Abstract
Bisphenol A (BPA) is a typical endocrine disruptor. Bisphenol S (BPS) has been widely used as a substitute for various plastic materials due to the limited application of BPA. However, it does not mean that BPS is a safe substitute due to the lack of effective evaluation of BPS. In this study, the clinical model of Caenorhabditis elegans (C. elegans) was used to study the effects of BPS on the locomotion behavior, growth, reproduction, lifespan and antioxidant system. Our study found that C. elegans exposed to 0.01 μM BPS could have significantly inhibited locomotion behavior and growth, as well as damaged reproductive and antioxidant systems and lifespan. It is interesting to note that in multi-generational exposure studies, we found that BPS exhibits complex genotoxicity. With the transmission to the offspring, BPS showed more significant inhibition of the head thrashes of the nematode, while the effect on the body bends and body length was gradually weakened. The effect of BPS on the brood size shows different rules according to different concentrations and offsprings. Therefore, the safety of BPS still needs further evaluation, especially the multi-generational genotoxicity.
1. Introduction
Bisphenol A (BPA), an endocrine-disrupting chemical, has been widely used in the synthesis of polycarbonates and epoxy resin.1–3 BPA is suspected to affect a variety of physiological functions and to be associated with many human diseases such as diabetes, obesity, reproductive disorders, cardiovascular disease, birth defects, and breast cancer.4–6 Since increasing pieces of evidence showed the negative effects of BPA on human health, it has been prompted to be removed from consumer products.7 As early as 2009, Canada was the first to ban the use of BPA in infant and baby bottle products. The United States (2010), the European Union (2011) and China (2011) have followed suit. France even began to ban the use of bisphenol A in food packaging materials in 2015.8–10
Bisphenol S is a structural analog of bisphenol A which is widely used as a substitute in a variety of products such as baby bottles, thermal receipt paper, food packaging materials and personal care products.2,11–13 In Europe, the annual production of BPS is between 1000 and 10 000 t, and steadily increases.14 In 2013, the detection concentration of BPS in Taihu Lake was 2 ng L–1. In 2016, BPS was detected again and the detection concentration was 6.4 ng L–1 which significantly increased compared to that reported in 2013.15–17 In 2015, the occurrence of BPS was determined in surface waters sampled from four Asian countries: China, India, Japan, and Korea.18 For indoor dust, the mean concentrations of BPS were found to be 220 ng g–1 in samples from 12 countries including China, Colombia, Greece, etc.19 Food and the environment are the main ways for humans to be exposed to BPS. The mean and median concentrations of BPS in 267 food samples in the United States were 0.13 and 0.005 ng g–1 (fresh weight), respectively.20 The occurrence of BPS in Chinese food was almost the same as that in the United States. 23% of BPS in 289 samples was detected, and the concentration was from ND to 42.3 ng per g(fresh weight).21 BPS has also been detected in urine from the citizens of 8 countries and within the same concentration ranges as BPA.12,22,23 Indeed, BPS has been detected in human blood and infant cord blood.24,25
Studies have shown that BPS has estrogen, antiandrogen and anti-thyroid hormone properties, which may adversely affect the reproductive system, endocrine system and nervous system of C. elegans and humans, and may cause oxidative stress.7 Ullah et al. showed that BPS caused a significant increase in reactive oxygen species and lipid peroxidation in rat testis, while the antioxidant enzyme activity and protein content were significantly reduced.26 Similarly, plasma and testicular testosterone concentrations are also reduced. Together with the modification of antioxidant system activity and induction of DNA fragmentation in vitro, BPS resulted in a decrease in daily sperm production and significant DNA damage in vivo.27,28 Recent studies showed that BPS could cause developmental toxicity, hormonal imbalance and reproductive failure in adult zebrafish.29,30 Exposure of F1 embryos to BPS resulted in worse hatchability and increased malformation rates compared to those without BPS exposure.31 BPS can alter the normal developmental timing of the critical neuroendocrine center, the consequences of which can lead to early synaptogenesis and improper fine-tuning of the brain later in development.32
With the increasing use of BPS, human beings were constantly exposed to an environment with BPS, which turns out to be unbeneficial. There are many indications that BPS has become a “regrettable substitution”. Compared with BPA, BPS has higher thermal stability, stronger skin permeability, and better absorption, and may cause heavier burden on the human body.22,33–35 At present, the toxicology of BPS has not been fully studied before application, so the safety of BPS has received more and more attention, especially the low toxicity effect of BPS. In this study, BPS was exposed to C. elegans to study its effects on the locomotion behavior, growth, reproduction, lifespan and oxidative stress. The genotoxic effects of BPS were studied by multi-generational exposure. Compared with other model organisms, C. elegans has the advantages of small size, strong reproductive ability, easy cultivation, and low cost.36C. elegans is one of the classic model organisms in toxicological evaluation which is sensitive to exogenous compounds. The objective of the current study is to more comprehensively evaluate the toxicity of BPS.
2. Materials and methods
2.1. Reagents and strains
BPS (purity ≥ 98%) was purchased from Sigma-Aldrich. BPS was dissolved in absolute ethanol and different BPS original solutions were obtained. BPS original solutions were diluted to 0.001, 0.01, 0.1, 1, 10 and 100 μM with M9 buffer before exposure. All absolute ethanol concentrations were 0.1% in final BPS solutions. C. elegans exposed to 0.1% absolute ethanol in M9 buffer served as the control, which was known as a nontoxic dose of absolute ethanol.
Nematodes used in the present study were wild-type N2, originally obtained from the Caenorhabditis Genetics Center (CGC). Nematodes were maintained on nematode growth medium (NGM) plates seeded with Escherichia coli OP50 at 20 °C as described in ref. 37. Gravid nematodes were lysed with a bleaching mixture (1 M NaOH, 10% NaHOCl) to obtain an age-synchronized population of L1-larvae. C. elegans was exposed to BPS from L4-larvae for 24 h. During the exposure duration, the BPS solutions were stable. Although we cannot guarantee the adequate uptake of BPS by nematodes, the previous study has demonstrated that this exposure route for drug administration was effective in nematodes.34 Nematodes were used for toxicity evaluation with lethality, locomotion behavior, growth, reproduction, lifespan, total superoxide dismutase (SOD) and intestinal reactive oxygen species (ROS) as the endpoints.
