Abstract
Despite decreasing incidence, gastric cancer remains a major health problem worldwide and is associated with poor survival. The poor survival is mainly attributed to delayed presentation which may cause local or systemic metastases. The standard of care for patients with metastatic gastric cancer (MGC) is palliative chemotherapy with best supportive care. Although the survival has improved owing to advances in chemotherapeutic agents, it is still unsatisfactory, and some perspective changes are needed in the management of MGC to improve the outcomes. Therefore, various alternative treatment strategies for MGC have formed the most important research topics. Liver-directed treatment (LDT) options such as liver resection, radiofrequency ablation (RFA), microwave ablation (MWA), and hepatic artery infusion chemotherapy (HAIC) have been studied in the management of liver metastasis from gastric cancer (LMGC). Intraperitoneal chemotherapy (IPC) in addition to cytoreductive surgery (CRS) aiming to remove all macroscopic tumor focus resulting from peritoneal dissemination is the treatment option for peritoneal metastasis, while para-aortic lymph node dissection is the treatment option for para-aortic lymph node metastasis which is considered to be M1 disease. Conversion surgery is a novel concept aiming at R0 resection for originally unresectable or marginally resectable tumors after a remarkably good response to the chemotherapy. Large amounts of data in the literature have demonstrated the benefits of individualized approaches such as the combination of systemic and local treatment options in selected patient groups. In this review, we aimed to explore the current and future treatment options by reviewing the literature on this controversial topic.
Keywords: Gastric cancer, metastasis, metastasectomy, hyperthermic intraperitoneal chemotherapy, conversion therapy
Gastric cancer remains a major health problem worldwide and has been considered as a dynamic disease that has potential to spread through various routes, such as hematogenous metastasis, lymphatic metastasis, or peritoneal seeding (1-4). Relatively aggressive behavior of gastric cancer may cause metastasis even in the early stages of the disease, and metastases may affect multiple focuses simultaneously (5,6).
A few decades ago, the only treatment option for metastatic gastric cancer (MGC) was best supportive care. With the increasing use of cytotoxic chemotherapy agents in various types of cancer, chemotherapy has also been used for MGC, and various studies have demonstrated the superiority of systemic chemotherapy over best supportive care (7-9). One of the well-known studies was published in 1997 and, for patients randomized to the chemotherapy group, overall survival (OS) tended to be longer compared to best supportive care in advanced gastric cancer (8 vs. 5 months; not statistically significant) (7). In the study, systemic chemotherapy provided longer quality-adjusted survival (median 6 vs. 2 months) and longer time to disease progression (median 6 vs. 2 months). Following the favorable outcomes of initial studies on survival and quality of life, palliative chemotherapy consisting of multi-agent regimens has been introduced as a first- and second-line therapy, as well as a subsequent treatment option, and palliative chemotherapy has become the main strategy in the management of MGC for both western and eastern treatment guidelines (10,11). However, despite the established efficacy of chemotherapeutic agents, prognosis is still poor with median survival being less than 1 year. Therefore, various alternative treatment strategies for MGC have become critical topics for research.
Palliative surgery in a subgroup of MGC patients who have life-threating symptoms such as bleeding and obstruction is a traditional and conventional approach (12). However, the role of reduction surgery is to reduce the tumor volume that has an immunosuppressive effect on patients; eliminating the source of further distant metastases with concern to asymptomatic MGC patients has been a subject of debate. Although published series have shown positive outcomes, study characteristics such as being single-center and retrospective nature inevitably resulted in selection bias (13-18).
A recent study named REGATTA investigated whether the addition of gastrectomy to chemotherapy improves survival for MGC patients with a single non-curable factor (19). Median survival was 16.6 months [95% confidence interval (CI), 13.7–19.8] for the chemotherapy alone group, and 14.3 months (95% CI, 11.8–16.3) for the gastrectomy plus chemotherapy group. In the gastrectomy plus chemotherapy group, Grade 2 surgery-related complications occurred in only 16% (14/87) of patients and no reoperation was required. However, the incidence of adverse events related to chemotherapy was higher in patients assigned to gastrectomy plus chemotherapy. Although the study was terminated early in the first interim analysis, the group of patients included in the study revealed that the addition of reduction gastrectomy to chemotherapy could not offer an advantage over chemotherapy alone.
There are also some points of the study that need to be underlined. Surgical treatment encompassed only gastrectomy with D1 lymph node dissection, while metastatic lesions remained untouched and combined resection of adjacent organs was not allowed. Although stratified randomization eliminated potential systematic bias between groups, a non-curable factor was peritoneal metastasis in the majority of patients (75%). Although subgroup analyses showed no survival difference when evaluated based on a non-curable factor, the low enrollment rate among eligible patients (34%), the early termination of the study, and the low number of patients in the subgroups, make it challenging to evaluate the influence of reduction surgery in the subgroup of patients. Quality of life, which is considered as an essential topic for MGC studies because of the limited lifespan of the patients, was not evaluated in this study. Moreover, as an additional finding, 5 patients from the chemotherapy alone group had the complete disappearance of metastasis, and gastrectomy with curative intent could be performed without any operative mortality.
Although the REGETTA trial demonstrated that reduction gastrectomy with non-curative/non-palliative intent has no role in the management of MGC, in the era of multimodal treatment, we still have no exact evidence to suggest halting surgery in some subgroups, and we also have no evidence to consider the outcomes of palliative chemotherapy as satisfactory (20,21). Investigations into the effects of multimodal treatment protocols and radical surgery including metastasectomy on prognosis for patients with MGC is still ongoing. In this review, we aimed to explore the current and future treatment options by reviewing the literature on this controversial topic.
