Abstract
The transmission of zoonotic pathogens associated with wildlife in peri-urban environments can be influenced by the interplay of numerous socioecological factors. Echinococcus granulosus is known to be common within peri-urban wild dog populations however knowledge of the factors that influence its presence is limited. We investigated the demographic distribution of adult cestode abundance (ACA: defined as the product between prevalence of infection and adult cestode infection intensity) and the role of the physical environment, climate and individual factors in determining the geographical variation of E. granulosus infection in wild dog populations from southeast Queensland and surrounds. Our results align with previous studies that show significant E. granulosus aggregation in that 15.8% of peri-urban wild dogs sampled were responsible for ∼70% of the total adult cestode infection intensity. On average, female dogs were found to have a higher ACA than male dogs, and the average ACA generally decreased with age. Significant geographical variation was found in the prevalence of E. granulosus, with a strong propensity for clustering. The average size of clusters was 22.5 km. The probability of finding E. granulosus infection significantly increased with maximum temperature, relative humidity, and rainfall, and after accounting for individual and climatic variables, the model accounted for the majority of the spatial dependence in prevalence. Our predictive map of E. granulosus prevalence in peri-urban wild dogs confirms that E. granulosus is highly endemic in the eastern Australia study area. The prediction map provides a useful tool for targeting potential disease management strategies in peri-urban areas, where broad scale management of wild dog populations is difficult to implement.
Keywords: Echinococcus granulosus, Wild dog, Zoonosis, Hydatid, Dingo, Public health
Graphical abstract
Highlights
-
•
E. granulosus is common in peri-urban wild dog populations.
-
•
E. granulosus worm burdens in peri-urban wild dogs are highly aggregated.
-
•
Bitches and pups have higher adult cestode infection intensity than males and older animals.
-
•
High endemic regions of E. granulosus are present within human developed environs.
1. Introduction
Echinococcus granulosus is a common zoonotic pathogen worldwide (Deplazes et al., 2017; Jenkins et al., 2005). In intermediate hosts (e.g. macropods, livestock), it presents as hydatid cysts in the internal organs, with humans acting as accidental hosts (Jenkins, 2006). In Australia, Echinococcus granulosus (sensu stricto) genotype G1 is currently the only Echinococcus taxa present. Transmission occurs through a predator-prey relationship, with dog-sheep, and/or dog-macropods comprising the primary domestic and sylvatic cycles, respectively. Wild dogs (Canis familiaris), including dingoes, feral domestic dogs, and various cross breeds (Jackson et al., 2017), consume the cysts of infected intermediate hosts, and as definitive hosts of the tapeworm, shed eggs into the environment which become infective to intermediate hosts (Jenkins and MacPherson, 2003). E. granulosus infection remains common in wild dog populations around Australia (Baldock et al., 1985; Grainger and Jenkins, 1996; Harriott et al., 2019b; Jenkins et al., 2008; Jenkins and Morris, 2003) but also occurs occasionally in rural farm dogs (Jenkins et al., 2014) and foxes (Vulpes vulpes) (Jenkins and Craig, 1992). Wild dogs are known to carry large adult cestode infection intensities in comparison to foxes and hence are considered to be the most significant definitive host in terms of transmission potential in Australia (Mackenstedt et al., 2015).
Human cases of hydatid disease (excluding NSW) were notifiable in Australia until 2000. Between 25 and 56 cases were reported annually from 1991 to 2000 (Lin et al., 2002). Little data on human infection of hydatid disease exists post the year 2000. From 2005 through 2018, serological testing at a large Australian private diagnostic laboratory suggests continuing cases. On average, 15 (range 8–26) individuals per year have high titres suggestive of hydatid disease (Jenny Robson, Sullivan Nicollaides Pathology, Brisbane, unpublished data). Many human infections diagnosed in Australia are acquired outside Australia in immigrant populations or travellers, although a significant number of infections from local sources do occur (David Looke, Infection Management Metro South, Princess Alexandra Hospital, Woolloongabba, personal communication). A case of hydatid infection in an urban child, whose only risk factor was an occasional bicycle ride within rural areas where wild dogs are present, has been recorded (Clare Nourse, Children's Health Queensland, Queensland Childrens Hospital, South Brisbane, personal communication). It remains likely that the current presence of the disease within the Australian public is under-reported (Jenkins and Power, 1996; O'Hern and Cooley, 2013).
Incursions of wild dogs into peri-urban environments has emphasised the importance of E. granulosus. Novel data have detailed the movement and home-range patterns of peri-urban wild dogs, assisting our understanding of potential impacts to human health (Allen et al., 2013; McNeill et al., 2016). Swamp wallabies are a staple food source for peri-urban wild dogs (Allen et al., 2016) and consumption of this prey has been found to be positively associated with E. granulosus infection in wild dogs (Harriott et al., 2019a). It is likely that climatic and physical environment variables, as well as individual level traits of each dog (i.e. age and sex) contribute to maintenance of the parasite in a given landscape. To explore these drivers, estimates of the adult cestode abundance (ACA) index were developed to profile environmental factors, dog age and sex groups as contributors to disease transmission. This method is used in schistosomiases studies (Vercruysse et al., 2001) and is beneficial as it takes into account both prevalence and intensity of infection. For the case of E. granulosus, intensity of infection is important, as a small number of animals can be responsible for large adult cestode infection intensities (Jenkins et al., 2008). Then, using model-based geostatistics and data on individual level factors, physical environment and climate, we investigate the spatial variation in the prevalence of E. granulosus within the peri-urban wild dog population. Together, this information will provide evidence for the design of management strategies targeting areas most at risk both at local government and regional levels.