2.2. Lethality
According to the experimental design, BPS was dissolved in absolute ethanol to prepare the original solution at concentrations of 0.25, 0.5, 1, 1.5 and 2 M and then diluted with S liquid medium to get the final BPS concentrations of 0.25, 0.5, 1, 1.5 and 2 mM; the final concentration of ethanol in the BPS solution was 0.1%. S liquid medium with 0.1% absolute ethanol served as the control. Since the high concentration of BPS solution was difficult to dilute, the preparation of the high concentration BPS solution was assisted by ultrasonic and water bath heating. A 200 μl aliquot of test solution for BPS was added to each well of the 96-well plates, which were subsequently loaded with 30 nematodes for each concentration. C. elegans was exposed from L4-larvae to day 1, and the inactive ones were scored under a dissecting microscope. Nematodes were judged to be dead if they did not respond to a stimulus using a small, metal wire.38 Lethality of nematodes was evaluated by the percentage of survival of C. elegans. Three replicates were examined per experiment.
2.3. Locomotion behavior
Locomotion behavior was evaluated by both the head thrash and the body bend. To assay head thrash, the examined nematodes were exposed to BPS from L4-larvae to day 1. Head thrash, defined as a change in the direction of bending at the mid body, was counted for 1 min. Body bend was counted as a change in the direction of the part of nematodes corresponding to the posterior bulb of the pharynx along the y-axis, assuming that the nematode was moving along the x-axis, and was counted for 20 s.39–41 Twenty nematodes were examined per experiment.
2.4. Growth alteration assays
Growth of nematodes was assessed by the body length. C. elegans was exposed to BPS from L4-larvae to day 1. Photographs were taken of the nematodes on the day 1 of adulthood, and the body length of each individual nematode was analyzed by using Image J software.40 The test was performed at least 3 times.
2.5. Reproduction assays
C. elegans was exposed to BPS from L4 larvae to day 1 and then individually transferred to a fresh plate each day until reproduction ceased. The offsprings of C. elegans were counted at the L3 stage. The test was performed at least 3 times.42–44
2.6. Lifespan assays
Lifespan analysis was performed in the same manner for all nematodes at 20 °C. The examined nematodes were transferred into new NGM plates daily from day 1 to day 4 after nematodes were acutely exposed to BPS. Nematodes would be transferred every 2 days into new NGM plates from day 5. Surviving C. elegans samples were counted daily (starting on the first day of adulthood) until all nematodes had died. C. elegans samples that did not move when gently prodded (with a platinum wire) were scored as dead. Nematodes suffering from internal hatch (a defect in egg laying) and those that crawled off the NGM plate were not included in the lifespan counts.42,45–47 The lifespan assays were performed at least 3 times.
2.7. Analysis of intracellular oxidative free radicals
To quantify whether the examined BPS treatment activated the oxidative damage, ROS production was assayed. Intracellular ROS was measured using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA). Non-fluorescent DCF-DA is a free cell permeable dye, which is readily converted to fluorescent 2′,7-dichlorofluorescein (DCF) due to the interaction with intracellular peroxide (H2O2). The nematodes exposed to BPS from L4-larve to day 1 were transferred to M9 buffer containing 1 M CM-H2DCFDA to pre-incubate for 3 h at 20 °C. The ROS level of each tested nematode was detected by using a multi-function fluorescence microplate reader. The excitation and emission wavelengths were set to 485 nm and 535 nm, respectively. The unit of the ROS level is defined as the relative fluorescence intensity per 50 tested nematodes.48 The test was performed at least 3 times.
2.8. Analysis of intracellular total superoxide dismutase
To further quantify whether the examined BPS treatment activated oxidative damage, SOD production was determined. The xanthine and xanthine oxidase reaction system produces superoxide anion radicals (O2–), which oxidize hydroxylamine to form nitrite, which is purple-red under the action of a color developer. SOD can inhibit superoxide anion radicals and reduce the formation of nitrite. The examined nematodes exposed to BPS from L4-larve to day 1 were ultrasonically disrupted and reacted with the kit. After the end of the reaction, the absorbance was measured at a wavelength of 550 nm.49 The test was performed at least 3 times.
2.9. Statistical analysis
All data were expressed as means ± standard error of the mean (SEM). Graphs were generated using GraphPad Primer 7; statistical analysis was performed using SPSS 19.0 software. One-way analysis of variance was used for comparison between the control and the exposed groups. Probability levels of 0.05 and 0.01 were considered statistically significant.
3. Results
3.1. LC50 of BPS acute exposure to C. elegans
To investigate the toxic effects of BPS, LC50 was tested by acute exposure of BPS to C. elegans for 24 h. Table 1 displays the survival after acute exposure to different concentrations of BPS. The calculation was performed using the PROBIT module in the SPSS software. Data analysis showed Sig = 0.000 < 0.05, indicating a good linear fit. According to the analytical equation, PROBIT(P) = –1.983 + 1.141X, when PROBIT = 0.5, the obtained LC50 = (0.5 + 1.983)/1.141, LC50 = 2.18 mM L–1, and converted to 545.59 mg L–1.
Table 1. Effects of BPS on the LC50 value of C. elegans after 24 h acute exposure.
| Concentration/mM | Total | Survival |
| 0 | 30 | 30 |
| 0.25 | 30 | 30 |
| 0.5 | 30 | 26 |
| 1 | 30 | 22 |
| 1.5 | 30 | 18 |
| 2 | 30 | 13 |
3.2. Effect of BPS on the locomotion behavior of C. elegans by acute exposure
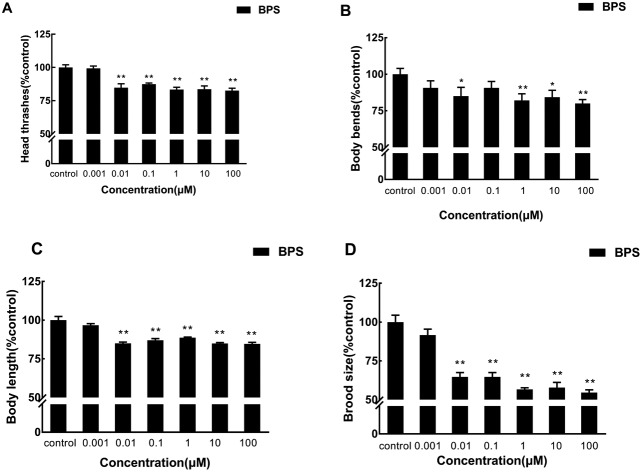
To investigate the effects of BPS on the locomotion behavior of C. elegans by acute exposure, we assayed the effects of BPS on head thrashes and body bends of nematodes (Fig. 1A and B; raw data, ESI, Table S1†). BPS could significantly (p < 0.01) decrease the head thrashes of C. elegans at concentrations above 0.01 μM. When BPS concentrations were 0.01 μM and 100 μM, the head thrashes of nematodes decreased by 15.21% and 17.4%, respectively, compared with the control. As for the body bends, there was a significant (1 μM, p < 0.01; 10 μM, p < 0.05; 100 μM, p < 0.01) decrease in the exposure groups from 1 μM to 100 μM. Body bends were decreased by 20% at a concentration of 100 μM compared with the control. With regard to the tested locomotion behavior, the exposure group of 100 μM showed the most negative effects.
