Abstract
Thirty per cent of all colorectal tumours develop in the rectum. The location of the rectum within the bony pelvis and its proximity to vital structures presents significant therapeutic challenges when considering neoadjuvant options and surgical interventions. Most patients with early rectal cancer can be adequately managed by surgery alone. However, a significant proportion of patients with rectal cancer present with locally advanced disease and will potentially benefit from down staging prior to surgery. Neoadjuvant therapy involves a variety of options including radiotherapy, chemotherapy used alone or in combination. Neoadjuvant radiotherapy in rectal cancer has been shown to be effective in reducing tumour burden in advance of curative surgery. The gold standard surgical rectal cancer management aims to achieve surgical removal of the tumour and all draining lymph nodes, within an intact mesorectal package, in order to minimise local recurrence. It is critically important that all rectal cancer cases are discussed at a multidisciplinary meeting represented by all relevant specialties. Pre-operative staging including CT thorax, abdomen, pelvis to assess for distal disease and magnetic resonance imaging to assess local involvement is essential. Staging radiology and MDT discussion are integral in identifying patients who require neoadjuvant radiotherapy. While Neoadjuvant radiotherapy is potentially beneficial it may also result in morbidity and thus should be reserved for those patients who are at a high risk of local failure, which includes patients with nodal involvement, extramural venous invasion and threatened circumferential margin. The aim of this review is to discuss the role of neoadjuvant radiotherapy in the management of rectal cancer.
Keywords: Rectal cancer, Neoadjuvant therapy, Low anterior resection syndrome, Stoma, Transanal endoscopic microsurgery, Trans-anal total mesorectal excision, Robotic surgery, Watch and wait
Core tip: Neoadjuvant radiotherapy aims to downstage tumours for a more effective oncological resection. Studies have shown that both long and short course pre-operative radiotherapy confers benefits to local recurrence. Some patients completely respond to radiotherapy and have been enrolled in surveillance programmes without undergoing surgery. It is essential to be aware of the disadvantages associated with radiotherapy. Radiation therapy increases the risk of anorectal and genitourinary dysfunction which have a deleterious impact on quality of life. Thus it is imperative to accurately identify patients who are likely to benefit from neoadjuvant radiotherapy in order to minimise morbidity and improve patient outcomes.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer diagnosed in both sexes in the Western World. In 2019 there were approximately 44180 new cases of rectal cancer diagnosed in the United States[1]. Several risk factors have been implicated in rectal tumorigenesis including genetics, age, obesity, smoking, and diet. Cancers of the rectum and rectosigmoid junction account for 30% of all CRC diagnosed. Rectal cancer is defined as tumours arising within 15 cm of the anal verge. While histologically similar to cancers occurring at other sites in the colon, rectal cancers, given the anatomical confinements of the bony pelvis, blood supply, lymphatic drainage and nervous innervation rectal cancer are considered a distinct entity, specifically in regards to the invasive growth pattern, surgical approach, and treatment outcomes[2,3]. The use of neoadjuvant chemoradiotherapy is recommended for all newly diagnosed rectal adenocarcinoma with a clinical (c) stage T3 or T4 based on transrectal endoscopic ultrasound (EUS) or pelvic magnetic resonance imaging (MRI). Neoadjuvant therapy may comprise of either radiotherapy alone or in combination with chemotherapy. Commonly prescribed chemotherapy agents include 5-Fluorouracil (5-FU) and Oxaliplatin. These agents act to limit tumour cell division in several ways. Oxaliplatin acts via the formation of DNA-platinum adducts which deprives tumour cells of the necessary building blocks for cell replication. Similarly, 5-FU prevents the formation of nucleosides essential for tumour cell division. Following the completion of neoadjuvant chemoradiotherapy, the patient proceeds to curative surgery. The overarching aim of rectal cancer management is surgical removal of the tumour and all draining lymph node basins, in an intact mesenteric package, in order to achieve an R0 resection, with negative resection margins, with the aim of reducing local recurrence rates. Radiotherapy plays an integral role, as it aids in downsizing or downstaging large tumours (cT3/T4) in the neoadjuvant setting. It is important however to note that not every patient responds favourably to radiotherapy and that treatment-related toxicity can occur, which negatively impact patients’ overall and health-related quality of life (QoL)[4]. Furthermore, neoadjuvant radiotherapy can cause excessive tissue oedema, leading to a loss of surgical planes, thereby posing an increased surgical challenge, especially in the narrow male pelvis[5].
The aim of this review is to discuss the role of radiotherapy for the management of rectal cancer in the neoadjuvant setting.
EVOLUTION OF SURGERY IN MANAGEMENT OF RECTAL CANCER
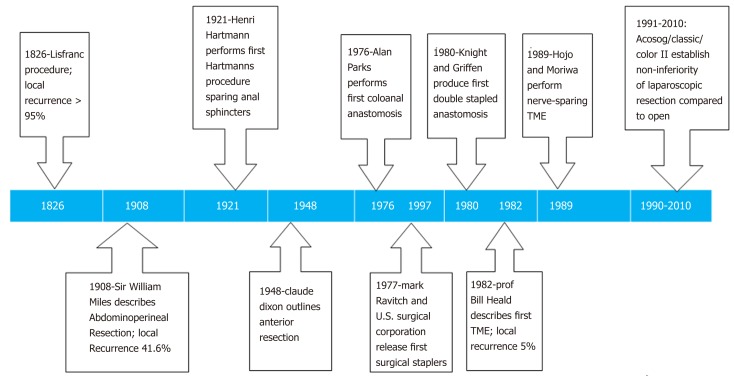
Surgery with curative intent provides the best chance of survival from rectal adenocarcinoma. Due to the challenges posed by the confinement of the bony pelvis, surgical approaches to rectal cancer have undergone several landmark technical milestones, which have lead to improved local recurrence rates and reduced overall morbidity and mortality. Historically rectal tumours were excised via a perineal approach, which was associated with poor mortality, morbidity, and local recurrence rates[6]. The first successful rectal resection was performed in 1826 by Lisfranc, where the rectum was everted and a minimal resection of the distal rectum was performed. There was no consideration for resection of the mesorectum and draining lymph nodes. As anaesthesia was still in the nascent stages, success was based primarily on patients’ survival and fitness for discharge. These procedures were principally performed with palliative intent. A review conducted by Vogel of 1500 cases performed in the 19th century found an average operative mortality rate of 20% and a local recurrence rate of 80%[6]. In 1908, the English surgeon William Ernest Miles described the first radical procedure using an abdominal and perineal approach, i.e. abdominoperineal resection (APR). This involved resection of the distal rectum and anal canal. The proximal rectum was exteriorized as an end colostomy. Miles published his case series between 1908 to 1923 and reported local recurrence in 5 patients of the 12 reported (41.6%)[7]. Miles influenced generations of future surgeons who adopted his technique. Subsequent improvements to the technique included performing a high-tie of the inferior mesenteric artery (IMA)[8]. This served to maximise lymph node yield and reduce local recurrence.
In 1938, Henri Hartmann published a case series of 38 patients with sigmoid tumours. Hartmann performed a sub-total colectomy and fashioned an end-colostomy, with oversewing of the rectal stump preserving anal anatomy. First described in 1921, the case series quoted a mortality rate of 8.8% which was a significant reduction, when compared to the 38% mortality rate associated with APR[9]. Hartmann did not advocate for the restoration of bowel continuity in his case series, as he felt the risk to the patient would be too high. This was challenged by the American surgeon Claude Dixon in 1948 when he published a series of 426 patients between 1930 and 1947 in which he performed an anterior resection. In this procedure, upper rectal tumours were resected with bowel continuity restored during the same procedure. A temporary diverting stoma may also be fashioned mitigating the clinical severity of any potential anastomotic leak. Dixon reported a mortality rate of 5.6% and a 5-year survival rate of 67.7% in 272 patients[10]. Dixon concluded that anterior resection was a safe and efficacious procedure for the treatment of upper rectal tumours.
In order to reduce local recurrence rates even further, Professor Richard J Heald developed the technique that is now known as total mesorectal excision (TME)[11]. This is a standardized and reproducible anatomical approach to pelvic dissection, which interrogates surgical planes in order to completely excise the lymphovascular fatty tissue surrounding the rectum and mesorectum under direct vision. Heald postulated that local recurrence was a result of leaving residual mesorectal tissue within the pelvis. In a case series performed at Basingstoke between 1978 and 1997, 519 patients underwent TME for rectal cancer. Neoadjuvant radiotherapy was administered to 49 of the patients in the series. The predominant surgical procedure performed was an anterior resection, although APR and Hartmann resections were also included. The findings of the case series demonstrated a 5-year cancer-specific survival rate of 68% for all patients. The local recurrence rate for curative resections, defined as disease-free proximal, distal and circumferential margins, was 3%. Local recurrence had been, on average, 20% before the publication of this study. Disease-free survival at 5 years was calculated at 80% for those patients treated with curative intent[12]. TME highlights the importance of utilizing natural anatomical planes and performing meticulous dissection during the surgical approach. TME is associated with the lowest rates of local recurrence and has become the surgical gold standard for the management of rectal cancer. Moreover, Quirke et al[13] examined 1156 surgical specimens from patients managed with TME. The authors graded the quality of the resections as Good (52%), Poor (13%) or Intermediate (38%), based on the integrity of the mesorectal envelope post-resection. The authors very elegantly demonstrated a significant direct correlation between a positive circumferential resection margin and rates of local recurrence thereby validating Heald’s embryological theory underpinning TME.
Restoration of intestinal continuity posed new challenges to rectal cancer management, principally the risk of anastomotic leakage. This feared complication occurs due to failure in the integrity of the anastomosis leading to an abnormal communication between the peritoneal cavity and the intraluminal contents of the bowel. Studies investigating anastomotic leaks have quoted incidence rates of 15%-20%[14,15]. To mitigate the severity of this event, a diverting stoma can be formed at the time of surgery. The creation of a diverting stoma does not reduce the incidence of anastomotic failure, however, it has been shown to minimize the risk of reoperation[14]. The fashioning of a stoma is not without risk. A meta-analysis comprising of 6 studies and 1063 patients demonstrated a complication rate of 18.2% for loop ileostomy and 30.6% for loop colostomy (P = 0.001)[16]. The authors found that rates of clinical dehydration (3.1% vs 0%, P = 0.13) and post-operative ileus (5.2% vs 1.7%, P = 0.02) were greater in those patients with a loop ileostomy. Emmanuel et al[14], published a study in 2018 investigating outcomes for rectal cancer patients with diverting stomas. The authors found that those with such stomas experienced a higher rate of post-operative complications (57.1% vs 34.9%, P = 0.003) and an increased average length of hospital stay (13 d vs 6.9 d, P = 0.005).
For the majority of these patients, diverting stomas are intended as a temporary measure. A prospective observational study of 275 patients with diverting stomas was published in 2017. Following an average follow-up of 4.9 years, the rate of permanent stoma formation was 16.7%[15]. A retrospective study in Sweden of 3564 patients with loop ileostomies outlined a 9-mo reversal rate of 68.4%. Risk factors for prolonged interval to reversal and for conversion to permanent stoma included, post-operative complications (HR = 0.67, 0.62-0.73), adjuvant chemotherapy (0.63, 0.57-0.69) and advanced cancer stage (Stage III 0.74, 0.66-0.83 and Stage IV 0.38, 0.32-0.46)[17] (Figure 1).
Figure 1.
