Abstract
Background: Several scoring systems are utilized to calculate the pre-test probability of heparin-induced thrombocytopenia (HIT). We hypothesize that a clinical-laboratory algorithm combining the 4Ts score with the optical density (OD) of anti-PF4-heparin antibody is more accurate than either the 4Ts or HIT expert probability (HEP) scores in the critical care setting. Methods: A single-institution retrospective review of adult patients admitted to the intensive care unit (ICU) that were evaluated for HIT was conducted. Two reviewers independently rated the proposed algorithm, 4Ts and HEP score. Summary, univariate and area under receiver operator characteristic analyses were performed. Results: A total of 88 patients with a mean (SD) age of 62 (15) years were included. The sensitivity, positive predictive value and negative predictive value were superior in our clinical-laboratory algorithm compared to the 4Ts score ≥ 4 and the HEP score ≥ 2. The algorithm’s specificity was non-inferior to the 4Ts score and HEP score. There was no significant difference between our clinical-laboratory algorithm and the 4Ts score or the HEP score in predicting HIT. Conclusion: Our study confirms that the combination of clinical and laboratory criteria is crucial in the presumable diagnosis of HIT. This is the first study that validates different HIT scores in an isolated ICU population.
Keywords: HIT, 4Ts score, HEP score, algorithms
Introduction
The diagnosis of HIT can be significantly challenging and both overdiagnosis and underdiagnoses pose potential dangers. Overdiagnosis tends to expose patients to unnecessary anticoagulation and a significant risk of bleeding. Underdiagnoses increase the risk of thrombosis, morbidity and mortality. Thrombotic events occur in up to 50% of untreated patients with HIT [1]. These are either arterial or venous clots presenting as deep venous thrombosis, pulmonary embolism, skin necrosis, or organ ischemia/infarction such as limb gangrene with approximately 10% of these patients requiring amputations [1,2]. The mortality rate can be as high as 20% in untreated HIT [2]. Thus, prompt accurate diagnosis and treatment of HIT are vital.
The presumptive diagnosis of HIT is based on clinical and immediately available laboratory findings. Immunoassays and functional assays are two major types of laboratory tests used in the diagnosis of HIT [3]. Immunoassays detect the presence of anti-PF4-heparin antibody and are reported in optical density (OD) units based on the concentration of antibodies presents [3]. The positive predictive value of these assays improves with increasing OD but only at the cost of decreased sensitivity and negative predictive value [4]. They are widely available, highly sensitive and fast however they have a high incidence of false positive results and are non-specific [3]. Functional assays, like serotonin release assay (SRA), remain the gold standard among diagnostic tests for HIT with sensitivities and specificities reaching more than 95%; however, they are not usually available at the time of decision making in the setting of HIT suspicion and take days to result [5].
In addition to laboratory testing, several clinical scoring systems have been utilized to calculate the pre-test probability of HIT to guide urgent management when HIT is suspected [6-8]. Most commonly used is the 4Ts score which quantifies the clinical features associated with HIT until laboratory findings are available [6]. The HIT expert probability (HEP) score is another score that was developed based on expert opinions and was found to have greater inter-observer agreement compared with the 4Ts score [7]. Only one small prospective study including 51 patients compared the 4Ts and the HEP score and found no significant difference in the scores compared with SRA in the diagnosis of HIT [8]. Lastly, the Lillo-Le Louet model is rarely used and is exclusively utilized in the post cardiopulmonary bypass setting [9]. None of these scores have been validated in critically ill patients. In this retrospective review of critically ill patients, we aim to i) validate and evaluate the diagnostic ability of our clinical-laboratory algorithm and ii) compare accuracy measures between our clinical-laboratory algorithm, the HEP and 4Ts scoring systems. We hypothesize that our clinical-laboratory algorithm combining the 4Ts score with the OD of anti-PF4-heparin antibody (Figure 1) is more accurate than either the 4Ts or HEP clinical scores.
Figure 1.