Liver metastasis
Liver metastasis is the most common pattern of hematogenous metastasis of gastric cancer. According to a recently published study from the Surveillance, Epidemiology, and End Results (SEER) Program database, the ratio of the presence of metastatic disease at the time of diagnosis (synchronous metastasis) was 41% (7,792 of 19,022 patients), and the majority of these were liver metastases (3,218 patients) (22). Among gastric cancer patients with synchronous liver metastasis, 70% (2,247 of 3,218 patients) of patients had liver-only metastasis, while the remaining patients had at least one additional hematogenous metastasis site.
The most common recurrence pattern of patients who had previously been curatively treated for gastric cancer were peritoneal metastasis and metachronous liver metastasis. In a prospectively maintained gastric cancer database from Memorial Sloan-Kettering Cancer Center which reported a 42% recurrence rate, distant sites were involved as a part of the recurrence in 51% of patients (188 patients with 245 specific sites among 367 patients), and the liver was the most common distant metastatic site (90 of 245 specific sites) (23). In a study by Yoo from Korea, the overall recurrence rate was 21% after curative gastrectomy, and liver metastasis was the second most common recurrence pattern (19%, 90 of 508 patients) after peritoneal metastasis (24).
Patients with liver metastases are traditionally treated with palliative chemotherapy. Because liver metastases are frequently encountered, several studies have focused on treating liver metastasis in light of the favorable outcomes of liver-directed treatment (LDT) options in the management of liver metastasis from colorectal and neuroendocrine cancer. Among LDT options, although liver resection is the most well-studied, radiofrequency ablation (RFA), microwave ablation (MWA), hepatic artery infusion chemotherapy (HAIC), transarterial chemoembolization (TACE), and stereotactic body radiotherapy (SBRT) have been used to treat liver metastasis from gastric cancer (LMGC) (25-34).
Irrespective of the LDT option, the metastatic disease should be limited to the liver, as LDT will be a local treatment specific to the liver. Unlike colorectal cancer liver metastasis, the aggressive biology of gastric cancer and the fact that metastases usually contain multiple sites/organs, restrict the number of appropriate patients to be treated with LDT. Because the data on LDT for LMGC are obtained only from the surgically treated patients, it is problematic to report the exact incidence of appropriate patients with LMGC. In Cheon’s study, among 10,259 patients with gastric cancer, 1,013 (9.9%) patients had liver metastasis. Only 41 (4% of patients with liver metastasis) of them underwent curative gastrectomy plus LDT with curative intent (35). LDT incidences in a different series were 0.2–3.3% among patients with a gastric cancer who underwent a gastrectomy, and 2.2–50% among patients with LMGC (Table 1). Because the LDT is seldom indicated for MGC, a high-quality randomized study has yet to be conducted; all that we know comes from the observational studies that have been done over an expansive time period: the data of the 809 patients presented in Table 1 (22 studies) were obtained from a database spanning 309 years (median 14 years) (25,26,28,35-52).
Table 1. Short- and long-term outcomes of the published studies evaluating liver-directed treatment for liver metastasis in gastric cancer.
| Author (reference) | Year | LDT option | Study period (years) | n of all GC/all LM | n of patients undergoing LDT | %LDT in GC†; %LDT in LM‡ | Major complication, n (%) | Mortality, n (%) | Median FU (months with range) | Recurrence, n (%) | Median survival (months) | Survival (%) | 5-year survivor, n (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-year | 3-year | 5-year | |||||||||||||
| Ambiru (36) | 2001 | LR | 24 | NR/NR | 40 | NA; NA | NR | 0 | 88 [4–296] | 31 (78%) | 12 | NR | NR | 18 | 6 (15%) |
| Okano (37) | 2002 | LR | 13 | 807/90 | 19 | 2.4%; 21.1% | NR | 0 | 36 [13–148] | 14 (74%) | NR | 77 | 34 | 34 | 3 (14%) |
| Zacherl (38) | 2002 | LR | 19 | NR/14% | 15 | NA; NA | 4 (27%) | 1 (7%) | NR | NR | 9 | 36 | 14 | 0 | 0 |
| Shirabe (39) | 2003 | LR | 22 | NR/NR | 36 | NA; NA | NR | 0 | NR | 30 (83%) | NR | 64 | 26 | 26 | 4 (11%) |
| Sakamoto (40) | 2003 | LR | 16 | 4,730/228 | 22 | 0.5%; 9.6% | NR | 1 (5%) | 17 [2–71] | 15 (68%) | 21 | 73 | 38 | 38 | 5 (20%) |
| Koga (41) | 2007 | LR | 20 | 5,520/247 | 42 | 0.8%; 17% | NR | 2 | 16 [1–86] | 28 (67%) | 34 | 76 | 48 | 42 | 8 (19%) |
| Thelen (42) | 2008 | LR | 14 | NR/NR | 24 | NA; NA | 5 (20%) | 1 (4%) | 9 [1–67] | 17 (73%) | 9 | 38 | 16 | 10 | 2 (8%) |
| Cheon (35) | 2008 | LR | 10 | 10,259/1,013 | 22 | 0.2%; 2.2% | NR | 1 (5%) | 16 [1–107] | 14 (64%) | 17 | 77 | 30 | 23 | 3 (14%) |
| Morise (43) | 2008 | LR | 15 | NR/NR | 18 | NA; NA | NR | NR | NR | NR | 13 | 56 | 27 | 27 | 3 (17%) |
