Abstract
Background: Coronary artery bypass grafting a frequent surgical procedure to treat coronary heart disease, uses the patient’s own veins or arteries to bypass narrowed areas and restore blood flow to heart muscle. Cardiac rehabilitation follows this procedure and includes psychological and nutritional support along with the regular practice of physical exercises. Objective: The aim of this study was to investigate the acute effects of the aerobic exercise on the blood pressure of patients after coronary artery bypass grafting. Methods: After 30 days of surgical procedure, 14 patients were assigned to the aerobic exercise group (exercise on the cycle ergometer for 35 minutes), while 8 patients were assigned to the control group (absolute rest for 35 minutes). Blood pressure was measured by a digital automatic device before and after 24 hours of the experiment in both groups. Results: Systolic (P = 0.639) and diastolic (P = 0.103) blood pressures were similar between CG and AEG at baseline. Regarding intragroup differences, no significant changes were observed after 24 hours for SBP in the CG (P = 0.999) and AEG (P = 0.244). On the other hand, significant changes were found for DBP after 24 hours for the CG (P = 0.007) and AEG (P = 0.015). When CG and AEG were compared after 24 hours, no significant differences were found for SBP (P = 0.999) and DBP (P = 0.054). Conclusions: We found decreased diastolic blood pressure in the aerobic exercise group when the results for pre-training and post-training were compared. However, to support our findings further research is needed, preferably using randomized controlled trials.
Keywords: Cardiovascular surgical procedures, rehabilitation, arterial pressure, prevention and control, exercise
Introduction
Coronary artery bypass grafting (CABG) a frequent surgical procedure to treat coronary heart disease, uses the patient’s own veins or arteries to bypass narrowed areas and restore blood flow to heart muscle [1,2]. The surgery is followed by a cardiac rehabilitation program, which includes psychological and nutritional support along with the regular practice of physical exercises [3,4].
In this sense, cardiac rehabilitation plays a fundamental role after CABG and recommendations for exercise protocols are based on the potential benefits, as they provide minimizing morbidity and mortality risks in elderly patients [5]. Although several studies have investigated the effects of a range of physical exercise programs on cardiac rehabilitation after myocardial revascularization (MR), few studies have focused on the acute effects of physical exercise on the blood pressure (BP) of patients following MR [6].
Reduction in BP after exercise (post-exercise hypotension) is defined as the decrease in BP at recovery after the physical exercise [7]. This decrease may be regarded as an important adjuvant against hypertension and may allow a better understanding of regulation of BP. In addition, some studies have demonstrated BP reduction after exercise training (ET) in healthy individuals, and/or in hypertensive patients [8,9]. However, the effects of ET in patients after CABG remain rather understudied. Since most of the individuals undergoing CABG are hypertensive, the impact of physical exercises in this population should be determined, along with their potential role as a non-pharmacological strategy to control BP. The aim of this study was to investigate the acute effects of the aerobic exercise on the BP of patients after CABG. We hypothesize that the practice of aerobic exercise may improve both systolic and diastolic blood pressure control in these patients.
Materials and methods
Patients
The study was performed according to the Helsinki Declaration of 1975 (1983 version). All the procedures here described were approved by the local ethics committee (no. 847485) and written, informed consent was obtained from all patients. This nonrandomized experimental pilot study was carried with middle-aged and elderly patients who underwent CABG, recruited from the waiting list of a cardiac rehabilitation program at the Department of Cardiac Surgery, University Hospital-HUUFMA, President Dutra Unit, São Luís, MA, Brazil. All patients signed an informed consent form after being properly instructed about the study proposal, the procedures they would have to undergo, and their potential risks and benefits.
The inclusion criteria were as such: patients who underwent a successful CABG (no complications during surgery and/or in the following weeks), with normal ejection fraction (> 50%), Class I according to New York Heart Association, and participated in phases I and II of cardiac rehabilitation. Patients were excluded if they had a clinical diagnosis of diabetes mellitus (uncontrolled), hypertension (uncontrolled) according to medical records, presented any disabling pain during exercise performance, and were not able to perform the exercise sessions and/or any of the evaluations. After selecting those who met the inclusion criteria 30 days after CABG, 14 patients were assigned to the aerobic exercise group (AEG), and 8 patients to the control group (CG) according to the characteristics presented in Table 1.