Timeline of surgical innovations in the treatment of rectal cancer[81-87]. TME: Total mesorectal excision.
RADIOTHERAPY
Staging
Neoadjuvant therapy comprises a combination of radiotherapy and chemotherapy. The European Society of Medical Oncology (ESMO) recommend neoadjuvant therapy in cases of advanced disease (> T3), lymph node involvement on imaging and where the adequacy of TME surgery is in question (circumferential resection margin)[18]. The goal of neoadjuvant therapy is to downsize or downstage the tumour in anticipation of surgical resection. In instances where there is involvement of the anal sphincters, successful neoadjuvant therapy can potentially downsize a tumour, to allow for the creation of a safe resection margin thereby preserving the anal sphincters and maintaining anal continence. In certain cases, tumours may completely respond to neoadjuvant therapy. Complete Response is defined as the replacement of tumour with fibrous tissue post-radiotherapy. Analysis of the National Cancer Database in 2017 detailed a pathologic complete response (pCR) rate of 13% in an overall patient cohort of 27532[19]. The decision to treat a patient with neoadjuvant therapy is dependent on the clinical tumour stage at presentation. This entails taking a full medical history and clinical examination, including digital rectal examination (DRE), and radiological examinations. Local staging is performed through MRI of the pelvis and EUS of the rectal lesion. MRI provides detailed images of the pelvis allowing for accurate staging of the tumour and facilitating pre-operative planning. Furthermore, MRI aids in assessing the circumferential resection margin (CRM) status. In a prospective observational study of 408 patients, 87% (95%CI: 83%-90%) had clear margins on MRI. Surgical resection specimens of this cohort demonstrated clear margins in 94% (95%CI: 93%-96%). Specificity was found to be 92% (95%CI: 90%-95%)[20]. EUS is effective at measuring the depth of tumour invasion in early rectal cancers[21]. Accuracy in assessing T stage for EUS has been quoted in the range of 85%-90%[22]. Computed tomography (CT) of the Thorax, Abdomen, and Pelvis is useful for both local and distant staging. CT has an accuracy rate of 85.1%, a positive predictive value of 96.1% and a negative predictive value of 3.9% in detecting hepatic metastases[23].
Short course vs long course neoadjuvant radiotherapy
The clear advantages of neoadjuvant radiotherapy were first recognised in 1997 by the Swedish Rectal Cancer Study Group[24]. Between 1987 and 1990 1168 patients diagnosed with rectal cancer were randomly assigned to an intervention arm, i.e., patients received neoadjuvant therapy prior to surgery and a control arm defined as those patients who underwent surgery alone. The neoadjuvant regime involved 25 Gy of radiotherapy in 5 fractions over the duration of one week. These patients were operated one week after completing neoadjuvant therapy. This study found that there was a significant reduction in local recurrence rates between intervention and control (11% vs 27%, P < 0.001). The overall rate of local recurrence reduction in patients who received radiotherapy was 58% (95%CI: 46%-69%). Even though neoadjuvant therapy had no bearing on postoperative mortality the 5-year survival was significantly higher in the radiotherapy group (58% vs 48%). This landmark study was the first to demonstrate improved overall survival in those patients receiving radiotherapy prior to undergoing curative surgery.
In 2001, The Dutch Rectal Cancer Study Group performed a randomized control trial comparing the effects of pre-operative radiotherapy and TME surgery in 1861 patients[25]. The protocol for neoadjuvant therapy involved 5 Gy of radiotherapy per day for five days which was followed by TME surgery. Patients were regularly followed up every three months for one year and annually thereafter for at least two years. The overall rate of local recurrence was found to be 5.3%. The cohort treated with radiotherapy and surgery exhibited local recurrence in 2.4% of cases vs 8.2% in the surgery only group (P < 0.001). Unlike the Swedish trial, however, there was no difference in overall survival between the two study arms.
Sebag-Montefiore et al[26] performed a multicentre, randomised, control trial comparing preoperative radiotherapy vs selective postoperative chemoradiotherapy in patients with rectal cancer. This study encompassed 80 centres spanning four countries. A total of 1350 patients with locally advanced adenocarcinoma of the rectum were randomly assigned to a short-course preoperative radiotherapy (25 Gy in five fractions; n = 674) arm vs surgery with selective postoperative chemoradiotherapy (45 Gy in 25 fractions with concurrent 5-FU) arm, restricted to patients with a positive circumferential resection margin (n = 676). The primary outcome was local recurrence and the median follow-up was 4 years. Ninety-nine patients had developed a local recurrence (27 in the preoperative radiotherapy group vs 72 in the selective postoperative chemoradiotherapy cohort). The authors noted a reduction of 61% in the relative risk of local recurrence for patients receiving preoperative radiotherapy (95%CI: 0.27-0.58, P < 0.0001), and an absolute difference at 3-years of 6.2% (95%CI: 5.3-7.1). Moreover, there was a relative improvement in disease-free survival of 24% in patients who received preoperative radiotherapy (HR = 0.76, 95%CI: 0.62-0.94, P = 0.013), and an absolute difference at 3-years of 6.0% (95%CI: 5.3-6.8) (77.5% vs 71.5%). Overall survival did not differ between the groups (HR 0.91, 95%CI: 0.73-1.13, P = 0.40). The authors were able to demonstrate an overall relative risk reduction of 61% in local recurrence for patients receiving neoadjuvant therapy. The rate of anastomotic leak in anterior resection patients was similar at one month (9% pre-op radiotherapy vs 7% post-op chemotherapy). Patients undergoing pre-operative radiotherapy were more likely to have poor perineal wound healing post-APR (35% vs 22%). Rates of CRM involvement were also similar between groups (10% vs 12%). Taken with results from other randomised trials, the MRC CR-07 findings provided convincing and consistent evidence that short-course preoperative radiotherapy is an effective treatment option for patients with locally advanced rectal cancer.
In 2004 Sauer et al[27] demonstrated favourable outcomes in relation to long-course combination therapy of chemotherapy and radiotherapy in the neoadjuvant setting (nCRT) for the management of rectal cancer. A total of 823 patients with T3/T4 rectal adenocarcinoma were randomised to either a neoadjuvant long course chemoradiotherapy arm or an adjuvant chemoradiotherapy arm. Neoadjuvant therapy involved 28 fractions totalling 50.4 Gy. This was supplemented with Fluorouracil (5-FU) infusions at weeks one and five. Surgery was performed 6-wk followed by four cycles of 5-FU at one month post-operatively. Adjuvant patients underwent the same adjuvant regimen except for the addition of 540-cGy boost of radiation. The results confirmed an improvement in 5-year local recurrence rates for the pre-operative treatment (13% vs 6%) arm. Moreover, 5-year survival rates between the two arms were not dissimilar (76% vs 74%, P = 0.8). Overall morbidity rates were 36% in the pre-operative arm and 34% in the post-operative arm (P = 0.68). Incidence of anastomotic leak (11% vs 12%, P = 0.77), post-operative ileus (2% vs 1%, P = 0.26), post-operative bleeding (3% vs 2%, P = 0.5) and sacral wound healing (10% vs 8%, P = 0.1) demonstrated no significant difference. This study utilised not only long-course neoadjuvant therapy but also combined chemoradiotherapy in the neoadjuvant phase. The benefits of combined chemoradiotherapy had been previously described by Fryckholm et al[28] in 2001. In this study, 70 patients were divided into a combined therapy group and a radiotherapy monotherapy group. Both groups underwent surgery within 3-4 wk after completing neoadjuvant therapy. Combined therapy consisted of 40Gy of radiotherapy over 7 wk with weekly infusions of chemotherapy. The authors concluded that treatment with combined therapy resulted in improved local control. Post radical resection surgery, local recurrence rates were 4% and 35% for the combined group compared to the radiotherapy alone group respectively (P = 0.02). Even with this regimen, no significant difference was appreciated in 5-year survival between the two cohorts. The combined cohort had a five-year survival rate of 29% with the radiotherapy group at 18% (P = 0.3).
A recent meta-analysis comparing short-course with long-course preoperative neoadjuvant therapy for rectal cancer included eight robust studies[29]. The qualifying studies included a total of 1475 patients (short treatment: n = 665; long treatment: n = 810). No significant difference was detected in each outcome between the short- and long-course preoperative treatments. Interestingly, subgroup analysis indicated that the outcome of distant metastasis was significantly higher in long-course radiotherapy, compared with short-course radiotherapy (OR = 2.65, 95%CI: 1.05-6.68).
Total neoadjuvant therapy
Intensified treatment has been proposed, in certain cases, for patients who present with advanced local disease or those who are partial responders to neoadjuvant radiation. Studies have investigated whether the addition of further cycles of chemotherapy in the neoadjuvant phase, known as total neoadjuvant therapy (TNT), had any impact on response rates or long-term outcomes such as local recurrence and survival.
The GCR-3 trial was a Phase II randomised controlled trial incorporating 108 patients that were randomised to either receive neoadjuvant chemoradiotherapy and 4 cycles of adjuvant capecitabine/oxaliplatin (CAPOX) chemotherapy or receive 4 cycles of CAPOX in conjunction with radiation in the neoadjuvant phase. Both groups demonstrated similar pCR rates (13% vs 14%), 5- year overall survival (62% vs 64%) and 5-year disease free survival (77% vs 74%). Median follow-up was 69.5 months. The authors noted a significant reduction in the incidence of treatment toxicity (19% vs 54%, P = 0.004) and increased rate of therapy completion (91% vs 51%, P < 0.0001) in the TNT cohort[30].
INTERVAL TO SURGERY
To date, there is no consensus regarding the interval between the end of neoadjuvant chemoradiotherapy and time to surgery. In 1999, the Lyon R90-01 trial aimed to identify any benefits between short intervals to surgery (< 2 wk) and long intervals to surgery (6-8 wk) in 201 patients[31]. The trial demonstrated that a long interval was associated with a greater treatment response rate (53.1% vs 71.7%, P = 0.007). Furthermore, the long interval cohort had increased rates of downstaging relative to the short interval cohort (26% vs 10.3%, P = 0.0054). Patients were routinely followed up twice a year for 5 years. The median follow-up was 33.5 mo (range, 1-79 mo) The overall local recurrence rate was 9%. Both study arms had similar rates of local recurrence. There was no significant difference in overall survival between both study arms. The 3-year survival was 78% and 73% for the short interval and long interval group respectively. In 2016, patient outcomes in this cohort were reanalyzed post follow-up of 15 years[32]. The long interval group demonstrated superior pathological response rates (26% vs 10.3%, P = 0.015). Pathological response was related with improved survival outcomes for patients (P-0.0048). No differences were noted between both study arms in relation to local recurrence or survival. Of note, the majority of local recurrences presented within 5 years of treatment (96%). In 2017, the Stockholm III trial results were published in the Lancet[33]. This multicentre, randomised, non-blinded, non-inferiority trial aimed to determine the optimal interval to surgery between neoadjuvant therapy and upfront surgery in 840 patients. Furthermore, the study also sought to determine whether the short course or long course neoadjuvant therapy had a stronger impact on local recurrence. The first study arm received 5 fractions of 5Gy radiation followed by surgery within one week, i.e., the short course group. The second study arm received a similar dose of radiation with surgery performed between 4-8 wk, the delayed short course group. The final study group underwent 25 fractions of 2 Gy radiation with surgery carried out after 4-8 wk i.e., the delayed long course radiotherapy arm. The study demonstrated no significant difference in local recurrence between the three study arms. Interestingly there was an increased rate of post-operative complications in the short course cohort when compared to the delayed short course group (53% vs 41%, P = 0.001) in a pooled analysis. The overall complication rate was 50% for the Short Course Group, 38% for the Short Course Delayed Group and 39% for the Long Course Group. Patients who received short-course therapy had a reoperation rate of 11% vs 7% for the other intervention arms. Surgical complications occurred in 31% of short course patients with a rate of 26% and 23% for the short course delayed and long course groups, respectively. Surgical complications were defined as surgical site infections (SSI), post-operative bleeding, anastomotic leak, wound dehiscence, etc.