Fig. 1. Toxicity evaluation of physiological traits after acute exposure to BPS. A: Comparison of head thrashes of the exposed C. elegans; B: comparison of body bends of the exposed C. elegans; C: comparison of the body length of the exposed C. elegans; D: comparison of the brood size of the exposed C. elegans. Data (mean ± SEM) are expressed as the percentage value compared to the control group. The asterisks indicate significant differences between the exposure group and control group. *p < 0.05, **p < 0.01.
3.3. Effect of BPS on the body length of C. elegans by acute exposure
The body length is an important manifestation of nematode growth and development; Fig. 1C (raw data, ESI, Table S2†) displays the effect of different concentrations of BPS on the body length of C. elegans by acute exposure. Compared with the control, the body length was significantly (p < 0.01) decreased in the exposure groups from 0.01 μM to 100 μM. The body length was decreased by 15.00% at a concentration of 0.01 μM compared with the control. The exposure group of 100 μM showed the most negative effects, and the body length was decreased by 15.27% compared with the control.
3.4. Effect of BPS on the brood size of C. elegans by acute exposure
To investigate the effects of BPS on the reproductive toxicity of C. elegans by acute exposure, we assayed the effects of BPS on the brood size of nematodes (Fig. 1D; raw data, ESI, Table S2†). The brood size was significantly (p < 0.01) decreased in the exposure groups from 0.01 μM to 100 μM. When the exposure concentration of BPS was 100 μM, the brood size decreased most significantly, which decreased 45.39% compared with the control.
3.5. Effect of BPS on the lifespan of C. elegans by acute exposure
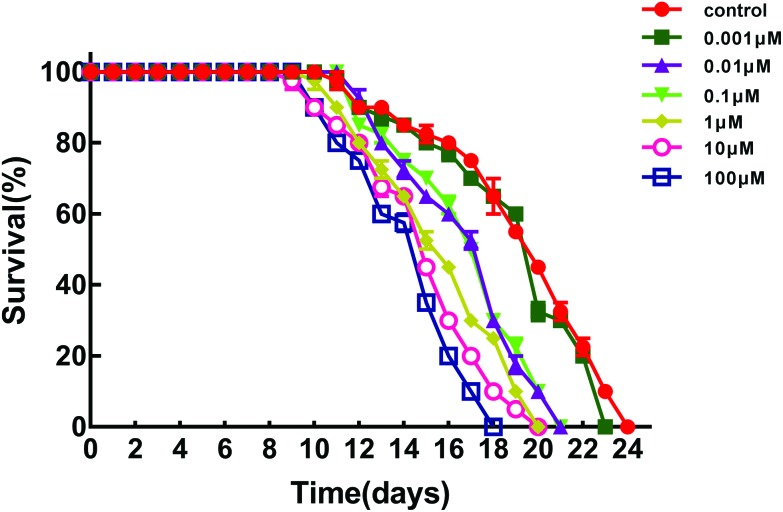
We acutely exposed C. elegans to different concentrations of BPS to study its effect on the lifespan and produced a lifespan curve as shown in Fig. 2 (raw data, ESI, Table S2†). The lifespan of C. elegans was significantly inhibited in the exposure groups from 0.01 μM to 100 μM. The lifespan of the control was 24 days, and the lifespan was decreased to 21 days when the BPS concentration was 0.01 μM. Compared with the control, the lifespan was reduced by 12.50%. When the concentration of BPS was 100 μM, the lifespan of nematodes decreased the most, which was decreased by 15.27% compared with the control.
Fig. 2. Toxicity evaluation of BPS acute exposure to C. elegans. Comparison of the lifespan of the exposed C. elegans. Data (mean ± SEM) are expressed as the percentage value compared to the control group. The asterisks indicate significant differences between the exposure group and control group. *p < 0.05, **p < 0.01.
3.6. Effect of acute exposure to BPS on the biochemical criterion of C. elegans
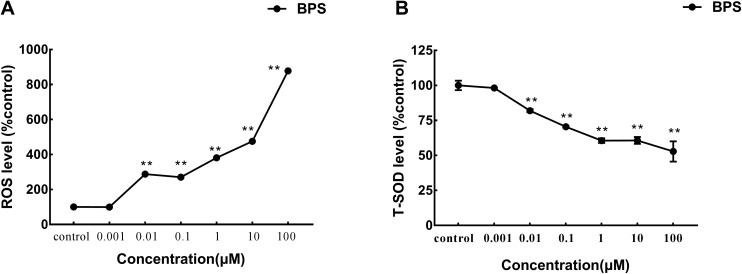
We evaluated whether BPS treatment could cause damage to the oxidation system. ROS levels were examined in C. elegans exposed to BPS (Fig. 3A; raw data, ESI, Table S3†). We found that ROS levels were significantly (p < 0.01) increased in the exposure groups from 0.01 μM to 100 μM. Compared with the control group, the ROS was increased by 778.13%. SOD levels were also examined in C. elegans exposed to BPS (Fig. 3B; raw data, ESI, Table S3†). We found that SOD levels were significantly (p < 0.01) reduced in the exposure groups from 0.01 μM to 100 μM. Compared with the control group, the SOD was reduced by 47.17%. The results of acute exposure to BPS on superoxide dismutase (SOD) in C. elegans complemented the results of the reactive oxygen species (ROS) study. Both results indicated that BPS could cause oxidative damage to nematodes.
Fig. 3. Toxicity evaluation of BPS acute exposure to C. elegans. A: Comparison of the ROS level of the exposed C. elegans; B: comparison of the SOD level of the exposed C. elegans. Data (mean ± SEM) are expressed as the percentage value compared to the control group. The asterisks indicate significant differences between the exposure group and control group. *p < 0.05, **p < 0.01.
