Abstract
Background:
Dogs can act as reservoirs of canine leishmaniasis, caused by Leishmania species. The aims of this study were to determine the prevalence of canine leishmaniasis using a PCR technique among stray dogs living in three provinces of Saudi Arabia, Riyadh, Al-Ahsa Oasis and Al-Qaseem, where the disease is endemic; and to identify and document different Leishmania to species levels
Methods:
This cross-sectional investigation was conducted, from Mar 2016 to Apr 2018, in three parts of Saudi Arabia: Central province (Riyadh), Eastern province (Al-Ahsa Oasis) and Al-Qaseem province. Blood samples were collected from 526 dogs; 40 presented cutaneous nodules so were suspected clinically of cutaneous leishmaniasis. Biopsy tissue collections and parasite cultures were performed. A generic kDNA was performed using different primers for Leishmania differentiation.
Results:
All blood samples were negative for Leishmania infantum infection by molecular analysis, though forty dogs had thick cutaneous lesions in different parts of their body. Four dogs’ skin lesions were associated with dermatitis, splenomegaly and lymphadenomegaly. Parasite culture was used to diagnose cutaneous leishmaniasis, identifying 31/40 (77.5%) positive samples. Overall, of 526 samples, the prevalence of L. major and L. tropica was found to be 4% and 1.9%, respectively. Gender and age had a significant effect on Leishmania prevalence: (P=0.0212 and 0.0357), respectively.
Conclusion:
This was the first molecular study of dog leishmaniasis from Saudi Arabia of dogs confirmed to have cutaneous leishmaniasis. Further epidemiological and molecular investigations of domestic and wild canine infections with L. major, L. tropica and L. infantum in endemic and nonendemic areas of Saudi Arabia are required, for leishmaniasis control.
Keywords: Leishmania, Dogs, kDNA, Saudi Arabia
Introduction
Leishmaniasis is a disease complex of various clinical manifestations caused by protozoan parasite infection (Leishmania spp.), transmitted through infected female sandfly bites (Phlebotomus spp. or Lutzomyia spp.) (1). This widespread disease was reported as one of the nine parasitic infections by WHO and is commonly seen in tropical and subtropical countries (2, 3). Epidemiological studies in the Middle East showed that anthroponotic cutaneous leishmaniasis caused by L.tropica and zoonotic cutaneous leishmaniasis caused by L. major occur in Saudi Arabia, Iraq, Iran, Afghanistan, Pakistan and Yemen (4–6), while visceral leishmaniasis caused by Leishmania infantum is reported in many countries such as Saudi Arabia, Iran, Afghanistan, Egypt, Iraq, and Yemen (5–7).
In Saudi Arabia, cutaneous leishmaniasis (CL) is endemic in different regions (8). The anthroponotic CL - caused by L tropica, transmitted by Ph. sergenti - is more endemic in the southwest and northwest, whereas L. major that causes zoonotic CL (ZCL) - transmitted by sand fly Ph. papatasi, possible reservoir host Psammomys obesus - is more seen in central and eastern regions like Al-Ahsa, Riyadh and Al-Qaseem (9–12). Visceral leishmaniasis (VL) is only reported in southwest Saudi Arabia (13–14).
Infected dogs are potential primary reservoirs (15). Cases of cutaneous and visceral infections caused by L. tropica were reported in Iran and Israel (16–18). Additionally, cases of cutaneous infection in L. major infected dogs have been reported in Saudi Arabia, Egypt, Iraq and Israel (19–22). In Saudi Arabia, although stray dogs have presented with clinical disease associated with Leishmania species infection, there is a lack of information on leishmaniasis, particularly in molecular levels. Most previous studies were epidemiological, clinical, histopathological and biochemical (23,24). Since dogs can be infected by sand flies in endemic areas, infected stray dogs may be a mobile risk for nonendemic surrounding areas (25).