A comprehensive meta-analysis and systematic review was conducted by Donlin Du et al[34] in 2018. This review sought to determine if an extended interval to surgery (≥ 8 wk) influenced patient outcomes, in particular, pathological complete response (pCR) rates (defined as the replacement of tumour cells with fibrous tissue on a resected pathological specimen after neoadjuvant therapy). Thirteen studies involving 19652 patients were included. The meta-analysis demonstrated that pCR was significantly increased in patients with locally advanced rectal cancer and a waiting interval of ≥ 8 wk between preoperative nCRT and surgery compared to a waiting interval of < 8 wk, or a waiting interval of > 8 wk compared to ≤ 8 wk (risk ratio ¼ 1.25; 95%CI: 1.16-1.35; P < 0.0001). There were no significant differences in overall survival, disease-free survival, operative time, or incidence of local recurrence, postoperative complications, or sphincter-preserving surgery. This study revealed that performing surgery after a waiting interval of 8 wk after the end of preoperative nCRT is safe and efficacious for patients with locally advanced rectal cancer, significantly improving pCR without increasing operative time or incidence of postoperative complications when compared to a waiting interval of 8 wk.
Moreover in 2018 Kim et al[35] analysed outcomes for rectal cancer patients who received differing intervals to surgery after completion of neoadjuvant therapy. The primary outcomes measured were pCR and tumour downstaging. Overall 249 patients with differing intervals to surgery were included. The majority (45.4%) underwent surgery within 7 to 9 wk. The shortest time to surgery was within 5 wk whereas some patients’ surgery was performed over 11 wk after neoadjuvant therapy was completed. The authors noted a higher rate of pCR in the 9 to 11-wk interval with a pCR of 8.6% (P = 0.886). Downstaging occurred most frequently in the 7 to 9-wk cohort with a downstaging rate of 52.9% (P = 0.087).
A meta-analysis incorporating 3584 patients examined the correlations between interval to surgery and the rate of pCR[36]. The control for this study was patients treated with surgery 6 to 8 wk after neoadjuvant therapy. There was a higher rate of pCR in patients operated on after 8 wk (P < 0.0001). The rates of pCR were found to increase from 13.7% to 19.5%. Other patient outcomes such as survival, local recurrence, and post-operative complication rates were similar between both groups.
A further multicentre study investigated outcomes for rectal cancer patients treated with surgery over 12 wk after completing neoadjuvant therapy[37]. Seventy-six patients were enrolled in the long interval group, with 48 patients undergoing surgery within 12 wk. There was no statistically significant difference between both groups regarding post-operative complications (P = 0.547), readmission rates post-operatively (P = 0.183) and 30-d mortality (0.148). Histopathological analysis of the resected surgical specimens demonstrated a pCR rate of 8.3% for those undergoing surgery within 12 wk and 15.8% in those with an extended interval to surgery (P = 0.28). Similarly, there were no significant differences found regarding morbidity and mortality in either group.
Overall, debate still continues as to the benefit of long vs short interval to surgery post neoadjuvant therapy. Patients who undergo prompt resection post neoadjuvant therapy (< 6 wk) have a shorter duration of treatment yet are at a higher risk of post-operative complication and downstaging of the tumour. Alternatively, patients with prolonged interval to surgery (> 8 wk) have a reduced rate of post-operative complications with a higher incidence of treatment response and downstaging. If rectal preservation is the aim of treatment, then long-course radiotherapy is essential (Table 1).
Table 1.
Impact of radiotherapy on local recurrence and survival
| Study | n | Interventions | Local recurrence | Overall survival | 5-yr disease free survival |
| Swedish Rectal Cancer Trial, NEJM, 1997[24] | 1168 | 25 Gy in 5 fractions in one week surgery | 27% 11% (P ≤ 0.001) | 58% 48% (P = 0.004) | 74% 65% (after nine years) (P = 0.002) |
| Dutch Rectal Cancer Trial, NEJM, 2001[25] | 1861 | 25 Gy in one week TME surgery | 2.4% 8.2% (P ≤ 0.001) | 82% 81.8% (P = 0.2) | N/A |
| MRC CR-07, Lancet, 2009[26] | 1350 | 25 Gy in one week TME surgery and adjuvant therapy | 27 (674) = 4% 72 (676) = 10.7% | 70.3% 67.9% (P = 0.4) | 73.6% 66.7% (P = 0.013) |
| Sauer et al[27], NEJM, 2004 | 850 | 50.4 Gy over 5 wk with 5-FU TME surgery | 6% 13% | 76% 74% | 68% 65% |
| Fryckholm et al[28], 2001 | 70 | 40 Gy and 5-FU 40 Gy | 4% 35% (P = 0.02) | 66% 38% (P = 0.03) | 29% 18% (P = 0.3) |
| Stockholm III trial, 2017[33] | 840 | Short course Short course w/ delay Long course w/ delay | 2.24% 2.8% 5.5% | 73% 76% 78% | 65% 64% 65% |
| Bujko et al[88], 2016 | 515 | 5 × 5 Gy and FOLFOX 50.4 Gy in 28 fractions w/ 5-FU | 22% 21% (P = 0.82) | 73% 64.5% (P = 0.055) | 53% 52% (P = 0.74) |
| Trans-Tasman Oncology Group, 2012[89] | 326 | 5 × 5 Gy in 1 wk 50.4 Gy in 5 wk | 7.5% 4.4% (P = 0.24) | 74% 70% (P = 0.62) | N/A |
| Wawok et al[90], 2018 | 51 | 5 × 5 Gy 50.4 Gy w/5-FU | 35% 5% (P = 0.036) | 47% 86% (P = 0.009) | N/A |
| German CAO/ARO/AIO-04 study, 2012[91] | 1236 | 50.4 Gy w/ 5-FU (Control) 50.4 Gy w/5-FU and Oxaliplatin | 4.6% 2.9% | 88% 88.7% | 71.2% 75.9% |
TME: Total mesorectal excision; FU: Fluorouracil; FOLFOX: Folinic Acid, Fluorouracil, Oxaliplatin; 5-FU: 5-Fluorouracil.
COMPLICATIONS OF RADIOTHERAPY
The introduction of neoadjuvant radiotherapy to the management of rectal cancer has resulted in improved outcomes for patients. This has now been demonstrated by multiple studies, with all reporting reduced rates of local recurrence. It has been suggested that patients who receive a complete pathological response to radiotherapy could potentially avoid surgery and the morbidities associated with surgery or at the very least the adjuvant chemotherapy limb of the current neoadjuvant protocols. The survival outcome data from these studies are ambiguous, however. The potential benefit of radiotherapy in treating a rectal tumour must also be balanced against the risk of patients developing serious side effects secondary to radiation exposure. Numerous side effects, complications, and toxicities from radiotherapy have been reported, ranging from immediate complications such as wound dehiscence, surgical site infection and anastomotic leak to long-term functional disorders such as low anterior resection syndrome (LARS) and genitourinary dysfunction.
Radiotherapy toxicity
Radiation toxicity has been recognised since the discovery of radiation in the early 20th century. Symptoms of toxicity are manifold and of variable severity. In order to accurately quantify and measure such adverse events, a grading system was devised by the Radiation Therapy Oncology Group (RTOG) and the European Organisation for the Research and Treatment of Cancer (EORTC). This grading system is specific to each system or organ exposed to radiation (Table 2).
Table 2.
RTOG/EORTC radiation toxicity grading system for lower gastrointestinal tract
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Early radiation toxicity (< 6 mo post radiotherapy) | Increased frequency of bowel movements not requiring medical therapy | Increased frequency of bowel movements requiring medication or causing abdominal pain | Diarrhoea requiring IV treatment, mucous or bloody discharge PR, abdominal distention | Acute/subacute bowel obstruction, fistula formation, GI bleed requiring transfusion, abdominal pain requiring tube decompression |
| Late radiation toxicity (> 6 mo post radiotherapy) | Bowel movements of 5 per day, mild abdominal cramping, mild PR bleeding | Bowel movements > 5 per day, increased mucous PR, intermittent PR bleeding | Obstruction or bleeding requiring operative management | Necrosis, perforation, fistula formation |
PR: Per rectum; IV: Intravenous; GI: Gastrointestinal.
In 2004 Sauer et al[27] recorded all incidences of Grades 3 and 4 toxicity in their patient cohort. In the acute phase, 27% of neoadjuvant patients experienced Grade 3-4 toxicity with 12% of neoadjuvant patients reporting diarrhoea. Long-term data on the same cohort demonstrated an incidence rate of 14% for Grade 3-4 toxicity. This included 4% of neoadjuvant patients developing a stricture at their anastomosis site. Of note, the incidence of toxicity was greater in the adjuvant cohort (40% in acute vs 24% in long-term).
The Stockholm III trial reported on the frequency of post-operative complications and found that the rate of complications was similar overall between patients who received long-course therapy and those who received a short course[33]. The authors did note, that in a pooled analysis, there was an increased risk of post-operative complications in the cohort of patients who received short-course radiotherapy without a delay to surgery (53% vs 44%, P = 0.001).
Differences in immediate post-operative outcomes between short course and long course neoadjuvant patients were analysed by the Trans-Tasman Oncology Group in 2017[38]. The findings of this study indicated increased rates of Grade 3 events in patients who underwent short-course radiotherapy. These adverse events included proctitis (0% vs 3.7%, P = 0.016) and diarrhoea (1.3% vs 14.2%, P < 0.001). Conversely, patients who were administered radiotherapy over a longer course were at higher risk of developing an anastomotic leak (7.1% vs 3.5%) and perineal wound breakdown (50% vs 38.3%), however, neither of these were found to be statistically significant.
Anorectal dysfunction and LARS
As noted in the Sauer and Trans-Tasman studies above[27,38], one of the most frequent and often most distressing side effects of radiotherapy for patients was diarrhoea. Patients who receive neoadjuvant treatment and undergo anterior resection for distal rectal tumours are at risk of developing LARS. LARS can present with a myriad of symptoms including faecal incontinence, faecal urgency and abdominal bloating. The prevalence of LARS was found to be 42%[39]. The pathophysiology of this syndrome is attributed to impaired function of the anal sphincters, colonic dysmotility, and dysfunction of the neorectal reservoir. The causes of this condition are thought to be secondary to physical and neural factors. It is postulated that a reduction in the volume of rectum post-resection contributes to reduced colonic transit times and therefore increased the frequency of bowel motions. A systematic review in 2008 investigated bowel function outcomes after alternative rectal reconstructive techniques. Only two studies included in this review investigated long-term bowel function outcomes in patients post rectal surgery. The authors concluded that patients who received a Colonic J Pouch (CJP) demonstrated better outcomes in bowel function than their counterparts who received a Straight Coloanal Anastomosis (SCA) (P < 0.05[40], P < 0.001)[41]. The authors noted, however, that these benefits were only apparent for the first 18 mo post-operatively[42].
