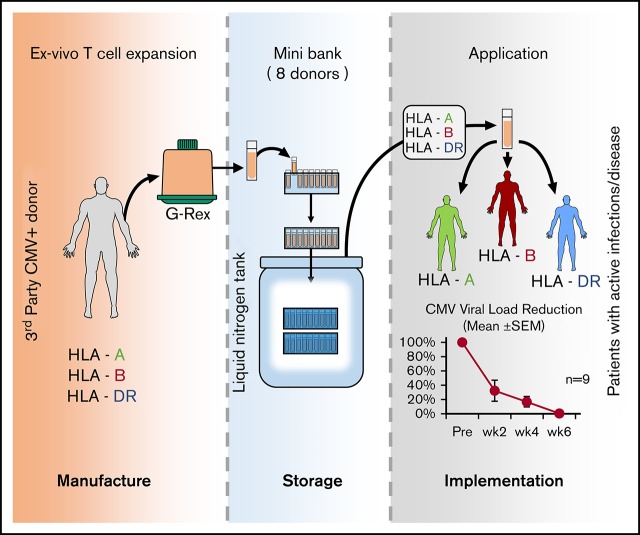
Key Points
CMV-specific T cells generated from a pool of just 8 donors provide an appropriately matched cell line for the majority of patients.
“Off-the-shelf” CMV-specific T cells are effective, readily available, and lack associated toxicities seen with standard therapies.
Abstract
Cytomegalovirus (CMV) infections remain a major cause of morbidity and mortality after allogeneic hematopoietic stem cell transplantation (HSCT), and standard antiviral therapies are associated with significant side effects and development of drug-resistant mutants. Adoptively transferred donor-derived CMV-specific T cells (CMVSTs) can provide an alternative treatment modality with few side effects but are not widely available due to their patient-specific nature. Here we report the establishment and use of a bank of CMVSTs derived from just 8 CMV-seropositive donors, with HLA types representing the diverse US population, as an “off-the-shelf” therapy to treat drug-refractory infections. To date, we have screened 29 patients for study participation and identified a suitable line, with ≥2 of 8 shared HLA antigens, for 28 (96.6%) patients with a median of 4 shared HLA antigens. Of these, 10 patients with persistent/refractory CMV infections or disease were eligible for treatment; a single infusion of cells produced 3 partial responses and 7 complete responses, for a cumulative response rate of 100% (95% confidence interval, 69.2-100) with no graft-versus-host disease, graft failure, or cytokine release syndrome. Potential wider use of the tested CMVSTs across transplant centers is made more feasible by our ability to produce sufficient material to generate cells for >2000 infusions from a single donor collection. Our data indicate that a “mini” bank of CMVSTs prepared from just 8 well-chosen third-party donors can supply the majority of patients with an appropriately matched line that produces safe and effective anti-CMV activity post-HSCT.
Visual Abstract
Introduction
Cytomegalovirus (CMV) remains the most clinically significant infection following allogeneic hematopoietic stem cell transplantation (HSCT). Center for International Blood and Marrow Transplant Research data show that early posttransplantation CMV reactivation occurs in >30% of CMV-seropositive HSCT recipients and can result in colitis, retinitis, pneumonitis, and death.1 Although antiviral agents, including ganciclovir, valganciclovir, letermovir, foscarnet, and cidofovir, have been used both prophylactically and therapeutically, they are not always effective and are associated with significant toxicities such as bone marrow suppression, renal toxicity, and, ultimately, nonrelapse mortality.2 Because immune reconstitution remains paramount to infection control,3,4 the adoptive transfer of ex vivo expanded/isolated CMV-specific T cells (CMVSTs) has emerged as an effective means of providing antiviral benefit.
Early immunotherapies targeting CMV focused on stem cell donor–derived T-cell products, which proved both safe and effective in a series of academic phase 1/2 studies spanning >20 years.5-16However, the personalized nature of the therapy and the requirement for virus-immune donors (an important issue given the benefits of using younger donors who are more likely virus naive)17 have emerged as barriers that preclude broad implementation. Thus, more recently, partially HLA-matched, third party–derived, virus-specific T cells (VSTs), which can be prepared prospectively and banked in advance of clinical need, have been investigated as a therapeutic modality. These VSTs have proved safe and effective against a spectrum of viruses, including Epstein-Barr virus, CMV, adenovirus, HHV6, and BK virus in >150 HSCT or solid organ transplant recipients with drug-refractory infections/disease.18-28 These studies prompted interest in advancing “off-the-shelf” VSTs toward pivotal studies and subsequent commercialization, with the remaining questions relating to the number of cell lines required to accommodate the racially and ethnically diverse transplant population, and establishing criteria for line selection to assure clinical benefit.
We chose to address these issues in a clinical trial using third-party T cells to treat CMV, a ubiquitous virus that remains a major cause of posttransplantation morbidity and mortality. The current article describes the establishment and clinical use of a bank of CMVSTs derived from just 8 well-chosen third-party donors to treat drug-refractory infections.
Materials and methods
Selection of donors for CMVST generation
To ensure that we could provide a clinically effective line for the majority of the allogeneic HSCT patient population, the HLA types of 666 allogeneic HSCT recipients treated in the Houston, TX, region (Houston Methodist or Texas Children’s Hospital), which has a diverse ethnic composition that is similar to the United States as a whole, were analyzed. These HSCT recipient HLAs were then compared with the HLA types of a pool of diverse, healthy, eligible CMV-seropositive donors. As an initial step, we identified the healthy donor whose HLA profile accommodated the greatest number of patients with a CMVST product. This donor was removed from the general donor pool; all patients accommodated by this donor were also removed from the unmatched patient population. Subsequently, we repeated these steps with a second, third, and so forth, donor, each time identifying the donor who best covered the remaining patients and then removed both the donor and accommodated patients from further consideration, until a panel was generated that covered at least 95% of the patients analyzed. This procedure was then repeated a second time to ensure that patients would have >1 potential donor option. Using this model, we found that only 8 well-selected donors would provide >95% of the patient population with a T-cell product that was matched on at least 2 HLA antigens; further increasing the donor pool would not significantly increase the number of matches. Eight of these donors were then selected with the goal to provide a coverage-suitable CMVST line (≥2 shared HLA antigens) with confirmed CMV activity to ≥95% of this diverse population of allogeneic HSCT recipients.