| Garancini (44) | 2010 | LR | 9 | 984/67 | 21 | 2.1%; 31.3% | 4 (19%) | 0 | 20 [6–90] | 14 (66%) | 11 | 68 | 31 | 19 | 3 (14%) |
| Makino (45) | 2010 | LR | 15 | 1,608/63 | 16 | 1%; 25.4% | NR | NR | 16 [1–127] | 8 (50%) | 31 | 81 | 46 | 37 | 4 (25%) |
| Miki (46) | 2012 | LR | 14 | NA/50 | 25 | NA; 50% | NR | NR | NR | 18 (72%) | 33 | 74 | 43 | 37 | NR |
| Chen (47) | 2013 | LR | 5 | 1,821/114 | 20 | 1.1%; 17.5% | 6 (30%) | 0 | 10 [2–53] | NR | 22 | 70 | 20 | 20 | 3 (15%) |
| Tiberio (48) | 2015 | LR | 14 | NR/212 | 53 | NA; 25% | 9 (17%) | 0 | NR | 49 (92%) | 13 | 50 | 14 | 9 | 3 (5.6%) |
| Guner (25) | 2016 | LR | 15 | NR/NR | 68 | NA; NA | 12 (18%) | 1 (2%) | 24 [4–189] | 41 (60%) | 24 | 79 | 41 | 30 | NR |
| Oki (49) | 2016 | LR | 10 | NR/NR | 69 | NA; NA | NR | NR | 25 [1–142] | NR | 41 | 87 | 51 | 42 | NR |
| Tatsubayashi (50) | 2017 | LR | 10 | NR/NR | 28 | NA; NA | 11 (39%) | 0 | 26 | 17 (61%) | 49 | NR | NR | 32 | NR |
| Ministrini (51) | 2018 | LR | 27 | NR/NR | 144 | NA; NA | 31 (22%) | 3 (2%) | NR | 115 (81%) | 12 | 50 | 19 | 11 | 26 (18%) |
| Chen (52) | 2013 | RFA | 5 | NR/NR | 21 | NA; NA | 1 (5%) | 0 | 19 | 6 (29%) | 14 | 70 | 5 | 3 | 3 (14%)§ |
| Hwang (26) | 2014 | RFA | 9 | 1,342/229 | 44 | 3.3%; 19.2% | 0 | 0 | 32 [11–57] | NR | 15 | NR | NR | NR | NR |
| Guner (25) | 2016 | RFA | 15 | NR/NR | 30 | NA; NA | 3 (10%) | 0 | 24 [4–189] | 17 (57%) | 23 | 73 | 43 | 34 | NR |
| Zhou (28) | 2017 | MWA | 8 | NR/108 | 32 | NA; 29.6% | 0 | 0 | 15 [2–68] | 24 (75%) | 25 | 81 | 31 | 17 | NR |
†, %LDT in GC indicates the percentage of patients who underwent LDT in all patients with gastric cancer; ‡, %LDT in LM indicates the percentage of patients who underwent LDT in all patients with liver metastasis; §, 4-year survivor. LDT, liver-directed treatment; GC, gastric cancer; LM, liver metastasis; LR, liver resection; RFA, radio-frequency ablation; MWA, microwave ablation; FU, follow-up; NA, not available; NR, not reported.
Indications for LDT
The two most crucial points for LDT are proper patient selection and choice of appropriate modality. Although there are some minor variations on indications for LDT, common indications of LDT for LMGC are the following (1,39,53-55):
❖ no extrahepatic hematogenous metastasis;
❖ no peritoneal dissemination;
❖ the possibility of complete eradication of liver metastasis after LDT;
❖ adequate primary tumor control with complete removal of primary gastric tumor and lymph nodes for synchronous metastasis.
In addition to these indications, the main factors affecting the treatment decision are the following: patient-specific features (performance status, age, willingness), metastasis-specific features (location, size, distribution, number, timing), and treatment-specific features (adequate hepatic reserve for liver resection, <5 cm in size for RFA and MWA).
Liver resection as a LDT option
Liver resection has been the most studied treatment modality for LMGC. Two meta-analyses that were published in 2015 concluded that liver resection is associated with increased survival and could be offered to selected patients (56,57). The most recent systematic review and pooled analysis was published in 2016 and a total of 991 patients from 39 studies (30 from the East Asia and 9 from the West) who underwent liver resection for LMGC were included (58). The median number of patients in the studies was 21 (range from 10 to 64), median 30-day morbidity was 24% (range, 0–47%), and the median mortality was 0% (range, 0–30%). The 1-, 3-, and 5-year survival rates were 68%, 31%, and 27%, respectively, with a median survival of 21 months (range, 9–52) for patients undergoing resection of LMGC. However, despite the acceptable and improved outcomes of the studies, the results of pooled analysis should be interpreted carefully because of the limitations stated by the authors. The authors concluded that in selected patients such as the patients with solitary and unilobar liver-only metastasis, liver resection may provide survival improvement, particularly in patients with R0 resection.
Short- and long-term outcomes of the published studies evaluating LDT for the LMGC are presented in Table 1. Short-term outcomes were acceptable and comparable among studies demonstrating major complication rates of 17–27% and operative mortality of 0–7%. Median survival ranged from 9 to 49 months, and the 1-, 3-, and 5-year survival were 36–87%, 14–51%, and 0–42% respectively. Although survival data show improved outcomes, results may also reflect the variation in patient selection criteria among studies. Of further note, recurrence rates are as high as 50–92%, and the recurrence mostly occurs within the liver or systemic metastasis along with the liver metastasis. This finding may support the idea of combined therapy (systemic chemotherapy in addition to the LDT), rather than applying LDT alone in the management of liver-only metastasis.