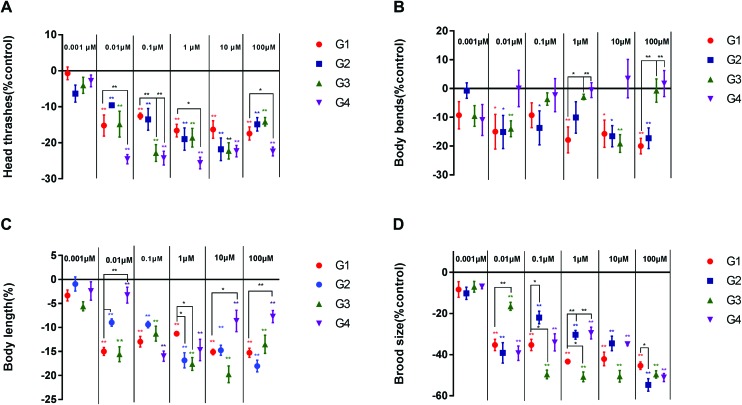
3.7. Effect of BPS on the locomotion behavior of C. elegans by multi-generational acute exposure
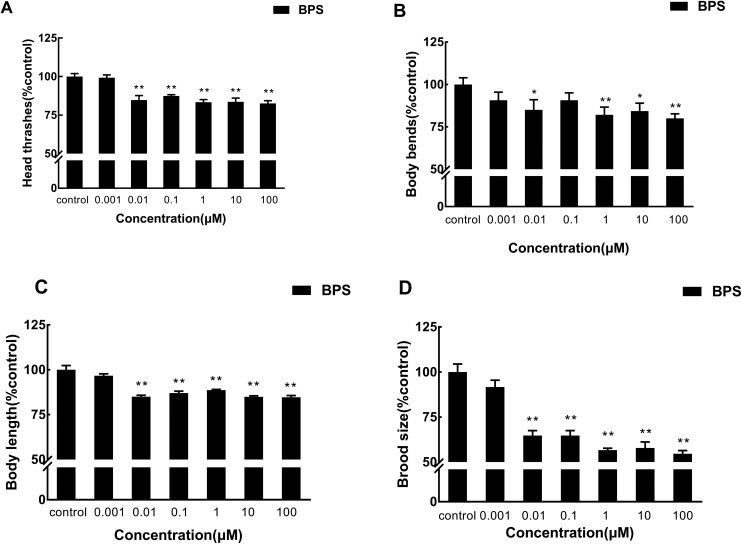
In the above study, we examined the effects of acute BPS exposure on the locomotion behaviors (head thrashes and body bends), growth, and reproduction of C. elegans in the G1 generation. We found that BPS could cause a negative impact on C. elegans. On this basis, we further studied the influence of BPS on nematodes by multi-generational exposure. As shown in Fig. 4A (raw data, ESI, Table S4†), the head thrashes significantly decreased from 0.01 μM to 100 μM in four generations. At concentrations of 0.01 μM and 100 μM, the head thrashes of G4 generation nematodes showed a significant (p < 0.01) decrease compared with those of the G1 generation. At the G4 generation, head thrashes showed a significant (p < 0.01) decrease compared to the G1 generation from 0.1 μM to 1 μM. Fig. 4B (raw data, ESI, Table S4†) shows that the body bends significantly (G1 and G2, p < 0.05; G3, p < 0.01) decrease compared to the control from G1 to G3. There was no significant effect in the G4 generation compared to the control. At the concentration of 1 μM, body bends showed a significant (G3, p < 0.05; G4, p < 0.01) increase compared to the control from G3 to G4. The concentration of 100 μM had similar results compared to 1 μM.
Fig. 4. Toxicity evaluation of BPS in multiple generations of continuous exposure to C. elegans. A: Comparison of head thrashes of the exposed C. elegans; B: comparison of body bends of the exposed C. elegans; C: comparison of the body length of the exposed C. elegans; D: comparison of the brood size of the exposed C. elegans. Data (mean ± SEM) are expressed as the percentage value compared to the control group. The asterisks indicate significant differences between the exposure group and control group. *p < 0.05, **p < 0.01.
3.8. Effect of BPS on the body length of C. elegans by multi-generational acute exposure
Growth was investigated by measuring the body length (Fig. 4C; raw data, ESI, Table S4†). At the lowest tested concentration (0.001 μM), BPS did not significantly affect growth over four generations. The body length of the four generations of C. elegans was significantly reduced at a concentration of 0.01 to 100 μM compared with the control. However, compared with the G1 generation, the G4 generation body length increased significantly (0.01 and 100 μM, p < 0.05; 10 μM, p < 0.01) at concentrations of 0.01 μM, 10 μM and 100 μM. This result indicates that the effect of BPS on the body length of C. elegans was gradually weakened with the passage through different generations.
3.9. Effect of BPS on the brood size of C. elegans by multi-generational acute exposure
Reproduction was assessed by the brood size (Fig. 4D; raw data, ESI, Table S4†). Similar to the growth assay, BPS exposure at a concentration of 0.001 μM did not significantly affect the brood size from G1 to G4. However, compared with the control, the brood size of the four generations of C. elegans was significantly reduced at a concentration of 0.01 to 100 μM. At a concentration of 0.01 μM, the G3 generation showed there were significant (p < 0.01) effects on nematodes compared with the G1 generation. Different to the concentration of 0.01 μM, at concentrations of 0.1 μM and 1 μM, the G2 generation showed a significant (0.1 μM, p < 0.05; 1 μM, p < 0.01) increase and the G3 generation showed a significant (p < 0.05) decrease compared with the G1 generation. At the highest concentration of 100 μM, the G2 generation showed a significant (p < 0.05) decrease compared with the G1 generation.
4. Discussion
As a substitute for BPA, BPS is widely used in food packaging materials. For the first time, we used C. elegans as a model organism to comprehensively assess the effects of BPS on locomotion behavior, growth, reproduction, lifespan, and antioxidant systems. We specifically studied the effects of acute exposure to BPS on the first generation, and on this basis, the effects of BPS on multiple generations were studied. In the present study, we found that BPS could cause negative effects on C. elegans.
