Abstract
Background: Obese patients with chronic HF have a lower mortality than do non-obese patients with heart failure (HF) i.e. “obesity paradox”. We sought to determine the relationship between obesity (defined by body-mass index; BMI) and survival in inotrope-dependent patients with Stage D HF. Methods and Results: We screened the medical records of adults with ACC/AHA stage D HF who were admitted to our institution between January, 2010 and July, 2018 who were both initiated and discharged on continuous intravenous milrinone. Patients were divided into three groups: non-obese patients (Nob-BMI < 30 kg/m2), Class 1 obese patients (Ob1-BMI 30 to 34.9 kg/m2), and class 2/3 obese patients (Ob2/3-BMI ≥ 35 kg/m2). The primary endpoint was all-cause mortality. Of the 233 patients included in the study, 154 were NOb, 39 were Ob1, and 40 were OB2/3. Age and baseline comorbidities did not differ significantly among the groups. Mean follow up was 21.8 months (Median: 12.4, IQ range: 3.6-31.3). Compared to the NOb, relative mortality (HR) was 0.68 for Ob1 patients and 1.21 for Ob2/3 patients (P = 0.30). Adjusting for age, sex, race, and medical comorbidities, relative mortality was 0.85 in the Ob1 and 1.77 in the Ob2/3 (P = 0.08). Conclusion: In this retrospective study of stage D inotrope-dependent HF patients, there was trend of an “obesity paradox” with higher survival in the Ob1 group patients compared to NOb and Ob2/3 patients. Ob2/3 patients had the worst survival.
Keywords: Heart failure, inotropes, left ventricular assist device, milrinone, obesity
Introduction
The age-adjusted prevalence of obesity in the US for 2009 and 2010 was 35.5% and 25.8% among adult men and women, respectively [1]. Obesity adversely affects left ventricular diastolic functions and is a well-recognized independent risk factor for heart failure (HF) [2,3]. Although obesity-associated diseases, such as diabetes mellitus, dyslipidemia, and hypertension, are well-known risk factors for coronary artery disease and ischemic cardiomyopathy, the association of isolated obesity with marked LV systolic dysfunction or dilated cardiomyopathy is unclear [4,5]. Although obesity is associated with a higher incidence of HF, several studies have found that obese patients have lower mortality rates than do non-obese HF patients, once they have an established diagnosis of chronic HF. This relationship has been termed the “obesity paradox” [6,7].
The obesity paradox has been described in patients with HF but not in inotrope-dependent patients with American College of Cardiology/American Heart Association (ACCF/AHA) stage D HF. The 2013 ACCF/AHA guidelines describe stage D HF patients as a subset of patients with chronic HF who continue to progress and develop persistently severe symptoms, despite maximum guideline-directed medical therapy [8]. We sought to determine the relationship of obesity in this subset of end stage D HF patients.
Methods
The Institutional Review Board of The Medical College of Wisconsin approved the study. No informed consent was required.
We reviewed the medical records of all adults with ACC/AHA stage D HF who were admitted to our tertiary care academic hospital between January 2010 and July 2018 and who were both initiated and discharged on continuous intravenous milrinone infusion. Patients were included whether or not they were candidates for advanced heart failure therapies. Patients who were weaned off milrinone before being discharged from the index admission were excluded.
Standard demographic data were obtained from the index admission, when continuous intravenous milrinone was begun. Data on comorbidities, including coronary artery disease, ischemic cardiomyopathy, cerebrovascular disease, chronic kidney disease, chronic obstructive pulmonary disease, and diabetes mellitus were obtained from chart review.
Body mass index (BMI) has traditionally been used as a marker of obesity in clinical trials because it correlates well with the percentage of body fat and body fat mass [9]. Patients were divided into three groups based on their BMI according to the Center for Disease Control and Prevention definition of obesity; (1) Non-obese (NOb) with a BMI < 30 kg/m2, (2) Class 1 Obesity (Ob1) with a BMI 30-34.9 kg/m2, and (3) Class 2 and 3 obesity (Ob2/3) with a BMI ≥ 35 kg/m2. Patients with class 2 and 3 obesity were grouped together due to the small sample size. The endpoint for this study was all-cause mortality.