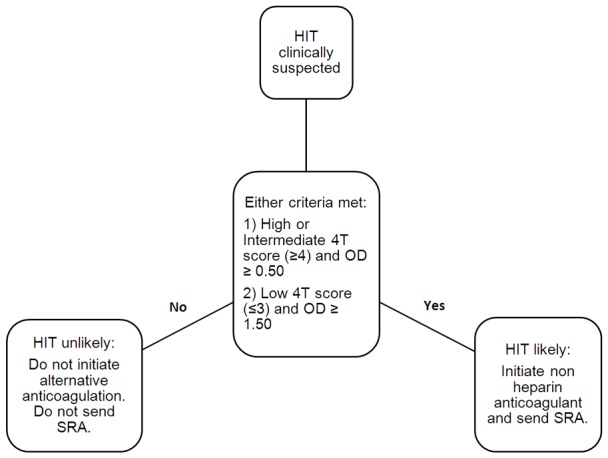
Our proposed clinical-laboratory algorithm combining the 4Ts scoring system along with the optical density of anti-PF4-heparin antibody in the management of HIT. OD: Optical Density. HIT: Heparin-Induced Thrombocytopenia. SRA: Serotonin Release Assay.
Materials and methods
Institutional review board approval was obtained. A single-institution retrospective analysis was conducted on patients admitted to the ICU. Our institution recently introduced a clinical-laboratory algorithm to help facilitate the management of patients with suspected HIT (Figure 1). This algorithm incorporates the 4Ts scoring system along with the OD of anti-PF4-heparin antibody. As these are immediately available at our institution, the algorithm assists in making a presumptive diagnosis of HIT that is eventually either confirmed or disproved once definitive functional assays (SRA) result. According to the algorithm proposed, HIT will be considered likely if; 4Ts score is high or intermediate (≥ 4) with an OD ≥ 0.50, or 4Ts score is low with an OD ≥ 1.50, otherwise, it is considered unlikely. At our institution, a confirmatory SRA is ordered on all patients with positive heparin antibody with an OD ≥ 0.50 regardless of the clinical likelihood of HIT.
All adult (age ≥ 18 years) patients admitted to the ICU between 11/01/2006 to 05/01/2016, who were exposed to heparin and evaluated for HIT, using both the anti-PF4-heparin antibody and the functional assay, were included in the study. We excluded subjects who were less than 18 years old, did not have the anti-PF4-heparin antibody nor the functional assay ordered, had a history of HIT, or lacked appropriate documentation to calculate clinical scores. The following demographic data were collected: age, sex, race, ICU diagnosis and body mass index (BMI). Race was classified as African American, Caucasian or other, which included Asian, American Indian, native Hawaiian or unknown. Patients were subcategorized based on their ICU diagnosis into either medical or surgical patients and subsequently grouped based on the diagnosis etiology into either sepsis, cardiac, respiratory failure or other. The following data points were recorded: indication for heparin administration, the heparin formulation used, surgery performed (when applicable), arterial or venous thromboembolic complications that developed during the hospital stay following heparin administration, the platelet count baseline, the platelet count nadir, the anti-PF4-heparin antibody OD, the result of the functional assay and alternative anticoagulation use. Alternative anticoagulation at our institution includes all direct thrombin inhibitors. Argatroban is the most common agent used at our institution with only a few cases treated using bivalirudin or lepirudin. At our institution, the enzyme-linked immunosorbent assay ELISA used to detect the anti-PF4-heparin antibody is the ZYMUTEST PF4 from Aniara Diagnostica in Ohio, USA. Also, the functional assay used is the SRA; the assay is analyzed at an outside referral laboratory and a ≥ 20% release of serotonin with low dose heparin plus a < 20% release in the presence of a high concentration of heparin was considered positive. A diagnosis of HIT was confirmed or excluded based on the results of the SRA. Daily complete blood count is the routine practice at the studied ICUs. All data points were collected through chart review using EPIC.
A medical resident and a critical care fellow independently rated the 4Ts score and the HEP score retrospectively for each individual patient, the scores were then averaged to facilitate comparison. Initially, the evaluators blinded themselves to the results of the HIT laboratory testing to calculate the 4Ts and HEP clinical scores. The evaluators then reviewed the results of heparin antibody to evaluate HIT using the proposed algorithm. Finally, SRA results were revealed and collected. A kappa test with 95% confidence intervals (CI) was utilized to estimate the degree of agreement between reviewers for the 4Ts score and the HEP score.
