Key Points
IA procedure decrease anti-AAV5 NABs to levels compatible with a successful AAV5 vector readministration.
Patients positive for anti-AAV antibodies could benefit from AAV-based gene therapy after IA treatment.
Abstract
Adeno-associated virus (AAV)–based liver gene therapy has been shown to be clinically successful. However, the presence of circulating neutralizing antibodies (NABs) against AAV vector capsids remains a major challenge as it may prevent successful transduction of the target cells. Therefore, there is a need to develop strategies that would enable AAV-mediated gene delivery to patients with preexisting anti-AAV NABs. In the current study, the feasibility of using an immunoadsorption (IA) procedure for repeated, liver-targeted gene delivery in nonhuman primates was explored. The animals were administered IV with recombinant AAV5 (rAAV5) carrying the reporter gene human secreted embryonic alkaline phosphatase (hSEAP). Seven weeks after the first rAAV treatment, all of the animals were readministered with rAAV5 carrying the therapeutic hemophilia B gene human factor IX (hFIX). Half of the animals administered with rAAV5-hSEAP underwent IA prior to the second rAAV5 exposure. The transduction efficacies of rAAV5-hSEAP and rAAV5-hFIX were assessed by measuring the levels of hSEAP and hFIX proteins. Although no hFIX was detected after rAAV5-hFIX readministration without prior IA, all animals submitted to IA showed therapeutic levels of hFIX expression, and a threshold of anti-AAV5 NAB levels compatible with successful readministration was demonstrated. In summary, our data demonstrate that the use of a clinically applicable IA procedure enables successful readministration of an rAAV5-based gene transfer in a clinically relevant animal model. Finally, the analysis of anti-AAV NAB levels in human subjects submitted to IA confirmed the safety and efficacy of the procedure to reduce anti-AAV NABs. Furthermore, clinical translation was assessed using an immunoglobulin G assay as surrogate.
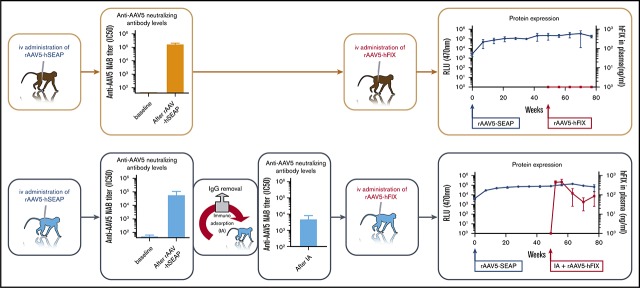
Visual Abstract
Introduction
Adeno-associated virus (AAV) vectors have been successfully used for the treatment of genetic disorders in preclinical studies as well as in clinical trials. Those clinical studies included hundreds of patients and demonstrated efficacy and safety of AAV vectors for gene therapy in humans with long-term therapeutic benefit.1-3 Particularly, recent data confirm the effectiveness and safety of gene therapy in hemophilia B (Hem B).4,5 However, it has been reported that even low levels of anti-AAV neutralizing antibodies (NABs; 1:5 to 1:10) can have an impact on transduction of high doses of AAV vectors.4,6-12 NABs can be the result of naturally acquired AAV infections and AAV-based gene therapies usually induce high serotype-specific NAB titers, precluding effective readministration. Patients, including children treated with AAV-based therapy, may experience a gradual decrease of therapeutic protein expression due to the natural turnover of transduced cells or because of insufficient initial transduction efficacy and may require a second treatment. Hence, there is a need to develop strategies that enable AAV-mediated gene delivery to patients with anti-AAV NABs.
Various approaches have been pursued to overcome the inhibitory effect of NABs such as dose fitting, alternating AAV serotypes, AAV-vector delivery directly to hepatic tissue, capsid engineering of the vector, or use of capsid decoys.2,13-18 However, those strategies have limitations because they show limited efficacy in the presence of high NABs titers, require the development of another product, or have safety issues.19,20
Plasmapheresis and immunoadsorption (IA) are extracorporeal blood-purification techniques that have been developed to remove large-molecular-weight substances from the plasma. Importantly, these techniques are safe medical procedures performed worldwide both in adults and children.21 The plasmapheresis procedure is a nonselective removal of all plasma components and can remove ∼50% to 75% of antibodies and other plasmatic factors.22 In contrast, IA results in the selective removal of immunoglobulins from separated plasma through high-affinity adsorbers and allows near complete clearance of circulating immunoglobulins of all types and subtypes. IA has high therapeutic efficacy even in diseases for which plasma exchange is not effective,23-28 and is currently clinically used to treat multiple antibody-mediated or immunological medical conditions.29-31
Different research groups have reported that extensive plasmapheresis may reduce NABs to undetectable levels only when initial anti-AAV NAB titers are low.32,33 However, this approach cannot be used when anti-AAV NABs titers are high, as is common following AAV-based gene therapy.
In the current study, the feasibility of using an IA procedure for repeated, liver-targeted AAV-based gene delivery in nonhuman primates (NHPs) was explored using recombinant AAV5 (rAAV)–based vectors of the same serotype. Furthermore, the safety and efficacy of the procedure to decrease anti-AAV NABs was investigated in humans.
Materials and methods
Animal welfare
Animal studies were approved by the ethics committee for animal testing of the University of Navarra and by the Department of Health of the government of Navarra (Comité de Ética para la Experimentación Animal [CEEA] 038/15) and performed according to the guidelines from the institutional ethics commission.
Experimental design
Six female Macaca fascicularis NHPs (1-6) negative for anti-AAV5 NABs were administered by IV injection with rAAV5-hAAT-human secreted embryonic alkaline phosphatase (hSEAP; 1 × 1013 genome copies [gc]/kg). Seven weeks after this administration, 3 animals (NHPs 4-6) randomly selected were submitted to IA and readministered (IV) with rAAV5-LP1-hFIX (3 × 1013 gc/kg) 15 minutes after completion of the procedure. Three animals (NHPs 1-3) were readministered with rAAV5-LP1-hFIX (3 × 1013gc/kg) without prior procedure. Four weeks after the rAAV5-LP1-hFIX readministration, the animals were euthanized, and blood and tissues were harvested for further analyses. Eight additional NHPs negative for anti-AAV5 NABs (female, NHPs 7-14), were administered with rAAV5-hSEAP at a dose of 1 × 1013 gc/kg. Seven weeks after AAV5-hSEAP administration, all animals were submitted to IA before readministration with AAV5-hFIX (3 × 1013 gc/kg). Two control animals (controls 1 and 2) were administered with AAV5-hFIX (3 × 1013 gc/kg) and euthanized 4 weeks after.