2. Materials and methods
2.1. Study population
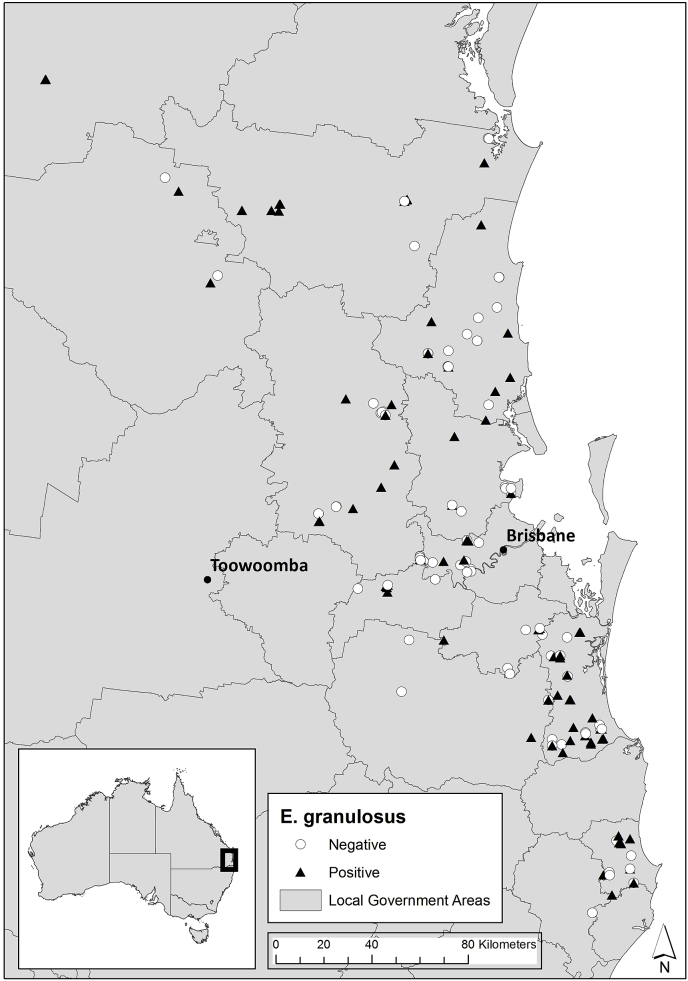
Two hundred and one wild dog carcasses were supplied from local government or private pest management programs undertaken within southeast Queensland and northern New South Wales between August 2012 and May 2015 (Harriott, 2018). The study area and locations of captures can be viewed in Fig. 1. All wild dogs were culled as part of routine pest management programs, and the supply of carcasses approved for necropsy by the University of Queensland Animal ethics committee (approval number SVS/145/13).
Fig. 1.
Geographical locations of trapped peri-urban wild dogs with (▲) or without (○) E. granulosus infections.
2.2. Sources of data
2.2.1. Echinococcus granulosus data
Intestinal contents of peri-urban wild dogs were flushed into a dish and sieved. Adult E. granulosus worms were expelled, with water into a 1000 ml beaker and two 50 ml subsamples were counted under a dissecting microscope, with the subsequent counts corrected to provide the number of worms per 1L of intestinal content (Jenkins et al., 2008). To determine if age or sex groups differ in their adult cestode infection intensities, we calculated the index of adult cestode abundance (ACA) We utilised the methods presented in schistosomiases studies (Vercruysse et al., 2001) and adapted for use with worm burden estimates. The ACA indexconsiders both prevalence and infection intensity. Where previous studies have applied egg counts (eggs per gram of faeces) to calculate an index of potential contamination (IPC), we have applied worm burden data. Adaption of IPC to ACA for E. granulosus data allows for comparison of age and sex groups on their potential to contaminate the environment through their ability to shed infective eggs according to their worm burden. The geometric mean (GM+) of the adult cestode infection intensities was calculated for each age and sex group. To calculate the crude abundance, the prevalence of each age and sex group was multiplied by their corresponding geometric mean. ACA was then calculated by dividing the crude abundance for the age or sex group of interest by the total crude abundance.
2.2.2. Wild dog age
At necropsy, the top jaw of each dog sampled was removed and stored at 4 °C until processing. Jaws were boiled and the canine teeth extracted. Teeth were examined via x-ray (exposure settings 48KVP 1.25 mAs) at the University of Queensland Veterinary Medical Centre. Individual teeth on the x-ray film were analysed digitally using the ‘measure line segment’ tool in SYNAPSE PD-S viewer (Fujifilm®) according to the methods of Knowlton and Whittemore (2001). An estimation of age in months was the calculated as described in Kershaw et al. (2005).
2.2.3. Climate data
Monthly rainfall data of the study area were provided from the Bureau of Meteorology (www.bom.gov.au). Monthly relative humidity (%) and temperature (°C) were provided by collaborating partners Biosecurity Queensland. Data was provided for the states of Queensland and New South Wales.