Therefore, the objectives of this study were to detect the prevalence rates of CL and VL based on conventional (PCR) among stray dogs in three endemic Saudi provinces - Riyadh, Al-Ahsa Oasis and Al-Qaseem - and to identify and document different Leishmania at species level using a PCR technique.
Materials and Methods
Study areas
The investigation was conducted from Mar 2016 to Apr 2018 in three parts of Saudi Arabia; central province (Riyadh), Eastern province (Al-Ahsa Oasis) and Al-Qaseem province (Fig. 1). Stray dogs were trapped by live bait straps and selected randomly. A filed examination was set up at each location to examine the dogs. Additionally, dogs at veterinary clinics or pre-euthanasia at the municipal from those areas were included.
Fig. 1:
Map of the study area locations in Saudi Arabia
Blood collections
Blood samples from a total of 526 dogs (279 males and 247 females) of varying ages (0≤1 yr to >1 yr old) were collected; a sample of 2–5 ml from each dog from the cephalic vein into EDTA vacuum tubes (BD Vacutainer® Tube, Gribbles Pathology, VIC, Australia) and transported to the parasitological laboratory, Shaqra University for DNA extraction.
Biopsy tissue collections and parasites cultures
Of 526 dogs, forty dogs were suspected for CL and diagnosed clinically by cutaneous nodules or ulcerated lesions present on skin. Skin biopsies of diameter 5mm were taken under sterile conditions from the border of the ulcer, inoculated into medium M199 supplemented (Gibco, Life Technologies, Germany) with 25 mmol/L HEPES (pH:7.5) and 20% fetal bovine serum (Gibco, Germany) followed by incubation (24 °C). Ten days later, parasites were harvested, washed with ice-cold phosphate-buffered saline (PBS, pH: 7.4) and stored in −20 °C before DNA isolation.
Blood and parasites DNA extraction
DNA from parasite cultures and lesion biopsies was isolated by overnight lysis in NET buffer containing Proteinase K (Sigma) and 1% sodium dodecyl sulfate as described previously (26). For blood samples, a total genomic DNA (gDNA) was isolated using DNeasy Blood and Tissue kit (Qiagen, Hilden, Germany), eluted in 50/100 μl of elution buffer as per manufacturer’s instruction. An aliquot (50 μl − 100 μl of gDNA from each sample) was stored at −80 °C prior to sending to the Molecular laboratory, Plymouth University, for PCR analysis. There, gDNA was stored at −20 °C for up to 1 month prior to diagnostics.
Leishmania PCR
For PCR amplification of multiple pathogenic Leishmania species in a single reaction, a generic kDNA was initially performed to amplify a ∼145 bp region of kDNA minicircles (27). The samples positive for L. infantum, L. donovani, L. major and L. tropica by kDNA-PCR were subjected to further rounds using species-specific primers to distinguish Leishmania at species level (Table 1).