Table 1.
Characteristics of patients participating in the study (n = 22)
| Baseline characteristics | CG (n = 8) | AEG (n = 14) |
|---|---|---|
| Age (years) | 62.7 ± 4.4 | 62.0 ± 6.6 |
| Gender, M/F, n (%) | 07 (87.5)/01 (12.5) | 11 (78.6)/03 (21.4) |
| Body mass index (Kg/m2) | 27.7 ± 1.7 | 27.7 ± 1.1 |
| Diabetes, n (%) | 02 (25) | 01 (7.1) |
| Hypertension, n (%) | 07 (87.5) | 10 (71.4) |
| Medication, n (%) | ||
| Diuretics | 02 (25) | 03 (21.4) |
| Beta-blockers | 04 (50) | 05 (35.7) |
| Angiotensin-converting enzyme inhibitor | 02 (25) | 03 (21.4) |
| Time after surgery (hours) (M ± SD) | 51.5 ± 10.7 | 51.5 ± 10.7 |
| Time of ECC (minutes) (M ± SD) | 123.2 ± 62.7 | 123.2 ± 62.7 |
| Time of Surgery (minutes) (M ± SD) | 257.8 ± 67.9 | 257.8 ± 67.9 |
| Time of hospitalization after surgery (days) (M ± SD) | 23.8 ± 18.9 | 23.8 ± 18.9 |
Data are expressed as mean ± standard deviation or absolute values and percentages. M: male; F: female. CG: Control Group. AEG: Aerobic Exercise Group. *Significant difference between groups.
Procedures
Measurements
From the first to the fifth day visit to the laboratory, anthropometric measurements were carried out, and participants were familiarized with BP measurements and the exercise protocol on the cycle ergometer.
Anthropometric measurements
Total body mass in kilograms (kg) and height in centimeters (cm) were measured using an anthropometric scale (PL-200, Filizola S.A. Pesagem e Automação São Paulo, SP, Brazil), with an accuracy of 50 g and 0.1 cm, properly calibrated (NBR ISO/IEC 17025:2005). Body mass index was determined by body mass (kg) divided by the square of height (m2).
Blood pressure
The procedures for measurement of blood pressure were based on the 7th Brazilian Arterial Hypertension Guidelines [10]. All subjects were instructed to refrain from exercising in the previous 48 hours and from drinking caffeinated beverages and/or alcohol for 24 hours before the evaluation. Briefly, the participants remained in a seated position on a comfortable recliner chair for 15 minutes in a quiet room. After this period, an appropriate cuff was placed at approximately the midpoint of the upper left arm (heart level). An automatic, non-invasive, calibrated, and validated arterial blood pressure monitor (BP785-Omron Healthcare Inc., Lake Forest, IL, USA) was used to measure systolic blood pressure (SBP) and diastolic blood pressure (DBP). Blood pressure was measured before and 24 hours after session (exercise).
Experimental protocol
All AEG patients underwent an aerobic exercise session on the cycle ergometer. The session consisted of the following: 5 minutes of low intensity warm-up (ranging from 7 to 9 CR-10 Borg scale); 25 minutes of moderate intensity exercise (resting heart rate +30 beats and ranging from 11 to 13 CR-10 Borg scale); 5 minutes of low intensity exercise (ranging from 7 to 9 CR-10 Borg scale). CG patients remained in absolute rest for 35 minutes [11,12].
Statistical analysis
Descriptive statistical analysis was performed using Prism software (GraphPad Inc., San Diego, CA, USA, Release 7.0). Continuous variables are presented as mean (± standard deviation) after checking data normality using the Shapiro-Wilk test. Comparisons between the means of the different moments were undertaken by t-test of Student paired t-test sample to compare the initial and final measures in each group and the unpaired t-test of Student to determine the differences between the two groups. All measurements were two-tailed, and p values were calculated with significance level set at < 0.05. Cohen’s effect size (ES) d was calculated to determine the magnitude of the difference between the variables. An effect size between 0.20 and 0.49 was considered small, 0.50 and 0.79 moderate, and an effect size ≥ 0.80 was considered the largest magnitude of effect [13].