2.3. Allocation of home-ranges to point data and data extraction from GIS
GPS tracking data of 28 peri-urban wild dogs from within the study area were provided by a concurrent study (McNeill et al., 2016). Home-ranges were calculated using minimum convex polygons in preference to kernel densities to ensure that the complete range of contact with different environmental sources were accounted for within the analyses. Home ranges were then allocated to individual waypoints of wild dog captures according to similarities of age, sex and season. The centroid of a home-range was placed on top of the point data and allocated a matching identifier. This was conducted for all peri-urban wild dogs (n = 201). Vector layers were first converted to raster format prior to extracting the values for both points locations of trapping sites and polygons of home-ranges. For each raster layer, data was extracted at each point utilising the ‘extract values to table’ spatial analyst tool in ArcGIS Desktop 10.5.1 (ESRI Inc, Redlands, CA, USA). The average values of the raster layers at each home-range polygon was also extracted utilising the ‘zonal statistics as table’ tool. The extracted values for point and polygons were correlated using statistical software STATA/IC 13.1 (StataCorp, 2013, College Station, TX, USA). A Pearson's correlation coefficient >0.7 was accepted as equivalent to the extracted value from the point, and the point data was selected to continue further analyses. All data was standardised by subtracting the mean from each individual value and dividing by the standard deviation for each category. The standardised values were then used in the analyses. Extractions of independent variables were conducted in ArcGIS.
2.4. Non-spatial statistical analysis
To investigate the spatial epidemiology of E. granulosus prevalence, the spatial analysis pipeline previously described by Magalhaes et al. (2011) was used. To investigate the role of physical environment and climate in the prevalence of E. granulosus carriage in wild dogs, non-spatial univariable and multivariable logistic regression models were utilised. Nine independent variables were selected, including climatic factors (maximum rain, minimum rain, average rain, minimum relative humidity, maximum relative humidity, minimum temperature, maximum temperature), and significant physical landscape factors (distance to roads, and distance to natural waterways). Independent variables for the climatic factors were calculated according to wet (November to March) and dry (April to October) season. Univariable non-spatial generalised linear model (GLM), (family: Bernoulli, link: logit) were developed using E. granulosus presence as the response variable, age and sex as individual factors, with the physical environment variables and climate data as predictors. Correlations between climatic covariates were investigated using Pearson's correlation coefficients. Variables significant at p < 0.20 were further considered in a multivariable model. The final multivariable model was determined by a backwards stepwise regression. Statistical analyses for the univariable and multivariable models were conducted in STATA/IC 13.1.
2.5. Analysis of spatial dependence
An empirical semivariogram was developed utilising the observed prevalence data at the point location of trapping sites to determine the extent of geographical clustering and the proportion of variance due to spatial factors. A semivariogram can be described by three parameters: the nugget, the partial sill and the range. The nugget represents the variance in the data that is due to non-spatial attributes (e.g. measurement error or random variation). The partial sill represents the variance in the data that is due to factors that determine spatial clustering. The range is the distance at which spatial correlation ends and signifies the average size of clusters of the pathogen. A maximum distance of approximately 55 km (0.5 decimal degrees) was utilised representing half the distance of the short side of the study area. Residuals from the final multivariable model were extracted and used as input to develop a residual semivariogram to address the implications on fitting individual factors, physical environment and climate on the geographical clustering of E. granulosus prevalence. Semivariograms were developed utilising the geoR package of R software (The R Foundation for Statistical Computing, Version 3.3.3).
2.6. Predictive mapping of E. granulosus prevalence
Based on the results of the residual semivariogram (Fig. 2) geostatistical models were built to account for residual spatial variation. A model-based geostatistical Bernoulli model of E. granulosus prevalence was built in OpenBUGS (Lunn et al., 2009). This model included all variables included in the final non-spatial multivariable logistic regression model (maximum rain, average rain, maximum relative humidity, and maximum temperature) individual-level covariates (age and sex) and a geostatistical random effect that accounts for spatial autocorrelation between pairs of locations of wild dogs (measured as by longitude and latitude). The parameter Phi indicates the rate of decay of spatial correlation (measured in decimal degrees, and 3/Phi determines the cluster size; where 1 decimal degree is approximately 111 km at the Equator). The parameter Tau indicates the variance of spatial random effect. Prediction locations and data values were then mapped and extracted in the GIS to be included in the final prediction model. The outputs of the Bayesian model (posterior distributions) included the parameter estimates and spatial prediction at non-sampled locations. The posterior distributions represent uncertainties that are associated with each parameter estimate. The mean of the posterior distribution and the standard deviation of the prediction were mapped in ArcGIS. Female peri-urban wild dogs within the 0–6 month age category were chosen as the age and sex category for spatial prediction, due to their significantly higher adult cestode infection intensities compared to other sex and age groups.
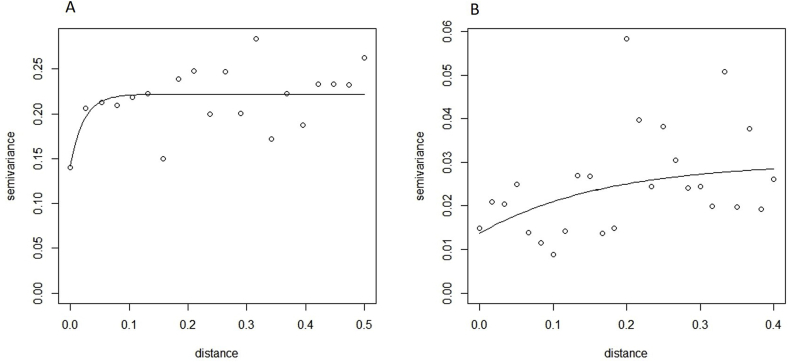
Fig. 2.
