Abstract
Background:
Recently, the use of common marmoset (Callithrix jacchus) has increased in biomedical research as an animal model. This study aimed to test fecal samples to monitor bacterial and parasite infections in common marmoset at the Laboratory Animal Center of Osong Medical Innovation Foundation in Korea.
Methods:
To monitor bacteria and parasites in common marmoset, we tested 43 fecal samples of 43 common marmosets by culture and parasitological test in 2014. Infection by Chilomastix mesnili was determined by PCR method.
Results:
We identified nonpathogenic bacteria such as Proteus mirabilis and Escherichia coli in feces of normal common marmosets. Interestingly, C. mesnili was isolated from a healthy common marmoset by fecal centrifugation concentration and PCR. The monkey infected with C. mesnili was treated with metronidazole. After the treatment, C. mesnili were not found in feces using fecal centrifugation concentration and PCR.
Conclusion:
This is the first case report of C. mesnili infection in common marmoset. Treatment with metronidazole is found to be highly effective in eradicating C. mesnili infection in common marmoset.
Keywords: Chilomastix mesnili, Common marmoset, Escherichia coli, Metronidazole, Proteus mirabilis
Introduction
Non-human primates (NHPs) are excellent experimental models for biomedical research such as physiology, anatomy, immunology, and neurology. The key advantage of using NHPs is that they share great genetic and physiological similarities with humans. Recently, the use of common marmoset (Callithrix jacchus) has increased in biomedical research as an animal model. Common marmoset is a member of the New World monkeys (1). Compared to rhesus monkey (Macaca mulatta) or cynomolgus monkey (M. fascicularis), common marmoset has several advantages as an animal model because it is small (adult: 350–400 g) and typically produces twins or triplets (1). Furthermore, it is easy to handle. Therefore, it is an economically attractive NHP species for biomedical research.
Animal experiments are essential to progress in biomedical science. Health surveillance is important for infection prevention and defining pathogen status. Colonies of research animals are susceptible to infection with a variety of pathogenic bacterial, viruses, and parasites (2, 3). In many instances, these agents produce no clinical signs. However, they result in physiologic changes that may alter, and in some cases, invalidate research carried out with infected animals (2–4). In addition, some zoonotic pathogens may infect researcher-using animals. Therefore, it is important to keep laboratory animals free from infectious pathogens (2, 3)
Over the past several decades, the health status of experimental animals has greatly improved because of resulting in increased availability of animals free of infectious agents. Health monitoring refers to the practice of regular and repeated inspection of laboratory animals to determine whether pathogens are infected (2). A program of health monitoring is essential to assure managers and researchers that the standards of animal health within the facility are maintained so that experiments will not be compromised by unexpected infections. In this study, we tested fecal samples to monitor bacterial and parasite infections in common marmoset.
Materials and Methods
Specimens
Total 43 fresh fecal samples of 43 common marmosets (C. jacchus) were obtained from the Laboratory Animal Center of Osong Medical Innovation Foundation (Chungbuk, Republic of Korea) in 2014. All common marmosets were housed in stainless steel cage. There were maintained in 12 h dawn and 12 h dusk conditions at a temperature of 24 °C and humidity 40% to 60% in the SPF animal facility. Common marmosets were provided with a standard marmoset diet (Clea New World Monkey Diet, CMS-1M; CLEA Japan Inc., Tokyo, Japan) and water ad libitum.
All protocols were approved by the Committee on Animal Care of the Laboratory Animal Center of Osong Medical Innovation Foundation.
Culture tests
Culture tests were performed to isolate Campylobacter jejuni, Salmonella spp., Shigella spp., Staphylococcus aureus, and Yersinia pseudotuberculosis. Fecal contents were streaked onto selective agars such as Campy-BAP (BD Diagnostics, MD, USA), Cefsulodin-Irgasan-Novobiocin (CIN, BD Diagnostics), DHL (Merck Millipore Corporation, Darmstadt, Germany), Salmonella-Shigella (SS, BD Diagnostics), and Volgel-Johnson (BD Diagnostics). After incubation of the agar plates at 37 °C for 24 or 48 h, colonies were recovered. We used the biochemical test and VITEK® 2 compact automated systems (bio-Mérieux, Inc., Marcy l’Etoile, France).
Parasitological tests
Parasitological tests were performed to isolate intestinal helminths and protozoa. Parasitological tests were used fecal flotation and fecal centrifugation concentration (FCC) by zinc sulfate (specific gravity: 1.18) method (5). We differentiated Entamoeba histolytica, Giardia spp. etc. by protozoal movement patterns and morphology.