Third-party CMVST bank preparation
All donors provided written informed consent on an institutional review board–approved protocol and met blood bank eligibility criteria. For manufacturing, a unit of blood was collected by peripheral blood draw and peripheral blood mononuclear cells (PBMCs) isolated by Ficoll gradient. Then, 10 × 106 PBMCs were seeded in a G-Rex 5 bioreactor (Wilson Wolf Corporation, Saint Paul, MN) and cultured in T-cell media (Advanced RPMI 1640 [HyClone Laboratories Inc., Logan, UT], 45% Click’s [Irvine Scientific, Santa Ana, CA], 2 mM GlutaMAX TM-I [Thermo Fisher Scientific, Waltham, MA], and 10% fetal bovine serum [HyClone Laboratories Inc.]) containing 800 U/mL interleukin-4 (IL-4) and 20 ng/mL IL-7 (R&D Systems, Minneapolis, MN) and IE1 and pp65 PepMixes (2 ng/peptide per mL) (JPT Peptide Technologies, Berlin, Germany). On days 9 to 12 after initiation, T cells were harvested, counted, and restimulated with autologous pepmix-pulsed irradiated PBMCs (1:4 effector: target [E:T] – 4 × 105 CMVSTs: 1.6 × 106 irradiated PBMCs/cm2) with IL-4 (800 U/mL) and IL-7 (20 ng/mL) in a G-Rex-100M. On days 3 to 4 of culture, the cells were fed with 200 ng/mL IL-2 (Prometheus Laboratories, San Diego, CA); 9 to 12 days after the second stimulation, T cells were harvested for cryopreservation. At the time of cryopreservation, each line was microbiologically tested, immunophenotyped (CD3, CD4, CD8, CD14, CD16, CD19, CD25, CD27, CD28, CD45, CD45RA, CD56, CD62L, CD69, CD83, HLA DR, and 7AAD; Becton Dickinson, Franklin Lakes, NJ) and evaluated for virus specificity by using the interferon-γ (IFN-γ) enzyme-linked immunospot (ELISPOT) assay. A cell line was defined as “reactive” when the frequency of reactive cells, as measured by using the IFN-γ ELISPOT assay, was >30 spot-forming cells (SFCs) per 2 × 105 input VSTs.
Clinical trial design
This single-center phase 1 study (NCT02313857) was conducted under an Investigational New Drug application from the US Food and Drug Administration and approved by the Baylor College of Medicine Institutional Review Board. The study was open to allogeneic HSCT recipients with CMV infections or disease that had persisted for at least 7 days despite standard therapy, defined as treatment with ganciclovir, foscarnet, or cidofovir. Exclusion criteria included treatment with prednisone (or equivalent) ≥0.5 mg/kg, respiratory failure with oxygen saturation of <90% on room air, other uncontrolled infections, and active graft-versus-host disease (GVHD) grade II or higher. Patients who received antithymocyte globulin, Campath, other T-cell immunosuppressive monoclonal antibodies, or a donor lymphocyte infusion within 28 days of the proposed administration date were also excluded from participation. Patients initially gave their consent to search for a suitable VST line (with ≥2 shared HLA antigens), and if available and if patients met eligibility criteria, they could be enrolled on the treatment portion of the study. Each patient received a single IV infusion of 2 × 107 partially HLA-matched VSTs/m2 with the option to receive a second infusion after 4 weeks and additional infusions at biweekly intervals thereafter. Therapy with standard antiviral medications could be administered at the discretion of the treating physician.
Safety end points
The primary objective of this pilot study was to determine the safety of CMVSTs in HSCT recipients with persistent CMV infections/disease. Toxicities were graded by using the National Cancer Institute’s Common Terminology Criteria for Adverse Events, v4.0X. Safety end points included acute GVHD grades III to IV within 42 days of the last CMVST dose, infusion-related toxicities within 24 hours of infusion, or grades 3 to 5 nonhematologic adverse events related to the T-cell product within 28 days of the last CMVST dose and not attributable to a preexisting infection, the original malignancy, or preexisting comorbidities. Acute and chronic GVHD, if present, were graded according to standard clinical definitions.29,30 The study was monitored by the Dan L. Duncan Cancer Center Data Review Committee.
Assessment of outcomes
CMV loads in peripheral blood were monitored by using quantitative polymerase chain reaction in Clinical Laboratory Improvement Amendments–approved laboratories. A complete response (CR) of the virus to treatment was defined as a decrease in viral load to below the threshold of detection according to quantitative polymerase chain reaction and resolution of clinical signs and symptoms of tissue disease (if present at baseline). A partial response was defined as a decrease in viral load of at least 50% from baseline. Clinical and virologic responses were assigned at week 6 after CMVST infusion.
Immune monitoring
ELISPOT analysis was used to determine the frequency of circulating T cells that secreted IFN-γ in response to CMV antigens and peptides. Clinical samples were collected before and at weeks 1, 2, 3, 4, 6, and 12 postinfusion. As a positive control, PBMCs were stimulated with Staphylococcal Enterotoxin B (1 μg/mL) (MilliporeSigma, St. Louis, MO). IE1 and pp65 PepMixes (JPT Technologies), diluted to 1000 ng/peptide per mL, were used to track donor-derived CMVSTs postinfusion. When available, peptides representing known epitopes (Genemed Synthesis Inc., San Antonio, TX), diluted to 1250 ng/mL, were also used in ELISPOT assays. For ELISPOT analyses, PBMCs were resuspended at 5 × 106/mL in T-cell medium and plated in 96-well ELISPOT plates. Each condition was run in duplicate. After 20 hours of incubation, plates were developed as previously described, dried overnight at room temperature in the dark, and then sent to ZellNet Consulting (New York, NY) for quantification. IFN-γ SFCs and input cell numbers were plotted, and the frequency of T cells specific for each antigen was expressed as specific SFCs per input cell numbers.