Neural factors also play a significant role in the development of LARS. Neural dysfunction can occur post-treatment either as a result of denervation post-surgery or as a consequence of radiotherapy. In a cross-sectional study on rectal cancer patients published in 2013, 41% of the total patient cohort of 938 experienced LARS[43]. The authors observed that those who received neoadjuvant therapy (long and short course) and TME surgery demonstrated an increased risk of developing LARS.
In a 14-year follow up study of patients enrolled in the Dutch Rectal Cancer Trial, the authors observed a 46% incidence of LARS in the 242 patients who responded to questionnaires[44]. Neoadjuvant radiotherapy and age < 75 years were found to be significant risk factors. Furthermore, LARS was also associated with a reduction in Health-Related Quality of Life (HRQOL). In a recent study by Kupsch et al[45,46], reported a significant reduction in HRQOL scores for patients reporting major LARS using the standardised EORCT-30 and CR38 questionnaire. Patients with major LARS scored 56 ± 19 compared to minor/no LARS who scored 67 ± 20 (P < 0.001).
Genitourinary dysfunction
Urinary and sexual dysfunction post-treatment for rectal cancer can be very distressing for patients and greatly impacts on their HRQOL. Dysfunction is secondary to autonomic nerve damage during surgery. The principal autonomic nerves damaged are the superior and inferior hypogastric plexus, the nervi erigenti and pudendal nerves. Nerve damage is attributed to several factors, including pre-operative radiotherapy resulting in inflammation of the local tissues. This makes delineating surgical planes difficult at the time of surgery. A retrospective study of 288 rectal cancer patients treated laparoscopically was conducted in 2017 in order to determine risk factors for prolonged pelvic pain post-treatment. Multivariate analysis demonstrated that extended operating time (P < 0.001) and resection margins in proximity to the anal verge (P < 0.001) were independent risk factors for prolonged pelvic pain[47]. Patients with distal tumours are also more likely to suffer some degree of genitourinary dysfunction post-operatively as the autonomic nerves are in close proximity to the rectum.
In a study by Hendren et al[48], questionnaires were sent to living rectal cancer patients who had been treated at Mount Sinai Hospital, Toronto, Canada between 1980 and 2003. The study found that 29% of women and 45% of men experienced some degree of sexual dysfunction after treatment. The authors described how radiation therapy had a strong association (P = 0.0001). The type of surgical procedure was also related to worse outcomes (P = 0.005) with most patients treated with APR reporting sexual dysfunction. Moreover, an observational retrospective study performed by Costa et al[49] in 2018 found the presence of a stoma post-operatively to be associated with sexual dysfunction. Attaallah et al[50] compared rates of sexual dysfunction in patients treated with laparoscopic TME and those treated with open TME in 187 patients and reported reduced rates of dysfunction in the laparoscopic arm compared to open. The authors noted that post-operative radiotherapy and chemotherapy was associated with male sexual dysfunction only on univariate analysis (P = 0.003, P = 0.03) however failed to maintain significance on multivariate analysis (P = 0.112, P = 0.818).
Urinary dysfunction encompasses a constellation of symptoms including urinary incontinence, difficulty in initiating micturition, and urinary retention. Similar to sexual dysfunction, urinary dysfunction most commonly occurs after neoadjuvant radiotherapy and surgery for distal tumours. A retrospective observational study in Sweden found that 36% of men and 57% of women reported urinary incontinence 3 years after undergoing APR[51].
Pelvic fractures
Insufficiency fractures in the pelvis are an underreported adverse event secondary to neoadjuvant therapy for rectal cancer. Stress fractures are commonly due to loss of mineralisation in the bone itself. This process is accentuated by radiotherapy which serves to exacerbate osteopenia via small vessel ischaemia in the bone[52].
A prospective case-control study involving 403 rectal cancer patients was published in 2018[53]. These patients underwent MRI pelvis imaging 3 years post resection of their rectal tumour to assess for local recurrence and the presence of pelvic insufficiency fractures. Fractures were identified in 49 patients with 39 of these patients having received neoadjuvant treatment (P < 0.001). Multivariate analysis demonstrated pre-operative CRT (OR: 14.2, 6.1-33.1), female gender (OR: 3.52, 1.7-7.5) and age over 65 (OR: 3.2, 1.5-6.9) to be significantly associated with the development of a pelvic fracture. Moreover, a retrospective review of 492 rectal cancer patients who received adjuvant radiotherapy was conducted with a median follow-up of 3.5 years[54]. The incidence of sacral fracture in this cohort was 7.1% and identified osteoporosis as a risk factor for the development of a sacral fracture (HR: 3.23, 1.23-8.5).
WATCH AND WAIT IN CLINICAL COMPLETE RESPONDERS
In those patients who receive neoadjuvant radiotherapy, there is a small cohort that has been shown to develop a complete pathological response. This occurs when the tumour cells are completely replaced with fibrous tissue. The relative extent of tumour response is objectively measured using the Mandard Tumour Regression Grade (TRG). Patients may also develop a Complete Clinical Response (cCR). cCR is defined in accordance with the Response Evaluation Criteria of Solid Tumours (RECIST)[55]. This defines cCR as the absence of tumour on clinical examination and endoscopy at least 4 wk after completion of neoadjuvant therapy. In 1998, Habr Gama et al[56] proposed that those patients who demonstrate a (cCR) to neoadjuvant radiotherapy could be managed by observation alone. When investigating the outcomes of combined neoadjuvant chemoradiotherapy on 118 patients, it was found that 30.5% exhibited a (cCR) after a follow-up of approximately 36 mo. Furthermore, 26.2% of patients did not require surgical management and 38.1% underwent sphincter-sparing management after diagnosis of low rectal cancer. In 2004, Habr-Gama published a controlled trial where complete clinical responders were followed up by surveillance and incomplete responders proceeded to surgery. The surveillance protocol consisted of monthly clinical examinations (including digital rectal examination), CEA levels and proctoscopy. Chest X-Rays in addition to CT imaging of the abdomen and pelvis were performed every 6 mo for the first year. Clinical follow-up frequency was increased to between two and six monthly visits after year one of surveillance. The long-term results of this study demonstrated local recurrence in 2 (cCR) patients (n = 99). Both patients underwent successful treatment with comparable survival outcomes to the incomplete responder group. It was noted that recurrence tended to occur after approximately 4-5 years indicating the need for prolonged surveillance. Distant recurrence was found to be higher in the surgery cohort (12.5% vs 6%). Finally, disease-specific mortality was found to be 8% in the surveillance group and 17% in the surgery cohort[57].
Long-term outcomes of watch and wait patients from multiple countries contributing to the International Watch and Wait Database (IWWD) were assessed in 2018[58]. Each patient included in the study had received neoadjuvant radiotherapy and were enrolled in frequent surveillance programmes. A total of 880 patients were included from 47 centres across 15 countries, 87% of which exhibited clinical complete response (cCR). Two-year cumulative rates of local regrowth were noted in 25.2%. Five-year overall survival was 85% with 5-year disease-free survival of 94%. The OnCore Project, published in 2016, was a propensity score-matched cohort analysis study[59]. Each patient underwent long course chemoradiotherapy. Patients who demonstrated (cCR) were offered surgery or surveillance. Overall, 129 patients were observed. Thirty-one patients were prospectively recruited with the remaining data obtained from a retrospective database of surveillance patients. The authors found that 34% of surveillance patients developed local regrowth with 88% requiring salvage surgery. There was no significant difference in 3-year overall survival in the matched analysis of the resection group and surveillance group (96% vs 87%, P = 0.024).
Innovative methods of delivering radiotherapy have demonstrated encouraging results in cCR rates of rectal cancer patients. An example of such a method is endocavitary irradiation. This involves the application of X-Ray radiation directly to the primary tumour, via a proctoscope, in addition to standard external beam radiotherapy (EBRT). In 1994, Gerard et al[60], published the results of a study investigating the outcomes of 414 patients with T2/T3 rectal cancers treated with this method. This technique resulted in a 91% local control rate in patients who did not undergo surgery with 90% local control in patients who went on to have curative surgery. The authors noted that 60% of patients with low/middle rectal tumours progressed to sphincter-sparing surgery. These results were replicated in a retrospective 1996 study where 25 patients long-term outcomes were assessed[61]. Within this cohort, 20 patients were managed with curative intent with the remaining 5 patients palliative cases. Local control was accomplished in 18 of the 20 curative patients and in 4 of the 5 palliative patients. In the curative study arm, 5-year local control was quoted at 89% with a 5-year survival rate of 76%.
The benefits of endocavitary radiation were confirmed in a Phase III randomised controlled trial in 2004[62]. Patients (n = 88) with low rectal tumours were randomised into receiving EBRT (39 Gy over 17 d) or EBRT with Contact X-Ray Radiotherapy boost (CXRT) of 85Gy in three fractions. Complete clinical response was greatly increased in patients who received endocavitary treatment compared to EBRT alone (24% vs 2%). There was also an increase in the rate of sphincter preserving surgeries performed on patients post endocavitary treatment (76% vs 44%, P = 0.004). These patients were followed up after a median follow-up of 132 mo[63] .
Local recurrence was lower in the CXRT group compared to EBRT (10% vs 15%, P = 0.69). Overall survival was similar between both study arms (53% vs 54%). Clinical response data demonstrated that a greater proportion of CXRT patients remained in a state of cCR after 10 years compared to EBRT (11 patients vs 1 patient). These studies highlighted the association between endocavitary radiation and cCR in patients with rectal cancer (Table 3).
Table 3.
Studies on watch and wait outcomes, n (%)
| Study | n | NA regime | Recurrence | Salvage therapy | Survival post salvage therapy | Survival |
| Habr-Gama et al[57], 2004 | 71 | Long-course radiotherapy w/ 5-FU | Local:2 Distant: 3 | 2 (100) | 100% | OS: 100% DFS: 92% |
| Habr-Gama et al[92], 2014 | 90 | Long course radiotherapy w/ 5-FU | Local: 28 (31%) | 26 (92.8) | OS: 94% | OS: 91% DFS: 68% |
| OnCore Project, 2016[59] | 129 | 45 Gy w/ 5-FU | Local: 44 (34%) | 36 (88) | N/A | OS: 96% at 3 yr DFS: 88% at 3 yr |
| IWWD Consortium, 2015[58] | 880 | Chemoradiotherapy: 91% | Local: 25.2% | 141 (69) | OS: 75.4% DFS: 84% | OS: 85% DFS: 94% |
| Appelt et al[93], 2015 | 40 | Chemoradiotherapy | Local: 25.9% at 2 yr | 9 | OS: 100% at 2 yr DFS: 100% at 2 yr | OS: 100% at 2 years DFS: 70% at 2 years |
| Smith et al[94], 2012 | 32 | Long-course chemoradiotherapy | Local: 6 (18.75) | 6 (100) | OS: 100% at 17 mo | OS: 96% DFS: 88% all at 17 mo |
| Smith et al[95], 2019 | 113 | Local: 22 (19.5) | 22 (100) | DFS: 91% | OS: 73% DFS: 75% | |
| Martens et al[96], 2016 | 100 | Long-Course: 95% Short Course: 5% | Local: 15% Distant: 5% | 13 | OS: 92.3% | OS: 96.6% DFS: 80.6% all after 3 yr |
| Lai et al[97], 2016 | 18 | Chemoradiotherapy | Local: 2 | 2 | 100% | OS: 100% |
| Rijkmans et al[98], 2017 | 38 | External beam radiotherapy and brachytherapy (iridium) | DFS: 42% OS: 63% | |||
| Vuong et al[99], 2007 | 100 | External beam radiotherapy with brachytherapy (iridium) | Local recurrence at 5 yr: 5% | DFS: 65% OS: 70% | ||
| Gerard et al[100], 2019 | 74 | Contact X-ray brachytherapy | 10% at 3 yr | 2 | DFS: 88% | |
| Sun Myint et al[101], 2018 | 83 | Contact X-ray brachytherapy | 13.2% after 2.5 yr (n = 7) | 6 | DFS: 83.1% | |
| Ortholan et al[63], 2012 | 45 | External beam radiotherapy with contact X-ray boost | DFS: 53% OS: 55% |
DFS: Disease-free survival; OS: Overall survival.
