Abstract
Background:
Anaplasmosis due to Anaplasma phagocytophilum is an important tick-borne zoonotic disease, which affects dogs, horses, cattle and human as well. This study aimed to probe the existence of this organism by means of molecular biology techniques for the first time in rural dogs of Khuzestan province, Southwestern Iran.
Methods:
During Sep 2014 to Apr 2015 blood samples of 103 apparently healthy rural dogs (60 males) were collected for A. phagocytophilum detection by light microscopical examination of Giemsa stained slides and Nested PCR on a fragment of 16S rRNA gene.
Results:
From the examined slides, 11.65% were positive for A. morulae while 57.28% of infection was revealed by Nested PCR method. There was no statistical difference between ages and sexes of dogs and infection in molecular survey of A. phagocytophilum.
Conclusion:
Molecular prevalence of A. phagocytophilum was noticeably high. It may cause the incidence of disease in human population.
Keywords: Anaplasma phagocytophilum, Dog, Iran, Nested-PCR
Introduction
Anaplasma phagocytophilum (Order, Rickettsiales; Family, Anaplasmataceae) is an emerging zoonotic tick-borne rickettsia, with veterinary and public health importance worldwide (1, 2). The organism is predominantly transmitted by Ixodes spp. hard ticks, but other questing hard ticks might act as vectors (3–5). A. phagocytophilum is closely related to the Gram-negative bacteria Ehrlichia and can be transmitted to wide range of animals causing granulocytic anaplasmosis that especially produce clinical signs in dogs, horses, cattle and human as well (6,7). Unlike A. marginale and A. centrale, which settle in erythrocytes, A. phagocytophilum prefers leukocytes as its favorite habitat named in this position as morulae (8). A. phagocytophilum causes human granulocytic anaplasmosis (HGA) that ranges in severity from asymptomatic seroconversion to a mild or severe febrile illness. In rare instances, severe disease can result in organ failure or death.
Nonspecific signs and symptoms including acute onset of fever, headache, malaise and myalgia are typical, even in severe infection. Less common symptoms include nausea, abdominal pain, diarrhea, and cough (3). HGA is considered a zoonosis of increasing concern (9–11). The disease manifests sub-clinical and occasionally fatal features and responsible for important economic losses to livestock industry (12,13). Afflicted dogs may develop either clinically acute febrile illness distinguished by fever, polyarthritis, thrombocytopenia, leucopenia, depression and anorexia or represent no distinct noticeable abnormalities and specific clinical signs (6, 12). However, some clinical features including lameness, coughing, polydipsia, intermittent vomiting, and hemorrhages are considered as less frequent sequela of this tick-borne fever in dogs (8).
In large-scale surveys, determination of A. phagocytophilum is based upon three distinct procedures, generally included microscopy by preparing thin layer Romanowsky-stained peripheral blood, serology and molecular techniques (8, 9, 12). Therefore, we utilized microscopy to detect morulae within neutrophils and specific Nested PCR to gain results that are more accurate.
Regardless of little scattered studies about A. phagocytophilum, there is no molecular-based documented data concerning the occurrence of this bacterium in dogs of Iran. Therefore, this study aimed to evaluate the prevalence of A. phagocytophilum infection in apparently healthy rural dogs from Khuzestan Province, Southwestern Iran by molecular and staining methods and to compare results from mentioned methods.
Materials and Methods
Sampling
During Sep 2014 to Apr 2015, 103 rural apparently healthy dogs (which consisted of 76 dogs equal and less than three years old and 27 dogs more than three years old and 60 male and 43 female) from Khuzestan Province in south-west of Iran were examined for A. phagocytophilum infection. Samples originated from four geographical regions within Khuzestan Province from villages around Shush (in North), Abadan (in South), Ramhormoz (East) and Hamidieh (South). Dogs were restrained and from each animal 2 ml of peripheral whole blood of saphenous or cephalic vein were taken and transferred to tubes containing coagulant for molecular investigation and Romanowsky-staining. In some cases, intra-muscular acepromazine (0.15 mg/kg) and ketamine (15 mg/kg) injection were used for relaxing of dogs.