IA procedure
Prior to IA, darbepoetin α (100 μg, Aranesp; 0.45 μg/kg, Amgen) in combination with ferric carboxymaltose (Ferinject, 10 mg/kg; Vifor Pharma) was given weekly for 3 weeks to the NHPs. IA was performed under anesthesia using ketamine (Imalgene 1000; Merial), midazolam (B. Braun), propofol (B. Braun), and sevofluorane (AbbVie). The adsorber filter TheraSorb–Ig Flex (Miltenyi Biotec) was used in combination with the IA device Life 18TM (Miltenyi Biotec). A single session of IA involving 3 consecutive cycles was performed. For detailed description of the IA procedure, see supplemental Data, "Detailed immunoadsorption (IA) procedure."
rAAV5 vectors
rAAV5 vectors were generated according to an adapted method.34 rAAV5-hSEAP carry the human SEAP transgene under the control of the liver promoter human α-1-antitrypsin (hAAT).35 rAAV5-hFIX carry the human factor IX transgene under the control of the liver promoter 1 (LP1).36
Analysis of the humoral immune response
Anti-AAV5 NABs
The level of NABs was determined as described previously.14 HEK293T cells (ATCC) were seeded in 96-well plates (Costar) coated with 0.25% poly l-lysine (Sigma-Aldrich) at a density of 0.5 × 105 cells per well in Dulbecco's modified Eagle medium 10% fetal bovine serum/1% penicillin/streptomycin (Gibco). Cells were incubated overnight at 37°C before addition of a mix of rAAV5-CMV-luc preincubated with heat-inactivated plasma (dilution range from 5 × 101 to 4 × 107). As a positive control, cells were incubated with rAAV5-CMV-luc; as a negative control, cells were incubated with medium only. After 20 hours at 37°C, the cells were washed with phosphate-buffered saline and lysed by addition of Glo Lysis Buffer (Promega). The luciferase expression was determined with the ONE-Glo Luciferase Assay (Promega) according to the manufacturer’s protocol. The anti-AAV NAB titer (half maximal inhibitory concentration [IC50]) was calculated with LabKey software. Plasma samples were considered to have neutralizing activity if the lowest plasma dilution inhibited vector transduction by at least 50%.
Total antibodies against AAV5
Levels of circulating antibodies were measured by enzyme-linked immunosorbent assay (ELISA). Ninety-six–well plates coated with rAAV5 capsid were incubated with plasma, and specific antibodies detected with an horseradish peroxidase–conjugated antibody against cynomonkey immunoglobulin G (IgG; Sigma-Aldrich) followed by colorimetric detection with 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma-Aldrich). Correlations between the levels of NAB and total anti-AAV antibody were determined by exponential regression.
ELISPOT assay
The enzyme-linked immunospot (ELISPOT) assay was performed using the human interferon-γ ELISPOT kit (BD) by incubating peripheral blood leukocytes obtained at days 14, 28, 42, 63, and 77 of the experiment with either AAV5 vector or medium as negative control or phorbol 12-myristate 13-acetate (50 ng/mL final) and ionomycin (500 ng/mL final) (Sigma-Aldrich) as positive controls. The analysis was done automatically using the CTL immunoSpot Analyzer and counted with CTL immunoSpot software.
Transgene expression
Secreted embryonic alkaline phosphatase (SEAP) activity/expression was measured using the chemiluminescent SEAP Reporter Gene Assay (Sigma-Aldrich). Analysis was performed according to the manufacturer.
Human factor IX (hFIX) expression was measured by a sandwich ELISA. Diluted plasma samples were added to a plate coated with a hFIX-specific monoclonal antibody (Hematologic Technologies Inc). Detection was performed using a peroxidase anti-FIX- mouse monoclonal antibody (Cedarlane Laboratories) followed by colorimetric detection using TMB (Sigma-Aldrich).
Quantification of AAV-vector DNA in the liver
Liver total DNA was prepared using the QIAamp DNA mini kit (Qiagen). Vector DNA levels were quantified by quantitative polymerase chain reaction (qPCR) with primers for hSEAP (forward, 5′-CCTGTTTGCTCCTCCGAT-3′; reverse, 5′-GGGTTCTCCTCCTCAACT-3) and hFIX (forward, 5′-CAAGTATGGCATCTACACCAAAGTCT-3′; reverse, 5′-GCAATAGCATCACAAATTTCACAAA-3′). Glyceraldehyde-3-phosphate dehydrogenase was used as reference (forward, 5′-GGTCGGAGTCAAACGGATTT-3′; reverse, 5′-CCAGCATCGCCCACTTGA-3′). Amplification was performed using iQ SYBR green supermix in an iQ5 real-time PCR detection system (Bio-Rad).
Statistics
Statistical significances were determined using GraphPad Prism 8 software and are described in the figure legends.
Results
IA procedure enables a significant reduction of anti-AAV5 NAB levels
NHPs (n = 6) testing negative for the presence of anti-AAV5 NABs were administered IV with rAAV5-hSEAP at a dose of 1 × 1013 gc/kg. Seven weeks after the first AAV administration, all animals were readministered with rAAV5-hFIX (dose of 3 × 1013 gc/kg). Fifteen minutes prior to readministration, 3 of the 6 animals were submitted to a single session of IA involving 3 consecutive cycles. Blood samples were collected for further analysis prior to the IA procedure and after every cycle (3) of the procedure.