Statistical analysis
Descriptive statistics were calculated to summarize data. Antiviral responses were summarized, and the response rate was estimated along with exact 95% binomial confidence intervals (CIs). Viral load and T-cell frequency data were plotted to graphically illustrate the patterns of immune responses over time. Comparisons of changes in viral load and T-cell frequency preinfusion and postinfusion were performed by using Wilcoxon signed rank tests. Data were analyzed by using SAS version 9.4 (SAS Institute, Inc., Cary, NC) and R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria). P < .05 was considered statistically significant.
Results
Third-party CMVST bank
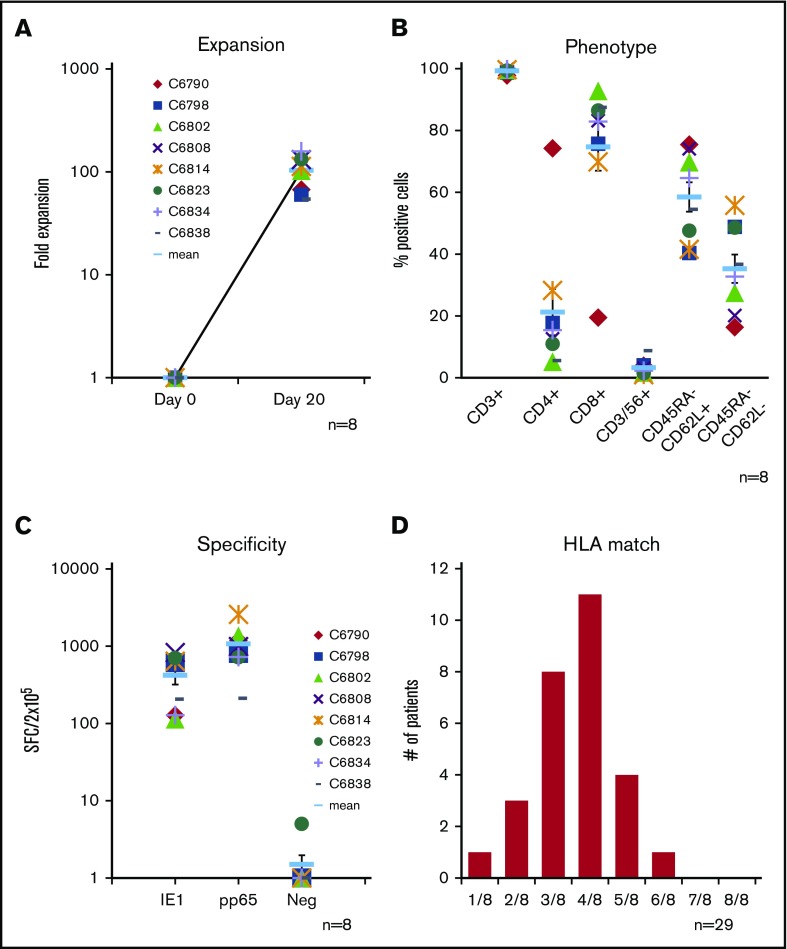
We generated a bank of CMVSTs from 8 CMV-seropositive donors chosen to represent the diverse HLA profile of the transplant population (Table 1). A median of 7.7 × 108 PBMCs (range, 4.6-8.8 × 108) were isolated from a single blood draw (median, 425 mL). To expand CMVSTs, PBMCs were exposed to PepMixes spanning pp65 and IE1, and over 20 days in culture a mean fold expansion of 102 ± 12 (Figure 1A) was achieved. The resulting cells were almost exclusively CD3+ (99.3% ± 0.4%), comprising both CD4+ (21.3% ± 7.5%) and CD8+ (74.7% ± 7.8%) subsets that expressed central CD45RA–/62L+ (58.5% ± 4.8%) and effector CD45RA–/62L– (35.3% ± 4.6%) memory markers (Figure 1B). All 8 lines were reactive against the stimulating CMV antigens (IE1 419 ± 100 and pp65 1069 ± 230, SFCs per 2 × 105) (Figure 1C). The characteristics of the cell lines are summarized in Table 1. Of these 8 lines, 6 products were administered to 10 treated study patients.
Table 1.
Characteristics of generated VST lines
| VST line compound no. | CMV specificity, SFC/1 × 105 IE1 | CMV specificity, SFC/1 × 105 pp65 | CD3, % | CD4, % | CD8, % | CD56, % | CD45RO+/CD62L+, % | CD45RO+/CD62L–, % | HLA-A | HLA-B | HLA-DR | HLA-DQ | No. of patients screened* | No. of patients treated |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 6790 | 127 | 1186 | 97.81 | 74.23 | 19.48 | 3.88 | 75.45 | 16.33 | 02,33 | 15,44 | 07,13 | 02,06 | 4 | 3 |
| 6798 | 612 | 805 | 98.79 | 17.75 | 75.73 | 4.05 | 40.3 | 44.83 | 02,02 | 40,52 | 04,08 | 03,03 | 6 | 4 |
| 6802 | 113 | 1354 | 99.66 | 5.20 | 92.82 | 1.69 | 69.75 | 27.51 | 11,23 | 35,57 | 01,07 | 03,05 | 1 | 0 |
| 6808 | 827 | 986 | 99.77 | 12.59 | 83.18 | 3.10 | 74.09 | 20.13 | 02,24 | 40,52 | 04,13 | 03,06 | 4 | 1 |
| 6814 | 639 | 2573 | 99.68 | 28.25 | 69.85 | 0.99 | 41.56 | 55.78 | 02, 24 | 08,14 | 01, 03 | 02,05 | 1 | 1 |
| 6823 | 700 | 717 | 99.39 | 10.99 | 86.49 | 1.51 | 47.59 | 48.59 | 11,68 | 07,35 | 03,07 | 02,02 | 3 | 1 |
| 6834 | 128 | 725 | 99.77 | 15.40 | 82.90 | 2.27 | 64.64 | 32.72 | 02,24 | 15,35 | 04,09 | 03,03 | 6 | 1 |
| 6838 | 205.5 | 211 | 99.75 | 5.57 | 87.46 | 8.76 | 54.50 | 36.42 | 02,30 | 13,35 | 07,08 | 02,06 | 1 | 0 |
Indicates how frequently the VST lines were determined to be the most suitable line for a screened patient.
