Key Points
Similar event-free survival after HLA-matched sibling and HLA-matched unrelated donor transplant.
High graft failure after HLA-mismatched related donor transplant.
Abstract
We studied 1110 patients with β-thalassemia major aged ≤25 years who received transplants with grafts from HLA-matched related (n = 677; 61%), HLA-mismatched related (n = 78; 7%), HLA-matched unrelated (n = 252; 23%), and HLA-mismatched unrelated (n = 103; 9%) donors between 2000 and 2016. Ninety percent of transplants were performed in the last decade. Eight-five percent of patients received ≥20 transfusions and 88% were inadequately chelated. All patients received myeloablative-conditioning regimen. Overall and event-free survival were highest for patients aged ≤6 years and after HLA-matched related and HLA-matched unrelated donor transplantation. The 5-year probabilities of overall survival for patients aged ≤6 years, 7 to 15 years, and 16 to 25 years, adjusted for donor type and conditioning regimen were 90%, 84%, and 63%, respectively (P < .001). The corresponding probabilities for event-free survival were 86%, 80%, and 63% (P < .001). Overall and event-free survival did not differ between HLA-matched related and HLA-matched unrelated donor transplantation (89% vs 87% and 86% vs 82%, respectively). Corresponding probabilities after mismatched related and mismatched unrelated donor transplantation were 73% vs 83% and 70% vs 78%. In conclusion, if transplantation is considered as a treatment option it should be offered early (age ≤6 years). An HLA-matched unrelated donor is a suitable alternative if an HLA-matched relative is not available.
Visual Abstract
Introduction
β-thalassemia major is a genetic defect that is associated with absent or impaired β-globin synthesis resulting in an imbalanced accumulation of α-globin chains and ineffective erythropoiesis with hemolysis.1 The hallmarks of supportive care include regular red blood cell transfusion with iron chelation therapy for transfusion-related iron overload.2 Allogeneic hematopoietic cell transplantation is a curative option for β-thalassemia major and ∼70% of transplants in the last decade used HLA-matched sibling donors.3 The availability of an HLA-matched sibling donor and transplantation-related risks (graft failure, acute and chronic graft-versus-host disease [GVHD]) has limited the broad use of allogeneic transplantation. In recent years, there has been an increase in the number of transplants from alternative donors and use of novel transplant-conditioning regimens to lower transplantation-related risks.4-8 A myeloablative dose of busulfan (Bu) with cyclophosphamide (Cy) used to be the predominant conditioning regimen. Although most conditioning regimens in recent years are also myeloablative in intensity, modifications include the addition of fludarabine (FLU) and/or thiotepa (TT) to Bu and Cy or treosulfan with FLU and/or TT with favorable outcomes.4-6
In addition to donor type and transplant-conditioning regimen, the timing of transplantation is also a significant contributor to the success of allogeneic transplantation for thalassemia. The Pesaro risk class that was developed over 30 years ago for children with β-thalassemia recorded higher mortality for those with risk class II and III compared with class I.9 Risk class was assigned based on the presence or absence of hepatomegaly and portal fibrosis. However, a liver biopsy that is needed to ascertain the presence or absence of portal fibrosis is not obtained for all patients who receive allogeneic transplantation. This could in part be attributed to: practice variation between geographic regions or transplant centers within a geographic region, morbidity, and a rare risk of mortality associated with the procedure, or a perception that risk class assignment is a prognostic tool unlikely to alter the decision to proceed to transplantation. Consequently, others have studied clinical risk factors associated with transplantation and recorded higher mortality in patients older than 6 years at transplantation and those with hepatomegaly at transplantation10,11 However, in both reports, age and hepatomegaly correlated with Pesaro risk class, implying that these clinical factors may be used to ascertain mortality risks when a liver biopsy is not obtained.
The recent reports on improved outcomes after allogeneic transplantation for thalassemia,4-7 with the exception of the report from the European Society for Blood and Marrow Transplantation (EBMT),3 included modest numbers of patients. The EBMT report studied the period 2000 to 2010, and included predominantly HLA-matched sibling donor transplants and patients aged <18 years. That report did not study outcomes for alternative donor transplantation.3 Therefore, in the current analyses, we sought to address outcomes after transplantation in children and young adults (age ≤25 years) and use of alternative donors relative to HLA-matched related donor transplantation in 3 geographic regions: China, India, and the United States. Although our study period included 2000 to 2016, 90% of transplants occurred between 2006 and 2016 thus representing current practice patterns.
Patients and methods
Data source
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a working group of >300 transplant centers that contribute data on consecutive allogeneic and autologous transplants. Fifty transplant centers in China, India, and the United States contributed data for the study. Patients are followed longitudinally until death or loss to follow-up. Accuracy of data reported to the CIBMTR and compliance is monitored by on-site audits. Consent is sought from patients and/or their legal guardians for research. The institutional review board of the National Marrow Donor Program approved the study.
Patients
Eligible were 1110 patients with β-thalassemia major aged ≤25 years who received their first allogeneic hematopoietic cell transplant between 2000 and 2016. Ninety percent of transplants were performed in the last decade. All patients received myeloablative-conditioning regimens and most regimens included Bu (dose ≥8.4 mg/kg and dose titrated based on pharmacokinetics).12 Two hundred fifteen patients (19%) have been reported previously. Excluded were patients aged >25 years (n = 1) and reduced-intensity conditioning regimen transplantation (n = 23).