Minimally invasive surgery
While radical resection provides the best chance for definitive management for rectal cancer it may also carry a high risk of poor functional outcome and quality of life for the patient. This is particularly pertinent for those rectal cancer patients diagnosed with early-stage disease (cT1-T2). New surgical techniques and surgical tools have been developed which aim to adequately resect and treat early rectal cancers whilst minimising the risk of poor functional outcomes post-operatively. Traditional transanal excision (TAE) is utilized for tumours that measure less than 3 cm or equal to 2 cm in diameter and located within 6-8 cm from the anal verge. It entails accessing the rectal lesion via the anal canal utilizing specialized laparoscopic equipment. Difficulties with resecting early rectal tumours via TAE have been noted in the literature[64,65]. TAE is only suitable for resection of distal tumours as access to proximal rectal lesions is limited. Precision of TAE is reduced, thereby, increasing rates of tumour fragmentation during resection. Tumour fragmentation during surgery increases the risk of incomplete resection and consequently local recurrence.
In 1983 Professor Gerhard Buess described transanal endoscopic microsurgery (TEMS) for resecting low rectal lesions[66]. The specialised equipment required for this procedure allows access to tumours up to 24 cm from the anal verge, greater precision in tumour resection and a magnified 3D view of the rectum. An endoscope is inserted in the anal canal to the level of the rectal lesion. This lesion is subsequently resected via electrocautery. In a single centre retrospective review, 92 TEMS patients were followed up for approximately 5 years[67]. The study detailed a post-operative complication rate of 10.9%, the most common being urinary retention and bleeding (both 4.3%). The overall recurrence rate stood at 6.7% with disease-free survival of 98.6% and overall survival of 89.4%[67].
Promising patient outcomes have been reported in those treated with neoadjuvant chemoradiotherapy preceding TEMS. The CARTS study (Chemoradiation Therapy for Rectal Cancer in the Distal Rectum followed by organ-sparing Transanal Endoscopic Microsurgery) followed neoadjuvant patients treated with TEMS for an average of 4.5 years[68]. Of the 55 patients enrolled in the study, 35 (74%) underwent TEMS with 16 patients receiving TME surgery. Local recurrence at 5 years was 7.7% with an overall survival of 82.8% and disease-free survival of 81.6%. The authors found that TEMS patients were more likely to gain improved QoL post-operatively. However, 78% of TEMS patients did report a degree of LARS in the aftermath of their procedure (50% major LARS, 28% minor LARS).
The outcomes of TEMS in incomplete responders to neoadjuvant therapy has also been studied. In a prospective single centre study, 53 patients who were restaged as T1-T2 after completing neoadjuvant therapy were offered TEMS. This cohort of patients was found to have a 3-year local recurrence rate of 23% (n = 12). Nine of these patients exhibited local recurrence and 8 were subsequently managed with salvage therapy[69] (Table 4).
Table 4.
Outcomes in transanal endoscopic microsurgery
| Study | n | Post-op complications | Local recurrence | Survival |
| Lee et al[71], 2017 | 247 | 11% | 7% | DFS: 80% |
| CARTS study, Stijns et al[68], 2019 | 47 | N/A | 7.7% | DFS: 81.6% OS: 82.8% |
| O’Neill et al[67], 2017 | 92 | 10.9% | 6.7% | DFS: 98.6% OS: 89.4% (after 3 yr) |
| Jeong et al[102], 2009 | 45 | 0 | 15.5% | DFS: 88.5% OS: 96.2% |
| Stipa et al[103], 2012 | 86 (T1 patients) | N/A | 11.6% (for T1 tumours) | OS: 92% (for T1 patients) |
| Baatrup et al[104],2009 | 143 | N/A | 18% | DFS: 87% OS: 66% |
| Van Den Eynde, 2019[105] | 53 | 40% | N/A | N/A |
DFS: Disease-free survival; OS: Overall survival.
The primary disadvantages of the TEMS procedure include the high cost of specialised equipment, in addition to the risk of anorectal dysfunction as outlined above. To mitigate this, a novel hybrid between single-port laparoscopy and TEM for transanal excision was introduced. Transanal minimally invasive surgery (TAMIS) involves access to the rectum via a single multichannel port with the use of ordinary laparoscopic instruments. In the original case series describing TAMIS, in 6 patients, with an average tumour location at 9.3 cm from the anal verge, were recruited[70]. When compared to TEM, the operative time for TAMIS was shorter compared to TEMS (86 min vs 120-140 min). Three of the patients were discharged on the same day. The longer length of hospital stay for some patients was primarily due to technical difficulties encountered during the procedure such as an anterior lying tumour and inadvertent violation of the peritoneum. There was no incidence of morbidity or mortality observed in the TAMIS patients after an average follow-up period of 6.2 wk.
A multi-institutional matched analysis study of both techniques was published in 2017 with the quality of excision examined[71]. Patients requiring excision of benign and malignant rectal lesions were included. Overall, 428 patients were enrolled and the quality of excision was assessed based on tumour fragmentation and positive resection margins. Both TEMS and TAMIS demonstrated similar rates of poor excision (8% vs 11%, P = 0.223). Post-operative complication rates were also similar between both groups (11% vs 9%, P = 0.477). Local recurrence in both cohorts was 7% (P = 0.864). The authors noted that TAMIS did allow for shorter operating times and a reduced length of hospital stay compared to TEMS. This study highlighted the non-inferiority of TAMIS excision compared to TEMS[71].
Several studies were subsequently published examining the adequacy of TAMIS excision. The primary determinant of excision quality was the presence of a positive excision margin on histological examination of resected specimens. Studies also examined the average distance of lesions from the anal verge, to analyse the extent of access TAMIS could achieve within the rectum. A systematic review of 390 TAMIS procedures conducted over three years was published in 2014[72]. The average distance of the tumour from the anal verge was 7.6 cm (3-15 cm). Of studies that recorded margin status, 4.36% of resected specimens demonstrated a positive margin on pathological analysis. Recurrence rates were recorded for 259 patients. The average rate of recurrence over a 7 mo period was 2.7%. Furthermore, a prospective observational study of 50 TAMIS patients was published in 2013[73]. Patients underwent TAMIS for both benign (n = 25) and malignant (n = 25) rectal lesions. Patients were recruited between 2009 and 2011 and received an average follow-up of 20 mo. The average distance of tumour to the anal verge was 8.1 cm (3-14 cm). The rate of positive margins on histology was 6%. There was a 4% recurrence rate documented after 20 mo of follow-up.
A larger study published in 2016 involved 75 patients[74]. The majority of lesions excised via TAMIS were benign with 17 patients treated for malignant lesions via TAMIS [59 benign (77.3%), 17 malignant (22.7%)]. The average distance from the anal verge was 10 cm (6-16 cm). Of note, two patients required temporary ileostomies after the peritoneal cavity was inadvertently entered. Average follow-up was over 39.5 mo. Of the 17 patients treated for rectal cancer, 5 (29%) had positive margins on pathology. Within this group, 2 patients went on to have a radical resection, 1 patient was deemed too high risk for radical surgery whilst another declined further surgery altogether. The fifth patient underwent a period of surveillance and was referred to medical oncology. Only one patient treated for rectal cancer and with negative margins on histology developed local recurrence and underwent an APR. This study was unique relative to those described above as it detailed the frequency and severity of post-operative complications from TAMIS. The common theme of the studies outlined above is that rectal lesions, both benign and early malignant tumours, can be safely and adequately resected via TAMIS. The average local recurrence rate for TAMIS resections is similar to those resected via traditional TME. It is essential however that appropriate patient selection is conducted in advance of any TAMIS procedure in order to further minimise the incidence of local recurrence.
The description of techniques such as TAMIS, TEMS, and TAE is in keeping with the global focus on minimally invasive surgery. The trials described above serve to demonstrate that minimally invasive surgery is a safe and effective means of surgically managing early, localised rectal cancer. Further advances in this field are being achieved through the use of robotics and novel techniques such as transanal total mesorectal excision (taTME). Robotic transanal surgery (RTS) involves multiple robotic arms being utilised to resect a rectal lesion via a transanal approach. The robotic arms are introduced transanally through a multichannel port. Robotic Transanal Surgery was first described in 2011[75]. Initial studies were performed in a dry lab setting, to assess feasibility. Later studies were performed on cadaveric models. The first documented description of RTS on a human patient was performed in 2012[76]. There were no immediate post-operative complications and the patient was discharged home on day one. The patient was followed up for 6 wk. In 2019 Tomassi et al performed a retrospective study of 58 patients who underwent RTS[77]. Within this cohort, 28 patients were operated for early localised rectal cancer, 11 for rectal carcinoid, 1 patient for rectal GI stromal tumour and the remainder for excision of rectal polyps. Specimen fragmentation was recorded in 1.7% of cases and 94.8% demonstrated negative margins on histopathology. After a mean follow-up of 11.5 mo (range, 0.3-33.3 mo), 3 patients (5.5%) demonstrated local recurrence with all 3 patients proceeding to salvage surgery.
taTME involves resecting rectal tumours via a transanal and transabdominal approach. The transabdominal approach involves an operating team mobilising the sigmoid colon and resecting the rectum proximal to the tumour allowing for adequate margins. A multichannel port is inserted into the anal canal by a second operating team with dissection proceeding distal to the rectal tumour. The transanal dissection proceeds proximally with simultaneous abdominal dissection distally[78]. A long-term follow-up of 373 patients treated with taTME was performed in 2017[79]. The majority of patients were treated for distal rectal tumours (91%) and received long-course neoadjuvant therapy preceding resection (97.7%). Good quality TME was performed in 96% of cases with a negative circumferential resection margin documented in 94% of patients. Morbidity and mortality rates following the procedure were 13.4% and 0.3% respectively. Local recurrence rates in this cohort were 7.4% with a 5-year survival rate of 90%. Furthermore, a systematic review and meta-analysis was conducted comparing outcomes between rectal cancer patients treated with open, laparoscopic, robotic and transanal excision of their tumours[80]. Overall, 29 studies were included incorporating 6237 patients. Post-operative morbidity was decreased in patients treated via laparoscopic and robotic surgery when compared to open. Similar findings were demonstrated in regards to the length of hospital stay. Quality of TME resection was found to be higher in open (OR = 1.52, 1.19-1.93) and transanal resections compared to laparoscopy. No significant differences were described regarding the incidence of anastomotic leaks, local recurrence rates and 5-year survival among patients (Table 5).
Table 5.