Figure 1.
Characteristics of generated CMVST lines and degree of matching with screened subjects. (A) T-cell expansion of CMVSTs achieved over a 20-day period based on cell counting using trypan blue exclusion (n = 8). Phenotype of the expanded CMVST lines on the day of cryopreservation (mean ± standard error of the mean [SEM], n = 8) (B) and frequency of antigen-specific T cells as determined by IFN-γ ELISPOT assay (C) after overnight stimulation of CMVSTs with IE1 and pp65 antigen-spanning PepMixes. Results are reported as SFCs per 2 × 105 VSTs plated. CMVST lines with a total of ≥30 SFCS per 2 × 105 were considered to be positive (n = 8). (D) Number of matching HLA antigens (of 8 total) of CMVST lines identified for clinical use with recipient HLA of screened patients (n = 29).
Screening
Twenty-nine allogeneic HSCT recipients with CMV infections were referred by their primary bone marrow transplant providers for study participation. From our bank of 8 lines, we identified a suitable product (minimum 2 of 8 HLA match threshold) for infusion in 28 cases (96.6%; 95% CI, 82.2-99.9). A 2-of-8 HLA match threshold was established based on retrospective analysis performed on our previous third-party study,27 which showed clinical benefit associated with the administration of such HLA-matched products. HLA class I or II matching did not seem to influence outcome. Of note, most products in the current study were matched at ≥4 antigens (Figure 1D). Of the 28 patients with available lines, 17 patients did not receive cells because they responded to standard antiviral treatment, and 1 patient was ineligible due to a recent donor lymphocyte infusion.
Characteristics of treated patients
The characteristics of the 10 patients (pediatric, n = 7; adult, n = 3) treated for persistent infections are summarized in Tables 2 and 3. The group included 2 African American recipients, 3 patients of white Hispanic origin, and 5 non-Hispanic white recipients. Three of the 10 patients had confirmed virus-associated disease (ie, CMV retinitis [n = 1], diarrhea attributed to CMV colitis [n = 2]). CMVSTs (matching at 2-6 of 8 HLA antigens) were administered between days 46 and 365 (median, day 133) posttransplant. Seven patients had infections that were refractory to standard antiviral treatment of a median of 24 days (mean, 48 days; range, 14-211 days), and 3 of the patients harbored viruses with mutations that conferred resistance to conventional antiviral therapy. Before immunotherapeutic intervention, 6 of these patients had experienced severe adverse events associated with conventional antiviral agents that included acute kidney injury (n = 4), foscarnet-induced renal tubulopathy (n = 1), and severe foscarnet-associated pancreatitis (n = 1), which in 3 cases precluded further treatment with any conventional drugs.
Table 2.
Patient demographic characteristics
| Patient ID no. | Sex | Age, y | Ethnicity | Race | Diagnosis | Type of transplant | R/D CMV serostatus | No. of infusions | Days posttransplant |
|---|---|---|---|---|---|---|---|---|---|
| 3910 | Male | 12 | Non-Hispanic | African American | Sickle cell anemia | MRD | Negative/positive | 1 | 61 |
| 3944 | Female | 45 | Hispanic | White | AML | UCB | Positive/negative | 1 | 197 |
| 3976 | Male | 13 | Hispanic | White | ALL | MUD | Positive/positive | 2 | 46 |
| 3762 | Female | 10 | Hispanic | White | HLH | MMUD | Positive/negative | 1 | 161 |
| 3967 | Male | 51 | Non-Hispanic | White | AML | UCB | Positive/negative | 1 | 365 |
| 4091 | Male | 70 | Non-Hispanic | White | CTCL | Haplo | Positive/positive | 1 | 215 |
| 4115 | Female | 3 | Non-Hispanic | White | Fanconi anemia | MUD | Positive/positive | 1 | 105 |
| 4170 | Male | 3 | Non-Hispanic | African American | Sickle cell anemia | MRD | Negative/positive | 1 | 76 |
| 4134 | Female | 16 | Non-Hispanic | White | SCID | MUD | Positive/positive | 1 | 218 |
| 4201 | Male | 11 | Non-Hispanic | White | Anaplastic large-cell lymphoma | MUD | Positive/negative | 3 | 70 |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CTCL, cutaneous T-cell lymphoma; Haplo, haploidentical; HLH, hemophagocytic lymphohistiocytosis; MMUD, mismatched unrelated donor; MUD, matched unrelated donor; MRD, matched related donor; R/D, recipient/donor; SCID, severe combined immunodeficiency; UCB, umbilical cord blood.
Table 3.