Giemsa stain
Giemsa stain was used in histopathological diagnosis of intestinal helminths and protozoa. A thin film of the specimen on a microscope slide was fixed in methanol for 5 min. The slide was immersed in a freshly prepared 20% Giemsa stain solution (Sigma-Aldrich, Inc., MO, USA) for 10 min, then rinse slides with tap water, air dry and evaluate.
PCR amplifications
Fecal DNA was extracted using an AccuPrep genomic DNA extraction kit (Bioneer, Daejeon, Republic of Korea). PCR was performed to identify intestinal protozoa. The Chilomastix mesnili specific PCR primers 5′ GCA GTT CTT TCG TGA TTG TGA 3′ and 5′ GAG GTC TCG TCC GTT ATC G 3′ designed to small subunit ribosomal RNA gene (94 bp, GenBank accession number EU009465). For PCR, DNA was denatured at 95 °C for 15 sec, annealed at 60 °C for 10 sec, and extended at 72 °C for 30 sec. This process was repeated for 35 cycles. Detection for Giardia intestinalis, Entamoeba histolytica, and Pentatrichomonas hominis were performed with primers described by previous reports (6).
PCR amplification was performed to differentiate Helicobacter spp. We used species-specific PCR primer 5′ CTA TGA CGG GTA TCC GGC 3′ and 5′ ATT CCA CCT ACC TCT CCC A 3′ designed to 16S ribosomal RNA gene sequence (7). For PCR, DNA was denatured at 94 °C for 2 sec, annealed at 53 °C for 2 sec, and extended at 72 °C for 30 sec. This process was repeated for 35 cycles. All PCR products were separated on a 1.8% agarose gel and stained with ethidium bromide.
Metronidazole treatment
Metronidazole is on the WHO’s List of Essential Medicines, a list of the most important medication needed in a basic health system. The monkey infected with C. mesnili was treated orally with metronidazole (30 mg/kg body weight) for 1 week (daily, 3 times), immediately after detection of parasite infection.
Results
Bacteria detection in feces
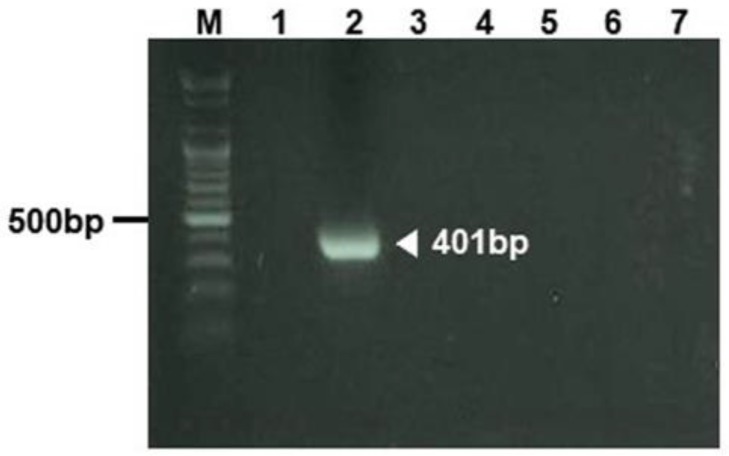
To monitor pathogenic bacteria such as Campylobacter jejuni, Helicobacter spp., Salmonella spp., Shigella spp., S. aureus, and Yersinia pseudotuberculosis, we tested 43 fecal samples of 43 common marmosets. We used culture test to detect pathogenic bacteria except for Helicobacter spp. To detect Helicobacter spp. in feces, PCR method was used because of the most efficient and fast method. Pathogenic bacteria were not identified in all feces from 43 common marmosets (Table 1 and Fig. 1). However, nonpathogenic bacterium Proteus mirabilis was isolated from 4 common marmosets. Prevalence of P. mirabilis was approximately 9.3%. Nonpathogenic Escherichia coli was isolated from most common marmosets.
Table 1:
Prevalence of bacteria in common marmosets. Culture and PCR tests were performed
| Organism | Sample Tested | Test Method | Result |
|---|---|---|---|
| Campylobacter jejuni | Feces | Culture | 0/43 |
| Helicobacter spp. | Feces | PCR | 0/43 |
| Nonpathogenic E. coli | Feces | Culture | 31/43 |
| Proteus mirabilis | Feces | Culture | 4/43 |
| Salmonella spp. | Feces | Culture | 0/43 |
| Shigella spp. | Feces | Culture | 0/43 |
| Staphylococcus aureus | Feces | Culture | 0/43 |
| Yersinia pseudotuberculosis | Feces | Culture | 0/43 |
Fig. 1:
Helicobacter genus-specific DNA amplified by PCR in fecal sample. M; marker, lane 1; negative control, lane 2; positive control, lanes 3 and 7; fecal samples collected from common marmoset number 1 to 5
Parasite detection in feces
To monitor eggs of intestinal helminths and protozoa, we tested 43 fecal samples of 43 common marmosets using fecal flotation and fecal centrifugation concentration (FCC) method. Intestinal protozoa C. mesnili was isolated from one common marmoset (animal number 5). Prevalence of C. mesnili was approximately 2.3%. Morphology of C. mesnili was observed by Giemsa stain (Fig. 2). The identification of C. mesnili was also confirmed by PCR (Fig. 3).