Results
Systolic (P = 0.639) and diastolic (P = 0.103) blood pressures were similar between CG and AEG at baseline (Figure 1; Table 2). Regarding intragroup differences, no significant changes were observed after 24 hours for SBP in the CG (P = 0.999) and AEG (P = 0.244) (Figure 1; Table 2). On the other hand, significant changes were found for DBP after 24 hours for the CG (P = 0.007) and AEG (P = 0.015) (Figure 1; Table 2). When CG and AEG were compared after 24 hours, no significant differences were found for SBP (P = 0.999) and DBP (P = 0.054) (Figure 1; Table 2).
Figure 1.
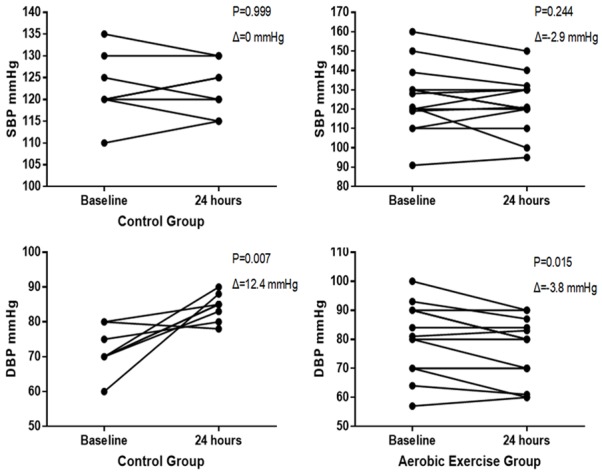
Changes in blood pressure for each participant between the assessment times (Baseline and after 24 hours). Abbreviations: SBP: systolic blood pressure; DPB: diastolic blood pressure.
Table 2.
Comparison and effect size for blood pressure measures baseline and 24 hours after exercise
| Blood Pressure | CG (n = 8) | CG (n = 8) | AEG (n = 14) | AEG (n = 14) | AEG vs. CG | AEG vs. CG |
|---|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | 24 hours | 24 hours | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ∆ | ES | |
| SBP mmHg | 122.5 ± 7.6 | 122.5 ± 6.0 | 125.6 ± 17.2 | 122.7 ± 14.6 | 0.2 | Small |
| DBP mmHg | 71.9 ± 6.5 | 84.3 ± 3.9* | 79.9 ± 12.3 | 76.1 ± 10.8* | -8.2 | Large |
Abbreviations: SD: standard deviation of the mean; CG: control group; AEG: aerobic exercise group; ES: effect size; SBP: systolic blood pressure; DBP: diastolic blood pressure.
Significant difference between Baseline and post moments.
Discussion
The findings of this study point to a reduction in DBP in the population investigated after aerobic exercise of moderate intensity. This effect was demonstrated by a significant decrease in DBP 24 hours after an exercise session. We should to make it clear that we only observed a statistical difference of DBP for the AEG when we compared the measurements before and after 24 hours [(P = 0.015) (Δ = -3.85 mmHg)]. When we compared the groups after 24 hours, no significant difference was found for DBP (P = 0.054); however, we observed a significant decrease in this variable (Δ = -8.17 mmHg). Thus, our findings lend partial support to our hypothesis, given that that we did not find any positive effects of aerobic exercise on SBP in patients after CABG.
On the other hand, Ghashghaei FE, Sadeghi M, Marandi SM, Ghashghaei SE [14] have shown positive effects on SBP (Δ = -14.70 mmHg) and DBP (Δ = -7.64 mmHg) in post-CABG patients after performing 24 combined training sessions (aerobic + resistance). To our knowledge, that is the only study that investigated the acute effects of aerobic exercise on BP in this population. While few studies in the literature investigated acute and chronic effects of exercise on BP in patients after CABG there are several studies demonstrating hypotensive effects in other populations.
