam G, Vilstrup H, Watson H, Jepsen P. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology. 2016;64:1265–1272. doi: 10.1002/hep.28737. [DOI] [PubMed] [Google Scholar]
- 11.Weersink RA, Bouma M, Burger DM, Drenth JPH, Harkes-Idzinga SF, Hunfeld NGM, Metselaar HJ, Monster-Simons MH, van Putten SAW, Taxis K, Borgsteede SD. Safe use of proton pump inhibitors in patients with cirrhosis. Br J Clin Pharmacol. 2018;84:1806–1820. doi: 10.1111/bcp.13615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bajaj JS, Acharya C, Fagan A, White MB, Gavis E, Heuman DM, Hylemon PB, Fuchs M, Puri P, Schubert ML, Sanyal AJ, Sterling RK, Stravitz TR, Siddiqui MS, Luketic V, Lee H, Sikaroodi M, Gillevet PM. Proton Pump Inhibitor Initiation and Withdrawal affects Gut Microbiota and Readmission Risk in Cirrhosis. Am J Gastroenterol. 2018;113:1177–1186. doi: 10.1038/s41395-018-0085-9. [DOI] [PubMed] [Google Scholar]
- 13.Cole HL, Pennycook S, Hayes PC. The impact of proton pump inhibitor therapy on patients with liver disease. Aliment Pharmacol Ther. 2016;44:1213–1223. doi: 10.1111/apt.13827. [DOI] [PubMed] [Google Scholar]
- 14.Yang YX, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology. 2010;139:1115–1127. doi: 10.1053/j.gastro.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 15.Lodato F, Azzaroli F, Di Girolamo M, Feletti V, Cecinato P, Lisotti A, Festi D, Roda E, Mazzella G. Proton pump inhibitors in cirrhosis: tradition or evidence based practice? World J Gastroenterol. 2008;14:2980–2985. doi: 10.3748/wjg.14.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dultz G, Piiper A, Zeuzem S, Kronenberger B, Waidmann O. Proton pump inhibitor treatment is associated with the severity of liver disease and increased mortality in patients with cirrhosis. Aliment Pharmacol Ther. 2015;41:459–466. doi: 10.1111/apt.13061. [DOI] [PubMed] [Google Scholar]
- 17.Hung TH, Lee HF, Tseng CW, Tsai CC, Tsai CC. Effect of proton pump inhibitors in hospitalization on mortality of patients with hepatic encephalopathy and cirrhosis but no active gastrointestinal bleeding. Clin Res Hepatol Gastroenterol. 2018;42:353–359. doi: 10.1016/j.clinre.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Hung TH, Tseng CW, Lee HF, Tsai CC, Tsai CC. Effect of Proton Pump Inhibitors on Mortality in Patients with Cirrhosis and Spontaneous Bacterial Peritonitis. Ann Hepatol. 2018;17:933–939. doi: 10.5604/01.3001.0012.7193. [DOI] [PubMed] [Google Scholar]
- 19.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–2969. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall RJ. Assessment of exposure misclassification bias in case-control studies using validation data. J Clin Epidemiol. 1997;50:15–19. doi: 10.1016/s0895-4356(96)00315-0. [DOI] [PubMed] [Google Scholar]
- 21.European Association for the Study of the Liver. European Association for the Study of the Liver, EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Dafni U. Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4:363–371. doi: 10.1161/CIRCOUTCOMES.110.957951. [DOI] [PubMed] [Google Scholar]
- 23.WHO Collaborating Centre for Drug Statistics Methodology. 2014. Guidelines for ATC classification and DDD assignment, 2015. Oslo. Available from: http://www.whocc.no/filearchive/publications/2015_guidelines.pdf. [Google Scholar]
- 24.Xie Y, Bowe B, Li T, Xian H, Yan Y, Al-Aly Z. Risk of death among users of Proton Pump Inhibitors: a longitudinal observational cohort study of United States veterans. BMJ Open. 2017;7:e015735. doi: 10.1136/bmjopen-2016-015735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellhöner F, Döscher N, Marius V, Plumeier I, Kahl S, Potthoff A, Manns MP, Dietmar P, Wedemeyer H, Cornberg M, Heidrich B. The Impact of proton pump inhibitors on the intestinal microbiota in chronic hepatitis C patients. Journal of hepatology. doi: 10.1080/00365521.2019.1647280. [DOI] [PubMed] [Google Scholar]
- 26.Wiest R, Lawson M, Geuking M. Pathological bacterial translocation in liver cirrhosis. J Hepatol. 2014;60:197–209. doi: 10.1016/j.jhep.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 27.Freedberg DE, Kim LS, Yang YX. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice From the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Scarpignato C, Gatta L, Zullo A, Blandizzi C SIF-AIGO-FIMMG Group; Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. doi: 10.1186/s12916-016-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen P, Stärkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883–894. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061–8071. doi: 10.3748/wjg.v20.i25.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.European Association for the Study of the Liver. European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70:172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altamirano J, Bataller R. Cigarette smoking and chronic liver diseases. Gut. 2010;59:1159–1162. doi: 10.1136/gut.2008.162453. [DOI] [PubMed] [Google Scholar]