Thermal ablative therapies as a LDT option
Localized application of thermal energy, including RFA and MWA have been developed for the treatment of primary and metastatic liver tumors (59-61). Although thermal ablative therapies are used in the treatment of liver metastasis from colorectal cancer, the data concerning the efficiency for LMGC are limited. RFA transmits electric currents while MWA transmits microwave energy through the needle, and both induce tumor cell destruction via coagulation necrosis. The features that highlight these techniques among LDT options are their less invasive, safe, and repeatable characteristics. Although RFA is less invasive compared to liver resection, minor complications such as transient fever, bacteremia, liver enzyme elevation and major complications such as abdominal bleeding due to liver mass rupture, liver abscess, and even treatment-related death were reported after RFA (52,62). Compared with RFA, MWA has the advantage of larger ablation volumes, no charring at the ablation site, and lower rates of major complications (63).
In a comparative study, RFA showed satisfactory and comparable short- and long-term outcomes compared to liver resection in patients with LMGC. Median OS after RFA was 23 months, 5-year OS and progression-free survival rates were 34% and 33%, respectively (25). In patients with liver metastasis from colorectal cancer, MWA provided lower ablation-site recurrence rates compared to RFA [20% vs. 6% (63)]. In the largest series of MWA for LMGC (n=32), there was no recurrence at ablation site while 24 patients experienced recurrence at other than ablation site or extrahepatic sites (28). A recent systematic review and pooled analysis of RFA and MWA for LMGC (12 studies including 226 patients) showed that thermal ablative therapies may provide a survival benefit and should be considered an alternative option for the treatment of LMGC (64).
Best candidate for LDT
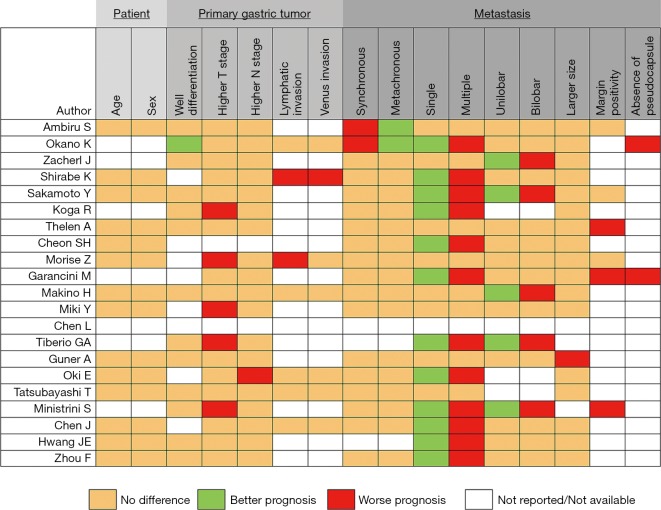
Despite the lack of high-level evidence, some published series have demonstrated that LDT was effective and provided better oncological outcomes compared to systemic chemotherapy in a selected patient group. The key point is to define the selected patient group. Given the variation of treatment protocols among studies, it seems unlikely that a definite judgment regarding proper patient selection can be made. As shown in Figure 1, the presence of a single metastasis is the independent factor for a better prognosis in the majority of the studies and can be used as the main selection criteria in combination with the absence of the extrahepatic metastasis. Moreover, the addition of adjuvant/neoadjuvant chemotherapy to the LDT will likely improve outcomes. Liver resection can be suggested as the main strategy for selected groups of patients; however, when it is not feasible to perform liver resection, the complementary role of the thermal therapies should be remembered.
Figure 1.
Factors affecting prognosis in patients undergoing liver-directed treatment of gastric cancer liver metastasis. A green box indicates a better prognostic factor, while a red box indicates a worse prognostic factor in patients who received liver-directed treatment.
Peritoneal metastasis
Peritoneal metastasis is a common problem in the course of gastric cancer and may be considered a leading factor in the poor outcome of gastric cancer. In Thomassen’s study, 2,029 of 5,220 (39%) newly diagnosed gastric cancer patients presented with metastatic disease (65). Of these, 706 patients had synchronous peritoneal metastasis and 491 of them had peritoneal metastasis only, while the remaining 215 patients had another metastasis besides peritoneal metastasis. In a metachronous manner, peritoneal metastasis is a common site of treatment failure for gastric cancer after curative treatment, and 10–46% of gastric cancer patients develop peritoneal recurrence after the surgery, accounting for 36–45% of all recurrences (24,65,66). Both the presence of peritoneal metastasis and the detrimental complications resulting from peritoneal metastasis including malignant ascites, malign bowel obstruction, and nutritional impairment, caused both poor survival and unfavorable quality of life (67). The median 6-month and 5-year survival probability of 0% in published reports reflects a rather poor prognosis for peritoneal metastasis from gastric cancer (PMGC) (68,69).