Normally distributed continuous variables were reported as means ± standard deviation (SD), nonparametric data were reported as medians with interquartile range (IQR), and categorical variables were reported as counts and percentages. Chi-square test was used to compare categorical variables between the two groups, and Student’s t-test was used to compare the continuous outcomes between the two groups for uniformly distributed variables. When data were not distributed uniformly, the Wilcoxon rank-sum test was used to compare the outcomes between the two groups. The 4Ts score cut off used in our study was ≥ 4 (14). The HEP score cut off used was ≥ 2 [7]). The following ROC statistics with 95% CIs were reported for the established 4Ts score, HEP score and our algorithm’s cut-offs in the prediction of HIT: sensitivity, specificity, positive predictive value, negative predictive, positive likelihood ratio and negative likelihood ratio. Area under receiver operative characteristic (AUROC) analyses were used to assess the predictive ability of our clinical-laboratory algorithm to diagnose HIT in comparison with the 4Ts score and the HEP score. All tests were two-sided with an α level set at 0.05 for statistical significance. Data analysis was performed using JMP Pro version 10.0 (SAS Institute Inc., Cary, NC, USA).
Results
A total of 101 ICU patients were exposed to heparin and evaluated for HIT, using both the anti-PF4-heparin antibody and the functional assay. Thirteen patients were excluded; two patients had a history of HIT and 11 patients lacked appropriate documentation to calculate clinical scores. A final total of 88 patients were included with a mean (SD) age of 62 (15) years; 56% (n=49) were male. There were 21 patients (24%) with a confirmed diagnosis of HIT (positive SRA). The majority (n=66; 75%) of our patient population were Caucasian with 15 patients (17%) of African American and 7 patients (8%) of “other” race. Surgical interventions were performed in 24 patients (27%); of which, 18 (75%) were cardiac surgeries. There was a significantly higher proportion of patients that underwent any surgical intervention in patients with a confirmed diagnosis of HIT compared with patients without HIT {n=10, 48% vs n=14, 21%; P=0.02} (Table 1). The most common ICU diagnosis was cardiac (n=46; 52%) followed by sepsis (n=17; 19%), other etiologies (n=13; 15%) and isolated respiratory failure (n=12; 14%). All patients received either therapeutic heparin (n=61; 69%) or prophylactic heparin (n=27; 31%); intravenous (IV) unfractionated heparin (UH) with a bolus (n=47; 53%), IV UH without a bolus (n=12; 14%), subcutaneous UH (n=20; 23%) or low molecular weight heparin (LMWH) (n=9; 10%). Patients with a confirmed diagnosis of HIT were more likely to have received a therapeutic dose of heparin compared with patients without HIT {n=16, 76% vs n=33, 49%; P=0.04} (Table 1). However, there was no significant difference in the heparin formulation administered between both groups (P=0.38). The median (IQR) baseline platelet count/microL was 200 (138-255). The median (IQR) platelet count/microL nadir was 60 (41-80). Most patients (n=71; 81%) did not develop any thromboembolic phenomena; while 17 patients (19%) developed either venous thrombosis (n=9, 10%), arterial thrombosis (n=7; 8%) or both a venous and arterial thrombosis (n=1; 1%). Alternative anticoagulation was initiated in 52 patients (60%). Patients with HIT were more likely to receive alternative anticoagulation {n=19, 90% vs n=33, 49%; P=0.0007} (Table 1). Only 2 patients out of the 21 patients with confirmed HIT did not receive alternative anticoagulation. These two patients did not develop any thrombosis throughout their hospital stay. Most patients (n=13, 76%) that developed a thrombus were started on alternative anticoagulation. Only four out of the seventeen patients with a thrombus did not receive alternative anticoagulation. HIT was excluded in these four patients. There was no significant difference in age, race, sex, ICU diagnosis, type of heparin received, baseline platelet count, platelet count nadir and thromboembolic events between both groups (Table 1).
Table 1.