Table 1:
Summary of primers used with sequences (5′-3) in the PCR assay
| Primer name | Primer sequence (5′-3′) | Annealing temperature | Specificity | Reference |
|---|---|---|---|---|
| RV1/RV2 | F: 5′-CTTTTCTGGTCCCGCGGGTAGG-3′ R: 5′-CCACCTGGCCTATTTTACAC-3′ |
62 °C | Leishmania spp | 27 |
| MC1/MC2 | F: 5′-GTTAGCCGATGGTGGTCTTG-3′ R: 5′-CACCCATTTTTCCGATTTTG-3′ |
50 °C | L.infantum/donovani | 36 |
| F/R | F: 5′- TCGCAGAACGCCCCTACC-3′ R: 5′-AGGGGTTGGTGTAAAATAGGC-3′ |
60 °C | L.major /L. tropica | 37 |
PCR reaction was performed in a total volume of 50 μL, containing 10 mM Tris-HCl, (pH 9.0), 1.5 mM MgCl2, 50 mM KCl, 200 μM of each deoxynucleotide triphosphate, 1.5 units of Dream Taq DNA polymerase, (Thermo Scientific™, Nalgene, UK), 1 μM of each related primer (RV1/RV2, MC1/MC2 and F/R), and 2 μL of template DNA (10–20 ng of DNA). The reaction was brought to 50 μL total volume with PCR grade water (Invitrogen, Paisley, UK). Leishmania positive controls were used as reference strains, including; L. tropica (MHOM /Sudan/ 58 OD strain), L. major (MHOM/IL/67/Jericho-II), L. infantum (MHOM/ TN/80/IPT1). Reactions were performed in automated thermal cycler (Veriti; Applied Biosystems, Foster City, USA) and consisted of an initial denaturation step (94 °C/10 min), then 45 cycles of denaturation (95 °C/1 min), primer annealing (62 °C/1.5 min), with an exception for MC1/MC2 primers (50 °C/1.5 min) and F/R primers (62 °C/1 min) and primer extension (72 °C/30 sec). A final extension (72 °C/10 min) was performed and samples held at 4°C. Aliquots of 15 μL PCR product were electrophoresed on a 1.7% agarose gel containing 1μL/mL Syber safe (Thermo Scientific™, UK) in Tris-acetate–EDTA buffer (100V/45 min) and visualized under UV imaging system (ImageQuant Laz4000, GE Healthcare Life Science, UK). The size of each product (e.g.: kDNA minicircles region for L. infantum/donovani complex =447 bp) was estimated by comparison with Gene Ruler 100 bp DNA Ladder Marker (Thermo Scientific™, UK).
Sequencing of Leishmania kDNA
Leishmania species were determined by sending positive samples to Macrogen Europe (Netherlands) to sequence kDNA minicircles using forward primers. Results were compared with sequences available at GenBank database using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The data will be uploaded onto GenBank for future studies.
Statistical analysis
Statistical analyses were performed with SPSS package (ver. 17.0; IBM, New York, USA). The relationship between infection rates and risk factors, such as gender and age, was analyzed using a chi-squared test of significant P-values (P<0.05).
Results
Forty dogs had thick cutaneous lesions (1.5×3 cm) in areas such as mouth, nose, ear, muzzle, abdomen, legs, and between fingers. Ulceration in the left hind foot was noted in a few dogs. Four dogs’ skin lesions were associated with dermatitis, splenomegaly and lymphadenomegaly. Parasite culture was used to diagnose cutaneous leishmaniasis, identified in 31/40 (77.5%) of confirmed positive samples.
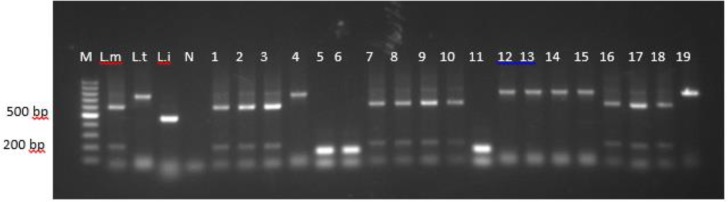
Results obtained from PCR using the RV1/RV2 primer set showed 31 (5.9%) of 526 samples were positive for genus Leishmania. Two of 198 Riyadh dogs identified as infected with Leishmania spp., with 18 of 175 and 11 of 153 found positive in Al-Ahsa Oasis and Al-Qaseem, respectively. Further PCR analysis using MC1/MC2 primers revealed negative infection with the L. infantum/donovani complex. Additional PCR analysis using F/R primers confirmed positive infection for L. major with two amplification bands (∼200 bp and ∼620 bp respectively), and L. tropica in predictable amplification at ∼738 bp (Fig. 2).