The animals were handled according to the recommendation of the Ethics Committee, Shahid Chamran University of Ahvaz, Ahvaz, Iran (Ethical Approval Number 2/3/93, 23 July 2014).
Microscopy
From each sample, thin blood smear was prepared following by methanol fixation and to stain with 10% of Giemsa (Merck, USA) for 30 min. Slides were then examined for detection of morulae of A. phagocytophilum using ×1000 magnification of light microscopy by searching all area of slide.
PCR
DNA was exploited by application of the genomic DNA extraction Kit (Cinnagen, Iran). Species specification was accomplished by Nested PCR according to amplification of 16S rRNA gene conserved for all Anaplasma species. PCR protocol and primers were adopted from description (14). Briefly, amplification was performed in 25 μl reaction volumes including 5 μl of DNA template, 5 pmol of forward and reverse primers (each 1 μl), 12.5 μl of master mix (Ampliqon, Denmark) containing 3 mM MgCl2, 0.4 mM of each dATP, dCTP, dGTP and dTTP and 0.08 U/ml Taq DNA polymerase in reaction buffer. Forward and reverse genus-specific primer sequences were 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-AGCACTCATCGTTTACAGCG-3′, respectively. Positive samples showed a band of approximately 781 bp. Nest 2 reactions were carried out on products of prime PCR by forward and reverse primers of 5′-GCAAGCTTAACACATGCAAGTC-3′ and 5-GTTAAGCCCTGGTATTTCAC-3′ for Nested PCR to confirm the Anaplasma spp. and 5′-CTTTATAGCTTGCTATAAAGAA-3′ and 5′-GTTAAGCCCTGGTATTTCAC-3′ for specific Nested PCR to confirm A. phagocytophilum. Positive samples showed a band of approximately 543 and 504 bp in Nested PCR and specific Nested PCR, respectively. The thermal program of all PCRs was as follows: 95 °C for 5 min, 35 cycles of 94 °C for 45 sec, annealing at 56 °C for 45 sec, and 72 °C for 45 sec, followed by a final extension step at 72 °C for 5 min.
Statistical analyses
The data were correlated with sex and age of the dogs and the statistical significances of their relation or independence were evaluated by Chi-square test (χ2), Fisher’s exact test and logistic regression with 95% of confidence level. Analyses were performed by SPSS 18.0 software (Chicago, IL, USA) for Windows 8, with a P-value <0.05 as statistically significant. Confidence limits for the proportions were established by exact binomial test with 95%.
Results
From 103 prepared slides of examined rural dogs, 11.65% (12 from 103) were microscopically positive for morulae of Anaplasma (Fig. 1).
Fig. 1:
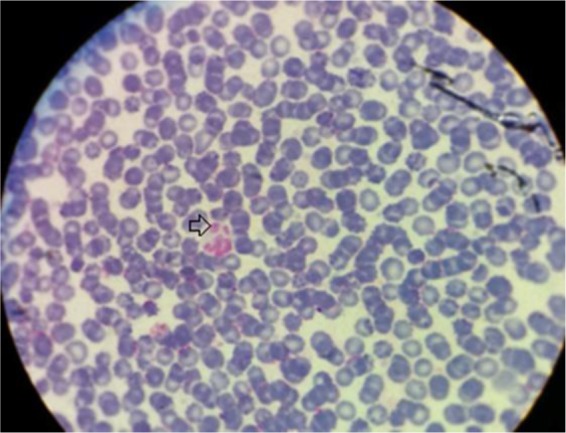
Morulae of Anaplasma phagocytophilum in a neutrophil of a dog (Arrow)
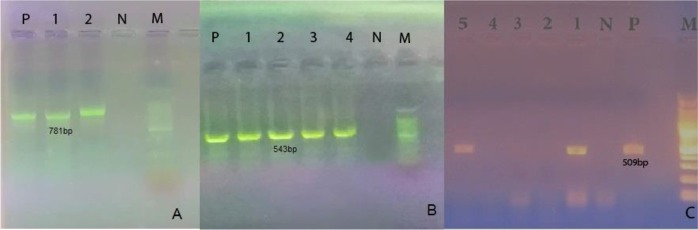
All of the approximately 781bp bands amplified in prime PCR were confirmed by detecting of expected 544 bp length bands (Fig. 2). Specific Nested PCR revealed high prevalence of infection, that specific band with 509 bp length bands were exhibited in 57.28% of cases (59 of 103) in electrophoresis (Fig. 2). Molecular method diagnosed more infected dogs than staining method.