A comparison of patient clinical and laboratory characteristics in patients with and without HIT
| Characteristics | HIT confirmed (n=21) | HIT excluded (n=67) | p value |
|---|---|---|---|
| Age, mean (SD) | 64 (15) | 62 (16) | 0.60 |
| Sex, n (%) | Male: 13 (62) | Male: 36 (54) | 0.62 |
| Female: 8 (38) | Female: 31 (46) | ||
| Race, n (%) | Caucasian: 19 (90) | Caucasian: 47 (70) | 0.16 |
| African American: 1 (5) | African American: 14 (21) | ||
| Other: 1 (5) | Other: 6 (9) | ||
| Probability based on the 4Ts score, n (%) | Low: 6 (29) | Low: 35 (52) | 0.13 |
| Intermediate: 12 (57) | Intermediate: 28 (42) | ||
| High: 3 (14) | High: 4 (6) | ||
| OD ≥ 0.50, n (%) | 20 (95) | 54 (81) | 0.17 |
| OD ≥ 1.00, n (%) | 14 (67) | 22 (33) | 0.01 |
| OD ≥ 1.50, n (%) | 14 (67) | 14 (21) | 0.00 |
| OD ≥ 2.0, n (%) | 12 (57) | 4 (6) | 0.00 |
| Medical vs surgical, n (%) | Medical: 11 (52) | Medical: 53 (79) | 0.02 |
| Surgical: 10 (48) | Surgical: 14 (21) | ||
| ICU diagnosis, n (%) | Cardiac: 11 (52) | Cardiac: 35 (52) | 0.88 |
| Sepsis: 4 (19) | Sepsis: 13 (19) | ||
| Respiratory: 2 (10) | Respiratory: 10 (15) | ||
| Other: 4 (19) | Other: 9 (13) | ||
| Heparin formulation, n (%) | IV UH w bolus: 13 (62) | IV heparin w bolus: 34 (51) | 0.38 |
| IV UH w/o bolus: 4 (19) | IV heparin w/o bolus: 8 (12) | ||
| LMWH: 2 (10) | LMWH: 7 (10) | ||
| Sc UH: 2 (10) | Sc heparin: 18 (27) | ||
| Heparin indication, n (%) | Prophylactic: 5 (24) | Prophylactic: 34 (51) | 0.04 |
| Therapeutic: 16 (76) | Therapeutic: 33 (49) | ||
| Baseline platelet count, median (IQR) | 219 (176-305) | 183 (125-246) | 0.05 |
| Platelet count nadir, median (IQR) | 61 (43-80) | 59 (41-80) | 0.98 |
| Alternative anticoagulation initiated, n (%) | 19 (90) | 33 (49) | 0.0007 |
| Thromboembolic events, n (%) | Arterial: 2 (10) | Arterial: 5 (7) | 0.11 |
| Venous: 4 (19) | Venous: 5 (7) | ||
| Arterial and venous: 1 (5) | Arterial and venous: 0 (0) |
OD: optical density, ICU: intensive care unit, IV: intravenous, UH: unfractionated heparin, w: with, w/o; without, Sc: subcutaneous, LMWH: low molecular weight heparin.
Using our clinical-laboratory algorithm, a total of 54 patients (61%) from our cohort were likely to have HIT. Out of these 54 patients, 14 patients (26%) had 4Ts score ≤ 3 and OD ≥ 1.5 while 40 patients (74%) had 4Ts score > 3 and OD ≥ 0.5. There was no significant difference in terms of age, sex, race, diagnosis, medical vs surgical, thrombosis risk or alternative anticoagulation use between patients that had 4Ts score ≤ 3 and OD ≥ 1.5 versus patients that had 4Ts score > 3 and OD ≥ 0.5 (P > 0.05). Patients with a confirmed diagnosis of HIT had a significantly higher proportion of patients that scored “HIT likely” compared to patients without HIT {n=19, 90% vs n=33, 49%; P=0.0007}. The median (IQR) 4Ts score was 3.5 (2.5-4.5). HIT pre-test probability was low (4Ts score ≤ 3) in 41 patients (47%), intermediate (3 < 4Ts score < 6) in 40 patients (45%) and high (4Ts score ≥ 6) in 7 patients (8%). The median (IQR) 4Ts score was significantly higher in patients with a confirmed diagnosis of HIT compared to patients were HIT was excluded {4 (3-5) vs 3 (2-4.5); P=0.04}. However, there was no significant difference in the proportion of patients that had a 4Ts score ≥ 4 in patients with a confirmed diagnosis of HIT compared to patients where HIT was excluded {n=15, 71% vs n=32, 48%; P=0.08}. Using 4Ts score ≥ 4 as a cut-off, an interobserver agreement between reviewer 1 and 2 was moderate with a kappa coefficient {0.60, 95% CI: 0.43-0.77; P < 0.0001}. The median (IQR) HEP score was 3 (1-6). HIT pre-test probability was likely in 65 patients (74%) based on HEP scores ≥ 2. There was also a significantly higher median (IQR) HEP score in patients with confirmed HIT {6 (3-9) vs 3 (1-5); p=0.003}. However, there was no significant difference in the proportion of patients that had a HEP score ≥ 2 in patients with a confirmed diagnosis of HIT compared to patients where HIT was excluded {n=17, 81% vs n=48, 72%; P=0.57}. Using HEP scores ≥ 2 as a cut-off, an interobserver agreement between reviewer 1 and 2 was weak with a kappa coefficient {0.53, 95% CI: 0.33-0.73; P < 0.0001}. The median (IQR) anti-PF4-heparin antibody OD 0.8 (0.6-1.7). The median (IQR) anti-PF4-heparin antibody OD was also significantly higher in patients with confirmed HIT {2.1 (0.8-2.4) vs 0.7 (0.6-1.4); P < 0.0001}. Lastly, there was a significant mildly positive correlation between anti-PF4-heparin antibody OD and both the HEP score {r: 0.21, 95% CI: 0.0004-0.40; P=0.0003} and 4Ts score {r: 0.09, 95% CI: -0.1-0.30, P < 0.0001}.