The acute toxicity test showed that 24 h-LC50 BPS response of C. elegans was 545.59 mg L–1. 24 h LC50 BPA response of C. elegans has been reported in the literature as 327.24 mg L–1.50 Another study showed that the 24 h and 48 h EC50 values of Daphnia magna in response to BPS were 76 and 55 mg L–1, respectively; however, the 24 h and 48 h EC50 values of Daphnia magna in response to BPA were 24 and 10 mg L–1.51 The 96 h LC50 of the freshwater amphibian (Pelophylax nigromaculatus) in response to BPS was >100 mg L–1.52 Crump et al. injected BPS into the air chamber of unincubated fertilized chicken embryos and assessed that the LD50 of acute BPS exposure to chicken embryos was approximately 279 mg g–1.53 Different species show different resistance to the toxicity of BPS. Our study found that nematodes can show more sensitive responses to the toxicity of BPS, which may be related to the nematode's rich nervous system.36 It could also be seen that the toxicity of BPS was lower than that of BPA, but it does not mean that BPS is safer than BPA. BPS still lacks an effective safety assessment, especially its potential toxicity. Many investigations have shown that we are in a relatively safe concentration exposure range. According to the concentration of BPS in urine, Liao et al. estimated the concentrations of BPS exposed to humans in the United States and seven Asian countries. The levels of estimated daily intake in Japan (1.67 μg day–1, median), China (0.339 μg day–1), the US (0.316 μg day–1) and Kuwait (0.292 μg day–1) were higher than those of other target countries.23 However, low concentrations of BPS do not imply a safe concentration, and many studies have shown that BPS displays comparable biological effects even at much lower doses. Furthermore, BPS may be more chemically stable and less biodegradable, and have a higher skin penetration level than BPA.22,33,34,54 Therefore, based on the actual concentration of people's daily exposure, we chose low concentrations of BPS exposed to C. elegans to study the effects.
In the present study, endpoints of locomotion behavior were used to evaluate the neurotoxicity of C. elegans. The results indicated that BPS could have negative impacts on the locomotion behavior of C. elegans, especially on the head thrashes. The head thrash was most sensitive among the tested endpoints for assessing the toxicity of BPS. Interestingly, our data suggest that BPS has nonlinear effects on the locomotion behavior of C. elegans, and low concentrations (0.01 μM) of BPS can significantly inhibit nematode head thrashes and body bends. Its structural analog BPA has been used as one of the most common models for demonstrating the low dose and non-monotonic nature of hormones (and EDCs) that regulate or affect the endocrine system.55 Thus, BPS may also have this property, which produces a more significant effect at low concentrations. Endocrine disruptors, similar to endogenous structures, may alter the biosynthesis, degradation, or excretion of hormones in the organism, causing changes in the homeostasis of the organism.56,57 Studies have shown that BPA exposure as a typical endocrine disruptor was associated with a variety of health symptoms, including changes in neurological disorders, dysplasia, and reproductive disorders.58–60 We suspect that BPS may have similar effects. Kinch et al. found that BPS can alter the normal developmental timing of the critical neuroendocrine center, the consequences of which can lead to early synaptogenesis and improper fine-tuning of the brain later in development.30 Pregnant and lactating mice are sensitive to endocrine disruption. Studies have found that BPS could induce neurological and behavioral changes in perinatal mice, including maternal behavior and even affecting offspring's behavior and neurodevelopment.60,61 Mersha et al. found that early exposure of embryonic nematodes to BPS and BPA affects adult behavior.62
There is a complex relationship between normal growth and thyroid hormone homeostasis, and the destruction of thyroid hormone homeostasis could affect the normal growth and metabolism of vertebrates.53,63 We found that the body length of C. elegans was significantly inhibited when the BPS concentration was ≥0.01 μM, which may be related to BPS interference with thyroid hormone homeostasis. Previous studies have demonstrated that BPA adversely affected post-embryonic development via disrupting thyroid hormone signaling in vertebrates.64 Zhang et al. showed that BPS, similar to BPA, may also inhibit the growth and development of C. elegans via interfering with the signaling pathway of thyroid hormone signaling.65
In the present study, we also evaluated the effects of BPS on the reproduction of C. elegans. The study found that BPS could significantly reduce the brood size of C. elegans. The results of the study were consistent with existing research data, and a large number of studies have shown that BPS could destroy the reproductive system. BPS could cause a decrease in egg production and hatching rate and the teratogenic rate increased significantly in the study of BPS exposure to zebrafish.66 BPS may disrupt the balance of sex hormones and adversely affect sexual development or reproduction.29,67 Low levels of BPS may influence the feedback regulation loop of the hypothalamic-pituitary-gonadal (HPG) axis and impair the development of offspring.32 Mammal studies have shown that BPS could induce testicular oxidative stress and exhibit antiandrogenic properties.68,69 As a result, the epithelial height of the seminiferous tubules was thin and the number of sperms was reduced.29In vitro studies have found that mammalian oocytes were highly sensitive to the effects of BPS. BPS has a significant negative impact on the maturation of pig oocytes in vitro.70 BPS could disrupt oocyte meiosis progression and spindle formation, and alter cumulus cell gene expression and hyaluronic acid production.71 The effects of BPS on the ovaries are largely unknown. Oestradiol produced by the granulosa cells is a modulator of oocyte developmental competence and is also an important component of the HPG axis.72 Therefore we suspect that BPS may alter plasma estradiol levels and reduce the number of eggs laid. Campen et al. verified this hypothesis, and they found that 100 μM BPS could induce an increase of basal estradiol in bovine granulosa cells.73
It was noticed that our study found that the lifespan of C. elegans with BPS concentrations ≥0.01 μM was significantly inhibited. Factors affecting the lifespan are complex, and apoptosis may be one of the factors. Previous studies have demonstrated that BPA induces apoptosis in various cell types via the mitochondrial pathway.74,75 There is also evidence that BPS may have a certain effect on the endoplasmic reticulum, mitochondria and cell membrane, which can regulate caspase activation, leading to apoptosis.76–78 The study found that oxidative stress induced by reactive oxygen species was an important link in apoptosis.79 Oxidative stress has been shown to be involved in aging and various disease developments.80–82 Our study found that BPS exposure to C. elegans could significantly induce an increase in ROS levels and a decrease in SOD levels. This indicated that BPS could damage the intracellular antioxidant system and cause oxidative damage. The production and elimination of active oxygen in the body are in a state of dynamic equilibrium under normal circumstances, which has no harmful effect on the body. However, if the balance is broken, and the active oxygen is generated in a large amount and exceeds the scavenging ability of the antioxidant system, the body will form an oxidative stress state.83 Qiu et al. also found that BPS could cause severe oxidative stress at higher BPS exposure concentrations, subsequently destroying cell homeostasis and promoting apoptosis.13 Based on our studies, we hypothesized that BPS may affect apoptosis by oxidative stress and thus affect the lifespan of C. elegans.