Fig. 2:
Agarose gel electrophoresis of PCR of 16S rRNA gene (A), Nested PCR (B) and Specific Nested PCR (C) products. A. 1,2: positive samples for Anaplasma spp. P: positive control, N: negative control (781 bp). B. 1–4: positive samples for Anaplasma spp. P: positive control, N: negative control (543 bp). C. 1,5: positive samples for A. phagocytophilum. P: positive control, N: negative control (509 bp). M is a 100 bp ladder
In terms of sex, there was no significant difference between the prevalence percentage of infection in males and females (P=0.58). Age was not identified as a risk factor for A. phagocytophilum infection in this study (P=0.5). There was not a significant geographical variation in the prevalence of A. phagocytophilum infection, ranging from 45.83% in Shush to 69.23% in Ramhormoz (CI, 2.46%–76.82%). The odds of infection in dogs in west were 2.6 times greater than in animals kept in north (Table 1).
Table 1:
Prevalence of A. phagocytophilum in dogs from Khuzestan province, southwest of Iran and related factors
| Factor | No. examined | Positive | % | P-value | OR | CI 95% |
|---|---|---|---|---|---|---|
| Prevalence | 103 | 59 | 57.28 | - | - | - |
| Gender | ||||||
| Male | 60 | 33 | 55 | 0.58 | 1.25 | 0.56–2.7 |
| Female | 43 | 26 | 60.46 | |||
| Age | ||||||
| ≤3 yr old | 76 | 45 | 59.2 | 0.5 | 1.31 | 0.54–3.19 |
| >3 yr old | 27 | 14 | 51.8 | |||
| Locality | ||||||
| Shush | 24 | 11 | 45.83 | - | - | - |
| Abadan | 26 | 15 | 57.69 | 0.53 | 1.6 | 0.52–5.15 |
| Ramhormoz | 26 | 18 | 69.23 | 0.42 | 1.8 | 0.58–5.5 |
| Hamidieh | 27 | 15 | 55.5 | 0.55 | 2.6 | 0.83–8.45 |
Discussion
Clinical manifests of A. phagocytophilum are not always pathognomic, therefore diagnosis of this infection is basically based upon paraclinical aspects of the infection. For this purpose, many diagnostic approaches including microscopy to recognize morulae in leukocytes, different serologic procedures and tracing DNA of rickettsia from blood, buffy coat, bone marrow or spleen are developed (8). Intra-leukocytic morulae are indiscernible from those of Ehrlichia species. On the other sides, serology is along with cross-reacting phenomenon, seronegative response in the primary phase and indiscrimination between recent and former infections, since anaplasmosis due to A. phagocytophilum is normally a self-limiting infection (15, 16). Therefore, in such cases sensitive and specific molecular recognition of the organism is method of choice (15). Among the different targeted genes for amplification in PCR, 16S rRNA gene with highly variable nucleotide region appears more reliable as a matter of A. phagocytophilum detection (4, 17,18). However, we applied both specific PCR and microscopy to establish a comparison on this issue.
In Iran, for the first-time detection of A. phagocytophilum was reported (14). 16S rRNA and MSP4 genes of A. phagocytophilum were analyzed by Nested-PCR method and overall 1.08% (4/370) of domesticated small ruminants was positive for A. phagocytophilum infection (19).
By molecular approach, we determined the infection rate of 57% in dogs, whereas microscopy indicated 11.65% of infection. In 18% of dogs from Tehran A. phagocytophilum morula as forms were detected (20). Four percent of A. phagocytophilum infection was found in dogs from Turkey by molecular method (21). Surprisingly, according to current work molecular prevalence of A. phagocytophilum in the zone of study is considerably high in comparison with other countries and this infection is hyper enzootic in this area. In a Brazilian survey, 6.03% of dogs were infected with this rickettsia (22). In central Italy, only 0.9% of prevalence were in hunter dogs (23). Latter two studies were performed by PCR based on 16S rRNA gene.