Patient clinical characteristics
| Patient ID no. | Infused VSTs | HLA matching | Viral disease | Reduction in immuno-suppression | Prior antiviral(s) | Duration prior antiviral therapy | Antiviral drug intolerance | CMV resistance | Change in antiviral post-VST | Response by 6 wk | Overall outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 3910 | C6790 | 2/8 | No | No | Ganciclovir | 17 | No | No | No | CR | Sustained CR |
| 3944 | C6798 | 4/8 | No | No | Leflunomide | 94 | AKI, cytopenia | Yes | No | CR | Sustained CR |
| 3976 | C6798 | 6/8 | No | Yes, reduction in tacrolimus between wk 4 and 6 | Foscarnet, ganciclovir | 14 | Renal tubulopathy | Yes (U97 mutation) | No | CR | Sustained CR after second infusion |
| 3762 | C6808 | 5/8 | CMV retinitis | No | None | Unable to tolerate | Poor graft function | Not done | Yes, ganciclovir administered for 1 wk | CR | Sustained CR |
| 3967 | C6823 | 2/8 | CMV colitis (diarrhea) | No | Foscarnet | 211 | AKI | Yes (U97 mutation) | No | PR | Died of renal failure and AdV infection |
| 4091 | C6790 | 5/8 | No | No | Foscarnet, cidofovir, leflunomide | 29 | AKI | Not done | No | CR | Died of renal failure |
| 4115 | C6834 | 5/8 | No | No | Foscarnet | 24 | AKI | Not done | No | CR | Sustained CR |
| 4170 | C6790 | 3/8 | No | No | Ganciclovir, foscarnet | 42 | Pancreatitis | No | No | CR | Sustained CR |
| 4134 | C6798 | 5/8 | CMV colitis (diarrhea) | No | Foscarnet, ganciclovir | 17 | No | Not done | No | PR | Sustained CR |
| 4201 | C6814 | 3/8 | No | Yes, tacrolimus taper initiated before VST infusion | Foscarnet, ganciclovir | 41 | No | No | No | PR | Sustained CR after third infusion |
AdV, adenovirus; AKI, acute kidney injury; PR, partial response.
Clinical safety
All infusions were well tolerated. Except for 1 patient who developed a transient isolated fever 8 hours after infusion, no immediate toxicities were observed. One patient developed a mild maculopapular rash on his trunk, which appeared suggestive of a viral exanthem and spontaneously resolved within a few days without topical or systemic treatment. No cases of cytokine release syndrome or other toxicities related to the infused CMVSTs were observed, and none of the patients developed graft failure, autoimmune hemolytic anemia, or transplant-associated microangiopathy. Patients were followed up for 6 weeks for acute GVHD and 12 months for chronic GVHD. Despite the HLA disparity between the patients and the infused cells, none of the patients developed recurrent or de novo acute or chronic GVHD posttreatment (Table 4), including 3 patients with a history of acute GVHD (grade II [n = 2] or III [n = 1]).
Table 4.
GVHD preinfusion and postinfusion
| Patient ID no. | Prior GVHD | Baseline | GVHD Rx/PPx at infusion | aGVHD | cGVHD |
|---|---|---|---|---|---|
| 3910 | None | None | Cyclosporine | None | None |
| 3944 | None | None | Tacrolimus | None | None |
| 3976 | None | None | Tacrolimus | None | None |
| 3762 | None | None | None | None | None |
| 3967 | GI grade II | None | Sirolimus | None | None |
| 4091 | GI, skin grade II | None | Tacrolimus | None | None |
| 4115 | None | None | None | None | None |
| 4170 | None | None | Tacrolimus | None | None |
| 4134 | GI grade III | None | None | None | None |
| 4201 | None | None | Tacrolimus | None | None |
aGVHD, acute GVHD; cGVHD, chronic GVHD; GI, gastrointestinal; PPx, prophylaxis; Rx, treatment.
Clinical responses
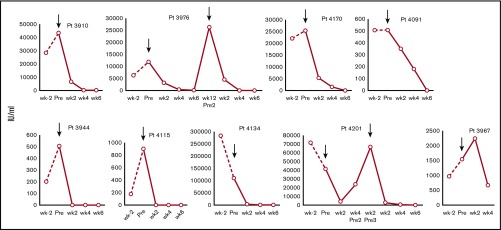
All 10 infused patients responded to CMVSTs with 7 CRs and 3 partial responses, for a cumulative response rate of 100% (95% CI, 69.2-100.0) by week 6. The average plasma viral load reduction at week 6 was 89.8% (±21.4%). Figure 2 summarizes the virologic outcomes of all treated patients based on sequential viral load measurements. Of note, clinical benefit was achieved not only in patients with refractory infections but also in the 3 individuals with tissue disease (ie, CMV retinitis [n = 1], diarrhea attributed to CMV colitis [n = 2]).
Figure 2.
Treatment outcomes in individual patients infected with CMV. Depiction of plasma CMV viral loads in patients 2 weeks before (viral load level closest to week −2), immediately before (pre), and after (post) infusion (weeks 2, 4, and 6) of CMVSTs. Arrows indicate infusion time points.