Outcomes
The primary outcome was event-free survival (death from any cause or graft failure). Graft failure was defined as: failure to achieve absolute neutrophil recovery (ANC) ≥0.5 × 109/L for 3 consecutive days; ANC decline to <0.5 × 109/L without recovery after having achieved ANC ≥0.5 × 109/L; myeloid donor chimerism (<5%); recurrence of transfusion dependence; or second transplant.13 Other outcomes studied were overall survival (death from any cause), neutrophil recovery (absolute neutrophil count, ≥0.5 × 109/L; and donor chimerism, >5%) and platelet recovery (≥20 × 109/L unsupported for 7 days), and acute and chronic GVHD graded using standard criteria.14,15 Surviving patients were censored at last follow-up.
Statistical methods
The probabilities of event-free survival and overall survival were calculated using the Kaplan-Meier estimator.16 The probability of graft failure was calculated using the cumulative incidence estimator to accommodate competing risks.17 Risk factors associated with event-free survival and overall survival were examined using the Cox proportional hazards model.18 Risk factors associated with graft failure and acute and chronic GVHD were examined using the Fine and Gray model.19 Variables considered included age, sex, red blood cell transfusion, adequacy of iron chelation, serum ferritin, hepatomegaly, donor type, graft type, conditioning regimen, in vivo T-cell depletion, GVHD prophylaxis, and transplant period (Table 1). The age groups (≤6 years vs 7-15 years vs 16-25 years) were determined statistically as the optimal cut points in the Cox regression model for overall survival. Models were built using stepwise forward selection and variables that met a significance level of ≥0.05 were held in the final model. There were no first-order interactions between the variables held in the final models. An effect of transplant center on survival was tested using the frailty model.20 All P values are 2-sided and all analyses were done using SAS version 9.3 (Cary, NC).
Table 1.
Patient and transplant characteristics
| No. (%) | |
|---|---|
| Age at transplantation, y | |
| ≤6 | 638 (57) |
| 7-15 | 439 (40) |
| 16-25 | 33 (3) |
| Sex | |
| Male/female | 696 (63)/414 (37) |
| Red blood cell transfusions prior to transplantation | |
| <20 | 40 (4) |
| 20-50 | 304 (27) |
| >50 | 634 (57) |
| Not reported | 132 (12) |
| Iron chelation | |
| Adequate | 117 (11) |
| Inadequate | 981 (88) |
| Not reported | 12 (1) |
| Hepatomegaly | |
| Absent | 280 (25) |
| Present | 806 (73) |
| Not reported | 24 (2) |
| Donor type | |
| HLA-matched relative | 677 (61) |
| HLA-mismatched relative | 78 (7) |
| HLA-matched unrelated | 252 (23) |
| HLA-mismatched unrelated | 103 (9) |
| Graft type | |
| Bone marrow | 321 (29) |
| Peripheral blood | 682 (61) |
| Cord blood | 107 (10) |
| In vivo T-cell depletion | |
| Yes/none | 827 (75)/283 (25) |
| Conditioning regimen | |
| BU/Cy/TT/FLU | 376 (34) |
| BU/Cy/FLU | 259 (23) |
| BU/Cy | 249 (22) |
| Treosulfan/TT/FLU | 169 (15) |
| BU or melphalan ± TT ± FLU | 57 (5) |
| GVHD prophylaxis | |
| Calcineurin inhibitor/methotrexate/mycophenolate | 187 (17) |
| Calcineurin inhibitor/methotrexate | 446 (40) |
| Calcineurin inhibitor/mycophenolate | 350 (32) |
| Calcineurin inhibitor alone | 31 (3) |
| Posttransplant Cy/cyclosporine/mycophenolate | 96 (9) |
Results
Patient, disease, and transplant characteristics
The characteristics of the 1110 children, adolescents, and young adults are presented in Table 1. The median age at transplantation was 6 years (range, <1-25 years). Although the cohort included young adults, they represent only 3% of the study population. All patients were on a chronic transfusion program at time of transplantation. Over one-half of patients had received over 50 red blood cell transfusions and almost 90% of patients were inadequately chelated. Adequacy of chelation was defined as daily chelation for at least 5 days per week beginning within 2 years of regular transfusion. Approximately one-half of patients had a serum ferritin ≥2500 ng/mL and over 70% of patients were recorded to have hepatomegaly. In a subset (526 of 1110; 47%), liver biopsy was obtained prior to transplantation to ascertain presence or absence of portal fibrosis. Consequently, we were able to assign Pesaro risk class for this subset: risk class I (39%), II (8%), and III (54%). All patients received nonirradiation myeloablative-conditioning regimens. Thirty-four percent of patients received Bu (8.4-13.2 mg/kg) plus Cy (100-120 mg/kg) plus TT (5 mg/kg) plus FLU (200 mg/m2), 23% received Bu (11.2-17.6 mg/kg) plus Cy (100-120 mg/kg) plus FLU (200 mg/m2), 22% received Bu (16 mg/kg) plus Cy (200 mg/kg), 15% received treosulfan (42 mg/m2) plus TT (8 mg/kg) plus FLU (120 mg/m2), and the remaining 5% received Bu (12 or 16 mg/kg) or melphalan (140 mg/m2) plus FLU (150-180 mg/m2) plus or minus TT (8-10 mg/kg). Bu dose was titrated based on pharmacokinetics for all transplants that included a Bu-containing regimen. In vivo T-cell depletion with antithymocyte globulin was common (75% of transplants). Donor, graft, and GVHD prophylaxis were confounded with transplant-conditioning regimen. For the Bu/Cy and treosulfan/TT/FLU regimens, the predominant donor type was matched related donor (85%). Peripheral blood was the predominant graft associated with Bu/Cy/TT/FLU-, Bu/Cy/FLU-, and treosulfan/TT/FLU-conditioning regimens. All patients received calcineurin inhibitor–containing regimens for GVHD prophylaxis and <10% of GVHD prophylaxis included posttransplant Cy. This GVHD prophylaxis regimen was used exclusively with the Bu/Cy/FLU regimen. Fifty-nine percent of transplants (657 of 1110) were performed in 2012 to 2016, 31% (339 of 1110) were performed in 2006 to 2011, and 10% (114 of 1110) were performed in 2000 to 2005. The median follow-up of surviving patients was 48 months (range, 3-193 months).