Statistical methods
Baseline characteristics were compared across groups using unpaired T tests and Chi-square tests, as appropriate. Survival estimates were calculated with the Kaplan-Meier method, and the resulting curves were compared with log-rank tests. Hazard ratios for overall survival were estimated with Cox proportional hazards models. The simultaneous effects of factors considered were estimated with a multivariate proportional hazards model for the incidence of death. The final multivariate model included age, sex, race, heart transplant status and the following medical comorbidities: diabetes mellitus, chronic kidney disease, atrial fibrillation, hypertension, hyperlipidemia, coronary artery disease, cerebrovascular disease and chronic obstructive pulmonary disease. Alpha was set at 0.05, and all tests were two-tailed. Data were analyzed with SAS version 9.4 (The SAS Institute, Cary, NC).
Results
Of the 233 patients enrolled in the study, 154 were NOb, 39 were Ob1, and 40 were Ob2/3. Mean follow up duration was 21.8 ± 20.0 months of follow up (Median: 12.4, IQ range: 3.6-31.3). Demographic and baseline comorbidities did not differ significantly among groups (Table 1). The percentage of patients in both obese groups with diabetes mellitus was significantly higher than that in the non-obese group.
Table 1.
Baseline Characteristics of 233 Patients with Stage D Heart Failure on Long-term Milrinone Infusion
| Characteristic | Overall n = 233 | NOb (BMI < 30 kg/m2) n = 90 | Ob1 (BMI 30 to <35 kg/m2) n = 39 | Ob2/3 (BMI ≥ 35 kg/m2) n = 39 | P value |
|---|---|---|---|---|---|
| Men, n (%) | 154 (66.1) | 105 (68.2) | 27 (69.2) | 21 (53.9) | 0.22 |
| Age, mean (SD), years | 60 (14.2) | 63 (13.9) | 58 (11.9) | 51 (13.6) | 0.001 |
| Race, n (%) | 0.39 | ||||
| White | 131 (56.2) | 89 (57.8) | 23 (59.0) | 18 (46.2) | |
| African American | 102 (43.8) | 65 (42.2) | 16 (41.0) | 21 (53.9) | |
| Body mass Index | 28.8 (8.5) | 24.2 (3.3) | 32.3 (1.5) | 43.3 (8.6) | < 0.001 |
| Ischemic cardiomyopathy, n (%) | 45 (19.3) | 29 (18.8) | 10 (25.6) | 6 (15.4) | 0.50 |
| Diabetes mellitus, n (%) | 79 (33.9) | 41 (26.6) | 18 (46.2) | 20 (51.3) | 0.003 |
| Atrial fibrillation, n (%) | 74 (31.8) | 53 (34.4) | 13 (33.3) | 8 (20.5) | 0.25 |
| Hyperlipidemia, n (%) | 82 (35.2) | 54 (35.1) | 12 (30.8) | 12 (30.8) | 0.63 |
| COPD, n (%) | 26 (11.2) | 16 (10.4) | 5 (12.8) | 5 (12.8) | 0.86 |
| CVD, n (%) | 14 (6.0) | 10 (6.5) | 3 (7.7) | 1 (2.6) | 0.58 |
COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease.
Outcomes
Among 154 NOb, 39 were Ob1, and 40 were Ob2/3 pts, heart transplants were performed in 23 (14.9%), 8 (20.5%), and 3 (7.7%) patients, respectively (P = 0.27), and 49 (31.8%), 13 (33.3%), and 15 (38.5%), respectively, received a left ventricular assist device (LVAD; P = 0.28; Table 2). Mean overall survival was 70.3%, 64.6%, and 55.4% at 6 months, 1 year, and 5 years respectively (Figure 1). Younger age was significantly associated with survival (P < 0.001). The presence of an LVAD resulted in a significantly lower hazard ratio for death (HR, 0.43; P < 0.001). Heart transplantation also had lower hazard ratio for mortality (HR, 0.20; P = 0.11) across all three groups.