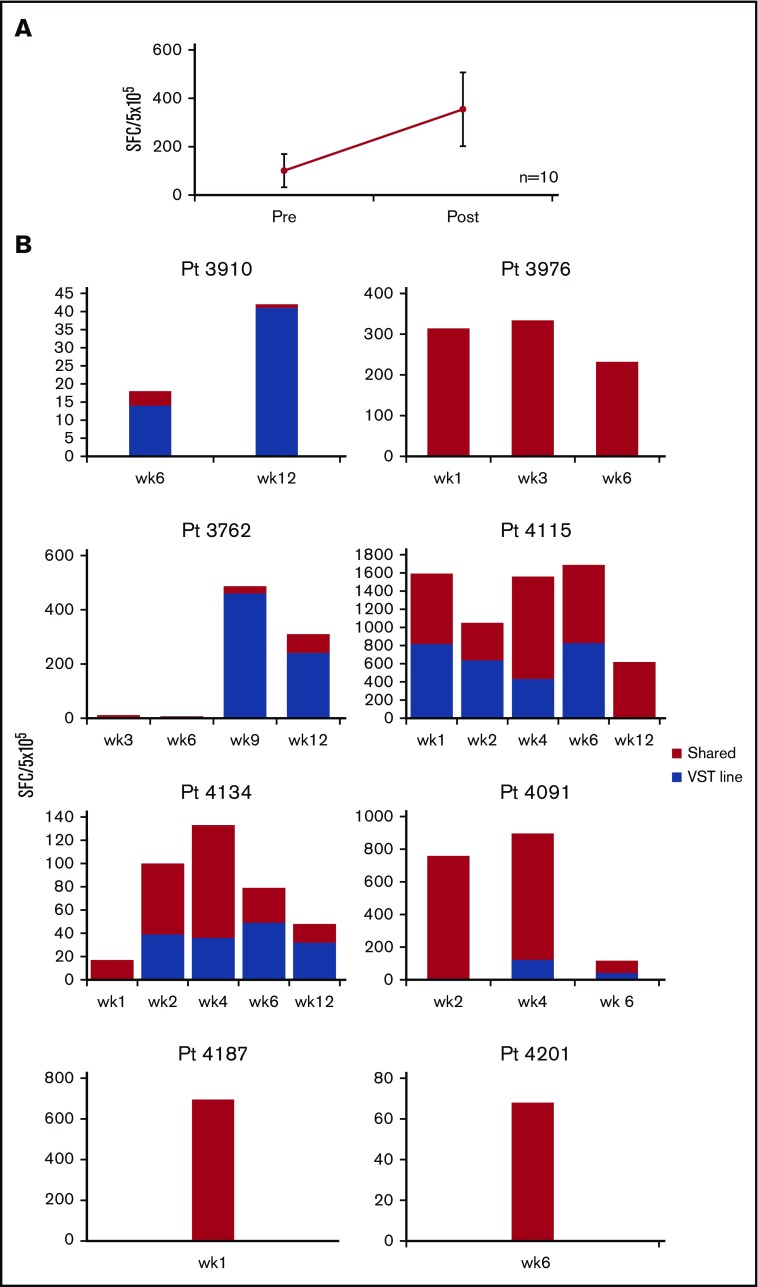
Eight patients received a single infusion of CMVSTs, 1 patient (no. 3976) had 2 infusions, and 1 patient (no. 4201) had 3 infusions of CMVSTs. Patient no. 3976 had a CR at week 6 but relapsed with increasing virus loads at week 10. Eighty days after the first infusion, he received a second infusion with the same CMVST line that resulted in a sustained CR. Patient no. 4201 received a second infusion of the same CMVSTs 28 days after the initial administration but failed to respond; 2 weeks later, the patient was therefore administered a third infusion with a different CMVST line and achieved a sustained CR. The clinical and virologic responses achieved in these patients were associated with an increase in virus-reactive CMVSTs in 8 of the 10 treated patients (increase from mean 126 ± 84 SFC preinfusion to peak of 443 ± 178 per 5 × 105 PBMCs (P = .023) (Figure 3A).
Figure 3.
Frequency of CMV-specific T cells in vivo. (A) Frequency of CMVSTs in the peripheral blood before (pre) infusion and after (post) infusion, as measured by using the IFN-γ ELISPOT assay after overnight stimulation with IE1 and pp65 viral PepMixes. Results are expressed as SFCs per 5 × 105 input cells (mean ± SEM, n = 10). (B) Persistence of infused CMVSTs in individual patients. Frequency of T cells in peripheral blood as measured by using the IFN-γ ELISPOT assay after stimulation with epitope-specific CMV peptides with restriction to HLA antigens exclusive to the CMVST line or shared between the recipient and the CMVST line.
T-cell persistence
To determine if the CMVST infusions contributed to the protective effects seen in these patients and to evaluate the in vivo longevity of these partially HLA-matched VSTs, the specificity of CMVSTs in patient PBMCs before and after infusion were examined by using HLA-restricted epitope peptides restricted to the line infused. Functional T cells of confirmed third-party origin were detected in 5 patients for whom we had available HLA-restricting peptide reagents, which persisted for up to 12 weeks; in all 8 patients, we observed antiviral responses restricted by the HLA alleles shared between the patient and the CMVST line (Figure 3B). Thus, we infer that the infused CMVSTs induced an antiviral effect resulting in the control of CMV infections.
Discussion
In this phase 1 trial, third-party CMVSTs were administered to treat CMV infections/disease in allogeneic HSCT recipients who had failed at least 14 days of treatment with ganciclovir and/or foscarnet or could not tolerate standard antiviral medications. Notable exclusion criteria were patients with active GVHD or receiving corticosteroids at moderate or high doses. We generated a bank of CMVSTs from just 8 healthy donors, which were carefully selected based on their HLA profile to provide broad coverage to a racially and ethnically diverse allogeneic HSCT patient population. Indeed, of the 29 patients screened for study participation, we were able to identify a suitable line (minimum 2 shared HLA antigen threshold) for 28 patients (96.6%; 95% CI, 82.2-99.9), attesting to the feasibility of providing broad patient coverage with a small, well-chosen cell bank. Of these 28 patients, we treated 10 from diverse backgrounds (2 African American, 3 of white Hispanic origin, and 5 non-Hispanic white), and all achieved virologic and clinical benefit, without experiencing acute or chronic GVHD or other toxicities. This outcome was notable because 6 had previously experienced serious adverse events, including acute kidney injury, renal tubulopathy, and pancreatitis, related to conventional antiviral medication.31 This phase 1 trial showcases the safety and clinical benefit associated with the administration of third-party, virus-reactive T cells, sourced from a small and carefully designed donor bank, for the treatment of refractory CMV infections.