Event-free survival
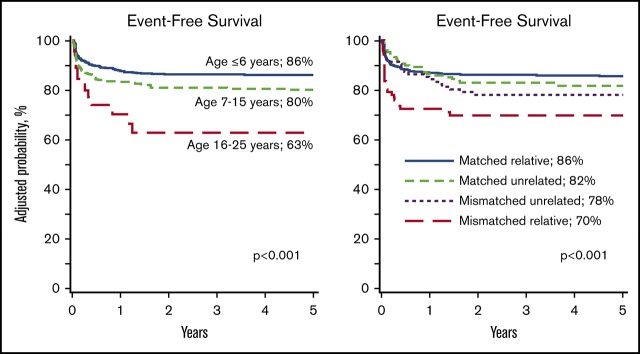
Event-free survival was lower in patients older than 6 years at transplantation compared with those aged ≤6 years, recipients of HLA-mismatched related compared with HLA-matched related donor transplant, and transplant period prior to 2012 (Table 2). Among patients older than 6 years, event-free survival was lower for patients aged 16 to 25 years compared with those aged 7 to 15 years (hazard ratio [HR], 2.08; 95% confidence interval [CI], 1.16-3.70; P = .01). Event-free survival was also lower after HLA-mismatched related donor compared with HLA-matched unrelated donor (HR, 2.20; 95% CI, 1.20-4.01; P = .01). Event-free survival did not differ between HLA-mismatched related and HLA-mismatched unrelated donor transplants (HR, 1.72; 95% CI, 0.92-3.20; P = .09). The 5-year probabilities of event-free survival by age and donor type are shown in Figure 1 and Table 3. Although all patients received myeloablative regimens, event-free survival was lower with conditioning regimens other than Bu/Cy/TT/FLU compared with Bu/Cy, treosulfan/TT/FLU and Bu or melphalan plus or minus TT plus or minus FLU (Table 2). Similarly, compared with the Bu/Cy/FLU regimen, event-free survival was lower after Bu/Cy (HR, 2.38; 95% CI, 1.39-4.00; P = .002), treosulfan/TT/FLU (HR, 2.13; 95% CI, 1.22-3.70; P = .008), and Bu or melphalan plus or minus TT plus or minus FLU (HR, 3.85; 95% CI, 2.13-7.14; P < .0001) regimens. Event-free survival did not differ between Bu/Cy/TT/FLU and Bu/Cy/FLU regimens (Table 2). The effect of conditioning regimen was independent of donor type. Additionally, among recipients of unrelated donor transplantation, event-free survival did not differ between the 2 predominant regimens: Bu/Cy/TT/FLU and Bu/Cy/FLU (91% [95% CI, 86-95] and 89% [95% CI, 83-94], respectively). The 5-year probabilities of event-free survival for patients aged ≤6 years, 7 to 15 years, and 16 to 25 years, adjusted for donor type and conditioning regimen were 86% (95% CI, 83-89), 80% (95% CI, 76-84), and 63% (95% CI, 48-78), respectively (P < .001).
Table 2.
Risks factors for event-free survival and overall survival
| Outcomes | Events/evaluable | HR (95% CI) | P |
|---|---|---|---|
| Event-free survival | |||
| Age at transplantation, y | |||
| ≤6 | 84/638 | 1.00 | <.001 |
| 7-15 | 87/439 | 1.46 (1.07-2.00) | .017 |
| 16-25 | 14/33 | 3.03 (1.68-5.47) | <.001 |
| Donor type | |||
| HLA-matched relative | 108/677 | 1.00 | <.001 |
| HLA-mismatched relative | 29/78 | 2.75 (1.81-4.16) | <.001 |
| HLA-matched unrelated | 26/252 | 1.25 (0.75-2.07) | .39 |
| HLA-mismatched unrelated | 22/103 | 1.60 (0.95-2.71) | .08 |
| Conditioning regimen | |||
| Bu/Cy/TT/FLU | 28/376 | 1.00 | <.001 |
| Bu/Cy/FLU | 27/259 | 1.32 (0.78-2.25) | .31 |
| Bu/Cy | 71/249 | 3.11 (1.80-5.40) | <.001 |
| Treosulfan/TT/FLU | 38/169 | 2.79 (1.61-4.83) | <.001 |
| Bu or melphalan ± TT ± FLU | 21/57 | 5.09 (2.81-9.21) | <.001 |
| Transplant period | |||
| 2012-2016 | 74/657 | 1.00 | .008 |
| 2006-2011 | 73/339 | 1.66 (1.17-2.35) | .01 |
| 2000-2005 | 38/114 | 1.96 (1.16-3.33) | .01 |
| Overall survival | |||
| Age at transplantation, y | |||
| ≤6 | 57/638 | 1.00 | <.001 |
| 7-15 | 73/439 | 1.84 (1.29-2.63) | <.001 |
| 16-25 | 12/33 | 4.35 (2.28-8.30) | <.001 |
| Donor type | |||
| HLA-matched relative | 83/677 | 1.00 | <.001 |
| HLA-mismatched relative | 25/78 | 3.30 (1.93-4.77) | <.001 |
| HLA-matched unrelated | 19/252 | 1.13 (0.64-2.02) | .67 |
| HLA-mismatched unrelated | 15/103 | 1.56 (0.85-2.88) | .16 |
| Conditioning regimen | |||
| Bu/Cy/TT/FLU | 25/376 | 1.00 | <.001 |
| Bu/Cy/FLU | 22/259 | 1.32 (0.74-2.34) | .34 |
| Bu/Cy | 55/249 | 3.61 (2.15-6.07) | <.001 |
| Treosulfan/TT/FLU | 30/169 | 2.22 (1.23-4.030 | .008 |
| Bu or melphalan ± TT ± FLU | 10/57 | 2.55 (1.18-5.50) | .02 |
Figure 1.