BPS has been widely used as a substitute for BPA in the production of baby bottles. One recent study reported that BPS was found in four maternal and seven cord sera samples collected from China.84 Another study in the United States also reported that the estimated daily intake of BPS in young children was higher than that in other age groups.22 Therefore it raised our concern whether BPS would have a more profound impact on the offspring. Zhou et al. have found that continuous exposure of BPA for multiple generations still has a negative impact on the locomotion behavior, body length and lifespan of C. elegans.85 We continuously exposed four generations based on the first generation results to study the effects of BPS on the locomotion behavior, growth and reproduction of C. elegans. Our study found that with the transmission to offspring, BPS has a more significant inhibition on the head thrashes of the fourth generation of C. elegans, and the influence on the body bends and body length was gradually weakened. The effect of BPS on the brood size of C. elegans showed different rules according to different concentrations and generations, which indicated that BPS showed more complicated effects on reproductive toxicity. In experiments with BPS exposure to zebrafish, Ji et al. found that parental exposure to environmentally relevant concentrations of BPS resulted in delayed and damaged hatching and increased malformation rates of the offspring generation.66 Pregnant and lactating mothers may be sensitive and vulnerable to endocrine disruptors. The effects of BPS on mothers may affect the behavior and neurodevelopment of her offspring.61,86 More and more research evidence indicates that the genetic toxicity of BPS deserves our attention.
5. Conclusion
To the best of our knowledge, our research is the first to use C. elegans for the safety assessment of BPS and the first to assess the genotoxic effects of multiple exposures of BPS. Our study demonstrates that BPS could have a negative impact on the locomotion behavior, growth, reproduction, lifespan and antioxidant system of C. elegans. It is worth noting that in the multi-generation continuous exposure experiment, BPS could still have a negative effect on C. elegans. BPS may not be a safe substitute for BPA, which may have similar toxic effects to BPA. The safety of BPS needs further evaluation, especially the potential genotoxic effects.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Supplementary Material
Acknowledgments
This research was supported by Grants from the National Natural Science Foundation of China (Grant 31501569, 31701598 and 31701605).
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c9tx00055k
References
- Liao C., Liu F., Kannan K. Environ. Sci. Technol. 2012;46:6515–6522. doi: 10.1021/es300876n. [DOI] [PubMed] [Google Scholar]
- Liao C., Kannan K. J. Agric. Food Chem. 2013;61:4655–4662. doi: 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- Zhao F., Jiang G., Wei P., Wang H., Ru S. Ecotoxicol. Environ. Saf. 2017;147:794–802. doi: 10.1016/j.ecoenv.2017.09.048. [DOI] [PubMed] [Google Scholar]
- Giulivo M., Lopez de. A. M., Capri E., Barceló D. Environ. Res. 2016;151:251–264. doi: 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Ziv-Gal A., Flaws J. A. Fertil. Steril. 2016;106:827–856. doi: 10.1016/j.fertnstert.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Ren X. M., Li Y. Y., Yao X. F., Li C. H., Qin Z. F., Guo L. H. Environ. Pollut. 2017;237:1072–1079. doi: 10.1016/j.envpol.2017.11.027. [DOI] [PubMed] [Google Scholar]
- Rochester J. R., Bolden A. L. Environ. Health Perspect. 2015;123:643–650. doi: 10.1289/ehp.1408989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallart-Ayala H., Moyano E., Galceran M. T. Anal. Chim. Acta. 2011;683:227–233. doi: 10.1016/j.aca.2010.10.034. [DOI] [PubMed] [Google Scholar]
- Liao C., Kannan K. Food Addit. Contam. 2014;31:319–329. doi: 10.1080/19440049.2013.868611. [DOI] [PubMed] [Google Scholar]
- Liao C., Kannan K. Environ. Sci. Technol. 2012;46:5003–5009. doi: 10.1021/es300115a. [DOI] [PubMed] [Google Scholar]
- Mesnage R., Phedonos A., Arno M., Balu S., Christopher C. J., Antoniou M. N. Toxicol. Sci. 2017;158:431–443. doi: 10.1093/toxsci/kfx101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C., Liu F., Moon H. B., Yamashita N., Yun S., Kannan K. Environ. Sci. Technol. 2012;46:11558–11565. doi: 10.1021/es303191g. [DOI] [PubMed] [Google Scholar]
- Qiu W., Yang M., Liu S., Lei P., Hu L., Chen B., Wu M., Wang K. J. Environ. Sci. Technol. 2018;52:831–838. doi: 10.1021/acs.est.7b04226. [DOI] [PubMed] [Google Scholar]
- Ivry D. M. L., Le C. L., Poirier H., Niot I., Truntzer T., Merlin J. F., Rouimi P., Besnard P., Rahmani R., Chagnon M. C. Toxicology. 2016;357–358:11–20. doi: 10.1016/j.tox.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhang S., Song N., Guo R., Chen M., Mai D., Yan Z., Han Z., Chen J. Sci. Total Environ. 2017;s599–600:1090–1098. doi: 10.1016/j.scitotenv.2017.05.069. [DOI] [PubMed] [Google Scholar]
- Jin H., Zhu L. Water Res. 2016;103:343–351. doi: 10.1016/j.watres.2016.07.059. [DOI] [PubMed] [Google Scholar]
- Wu L. H., Zhang X. M., Wang F., Gao C. J., Chen D., Palumbo J. R., Guo Y., Zeng E. Y. Sci. Total Environ. 2017;615:87–98. doi: 10.1016/j.scitotenv.2017.09.194. [DOI] [PubMed] [Google Scholar]