Table 2.
Outcomes at hospital discharge for 233 patients with stage D heart failure on long-term milrinone infusion
| Outcome | Overall n = 233 | NOb (BMI < 30 kg/m2) n = 90 | Ob1 (BMI 30 to < 35 kg/m2) n = 39 | OB2/3 (BMI ≥ 35 kg/m2) n = 39 | P value |
|---|---|---|---|---|---|
| LVAD | 72 (30.9) | 43 (27.9) | 12 (30.8) | 17 (43.6) | 0.17 |
| Heart transplant | 34 (14.6) | 23 (14.9) | 8 (20.5) | 3 (7.7) | 0.27 |
| Death | 92 (39.5) | 61 (39.6) | 12 (30.8) | 19 (48.7) | 0.27 |
LVAD, left ventricular assist device.
Figure 1.
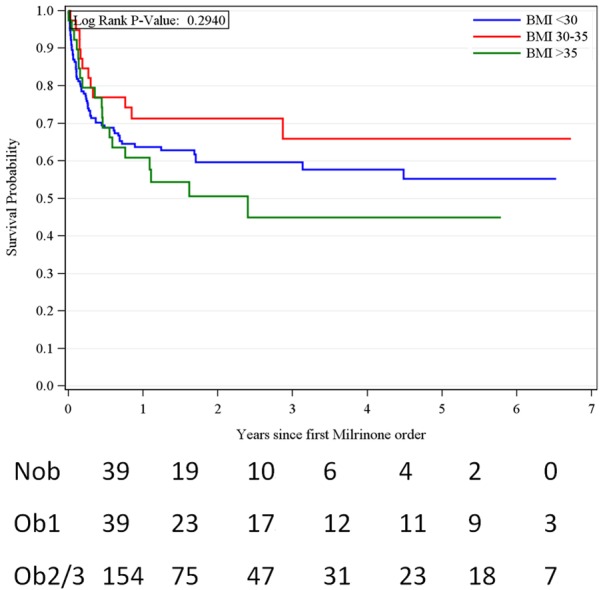
Survival of 233 patients with stage d heart failure on long-term milrinone infusion, by Body Mass Index.
In the unadjusted model, compared to the NOb patients, Ob1 had a mortality hazard ratio of 0.68 (95% CI, 0.37 to 1.27) while Ob2/3 was 1.21 (95% CI, 0.72 to 2.02); P = 0.30 (Table 3). In a multivariate model, after adjusting for age, sex, race, heart transplant and the number of medical comorbidities, the hazard ratio for mortality was 0.85 (95% CI, 0.44 to 1.64) in the Ob1 group and 1.77 (95% CI, 1.00 to 3.14) in the Ob 2/3 group (P = 0.08; Table 4).
Table 3.
Results of the Unadjusted Analysis for the Relationship Between Body Mass Index and Survival among 233 Patients with Stage D Heart Failure on Long-term Milrinone Infusion
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| BMI group (ref BMI < 30 kg/m2) | 0.30 | |
| BMI 30 to 35 kg/m2 | 0.68 (0.37-1.27) | |
| BMI > 35 kg/m2 | 1.21 (0.72-2.02) | |
| Male | 0.80 (0.52-1.21) | 0.29 |
| Age group, years (ref age < 55) | < 0.001 | |
| 55-69 | 1.76 (1.00-3.09) | |
| 70+ | 3.11 (1.73-5.60) | |
| Non-White race | 0.75 (0.49-1.14) | 0.18 |
| LVAD | 0.43 (0.26-0.72) | 0.001 |
| Heart Transplant | 0.20 (0.03-1.49) | 0.12 |
| DM | 1.10 (0.72-1.67) | 0.67 |
| CKD | 0.86 (0.31-2.34) | 0.76 |
| Atrial Fibrillation | 1.47 (0.96-2.24) | 0.07 |
| COPD | 0.54 (0.25-1.18) | 0.12 |
| History of CVA/TIA | 0.60 (0.22-1.64) | 0.32 |
BMI, body mass index; LVAD, left ventricular assist device; DM, diabetes melitis; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVA, cerebral vascular accident; TIA, transient ischemic attack.