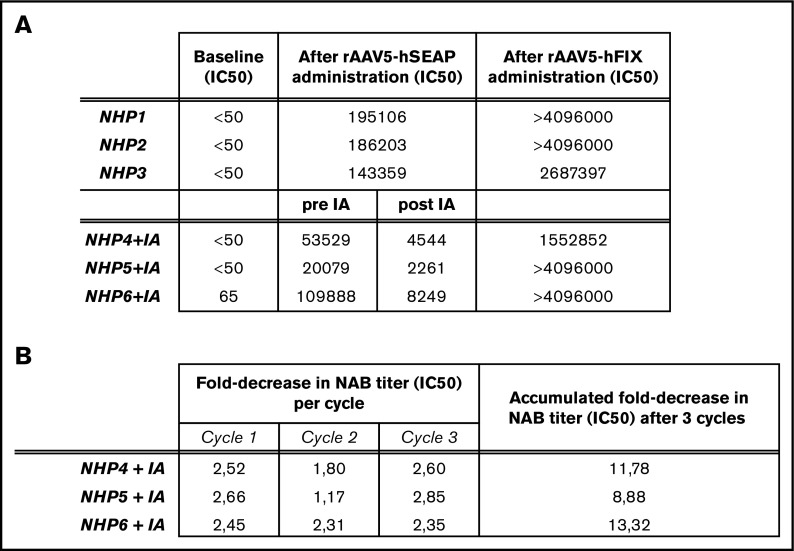
After administration of rAAV5-hSEAP, circulating anti-AAV5 NABs developed in all 6 NHPs (NHP 1-6; Figure 1A). Although the plasma levels of rAAV5-hSEAP vector genome copies of DNA measured 2 days after AAV vector administration were consistent between animals, indicating that all animals have been exposed to a similar dose of circulating vector capsids (supplemental Table 1), the levels of anti-AAV5 NABs following administration with rAAV5-hSEAP varied between animals, illustrating the natural variability of the antigenic immune responses in a genetically diverse population.37,38
Figure 1.
Reduction of anti-AAV5 NAB levels after IA procedure. (A). Levels of anti-AAV5 NAB (IC50) levels are reported at baseline (day 0), after rAAV5-hSEAP administration (day 49, NHPs 1-6), after IA (post IA, NHPs 4-6) and after rAAV5-hFIX readministration (day 77, NHPs 1-6). (B). Fold-decrease in anti-AAV5 NAB (IC50) levels measured in plasma samples taken after every cycle of the IA procedure.
After 7 weeks, 3 of the 6 animals (NHP 4-6) were submitted to IA, resulting in a decrease of the anti-AAV NAB titers from an average titer of 61 165 to final titers ranging from 2261 to 8249 (Figure 1A). The mean NAB titer decreased by >1 log following the total procedure with an average 2.3-fold reduction per cycle (Figure 1B). As expected, after the readministration (rAAV5-hFIX), all animals developed a secondary response against the rAAV5 vector, with an increase in the NAB titers (Figure 1A).
Successful readministration of rAAV5-vector after IA procedure
The impact of the IA procedure on the efficacy of liver transduction was determined by assessing transgene expression. Plasma samples were collected from animals throughout the experiment, and hSEAP and hFIX gene expression was analyzed at the circulating protein level.
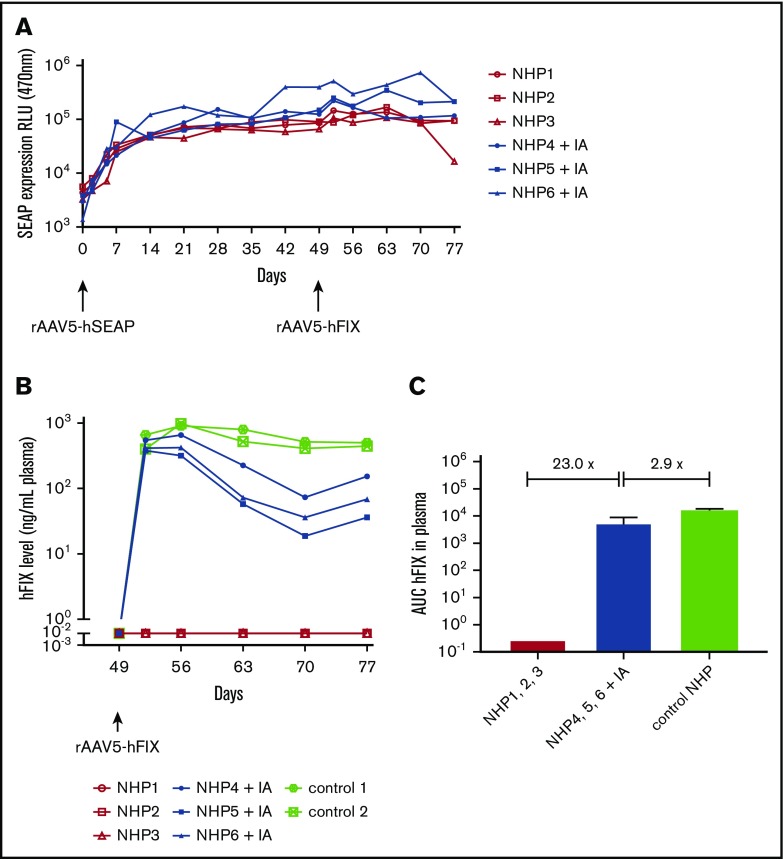
After administration with rAAV5-hSEAP, stable and comparable hSEAP activity was detected in the plasma of all animals (NHP 1-6) until euthanization, 77 days after the initiation of the study (Figure 2A). As expected, animals that were treated with rAAV5-hFIX without prior IA (NHP 1-3) did not have detectable expression of hFIX in the plasma (Figure 2B). rAAV5-hFIX administration had no effect on hSEAP activity levels resulting from the primary injection with rAAV5-hSEAP (Figure 2A).
Figure 2.
Dual hSEAP and hFIX transgene protein expression only in NHPs following IA. NHPs received a primary administration of rAAV5-hSEAP (day 0) and a secondary readministration of rAAV5-hFIX (day 49) with or without prior IA treatment (IA). (A) hSEAP protein expression was stable throughout the experiment and not impacted by the IA procedure. (B) hFIX protein was expressed successfully after IA. As a reference for hFIX levels, 2 NHPs that were administered with 3 × 1013 gc/kg rAAV5-hFIX were included. (C) The averages of the areas under the curves (AUC) ± standard deviation calculated for the hFIX expression over time showed an hFIX increase for the group submitted to IA (NHPs 4-6) when compared with the group without IA (NHPs 1-3). Furthermore, a decrease of 2.9 times was observed for the NHPs submitted to IA (NHPs 4-6) compared with the control animals (NHPs controls 1 and 2). RLU, relative light unit.