- Yamazaki E., Yamashita N., Taniyasu S., Lam J., Lam P. K. S., Moon H. B., Jeong Y., Kannan P., Achyuthan H., Munuswamy N. Ecotoxicol. Environ. Saf. 2015;122:565–572. doi: 10.1016/j.ecoenv.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Wang W., Abualnaja K. O., Asimakopoulos A. G., Covaci A., Gevao B., Johnson-Restrepo B., Kumosani T. A., Malarvannan G., Tu B. M., Moon H. B. Environ. Int. 2015;83:183–191. doi: 10.1016/j.envint.2015.06.015. [DOI] [PubMed] [Google Scholar]
- Liao C., Kannan K. J. Agric. Food Chem. 2013;61:4655–4662. doi: 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- Liao C. Y., Kannan K. Food Addit. Contam., Part A. 2014;31:319–329. doi: 10.1080/19440049.2013.868611. [DOI] [PubMed] [Google Scholar]
- Liao C., Liu F., Alomirah H., Loi V. D., Mohd M. A., Moon H. B., Nakata H., Kannan K. Environ. Sci. Technol. 2012;46:6860–6866. doi: 10.1021/es301334j. [DOI] [PubMed] [Google Scholar]
- Liao C., Liu F., Guo Y., Moon H. B., Nakata H., Wu Q., Kannan K. Environ. Sci. Technol. 2012;46:9138–9145. doi: 10.1021/es302004w. [DOI] [PubMed] [Google Scholar]
- Thayer K. A., Taylor K. W., Garantziotis S., Schurman S. H., Kissling G. E., Hunt D., Herbert B., Church R., Jankowich R., Churchwell M. I. Environ. Health Perspect. 2016;124:437–444. doi: 10.1289/ehp.1409427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li J., Wu Y., Zhao Y., Luo F., Li S., Lin Y., Moez E. K., Dinu I. A., Martin J. W. Environ. Sci. Technol. 2017;51:2456–2463. doi: 10.1021/acs.est.6b05718. [DOI] [PubMed] [Google Scholar]
- Ullah H., Jahan S., Ain Q. U., Shaheen G., Ahsan N. Chemosphere. 2016;152:383–391. doi: 10.1016/j.chemosphere.2016.02.125. [DOI] [PubMed] [Google Scholar]
- Ullah H., Ambreen A., Ahsan N., Jahan S. Toxicol. Environ. Chem. 2017;99:1–23. [Google Scholar]
- Hass U., Christiansen S., Boberg J., Rasmussen M. G., Mandrup K., Axelstad M. Andrology. 2016;4:594–607. doi: 10.1111/andr.12176. [DOI] [PubMed] [Google Scholar]
- Naderi M., Wong M. Y., Gholami F. Aquat. Toxicol. 2014;148:195–203. doi: 10.1016/j.aquatox.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Kinch C. D., Ibhazehiebo K., Jeong J. H., Habibi H. R., Kurrasch D. M. Proc. Natl. Acad. Sci. U. S. A. 2015;112:1475–1480. doi: 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K., Hong S., Kho Y., Choi K. Environ. Sci. Technol. 2013;47:8793–8800. doi: 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- Qiu W., Zhao Y., Yang M., Farajzadeh M., Pan C., Wayne N. L. Endocrinology. 2016;157:636–647. doi: 10.1210/en.2015-1785. [DOI] [PubMed] [Google Scholar]
- Ike M., Chen M. Y., Danzl E., Sei K., Fujita M. Water Sci. Technol. 2006;53:153–159. doi: 10.2166/wst.2006.189. [DOI] [PubMed] [Google Scholar]
- Danzl E., Sei K., Soda S., Ike M., Fujita M. Int. J. Environ. Res. Public Health. 2009;6:1472–1484. doi: 10.3390/ijerph6041472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélièstoussaint C., Peyre L., Costanzo C., Chagnon M. C., Rahmani R. Toxicol. Appl. Pharmacol. 2014;280:224–235. doi: 10.1016/j.taap.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Corsi A. K., Bruce W., Martin C. Genetics. 2015;200:387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi A. K., Wightman B., Chalfie M. Genetics. 2015;201:339–339. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Zhao Y., Li Y., Wang D. Nanomedicine. 2014;10:1263–1271. doi: 10.1016/j.nano.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Qiao Y., Zhao Y., Wu Q., Sun L., Ruan Q., Chen Y., Wang M., Duan J., Wang D. PLoS One. 2014;9:e91825. doi: 10.1371/journal.pone.0091825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Lv T., Li M., Wu Q., Yang L., Liu H., Sun D., Sun L., Zhuang Z., Wang D. PLoS One. 2013;8:e74553. doi: 10.1371/journal.pone.0074553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju J., Ruan Q., Li X., Liu R., Li Y., Pu Y., Yin L., Wang D. Environ. Sci. Pollut. Res. 2013;20:1823–1830. doi: 10.1007/s11356-012-1151-2. [DOI] [PubMed] [Google Scholar]
- Liao V. H., Yu C. W., Chu Y. J., Li W. H., Hsieh Y. C., Wang T. T. Mech. Ageing Dev. 2011;132:480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Rui Q., Zhao Y., Wu Q., Tang M., Wang D. Chemosphere. 2013;93:2289–2296. doi: 10.1016/j.chemosphere.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Wang D., Xing X. Ecotoxicol. Environ. Saf. 2010;73:423–429. doi: 10.1016/j.ecoenv.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Shen L., Xiao J., Ye H. Y., Wang D. Y. Environ. Toxicol. Pharmacol. 2009;28:125–132. doi: 10.1016/j.etap.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Apfeld J., Kenyon C. Nature. 1999;402:804–809. doi: 10.1038/45544. [DOI] [PubMed] [Google Scholar]
- Lakowski B., Hekimi S. Proc. Natl. Acad. Sci. U. S. A. 1998;95:13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wu Q., Meng T., Wang D. Nanomedicine. 2014;10:89–98. doi: 10.1016/j.nano.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Ye K., Ji C. B., Lu X. W., Ni Y. H., Gao C. L., Chen X. H., Zhao Y. P., Gu G. X., Guo X. R. J. Radiat. Res. 2010;51:473–479. doi: 10.1269/jrr.10009. [DOI] [PubMed] [Google Scholar]
- Ura K., Kai T., Sakata S., Iguchi T., Arizono K. J. Health Sci. 2002;48:583–586. [Google Scholar]
- Chen M. Y., Ike M., Fujita M. Environ. Toxicol. 2002;17:80–86. doi: 10.1002/tox.10035. [DOI] [PubMed] [Google Scholar]
- Li Y., Fu X., Zhao Y., Su H., Qin Z. Asian J. Ecotoxicol. 2015;10(2):251–257. [Google Scholar]
- Crump D., Chiu S., Williams K. L. Environ. Toxicol. Chem. 2015;35:1541–1549. doi: 10.1002/etc.3313. [DOI] [PubMed] [Google Scholar]