Fig. 2:
Agarose gel electrophoresis (1.7%) of amplified DNA from collected samples using F/R. M, 100 bp molecular size marker (Generuler); Lane L.m, L. major-positive control DNA (200 bp and 620bp); L.t L. tropica-positive control DNA (738bp); Li, L. infantum-positive control DNA (447bp); N, negative PCR control (water); Lanes1–19, template DNA isolated from stray dog tissue samples from areas during this study shows positive infection with L. major and L. tropica and negative infection with L. infantum
Blast results showed kDNA sequences of L. major and L. tropica - identified from cutaneous lesions - of 99.9% and 99.8%, similar to Pakistan L. major and Iraq L. tropica kDNA with accession numbers HQ727556.1 and MF166799, respectively. The results showed Al-Ahsa Oasis as the most endemic area, with prevalence of L. major (6.3%) and L. tropica (4%). Al-Qaseem was second: L. major (5.2%), L. tropica (2%) (Table 2). Male dogs were most infected with L. major (5.2%), whereas female dogs were most infected with L. tropica (2.4%). Moreover, most infections with L. major and L. tropica were in dogs over one year old (8.1% and 4.5%, respectively). Dog gender and age had significant effects on parasite prevalence: (P=0.0212 and 0.0357), respectively (Table 3).
Table 2:
Prevalence of Leishmania species infecting stray dogs in different regions of Saudi Arabia
| Location | Leishmania spp. | |||||
|---|---|---|---|---|---|---|
| L. major | L. tropica | L. infantum | ||||
| Positive No (%) | Negative (%) | Positive No (%) | Negative (%) | Positive No (%) | Negative (%) | |
| Riyadh | 2 (1.0) | 196 (99) | 0 | 198 (100) | 0 | 198 (100) |
| Al-Ahsa Oasis | 11 (6.3) | 164 (93.75) | 7 (4.0) | 168 (96.0) | 0 | 175 (100) |
| Al-Qaseem | 8 (5.2) | 145 (94.8) | 3 (2.0) | 150 (98.0) | 0 | 153 (100) |
| Total | 21 (4.0) | 505 (96.0) | 10 (1.9) | 516 (98.1) | 0 | 526 (100) |
Table 3:
Prevalence and Risk factors associated with Leishmania spp. infection among stray dogs in different regions of Saudi Arabia
| Risk Factor | Leishmania spp. | P-value | |||||
|---|---|---|---|---|---|---|---|
| L. major | L. tropica | L. infantum | |||||
| Positive No (%) | Negative (%) | Positive No (%) | Negative (%) | Positive No (%) | Negative (%) | ||
| Gender | 0.02 | ||||||
| Male | 16 (5.7.) | 263 (94.2) | 4 (1.4) | 275 (98.6) | 0 | 279 (100) | |
| Female | 5 (2.0) | 242 (98.0) | 6 (2.4) | 241 (97.6) | 0 | 247 (100) | |
| Total | 21 (4.0) | 505 (96.0) | 10 (1.9) | 516 (98.1) | 0 | 526 (100) | |
| Age group | 0.03 | ||||||
| ≤1 year | 3 (1.0) | 301 (99.0) | 0 | 304 (100) | 0 | 304 (100) | |
| >1 year | 18 (8.1) | 204 (91.9) | 10 (4.5%) | 212 (95.5) | 0 | 222 (100) | |
| Total | 21 (4.0) | 505 (96.0) | 10 (1.9) | 516 (98.1) | 0 | 526 (100) | |
Discussion
This report was the country’s first extensive molecular and epidemiological study describing dog infections with L. major and L. tropica, common agents of cutaneous leishmaniasis in Saudi Arabian and Middle Eastern people (2, 15–17).
The study showed cutaneous lesions are the most common manifestation of leishmaniasis in dogs, in agreement with previous studies (28–30). These dogs had cutaneous involvement in different areas. However, the diagnosis of canine leishmaniasis based on clinical features is difficult, needing specific laboratory tests (31,32). Due to few reports of L. major and L. tropica infections in dogs, more investigations are needed to confirm accurate clinical signs, standardized serological/hematological methods, and chemical/biochemical parameters. We did not attempt histopathological and microscopic examinations due to the limitation of this study.