(A) Raw semivariogram (B) Residual semivariogram. Distance is measured in Decimal Degrees (1 decimal degree = 110 km at the equator).
3. Results
3.1. Characteristics of peri-urban wild dogs
Males and females were equally represented within the sampled population. Dogs aged below 12 months old were most frequent within the population (57%) with 1–2 year olds, 2–5 year olds and greater than 5 year olds represented at 22%, 10% and 10% respectively. Wild dogs were captured throughout all months of the year with 58% captured during the wet season and 42% captured during the dry season.
3.2. Echinococcus granulosus prevalence and intensity of infection
Wild dogs infected with E. granulosus were caught throughout all regions of the eastern Australia study area. Geographical locations of infected and non-infected wilds dogs are shown in Fig. 1. In total, 101 from 201 (50.7%) of wild dogs within the study area were infected with adult worms within their small intestines. An undetermined proportion of tapeworms had gravid segments. Adult cestode infection intensities recovered from the wild dogs ranged from 40 to 85,950 worms (Table 1). The majority of dogs sampled (∼73%) carried less than 10,000 worms each, which represented only 16.9% of the total worms recovered. A much smaller proportion (15.8%) of dogs carried above 20,000 worms, which represented nearly 70% of total worms recovered. The total adult cestode infection intensity carried by infected wild dogs was 998,856.
Table 1.
Distribution of pathogen intensity in peri-urban wild dogs across southeast Queensland and northeast New South Wales.
| Group | Adult cestode infection intensity | Group size (% of infected animals) | No. of adult cestodes carried by each group (% adult cestodes recovered) |
|---|---|---|---|
| 1 | 1–999 | 30 (29.7%) | 9920 (1%) |
| 2 | 1,000–9,999 | 44 (43.6%) | 158,905 (15.9%) |
| 3 | 10,000–19,999 | 11 (10.9%) | 164,180 (16.4%) |
| 4 | 20,000–90,000 | 16 (15.8%) | 665,860 (66.7%) |
Male and female wild dogs were equally likely to be infected with E. granulosus. However, females carried much higher adult cestode infection intensities, and the ACA index suggests that females contribute to 66% of the adult cestode abundance of E. granulosus (Table 2). ACA values indicate that wild dogs greater than two years of age have a much reduced contribution to adult cestode abundance compared to younger animals (Table 3).
Table 2.
Index of Adult Cestode Abundance (ACA) for E. granulosus for male and female peri-urban wild dogs.
| Sex | n (dogs) | n (infected) | Prevalence (%) | Adult cestode infection intensity (GM+) | Crude ACA | Relative ACA (%) |
|---|---|---|---|---|---|---|
| Male | 97 | 51 | 52.6 | 1785.3 | 93865 | 34.23 |
| Female | 101 | 48 | 47.5 | 3794.4 | 180328 | 65.77 |
Table 3.
Index of Adult Cestode Abundance (ACA) for E. granulosus across five age categories.
| Age Group | n (dogs) | n (infected) | Prevalence (%) | Adult cestode infection intensity (GM+) | Crude ACA | Relative ACA (%) |
|---|---|---|---|---|---|---|
| <6 months | 42 | 22 | 52.4 | 4299.2 | 225195 | 33.9 |
| 6–12 months | 56 | 25 | 44.6 | 3041.6 | 135784 | 20.5 |
| 1–2 years | 36 | 18 | 50.0 | 3552.0 | 177602 | 26.8 |
| 2–5 years | 21 | 12 | 57.1 | 1085.0 | 61999 | 9.3 |
| >5 years | 19 | 12 | 63.2 | 995.2 | 62854 | 9.5 |
3.3. Spatial dependence in E. granulosus prevalence
The raw semivariogram shows significant tendency for E. granulosus clustering with 14% of spatial variance explained by geographical location (Fig. 2A). The semivariogram of the model residuals shows less spatial clustering after fitting the climatic variables (Fig. 2B). The residual semivariogram explains most (92%) of the spatial variation suggesting that other factors remain unaccounted for in the model.