Table 4.
Results of the adjusted analysis for the relationship between body mass index and survival among 233 patients with stage D Heart Failure on Long-term Milrinone Infusion
| Variable | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| BMI group (ref BMI < 30 kg/m2) | 0.08 | |
| BMI 30 to 35 kg/m2 | 0.85 (0.44 to 1.64) | |
| BMI > 35 kg/m2 | 1.77 (1.00 to 3.14) | |
| Male | 0.73 (0.47 to 1.15) | 0.17 |
| Age group (ref age < 55) | < 0.001 | |
| 55-69 | 2.37 (1.28 to 4.37) | |
| 70+ | 3.71 (1.90 to 7.26) | |
| Non-White race | 0.82 (0.52 to 1.29) | 0.38 |
| Heart Transplant | 0.22 (0.03 to 1.66) | 0.14 |
Discussion
In this retrospective study, we sought to determine the relationship between BMI and survival in inotrope-dependent patients with ACC/AHA stage D HF. Ob1 patients tended to live longer than NOb patients, a finding consistent with the “obesity paradox” observed in the general population of patients with HF [6,10-12]. The significantly higher mortality in Ob2/3 (morbidly obese) patients is also consistent with previous studies [13]. Heart transplantation and LVAD implantation were associated with markedly improved survival across all BMI groups [14].
Our results are similar to previous studies of the paradoxical relationship between obesity and HF mortality [7,10,11,13,15,16] and cardiovascular outcomes in general [17] in patients with HF. For example, in a meta-analysis (9 studies, 28,209 patients), both obesity (BMI > 30 kg/m2; adjusted HR, 0.88; 95% CI 0.83 to 0.93) and overweight (BMI 25.0 to 29.9 kg/m2; adjusted HR, 0.93; 95% CI 0.89 to 0.97) protected against death in risk-adjusted sensitivity analysis [15]. Another meta-analysis of 6 studies found a similar outcome for 3,000 patients with chronic HF after a mean follow up of 33 years; overweight patients had the lowest total and cardiovascular-related mortality rate, and underweight patients had the highest rate [12]. The obesity paradox is more pronounced in women with advanced HF [18].
The relationship of obesity and survival in advanced HF patients is not well studied. In one study of 501 patients who were referred to heart failure clinic for heart transplant, after a maximum follow up of 3.8 years, event-free survival rates were 48.4%, 57.4%, and 28.6% in the nonobese, obese, and morbidly obese groups, respectively. The non-obese group (HR, 1.44; 95% CI, 1.09 to 1.91; P = 0.01) and the morbidly obese group (HR, 2.46; 95% CI, 1.40 to 4.30; P = 0.002) had markedly higher risks of all-cause mortality than that of the obese group, suggesting. “Obesity paradox” This difference persisted after adjusting for confounding factors. Unlike this study, where patients with difference stages of advanced HF were included only, our study only had a cohort of inotrope-dependent patients only, making our patients significantly sicker. However, similar relationship between obesity and mortality was noted with better prognosis in obese when compared to non-obese.
In our study, a higher percentage of morbidly obese patients received an LVAD, and a higher BMI after LVAD implant was not associated with increased mortality. Others have reported similar findings. Among 222 patients who received LVADs (190 as bridge-to-transplant [BTT] and 32 as destination therapy), with a composite endpoint of survival on LVAD and within 30 days posttransplant among BTT patients, a higher BMI (> 29.4 kg/m2) did not adversely affect survival after LVAD implantation [14]. In general, LVAD therapy is well tolerated in morbidly obese patients, in contrast to outcomes in this population after heart transplantation [19].