A critical initiation factor in the formation of peritoneal metastasis, whether it is trans-mesothelial or trans-lymphatic, is the spreading and persistence of the intraperitoneal free cancer cells (IFCCs) into the peritoneal cavity. The peritoneal-plasma barrier converts the peritoneal cavity into a closed environment far from blood flow, making it difficult for systemic chemotherapeutic agents to reach the IFCCs in the peritoneal cavity (70-72). Efforts to treat the peritoneal cavity locally, which is difficult to reach systemically, have given rise to treatment options for peritoneal metastasis (71,73). These options are cytoreductive surgery (CRS) which aims to remove all macroscopic tumor focus and intraperitoneal chemotherapy (IPC) and provides high peritoneal concentration to clear tumor cells which may persist after a curative resection in the peritoneal cavity. During IPC, through the use of hyperthermic chemotherapy, several benefits such as direct cytotoxicity, increased drug penetration, increased drug uptake, and increased chemosensibility of tumor cells are intended (68). Hyperthermic IPC’s acronym, HIPEC, is now used as the standard nomenclature for this technique (74).
IPC with or without CRS may be chosen for four purposes:
❖ Prophylactic IPC: to prevent peritoneal metastasis that may develop in high-risk patients after curative surgery;
❖ Therapeutic IPC: to treat peritoneal metastasis in patients with known peritoneal metastases including patients with positive peritoneal cytology;
❖ Palliative IPC: to control symptoms in patients with malignant ascites which result from peritoneal metastasis;
❖ Neoadjuvant IPC: as a part of neoadjuvant therapy in patients with peritoneal metastasis.
Prophylactic IPC
Some patients have an increased risk of peritoneal metastasis. Although many risk factors have been defined for peritoneal metastasis, the most important are the depth of gastric tumor, lymph node status, and some histological features (75,76). In the multivariate logistic regression analysis of Roviello’s study, relative risk (RR) was 4.31 for diffuse-mixed type histology, RR for serosal involvement was 3.36, and RR for lymph node positivity was 2.67 (76). Also, 69% of the 5-year cumulative risk was observed in patients with both diffuse-mixed type histology and serosal involvement, while only 4% of the cumulative risk was noted in cases with an absence of both features.
Prophylactic IPCs were introduced in the 1980s with studies demonstrating that curative surgery plus HIPEC improves survival and decreases peritoneal recurrence rates in high risk patients (77-80). With the favorable outcomes of early reports, several randomized controlled studies (RCTs) comparing curative surgery versus curative surgery plus IPC were conducted (68,81-83). Both HIPEC and Early Postoperative Intraperitoneal Chemotherapy (EPIC) were used in the experimental arms of these studies (81). In a meta-analysis of studies using HIPEC (16 RCTs including 1,906 patients), surgery plus HIPEC was associated with a significant improvement in survival rate at 1-year [hazard ratio (HR): 2.99; 95% CI, 2.21–4.05], 3-year (HR: 2.63; 95% CI, 2.17–3.20), and 5-year (HR: 2.49; 95% CI, 1.97–3.14) compared with surgery alone. Surgery plus HIPEC was also associated with a significant reduction in recurrence rate at 2 years (RR: 0.42; 95% CI, 0.29–0.61) and 5 years (RR: 0.47; 95% CI, 0.39–0.56). There was no difference between groups in terms of major surgery-related complications, and the authors concluded that HIPEC improves survival rates and reduces the recurrence rates, with an acceptable safety profile (84). In another meta-analysis that includes various types of IPC, an improved 1-, 2- and 3-year OS and positive effect on peritoneal recurrence was reported, with an increased morbidity and no survival difference at 5 years (81).
Therapeutic IPC
The local treatment approach is seen as a rational approach in the case of PMGC in which the systemic chemotherapy has limited effect due to factors such as the peritoneal-plasma barrier. Yonemura performed radical surgery (resection of primary tumor, lymph nodes, and peritoneal metastases) and subsequent HIPEC in 83 patients with peritoneal metastasis, and reported a 43% 1-year OS and an 11% 5-year OS, with 5 patients surviving more than 5 years (85). Although these outcomes seem to be poor, they were the first improved outcomes reported in patients with PMGC. Moreover, achieving complete response in patients with residual peritoneal metastasis despite radical surgery and better prognosis in patients who have complete resection or complete response, has opened a new awareness for using CRS plus HIPEC in selected PMGC patients.
One of the early studies compared 18 patients who underwent CRS versus 48 patients who underwent CRS plus HIPEC (86). For HIPEC, solution containing mitomycin-C was circulated for 2 hours in the peritoneal cavity. Abdominal effusion disappeared after treatment in all patients in the HIPEC group, while this occurred in only 5% (1/18) of patients in the CRS only group. The 5- and 8-year survival rates were 31% and 25% in the HIPEC group respectively, while the maximal surviving time in the CRS only group was just 17 months. Moreover, improvement was more noticeable in patients with limited peritoneal disease, and the authors concluded that CRS plus HIPEC may offer hope for patients with PMGC.
The first randomized phase III study was published in 2011 in China (87). Sixty-eight patients with PMGC were randomized into CRS alone and the CRS plus HIPEC groups (34 patients in each group). The HIPEC group received mitomycin-C and cisplatin at 43 °C for 60–90 minutes. Median survival was significantly longer in the HIPEC group (11 months; 95% CI, 10–11.9) compared to the CRS only group (6.5 months; 95% CI, 4.8–8.2). Serious complications including sepsis, respiratory problems, gastrointestinal bleeding, and intestinal obstruction were comparable (14.7% in the CRS plus HIPEC group versus 11.7% in the CRS only group). The authors further explored the influences of the timing of metastasis, completeness of cytoreduction (CC), and peritoneal carcinomatosis index (PCI). CRS plus HIPEC, synchronous peritoneal metastasis, CC 0–1 (no or less than 2.5 mm peritoneal residual nodule), and receiving systemic chemotherapy over 6 cycles were identified as major independent predictors for better survival.