Several biochemical, histopathological and serological methods have been used to differentially diagnose Leishmania species, but there are drawbacks: being intensive, time-consuming, and requiring estimating 10–20 enzymes and specific parasite isolating media (33). PCR assays have been proven as appropriate tools for Leishmania species identification (34). However, in endemic regions where more than one Leishmania species exists, diagnostic tools are required to detect and distinguish all suspected cases at species level (35).
In this study, electrophoresis data displayed a product size of approximately 145 bp for RV1/RV2 and 620 bp and 780 bp for F/R primers pairs regions of the kDNA of Leishmania spp and of the L. major and L. tropica, respectively. MC1/MC2 primers pairs were used to amplify 447 bp of the L. infantum/donovani complex. These primers were used in differential molecular diagnosis of Leishmania species causing visceral and cutaneous leishmaniasis (36–39). Targeting a region of kDNA minicircles appeared effective for detecting Leishmania spp. because of high copy numbers, variability in amplicon size and detection ability <1pg of Leishmania parasite DNA (40–42).
Overall, prevalence of dog CL in these regions was determined at 31/526 dogs (21 cases L. major and 10 cases L. tropica). Two previous Saudi studies that reported natural infections of L. major in dogs using enzymatic biochemical methods (19, 23), but no clinical information was available, no serology was performed or molecular confirmation done as it was unavailable hence, no treatment has been reported. To our knowledge, few studies have determined L. major as a cause of cutaneous manifestations in dogs; though Egyptian, Iranian, and Iraqi studies have confirmed infection with L. major by enzymatic biochemical and serological methods and molecular tools (20, 21, 36, 38). L. tropica infection in dogs has been reported in few countries such as Morocco (43) and Iran (16,44).
This study found all dogs’ samples were negative of L. infantum infection in examined areas. Earlier studies reported L. infantum has been endemic in the South of Saudi Arabia but has not been reported in the other parts of the country (13,14), suggesting that dogs could be reservoirs of visceral leishmaniasis in this region. However, L. infantum - spread through Phlebotomus species with dogs acting as reservoirs - are the largest source of human L. infantum infection (45, 46). However, in Saudi Arabia, the role of Phlebotomus species in VL transmission is still unknown and surveys that are more molecular are needed.
Our results showed that gender and age significantly affect the prevalence of canine Leishmania infection, with males more infected than females (P>0.0212) and adults more than younger dogs (P>0.0357). Other studies reported dogs under a year old being infected with L. major (22, 29) and both young infected with L. major and L. tropica (16, 30) and dogs over 5 yr old infected with L. tropica (45). The higher leishmaniasis in male and adult dogs in this study might be due to primarily examining males, or attributed to several factors such as dog migration or traveling to regions where flies are common, poor management or body condition leading to weak immunity, stress, and fly preference. However, investigations that are more molecular are required on domestic and wild animals infected with L. major and L. tropica may be more prevalent in endemic areas. Moreover, epidemiological surveys are needed on the role of Phlebotomus species cutaneous and visceral leishmaniasis transmission in Saudi Arabia.
Conclusion
This is a first molecular study of dog leishmaniasis from Saudi Arabia confirmed to have cutaneous leishmaniasis caused by L. major and L. tropica. The relationship between the disease vectors and reservoirs with transmission cycle in endemic areas of Saudi Arabia still unknown. Further epidemiological and molecular investigations are required for implementation of future cutaneous leishmaniasis control programs.