Transanal minimally invasive surgery studies
| Study | Pt numbers (n) | Average distance from Anal Verge (cm) | Positive margins | Local recurrence | Average length of follow-up |
| Atallah et al[70], 2010 | 6 | 9.3 | 0 | N/A | N/A |
| Albert et al[73], 2013 | 50 | 8.1 | 6% | 2 (4%) | N/A |
| Keller et al[74], 2016 | 75 17 (malignant) 58 (benign) | 10 | 5 | ||
| Garcia-Florez et al[106], 2017 | 32 | 5.6 | 1 | 10.3% | 26 mo |
| Van den Eynde et al[105], 2019 | 68 | 6 | 12% | N/A | 30 d |
| Melin et al[107], 2016 | 29 | 6.79 | 3 | 1 | Retrospective study |
CONCLUSION
Management of rectal cancer has evolved significantly over the course of the past century. Local recurrence rates and overall survival have increased progressively as a consequence of refinements in surgical techniques and instrumentation, culminating with the description of the TME. Studies outlining novel minimally invasive approaches to accessing rectal lesions are producing intriguing results. These newer approaches require strict criteria for patient selection and are most effective for treating early, localised rectal cancers. The advent of neoadjuvant therapy, and neoadjuvant radiotherapy, in particular, has resulted in further improvements in local recurrence. There have been numerous studies examining the benefit in enrolling patients with a complete response to radiotherapy into surveillance programmes. Medical professionals must be mindful of the side effect profile of radiotherapy such as long-term genitourinary and anorectal dysfunction. Therefore, it is essential that the nomination of patients for neoadjuvant radiotherapy should occur only after careful consideration and discussion by a multidisciplinary team of rectal cancer specialists.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior authors or other coauthors contributed their efforts in this manuscript.
Peer-review started: May 10, 2019
First decision: July 21, 2019
Article in press: August 7, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: Ireland
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brisinda G, Gerard JP, Horne J, Perse M S-Editor: Ma RY L-Editor: A E-Editor: Ma YJ
Contributor Information
Gerard Feeney, Department of General/Colorectal Surgery, Galway University Hospital, Galway H91 YR71, Ireland. g.feeney3@outlook.com.
Rishabh Sehgal, Department of General/Colorectal Surgery, Galway University Hospital, Galway H91 YR71, Ireland.
Margaret Sheehan, Department of Histopathology, Galway University Hospital, Galway H91 YR71, Ireland.
Aisling Hogan, Department of General/Colorectal Surgery, Galway University Hospital, Galway H91 YR71, Ireland.
Mark Regan, Department of General/Colorectal Surgery, Galway University Hospital, Galway H91 YR71, Ireland.
Myles Joyce, Department of General/Colorectal Surgery, Galway University Hospital, Galway H91 YR71, Ireland.
Michael Kerin, Department of General/Colorectal Surgery, Galway University Hospital, Galway H91 YR71, Ireland.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Tamas K, Walenkamp AM, de Vries EG, van Vugt MA, Beets-Tan RG, van Etten B, de Groot DJ, Hospers GA. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat Rev. 2015;41:671–679. doi: 10.1016/j.ctrv.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Paschke S, Jafarov S, Staib L, Kreuser ED, Maulbecker-Armstrong C, Roitman M, Holm T, Harris CC, Link KH, Kornmann M. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19092577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couwenberg AM, Burbach JPM, van Grevenstein WMU, Smits AB, Consten ECJ, Schiphorst AHW, Wijffels NAT, Heikens JT, Intven MPW, Verkooijen HM. Effect of Neoadjuvant Therapy and Rectal Surgery on Health-related Quality of Life in Patients With Rectal Cancer During the First 2 Years After Diagnosis. Clin Colorectal Cancer. 2018;17:e499–e512. doi: 10.1016/j.clcc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Ashburn JH, Kalady MF. Radiation-Induced Problems in Colorectal Surgery. Clin Colon Rectal Surg. 2016;29:85–91. doi: 10.1055/s-0036-1580632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lirici MM, Hüscher CG. Techniques and technology evolution of rectal cancer surgery: a history of more than a hundred years. Minim Invasive Ther Allied Technol. 2016;25:226–233. doi: 10.1080/13645706.2016.1198381. [DOI] [PubMed] [Google Scholar]
- 7.Miles WE. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon (1908) CA Cancer J Clin. 1971;21:361–364. doi: 10.3322/canjclin.21.6.361. [DOI] [PubMed] [Google Scholar]
- 8.Moynihan B. The surgical treatment of cancer of the sigmoid flexure and rectum. Surg Gynecol Obstet. 1908;6:463. [Google Scholar]
- 9.Ronel DN, Hardy MA. Henri Albert Hartmann: labor and discipline. Curr Surg. 2002;59:59–64. doi: 10.1016/s0149-7944(01)00572-4. [DOI] [PubMed] [Google Scholar]
- 10.Dixon CF. Anterior Resection for Malignant Lesions of the Upper Part of the Rectum and Lower Part of the Sigmoid. Ann Surg. 1948;128:425–442. doi: 10.1097/00000658-194809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 12.Heald RJ, Moran BJ, Ryall RD, Sexton R, MacFarlane JK. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 13.Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O'Callaghan C, Myint AS, Bessell E, Thompson LC, Parmar M, Stephens RJ, Sebag-Montefiore D MRC CR07/NCIC-CTG CO16 Trial Investigators; NCRI Colorectal Cancer Study Group. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821–828. doi: 10.1016/S0140-6736(09)60485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emmanuel A, Chohda E, Lapa C, Miles A, Haji A, Ellul J. Defunctioning Stomas Result in Significantly More Short-Term Complications Following Low Anterior Resection for Rectal Cancer. World J Surg. 2018;42:3755–3764. doi: 10.1007/s00268-018-4672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miura T, Sakamoto Y, Morohashi H, Yoshida T, Sato K, Hakamada K. Risk factor for permanent stoma and incontinence quality of life after sphincter-preserving surgery for low rectal cancer without a diverting stoma. Ann Gastroenterol Surg. 2017;2:79–86. doi: 10.1002/ags3.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chudner A, Gachabayov M, Dyatlov A, Lee H, Essani R, Bergamaschi R. The influence of diverting loop ileostomy vs. colostomy on postoperative morbidity in restorative anterior resection for rectal cancer: a systematic review and meta-analysis. Langenbecks Arch Surg. 2019;404:129–139. doi: 10.1007/s00423-019-01758-1. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson CP, Gunnarsson U, Dahlstrand U, Lindforss U. Loop-ileostomy reversal-patient-related characteristics influencing time to closure. Int J Colorectal Dis. 2018;33:593–600. doi: 10.1007/s00384-018-2994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 19.Lorimer PD, Motz BM, Kirks RC, Boselli DM, Walsh KK, Prabhu RS, Hill JS, Salo JC. Pathologic Complete Response Rates After Neoadjuvant Treatment in Rectal Cancer: An Analysis of the National Cancer Database. Ann Surg Oncol. 2017;24:2095–2103. doi: 10.1245/s10434-017-5873-8. [DOI] [PubMed] [Google Scholar]
- 20.MERCURY Study Group. Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown G. Staging rectal cancer: endoscopic ultrasound and pelvic MRI. Cancer Imaging. 2008;8 Spec No A:S43–S45. doi: 10.1102/1470-7330.2008.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiqui AA, Fayiga Y, Huerta S. The role of endoscopic ultrasound in the evaluation of rectal cancer. Int Semin Surg Oncol. 2006;3:36. doi: 10.1186/1477-7800-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valls C, Andía E, Sánchez A, Gumà A, Figueras J, Torras J, Serrano T. Hepatic metastases from colorectal cancer: preoperative detection and assessment of resectability with helical CT. Radiology. 2001;218:55–60. doi: 10.1148/radiology.218.1.r01dc1155. [DOI] [PubMed] [Google Scholar]
- 24.Swedish Rectal Cancer Trial. Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–987. doi: 10.1056/NEJM199704033361402. [DOI] [PubMed] [Google Scholar]
- 25.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 26.Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, Bessell E, Griffiths G, Thompson LC, Parmar M. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 28.Frykholm GJ, Påhlman L, Glimelius B. Combined chemo- and radiotherapy vs. radiotherapy alone in the treatment of primary, nonresectable adenocarcinoma of the rectum. Int J Radiat Oncol Biol Phys. 2001;50:427–434. doi: 10.1016/s0360-3016(01)01479-1. [DOI] [PubMed] [Google Scholar]
- 29.Chen K, Xie G, Zhang Q, Shen Y, Zhou T. Comparison of short-course with long-course preoperative neoadjuvant therapy for rectal cancer: A meta-analysis. J Cancer Res Ther. 2018;14:S224–S231. doi: 10.4103/0973-1482.202231. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, Safont MJ, Salud A, Vera R, Massuti B, Escudero P, Alonso V, Bosch C, Martin M, Minsky BD. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial†. Ann Oncol. 2015;26:1722–1728. doi: 10.1093/annonc/mdv223. [DOI] [PubMed] [Google Scholar]
- 31.Francois Y, Nemoz CJ, Baulieux J, Vignal J, Grandjean JP, Partensky C, Souquet JC, Adeleine P, Gerard JP. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 32.Cotte E, Passot G, Decullier E, Maurice C, Glehen O, François Y, Lorchel F, Chapet O, Gerard JP. Pathologic Response, When Increased by Longer Interval, Is a Marker but Not the Cause of Good Prognosis in Rectal Cancer: 17-year Follow-up of the Lyon R90-01 Randomized Trial. Int J Radiat Oncol Biol Phys. 2016;94:544–553. doi: 10.1016/j.ijrobp.2015.10.061. [DOI] [PubMed] [Google Scholar]
- 33.Erlandsson J, Holm T, Pettersson D, Berglund Å, Cedermark B, Radu C, Johansson H, Machado M, Hjern F, Hallböök O, Syk I, Glimelius B, Martling A. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol. 2017;18:336–346. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 34.Du D, Su Z, Wang D, Liu W, Wei Z. Optimal Interval to Surgery After Neoadjuvant Chemoradiotherapy in Rectal Cancer: A Systematic Review and Meta-analysis. Clin Colorectal Cancer. 2018;17:13–24. doi: 10.1016/j.clcc.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Kim MJ, Cho JS, Kim EM, Ko WA, Oh JH. Optimal Time Interval for Surgery After Neoadjuvant Chemoradiotherapy in Patients With Locally Advanced Rectal Cancer: Analysis of Health Insurance Review and Assessment Service Data. Ann Coloproctol. 2018;34:241–247. doi: 10.3393/ac.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the Interval Between Neoadjuvant Chemoradiotherapy and Surgery in Rectal Cancer: A Meta-analysis of Published Studies. Ann Surg. 2016;263:458–464. doi: 10.1097/SLA.0000000000000368. [DOI] [PubMed] [Google Scholar]