- žalmanová T., Hošková K., Nevoral J., Prokešová Š., Zámostná K., Kott T., Petr J. Czech J. Anim. Sci. 2016;61:433–449. [Google Scholar]
- Vandenberg L. N., Zoeller R. T., Welshons W. V., Myers J. P., Colborn T., Hayes T. B., Heindel J. J., Jacobs Jr. D. R., Lee D.-H., Shioda T., Soto A. M., vom Saal F. S. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreman J., Lee O., Trznadel M., David A., Kudoh T., Tyler C. R. Environ. Sci. Technol. 2017;51:12796–12805. doi: 10.1021/acs.est.7b03283. [DOI] [PubMed] [Google Scholar]
- Gore A. C., Chappell V. A., Fenton S. E., Flaws J. A., Nadal A., Prins G. S., Toppari J., Zoeller R. T. Endocr. Rev. 2015;36:E1–E150. doi: 10.1210/er.2015-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto V. Y., Kim D., vom Saal F. S., Lamb J. D., Taylor J. A., Bloom M. S. Fertil. Steril. 2011;95:1816–1819. doi: 10.1016/j.fertnstert.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Kajta M., Wójtowicz A. K. Pharmacol. Rep. 2013;65:1632–1639. doi: 10.1016/s1734-1140(13)71524-x. [DOI] [PubMed] [Google Scholar]
- Miao M., Yuan W., Zhu G., He X., Li D. K. Reprod. Toxicol. 2011;32:64–68. doi: 10.1016/j.reprotox.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Laplante C. D., Catanese M. C., Bansal R., Vandenberg L. N. Endocrinology. 2017;158:3448–3461. doi: 10.1210/en.2017-00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersha M. D., Patel B. M., Patel D., Richardson B. N., Dhillon H. S. Behav. Brain Funct. 2015;11(1):27. doi: 10.1186/s12993-015-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. Y., Leonard J. L., Davis P. J. Endocr. Rev. 2010;31:139–170. doi: 10.1210/er.2009-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimeier R. A., Das B., Buchholz D. R., Shi Y. B. Endocrinology. 2009;150(6):2964–2973. doi: 10.1210/en.2008-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Ren X. M., Li Y. Y., Yao X. F., Li C. H., Qin Z. F., Guo L. H. Environ. Pollut. 2017;237:1072–1079. doi: 10.1016/j.envpol.2017.11.027. [DOI] [PubMed] [Google Scholar]
- Ji K., Hong S., Kho Y., Choi K. Environ. Sci. Technol. 2013;47:8793–8800. doi: 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- Horan T. S., Pulcastro H., Lawson C., Gerona R., Martin S., Gieske M. C., Sartain C. V., Hunt P. A. Curr. Biol. 2018;28:2948–2953. doi: 10.1016/j.cub.2018.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofs M. J., Van d. B. M., Bovee T. F., Piersma A. H., van Duursen M. B. Toxicology. 2015;329:10–20. doi: 10.1016/j.tox.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Ullah H., Jahan S., Ain Q. U., Shaheen G., Ahsan N. Chemosphere. 2016;152:383–391. doi: 10.1016/j.chemosphere.2016.02.125. [DOI] [PubMed] [Google Scholar]
- žalmanová T., Hošková K., Nevoral J., Adámková K., Kott T., Šulc M., Kotíková Z., Prokešová Š., Jílek F., Králíčková M., Petr J. Sci. Rep. 2017;7:485. doi: 10.1038/s41598-017-00570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevoral J., Kolinko Y., Moravec J., Zalmanova T., Hoskova K., Prokesova S., Klein P., Ghaibour K., Hosek P., Stiavnicka M., Rimnacova H., Tonar Z., Petr J., Kralickova M. Reproduction. 2018;156:47–57. doi: 10.1530/REP-18-0092. [DOI] [PubMed] [Google Scholar]
- Xi W., Lee C. K. F., Yeung W. S. B., Giesy J. P., Wong M. H., Zhang X., Hecker M., Wong C. K. C. Reprod. Toxicol. 2011;31:409–417. doi: 10.1016/j.reprotox.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Campen K. A., Lavallee M., Combelles C. Reprod. Domest. Anim. 2018;53:450–457. doi: 10.1111/rda.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Sun X., Qiu L., Wei J., Huang Q., Fang C., Ye T., Kang M., Shen H., Dong S. Cell Death Dis. 2013;4:e460. doi: 10.1038/cddis.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokra K., Kocia M., Michałowicz J. Food Chem. Toxicol. 2015;84:79–88. doi: 10.1016/j.fct.2015.08.007. [DOI] [PubMed] [Google Scholar]
- Zamzami N., Kroemer G. Nat. Rev. Mol. Cell Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- Idriss H. T., Naismith J. H. Microsc. Res. Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Zhang K., Kaufman R. J. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka R. B., Chandel N. S. Trends Biochem. Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A. H., Chung K. K. Biochim. Biophys. Acta, Mol. Basis Dis. 2009;1792:643–650. doi: 10.1016/j.bbadis.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Zhu X., Smith M. A., Honda K., Aliev G., Moreira P. I., Nunomura A., Casadesus G., Harris P. L. R., Siedlak S. L., Perry G. J. Neurol. Sci. 2007;257:240–246. doi: 10.1016/j.jns.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey K. B., Rizvi S. I. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. Repub. 2011;155:131–136. doi: 10.5507/bp.2011.027. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Doita M., Nishida K., Yamamoto T., Sumi M., Kurosaka M. Spine. 2006;31:4–9. doi: 10.1097/01.brs.0000192682.87267.2a. [DOI] [PubMed] [Google Scholar]
- Liu J., Li J., Wu Y., Zhao Y., Luo F., Li S., Yang L., Moez E. K., Dinu I., Martin J. W. Environ. Sci. Technol. 2017;51:2456–2463. doi: 10.1021/acs.est.6b05718. [DOI] [PubMed] [Google Scholar]
- Zhou D., Yang J., Li H., Lu Q., Liu Y. D., Lin K. F. Environ. Pollut. 2016;208:767–773. doi: 10.1016/j.envpol.2015.10.057. [DOI] [PubMed] [Google Scholar]
- Catanese M. C., Vandenberg L. N. Endocrinology. 2017;158:516–530. doi: 10.1210/en.2016-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.