3.4. Bayesian model-based geostatistical model of E. granulosus prevalence
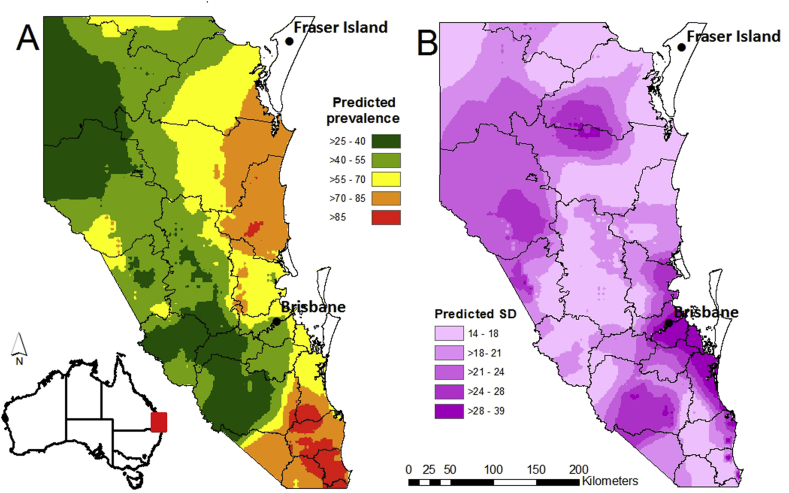
Table 4 presents the estimates of posterior mean for E. granulosus prevalence. After accounting for the covariates the radii of the clusters were 22.5 km. The probability of finding infection increased as maximum rainfall, maximum relative humidity, and maximum temperature increased. However, the results suggest that maximum rainfall was the greatest environmental factor of all the variables. The probability of finding infection also increased in dogs aged one to two years, compared to dogs aged less than six months. The predictive map of E. granulosus infection indicates that prevalence tends to increase from the west towards the east coast (Fig. 3A). Clusters of high prevalence of E. granulosus infection (prevalence >85%) are present in at least three local government regions within the study area. The areas predicted with high prevalence correlate with areas of low uncertainty represented by small standard deviations (Fig. 3B). Large areas of moderate prevalence (>55–70%) correlate with areas of high uncertainty represented by larger standard deviation.
Table 4.
Estimates of posterior mean (in the log odds scale) for E. granulosus prevalence across south eastern Queensland and northern New South Wales, based on Bayesian geostatistical logistic regression models.
| Variable | Posterior mean (95% CI) |
|---|---|
| Female (vs male) | −0.61 (−0.58, 0.02) |
| 6–12 months (vs < 6 months) | −1.17 (−1.05, 0.05) |
| 1–2 years (vs < 6 months) | 0.22 (0.03, 0.19) |
| >2 years (vs < 6 months) | −0.13 (−0.08, 0.035) |
| Maximum Rain | 1.6 (0.17, 1.36) |
| Average Rain | −1.02 (−0.80, 0.15) |
| Maximum RH | 0.59 (0.02, 0.56) |
| Maximum Temperature | 0.39 (0.02, 0.36) |
| Intercept | 0.89 (0.05, 0.82) |
| Rate of decay of spatial autocorrelation (Phi) | 15.21 (0.15, 16) |
| Variance of spatial random effect | 0.89 (0.04,1.13) |
Fig. 3.
(A) Predicted posterior mean prevalence of E. granulosus infection in female peri-urban wild dogs aged 0–6 months. (B) Predicted posterior mean standard deviation.
4. Discussion
Echinococcus granulosus infection is present in half of all sampled peri-urban wild dogs in southeast Queensland and surrounding areas. These results demonstrate that E. granulosus carriage is not associated with sex differences of peri-urban wild dogs. However, there is a slightly higher probability of finding infection in dogs aged between one and two years old, compared to dogs aged less than six months. Although the presence of E. granulosus infection in wild dogs is important, a potentially more significant factor is the adult cestode infection intensity carried by an infected animal, as this relates to their ability to shed large numbers of eggs into the environment. The intensity of infection is greater in female compared to male dogs, and greater in pups (less than six months of age) than animals greater than six months of age. Such high adult cestode infection intensities in females and pups may be related to immunosuppression, due to stress of reproduction or an immature immune system, respectively. It is also possible that the feeding behaviour of wild dogs has a role in E. granulosus infection in wild dogs. Dominant wild dogs will often have first access to the carcass and as a result will consume the preferred organs, such as liver and lungs of the prey. It is possible that females, particularly lactating females will have priority to consume these organs when packs share a prey animal. Hence, males would then consume the remaining tissue that are less likely to be infected by the intermediate stage of the parasite resulting in a reduced ability for heavy infections to develop. Females will also regurgitate their food to feed their young pups (Thomson, 1992) which combined with an immature immune system, may explain the high adult cestode infection intensities found in younger animals. Dominant bitches can be difficult to remove by trapping (the main control technique for peri-urban wild dog populations) and other control techniques are limited in such environs. Greater survivorship of these animals in peri-urban environs could be a significant contributing factor to the ability of the pathogen to survive within the population.
Climatic variables are known to influence the prevalence of infectious pathogens in animals (Dybing et al., 2013; Gordon, 1948). The thick walled characteristics of E. granulosus eggs means that they are highly resistant to adverse climatic effects (Thevenet et al., 2005). The observed prevalence of E. granulosus infection was highly spatially auto correlated which was significantly reduced once the climatic co-variates were fitted. However, not all variables were accounted for and some minor spatial dependence remained. Spatial dependence is common in ecology where sampled values located nearby are more similar than those further apart (Dormann, 2007). Spatial dependence may be reliant on exogenous or endogenous factors (Dormann, 2007). Exogenous factors include climate and other physical environmental structures. Endogenous factors are those often influenced by the biology or ecology of the host species, and are typically more difficult to quantify. Although consideration was given to the variation in home-ranges of peri-urban wild dogs, there are no data available on wild dog densities suitable for model input. The lack of standard measures and methodological inconsistencies for wild dog populations makes it difficult to produce density estimates (Allen et al., 2011). Further attempts to reduce spatial dependence in the current dataset are hampered given the lack of data on density of both wild dogs and intermediate hosts and other unpredictable factors, such as individual behaviour of wild dogs and influence on use of landscape features for denning sites that may affect results. As the values of maximum rainfall, maximum relative humidity and maximum temperature increase, so does the probability of finding E. granulosus infection. However, rainfall is the greatest environmental factor of all the climatic variables. It would be beneficial to examine climatic factors at larger geographical scale. Our results do suggest that E. granulosus appears to be suited to the sub-tropical climate of southern Queensland, in contrast to the tropical regions of the state (northern Queensland) where it has not been detected in wild dogs (Smout et al., 2013, 2018).