Readministration of NHPs 4 to 6 with rAAV5-hFIX after IA at day 49 resulted in plasma hFIX protein expression in a range of 115 to 783 ng/mL (2.3% to 15.66% of 5 μg/mL) consistent with therapeutic efficacy (average level of 401 ng/mL at the peak of expression, day 56). When considering the area under the curves, a 23-fold increase of hFIX was measured when compared with NHPs 1 to 3 readministered with rAAV5-hFIX without IA. The hFIX levels achieved, however, were 2.4 times lower than observed in NHPs after primary injection with rAAV5-hFIX at the same dose of 3 × 1013 gc/kg (average level of 984 ng/mL at the peak of expression, day 56) (Figure 2B). The difference in expression was 2.9 times greater when considering the area under the curves (Figure 2C). As has been reported by us and others, the AAV-mediated hFIX kinetic expression pattern in NHP plasma is characterized by a peak of expression at 7 days (day 56) after administration followed by a decrease and a subsequent stabilization over time, and the expression of hFIX in the NHPs subjected to IA showed similar kinetics.14,39,40
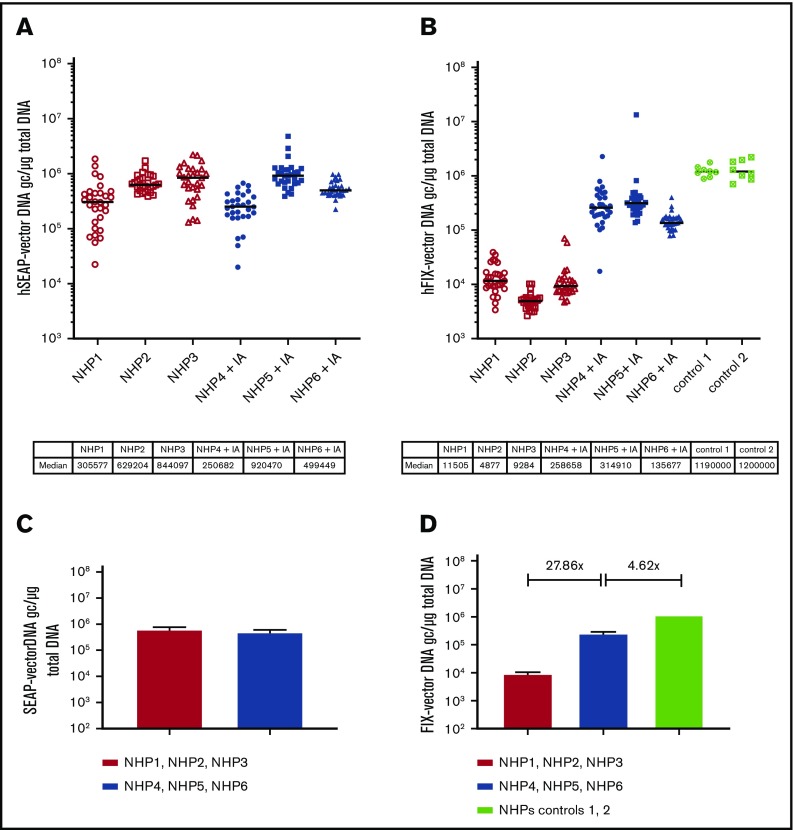
Following sacrifice, 30 tissue samples were taken throughout the liver for each animal and rAAV5-hSEAP and rAAV5-hFIX-vector DNA copy numbers were determined by qPCR. Similar levels of rAAV-hSEAP-vector DNA copies were measured in the liver tissues of all animals (Figure 3A-B) and consistent with the hFIX protein data, substantially higher levels (27.86 times) of hFIX-vector DNA copies were detected in animals that underwent the IA procedure prior to readministration with rAAV5-hFIX compared with the untreated group (Figure 3C-D). As previously observed for the circulating hFIX protein, the levels of hFIX vector DNA copies measured in liver after IA (median of 2.6 × 105 gc/μg total DNA) were lower (4.62 times) than after primary administration with rAAV5-hFIX at the same dose of 3 × 1013 gc/kg (median of 1.2 × 106 gc/μg total DNA) of the control animals.
Figure 3.
Increase of hFIX-vector DNA in liver following IA. Levels of hSEAP- and hFIX-vector DNA were monitored by qPCR in the liver tissue at euthanization. NHPs received a primary administration of rAAV5-hSEAP (day 0) and a second administration of rAAV5-hFIX (day 49) with or without pretreatment with IA (IA). Per animal, 30 different liver samples were analyzed. The hSEAP- (A) and hFIX-vector (B) DNA levels are plotted per animal with indication of the medians. (C-D) Medians and range per group are reported. Although animal groups with or without an IA procedure had similar levels of hSEAP-vector DNA, an increase of 27.86 times in genome copy number of hFIX-vector DNA was measured in the animal group that underwent the IA. The levels of hFIX-vector DNA measured in the control group, primary administered with rAAV5-hFIX at the same dose of 3 × 1013 gc/kg, were 4.62 times higher than the group that underwent IA.
Overall, the results suggest that the presence of residual anti-AAV5 NABs after IA at titers ranging from 2261 to 8249 impact only partially AAV5 liver transduction, allowing therapeutic levels of hFIX expression.
The IA procedure was well tolerated
The IA procedure was well tolerated by all the animals. No changes of body temperature, activity, general health, and skin and mucosa color were observed. Clinical laboratory parameters, including hematological parameters, coagulation tests, urine analysis, renal and liver function tests, and complete blood count remained within the normal range through the experiment. Only mild and transient alterations in serum biochemistry (alanine aminotransferase elevation of 3 to 4 times the basal levels) occurred 3 days after IA and reversed to baseline within a week in all animals (data not shown). Histology of liver tissues showed that neither AAV vector administration nor the IA procedure caused any pathological changes (data not shown).
Lack of T-cell response against AAV5 capsid
To get more insight into the immune responses occurring after AAV delivery, the cellular immune response against the rAAV5 viral capsid proteins was analyzed for NHPs 1 to 6. Peripheral blood mononuclear cells isolated after rAAV5-hSEAP primary administration (days 14 and 28) and readministration with rAAV5-hFIX (days 42, 63, and 77) were screened for T cells against AAV5 capsid antigens, using an ELISPOT assay (supplemental Figure 1). No cellular immune responses were observed at the time points analyzed.