The sensitivity, positive predictive value, negative predictive value and positive likelihood ratio were superior in our clinical-laboratory algorithm compared to the 4Ts score ≥ 4 and the HEP score ≥ 2. In addition, the algorithm’s specificity was non-inferior compared with the 4Ts score and HEP score. The specificity and positive predictive value increase with higher cutoffs of OD while maintaining a high negative predictive value. The current cut off for a positive heparin antibody at our institution is 0.50, which has a high false positive rate of 81%. Table 2 demonstrates all accuracy measures. AUROC analysis demonstrated that our clinical-laboratory algorithm was non-inferior in comparison to the 4Ts and the HEP score (Figure 2). Our clinical-laboratory algorithm’s AUROC was 0.71 (95% CI: 0.60-0.80). There was no significant difference between our clinical-laboratory algorithm and the 4Ts score {AUC: 0.64, 95% CI: 0.53-0.74; P=0.35} or the HEP score {AUC: 0.71, 95% CI: 0.61-0.81; P=0.87} in detecting HIT. There was no significant difference between the 4Ts score and the HEP score in detecting HIT either (P=0.14).
Table 2.
Sensitivity, specificity, positive and negative predictive value and positive and negative likelihood ratios for the clinical-laboratory algorithm, 4Ts score ≥ 4, HEP score ≥ 2 and several OD cutoffs
| Statistic | Clinical-laboratory algorithm | 4Ts score ≥ 4 | HEP score ≥ 2 | OD ≥ 0.5 | OD ≥ 1.0 | OD ≥ 1.50 | OD ≥ 2.0 |
|---|---|---|---|---|---|---|---|
| Sensitivity (95% CI) | 0.90 (0.70-0.99) | 0.71 (0.48-0.89) | 0.81 (0.58-0.95) | 0.95 (0.76-1.00) | 0.67 (0.43-0.85) | 0.67 (0.43-0.85) | 0.57 (0.34-0.78) |
| Specificity (95% CI) | 0.51 (0.38-0.63) | 0.52 (0.40-0.65) | 0.28 (0.18-0.41) | 0.19 (0.11-0.31) | 0.67 (0.55-0.78) | 0.79 (0.67-0.77) | 0.94 (0.85-0.98) |
| Positive predictive value (95% CI) | 0.37 (0.30-0.43) | 0.32 (0.24-0.40) | 0.26 (0.22-0.31) | 0.27 (0.24-0.30) | 0.39 (0.29-0.50) | 0.50 (0.36-0.64) | 0.75 (0.52-0.89) |
| Negative predictive value (95% CI) | 0.94 (0.82-0.98) | 0.85 (0.74-0.92) | 0.83 (0.65-0.93) | 0.93 (0.64-0.99) | 0.50 (0.26-0.93) | 0.88 (0.80-0.93) | 0.88 (0.81-0.92) |
| Positive likelihood ratio (95% CI) | 1.84 (1.39-2.43) | 1.50 (1.03-2.16) | 1.13 (0.87-1.46) | 1.18 (1.02-1.37) | 2.0 (1.3-3.2) | 3.2 (1.8-5.6) | 9.6 (3.5-26.5) |
| Negative likelihood ratio (95% CI) | 0.19 (0.05-0.72) | 0.55 (0.27-1.12) | 0.67 (0.26-1.75) | 0.25 (0.03-1.77) | 0.50 (0.26-0.93) | 0.42 (0.23-0.78) | 0.46 (0.28-0.75) |
Figure 2.
