See Clinical Research on Page 1235
Patent protection for pharmaceuticals in the United States is very robust and perhaps there is no greater example than epoetin alfa. Since the approval of epoetin alfa by the Food and Drug Administration (FDA) in 1989, the developer, Amgen (Thousand Oaks, CA), has successfully defended its patent against competing agents, such as epoetin beta (Chugai-Upjohn, Rosemont, IL), epoetin delta (Shire, Lexington, MA), and methoxy polyethylene glycol-epoetin beta (CERA; Roche, Basel, Switzerland). The US patent on epoetin alfa expired in 2015, opening the way for competition by products other than Amgen’s own darbepoetin. The first non-Amgen erythropoiesis-stimulating agent (ESA) to enter the US market was CERA, which had previously been approved by the FDA as a new drug under a biologic license application 351(a). Drugs approved through the 351(a) pathway must undergo expensive clinical testing, the cost of which is ultimately passed on to the consumer. ESAs are biologic drugs, defined by the FDA as “a virus, therapeutic serum, toxin, antitoxin, blood, blood component or derivative, allergenic product, protein (except any chemically synthesized polypeptide), or analogous product . . . applicable to the prevention, treatment of cure of a disease or condition of human beings.”
Because they are more structurally complex than small-molecule drugs, biologics are considerably more expensive to develop and produce; those costs also are passed on to the consumer, which limits patient access to important therapeutic agents. A biosimilar agent is defined by the FDA as “a biologic that is highly similar to the reference or originator product, with no clinically meaningful differences in terms of the safety, purity, and potency regardless of minor differences in clinically inactive components.” Biosimilar agents also are expensive to produce, but some of their cost can be decreased by an expedited regulatory approval process in the United States: the biologic license application for biosimilar agents, 351(k) enacted in 2009, which decreases the time and cost burden of the extensive clinical testing required under the 351(a) pathway. The 351(k) pathway is based on the “comparability principle”: if A leads to C, and B is comparable to A, then B leads to C. The burden on the developer of a biosimilar agent is to demonstrate structural, functional, pharmacokinetic, and pharmacodynamic high similarity with the reference biologic. If this is achieved, the 351(k) pathway allows for a significantly lower burden of clinical trial data than that was required for the reference biologic under the 315(a) pathway.1 The enactment of the 351(k) pathway in the United States was driven by very favorable results of a similar pathway to expedite the approval process of lower-cost biosimilar agents in the European Union (EU) where patent protection is not as robust as in the United States. The first biosimilar ESAs were approved in the EU in 2007; it has been estimated that the use of these agents has led to 15% to 30% cost savings versus originator ESAs.2
The primary safety concern with ESAs in general, and biosimilar ESAs in particular, is pure red cell aplasia (PRCA), which results from the development of antibodies against the ESA that cross react with native erythropoietin, leading to the loss of red blood cell precursors in the bone marrow and severe anemia. PRCA has been a significant issue in countries with poor regulation of pharmaceuticals where biosimilar-like (not truly biosimilar by the strict definition of the word) ESAs have entered the market without adequate safeguards regarding manufacturing, packaging, and distribution. Praditpornsilpa et al.3 noted an alarming increase in the prevalence of PRCA in Thailand to 1 in 2068 patients at risk, concomitant with the higher penetration of poorly regulated biosimilar-like epoetins into the market. Such reports have undeservedly tarnished the excellent safety record of true biosimilar ESAs. Nonetheless, clusters of PRCA have been associated with originator ESAs and biosimilar ESAs, usually attributable to packaging issues and not to the inferiority of the ESA molecule. Eprex, an originator epoetin alfa product sold in Europe, was associated with a PRCA cluster that was attributed to the interaction between polysorbate-80 (a stabilizer that replaced human albumin because of concerns regarding prion transmission by a human protein) and the rubber in the gasket of prefilled syringes. Once the problem was identified and the gasket was coated with latex, the cluster of PRCA was arrested.4 Two cases of PRCA occurred with a biosimilar version of epoetin alfa approved in the EU, Binocrit, which interacted with tungsten used to manufacture prefilled syringes.5 Virtually all cases of PRCA occur with subcutaneous (s.c.) administration of the ESA. Following their respective PRCA clusters, s.c. administration of Eprex was contraindicated from 2002 to 2006, and the EU withheld approval for s.c. administration of Binocrit from 2008 to 2016. Therefore, it is vitally important for all newly approved ESAs to demonstrate their safety when administered s.c., particularly with regard to immunogenicity.