In a European multicenter study, HIPEC was used intraoperatively and/or EPIC was used for 5 days postoperatively; the median survival was 9.2 months and the 5-year survival was 13% (88). The most important predictor of survival was the CC, and median survival in patients in whom complete cytoreduction was achieved was 15 months with a 23% 5-year survival rate. There were some important points in the study indicating the risks of postoperative adverse events and the importance of the experience and learning curve. Operative mortality rate was 6.5%, major complication rate was 27.8%, reoperation rate was 14%, intestinal fistula rate was 15.9%, and mean length of hospital stay was 24. A critical relationship was observed between the experience of the center and morbidity/mortality/survival.
The most recent meta-analysis, which included 11 randomized and 21 non-randomized comparative studies, demonstrated median survival benefit in favor of the CRS plus HIPEC group (11.1 months) versus the control group (7.06 months) (weighted mean difference 4.04 months, 95% CI, 2.40–5.67) (67). However, in the HIPEC group, consistent with RCTs and NRCTs, there was a significantly higher risk of postoperative complications (RR: 2.15; 95% CI, 1.29–3.58), particularly respiratory and renal failure.
Short- and long-term outcomes of some published studies evaluating therapeutic IPC in patients with PMGC are presented in Table 2 (87-98). As experienced in the LMGC, patient selection is the key for the potential benefit of therapeutic IPC. Achieving no/minimal residual disease is important for success. If maximum cytoreduction can be achieved with acceptable morbidity, it seems sensible to expect a survival benefit and better quality of life.
Table 2. Short- and long-term outcomes of some published studies evaluating therapeutic IPC in GC patients with peritoneal metastasis.
| Author (reference) | Year | Type of study | Selection Criteria | n of patients in study group | n of patients in control group | Drug | Completeness of cytoreduction, n (%) | Morbidity, n (%) | Mortality, n (%) | Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Shimada (89) | 2002 | Retrospective | Cy (+) | 7 EIPL+ IPC+ CRS and 7 IPC+ CRS | 8 Gastrectomy | NR | NR | NR | NR | 2-y: EIPL + IPC: 57.1%; IPC: 14.3%; gastrectomy: 0% |
| Hall (90) | 2004 | Retrospective | Cy (+) | 34 HIPEC + CRS | 40 gastrectomy | MMC | HIPEC: 7 (21%); gastrectomy: 20 (58%) | HIPEC: 12 (35%); gastrectomy: 7 (17.5%) |
HIPEC: 1 (2.9%); gastrectomy: 6 (15%) | Median survival for R0: HIPEC: 36.3 months; gastrectomy: 23.3 months |
| Glehen (91) | 2004 | Prospective | PC | 49 HIPEC + CRS | – | MMC | CC 0: 5; CC 1: 20; CC 2: 24 | 13 (27%) | 2 (4%) | Median: CCR 0-1: 21.3 months; CCR 2: 6.1 months |
| Kuramoto (92) | 2009 | RCT | Cy (+) | 30 EIPL + IPC + CRS and 29 IPC + CRS | 29 Gastrectomy | NR | NR | NR | NR | 5-y: EIPL + IPC: 43.8%; IPC: 4.6%; gastrectomy: 0% |
| Glehen (88) | 2010 | Retrospective | PC | 159 HIPEC and/or EPIC | – | HIPEC: MMC/Oxaliplatin; EPIC: MMC+5-FU |
CC 0: 89 (56.0%); CC 1: 40 (25.2%); CC 2: 30 (18.8%) |
38 (27.8%) | 10 (6.5%) | Median: 9.2 months; 1-y: 43%; 3-y: 18%; 5-y: 13% |
| Yang (93) | 2010 | Retrospective | PC and/or Ascites | 32 HIPEC + CRS | – | MMC | CC 0: 11 (39.2%); CC 1: 6 (21.4%); CC 2: 8 (28.8%); CC 3: 3 (10.6%) |
4 (14.3%) | 0% | 6-month: 75%; 12-month: 50%; 18-month: 43%; 24-month: 43% |
| Yang (87) | 2011 | RCT | PC | 34 HIPEC + CRS | 34 CRS | MMC and Cisplatin | HIPEC+CRS: 20 (58.8%); CRS: 20 (58.8%) |
HIPEC + CRS: 5 (14.7%); CRS: 4 (11.7%) |
NR | Median: HIPEC + CRS: 11 months; CRS: 6.5 months |
| Rudloff (94) | 2014 | RCT | PC | 9 HIPEC + CRS + systemic CT | 7 systemic CT | Oxaliplatin | CC 0-1: 8 (88.8%) | HIPEC+ CRS+ systemic CT: 8 (88.8%) | HIPEC+ CRS+ systemic CT: 3 (33.3%) | Median: HIPEC+ CRS+ systemic CT: 11.3 months; systemic CT: 4.3 months |
| Magge (95) | 2014 | Prospective | PC | 23 HIPEC + CRS | – | MMC | CC 0: 17 (73.9%); CC 1: 5 (21.7%) |
12 (52.2%) | 1 (4.4%) | Median: 9.5 months; 1-y: 49.6%; 5-y: 17.9% |
| Topal (96) | 2014 | Prospective | PC | 32 HIPEC + CRS | – | Cisplatin | CC 0: 100% | 23 (72%) | 0% | 1-y: 90%; 2-y: 55%; 5-y: 5.6% |
| Rihuete (97) | 2018 | Prospective | PC | 35 HIPEC + CRS | – | Cisplatin+ Doxorubicin | NR | 9 (25.7%) | 2 (5.7%) | Median: 16 months; 1-y: 70.8%; 3-y: 21.3%; 5-y: 21.3% |
| Kim (98) | 2018 | Prospective | PC | 38 HIPEC + CRS | – | MMC+ Cisplatin | 21 (55.2%) | 16 (42.1%) | 2 (5.7%) | Median: 19 months |
Cy, Cytology; EIPL, extensive intraoperative peritoneal lavage; IPC, intraperitoneal chemotherapy; CRS, cytoreductive surgery; MMC, mitomycin C; 5-FU, 5-Fluorouracil; PC, peritoneal carcinomatosis; HIPEC, hyperthermic intraperitoneal chemotherapy; CC, completeness of cytoreduction; EPIC, early postoperative intraperitoneal chemotherapy; CT, chemotherapy; CR, curative resection; RCT, randomized controlled trial; NR, not reported.