Event-free survival. (A) Event-free survival by age at transplantation. The 5-year probabilities of event-free survival for patients aged ≤6 years, 7 to 15 years, and 16 to 25 years were 86% (83% to 89%), 80% (76% to 84%), and 63% (48% to 78%), respectively. (B) Event-free survival by donor type. The 5-year probabilities of event-free survival after HLA-matched relative, HLA-mismatched relative, HLA-matched unrelated, and HLA-mismatched unrelated donor transplants were 86% (83% to 88%), 70% (61% to 79%), 82% (75% to 89%), and 78% (70% to 86%), respectively.
Table 3.
Five-year probabilities of event-free and overall survival by age and donor type
| Donor type | Event-free survival (95% CI), % | Overall survival (95% CI), % |
|---|---|---|
| Age, ≤6 y | ||
| HLA-matched relative | 88 (84-91) | 92 (89-95) |
| HLA-mismatched relative | 73 (59-86) | 75 (61-87) |
| HLA-matched unrelated | 89 (83-93) | 93 (88-96) |
| HLA-mismatched unrelated | 83 (73-91) | 89 (81-96) |
| Age, 7-15 y | ||
| HLA-matched relative | 80 (75-85) | 84 (79-88) |
| HLA-mismatched relative | 56 (39-73) | 62 (44-78) |
| HLA-matched unrelated | 89 (82-95) | 91 (83-97) |
| HLA-mismatched unrelated | 71 (54-85) | 79 (63-91) |
Patients aged 16-25 years: N = 24 patients who received HLA-matched related donor transplantation with event-free survival of 58% (14 of 24) and overall survival of 63% (15 of 24). N = 4 patients received HLA-mismatched related donor transplantation and only 2 patients are alive. N = 4 patients received HLA-matched unrelated donor transplantation and all patients are alive. N = 1 patient received HLA-mismatched unrelated donor transplantation and is death.
Overall survival
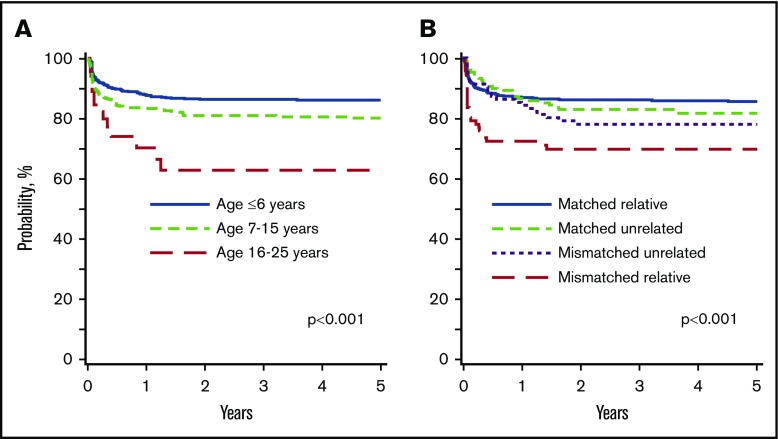
Age at transplantation was associated with overall survival. Compared with patients aged ≤6 years, survival was lower for patients aged 7 to 15 years and 16 to 25 years at transplantation (Table 2). Among patients older than 6 years at transplantation, survival was lower in patients aged 16 to 25 years compared with 7 to 15 years (HR, 2.38; 95% CI, 1.27-4.35; P = .006). Donor type was also associated with survival. Survival was lower after HLA-mismatched related donor compared with HLA-matched related donor (Table 2) and HLA-matched unrelated donor (HR, 2.38; 95% CI, 1.70-4.35; P = .006). Survival did not differ between HLA-matched unrelated and HLA-matched related (Table 2) and HLA-mismatched unrelated donor transplants (HR, 1.37; 95% CI, 0.67-2.78; P = .38). The 5-year probabilities of overall survival by age and donor type are shown in Figure 2 and Table 3. Overall survival was also associated with conditioning regimen. Compared with the Bu/Cy/TT/FLU regimen, survival was lower after other regimens except Bu/Cy/FLU (Table 2). Similarly, compared with the Bu/Cy/FLU regimen, survival was lower after Bu/Cy (HR, 2.70; 95% CI, 1.62-4.76; P = .0002) and treosulfan/TT/FLU (HR, 1.62; 95% CI, 1.02-2.58; P = .04) regimens but not Bu or melphalan plus or minus TT plus or minus FLU. The effect of conditioning regimen was independent of donor type. The 5-year probabilities of overall survival for patients aged ≤6 years, 7 to 15 years, and 16 to 25 years, adjusted for donor type and conditioning regimen were 90% (95% CI, 88-93), 84% (95% CI, 80-87), and 63% (95% CI, 45-82), respectively (P < .001). There were a total of 142 deaths. Infection (34%), GVHD (24%), and graft failure (13%) were the main causes of death. Other causes of death included veno-occlusive disease (6%), interstitial pneumonitis (3%), organ failure (11%), hemorrhage (7%), secondary malignancy (1%), and other causes (1%).
Figure 2.
Overall survival. (A) Overall survival by age at transplantation The 5-year probabilities of overall survival for patients aged ≤6 years, 7 to 15 years, and 16 to 25 years were 90% (88% to 93%), 84% (80% to 87%), and 63% (45% to 82%), respectively. (B) Overall survival by donor type. The 5-year probabilities of overall survival after HLA-matched relative, HLA-mismatched relative, HLA-matched unrelated, and HLA-mismatched unrelated donor transplants were 89% (87% to 91%), 73% (64% to 82%), 87% (81% to 93%), and 83% (74% to 91%), respectively.