Strengths and limitations of the study
Our study is the first to study relationship between obesity and inotrope-dependent patients with ACC/AHA stage D HF. However, this analysis has limitations of small cohort and biases of retrospective study. In addition, the role of BMI to assess obesity has been questioned as it reflects both fat mass and non-fat mass (mostly muscle and skeletal mass); thus, patients with high BMIs might have greatly different body fat compositions. Alternative methods to define obesity, including waist circumference, waist-to-hip-ratio, and percent body fat, may better reflect metabolically unhealthy obesity [9,20,21]. Some studies also suggest that cardiorespiratory fitness should be considered when studying the complications of obesity [22,23]. Nonetheless, our analysis showed that the relationship between obesity and outcomes in HF is complex and the obesity paradox that was noted in general HF patients was also noted in sicker inotrope dependent stage D HF patients. This helps clinicians in prognosticating and triaging patients towards advanced HF therapies such as LVAD and orthotropic heart transplant.
In conclusion, in this retrospective analysis of inotrope-dependent patients with ACC/AHA stage D HF, higher survival in Class 1 obesity compared to non-obese and Class 2/3 obese patients. Class 2/3 (morbidly) obese had the worst survival.
Disclosure of conflict of interest
None.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Reddy YNV, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High-output heart failure: a 15-year experience. J Am Coll Cardiol. 2016;68:473–482. doi: 10.1016/j.jacc.2016.05.043. [DOI] [PubMed] [Google Scholar]
- 4.Pascual M, Pascual DA, Soria F, Vicente T, Hernandez AM, Tebar FJ, Valdes M. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–1156. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan MF, Movahed MR. Obesity cardiomyopathy and systolic function: obesity is not independently associated with dilated cardiomyopathy. Heart Fail Rev. 2013;18:207–217. doi: 10.1007/s10741-012-9320-4. [DOI] [PubMed] [Google Scholar]
- 6.Kenchaiah S, Gaziano JM, Vasan RS. Impact of obesity on the risk of heart failure and survival after the onset of heart failure. Med Clin North Am. 2004;88:1273–1294. doi: 10.1016/j.mcna.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Bozkurt B, Deswal A. Obesity as a prognostic factor in chronic symptomatic heart failure. Am Heart J. 2005;150:1233–1239. doi: 10.1016/j.ahj.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 9.De Schutter A, Lavie CJ, Gonzalez J, Milani RV. Body composition in coronary heart disease: how does body mass index correlate with body fatness? Ochsner J. 2011;11:220–225. [PMC free article] [PubMed] [Google Scholar]
- 10.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 11.Davos CH, Doehner W, Rauchhaus M, Cicoira M, Francis DP, Coats AJ, Clark AL, Anker SD. Body mass and survival in patients with chronic heart failure without cachexia: the importance of obesity. J Card Fail. 2003;9:29–35. doi: 10.1054/jcaf.2003.4. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Lavie CJ, Borer JS, Vallakati A, Goel S, Lopez-Jimenez F, Arbab-Zadeh A, Mukherjee D, Lazar JM. Meta-analysis of the relation of body mass index to all-cause and cardiovascular mortality and hospitalization in patients with chronic heart failure. Am J Cardiol. 2015;115:1428–1434. doi: 10.1016/j.amjcard.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 13.Nagarajan V, Cauthen CA, Starling RC, Tang WH. Prognosis of morbid obesity patients with advanced heart failure. Congest Heart Fail. 2013;19:160–164. doi: 10.1111/chf.12038. [DOI] [PubMed] [Google Scholar]