- 37.Figueiredo N, Panteleimonitis S, Popeskou S, Cunha JF, Qureshi T, Beets GL, Heald RJ, Parvaiz A. Delaying surgery after neoadjuvant chemoradiotherapy in rectal cancer has no influence in surgical approach or short-term clinical outcomes. Eur J Surg Oncol. 2018;44:484–489. doi: 10.1016/j.ejso.2018.01.088. [DOI] [PubMed] [Google Scholar]
- 38.Ansari N, Solomon MJ, Fisher RJ, Mackay J, Burmeister B, Ackland S, Heriot A, Joseph D, McLachlan SA, McClure B, Ngan SY. Acute Adverse Events and Postoperative Complications in a Randomized Trial of Preoperative Short-course Radiotherapy Versus Long-course Chemoradiotherapy for T3 Adenocarcinoma of the Rectum: Trans-Tasman Radiation Oncology Group Trial (TROG 01.04) Ann Surg. 2017;265:882–888. doi: 10.1097/SLA.0000000000001987. [DOI] [PubMed] [Google Scholar]
- 39.Croese AD, Lonie JM, Trollope AF, Vangaveti VN, Ho YH. A meta-analysis of the prevalence of Low Anterior Resection Syndrome and systematic review of risk factors. Int J Surg. 2018;56:234–241. doi: 10.1016/j.ijsu.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 40.Ho YH, Seow-Choen F, Tan M. Colonic J-pouch function at six months versus straight coloanal anastomosis at two years: randomized controlled trial. World J Surg. 2001;25:876–881. doi: 10.1007/s00268-001-0044-1. [DOI] [PubMed] [Google Scholar]
- 41.Lazorthes F, Chiotasso P, Gamagami RA, Istvan G, Chevreau P. Late clinical outcome in a randomized prospective comparison of colonic J pouch and straight coloanal anastomosis. Br J Surg. 1997;84:1449–1451. [PubMed] [Google Scholar]
- 42.Brown CJ, Fenech DS, McLeod RS. Reconstructive techniques after rectal resection for rectal cancer. Cochrane Database Syst Rev. 2008:CD006040. doi: 10.1002/14651858.CD006040.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2013;15:1130–1139. doi: 10.1111/codi.12244. [DOI] [PubMed] [Google Scholar]
- 44.Chen TY, Wiltink LM, Nout RA, Meershoek-Klein Kranenbarg E, Laurberg S, Marijnen CA, van de Velde CJ. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. 2015;14:106–114. doi: 10.1016/j.clcc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Kupsch J, Jackisch T, Matzel KE, Zimmer J, Schreiber A, Sims A, Witzigmann H, Stelzner S. Outcome of bowel function following anterior resection for rectal cancer-an analysis using the low anterior resection syndrome (LARS) score. Int J Colorectal Dis. 2018;33:787–798. doi: 10.1007/s00384-018-3006-x. [DOI] [PubMed] [Google Scholar]
- 46.Kupsch J, Kuhn M, Matzel KE, Zimmer J, Radulova-Mauersberger O, Sims A, Witzigmann H, Stelzner S. To what extent is the low anterior resection syndrome (LARS) associated with quality of life as measured using the EORTC C30 and CR38 quality of life questionnaires? Int J Colorectal Dis. 2019;34:747–762. doi: 10.1007/s00384-019-03249-7. [DOI] [PubMed] [Google Scholar]
- 47.Lee JY, Kim HC, Huh JW, Sim WS, Lim HY, Lee EK, Park HG, Bang YJ. Incidence and risk factors for rectal pain after laparoscopic rectal cancer surgery. J Int Med Res. 2017;45:781–791. doi: 10.1177/0300060517693421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendren SK, O'Connor BI, Liu M, Asano T, Cohen Z, Swallow CJ, Macrae HM, Gryfe R, McLeod RS. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242:212–223. doi: 10.1097/01.sla.0000171299.43954.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Costa P, Cardoso JM, Louro H, Dias J, Costa L, Rodrigues R, Espiridião P, Maciel J, Ferraz L. Impact on sexual function of surgical treatment in rectal cancer. Int Braz J Urol. 2018;44:141–149. doi: 10.1590/S1677-5538.IBJU.2017.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Attaallah W, Ertekin SC, Yegen C. Prospective study of sexual dysfunction after proctectomy for rectal cancer. Asian J Surg. 2018;41:454–461. doi: 10.1016/j.asjsur.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Ledebo A, Bock D, Prytz M, Haglind E, Angenete E. Urogenital function 3 years after abdominoperineal excision for rectal cancer. Colorectal Dis. 2018;20:O123–O134. doi: 10.1111/codi.14229. [DOI] [PubMed] [Google Scholar]
- 52.Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003;41:208–211. doi: 10.1002/mpo.10338. [DOI] [PubMed] [Google Scholar]
- 53.Jørgensen JB, Bondeven P, Iversen LH, Laurberg S, Pedersen BG. Pelvic insufficiency fractures frequently occur following preoperative chemo-radiotherapy for rectal cancer - a nationwide MRI study. Colorectal Dis. 2018;20:873–880. doi: 10.1111/codi.14224. [DOI] [PubMed] [Google Scholar]
- 54.Kim HJ, Boland PJ, Meredith DS, Lis E, Zhang Z, Shi W, Yamada YJ, Goodman KA. Fractures of the sacrum after chemoradiation for rectal carcinoma: incidence, risk factors, and radiographic evaluation. Int J Radiat Oncol Biol Phys. 2012;84:694–699. doi: 10.1016/j.ijrobp.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 56.Habr-Gama A, de Souza PM, Ribeiro U, Jr, Nadalin W, Gansl R, Sousa AH, Jr, Campos FG, Gama-Rodrigues J. Low rectal cancer: impact of radiation and chemotherapy on surgical treatment. Dis Colon Rectum. 1998;41:1087–1096. doi: 10.1007/BF02239429. [DOI] [PubMed] [Google Scholar]
- 57.Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U, Jr, Silva e Sousa AH, Jr, Campos FG, Kiss DR, Gama-Rodrigues J. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–7; discussion 717-8. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Valk MJM, Hilling DE, Bastiaannet E, Meershoek-Klein Kranenbarg E, Beets GL, Figueiredo NL, Habr-Gama A, Perez RO, Renehan AG, van de Velde CJH IWWD Consortium. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391:2537–2545. doi: 10.1016/S0140-6736(18)31078-X. [DOI] [PubMed] [Google Scholar]
- 59.Renehan AG, Malcomson L, Emsley R, Gollins S, Maw A, Myint AS, Rooney PS, Susnerwala S, Blower A, Saunders MP, Wilson MS, Scott N, O'Dwyer ST. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17:174–183. doi: 10.1016/S1470-2045(15)00467-2. [DOI] [PubMed] [Google Scholar]
- 60.Gerard JP, Coquard R, Fric D, Ayzac L, Romestaing P, Ardiet JM, Rocher FP, Baron MH, Trillet-Lenoir V. Curative endocavitary irradiation of small rectal cancers and preoperative radiotherapy in T2 T3 (T4) rectal cancer. A brief overview of the Lyon experience. Eur J Surg Oncol. 1994;20:644–647. [PubMed] [Google Scholar]
- 61.Schild SE, Martenson JA, Gunderson LL. Endocavitary radiotherapy of rectal cancer. Int J Radiat Oncol Biol Phys. 1996;34:677–682. doi: 10.1016/0360-3016(95)02098-5. [DOI] [PubMed] [Google Scholar]
- 62.Gerard JP, Chapet O, Nemoz C, Hartweig J, Romestaing P, Coquard R, Barbet N, Maingon P, Mahe M, Baulieux J, Partensky C, Papillon M, Glehen O, Crozet B, Grandjean JP, Adeleine P. Improved sphincter preservation in low rectal cancer with high-dose preoperative radiotherapy: the lyon R96-02 randomized trial. J Clin Oncol. 2004;22:2404–2409. doi: 10.1200/JCO.2004.08.170. [DOI] [PubMed] [Google Scholar]
- 63.Ortholan C, Romestaing P, Chapet O, Gerard JP. Correlation in rectal cancer between clinical tumor response after neoadjuvant radiotherapy and sphincter or organ preservation: 10-year results of the Lyon R 96-02 randomized trial. Int J Radiat Oncol Biol Phys. 2012;83:e165–e171. doi: 10.1016/j.ijrobp.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Pigot F, Bouchard D, Mortaji M, Castinel A, Juguet F, Chaume JC, Faivre J. Local excision of large rectal villous adenomas: long-term results. Dis Colon Rectum. 2003;46:1345–1350. doi: 10.1007/s10350-004-6748-1. [DOI] [PubMed] [Google Scholar]
- 65.Rai V, Mishra N. Transanal Approach to Rectal Polyps and Cancer. Clin Colon Rectal Surg. 2016;29:65–70. doi: 10.1055/s-0035-1570395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buess G, Theiss R, Günther M, Hutterer F, Pichlmaier H. [Transanal endoscopic microsurgery] Leber Magen Darm. 1985;15:271–279. [PubMed] [Google Scholar]
- 67.O'Neill CH, Platz J, Moore JS, Callas PW, Cataldo PA. Transanal Endoscopic Microsurgery for Early Rectal Cancer: A Single-Center Experience. Dis Colon Rectum. 2017;60:152–160. doi: 10.1097/DCR.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 68.Stijns RCH, de Graaf EJR, Punt CJA, Nagtegaal ID, Nuyttens JJME, van Meerten E, Tanis PJ, de Hingh IHJT, van der Schelling GP, Acherman Y, Leijtens JWA, Bremers AJA, Beets GL, Hoff C, Verhoef C, Marijnen CAM, de Wilt JHW CARTS Study Group. Long-term Oncological and Functional Outcomes of Chemoradiotherapy Followed by Organ-Sparing Transanal Endoscopic Microsurgery for Distal Rectal Cancer: The CARTS Study. JAMA Surg. 2019;154:47–54. doi: 10.1001/jamasurg.2018.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perez RO, Habr-Gama A, São Julião GP, Proscurshim I, Fernandez LM, de Azevedo RU, Vailati BB, Fernandes FA, Gama-Rodrigues J. Transanal Endoscopic Microsurgery (TEM) Following Neoadjuvant Chemoradiation for Rectal Cancer: Outcomes of Salvage Resection for Local Recurrence. Ann Surg Oncol. 2016;23:1143–1148. doi: 10.1245/s10434-015-4977-2. [DOI] [PubMed] [Google Scholar]
- 70.Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc. 2010;24:2200–2205. doi: 10.1007/s00464-010-0927-z. [DOI] [PubMed] [Google Scholar]
- 71.Lee L, Edwards K, Hunter IA, Hartley JE, Atallah SB, Albert MR, Hill J, Monson JR. Quality of Local Excision for Rectal Neoplasms Using Transanal Endoscopic Microsurgery Versus Transanal Minimally Invasive Surgery: A Multi-institutional Matched Analysis. Dis Colon Rectum. 2017;60:928–935. doi: 10.1097/DCR.0000000000000884. [DOI] [PubMed] [Google Scholar]
- 72.Martin-Perez B, Andrade-Ribeiro GD, Hunter L, Atallah S. A systematic review of transanal minimally invasive surgery (TAMIS) from 2010 to 2013. Tech Coloproctol. 2014;18:775–788. doi: 10.1007/s10151-014-1148-6. [DOI] [PubMed] [Google Scholar]
- 73.Albert MR, Atallah SB, deBeche-Adams TC, Izfar S, Larach SW. Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum. 2013;56:301–307. doi: 10.1097/DCR.0b013e31827ca313. [DOI] [PubMed] [Google Scholar]