Acknowledgements
This project was kindly supported by Shaqra and Plymouth Universities. The authors thank the Staff Members of the Biological Sciences Department, Shaqra University and of the Molecular Laboratory, Plymouth University for kind technical support.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Ready PD. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014; 6:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012; 7(5):e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Sustaining the drive to overcome the global impact of neglected tropical diseases. Second Who Report on Neglected Tropical Diseases 2013;67–71. [Google Scholar]
- 4.Gonzalez U, Pinart M, Reveiz L, Alvar J. Intervention for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2008. October 8;(4):CD005067. PMID:18843677. [DOI] [PubMed] [Google Scholar]
- 5.Postigo JA. Leishmaniasis in the World Health Organization Eastern Mediterranean Region. Int J Antimicrob Agents. 2010; 36 Suppl 1:S62–5. [DOI] [PubMed] [Google Scholar]
- 6.Control of the leishmaniases. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010 2010. WHO Technical Report Series 949. Geneva: World Health Organization; 2010. (https://apps.who.int/iris/bitstream/handle/10665/44412/WHO_TRS_949_eng.pdf;jsessionid=53910B827409E2DC867730C1DB8F19F 7?sequence=1 (accessed 29 June 2015). [Google Scholar]
- 7.Shirzadi MR, Esfahania SB, Mohebalia M, et al. Epidemiological status of leishmaniasis in the Islamic Republic of Iran, 1983–2012. East Mediterr Health J. 2015; 21(10):736–42. [DOI] [PubMed] [Google Scholar]
- 8.Abuzaid AA, Abdoon AM, Aldahan MA, et al. Cutaneous Leishmaniasis in Saudi Arabia: A Comprehensive Overview. Vector Borne Zoonotic Dis. 2017; 17(10):673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Qurashi AR, Ghandour AM, Osman M, Al-Juma M. Dissemination in cutaneous leishmaniasis due to Leishmania major in different ethnic groups in Saudi Arabia. Int J Dermatol. 2000; 39(11):832–6. [DOI] [PubMed] [Google Scholar]
- 10.Amin TT, Al-Mohammed HI, Kaliyadan F, Mohammed BS. Cutaneous leishmaniasis in Al Hassa, Saudi Arabia: epidemiological trends from 2000 to 2010. Asian Pac J Trop Med. 2013; 6(8):667–72. [DOI] [PubMed] [Google Scholar]
- 11.Al-Tawfiq JA, AbuKhamsin A. Cutaneous leishmaniasis: a 46-year study of the epidemiology and clinical features in Saudi Arabia (1956–2002). Int J Infect Dis. 2004; 8(4):244–50. [DOI] [PubMed] [Google Scholar]
- 12.Alanazi AD, Alyousif MS, Saifi MA, Alanazi IO. Epidemiological studies on cutaneous leishmaniasis in Ad-Dawadimi District, Saudi Arabia. Trop J Pharm Res 2016; 15(12):2709–2712. [Google Scholar]
- 13.Al-Zahrani M, Peters W, Evans D, et al. Leishmania infecting man and wild animals in Saudi Arabia. 6. Cutaneous leishmaniasis of man in the southwest. Trans R Soc Trop Med Hyg 1989; 83(5):621–628. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim EA, al-Zahrani MA, al-Tuwaigri AS, et al. Leishmania infecting man and wild animals in Saudi Arabia. 9. The black rat (Rattus rattus) a probable reservoir of visceral leishmaniasis in Gizan province, southwest Saudi Arabia. Trans R Soc Trop Med Hyg. 1992; 86(5):513–4. [DOI] [PubMed] [Google Scholar]
- 15.Dantas-Torres F, de Brito ME, Brandão-Filho SP. Seroepidemiological survey on canine leishmaniasis among dogs from an urban area of Brazil. Vet Parasitol. 2006; 140(1–2):54–60. [DOI] [PubMed] [Google Scholar]