Besides reducing the BP of individuals with chronic degenerative diseases, exercise practice also seems to be beneficial to healthy and prehypertensive individuals. In this sense, Cunha RM, Costa AM, Silva CNF, Póvoa TIR, Pescatello LS, & Lehnen AM, [15] have demonstrated that aquatic exercises improves BP in elderly hypertensive women. The researchers found that both of SBP (Δ = -5.1 mmHg) and DBP (Δ = -1.2 mmHg) were decreased in the exercise group when compared to the control group 21 hours after training. A meta-analysis carried out by de Sousa EC, Abrahin O, Ferreira ALL, Rodrigues RP, Alves EAC, & Vieira RP, [16] have found that the practice of resistance training reduced systolic and diastolic blood pressure in prehypertensive and hypertensive individuals. Similarly, Gambassi BB, Rodrigues B, Almeida FDJF, Seguins Sotão S, Sousa TMS, Costa Chaves LF, Ascar Sauaia B, Schwingel PA, Bentivi Pulcherio JO, Mostarda CT, [8] have demonstrated a reduction in SBP (Δ = -16.3 mmHg) and DBP (Δ = -4.8 mmHg) in healthy elderly women using a protocol of resistance exercises with no rest intervals. In addition, a meta-analysis carried out by MacDonald HV, Johnson BT, Huedo-Medina TB, Livingston J, Forsyth KC, Kraemer WJ, Farinatti PTV, Pescatello LS, [17] have shown a beneficial effect of resistance training in adults with hypertension.
Regarding the alterations observed in the present study, we hypothesize that the decrease in DBP may be caused by the reduction in peripheral resistance. This in turn may be due to the increase in nitric oxide production and consequent vascular stress produced by/during training. In this sense, some researchers have focused on the effects of exercise training on peripheral vascular resistance which is directly related to changes in BP. In line with this, de Freitas Brito A, de Oliveira CVC, do Socorro Brasileiro-Santos M, da Cruz Santos A. [18] have observed decreased BP with increased blood flow and resistance reduction in the forearm vascular resistance of elderly individuals after resistance training. However, more research is needed to determine the mechanisms underlying these observed changes.
Our study has some limitations that should be mentioned. The lack of randomization, the small sample size, and the lack of BP control on an ongoing basis may be regarded as limitations. Most of the studies involving exercise and the hypotensive effect of BP are measured continuously (every 10 minutes) after exercise. However, the daily work routine at the Department of Cardiac Surgery, at the University Hospital, associated with poor availability of patients’ time and lack of resources/funding, made it impossible for us to measure BP continuously.
Conclusions
We found decreased DBP in the aerobic exercise group when we compared pre-training to post-training results. However, our findings need further investigation, using randomized controlled trials with continuous monitoring to confirm the benefits of aerobic training to the BP of patients after CABG.
Acknowledgements
Fabiano de Jesus Furtado Almeida and Vinicius José Nina are grateful to the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA). Vinicius José da Silva Nina received financial support from FAPEMA (Grant: APP-Universal 01019/13).
Disclosure of conflict of interest
None.