Despite decreasing rates of disease in recent decades, CMV remains a major cause of morbidity after allogeneic HSCT; in a Center for International Blood and Marrow Transplant Research report, data from 9469 patients (undergoing transplant from 2003-2010 for acute myeloid leukemia [n = 5310], acute lymphoblastic leukemia [n = 1883], chronic myeloid leukemia [n = 1079], and myelodysplastic syndrome [n = 1197]) were interrogated, and CMV reactivation was associated with higher nonrelapse mortality as well as lower overall survival among all 4 disease categories.14 Furthermore, in a recent study of 208 patients who underwent transplantation between 2008 and 2013, the average length of in-hospital stay was found to be prolonged by 26 days in patients with CMV reactivation, whereas the occurrence of >1 CMV reactivation episode increased the costs of an allogeneic HSCT by 25% to 30% (P < .0001), highlighting the economic burden of CMV management.32
Foscarnet and ganciclovir are frequently used to treat CMV infections after HSCT. However, other than ganciclovir for CMV retinitis, their use is off-label, and both drugs are associated with significant side effects, particularly renal disease and graft suppression. When used prophylactically, letermovir, a cytomegalovirus DNA terminase complex inhibitor, decreased the incidence of CMV infection/reactivation posttransplant,33 and since US Food and Drug Administration approval (for CMV prophylaxis in adult HSCT patients) in 2017, is increasingly used in high-risk patients. However, the CMV Resistance Working Group of the multidisciplinary CMV Drug Development Forum expects that the wider prophylactic use of letermovir will increase the emergence of organisms that are resistant to conventional antiviral medication if a CMV breakthrough infection does occur.34 Indeed, letermovir-resistant CMV strains are increasingly reported,35,36 and clinical outcomes in those with resistant disease are poor and associated with progressive tissue disease and mortality.37
CMVSTs provide an alternative strategy to target both initial reactivations as well as drug-resistant viral strains, as previously reported by our group and others.27,38 Thirty percent of the patients treated with CMVSTs in the current study were infected with viral strains confirmed to be resistant to one or more conventional antiviral drugs.
One goal of the current study was to prepare a CMV-specific T-cell bank with sufficient diversity to cover the majority of allogeneic HSCT recipients referred for treatment. Thus, we prospectively compared the HLA types of >600 allogeneic HSCT recipients with a pool of diverse healthy, eligible (CMV-seropositive) donors from whom CMVSTs could be generated to identify the minimum cohort that would provide our patients with well-matched products. Using this model, we found that only 8 well-selected donors would provide >95% of the patient population with a T-cell product that was matched on at least 2 HLA antigens; further increasing the donor pool would not significantly increase the number of matches. The current study, in which a suitable line was identified for 28 (96.5%) of 29 patients screened for clinical participation, supports our theory that such a donor bank could effectively supply the majority of the allogeneic HSCT patient population.
We compared the racial and ethnic diversity within our transplant patient population with that of the US transplant population (supplemental Table 1). This comparison revealed that the diversity within our patient population was similar if not slightly more diverse than the US population. This scenario suggests that the small cell bank developed for the current study could be broadly applied for clinical use across the country, but additional data will be needed to confirm efficacy in smaller ethnic groups (eg, Asian-American subjects or patients of mixed heritage). Universal use of the tested CMVSTs across transplant centers is made more feasible by our ability to produce sufficient material to generate cells for >2000 infusions from a single donor collection. Thus, one could envisage a decentralized distribution model of “off-the-shelf,” third-party, virus-reactive T cells, ensuring on-demand availability of cells.
In conclusion, our data indicate that a well-characterized bank of CMV-reactive T cells prepared from just 8 well-chosen third-party donors can supply the majority of patients with refractory CMV infections with an appropriately matched line that can provide safe and effective antiviral activity.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all those who participated in this trial, clinicians, their staff, and patients. They also thank Amy Reyna and Catherine Robertson for study coordination, Sara Richman and Deborah Lyon for Quality Assurance/Quality Control, Huimin Zhang and her team for cell processing, and Bridget Medina for regulatory assistance.
Funds for CMVST generation and conduct of the clinical trial were provided by Viracyte. B.O. was supported by an educational National Institutes of Health K12 grant at Texas Children’s Hospital.
The funding sources (including Viracyte) had no role in data collection, interpretation of data, or decision to publish.
Authorship
Contribution: I.T., H.E.H., and B.O. conceived and designed the study; S.N., K.S.L., C.M., G.S., R.A.K., S.G., A.P.G., and B.O. provided study materials or patients; I.T., A.W., M.W., R.D., and M.K. collected and assembled the data; S.N., K.S.L., C.M., G.S., R.A.K., M.W., S.G., and B.O. analyzed and interpreted the data; and all authors were involved in writing and final approval of the manuscript.
Conflict-of-interest disclosure: H.E.H. has equity interest in Viracyte, the sponsor of the study; and designed the clinical trial but had no role in data collection, interpretation of data, or decision to publish. I.T. and S.G. are paid consultants for Viracyte. S.G. receives research support from TESSA Therapeutics that is unrelated to this project; has patents and patent applications in the field of T-cell therapy and gene therapy for cancer; and is a member of the data safety monitoring board of Immatics US, Inc. The remaining authors declare no competing financial interests.
Correspondence: Bilal Omer, Center for Cell and Gene Therapy, Feigin Tower, Suite 1640.16, 1102 Bates Ave, Houston, TX 77030; e-mail: baomer@txch.org.