E. granulosus infection risk in peri-urban wild dogs is remarkably clustered within defined areas. Areas of high endemicity (with high certainty) have been located within at least three different local government areas in southeast Queensland and northern New South Wales (Fig. 3). Management programs for rural wild dogs utilise a nil-tenure approach where the strategy for control is a broad collaborative effort across all land tenures, based on reduction in overall population numbers (WoolProducers, 2014). Wild dogs living within peri-urban regions are capable of residing within small fragments of urban bushland and are known to have flexible spatial requirements (McNeill et al., 2016). Traditional methods of control are often limited within peri-urban zones, due to legislative restrictions on the use of toxins (Australian Pesticides and Veterinary Medicine Authority, 2008). Importantly, reductions in overall population levels may not have a significant impact on the specific individuals or small groups of wild dogs that post the greatest risk to public health. To manage potential public health issues from E. granulosus, targeted control should be implemented in the predicted high-risk zones. However, culling of wild dogs may not necessarily be the most effective method to cause a reduction of infective eggs in the environment, as a reduction in the general wild dog population is not likely to target the specific age-sex groups that have the highest adult cestode infection intensities. Maintenance of fox populations with targeted praziquantel baiting has proven to be effective in significantly reducing the presence of E. multilocularis eggs within high-risk urban environments across Europe (Hegglin et al., 2003). The predicted areas of high prevalence in southeast Queensland would provide an ideal landscape for targeted praziquantel baiting to reduce E. granulosus prevalence within the peri-urban wild dog population. However, to remain effective, praziquantel baiting would require an ongoing program and would still fail to treat all animals in the population. The distribution of endemicity, but not risk, will differ between ages and sexes. The risk map presented in this study should therefore be considered a conservative estimate of the predicted prevalence of E. granulosus within the population of peri-urban wild dogs, and focuses on the most significant sex and age combination for potential environmental/public health impact.
Quantifying the potential risk to humans is difficult. Direct contact between humans and wildlife is usually limited, especially within urban regions. Data on peri-urban wild dog movements and home-ranges within southeast Queensland has revealed, relatively small home-ranges in comparison to rural living wild dogs, an average travel of 6.86 km per day, and recorded dispersal distances of 25–35 km. However, some peri-urban wild dogs lived in pockets of bushland surrounded by built up regions that provided limited opportunities for dispersal (Allen et al., 2013; McNeill et al., 2016). They utilised town parklands, school grounds, residential yards as well as adjacent areas of bushland and other corridors of vegetation. These findings suggest a potential pathway for transmission of hydatid disease from wild dogs to humans. An increase in reported cases of human alveolar echinococcosis was detected in Switzerland 10–15 years after an increase in the urban fox population (Schweiger et al., 2007). Although current evidence of hydatid disease in humans suggests that transmission is rare, it can take several years to manifest and detect. Hence, long term surveillance and reporting of new cases in urban patients is required to understand if there are any developing impacts from the high prevalence of E. granulosus within the peri-urban wild dog population.
Our findings are important but need to be interpreted with some limitations. Firstly, despite our best efforts, we were unable to collect samples from across all landscapes given the reliance on captures from current management programs which are often conducted in response to public complaints. Secondly, the climate data (temperature and humidity) was based on air measurements and not soil microclimate, which would be of greater relevance to egg survival, but is not available across this landscape. Thirdly, the prevalence risk map is a conservative estimate and does not present the worst case scenario; rather, it represents the sex-age combination of the most significant risk group (female: ≤6 months of age) based on ACA. Finally, a map of ACA could not be produced due to the dispersal of the data set. Mapping the ACA would identify the highest potential environmental contamination areas and hence, would provide an additional, valuable tool for implementation of targeted management programs. Future research should consider their ability to implement mapping of adult cestode abundance in their initial study design.
5. Conclusion
Our results demonstrate that E. granulosus is common in peri-urban wild dog populations across southeast Queensland and surrounding regions including an adjacent area of northern New South Wales. The highest adult cestode abundance of E. granulosus are present in females and young pups. As a result, they are considered to have the greatest potential to contribute to environmental contamination. E. granulosus prevalence in wild dog populations is not associated with the physical environment. However, climatic factors can increase the probability of detecting infection and they account for the majority of spatial dependence in the data. The data presented here describes the locations of clusters with high predicted prevalence. These locations could be candidate areas for targeted management programs to reduce the high prevalence of E. granulosus infection in the wild dog population and reduce the risk of transmission to humans or domestic dogs. Continual monitoring of prevalence and intensity in wild dogs is important to understand the status of the parasite in the definitive host, and the effect of wild dog management programs. It would also be beneficial for future research to understand the greater lifecycle of the parasite, through environmental sampling of soils within the areas of high and low predicted prevalence. This would allow a greater understanding of the availability of infective eggs in the environment to intermediate hosts.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
Collection of peri-urban wild dog cadavers was assisted by local governments and other individuals including but not limited to Ben Allen, Paul Meek, Doug Campbell, Glen Alchin, Suzanne Kallenbach, Warren Driver, Ruth Vicary, Andrew Vicary, Chris Thomas, Darcy Bliesner, Darren Pointon, SEQ Water, Sunshine Coast, Moreton Bay, Brisbane City, Ipswich City, Logan City, Somerset Regional, Gympie Regional, Gold Coast City and Byron Bay Regional Council. Lyn Knott provided technical assistance. Helpful discussions on human hydatid disease were had with Jenny Robson, Clare Nourse and David Locke. Comments on a draft were provided by Segun Osunkoya. This research was conducted with the financial support from the Invasive Animal Co-operative Research Centre, Biosecurity Queensland and the University of Queensland.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.08.005.