References
- 1.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ. ESC Committee for Practice Guidelines et al: ESC guidelines on the management of stable coronary artery disease: the task force on the management of stable coronary artery disease. The Eur J Cardiothorac Surg. 2013;46:517–92. [Google Scholar]
- 2.Pêgo-Fernandes PM, Gaiotto FA, Guimarães-Fernandes F. Estado atual da cirurgia de revascularização do miocárdio. Ver Med. 87:92–98. [Google Scholar]
- 3.Ricardo DR, Araujo CGS. Revisão cardíaca com ênfase no exercício: uma revisão sistemática. Revista Brasileira de Medicina do Esporte. v. 12, n. 5, set./out. [Google Scholar]
- 4.Taylor RS, Brown A, Ebrahim S, Jolliffe J, Noorani H, Rees K, Skydmore B, Stone JA, Thompson DR, Oldridge N. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116:682–92. doi: 10.1016/j.amjmed.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germanò G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte Op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F Comitato per Linee Guida Pratiche (CPG) dell’ESC. European guidelines on cardiovascular disease prevention in clinical practice: the fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) G Ital Cardiol (Rome) 2013;14:328–92. doi: 10.1714/1264.13964. [DOI] [PubMed] [Google Scholar]
- 6.de Jesus Furtado F, Gambassi BB, Schwingel PA, Almeida AERAF, Sauaia BA, da Silva Sousa TM, Pulcherio JOB, Rodrigues B, Nina VJ, editors. Possible benefits of different physical exercise programs after coronary artery bypass graft surgery: a minireview of selected randomized controlled trials. Sport Sci Health. 2017;13:477–483. https://doi.org/10.1007/s11332-017-0400-7. [Google Scholar]
- 7.MacDonald JR. Potential causes, mechanisms, and implications of post exercise hypotension. J Hum Hypertens. 2002;16:225–36. doi: 10.1038/sj.jhh.1001377. [DOI] [PubMed] [Google Scholar]
- 8.Gambassi BB, Roddrigues B, de Jesus Furtado Almeida F, Sotão SS, da Silva Souza TM, Chaves LFC, Sauaia BA, Schwingel PA, Pulcherio JOB, Mostarda CT, editors. Acute effect of resistance training without recovery intervals on the blood pressure of comorbidity-free elderly women: a pilot study. Sport Sci Health. 2016;12:315–320. https://doi.org/10.1007/s11332-016-0290-0. [Google Scholar]
- 9.Cardoso CG Jr, Gomides RS, Queiroz AC, Pinto LG, da Silveira Lobo F, Tinucci T, Mion D Jr, de Moraes Forjaz CL. Acute and chronic effects of aerobic and resistance exercise on ambulatory blood pressure. Clinics (Sao Paulo) 2010;65:317–25. doi: 10.1590/S1807-59322010000300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malachias MVB, Souza WKSB, Plavnik FL, Rodrigues CIS, Brandão AA, Neves MF. 7ª Diretriz brasileira de hipertensão arterial. Arq Bras Cardiol. 107:1–103. [Google Scholar]
- 11.Borg G. Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics; pp. 1–104. [Google Scholar]
- 12.American College of Sports Medicine (ACSM) ACSM’s guidelines for exercise testing and prescription. 9th edition. Philadelphia: Lippincott Williams & Wilkins; [Google Scholar]
- 13.Cohen J. Lawrence Erlbaum Associate, Routledge, Hillsdale, Mich, USA. 2nd edition Statistical power analysis for the behavioral sciences. [Google Scholar]
- 14.Ghashghaei FE, Sadeghi M, Marandi SM, Ghashghaei SE. Exercise-based cardiac rehabilitation improves hemodynamic responses after coronary artery bypass graft surgery. ARYA Atheroscler. 2012;7:151–6. [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha RM, Costa AM, Silva CNF, Póvoa TIR, Pescatello LS, Lehnen AM. Postexercise hypotension after aquatic exercise in older women with hypertension: a randomized crossover clinical trial. Am J Hypertens. 2018;31:247–252. doi: 10.1093/ajh/hpx165. [DOI] [PubMed] [Google Scholar]
- 16.de Sousa EC, Abrahin O, Ferreira ALL, Rodrigues RP, Alves EAC, Vieira RP. Resistance training alone reduces systolic and diastolic blood pressure in prehypertensive and hypertensive individuals: meta-analysis. Hypertens Res. 2017;40:927–931. doi: 10.1038/hr.2017.69. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald HV, Johnson BT, Huedo-Medina TB, Livingston J, Forsyth KC, Kraemer WJ, Farinatti PT, Pescatello LS. Dynamic resistance training as stand-alone antihypertensive lifestyle therapy: a meta-analysis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brito Ade F, de Oliveira CV, Brasileiro-Santos Mdo S, Santos Ada C. Resistance exercise with different volumes: blood pressure response and forearm blood flow in the hypertensive elderly. Clin Interv Aging. 2014;9:2151–8. doi: 10.2147/CIA.S53441. [DOI] [PMC free article] [PubMed] [Google Scholar]