Correlation between anti-AAV5 NAB levels and total anti-AAV5 antibodies
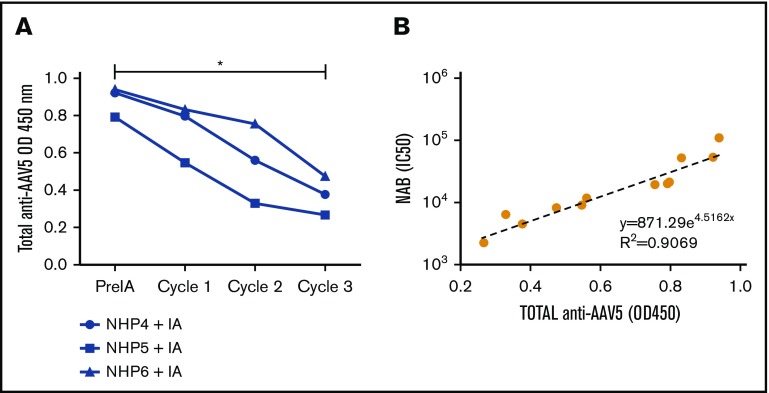
To further assess the impact of the IA on the circulating antibodies, a total anti-AAV5 antibody ELISA was performed on samples collected before, during, and after the procedure. Similar to the results obtained for the anti-AAV5 NABs, the levels of total anti-AAV5 antibody decreased after the procedure (Figure 4A) and a correlation (R2 = 0.9069) was established between the levels of total antibodies and NABs throughout the IA procedure (Figure 4B).
Figure 4.
Correlation between anti-AAV5 NABs and total antibodies. (A) Total anti-AAV5 antibodies were measured by ELISA on plasma samples collected before, during, and after the IA procedure. A significant decrease (*0.0266) was realized between the OD450 before and after the IA procedure using a Friedman test and a Dunn multiple comparison test. (B) A correlation was established by exponential regression between the levels of total and anti-AAV5 NABs just before and throughout the IA procedure (pre IA, cycle 1, 2, 3).
Determination of anti-AAV5 NAB threshold compatible with readministration
To determine the anti-AAV5 NAB threshold compatible with an effective rAAV5 readministration, 8 additional NHPs testing negative for the presence of anti-AAV5 NABs (NHPs 7-14) were administered with rAAV5-hSEAP at a dose of 1 × 1013 gc/kg. Seven weeks following administration, all animals developed titers of anti-AAV5 NABs in a range of 15 720 to 656 040 and were submitted to a single session of IA before readministration with rAAV5-hFIX (3 × 1013 gc/kg).
The decrease of anti-AAV5 NAB titer after the procedure was consistent with our previous observation with a mean 11-fold reduction per IA session, resulting in anti-AAV5 NAB titers ranging from 1772 to 24 713 after completion of the procedure (supplemental Table 2).
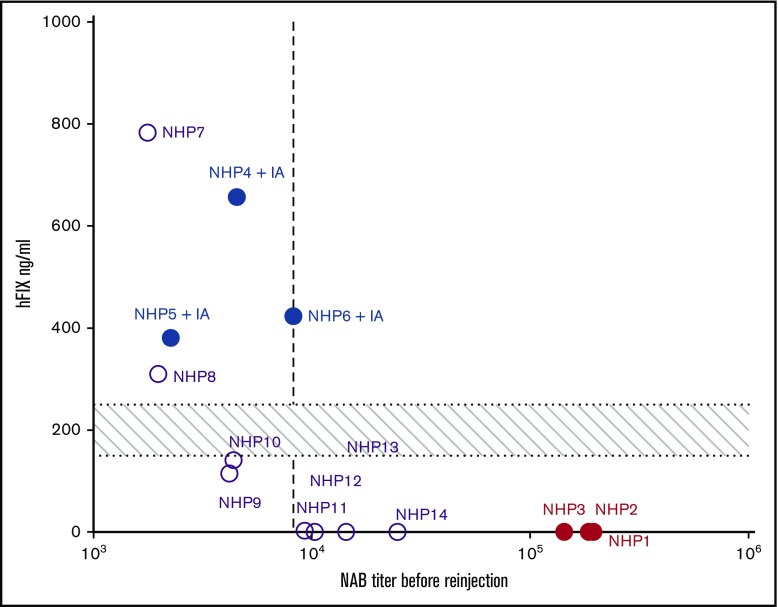
The efficacy of rAAV5-hFIX liver transduction at readministration as a function of preexisting anti-AAV5 NAB was determined by relating posttreatment levels of hFIX in plasma with pretreatment NAB titers. rAAV5-hFIX readministration resulted in expression of therapeutic levels of hFIX in the presence of anti-AAV5 NAB titers up to 8250 (Figure 5 dotted line) and no correlation could be established between the anti-AAV5 NAB titer at the time of readministration and the hFIX level measured at the peak of expression at day 56 (supplemental Table 2). The same observation was made for the levels of anti-AAV5 NABs and the hFIX-vector DNA copies measured at euthanization.
Figure 5.
Anti-AAV5 NAB threshold compatible with readministration. The efficacy of rAAV5-hFIX liver transduction at readministration was determined as a function of anti-AAV5 NAB titers. Post-rAAV5-hFIX readministration levels of hFIX protein at day 56 were plotted against the anti-AAV5 NAB IC50 levels just before the rAAV5-hFIX readministration. The data for 14 NHPs are reported in the graph; NHPs 1 to 6 (closed symbols) and NHPs 7 to 14 (open symbols). The vertical dotted line represents the threshold of efficacy determined at an IC50 of 8250. The horizontal area represents the minimal therapeutic range of 150 to 250 ng/mL corresponding to 3% to 5% of 5 mg/mL.
Overall, these data demonstrate that an anti-AAV5 NAB threshold of 8250 is compatible with efficient rAAV5 vector readministration. Interestingly, at a similar NAB titer at readministration, the hFIX expression varied between animals.
Total IgG levels for clinical application
The clinical use of IA to decrease the levels of pathologic circulating antibodies has been repeatedly reported.27-31 Following IA, antibodies rapidly reappear, and a return to initial levels has been observed 6 to 8 hours following the procedure.41 Hence, clinical use of IA would require a marker for rapid assessment of the efficacy of the procedure to reduce antibody titers. Standard total IgG antibody detection assays are widely available and can be completed within 2 hours vs 3 days for NAB detection assays. To get some insight into the translatability of the readministration procedure to the clinic, the correlation between total IgG antibody levels and anti-AAV NAB levels was determined for NHPs 4 to 6 that were submitted to IA. IgG levels were measured prior, during, and after IA and a similar average decrease in NABs and IgG was observed during the procedure (supplemental Figure 2).
These data suggest that a standard IgG assay could be used as a NAB surrogate to assess the efficacy of the IA procedure prior to AAV readministration in patients.