Data availability
Data will be made available upon reasonable request.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Allen B.L., Carmelito E., Amos M., Goullet M.S., Allen L.R., Speed J., Gentle M., Leung L.K.P. Diet of dingoes and other wild dogs in peri-urban areas of north-eastern Australia. Sci. Rep. 2016;6:23028. doi: 10.1038/srep23028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen B.L., Engeman R.M., Allen L.R. Wild dogma: an examination of recent "evidence" for dingo regulation of invasive mesopredator release in Australia. Curr. Zool. 2011;57:568–583. [Google Scholar]
- Allen B.L., Goullet M., Allen L.R., Lisle A., Leung L.K.P. Dingoes at the doorstep: preliminary data on the ecology of dingoes in urban areas. Landsc. Urban Plan. 2013;119:131–135. [Google Scholar]
- Australian Pesticides and Veterinary Medicines Authority . environmental assessment.; Canberra, Australia: 2008. Review and Findings for Sodium Monofluroacetate: the Reconsideration of Registrations and Products Containing Sodium Monofloroacetate and Approvals of Their Associated Labels. [Google Scholar]
- Baldock F.C., Thompson R.C., Kumaratilake L.M., Shield J. Echinococcus granulosus in farm dogs and dingoes in south eastern Queensland. Aust. Vet. J. 1985;62:335–337. doi: 10.1111/j.1751-0813.1985.tb07653.x. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Rinaldi L., Alvarez Rojas C.A., Torgerson P.R., Harandi M.F., Romig T., Antolova D., Schurer J.M., Lahmar S., Cringoli G., Magambo J., Thompson R.C., Jenkins E.J. Global distribution of alveolar and cystic echinococcosis. Adv. Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Dormann C.F. Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Glob. Ecol. Biogeogr. 2007;16:129–138. [Google Scholar]
- Dybing N.A., Fleming P.A., Adams P.J. Environmental conditions predict helminth prevalence in red foxes in Western Australia. Int. J. Parasitol. Parasites Wildl. 2013;13:165–172. doi: 10.1016/j.ijppaw.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon H.M. The epidemiology of parasitic diseases, with special reference to studies with nematode parasites of sheep. Aust. Vet. J. 1948;24:17–45. [Google Scholar]
- Grainger H.J., Jenkins D.J. Transmission of hydatid disease to sheep from wild dogs in Victoria, Australia. Int. J. Parasitol. 1996;26:1263–1270. doi: 10.1016/s0020-7519(96)00109-9. [DOI] [PubMed] [Google Scholar]
- Harriott L. School of Veterinary Science, The University of Queensland; Gatton, Qld: 2018. Prevalence, Risk Factors and Geographical Distribution of Zoonotic Pathogens Carried by Peri-Urban Wild Dogs. PhD Thesis. [Google Scholar]
- Harriott L., Gentle M., Traub R., Soares Magalhães R.J., Cobbold R. The association between diet of periurban wild dogs and zoonotic pathogen carriage. Aust. Mammal. 2019 doi: 10.1071/AM18042. (in press) [DOI] [Google Scholar]
- Harriott L., Gentle M., Traub R., Soares Magalhães R.J., Cobbold R. Zoonotic and economically significant pathogens of peri-urban wild dogs across north-eastern New South Wales and south-eastern Queensland, Australia. Wildl. Res. 2019;46:212–221. [Google Scholar]
- Hegglin D., Ward P.I., Deplazes P. Anthelmintic baiting of foxes against urban contamination with Echinococcus multilocularis. Emerg. Infect. Dis. 2003;9:1266–1272. doi: 10.3201/eid0910.030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.M., Groves C.P., Fleming P.J.S., Alpin K.P., Eldridge M.D.B., Gonzalez A., Helgen K.M. The wayward dog: is the Australian native dog or Dingo a distinct species? Zootaxa. 2017;4317:201–224. [Google Scholar]
- Jenkins D.J. Echinococcus granulosus in Australia, widespread and doing well! Parasitol. Int. 2006;55(Suppl.):S203–S206. doi: 10.1016/j.parint.2005.11.031. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Allen L., Goullet M. Encroachment of Echinococcus granulosus into urban areas in eastern Queensland, Australia. Aust. Vet. J. 2008;86:294–300. doi: 10.1111/j.1751-0813.2008.00327.x. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Craig N.A. The role of foxes Vulpes vulpes in the epidemiology of Echinococcus granulosus in urban environments. Med. J. Aust. 1992;157:754–756. doi: 10.5694/j.1326-5377.1992.tb141276.x. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Lievaart J.J., Boufana B., Lett W.S., Bradshaw H., Armua-Fernandez M.T. Echinococcus granulosus and other intestinal helminths: current status of prevalence and management in rural dogs of eastern Australia. Aust. Vet. J. 2014;92:292–298. doi: 10.1111/avj.12218. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., MacPherson C.N.L. Transmission ecology of Echinococcus in wild-life in Australia and Africa. Parasitology. 