Palliative IPC
Malignant ascites in PMGC patients leads to a very poor quality of life and negatively impacts the remaining days of the patient. Although drainage techniques or some medications such as diuretics may help with symptomatic relief, this is mostly temporary. HIPEC with or without CRS has also been used in the palliation of the ascites for patients with PMGC (99-100). Complete disappearance of ascites and its related symptoms were reported in the majority of the published studies. Although a randomized study comparing intraperitoneal plus intravenous treatment versus intravenous chemotherapy failed to show any statistical superiority of intraperitoneal treatment, and subgroup analyses suggested a survival benefit for patients with a moderate amount of ascites (101). Recently, laparoscopic HIPEC has also been used to decrease surgical trauma, and ascites was controlled in the majority of patients without any major complications (102).
Novel approaches
In addition to the systemic chemotherapy, intraperitoneal administration of chemotherapy drugs in the preoperative period is an option for PMGC patients aiming to increase complete cytoreduction rates and to control peritoneal dissemination before CRS. This bidirectional approach is called neoadjuvant intraperitoneal-systemic chemotherapy (NIPS) (103). Yonemura used oral TS-1 and intraperitoneal (through the peritoneal port system) docetaxel and cisplatin in patients with primary or recurrent PMGC as NIPS (103,104). Overall, 41 of 79 patients receiving NIPS (52%) underwent surgery and complete cytoreduction was achieved in 32 of them (78% of patients underwent surgery and 41% of all NIPS patients). Positive cytology turned negative in 63% (41 of 65 patients with positive cytology) after NIPS. The patients receiving CRS survived significantly longer than those of the no operation group, while patients receiving CC-0 survived significantly longer than those of the CC-1 group, and patients with negative cytology after NIPS survived significantly longer than those with positive cytology. This study demonstrated the possibility of monitoring peritoneal cytology via peritoneal port catheter as well as the safety of the NIPS approach and also indicated in which patients an aggressive approach could be beneficial.
For patients who are not eligible for CRS plus HIPEC, pressurized intraperitoneal aerosol chemotherapy (PIPAC) is a relatively novel approach moving chemotherapeutic agents into the peritoneal cavity as a pressurized normothermic aerosol (105). A study from Germany, which consists of 46 PIPAC procedures in 24 patients, demonstrated that PIPAC is a safe and well-tolerated technique, and the ascites production can be controlled by PIPAC in patients with PMGC. Also, patients who received one or two PIPAC sessions had 121 days of median survival, and patients who received more than two sessions had 450 days of median survival (106). Given its safety and the efficacy in the control of ascites production, PIPAC is now considered a promising option for both palliative and neoadjuvant intent before CRS. Future trials are needed for its usage as a rescue or supportive therapy.
The concept of IPC is still evolving (107). Understanding the molecular mechanisms in gastric cancer, developing new drugs that are more efficient in systemic and IPC as well as the targeted immunotherapy agents, and achieving acceptable perioperative morbidity, will possibly lead to better survival outcomes.
Para-aortic lymph node (PALN) metastasis
It has been the subject of debate as to whether PALN metastasis should be accepted as local lymph node or distant metastasis in the management of gastric cancer. Studies investigating curative gastrectomy including the PALN dissection showed that 8–28% of patients had PALN metastasis (108). In Baba’s study, which evaluated proximal gastric tumors, the PALN metastasis rate was 28%, and interestingly, 24% of these patients did not have any metastasis in the celiac region (109). Likewise, in a study by Roviello, the PALN metastasis rate was 7% in patients with distal tumors, while it was 27% in patients with proximal tumors (110).
In the well-known phase III study on prophylactic PALN dissection, patients were randomized into either the D2 dissection or D2 plus PALN dissection group (108). Patients who had gastric adenocarcinoma that was considered potentially curable were enrolled, while patients with clinically PALN metastasis were excluded from the study. The primary end point was OS. While there were more minor perioperative complications in the PALN dissection group, there was no difference between major surgery-related complications between groups. The incidence for anastomotic leakage was 1.9%, 6.2% for pancreatic fistula, 5.8% for abdominal abscess, and 1.5% for pneumonia, while the overall incidence of surgery-related complications was 28.1% in the PALN dissection group. The 5-year OS rate for 22 of 260 patients (8.5%) who had histologically detected metastases in the para-aortic lymph nodes after undergoing D2 dissection plus PAND was 18.2% (95% CI, 5.7–36.3). No difference was observed in terms of OS or recurrence-free survival. In a comparative study of Yonemura et al., 269 patients were randomized into D2 dissection versus D2 plus PALN dissection (defined as D4 dissection) groups (111). Only patients having potentially curable gastric cancer were included and the enlargement of PALN on computed-tomography images was considered an exclusion criterion. PALN metastasis was detected in 9% of patients, and there was no difference in 5-year survival (52.6% after D2 surgery and 55% after D4 gastrectomy).