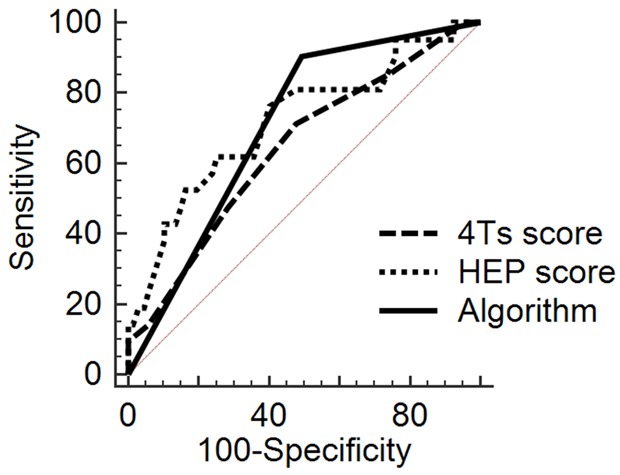
Comparison of the 4Ts score, HEP score and our clinical-laboratory algorithm’s receiver operating characteristic curves.
Our clinical-laboratory algorithm recommended alternative anticoagulation use in 19 out of the 21 patients (90%) with confirmed HIT and in 33 out of 67 patients (49%) without HIT. The algorithm would have avoided inappropriate alternative anticoagulation use in 15 out of 33 patients (45%) without HIT that was initiated on alternative anticoagulation.
Discussion
HIT is heparin’s most clinically relevant non-hemorrhagic complication. HIT can be lethal and early diagnosis and management are pivotal. To this date, HIT’s clinical diagnosis still poses a significant challenge and its dependent on a combination of clinical and laboratory variables. In the present study, we aimed to retrospectively validate a clinical-laboratory algorithm recently introduced to our institution in an ICU setting. In comparison with the 4Ts and the HEP score, the algorithm demonstrated higher sensitivity, positive predictive value and negative predictive value. Further, AUROC analyses demonstrated that the algorithm was non-inferior to both scores in predicting HIT. We conclude that our algorithm is advantageous over the 4Ts and the HEP score.
Several studies have revealed that a 4Ts score ≥ 4 is a good screening test with high sensitivity and negative predictive value, but it lacked specificity and had a low positive predictive value, resulting in high false positive rates [6,10]. This led to further efforts by 26 HIT experts to establish the HEP score that included variables not present in the 4Ts score [8]. According to Cuker et al who compared HEP scoring system with the 4Ts system, the specificity was higher for HEP when using the lower cut-off screening score of 2 or more (0.60, CI 0.45-0.75) compared to 4Ts score (0.44, CI 0.29-0.60) [7]. Given the lack of specificity of clinical scores and readily available laboratory tests; we attempted to combine clinical and laboratory findings to establish an improved screening test. Prior to this study, a pilot study was conducted and an OD ≥ 1.50 was found to have the highest concurrent sensitivity and specificity. Thus, we recommend the use of higher cut off values of OD test when screening for HIT in ICU patients with low probability 4Ts score especially if there is uncertainty about the 4Ts score calculation. Since uncertainty in the 4Ts score calculation is common we decided to include patients with low probability 4Ts score (≤ 3) in our algorithm. Combining low probability 4Ts score along with a high OD further increases sensitivity and decreases the rate of false negatives, that would otherwise be missed in the absence of anti-PF4-heparin antibody. Further, an OD cut-off of 0.4-0.5 has been used by many institutions, with good sensitivity and NPV, however, it lacks the needed specificity and PPV needed to reliably guide management before the result of SRA test is back [4,11]. Therefore, a lower OD threshold is preferred in patients with intermediate to high probability 4Ts score. The former served as our basis to set the criteria used in our algorithm. Multiple studies have previously combined clinical and laboratory criteria aiming to provide an enhanced screening tool [12,13]. Denys et al combined the 4Ts score ≥ 4 with an OD ≥ 0.5 to diagnose patients with suspected HIT [12]. In his study of 102 patients, he reported that his clinical-laboratory approach was an excellent screening method, with 100% NPV. In addition, it increased the pretest probability from 6.5% to 15.3% in intermediate risk patients and from 66.7% to 84% in high-risk patients [12]. Moreover, Ruf et al also used a clinical-laboratory algorithm in a retrospective review of 83 patients, 50 of which were ICU patients [13]. According to their algorithm, HIT was considered likely if the OD was ≥ 1 regardless of the 4 T, or OD > 0.4 with a high 4Ts score, otherwise HIT was considered unlikely. They found that the sensitivity of this approach was 90% and specificity of 82.2%. Mirroring these studies, our clinical-laboratory algorithm has demonstrated increased sensitivity of 90% with a negative predictive value of 94% while still maintaining moderate specificity comparable to both the HEP and 4Ts score. However, there was no significant difference in the AUC observed between all three scores. Our study is most likely underpowered to detect such a difference. Further, the interobserver agreement was slightly superior when calculating the 4Ts score in comparison with the HEP score suggesting that the application of the algorithm (comprises the 4Ts score) is easier. In contrast, Cuker A et.al found the HEP score to have greater inter-observer agreement compared with the 4Ts score [7]. Due to the heterogeneous results in the existing literature, the score with the higher interobserver agreement is yet to be elucidated.