In this issue of KI Reports, Fishbane et al.6 describe the results of phase 3 testing of a biosimilar epoetin alfa administered s.c. in hemodialysis patients as part of the US FDA approval process through the 351(k) pathway. This biosimilar epoetin alfa has been designated epoetin alfa-epbx by the nomenclature system recently announced by the FDA to distinguish multiple biosimilars from each other and from the reference product to promote pharmacovigilance. The agent was known as epoetin-hospira during the FDA approval process and has taken the brand name Retacrit in the United States, which is the same as its brand name in the EU, where it was approved in 2007 for both i.v. and s.c. administration. It was approved by the FDA on May 15, 2018, for i.v. and s.c. administration. The results of phase 3 testing of i.v.-administered epoetin alfa-epbx versus originator epoetin alfa in hemodialysis patients have previously been published7 and reveal no significant efficacy or safety differences between the 2 products. In the i.v. study of 612 patients, 5 tested positive for anti-recombinant human erythropoietin antibodies at baseline, and 2 additional patients (1 per study arm) developed anti-recombinant human erythropoietin antibodies while on study treatment. All patients tested negative for neutralizing antibodies, and no patient in either group experienced an event of PRCA. The s.c. study was required by the FDA because, as noted previously, the development of neutralizing antibodies and PRCA occurs almost exclusively in patients treated with s.c. ESAs. In the s.c. study,6 investigators randomized 246 hemodialysis patients who had previously been treated with epoetin alfa (i.v. or s.c.) to s.c. epoetin alfa-epbx or s.c. originator epoetin alfa. They were followed for 16 weeks on study drug; again, efficacy and safety were comparable between the 2 study arms. Three patients (1 treated with epoetin alfa-epbx and 2 treated with reference epoetin alfa) developed anti-recombinant human erythropoietin antibodies while receiving study drug; however, no patient in either treatment arm developed neutralizing antibodies, PRCA, or hypersensitivity consistent with immunogenic response to epoetin.
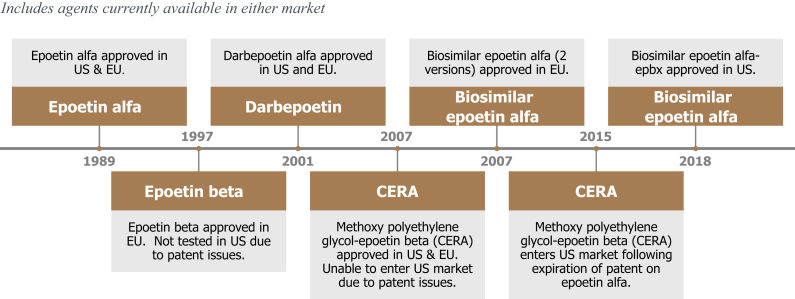
Despite reassurance regarding the lack of neutralizing antibody or PRCA development with a biosimilar ESA such as epoetin alfa-epbx in a highly regulated environment such as the United States or EU, there may be nephrologists who take a conservative approach to the adoption of such agents pending postmarketing experience with many more patients over longer periods of time. They cite the experience with peginesatide, a PEGylated peptide with erythropoietin receptor activity (not a biosimilar protein) that was voluntarily recalled following 49 cases of anaphylaxis-like reactions, including 7 fatalities. These reactions occurred exclusively with the multidose vial formulation of peginesatide, leading to suspicion that an interaction between one of the preservatives and the drug led to its immunogenicity.8 Although peginesatide was not a biosimilar, there remains some confusion if not distrust of the biosimilar category of agents because they have not undergone the same preapproval vetting by the FDA as originator biologics. To address these and other concerns regarding the use of biosimilar agents in nephrology, the National Kidney Foundation held a workshop in 2015 to demystify this class of agents and to provide context regarding their adoption.9 The workgroup acknowledged the need for evidence-based education of providers and patients regarding the advantages (primarily lower cost to patients and the health care system) and disadvantages (unknown risks due to relative lack of clinical experience with fewer patients over shorter duration) of biosimilars versus originator products; the need for postmarketing pharmacovigilance of at least 2 to 4 years (as is done in the EU) to detect safety issues that were not apparent in registration studies; the need for postmarketing studies with patient-reported outcomes that inform choice of therapeutic agent; and the need for increased clarity from the FDA regarding such issues as biosimilar interchangeability, substitution, and extrapolation. Epoetin alfa-epbx does not have an interchangeability designation (a designation the sponsor did not seek), so the pharmacist or prescription drug plan cannot substitute the biosimilar for the originator agent without informing the prescriber. Nonetheless, many prescription drug plans in the United States now have a prior approval process for ESAs that requires the prescriber to use epoetin alfa-epbx as first-line therapy for anemia in patients with non–dialysis-dependent chronic kidney disease. For patients on dialysis, the choice of ESA is driven by the formulary of the dialysis provider. In the United States, the 2 largest dialysis providers have long-term contracts for originator ESAs, so that leaves only 20% of the dialysis market open to penetration by a biosimilar product. Of note is a recent observational study from Japan that suggests the use of shorter-acting ESAs (epoetin alfa, epoetin beta, and a biosimilar of epoetin alfa) was associated lower death rates from all causes, cardiovascular disease, cardiac disease, stroke, non-cardiovascular disease, stroke, and malignancy, than longer-acting ESAs (darbepoetin and CERA).10 The reason for this difference in outcomes is unclear, but a perception that shorter-acting ESAs are safer could accelerate the adoption of the shorter-acting epoetin alfa-epbx over longer-acting alternatives that are also more expensive. The timeline of ESAs currently available in the United States and EU is shown in Figure 1.