References
- 1.Teira P, Battiwalla M, Ramanathan M, et al. Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood. 2016;127(20):2427-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan ST, Logan AC. The clinical impact of cytomegalovirus infection following allogeneic hematopoietic cell transplantation: why the quest for meaningful prophylaxis still matters. Blood Rev. 2017;31(3):173-183. [DOI] [PubMed] [Google Scholar]
- 3.Blyth E, Withers B, Clancy L, Gottlieb D. CMV-specific immune reconstitution following allogeneic stem cell transplantation. Virulence. 2016;7(8):967-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tormo N, Solano C, Benet I, et al. Lack of prompt expansion of cytomegalovirus pp65 and IE-1-specific IFNgamma CD8+ and CD4+ T cells is associated with rising levels of pp65 antigenemia and DNAemia during pre-emptive therapy in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2010;45(3):543-549. [DOI] [PubMed] [Google Scholar]
- 5.Walter EA, Greenberg PD, Gilbert MJ, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333(16):1038-1044. [DOI] [PubMed] [Google Scholar]
- 6.Einsele H, Roosnek E, Rufer N, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99(11):3916-3922. [DOI] [PubMed] [Google Scholar]
- 7.Leen AM, Myers GD, Sili U, et al. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12(10):1160-1166. [DOI] [PubMed] [Google Scholar]
- 8.Micklethwaite K, Hansen A, Foster A, et al. Ex vivo expansion and prophylactic infusion of CMV-pp65 peptide-specific cytotoxic T-lymphocytes following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(6):707-714. [DOI] [PubMed] [Google Scholar]
- 9.Peggs KS, Verfuerth S, Pizzey A, Chow SL, Thomson K, Mackinnon S. Cytomegalovirus-specific T cell immunotherapy promotes restoration of durable functional antiviral immunity following allogeneic stem cell transplantation. Clin Infect Dis. 2009;49(12):1851-1860. [DOI] [PubMed] [Google Scholar]
- 10.Scheinberg P, Melenhorst JJ, Brenchley JM, et al. The transfer of adaptive immunity to CMV during hematopoietic stem cell transplantation is dependent on the specificity and phenotype of CMV-specific T cells in the donor. Blood. 2009;114(24):5071-5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peggs KS, Thomson K, Samuel E, et al. Directly selected cytomegalovirus-reactive donor T cells confer rapid and safe systemic reconstitution of virus-specific immunity following stem cell transplantation. Clin Infect Dis. 2011;52(1):49-57. [DOI] [PubMed] [Google Scholar]
- 12.Blyth E, Clancy L, Simms R, et al. Donor-derived CMV-specific T cells reduce the requirement for CMV-directed pharmacotherapy after allogeneic stem cell transplantation. Blood. 2013;121(18):3745-3758. [DOI] [PubMed] [Google Scholar]
- 13.Gerdemann U, Katari UL, Papadopoulou A, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013;21(11):2113-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med. 2014;6(242):242ra83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmitt A, Tonn T, Busch DH, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51(3):591-599. [DOI] [PubMed] [Google Scholar]
- 16.Feucht J, Opherk K, Lang P, et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood. 2015;125(12):1986-1994. [DOI] [PubMed] [Google Scholar]
- 17.Mehta J, Gordon LI, Tallman MS, et al. Does younger donor age affect the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies beneficially [published correction appears in Bone Marrow Transplant. 2006;38(5): 397]? Bone Marrow Transplant. 2006;38(2):95-100. [DOI] [PubMed] [Google Scholar]
- 18.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110(4):1123-1131. [DOI] [PubMed] [Google Scholar]
- 19.Uhlin M, Okas M, Gertow J, Uzunel M, Brismar TB, Mattsson J. A novel haplo-identical adoptive CTL therapy as a treatment for EBV-associated lymphoma after stem cell transplantation. Cancer Immunol Immunother. 2010;59(3):473-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker JN, Doubrovina E, Sauter C, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. 2010;116(23):5045-5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qasim W, Derniame S, Gilmour K, et al. Third-party virus-specific T cells eradicate adenoviraemia but trigger bystander graft-versus-host disease. Br J Haematol. 2011;154(1):150-153. [DOI] [PubMed] [Google Scholar]
- 22.Uhlin M, Gertow J, Uzunel M, et al. Rapid salvage treatment with virus-specific T cells for therapy-resistant disease. Clin Infect Dis. 2012;55(8):1064-1073. [DOI] [PubMed] [Google Scholar]
- 23.Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012;119(11):2644-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vickers MA, Wilkie GM, Robinson N, et al. Establishment and operation of a Good Manufacturing Practice-compliant allogeneic Epstein-Barr virus (EBV)-specific cytotoxic cell bank for the treatment of EBV-associated lymphoproliferative disease. Br J Haematol. 2014;167(3):402-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallot G, Vollant S, Saïagh S, et al. T-cell therapy using a bank of EBV-specific cytotoxic T cells: lessons from a phase I/II feasibility and safety study. J Immunother. 2014;37(3):170-179. [DOI] [PubMed] [Google Scholar]
- 27.Tzannou I, Papadopoulou A, Naik S, et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein-Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2017;35(31):3547-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Withers B, Blyth E, Clancy LE, et al. Long-term control of recurrent or refractory viral infections after allogeneic HSCT with third-party virus-specific T cells. Blood Adv. 2017;1(24):2193-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant. 2005;11(12):945-956. [DOI] [PubMed] [Google Scholar]
- 30.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 31.Jacobsen T, Sifontis N. Drug interactions and toxicities associated with the antiviral management of cytomegalovirus infection. Am J Health Syst Pharm. 2010;67(17):1417-1425. [DOI] [PubMed] [Google Scholar]
- 32.Robin C, Hémery F, Dindorf C, et al. Economic burden of preemptive treatment of CMV infection after allogeneic stem cell transplantation: a retrospective study of 208 consecutive patients. BMC Infect Dis. 2017;17(1):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marty FM, Ljungman P, Chemaly RF, et al. Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med. 2017;377(25):2433-2444. [DOI] [PubMed] [Google Scholar]
- 34.Chemaly RF, Chou S, Einsele H, et al. ; Resistant Definitions Working Group of the Cytomegalovirus Drug Development Forum . Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials. Clin Infect Dis. 2019;68(8):1420-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cherrier L, Nasar A, Goodlet KJ, Nailor MD, Tokman S, Chou S. Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am J Transplant. 2018;18(12):3060-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chou S, Satterwhite LE, Ercolani RJ. New locus of drug resistance in the human cytomegalovirus UL56 gene revealed by in vitro exposure to letermovir and ganciclovir. Antimicrob Agents Chemother. 2018;62(9):e00922-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avery RK, Arav-Boger R, Marr KA, et al. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection. Transplantation. 2016;100(10):e74-e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prockop SE, Doubrovina E, Hasan AN, et al. Third party CMV-specific cytotoxic T cells for treatment of antiviral resistant CMV infection after hematopoietic stem cell transplant [abstract] Blood. 2016;128(22). Abstract 61. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.