Proof of concept in human subjects submitted to IA
Plasma samples obtained from 4 subjects treated for autoimmune diseases were obtained before and after a single session of IA involving 2 consecutive cycles and analyzed for the presence of NABs against rAAV2, rAAV5, rAAV9, and total IgG antibodies.
As expected, due to the low seroprevalence of NABs against AAV5 in the human population, none of the subjects were measured positive for anti-AAV5 NABs42 (supplemental Figure 3). However, 2 of 4 subjects showed a clear neutralizing activity against AAV2 and 1 subject against AAV9. After two consecutive cycles of IA a reduction in NABs against AAV2 was observed of threefold for subject 2 and of fivefold for subject 4. Similarly, in subject 4, a reduction of fivefold was observed in NABs against AAV9.
For all 4 subjects, a clear reduction of IgG concentration was observed with an average of 1.8-fold reduction per cycle in the same range than the results obtained in NHPs. The procedure was well tolerated in all cases, suggesting that additional sessions and/or cycles could be applied if needed to get to further reduction of NAB levels.
Discussion
Humoral immune responses against AAV vectors represent a major limitation for AAV therapeutic efficacy and NABs to AAV vectors can prevent or impair transgene delivery to the target organ.43-46 For this reason, patients with preexisting antibodies due to natural infection or AAV treatment are commonly excluded from AAV-based clinical trials.
We now report that an IA procedure significantly reduces the AAV5-neutralizing activity in NHP plasma enabling successful repeated rAAV5-based hepatic gene delivery. A single session of 3 IA cycles resulted in >1 log reduction of circulating NABs titers and in all animals with anti-AAV5 NAB levels below the threshold of a titer of 8250, sustained hSEAP and hFIX protein expression were measured in plasma after sequential administration of rAAV5-hSEAP followed by rAAV5-hFIX. DNA levels for both hSEAP and hFIX vector constructs were detected in the liver of the animals submitted successfully to the IA procedure.
Hem B is a monogenic disorder with a broad therapeutic window and, therefore, an ideal candidate for gene and cell therapy. The normal circulating levels of hFIX are reported to be in the range of 5 μg/mL. In severely affected patients, partial correction can already significantly lower bleed rates and improve quality of life. The hFIX levels needed to correct the Hem B disease phenotype are in the therapeutic range of 150 to 250 ng/mL, 3% to 5% of 5 μg/mL.47 We reached levels of hFIX plasma protein after readministration in a range of 115 to 783 ng/mL (2.3% to 15.66% of 5 μg/mL) compatible with therapeutic benefit in humans. These hFIX levels were achieved in presence of anti-AAV5 NABs remaining after IA at titers ranging from 2261 to 8249. Interestingly, at comparable NAB titers at readministration, some differences in hFIX levels of expression were observed between animals, indicating that (genetic) factors other than NAB determine the efficacy of AAV-mediated liver transduction in primates.
The IA procedure was well tolerated and only mild and transient alterations in serum biochemistry were associated with the procedure. As expected in a genetically diverse population,37,38 the levels of anti-AAV5 NABs achieved after primary administration with 1 × 1013 gc/kg rAAV5-hSEAP varied between animals. In the present study, the IA procedure consisted of a single session of 3 cycles of blood exposure to anti-human immunoglobulin columns (Ig-Therasorb), causing a consistent reduction of NAB titers in all animals the study. Because the efficacy of Ig-Therasorb columns has been shown to be similar in NHPs and humans,48 the average 11-fold reduction of anti-AAV NAB titers after a single session of 3 cycles constitutes a basis to predict the number of cycles and sessions needed to reduce anti-AAV5 NABs below the titer compatible with readministration. Such a tailored IA approach is currently used to treat several diseases including refractory rheumatoid arthritis, atopic dermatitis, cryoglobulinemia, and chronic focal encephalitis.49 The Ig-Therasorb specifically binds immunoglobulins and immune complexes by polyclonal IgG-binding antibodies. The matrix is Sepharose CL 4B coupled to specific antihuman-immunoglobulin sheep antibodies housed in a glass column. These columns remove not only IgG, IgA, IgM, and IgE from plasma, but also immune complexes such as complement components C3 and C4.50 We have previously shown that no impact of anti-AAV5 NABs on liver transduction was observed for NABs titers up to 1031 in NHPs and 340 in humans.51 In the present study, therapeutic levels of hFIX expression were achieved in presence of anti-AAV5 NABs remaining after IA at titers ranging from 2261 to 8249. However, the impact of individual complement factors as well as of IgM antibodies on the neutralization activity has been reported for viruses such as hepatitis C, vesicular stomatitis and adenovirus type 5.52-54 Therefore, it is conceivable that the decrease of NAB activity following IA is partially dependent on the depletion of non-IgG plasma proteins and further investigations are needed to address this point.
Low levels of anti-AAV2 or anti-AAV8 NAB have been related to a decrease or even total impairment of AAV liver transduction in both NHPs or humans,6,9,10 and patients with detectable anti-AAV NAB titers, as low as 1:5, are commonly excluded from clinical trials.55,56 However, the present results together with our previous findings57 suggest that differences in the neutralization ability of antibodies might exist between AAV serotypes. Even though anti-AAV5 NAB can be detected in plasma samples in vitro, those antibodies allow in vivo transduction of rAAV5-based vector up to a measured titer of 8250.
AAV serotypes such as 2 and 8 have been associated with cellular-mediated immune responses against the AAV5 capsid proteins.2,6 However, no T-cell–mediated immunity associated with AAV5 viral capsid was reported in humans submitted to AAV5-based therapy.5,14,58 Therefore, it cannot be excluded that IA may only be useful for serotypes that allow successful transduction in the presence of some titer of preexisting antibodies and that do not induce effector T cells upon readministration.
IA has been clinically applied59,60 and it is known that antibodies rapidly return to pretreatment levels.41 In general, 6 to 8 hours after IA a rebound of antibody plasma titers is seen due to redistribution of tissue-bound antibodies and antibodies from the lymphatic/interstitial compartment. Novel antibody synthesis may also occur, causing a restoration of antibody concentrations 7 to 14 days after treatment.23 The current study suggests that readministration of rAAV5 vectors would become feasible at NAB titers below 8250, allowing for a short readministration window. This would require a rapid surrogate test for the efficacy of the IA procedure, which could be extended if titers are too high. Because NAB assays can only be completed in days, we investigated the possibility of using a standard IgG ELISA as a surrogate test and establish a correlation between total IgG and NAB levels. Hence, a standard ELISA-based IgG assay can be used to assess the efficacy of IA prior to readministration of AAV5-based vectors. Importantly, the NAB levels in human samples submitted to only 2 cycles of IA corroborated the data obtained in NHPs with a reduction of NABs against AAV as well as of total IgG levels.