There was a wide gap between results of two applied procedures in the present survey (11.65% vs 57.28%), in which microcopy was very less sensitive than PCR. Such as all the infective organisms of circulating cells especially in low bacteremia period, detecting of A. phagocytophilum in neutrophils requires a professional microscopist thereupon maybe we missed these rickettsia in our slide probing with considering the short time of bacteremia pick of A. phagocytophilum (7).
All the examined dogs in this investigation were apparently healthy. Although based on living circumstances in rural districts in southwest of Iran, dogs are not always in very tight relation with owners so one recent illness may miss from attention. These animals have almost elapsed their whole life in such a condition in villages and unlike pets or even stray dogs are in close association with other domestic animals, which are hosts of numerous hard ticks and A. phagocytophilum in pastures (19).
In accordance with high prevalence of A. phagocytophilum infection in current survey, this infection is more likely present in its sub-clinical or silent mode in study. Unanimous with our discussion, most of A. phagocytophilum infections are sub-clinical in dogs of Germany (24). In dogs, pathogenicity of A. phagocytophilum is per se related to different factors such as concomitant infections, animal housekeeping, nutrition, immunity and variant of rickettsia. In Iran, rural dogs are in close confrontation with villagers. These domesticated animals are hardly ever considered for spraying or utilizing of pesticide compounds by owners and tick infestation is a current problem in them. On the other hand, unfortunately, there is no confirmative data about A. phagocytophilum infection of human population from Khuzestan, the area of this study, but considering that human granulocytic anaplasmosis is globally discussed as an emerging important zoonosis, and high-contaminated population of dogs is most probably increasing the incidence of disease in at risk public. However, different variants of A. phagocytophilum had inclination to distinct hosts. Correlation among host tropism and pathogenicity is also another issue which may impress the zoonotic potential of this organism (8, 25). As a zoonosis, the variants of A. phagocytophilum among domestic and wild life cycle of the organism should be crucially considered.
Conclusion
Molecular prevalence of A. phagocytophilum was noticeably high in dogs form Iran. It may cause the incidence of disease in human population.
Acknowledgements
This study was supported by Shahid Chamran University of Ahvaz and the authors wish to thank Vice-Chancellor for Research of the University for the Research.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Severo MS, Stephens KD, Kotsyfakis M, Pedra JH. Anaplasma phagocytophilum: deceptively simple or simply deceptive? Future Microbiol. 2012; 7: 719–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum – a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol. 2013; 3: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouqui P, Bacellar F, Baranton G, et al. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin Microbiol Infect. 2004; 10(12):1108–1132. [DOI] [PubMed] [Google Scholar]
- 4.Dumler JS, Barbet AF, Bekker CP, et al. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and “HGE agent” as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol. 2001; 51: 2145–2165. [DOI] [PubMed] [Google Scholar]
- 5.Woldehiwet Z. The natural history of Anaplasma phagocytophilum. Vet Parasitol. 2010; 167: 108–122. [DOI] [PubMed] [Google Scholar]
- 6.Rikihisa Y. Mechanisms of obligatory intracellular infection with Anaplasma phagocytophilum. Clin Microbiol Rev. 2011; 24: 469–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakken JS, Dumler JS. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci. 2006; 1078: 236–247. [DOI] [PubMed] [Google Scholar]