Graft failure
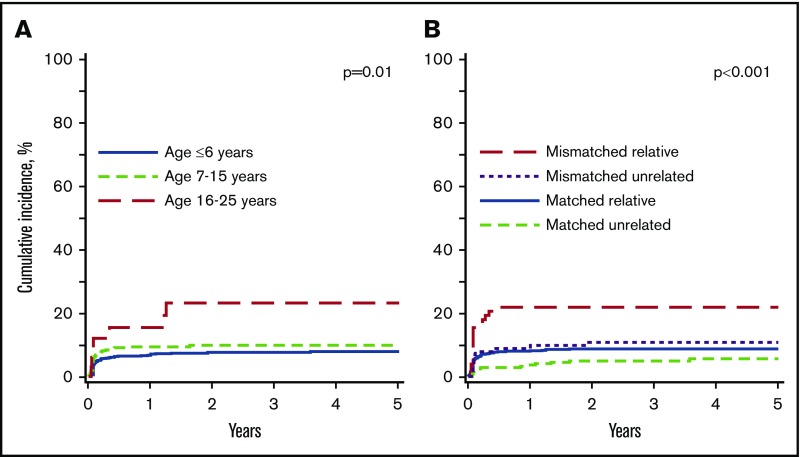
Ninety-nine patients reported graft failure; 56 were primary graft failure and 43 were secondary graft failure. The median time to secondary graft failure was 83 days (interquartile range, 42-220 days). Older age at transplantation and HLA-mismatched donor transplantations were associated with higher risk for graft failure. Patients older than 15 years at transplantation were at higher risk for graft failure compared with those ≤6 years (Table 4) and those aged 7 to 15 years (HR, 2.33; 95% CI, 1.04-5.26; P = .04). Graft failure was higher after HLA-mismatched relative compared with HLA-matched relative transplantation (Table 4). Graft failure was also higher after HLA-mismatched relative compared with HLA-matched unrelated donor (HR, 4.69; 95% CI, 2.27-9.67; P < .0001) and HLA-mismatched unrelated donor (HR, 2.17; 95% CI, 1.01-4.66; P = .05) transplantation. Similarly, graft failure was higher after HLA-mismatched unrelated compared with HLA-matched unrelated donor transplantation (HR, 2.17; 95% CI, 0.96-4.76; P = .06). Graft failure was lower with in vivo T-cell depletion (HR, 0.46; 95% CI, 0.30-0.70; P = .003). However, in vivo T-cell depletion was strongly correlated with age in that 80% of patients aged <7 years and 67% of patients aged 7 to 15 years received in vivo T-cell depletion compared with 58% of patients aged 16 to 25 years. The 5-year probability of graft failure by age at transplantation and donor type is shown in Figure 3.
Table 4.
Risks factors for graft failure, grade II-IV acute and chronic GVHD
| Outcomes | Events/evaluable | HR (95% CI) | P |
|---|---|---|---|
| Graft failure | |||
| Age at transplantation, y | |||
| ≤6 | 49/638 | 1.00 | .02 |
| 7-15 | 43/439 | 1.30 (0.86-1.96) | .21 |
| 16-25 | 7/33 | 3.03 (1.36-6.73) | .006 |
| Donor type | |||
| HLA-matched relative | 58/677 | 1.00 | <.001 |
| HLA-mismatched relative | 17/78 | 2.84 (1.65-4.88) | <.001 |
| HLA-matched unrelated | 13/252 | 0.61 (0.33-1.11) | .10 |
| HLA-mismatched unrelated | 11/103 | 1.31 (0.68-2.50) | .42 |
| Acute GVHD | |||
| Age at transplantation, y | |||
| ≤6 | 93/634 | 1.00 | .002 |
| 7-15 | 78/436 | 1.10 (0.71-1.70) | .66 |
| 16-25 | 10/33 | 2.23 (1.30-3.84) | .003 |
| Donor type | |||
| HLA-matched relative | 80/674 | 1.00 | <.001 |
| HLA-mismatched relative | 27/77 | 3.33 (2.15-5.17) | <.001 |
| HLA-matched unrelated | 54/251 | 2.44 (1.72-3.46) | <.001 |
| HLA-mismatched unrelated | 20/101 | 1.94 (1.19-3.16) | .008 |
| Transplant period | |||
| 2012-2016 | 85/653 | 1.00 | <.001 |
| 2006-2011 | 68/338 | 1.70 (1.23-2.35) | .001 |
| 2000-2005 | 28/112 | 2.50 (1.61-4.00) | <.001 |
| Chronic GVHD | |||
| Donor type | |||
| HLA-matched relative | 52/627 | 1.00 | <.001 |
| HLA-mismatched relative | 14/70 | 2.64 (1.42-4.89) | .002 |
| HLA-matched unrelated | 21/249 | 2.13 (1.13-4.00) | .02 |
| HLA-mismatched unrelated | 24/101 | 4.25 (2.43-7.44) | <.001 |
| Conditioning regimen | |||
| Bu/Cy/TT/FLU | 17/372 | 1.00 | <.001 |
| Bu/Cy/FLU | 24/255 | 2.03 (1.09-3.78) | .03 |
| Bu/Cy | 41/223 | 6.78 (3.59-12.81) | <.001 |
| Treosulfan/TT/FLU | 20/140 | 7.25 (3.41-15.41) | <.001 |
| Bu or melphalan ± TT ± FLU | 9/57 | 1.94 (0.82-4.60) | .13 |
| Recipient sex | |||
| Male | 82/659 | 1.00 | |
| Female | 29/388 | 0.59 (0.38-0.91) | .02 |
Figure 3.