Fig. 2:
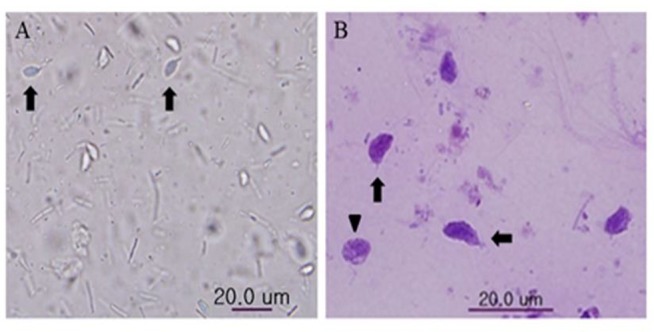
C. mesnili was isolated from a common marmoset by fecal centrifugation concentration technique. Trophozoite (arrow) and cyst (arrowhead) of C. mesnili from fecal sample (A) and stained with Giemsa stain (B).
Fig. 3:
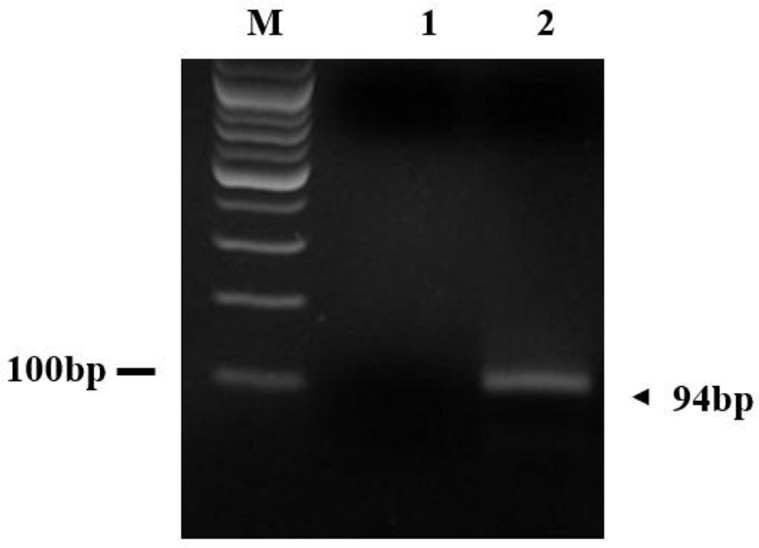
C. mesnili –specific DNA amplified by PCR in fecal sample. M; marker, lane 1; negative control, lane 2; fecal sample collected from common marmoset number 5
To remove protozoa, the common marmoset infected with C. mesnili was treated with metronidazole for one week. We monitored the common marmoset infected with C. mesnili every 3 months for 1.5 yr using FCC and PCR. After 1.5 yr, cysts, trophozoites, or PCR products of C. mesnili were not found in the feces. Therefore, C. mesnili was eradicated from this common marmoset
Discussion
We identified nonpathogenic bacteria such as P. mirabilis and E. coli in feces of common marmosets. In addition, C. mesnili was isolated from a healthy common marmoset by FCC technique and PCR. This is the first reported case of C. mesnili infection in a specific-pathogen-free common marmoset. The monkey infected with C. mesnili was treated orally with metronidazole. After treatment with metronidazole for one week, C. mesnili was not found in the feces anymore. P. mirabilis is a Gram-negative bacterium with swarming colony and urease activity. It is capable of causing human infection. Its infections have been reported in dog and human (8). However, it is not on the pathogenic bacteria list of New World monkey or Old World monkey (4).
C. mesnili is one of the zoonotic intestinal protozoa in Korea. In 2250 samples collected from 1969 to 1970 in the Republic of Korea, 0.5% of samples have been infected by C. mesnili (9). The prevalence of C. mesnili infection has been reported to be 21% in non-human primates of four zoological gardens in Belgium. However, New World monkeys including tamarin, marmoset, spider monkey, and squirrel monkey are not infected by C. mesnili in Belgium (10). Recently, the infectious rate of C. mesnili is reported to be 5.2% in wild chimpanzees (Pan troglodytes troglodytes) in southeast Cameroon (11). C. mesnili is not on the pathogenic protozoa list of New World monkey or Old World monkey (4). Even though C. mesnili is a commensal non-pathogenic protozoan, it is important to keep laboratory monkey free from parasites because of improved health and longevity of marmosets in SPF facility (12). To the best of our knowledge, this study is the first one that reports C. mesnili infection in common marmoset.