Another important aspect of the current procedure is the absence of rAAV5-specific T-cell responses. rAAV-capsid specific effector T cells are known to cause a reduction or complete annihilation of gene expression following successful transduction, and this often cannot be prevented by immune suppression. In contrast to other serotypes, T-cell responses to rAAV-5 capsid epitopes are infrequent in humans, even in individuals that were preexposed to AAV5 prior to gene therapy and were not observed upon second administration of rAAV-5 in the current primate study.
In summary, our study demonstrates that IA can be used for readministration of rAAV5-based therapeutic vectors when targeting the liver tissue. IA is widely clinically used and safe, and therefore this approach could be an option for both patients that have preexisting immunity toward AAV or patients that need to be readministered with the therapeutic AAV vector in case of decrease or loss of transgene activity.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Cristina Olaque and Miren Barberia for their experimental support.
Authorship
Contribution: D.S. performed the in vivo experiment; D.S. and K.L.K. performed the analyses and prepared the figures; V.F. and G.G.A. designed the study; V.F., G.G.A., and K.L.K. wrote the manuscript; H.P. and S.J.v.D. reviewed the manuscript; and N.Z. and A.B. participated in the experimental work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Valerie Ferreira, uniQure BV, Postbus 22506, 1100 DA Amsterdam, The Netherlands; e-mail: v.sier-ferreira@uniqure.com.
References
- 1.Grimm D, Zhou S, Nakai H, et al. . Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102(7):2412-2419. [DOI] [PubMed] [Google Scholar]
- 2.Nathwani AC, Tuddenham EG, Rangarajan S, et al. . Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaudet D, Méthot J, Déry S, et al. . Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther. 2013;20(4):361-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George LA, Sullivan SK, Giermasz A, et al. . Hemophilia B gene therapy with a high-specific-activity factor IX variant. N Engl J Med. 2017;377(23):2215-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miesbach W, Meijer K, Coppens M, et al. . Gene therapy with adeno-associated virus vector 5-human factor IX in adults with hemophilia B. Blood. 2018;131(9):1022-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response [published correction appears in Nat Med. 2006;12(5):592]. Nat Med. 2006;12(3):342-347. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Couto LB, Patarroyo-White S, et al. . Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108(10):3321-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scallan CD, Jiang H, Liu T, et al. . Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107(5):1810-1817. [DOI] [PubMed] [Google Scholar]
- 9.Hurlbut GD, Ziegler RJ, Nietupski JB, et al. . Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol Ther. 2010;18(11):1983-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Calcedo R, Bell P, et al. . Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther. 2011;22(11):1389-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mingozzi F. AAV immunogenicity: a matter of sensitivity. Mol Ther. 2018;26(10):2335-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao W, Gao G, Ling C, Herzog RW, Xiao X, Samulski RJ. Impact of neutralizing antibodies against AAV is a key consideration in gene transfer to nonhuman primates. Nat Med. 2018;24(6):699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao Y, Wang X, Yan R, et al. . Single point mutation in adeno-associated viral vectors -DJ capsid leads to improvement for gene delivery in vivo. BMC Biotechnol. 2016;16:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majowicz A, Salas D, Zabaleta N, et al. . Successful repeated hepatic gene delivery in mice and non-human primates achieved by sequential administration of AAV5ch and AAV1. Mol Ther. 2017;25(8):1831-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zabaleta N, Salas D, Paramo M, et al. . Improvement of adeno-associated virus-mediated liver transduction efficacy by regional administration in Macaca fascicularis. Hum Gene Ther Clin Dev. 2017;28(2):68-73. [DOI] [PubMed] [Google Scholar]
- 16.Mimuro J, Mizukami H, Hishikawa S, et al. . Minimizing the inhibitory effect of neutralizing antibody for efficient gene expression in the liver with adeno-associated virus 8 vectors. Mol Ther. 2013;21(2):318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lisowski L, Tay SS, Alexander IE. Adeno-associated virus serotypes for gene therapeutics. Curr Opin Pharmacol. 2015;24:59-67. [DOI] [PubMed] [Google Scholar]
- 18.Mingozzi F, Anguela XM, Pavani G, et al. . Overcoming preexisting humoral immunity to AAV using capsid decoys. Sci Transl Med. 2013;5(194):194ra92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meliani A, Boisgerault F, Hardet R, et al. . Antigen-selective modulation of AAV immunogenicity with tolerogenic rapamycin nanoparticles enables successful vector re-administration. Nat Commun. 2018;9(1):4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti M, Cleaver B, Clément N, et al. . Evaluation of readministration of a recombinant adeno-associated virus vector expressing acid alpha-glucosidase in Pompe disease: preclinical to clinical planning. Hum Gene Ther Clin Dev. 2015;26(3):185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer AL, Olthoff KM, Kim H, Rand E, Zamir G, Shaked A. Role of plasmapheresis in the management of acute hepatic failure in children. Ann Surg. 2001;234(3):418-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller F, Wagner K, Faber U, et al. . Elimination kinetics of plasma exchange. Klin Wochenschr. 1983;61(22):1115-1122. [DOI] [PubMed] [Google Scholar]
- 23.Schneider KM. Plasmapheresis and immunoadsorption: different techniques and their current role in medical therapy. Kidney Int Suppl. 1998;64:S61-S65. [PubMed] [Google Scholar]
- 24.Richter WO, Donner MG, Selmaier A, Hiller E, Schwandt P. Efficacy and safety of immunoglobulin apheresis. ASAIO J. 1997;43(1):53-59. [PubMed] [Google Scholar]
- 25.Knöbl P, Derfler K, Korninger L, et al. . Elimination of acquired factor VIII antibodies by extracorporal antibody-based immunoadsorption (Ig-Therasorb). Thromb Haemost. 1995;74(4):1035-1038. [PubMed] [Google Scholar]
- 26.Tribl B, Knöbl P, Derfler K, et al. . Rapid elimination of a high-titer spontaneous factor V antibody by extracorporeal antibody-based immunoadsorption and immunosuppression. Ann Hematol. 1995;71(4):199-203. [DOI] [PubMed] [Google Scholar]