Multiple risk factors have been associated with HIT. The currently identified risk factors include the use of UFH, higher doses and longer duration of heparin therapy, surgical interventions and female gender [14,15]. Coinciding with these studies, most patients in the confirmed HIT group were surgical patients. Furthermore, patients in the confirmed HIT group were more likely to have received therapeutic doses of heparin. There was otherwise no significant difference in the baseline patient characteristics between both groups dignifying a non-biased patient cohort. Despite a higher incidence of thromboembolic events in the confirmed HIT group, this difference was not statistically significant. This can be potentially explained by the early and high rate of alternative anticoagulation initiation in both groups (90% in confirmed HIT group vs 49% in the HIT excluded group). However, our clinical-laboratory algorithm would have avoided inappropriate alternative anticoagulation use in 15 out of 33 patients (45%) without HIT that was initiated on alternative anticoagulation. This is vital as it decreases the risk of complications associated with anticoagulation with these novel agents.
We acknowledge certain limitations of this study. Reviewing our inclusion criteria, only patients that had the HIT laboratory workup ordered were included in our patient population. This might have introduced a selection bias, where only patients at high risk for HIT were included in our study. This is further confirmed by a significantly higher median (IQR) 4Ts and HEP scores observed in our analyses. Also, there was no significant difference in the proportion of patients that had a 4Ts score ≥ 4 or a HEP score ≥ 2 in patients with a confirmed diagnosis of HIT compared to patients where HIT was excluded. Nevertheless, our patient population is consistent with the latest guidelines where HIT workup would be avoided in patients with low 4T score [16]. Also, our algorithm was not confirmed or evaluated by an expert committee or a second examiner. However, the proposed algorithm is pragmatic and would be easily utilized by clinicians at the bedside. Last, while our study provides useful comparative data, the results are limited by the single-center, retrospective design and small sample size. To be able to draw powerful conclusions, a larger multi-institutional prospective study is imperative.
Conclusion
In conclusion, our study confirms that the combination of clinical and laboratory criteria is crucial in the presumable diagnosis of HIT. This is the first study that validates a combined clinical-laboratory algorithm and compares different HIT evaluating clinical scores in an isolated ICU population. In comparison with the 4Ts and the HEP score, the algorithm demonstrated higher sensitivity, positive predictive value and negative predictive value confirming that our algorithm is a reliable strategy to rule in or rule out HIT. Further, the interobserver agreement was slightly more superior when calculating the 4Ts score in comparison with the HEP score. However, due to the limited number of patients in our cohort; larger multi-institutional studies are essential to validate this algorithm.
Acknowledgements
Dr. Moustafa Younis and the other authors did not receive any honoraria, grants, or other forms of payment to produce the manuscript.
Informed consent was obtained from all individual participants included in the study.
Disclosure of conflict of interest
None.