- 16.Mohebali M, Malmasi A, Hajjaran H, et al. Disseminated leishmaniasis caused by Leishmania tropica in a puppy from Karaj, Central Iran. Iran J Parasitol. 2011; 6(2):69–73. [PMC free article] [PubMed] [Google Scholar]
- 17.Bamorovat M, Sharifi I, Mohammadi MA, et al. Canine visceral leishmaniasis in kerman, southeast of Iran: aseroepidemiological, histopathological andmolecularstudy. Iran J Parasitol. 2014; 9(3):342–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Baneth G, Nasereddin A, Abdeen Z, Jaffe CL. Leishmania tropica infection in wild and domestic canines. Parasit Vectors 2014; 7(Suppl 1):O27.7/S1/O27 [Google Scholar]
- 19.Elbihari S, Kawasmeh ZA, Al Naiem AH. Possible reservoir host (s) of zoonotic cutaneous leishmaniasis in Al-Ahsa oasis, Saudi Arabia. Ann Trop Med Parasitol.1984; 78(5):543–5. [DOI] [PubMed] [Google Scholar]
- 20.Morsy TA, Schnur LF, Feinsod FM, et al. Natural infections of Leishmania major in domestic dogs from Alexandria, Egypt. Am J Trop Med Hyg. 1987; 37(1):49–52. [DOI] [PubMed] [Google Scholar]
- 21.Al-Bajalan MMM, Niranji SS, Al-Jaf SMA, Kato H. First identification of L. major in a dog in an endemic area of human cutaneous leishmaniasis in Iraq. molecular and phylogenetic studies. Parasitol Res. 2018; 117(2):585–590. [DOI] [PubMed] [Google Scholar]
- 22.Baneth G, Nachum-Biala Y, Simon MS, et al. Leishmania major infection in a dog with cutaneous manifestations. Parasites & Vectors 2016; 9(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters W, Elbihari S, Liu C, et al. Leishmania infecting man and wild animals in Saudi Arabia 1. General survey. Trans R Soc Trop Med Hyg 1985; 79(6):831–839. [DOI] [PubMed] [Google Scholar]
- 24.Elbihari S, Cheema AH, el-Hassan AM. Leishmania infecting man and wild animals in Saudi Arabia. 4. Canine cutaneous leishmaniasis in the Eastern Province. Trans R Soc Trop Med Hyg. 1987; 81(6):925–7. [DOI] [PubMed] [Google Scholar]
- 25.Silva RC, Richini-Pereira VB, Kikuti M, et al. Detection of Leishmania (L.) infantum in stray dogs by molecular techniques with sensitive species-specific primers. Vet Q. 2017; 37(1):23–30. [DOI] [PubMed] [Google Scholar]
- 26.Salotra P, Sreenivas G, Pogue GP, et al. Development of a species-specific PCR assay for detection of Leishmania donovani in clinical samples from patients with kala-azar and post-kala-azar dermal leishmaniasis. J Clin Microbiol. 2001; 39(3):849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.le Fichoux Y, Quaranta JF, Aufeuvre JP, et al. Occurrence of Leishmania infantum parasitemia in asymptomatic blood donors living in an area of endemicity in Southern France. J Clin Microbiol. 1999; 37(6):1953–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solano-Gallego L, Koutinas A, Miró G, et al. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniasis. Vet Parasitol. 2009; 165(1–2):1–18. [DOI] [PubMed] [Google Scholar]
- 29.Baneth G, Yasur-Landau D, Gilad M, Nachum-Biala Y. Canine leishmaniosis caused by Leishmania major and Leishmania tropica: comparative findings and serology. Parasit Vectors. 2017; 10(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baneth G, Zivotofsky D, Nachum-Biala Y, et al. Mucocutaneous Leishmania tropica infection in a dog from a human cutaneous leishmaniasis focus. Parasit Vectors. 2014; 7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohebali M, Edrissian GH, Shirzadi MR, et al. An observational study on the current distribution of visceral leishmaniasis in different geographical zones of Iran and implication to health policy. Travel Med Infect Dis. 2011; 9(2):67–74. [DOI] [PubMed] [Google Scholar]