Graft failure. (A) Graft failure by age at transplantation. The 5-year probabilities of graft failure for patients aged ≤6 years, 7 to 15 years, and 16 to 25 years were 8% (6% to 10%), 10% (7% to 12%), and 22% (7% to 34%), respectively. (B) Graft failure by donor type. The 5-year probabilities of graft failure after HLA-matched relative, HLA-mismatched relative, HLA-matched unrelated, and HLA-mismatched unrelated donor transplants were 9% (6% to 11%), 21% (12% to 29%), 6% (3% to 9%), and 11% (5% to 18%), respectively.
GVHD
Grade II-IV acute GVHD was higher in older patients, after transplantation of grafts from donors other than HLA-matched related donors and transplantations prior to 2012 (Table 4). Grade II-IV acute GVHD risks were not different between HLA-mismatched related, HLA-matched, and HLA-mismatched unrelated donor transplants (data not shown). In vivo T-cell depletion was not associated with grade II-IV acute GVHD (HR, 0.73; 95% CI, 0.43-1.26; P = .26). Age and donor type were also associated with grade III-IV acute GVHD. Grade III-IV acute GVHD was higher in patients aged 16 to 25 years compared with those aged <7 years (HR, 3.41; 95% CI, 1.40-8.28; P = .007) and those aged 7 to 15 years (HR, 3.33; 95% CI, 1.32-8.33; P = .01). Compared with HLA-matched related donor, grade III-IV acute GVHD was higher after HLA-mismatched related (HR, 4.40; 95% CI, 2.33-8.31; P < .0001), HLA-matched unrelated (HR, 2.43; 95% CI, 1.45-4.08; P = .0007), and HLA-mismatched unrelated donor (HR, 2.70; 95% CI, 1.38-5.28; P = .004) transplants. In vivo T-cell depletion was not associated with grade III-IV acute GVHD (HR, 0.70; 95% CI, 0.63-2.18; P = .62).
Chronic GVHD was also higher after transplantation of grafts from donors other than HLA-matched related donors (Table 4). Although the risk of chronic GVHD did not differ between HLA-mismatched related and HLA-matched unrelated donor transplants (HR, 1.24; 95% CI, 0.55-2.78; P = .61), risks were higher after HLA-mismatched unrelated compared with HLA-matched unrelated donor transplants (HR, 2.00; 95% CI, 1.04-3.85; P = .04). As recorded with acute GVHD, chronic GVHD risks were higher with Bu/Cy/FLU, Bu/Cy, and treosulfan/TT/FLU compared with the Bu/Cy/TT/FLU regimen (Table 3). Compared with Bu/Cy/FLU, risks were also higher with Bu/Cy (HR, 3.33; 95% CI, 1.82-6.25; P = .0001) and treosulfan/TT/FLU (HR, 3.57; 95% CI, 1.69-7.69; P = .0008) regimens. In vivo T-cell depletion was not associated with chronic GVHD (HR, 1.17; 95% CI, 0.63-2.18; P = .62).
Subset analysis
In the subset of patients for whom we could assign the Pesaro risk class (526 of 1110; 47%), treatment failure and overall mortality were higher for risk class II and III after adjusting for age at transplant, donor type, and conditioning regimen. Compared with risk class I, event-free survival was lower for risk class II (HR, 4.86; 95% CI, 1.86-12.72; P = .001) and for risk class III (HR, 4.76; 95% CI, 1.92-11.76; P < .0001). The corresponding hazards for overall survival were HR, 3.42; 95% CI, 1.03 to 11.29; P = .04 and HR, 5.41; 95% CI, 1.97 to 14.91; P = .001.
Discussion
We report on recent transplantations for β-thalassemia major outside of Europe, Iran, and Saudi Arabia.3 The current report extends our knowledge of transplantation for β-thalassemia major by including transplantations between 2012 and 2016, a period not previously reported by others. This allowed us to study outcomes after HLA-mismatched related donor and unrelated donor transplantation relative to outcomes after matched related donor transplantation, not previously reported. We confirm age at transplantation and donor type predict event-free survival, overall survival, and graft failure. The adverse effect of older age on event-free and overall survival confirm and extend published reports for β-thalassemia major6,9-11,21,22 and sickle cell disease.23 However, unlike most reports that have identified lower survival in patients older than 15 years, we identified 2 clinically relevant ages at transplantation, 6 years and 15 years that predict differences in event-free and overall survival. Graft failure was also substantially higher for patients older than 15 years at transplantation, underscoring the need for early transplantation if this treatment modality is being considered. With 5-year event-free survival of 86% for patients aged ≤6 years, 80% for patients aged 7 to 15 years, and 63% for patients aged 16 to 25 years, the optimal age for transplantation is ≤6 years. Most patients in the current analyses were inadequately chelated and consequently more likely to have hepatomegaly and portal fibrosis with increasing age. Inadequate chelation, hepatomegaly, and portal fibrosis are risk factors associated with allogeneic transplantation.24 Ours is the first report to demonstrate comparable event-free and overall survival after HLA-matched related and HLA-matched unrelated donor transplantation. Thus, our findings support initiating simultaneous search for HLA-matched related and unrelated donors early in the course of the disease, and transplantation being offered with an HLA-matched unrelated donor if an HLA-matched sibling is not available. Delaying transplantation beyond 15 years results in a 20% to 25% absolute decrement in event-free and overall survival. Although event-free and overall survival were lower after mismatched related and unrelated donor transplantation, our data support 1 HLA locus mismatched unrelated donor instead of an HLA-mismatched relative. Graft failure was highest after transplantation of grafts from an HLA-mismatched relative, and an absolute decrement of 10% to 15% in event-free survival in patients aged ≤6 years and 7 to 15 years after transplantation of grafts from HLA-mismatched relative compared with HLA-mismatched unrelated donor cannot be ignored.