- 8.Carrade DD, Foley JE, Borjesson DL, Sykes JE. Canine granulocytic anaplasmosis: a review. J Vet Intern Med. 2009; 23: 1129–41. [DOI] [PubMed] [Google Scholar]
- 9.Atif FA. Anaplasma marginale and Anaplasma phagocytophilum: Rickettsiales pathogens of veterinary and public health significance. Parasitol Res. 2015; 114: 3941–3957. [DOI] [PubMed] [Google Scholar]
- 10.Markiwicz M, Schotta AM, Wijnveld M, Stanek G. Human granulocytic anaplasmosis acquired in Connecticut, USA, diagnosed in Vienna, Austria, 2015. Diagn Microbiol Infect Dis. 2016; 84: 347–349. [DOI] [PubMed] [Google Scholar]
- 11.Alberdi P, Aylion N, C-Cruz A, et al. Infection of Ixodes spp. tick cells with different Anaplasma phagocytophilum isolates induces the inhibition of apoptotic cell death. Ticks Tick Borne Dis. 2015; 6: 758–767. [DOI] [PubMed] [Google Scholar]
- 12.Stuen S. Anaplasma phagocytophilum-the most widespread tick-borne infection in animals in Europe. Vet Res Commun. 2007; 31: 79–84. [DOI] [PubMed] [Google Scholar]
- 13.Foley J, Dreazenovich N, Leutenegger CM, Chomel BB. Association between polyarthritis and thrombocytopenia and increased prevalence of vector borne pathogens in Californian dogs. Vet Rec. 2007; 160: 159–162. [DOI] [PubMed] [Google Scholar]
- 14.Noaman V, Shayan P. Molecular detection of Anaplasma phagocytophilum in carrier cattle of Iran - first documented report. Iran J Microbiol. 2009; 1: 37–42. [Google Scholar]
- 15.Dumler JS, Madigan JE, Pusterla N, Bakken JS. Ehrlichiosis in humans: epidemiology, clinical presentation, diagnosis, and treatment. Clin Infect Dis. 2007; 45 Suppl 1: S45–51. [DOI] [PubMed] [Google Scholar]
- 16.Silaghi C, Kauffmann M, Passos LMF, et al. Isolation, propagation and preliminary characterization of Anaplasma phagocytophilum from roe deer (Capreolus capreolus) in the tick cell line IDE8. Ticks Tick Borne Dis. 2011; 2: 204–208. [DOI] [PubMed] [Google Scholar]
- 17.Goodman JL. Human granulocytic anaplasmosis (Ehrlichiosis). In: Goodman J.L., Dennis D.T., Sonenshine D.E. (Eds.), Tick-borne diseases of humans. ASM Press, Washington, DC, 2005; pp. 218–238. [Google Scholar]
- 18.Kohn B, Silaghi C, Galke D, et al. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci. 2011; 91: 71–76. [DOI] [PubMed] [Google Scholar]
- 19.Yousefi A, Rahbari S, Shayan P, et al. Molecular evidence of Anaplasma phagocytophilum: an emerging tick-borne pathogen in domesticated small ruminant of Iran; first report. Com Clin Pathol. 2017; 26(3): 637–642. [Google Scholar]
- 20.Rassouli M, Aghazamani G. Retrospective study of tick-borne pathogens and observation of Ehrlichia ewingii / Anaplasma phagocytophilum and hemotropic Mycoplasma spp. in dogs’ blood films. Anim Vet Sci. 2015; 3(6): 171–178 [Google Scholar]
- 21.Çetinkaya H, Matur E, Akyazi İ, et al. Serological and molecular investigation of Ehrlichia spp. and Anaplasma spp. in ticks and blood of dogs, in the Thrace Region of Turkey. Ticks Tick Borne Dis. 2016; 7(5):706–714. [DOI] [PubMed] [Google Scholar]
- 22.Santos HA, Thome SM, Baldani CD, et al. Molecular epidemiology of the emerging zoonosis agent Anaplasma phagocytophilum (Foggie, 1949) in dogs and ixodid ticks in Brazil. Parasit Vectors. 2013; 6: 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ebani VV, Bertelloni F, Turchi B, Cerri D. Serological and molecular survey of Anaplasma phagocytophilum in Italian hunting dogs. Ann Agric Environ Med. 2013; 20: 289–292. [PubMed] [Google Scholar]
- 24.Jensen J, Simon D, Murua Escobar H, et al. Anaplasma phagocytophilum in dogs in Germany. Zoonoses Public Health. 2007; 54: 94–101. [DOI] [PubMed] [Google Scholar]
- 25.Rejmanek D, Freycon P, Bradburd G, et al. Unique strains of Anaplasma phagocytophilum segregate among diverse questing and non-questing Ixodes tick species in the western United States. Ticks Tick Borne Dis. 2013; 4: 482–7. [DOI] [PMC free article] [PubMed] [Google Scholar]