Abbreviations
- HIT
Heparin-Induced thrombocytopenia
- OD
Optical density
- HEP
HIT expert probability
- UH
Unfractionated heparin
- LMWH
Low molecular weight heparin
- PF4
Platelet factor 4
- ICU
Intensive care unit
- SRA
Serotonin release assay
- BMI
Body mass index
- CI
Confidence intervals
- SD
Standard deviation
- IQR
Interquartile range
- AUROC
Area under receiver operative characteristic
References
- 1.Linkins LA, Dans AL, Morres LK, Bona R, Davidson BL, Schulman S, Crowther M. Treatment and prevention of heparin-induced thrombocytopenia: antithrombotic therapy and prevention of thrombosis, 9th edition: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2015;148:1529. doi: 10.1378/chest.12-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greinacher A, Lubenow N. Thrombosis: fundamental and clinical aspects. Leuven, Belgium: Leuven University Press; 2003. Heparin-induced thrombocytoepnia. [Google Scholar]
- 3.Warkentin TE, Greinacher A, Koster A, Lincoff AM. Treatment and prevention of heparin-induced thrombocytopenia: American college of chest physicians evidence-based clinical practice guidelines (8th Edition) Chest. 2008;133:340S–380S. doi: 10.1378/chest.08-0677. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6:1304–1312. doi: 10.1111/j.1538-7836.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 5.Napolitano LM, Warkentin TE, Almahameed A, Nasraway SA. Heparin-induced thrombocytopenia in the critical care setting: diagnosis and management. Crit Care Med. 2006;34:2898–2911. doi: 10.1097/01.CCM.0000248723.18068.90. [DOI] [PubMed] [Google Scholar]
- 6.Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4:759–765. doi: 10.1111/j.1538-7836.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 7.Cuker A, Arepally G, Crowther MA, Rice L, Datko F, Hook K, Propert KJ, Kuter DJ, Ortel TL, Konkle BA, Cines DB. The HIT expert probability (HEP) score: a novel pre-test probability model for heparin-induced thrombocytopenia based on broad expert opinion. J Thromb Haemost. 2010;8:2642–2650. doi: 10.1111/j.1538-7836.2010.04059.x. [DOI] [PubMed] [Google Scholar]
- 8.Joseph L, Gomes MP, Al Solaiman F, St John J, Ozaki A, Raju M, Dhariwal M, Kim ES. External validation of the HIT Expert Probability (HEP) score. Thromb Haemost. 2015;113:633–640. doi: 10.1160/TH14-05-0472. [DOI] [PubMed] [Google Scholar]
- 9.Lillo-Le Louet A, Boutouyrie P, Alhenc-Gelas M, Le Beller C, Gautier I, Aiach M, Lasne D. Diagnostic score for heparin-induced thrombocytopenia after cardiopulmonary bypass. J Thromb Haemost. 2004;2:1882–1888. doi: 10.1111/j.1538-7836.2004.00949.x. [DOI] [PubMed] [Google Scholar]
- 10.Strutt JK, Mackey JE, Johnson SM, Sylvia LM. Assessment of the 4Ts pretest clinical scoring system as a predictor of heparin-induced thrombocytopenia. Pharmacotherapy. 2011;31:138–145. doi: 10.1592/phco.31.2.138. [DOI] [PubMed] [Google Scholar]
- 11.Whitlatch NL, Perry SL, Ortel TL. Anti-heparin/platelet factor 4 antibody optical density values and the confirmatory procedure in the diagnosis of heparin-induced thrombocytopenia. Thromb Haemost. 2008;100:678–684. doi: 10.1160/th08-02-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denys B, Stove V, Philippe J, Devreese K. A clinical-laboratory approach contributing to a rapid and reliable diagnosis of heparin-induced thrombocytopenia. Thromb Res. 2008;123:137–145. doi: 10.1016/j.thromres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Ruf KM, Bensadoun ES, Davis GA, Flynn JD, Lewis DA. A clinical-laboratory algorithm incorporating optical density value to predict heparin-induced thrombocytopenia. Thromb Haemost. 2011;105:553–559. doi: 10.1160/TH10-09-0610. [DOI] [PubMed] [Google Scholar]
- 14.Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. 2005;106:2710–2715. doi: 10.1182/blood-2005-04-1546. [DOI] [PubMed] [Google Scholar]
- 15.Warkentin TE, Sheppard JA, Sigouin CS, Kohlmann T, Eichler P, Greinacher A. Gender imbalance and risk factor interactions in heparin-induced thrombocytopenia. Blood. 2006;108:2937–2941. doi: 10.1182/blood-2005-11-012450. [DOI] [PubMed] [Google Scholar]
- 16.Cuker A, Arepally GM, Chong BH, Cines DB, Greinacher A, Gruel Y, Linkins LA, Rodner SB, Selleng S, Warkentin TE, Wex A, Mustafa RA, Morgan RL, Santesso N. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2:3360–3392. doi: 10.1182/bloodadvances.2018024489. [DOI] [PMC free article] [PubMed] [Google Scholar]