Figure 1.
Timeline of erythropoiesis-stimulating agents (ESAs) in the United States and European Union (EU). CERA, methoxy polyethylene glycol-epoetin beta.
The future of all originator and biosimilar ESA therapy is unclear following the potential approval of the hypoxia-inducible factor stabilizer class of drugs, which are orally administered and often effective in patients who are ESA resistant. The oral route of hypoxia-inducible factor stabilizer administration may have the greatest appeal among non-hemodialysis patients, especially those who would otherwise be injecting shorter-acting ESAs, such as epoetin and its biosimilars. However, the value proposition of the hypoxia-inducible factor stabilizers has yet to be clarified, as pricing and long-term safety are not established. The safety of s.c.-administered epoetin alfa-epbx reported in this issue of KI Reports provides reassurance to prescribers, payers, and patients that this new less expensive option in the United States for the treatment of anemia of chronic kidney disease has a place in the current ESA-based paradigm.
Disclosure
JBW is a consultant/advisory board member for AstraZeneca, Akebia, Vifor, and Rockwell Medical. He previously served as a consultant to Hospira/Pfizer. He is on the speaker’s bureau for Akebia.
References
- 1.Wish J.B. The approval process for biosimilar erythropoiesis stimulating agents. Clin J Am Soc Nephrol. 2014;9:1645–1651. doi: 10.2215/CJN.01770214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh S.C., Bagnato K.M. The economic implications of biosimilars. Am J Manag Care. 2015;21(Suppl):s331–s340. [PubMed] [Google Scholar]
- 3.Praditpornsilpa K., Kupatawintu P., Jootar S. The immunogenicity of biosimilar recombinant human erythropoietin (r-HuEpo) administered by subcutaneous injection for the treatment of anemia in patients with chronic kidney disease. Kidney Int. 2011;80:88–92. doi: 10.1038/ki.2011.68. [DOI] [PubMed] [Google Scholar]
- 4.Boven K., Stryker S., Knight J. The increased incidence of pure red cell aplasia with an Eprex formulation in uncoated rubber stopper syringes. Kidney Int. 2005;67:2346–2353. doi: 10.1111/j.1523-1755.2005.00340.x. [DOI] [PubMed] [Google Scholar]
- 5.Seidl A., Hainzl O., Richter M. Tungsten-induced denaturation and aggregation of epoetin alfa during primary packaging as a cause of immunogenicity. Pharm Res. 2012;29:1454–1467. doi: 10.1007/s11095-011-0621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishbane S., Spinowitz B.S., Wisemandle W.A., Martin N.E. Randomized controlled trial of subcutaneous epoetin alfa-epbx versus epoetin alfa in end-stage kidney disease. Kidney Int Rep. 2019;4:1235–1247. doi: 10.1016/j.ekir.2019.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fishbane S., Singh B., Kumbhat S. Intravenous epoetin alfa-epbx versus epoetin alfa for treatment of anemia in end-stage kidney disease. Clin J Am Soc Nephrol. 2018;13:1204–1214. doi: 10.2215/CJN.11631017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotarek J., Stuart C., De Paoli S.H. Subvisible particle content, formulation, and dose of an erythropoietin peptide mimetic product are associated with severe adverse postmarketing events. J Pharm Sci. 2016;105:1023–1027. doi: 10.1016/S0022-3549(15)00180-X. [DOI] [PubMed] [Google Scholar]
- 9.Wish J.B., Charytan C., Chertow G.M. Introduction of biosimilar therapeutics into nephrology practice in the United States: report of a scientific workshop sponsored by the National Kidney Foundation. Am J Kidney Dis. 2016;68:843–852. doi: 10.1053/j.ajkd.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 10.Sakaguchi Y., Hamano T., Wada A., Masakane I. Types of erythropoietin-stimulating agents and mortality among patients undergoing hemodialysis. J Am Soc Nephrol. 2019;30:1037–1048. doi: 10.1681/ASN.2018101007. [DOI] [PMC free article] [PubMed] [Google Scholar]