- 74.Keller DS, Tahilramani RN, Flores-Gonzalez JR, Mahmood A, Haas EM. Transanal Minimally Invasive Surgery: Review of Indications and Outcomes from 75 Consecutive Patients. J Am Coll Surg. 2016;222:814–822. doi: 10.1016/j.jamcollsurg.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Atallah SB, Albert MR, deBeche-Adams TH, Larach SW. Robotic TransAnal Minimally Invasive Surgery in a cadaveric model. Tech Coloproctol. 2011;15:461–464. doi: 10.1007/s10151-011-0762-9. [DOI] [PubMed] [Google Scholar]
- 76.Hompes R, Rauh SM, Hagen ME, Mortensen NJ. Preclinical cadaveric study of transanal endoscopic da Vinci® surgery. Br J Surg. 2012;99:1144–1148. doi: 10.1002/bjs.8794. [DOI] [PubMed] [Google Scholar]
- 77.Tomassi MJ, Taller J, Yuhan R, Ruan JH, Klaristenfeld DD. Robotic Transanal Minimally Invasive Surgery for the Excision of Rectal Neoplasia: Clinical Experience With 58 Consecutive Patients. Dis Colon Rectum. 2019;62:279–285. doi: 10.1097/DCR.0000000000001223. [DOI] [PubMed] [Google Scholar]
- 78.Trépanier JS, Fernandez-Hevia M, Lacy AM. Transanal total mesorectal excision: surgical technique description and outcomes. Minim Invasive Ther Allied Technol. 2016;25:234–240. doi: 10.1080/13645706.2016.1199434. [DOI] [PubMed] [Google Scholar]
- 79.Marks JH, Myers EA, Zeger EL, Denittis AS, Gummadi M, Marks GJ. Long-term outcomes by a transanal approach to total mesorectal excision for rectal cancer. Surg Endosc. 2017;31:5248–5257. doi: 10.1007/s00464-017-5597-7. [DOI] [PubMed] [Google Scholar]
- 80.Simillis C, Lal N, Thoukididou SN, Kontovounisios C, Smith JJ, Hompes R, Adamina M, Tekkis PP. Open Versus Laparoscopic Versus Robotic Versus Transanal Mesorectal Excision for Rectal Cancer: A Systematic Review and Network Meta-analysis. Ann Surg. 2019;270:59–68. doi: 10.1097/SLA.0000000000003227. [DOI] [PubMed] [Google Scholar]
- 81.Ravitch MM, Steichen FM. A stapling instrument for end-to-end inverting anastomoses in the gastrointestinal tract. Ann Surg. 1979;189:791–797. doi: 10.1097/00000658-197906000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parks AG, Percy JP. Resection and sutured colo-anal anastomosis for rectal carcinoma. Br J Surg. 1982;69:301–304. doi: 10.1002/bjs.1800690602. [DOI] [PubMed] [Google Scholar]
- 83.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ COlorectal cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 84.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 85.Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR, Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hojo K, Sawada T, Moriya Y. An analysis of survival and voiding, sexual function after wide iliopelvic lymphadenectomy in patients with carcinoma of the rectum, compared with conventional lymphadenectomy. Dis Colon Rectum. 1989;32:128–133. doi: 10.1007/BF02553825. [DOI] [PubMed] [Google Scholar]
- 87.Knight CD, Griffen FD. An improved technique for low anterior resection of the rectum using the EEA stapler. Surgery. 1980;88:710–714. [PubMed] [Google Scholar]
- 88.Bujko K, Wyrwicz L, Rutkowski A, Malinowska M, Pietrzak L, Kryński J, Michalski W, Olędzki J, Kuśnierz J, Zając L, Bednarczyk M, Szczepkowski M, Tarnowski W, Kosakowska E, Zwoliński J, Winiarek M, Wiśniowska K, Partycki M, Bęczkowska K, Polkowski W, Styliński R, Wierzbicki R, Bury P, Jankiewicz M, Paprota K, Lewicka M, Ciseł B, Skórzewska M, Mielko J, Bębenek M, Maciejczyk A, Kapturkiewicz B, Dybko A, Hajac Ł, Wojnar A, Leśniak T, Zygulska J, Jantner D, Chudyba E, Zegarski W, Las-Jankowska M, Jankowski M, Kołodziejski L, Radkowski A, Żelazowska-Omiotek U, Czeremszyńska B, Kępka L, Kolb-Sielecki J, Toczko Z, Fedorowicz Z, Dziki A, Danek A, Nawrocki G, Sopyło R, Markiewicz W, Kędzierawski P, Wydmański Polish Colorectal Study Group. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol. 2016;27:834–842. doi: 10.1093/annonc/mdw062. [DOI] [PubMed] [Google Scholar]
- 89.Ngan SY, Burmeister B, Fisher RJ, Solomon M, Goldstein D, Joseph D, Ackland SP, Schache D, McClure B, McLachlan SA, McKendrick J, Leong T, Hartopeanu C, Zalcberg J, Mackay J. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol. 2012;30:3827–3833. doi: 10.1200/JCO.2012.42.9597. [DOI] [PubMed] [Google Scholar]
- 90.Wawok P, Polkowski W, Richter P, Szczepkowski M, Olędzki J, Wierzbicki R, Gach T, Rutkowski A, Dziki A, Kołodziejski L, Sopyło R, Pietrzak L, Kryński J, Wiśniowska K, Spałek M, Pawlewicz K, Polkowski M, Kowalska T, Paprota K, Jankiewicz M, Radkowski A, Chalubińska-Fendler J, Michalski W, Bujko K Polish Colorectal Cancer Study Group. Preoperative radiotherapy and local excision of rectal cancer: Long-term results of a randomised study. Radiother Oncol. 2018;127:396–403. doi: 10.1016/j.radonc.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 91.Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, Graeven U, Arnold D, Lang-Welzenbach M, Raab HR, Sülberg H, Wittekind C, Potapov S, Staib L, Hess C, Weigang-Köhler K, Grabenbauer GG, Hoffmanns H, Lindemann F, Schlenska-Lange A, Folprecht G, Sauer R German Rectal Cancer Study Group. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–687. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 92.Habr-Gama A, Gama-Rodrigues J, São Julião GP, Proscurshim I, Sabbagh C, Lynn PB, Perez RO. Local recurrence after complete clinical response and watch and wait in rectal cancer after neoadjuvant chemoradiation: impact of salvage therapy on local disease control. Int J Radiat Oncol Biol Phys. 2014;88:822–828. doi: 10.1016/j.ijrobp.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 93.Appelt AL, Pløen J, Harling H, Jensen FS, Jensen LH, Jørgensen JC, Lindebjerg J, Rafaelsen SR, Jakobsen A. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16:919–927. doi: 10.1016/S1470-2045(15)00120-5. [DOI] [PubMed] [Google Scholar]
- 94.Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, Temple LK, Nash GM, Paty PB. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256:965–972. doi: 10.1097/SLA.0b013e3182759f1c. [DOI] [PubMed] [Google Scholar]
- 95.Smith JJ, Strombom P, Chow OS, Roxburgh CS, Lynn P, Eaton A, Widmar M, Ganesh K, Yaeger R, Cercek A, Weiser MR, Nash GM, Guillem JG, Temple LKF, Chalasani SB, Fuqua JL, Petkovska I, Wu AJ, Reyngold M, Vakiani E, Shia J, Segal NH, Smith JD, Crane C, Gollub MJ, Gonen M, Saltz LB, Garcia-Aguilar J, Paty PB. Assessment of a Watch-and-Wait Strategy for Rectal Cancer in Patients With a Complete Response After Neoadjuvant Therapy. JAMA Oncol. 2019:e185896. doi: 10.1001/jamaoncol.2018.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martens MH, Maas M, Heijnen LA, Lambregts DM, Leijtens JW, Stassen LP, Breukink SO, Hoff C, Belgers EJ, Melenhorst J, Jansen R, Buijsen J, Hoofwijk TG, Beets-Tan RG, Beets GL. Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer. J Natl Cancer Inst. 2016;108 doi: 10.1093/jnci/djw171. [DOI] [PubMed] [Google Scholar]
- 97.Lai CL, Lai MJ, Wu CC, Jao SW, Hsiao CW. Rectal cancer with complete clinical response after neoadjuvant chemoradiotherapy, surgery, or "watch and wait". Int J Colorectal Dis. 2016;31:413–419. doi: 10.1007/s00384-015-2460-y. [DOI] [PubMed] [Google Scholar]
- 98.Rijkmans EC, Cats A, Nout RA, van den Bongard DHJG, Ketelaars M, Buijsen J, Rozema T, Franssen JH, Velema LA, van Triest B, Marijnen CAM. Endorectal Brachytherapy Boost After External Beam Radiation Therapy in Elderly or Medically Inoperable Patients With Rectal Cancer: Primary Outcomes of the Phase 1 HERBERT Study. Int J Radiat Oncol Biol Phys. 2017;98:908–917. doi: 10.1016/j.ijrobp.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 99.Vuong T, Devic S, Podgorsak E. High dose rate endorectal brachytherapy as a neoadjuvant treatment for patients with resectable rectal cancer. Clin Oncol (R Coll Radiol) 2007;19:701–705. doi: 10.1016/j.clon.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 100.Gérard JP, Barbet N, Gal J, Dejean C, Evesque L, Doyen J, Coquard R, Gugenheim J, Benizri E, Schiappa R, Baudin G, Benezery K, François E. Planned organ preservation for early T2-3 rectal adenocarcinoma: A French, multicentre study. Eur J Cancer. 2019;108:1–16. doi: 10.1016/j.ejca.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 101.Sun Myint A, Smith FM, Gollins S, Wong H, Rao C, Whitmarsh K, Sripadam R, Rooney P, Hershman M, Pritchard DM. Dose Escalation Using Contact X-ray Brachytherapy After External Beam Radiotherapy as Nonsurgical Treatment Option for Rectal Cancer: Outcomes From a Single-Center Experience. Int J Radiat Oncol Biol Phys. 2018;100:565–573. doi: 10.1016/j.ijrobp.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 102.Jeong WK, Park JW, Choi HS, Chang HJ, Jeong SY. Transanal endoscopic microsurgery for rectal tumors: experience at Korea's National Cancer Center. Surg Endosc. 2009;23:2575–2579. doi: 10.1007/s00464-009-0466-7. [DOI] [PubMed] [Google Scholar]
- 103.Stipa F, Giaccaglia V, Burza A. Management and outcome of local recurrence following transanal endoscopic microsurgery for rectal cancer. Dis Colon Rectum. 2012;55:262–269. doi: 10.1097/DCR.0b013e318241ef22. [DOI] [PubMed] [Google Scholar]
- 104.Baatrup G, Breum B, Qvist N, Wille-Jørgensen P, Elbrønd H, Møller P, Hesselfeldt P. Transanal endoscopic microsurgery in 143 consecutive patients with rectal adenocarcinoma: results from a Danish multicenter study. Colorectal Dis. 2009;11:270–275. doi: 10.1111/j.1463-1318.2008.01600.x. [DOI] [PubMed] [Google Scholar]
- 105.Van den Eynde F, Jaekers J, Fieuws S, D'Hoore AM, Wolthuis AM. TAMIS is a valuable alternative to TEM for resection of intraluminal rectal tumors. Tech Coloproctol. 2019;23:161–166. doi: 10.1007/s10151-019-01954-7. [DOI] [PubMed] [Google Scholar]
- 106.García-Flórez LJ, Otero-Díez JL, Encinas-Muñiz AI, Sánchez-Domínguez L. Indications and Outcomes From 32 Consecutive Patients for the Treatment of Rectal Lesions by Transanal Minimally Invasive Surgery. Surg Innov. 2017;24:336–342. doi: 10.1177/1553350617700803. [DOI] [PubMed] [Google Scholar]
- 107.Melin AA, Kalaskar S, Taylor L, Thompson JS, Ternent C, Langenfeld SJ. Transanal endoscopic microsurgery and transanal minimally invasive surgery: is one technique superior? Am J Surg. 2016;212:1063–1067. doi: 10.1016/j.amjsurg.2016.08.017. [DOI] [PubMed] [Google Scholar]