The 3 predominant conditioning regimens in the current analyses were Bu/Cy/TT/FLU, Bu/Cy/ FLU, and Bu/Cy accounting for 80% of transplantations. Although we recorded higher overall and event-free survival with Bu/Cy/TT/FLU and Bu/Cy/FLU regimens compared with the Bu/Cy regimen, the choice of conditioning regimen was directed by transplant center practice and/or physician choice. The excellent survival reported with Bu/Cy/TT/FLU is consistent with that reported from a single center.4 However, the majority of patients who received the Bu/Cy/TT/FLU in the current analyses were also transplanted at that center and may in part be attributed to center practice. In contrast, the Bu/Cy/FLU and Bu/Cy regimens were used in many more transplant centers and were more representative of clinical practice. The recorded higher overall and event-free survival after Bu/Cy/FLU compared with Bu/Cy cannot be attributed to age at transplantation or donor type. Approximately 60% of patients who received Bu/Cy/FLU and Bu/Cy were aged ≤6 years and 85% of patients who received the Bu/Cy regimen received grafts from an HLA-matched sibling compared with 50% of patients who received the Bu/Cy/FLU regimen. We hypothesize that the additional immunosuppression with the addition of FLU to Bu/Cy resulted in sustained donor engraftment and lower acute and chronic GVHD that resulted in higher overall and event-free survival. Others have reported excellent survival with the treosulfan/TT/FLU regimen5 and a single-center report recorded a survival advantage with treosulfan/TT/FLU compared with the Bu/Cy regimen in older patients.6 Our observations differ in that overall and event-free survival were substantially lower with the treosulfan/TT/FLU regimen compared with the Bu/Cy/FLU regimen. However, in our population, the treosulfan/TT/FLU regimen was used almost exclusively for older patients and those with Pesaro class III, making it a challenge to study this regimen in the context of the current analysis.
Although the data support an HLA-matched sibling as the ideal donor, availability is limited and strategies to overcome higher acute and chronic GVHD with alternative donors are highly desirable. Higher acute GVHD after mismatched related and unrelated donor transplants compared with matched sibling transplants is consistent with reports on the effect of donor-recipient HLA match on acute GVHD risks.25 Similarly, higher chronic GVHD after unrelated donor transplantation is also consistent with that reported by others.26 Grade II-IV and III-IV acute GVHD were higher in older patients consistent with published reports.27 With most patients in the current analyses aged <16 years, we were unable to record an effect of age on chronic GVHD.28
To our knowledge, this is the first study to analyze a large number of transplants for β-thalassemia that compared HLA-matched sibling and alternative donor transplants. Yet, there are limitations. We studied transplant strategies including timing, donor selection, and conditioning regimen retrospectively. Consequently, there are several unknown or unmeasured factors that may have influenced the findings reported herein. We were unable to study an effect of graft type in the current analyses. Graft correlated with transplant-conditioning regimen and the regimen (Bu/Cy/TT/FLU) most likely to use peripheral blood was not associated with lower event-free or overall survival. There are also limitations to conducting large-scale prospective clinical trials primarily attributed to costs and lengthy accrual periods.
A chronic transfusion program coupled with adherence to chelation and advances in supportive care has ensured that patients with thalassemia major are living longer with good quality of life.24,29,30 The decision to offer transplantation is determined by the treating physician in consultation with the patient and his or her family. If transplantation is determined as the treatment of choice for a given patient, with 5-year event-free survival approaching 90% after HLA-matched sibling and HLA-matched unrelated donor, the data support transplantation with either donor type in patients aged ≤6 years. Transplantation for older patients particularly those older than 15 years and with mismatched related or mismatched unrelated donors is less desirable and requires a careful evaluation of survival with best available treatment vs transplantation. Collectively, our data do not support the Bu/Cy regimen and favor the Bu/Cy/FLU regimen. However, we acknowledge that identifying an optimal conditioning regimen is best studied in the setting of carefully controlled prospective trials.
Acknowledgments
The Center for International Blood and Marrow Transplant Research was supported by U24-CA76518 from the National Institutes of Health, National Cancer Institute, National Heart, Lung, and Blood Institute, and National Institute of Allergy and Infectious Diseases, and HHSH 250201200016C from the Health Services Research Administration, Department of Health and Human Services.
The content is solely the responsibility of the authors and does not represent the official policy of the National Institutes of Health or the Health Resources and Services Administration or any other agency of the US government.
Authorship
Contribution: Chunfu Li, V.M., S.K., K.H., D.A.K., J.J.B., C.C.D., M.C.W., and M.E. designed the study; K.H., D.A.K., and S.K. conducted statistical analysis; and Chunfu Li, V.M., S.K., B.G., K.H., H.J., Changgang Li, Y.Z., D.A.K., J.J.B., C.C.D., R.A., J.J.A., R.K.G., R.H., K.K., S.S., A.R.S., M.C.W., and M.E. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary Eapen, Center for International Blood and Marrow Transplant Research, Department of Medicine, Medical College of Wisconsin, 9200 W Wisconsin Ave, Suite C5500, Milwaukee, WI 53226; e-mail: meapen@mcw.edu.