In the present study, we successfully detected small subunit ribosomal RNA gene of C. mesnili among intestinal protozoa in feces by PCR. PCR analysis has been used for the detection and identification of intestinal protozoa in fecal samples of human (6) and new world monkeys (13). Interestingly, Pentatrichomonas hominis has been found in both normal feces and diarrheal feces of laboratory-bred common marmosets (13). In this study, a common marmoset infected with C. mesnili was treated orally with metronidazole. Metronidazole is widely used to treat infections of protozoa in cats (14) and monkeys (15). We examined C. mesnili infection by FCC and PCR every 3 months for 1.5 year. Cysts, trophozoites, or PCR product of C. mesnili were not found in the feces after one-week treatment with metronidazole.
Conclusion
C. mesnili was isolated from a healthy common marmoset at the Laboratory Animal Center of Osong Medical Innovation Foundation by fecal centrifugation concentration and PCR. This is the first reported case of C. mesnili infection in common marmoset. Treatment with metronidazole was highly effective to eradicate C. mesnili infection.
Acknowledgements
This research was supported by a grant HO13C0005 from Osong Medical Innovation Foundation funded by the Ministry of Health & Welfare, the Republic of Korea.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Magden ER, Mansfield KG, Simmons JH, Abee CR. Nonhuman Primates. In: Fox JG, Anderson LC, Otto G, Pritchett-Corning KR, Whary MT, editors. Laboratory Animal Medicine. 3rd ed Academic Press; San Diego, USA; 2015. P. 771–822. [Google Scholar]
- 2.Nicklas W. International harmonization of health monitoring. ILAR J. 2008;49(3);338–346. [DOI] [PubMed] [Google Scholar]
- 3.Mähler C, Berard M, Feinstein R, et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim. 2014;48(3):178–192. [DOI] [PubMed] [Google Scholar]
- 4.Weber H, Berge E, Finch J, et al. Health monitoring of non-human primate colonies. Recommendations of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on non-human primate health accepted by the FELASA Board of Management, 21 November 1998. Lab Anim. 1999;33(Suppl 1):S1–18. [PubMed] [Google Scholar]
- 5.Parkinson CM, O’Brien A, Albers TM, et al. Diagnosis of ecto- and endoparasites in laboratory rats and mice. J Vis Exp. 2011; (55):e2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morimoto N, Korenaga M, Nishida Y, et al. PCR amplification and DNA sequence analysis of parasitic intestinal protozoa in specimens stained with Chlorazol Black E. Acta Parasitol. 2013;58(2):132–138. [DOI] [PubMed] [Google Scholar]
- 7.Riley LK, Franklin CL, Hook RR, Jr, Besch-Williford C. Identification of murine Helicobacter by PCR and restriction enzyme analyses. J Clin Microbiol. 1996;34(4):942–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz E, Haenni M, Mereghetti L, et al. Survey of multidrug resistance integrative mobilizable elements SGI1 and PGI1 in Proteus mirabilis in humans and dogs in France, 2010–13. J Antimicrob Chemother. 2015;70(9):2543–2546. [DOI] [PubMed] [Google Scholar]
- 9.Youn H. Review of zoonotic parasites in medical and veterinary fields in the Republic of Korea. Korean J Parasitol. 2009;47(Suppl):S133–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levecke B, Dorny P, Geurden T, et al. Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet Parasitol. 2007;148(3–4):236–46. [DOI] [PubMed] [Google Scholar]
- 11.Drakulovski P, Bertout S, Locatelli S, et al. Assessment of gastrointestinal parasites in wild chimpanzees (Pan troglodytes troglodytes) in southeast Cameroon. Parasitol Res. 2014; 113(7):2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross CN, Austad S, Brasky K, et al. The development of a specific pathogen free (SPF) barrier colony of marmosets (Callithrix jacchus) for aging research. Aging (Albany NY). 2017;9(12):2544–2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue T, Hayashimoto N, Yasuda M, et al. Pentatrichomonas hominis in laboratory-bred common marmosets. Exp Anim. 2015;64(4):363–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scorza AV, Lappin MR. Metronidazole for the treatment of feline giardiasis. J Feline Med Surg. 2004;6(3):157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labberton L, Bakker J, Klomp R, et al. Challenges in oral administration of metronidazole dissolved in drinking water to rhesus monkeys (Macaca mulatta). Lab Anim (NY). 2013;42(6):213–216. [DOI] [PubMed] [Google Scholar]