- 14.Butler J, Howser R, Portner PM, Pierson RN 3rd. Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg. 2005;79:66–73. doi: 10.1016/j.athoracsur.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 15.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. Am Heart J. 2008;156:13–22. doi: 10.1016/j.ahj.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Fall T, Hagg S, Magi R, Ploner A, Fischer K, Horikoshi M, Sarin AP, Thorleifsson G, Ladenvall C, Kals M, Kuningas M, Draisma HH, Ried JS, van Zuydam NR, Huikari V, Mangino M, Sonestedt E, Benyamin B, Nelson CP, Rivera NV, Kristiansson K, Shen HY, Havulinna AS, Dehghan A, Donnelly LA, Kaakinen M, Nuotio ML, Robertson N, de Bruijn RF, Ikram MA, Amin N, Balmforth AJ, Braund PS, Doney AS, Doring A, Elliott P, Esko T, Franco OH, Gretarsdottir S, Hartikainen AL, Heikkila K, Herzig KH, Holm H, Hottenga JJ, Hypponen E, Illig T, Isaacs A, Isomaa B, Karssen LC, Kettunen J, Koenig W, Kuulasmaa K, Laatikainen T, Laitinen J, Lindgren C, Lyssenko V, Laara E, Rayner NW, Mannisto S, Pouta A, Rathmann W, Rivadeneira F, Ruokonen A, Savolainen MJ, Sijbrands EJ, Small KS, Smit JH, Steinthorsdottir V, Syvanen AC, Taanila A, Tobin MD, Uitterlinden AG, Willems SM, Willemsen G, Witteman J, Perola M, Evans A, Ferrieres J, Virtamo J, Kee F, Tregouet DA, Arveiler D, Amouyel P, Ferrario MM, Brambilla P, Hall AS, Heath AC, Madden PA, Martin NG, Montgomery GW, Whitfield JB, Jula A, Knekt P, Oostra B, van Duijn CM, Penninx BW, Smith GD, Kaprio J, Samani NJ, Gieger C, Peters A, Wichmann HE, Boomsma DI, de Geus EJ, Tuomi T, Power C, Hammond CJ, Spector TD, Lind L, Orho-Melander M, Palmer CN, Morris AD, Groop L, Jarvelin MR, Salomaa V, Vartiainen E, Hofman A, Ripatti S, Metspalu A, Thorsteinsdottir U, Stefansson K, Pedersen NL, McCarthy MI, Ingelsson E, Prokopenko I European Network for Genetic and Genomic Epidemiology (ENGAGE) consortium. The role of adiposity in cardiometabolic traits: a Mendelian randomization analysis. PLoS Med. 2013;10:e1001474. doi: 10.1371/journal.pmed.1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma A, Vallakati A, Einstein AJ, Lavie CJ, Arbab-Zadeh A, Lopez-Jimenez F, Mukherjee D, Lichstein E. Relationship of body mass index with total mortality, cardiovascular mortality, and myocardial infarction after coronary revascularization: evidence from a meta-analysis. Mayo Clin Proc. 2014;89:1080–1100. doi: 10.1016/j.mayocp.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Vest AR, Wu Y, Hachamovitch R, Young JB, Cho L. The heart failure overweight/obesity survival paradox: the missing sex link. JACC Heart Fail. 2015;3:917–926. doi: 10.1016/j.jchf.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Foroutan F, Doumouras BS, Ross H, Alba AC. Impact of pretransplant recipient body mass index on post heart transplant mortality: a systematic review and meta-analysis. Clin Transplant. 2018;32:e13348. doi: 10.1111/ctr.13348. [DOI] [PubMed] [Google Scholar]
- 20.De Schutter A, Lavie CJ, Arce K, Menendez SG, Milani RV. Correlation and discrepancies between obesity by body mass index and body fat in patients with coronary heart disease. J Cardiopulm Rehabil Prev. 2013;33:77–83. doi: 10.1097/HCR.0b013e31828254fc. [DOI] [PubMed] [Google Scholar]
- 21.Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–239. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- 22.Clark AL, Fonarow GC, Horwich TB. Impact of cardiorespiratory fitness on the obesity paradox in patients with systolic heart failure. Am J Cardiol. 2015;115:209–213. doi: 10.1016/j.amjcard.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 23.Lavie CJ, Cahalin LP, Chase P, Myers J, Bensimhon D, Peberdy MA, Ashley E, West E, Forman DE, Guazzi M, Arena R. Impact of cardiorespiratory fitness on the obesity paradox in patients with heart failure. Mayo Clin Proc. 2013;88:251–258. doi: 10.1016/j.mayocp.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