2003;127:S63–S72. doi: 10.1017/s0031182003003871. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Morris B. Echinococcus granulosus in wildlife in and around the Kosciuszko National Park, south-eastern Australia. Aust. Vet. J. 2003;81:81–85. doi: 10.1111/j.1751-0813.2003.tb11440.x. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Power K. Human hydatidosis in New South Wales and the Australian capital territory, 1987-1992. Med. J. Aust. 1996;164:18–21. doi: 10.5694/j.1326-5377.1996.tb94103.x. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Romig T., Thompson R.C.A. Emergence/re-emergence of Echinococcus spp. A global update. Int. J. Parasitol. 2005;35:1205–1219. doi: 10.1016/j.ijpara.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Kershaw K., Allen L., Lisle A., Withers K. Determining the age of adult wild dogs (Canis lupus dingo, C. l. domesticus and their hybrids). I. Pulp cavity : tooth width ratios. Wildl. Res. 2005;32:581–585. [Google Scholar]
- Knowlton F., Whittemore S. Pulp cavity-tooth width ratios from known-age and wild-caught coyotes determined by radiography. Wildl. Soc. Bull. 2001;29:239–244. [Google Scholar]
- Lin M., Roche P., Spencer J., Milton A., Wright P., Witteveen D., Leader R., Merianos A., Bunn C., Gidding H., Kaldor J., Kirk M., Hall R., Della-Porta T. Australia's notifiable disease status, 2002. Annual report of the National Notifiable Diseases Surveillance System. Comm. Dis. Intell. 2002;26:118–203. doi: 10.33321/cdi.2002.26.14. [DOI] [PubMed] [Google Scholar]
- Lunn D., Spiegelhalter D., Thomas A., Best N. The BUGS project: evolution, critique and future directions. Stat. Med. 2009;28:3049–3067. doi: 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- Mackenstedt U., Jenkins D., Romig T. The role of wildlife in the transmission of parasitic zoonoses in peri-urban and urban areas. Int. J. Parasitol. Parasites Wildl. 2015;4:71–79. doi: 10.1016/j.ijppaw.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes R.J., Clements A.C., Patil A.P., Gething P.W., Brooker S. The applications of model-based geostatistics in helminth epidemiology and control. Adv. Parasitol. 2011;74:267–296. doi: 10.1016/B978-0-12-385897-9.00005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill A.T., Leung L.K., Goullet M.S., Gentle M.N., Allen B.L. Dingoes at the doorstep: home range sizes and activity patterns of dingoes and other wild dogs around urban areas of north-eastern Australia. Animals (Basel) 2016;6 doi: 10.3390/ani6080048. e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hern J.A., Cooley L. A description of human hydatid disease in Tasmania in the post-eradication era. Med. J. Aust. 2013;199:117–120. doi: 10.5694/mja12.11745. [DOI] [PubMed] [Google Scholar]
- Schweiger A., Ammann R.W., Candinas D., Clavien P.-A., Eckert J., Gottstein B., Halkic N., Muellhaupt B., Prinz B.M., Reichen J., Tarr P.E., Torgerson P.R., Deplazes P. Human alveolar echinococcosis after fox population increase, Switzerland. Emerg. Infect. Dis. 2007;13:878–882. doi: 10.3201/eid1306.061074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout F.A., Thompson A.R.C., Skerratt L.F. First report of Ancylostoma ceylanicum in wild canids. Int. J. Parasitol. Parasites Wildl. 2013;2:173–177. doi: 10.1016/j.ijppaw.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smout F.A., Skerratt L.F., Johnson C.N., Butler J.R.A., Congdon B.C. Zoonotic helminth diseases in dogs and dingoes utilising a shared resource in an Australian aboriginal community. Trav. Med. Infect. Dis. 2018;3:110–124. doi: 10.3390/tropicalmed3040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenet P.S., Jensen O., Drut R., Cerrone G.E., Grenovero M.S., Alvarez H.M., Targovnik H.M., Basualdo J.A. Viability and infectiousness of eggs of Echinococcus granulosus aged under natural conditions of inferior and climate. Vet. Parasitol. 2005;133:71–77. doi: 10.1016/j.vetpar.2005.05.048. [DOI] [PubMed] [Google Scholar]
- Thomson P.C. The behavioural ecology of dingoes in north-western Australia. III. Hunting and feeding behaviour, and diet. Wildl. Res. 1992;19:531–541. [Google Scholar]
- Vercruysse J., Shaw D.J., De Bont J. Index of potential contamination for schistosomiasis. Trends Parasitol. 2001;17:256–261. doi: 10.1016/s1471-4922(01)01937-7. [DOI] [PubMed] [Google Scholar]
- WoolProducers . 2014. National Wild Dog Action Plan: Promoting and Supporting Community-Driven Action for Landscape-Scale Wild Dog Management. (Barton, Australia) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request.