- 32.Sabzevari S, Razmi G, Naghibi A, Khoshnegah J. A study of visceral leishmaniasis in owned dogs with dermal lesions in Mashhad area, Khorasan Razavi province. Vet Res Forum. 2016: 7(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 33.Grimaldi G, Jr, David JR, McMahon-Pratt D. Identification and distribution of new world Leishmania species characterized by serodeme analysis using monoclonal antibodies. Am J Trop Med Hyg. 1987;36(2):270–87. [DOI] [PubMed] [Google Scholar]
- 34.Gadisa E, Genetu A, Kuru T, et al. Leishmania (Kinetoplastida): species typing with isoenzyme and PCR–RFLP from cutaneous leishmaniasis patients in Ethiopia. Exp Parasitol. 2007;115(4):339–43. [DOI] [PubMed] [Google Scholar]
- 35.Al-Jawabreh A, Schoenian G, Hamarsheh O, Presber W. Clinical diagnosis of cutaneous leishmaniasis: a comparison study between standardized graded direct microscopy and ITS1– PCR of Giemsa-stained smears. Acta Trop. 2006;99(1):55–61. [DOI] [PubMed] [Google Scholar]
- 36.Mahboudi F, Abolhassan M, Yaran M, et al. Identification and differentiation of Iranian Leishmania species by PCR amplification of kDNA. Scand J Infect Dis. 2001;33(8):596–8. [DOI] [PubMed] [Google Scholar]
- 37.Cortes S, Rolão N, Ramada J, Campino L. PCR as a rapid and sensitive tool in the diagnosis of human and canine leishmaniasis using Leishmania donovani sl-specific kinetoplastid primers. Trans R Soc Trop Med Hyg. 2004; 98(1):12–7. [DOI] [PubMed] [Google Scholar]
- 38.Hajjaran H, Mohebali M, Mamishi S, et al. Molecular identification and polymorphism determination of cutaneous and visceral leishmaniasis agents isolated from human and animal hosts in Iran. Biomed Res Int 2013;2013: 789326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bakirci S, Bilgiç HB, Köse O, et al. Molecular and seroprevalence of canine visceral leishmaniasis in West Anatolia, Turkey. Biomed Res Int. 2013;2013:789326.24286085 [Google Scholar]
- 40.Ozerdem D, Eroglu F, Genc A, et al. Comparison of microscopic examination, rK39, and PCR for visceral leishmaniasis diagnosis in Turkey. Parasitol Res. 2009; 106(1):197–200. [DOI] [PubMed] [Google Scholar]
- 41.Anders G, Eisenberger CL, Jonas F, Greenblatt CL. Distinguishing Leishmania tropica and Leishmania major in the Middle East using the polymerase chain reaction with kinetoplast DNA-specific primers. Trans R Soc Trop Med Hyg. 2002;96 Suppl 1:S87–92. [DOI] [PubMed] [Google Scholar]
- 42.Bensoussan E, Nasereddin A, Jonas F, et al. Comparison of PCR assays for diagnosis of cutaneous leishmaniasis. J Clin Microbiol. 2006; 44(4):1435–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dereure J, Rioux JA, Gallego M, et al. Leishmania tropica in Morocco: infection in dogs. Trans R Soc Trop Med Hyg. 1991; 85(5):595. [DOI] [PubMed] [Google Scholar]
- 44.Bamorovat M, Sharifi I, Dabiri S, et al. Leishmania tropica in stray dogs in southeast Iran. Iran J Public Health. 2015;44(10):1359–66. [PMC free article] [PubMed] [Google Scholar]
- 45.Azizi K, Rassi Y, Javadian E, et al. Phlebotomus (Paraphlebotomus) alexandri: a probable vector of Leishmania infantum in Iran. Ann Trop Med Parasitol. 2006;100(1):63–8. [DOI] [PubMed] [Google Scholar]
- 46.Mohebali M, Hajjaran H, Hamzavi Y, et al. Epidemiological aspects of canine visceral leishmaniosis in the Islamic Republic of Iran. Vet Parasitol. 2005;129(3–4):243–51. [DOI] [PubMed] [Google Scholar]