- 27.Rummler S, Barz D. Plasma exchange and immunoadsorption of patients with thoracic organ transplantation. Transfus Med Hemother. 2012;39(4):234-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stummvoll G, Aringer M, Handisurya A, Derfler K. Immunoadsorption in autoimmune diseases affecting the kidney. Semin Nephrol. 2017;37(5):478-487. [DOI] [PubMed] [Google Scholar]
- 29.Hohenstein B, Bornstein SR, Aringer M. Immunoadsorption for connective tissue disease. Atheroscler Suppl. 2013;14(1):185-189. [DOI] [PubMed] [Google Scholar]
- 30.Klingel R, Heibges A, Fassbender C. Plasma exchange and immunoadsorption for autoimmune neurologic diseases - current guidelines and future perspectives. Atheroscler Suppl. 2009;10(5):129-132. [DOI] [PubMed] [Google Scholar]
- 31.Hickstein H, Koball S, Lehmann R, et al. . AB0 incompatible kidney transplantation using unspecific immunoadsorption. Transfus Apheresis Sci. 2014;50(2):263-266. [DOI] [PubMed] [Google Scholar]
- 32.Monteilhet V, Saheb S, Boutin S, et al. . A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19(11):2084-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chicoine LG, Montgomery CL, Bremer WG, et al. . Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol Ther. 2014;22(2):338-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13(16):1935-1943. [DOI] [PubMed] [Google Scholar]
- 35.Hafenrichter DG, Wu X, Rettinger SD, Kennedy SC, Flye MW, Ponder KP. Quantitative evaluation of liver-specific promoters from retroviral vectors after in vivo transduction of hepatocytes. Blood. 1994;84(10):3394-3404. [PubMed] [Google Scholar]
- 36.Nathwani AC, Gray JT, Ng CY, et al. . Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107(7):2653-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ovsyannikova IG, Dhiman N, Jacobson RM, Poland GA. Human leukocyte antigen polymorphisms: variable humoral immune responses to viral vaccines. Expert Rev Vaccines. 2006;5(1):33-43. [DOI] [PubMed] [Google Scholar]
- 38.Haralambieva IH, Kennedy RB, Ovsyannikova IG, Whitaker JA, Poland GA. Variability in humoral immunity to measles vaccine: new developments. Trends Mol Med. 2015;21(12):789-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathwani AC, Davidoff AM, Hanawa H, et al. . Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100(5):1662-1669. [DOI] [PubMed] [Google Scholar]
- 40.Nathwani AC, Rosales C, McIntosh J, et al. . Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther. 2011;19(5):876-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blume OR, Yost SE, Kaplan B. Antibody-mediated rejection: pathogenesis, prevention, treatment, and outcomes. J Transplant. 2012;2012:201754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boutin S, Monteilhet V, Veron P, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704-712. [DOI] [PubMed] [Google Scholar]
- 43.Halbert CL, Standaert TA, Aitken ML, Alexander IE, Russell DW, Miller AD. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J Virol. 1997;71(8):5932-5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halbert CL, Rutledge EA, Allen JM, Russell DW, Miller AD. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J Virol. 2000;74(3):1524-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73(5):3994-4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivière C, Danos O, Douar AM. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 2006;13(17):1300-1308. [DOI] [PubMed] [Google Scholar]
- 47.Ramaswamy S, Tonnu N, Menon T, et al. . Autologous and heterologous cell therapy for hemophilia B toward functional restoration of factor IX. Cell Reports. 2018;23(5):1565-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leventhal JR, John R, Fryer JP, et al. . Removal of baboon and human antiporcine IgG and IgM natural antibodies by immunoadsorption. Results of in vitro and in vivo studies. Transplantation. 1995;59(2):294-300. [PubMed] [Google Scholar]
- 49.Pham HP. Immunoadsorption In: Shaz BH, ed. Transfusion Medicine and Hemostasis, Amsterdam, The Netherlands; 2013:525-527. Available from: 10.1016/B978-0-12-397164-7.00079-3 [DOI] [Google Scholar]
- 50.Brenner P, Hinz M, Huber H, et al. . The influence of antibody and complement removal with a Ig-Therasorb column in a xenogeneic working heart model. Eur J Cardiothorac Surg. 1999;15(5):672-679. [DOI] [PubMed] [Google Scholar]
- 51.Majowicz A, Nijmeijer B, Lampen MH, et al. . Therapeutic hFIX Activity Achieved after Single AAV5-hFIX Treatment in Hemophilia B Patients and NHPs with Pre-existing Anti-AAV5 NABs. Mol Ther Methods Clin Dev. 2019;14:27-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer K, Basu A, Przysiecki CT, et al. . Complement-mediated enhancement of antibody function for neutralization of pseudotype virus containing hepatitis C virus E2 chimeric glycoprotein. J Virol. 2002;76(5):2150-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beebe DP, Cooper NR. Neutralization of vesicular stomatitis virus (VSV) by human complement requires a natural IgM antibody present in human serum. J Immunol. 1981;126(4):1562-1568. [PubMed] [Google Scholar]
- 54.Qiu Q, Xu Z, Tian J, et al. . Impact of natural IgM concentration on gene therapy with adenovirus type 5 vectors. J Virol. 2015;89(6):3412-3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang M, Crosby A, Hastie E, et al. . Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther. 2015;22(12):984-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falese L, Sandza K, Yates B, et al. . Strategy to detect pre-existing immunity to AAV gene therapy. Gene Ther. 2017;24(12):768-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Majowicz A, Nijmeijer B, Lampen MH, et al. Therapeutic factor IX (FIX) activity after single treatment with AMT-060 (AAV5-FIX) in hemophilia B patients with pre-existing anti-AAV5 humoral immunity. American Society of Gene & Cell Therapy (ASGCT) 21st annual meeting; 16-19 May 2018; Chicago, IL. [Google Scholar]
- 58.D’Avola D, López-Franco E, Sangro B, et al. . Phase I open label liver-directed gene therapy clinical trial for acute intermittent porphyria. J Hepatol. 2016;65(4):776-783. [DOI] [PubMed] [Google Scholar]
- 59.Hohenstein B, Passauer J, Ziemssen T, Julius U. Immunoadsorption with regenerating systems in neurological disorders--a single center experience. Atheroscler Suppl. 2015;18:119-123. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez AP, Cunard R, Ward DM. The selective therapeutic apheresis procedures. J Clin Apher. 2013;28(1):20-29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.