References
- 1.Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353(11):1135-1146. [DOI] [PubMed] [Google Scholar]
- 2.Borgna-Pignatti C, Rugolotto S, De Stefano P, et al. . Survival and complications in patients with thalassemia major treated with transfusion and deferoxamine. Haematologica. 2004;89(10):1187-1193. [PubMed] [Google Scholar]
- 3.Baronciani D, Angelucci E, Potschger U, et al. . Hemopoietic stem cell transplantation in thalassemia: a report from the European Society for Blood and Bone Marrow Transplantation Hemoglobinopathy Registry, 2000-2010. Bone Marrow Transplant. 2016;51(4):536-541. [DOI] [PubMed] [Google Scholar]
- 4.Li C, Wu X, Feng X, et al. . A novel conditioning regimen improves outcomes in β-thalassemia major patients using unrelated donor peripheral blood stem cell transplantation. Blood. 2012;120(19):3875-3881. [DOI] [PubMed] [Google Scholar]
- 5.Bernardo ME, Piras E, Vacca A, et al. . Allogeneic hematopoietic stem cell transplantation in thalassemia major: results of a reduced-toxicity conditioning regimen based on the use of treosulfan. Blood. 2012;120(2):473-476. [DOI] [PubMed] [Google Scholar]
- 6.Mathews V, George B, Viswabandya A, et al. . Improved clinical outcomes of high risk β thalassemia major patients undergoing a HLA matched related allogeneic stem cell transplant with a treosulfan based conditioning regimen and peripheral blood stem cell grafts. PLoS One. 2013;8(4):e61637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaziev J, Marziali M, Isgrò A, et al. . Bone marrow transplantation for thalassemia from alternative related donors: improved outcomes with a new approach. Blood. 2013;122(15):2751-2756. [DOI] [PubMed] [Google Scholar]
- 8.Gaziev J, Isgrò A, Sodani P, et al. . Optimal outcomes in young class 3 patients with thalassemia undergoing HLA-identical sibling bone marrow transplantation. Transplantation. 2016;100(4):925-932. [DOI] [PubMed] [Google Scholar]
- 9.Lucarelli G, Galimberti M, Polchi P, et al. . Bone marrow transplantation in patients with thalassemia. N Engl J Med. 1990;322(7):417-421. [DOI] [PubMed] [Google Scholar]
- 10.Sabloff M, Chandy M, Wang Z, et al. . HLA-matched sibling bone marrow transplantation for β-thalassemia major. Blood. 2011;117(5):1745-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathews V, George B, Deotare U, et al. . A new stratification strategy that identifies a subset of class III patients with an adverse prognosis among children with β thalassemia major undergoing a matched related allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(8):889-894. [DOI] [PubMed] [Google Scholar]
- 12.Bacigalupo A, Ballen K, Rizzo D, et al. . Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsson R, Remberger M, Schaffer M, et al. . Graft failure in the modern era of allogeneic hematopoietic SCT [published correction appears in Bone Marrow Transplant. 2013;48(4):616]. Bone Marrow Transplant. 2013;48(4):537-543. [DOI] [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, et al. . 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 15.Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM; Committee of the International Bone Marrow Transplant Registry . Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft versus host disease. Bone Marrow Transplant. 1989;4(3):247-254. [PubMed] [Google Scholar]
- 16.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901-910. [DOI] [PubMed] [Google Scholar]
- 17.Cox DR. Regression model and life-tables. J R Stat Soc B. 1972;34(2):187-200. [Google Scholar]
- 18.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88(2):95-101. [DOI] [PubMed] [Google Scholar]
- 19.Fine JR, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 20.Andersen PK, Klein JP, Zhang MJ. Testing for centre effects in multi-centre survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18(12):1489-1500. [DOI] [PubMed] [Google Scholar]
- 21.Horan J, Wang T, Haagenson M, et al. . Evaluation of HLA matching in unrelated hematopoietic stem cell transplantation for nonmalignant disorders. Blood. 2012;120(14):2918-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eapen M, Wang T, Veys PA, et al. . Allele-level HLA matching for umbilical cord blood transplantation for non-malignant diseases in children: a retrospective analysis. Lancet Haematol. 2017;4(7):e325-e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gluckman E, Cappelli B, Bernaudin F, et al. ; Eurocord, the Pediatric Working Party of the European Society for Blood and Marrow Transplantation, and the Center for International Blood and Marrow Transplant Research . Sickle cell disease: an international survey of results of HLA-identical sibling hematopoietic stem cell transplantation. Blood. 2017;129(11):1548-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rachmilewitz EA, Giardina PJ. How I treat thalassemia. Blood. 2011;118(13):3479-3488. [DOI] [PubMed] [Google Scholar]
- 25.Jagasia M, Arora M, Flowers MED, et al. . Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arora M, Klein JP, Weisdorf DJ, et al. . Chronic GVHD risk score: a Center for International Blood and Marrow Transplant Research analysis. Blood. 2011;117(24):6714-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qayed M, Wang T, Hemmer MT, et al. . Influence of age on acute and chronic GVHD in children undergoing HLA-identical sibling bone marrow transplantation for acute leukemia: implications for prophylaxis. Biol Blood Marrow Transplant. 2018;24(3):521-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zecca M, Prete A, Rondelli R, et al. ; AIEOP-BMT Group, Italian Association for Pediatric Hematology and Oncology-Bone Marrow Transplant . Chronic graft-versus-host disease in children: incidence, risk factors, and impact on outcome. Blood. 2002;100(4):1192-1200. [DOI] [PubMed] [Google Scholar]
- 29.Angelucci E, Matthes-Martin S, Baronciani D, et al. ; EBMT Inborn Error and EBMT Paediatric Working Parties . Hematopoietic stem cell transplantation in thalassemia major and sickle cell disease: indications and management recommendations from an international expert panel. Haematologica. 2014;99(5):811-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caocci G, Orofino MG, Vacca A, et al. . Long-term survival of beta thalassemia major patients treated with hematopoietic stem cell transplantation compared with survival with conventional treatment. Am J Hematol. 2017;92(12):1303-1310. [DOI] [PubMed] [Google Scholar]