These randomized studies that include patients with no lymphadenopathy in the para-aortic region showed that systematic and prophylactic PALN dissection do not provide survival benefit, and PALN involvement was defined as metastatic disease in both NCCN and Japanese guidelines. It should be remembered that the patients in these trials had no known PALN involvement, did not receive chemotherapy in the preoperative or postoperative period, and were treated only with surgery. Therefore, further studies are needed to answer these two questions: can PALN dissection improve the survival outcomes in patients with known PALN metastasis? And, can a multimodal approach such as surgery combined with perioperative chemotherapy contribute to survival?
Overall, 178 patients with positive PALN confirmed by pathological examination and who underwent curative resection were evaluated in Tokunaga’s study (112). The 3- and 5-year survival rates were 20.9% and 13%, respectively. Subgroup analysis showed that the 5-year survival was significantly higher in patients with 15 positive nodes compared to >15 positive nodes (28.9% vs. 6.4%), and in patients with a macroscopic type other than 4 compared to type 4 (17% vs. 3.8%). The authors concluded that curative resection including PALN retrieval might be beneficial in selected group of patients with pathologically positive PALN. In parallel time frames, many other studies evaluating PALN dissection in patients with pathological PALN metastasis provided similar 5-year survival rates, specifically 18.5% in Sasako’s study, 25% in Yonemura’s study, 17% in Roviello’s study, and 22% in Fujimura’s study (108,110,111,113).
To find the answer for the second question, preoperative chemotherapy was investigated with the combination of curative surgery including PALN dissection. Preoperative chemotherapy provided a high clinical and pathological response rate of 68.8% and 87.5% respectively (114). Recurrence rates and 2-year survival rates were 85.7% and 32.9% in patients who did not receive pre-operative chemotherapy, and 31.2% and 93.8% in patients who did receive them, respectively. In a study of Tsubaraya, evaluating the influence of preoperative chemotherapy followed by curative gastrectomy with PALN dissection demonstrated a 57% 5-year survival rate for patients with PALN metastasis without bulky N2 lymph nodes and a 17% rate in those patients with both bulky N2 lymph nodes and PALN metastasis (115). A series of Japanese studies tested the preoperative chemotherapy for patients with resectable PALN metastasis, and found that neoadjuvant chemotherapy followed by gastrectomy with D2 plus PALN dissection was a promising option (114-119).
Although more data are needed to clarify the indications, proper patient selection, the best drug combination, and duration of chemotherapy, preoperative chemotherapy followed by PALN dissection performed by experienced surgeons in dedicated centers is considered a therapeutic option for patients with PALN metastasis.
Metastasis to other organs
We have limited data on distant metastasis to organs other than the liver, peritoneum, and PALNs. The largest series in this regard are synchronous or metachronous ovarian metastasis (Krukenberg tumors of gastric origin). Among 1,235 female patients who had curative gastrectomy for gastric cancer, 54 (4.4%) patients developed ovarian-only metastasis as a first recurrence (120). Some patients were treated with metastasectomy and systemic chemotherapy while others received only chemotherapy or supportive care. The median survival time in the metastasectomy group was significantly longer than that in the patients without metastasectomy (17 vs. 3 months). Another series comparing metastasectomy combined with chemotherapy vs. chemotherapy alone in patients with ovarian metastasis showed an association with survival benefit (121-123). In a multicenter western experience, among 2,515 female patients diagnosed with gastric cancer, 63 (2.5%) presented with ovarian metastasis (30 synchronous and 33 metachronous metastasis as recurrence) (124). Patients were treated with surgery (salpingo-oophorectomy, total abdominal hysterectomy ± peritonectomy), HIPEC, adjuvant, or palliative chemotherapy. Patients with metachronous metastasis demonstrated significantly longer median survival time compared to synchronous metastasis (36 vs. 17 months respectively). In addition to the timing of metastasis, the extent of resection and the use of adjuvant therapies have been found to be independent factors for survival. Patients with R0 resection had 34 months of median survival while patients with R1/R2 resection had 11 months of survival. Median survival for patients who underwent HIPEC plus systemic chemotherapy, surgery plus systemic chemotherapy, and palliative chemotherapy alone was 33, 20, and 10 months, respectively. Although studies attempted an explanation in favor of metastasectomy plus systemic chemotherapy, it should be kept in mind that all published studies are retrospective series that likely have severe selection bias. In order to benefit from the possible treatment strategy, a satisfying preoperative evaluation should be accomplished, and the extent of the disease should be determined properly.
Although pulmonary metastasectomy has been realized as a part of a multimodal treatment for a number of solid tumors, metastasectomy for gastric cancer lung metastasis has been reported only in a few case reports or small case series thus far (125,126). A recent systematic review evaluated 10 studies including a total of 44 patients (127). Median overall and disease-free survival were reported as 45 and 9 months in patients with lung metastasis from gastric cancer, respectively. However, no factor was found to be related to survival, and the authors concluded that metastasectomy has no role in the standard management of gastric cancer and should be suggested only in highly selected cases.
In a SEER database study, 5.1% of patients had bone metastasis and 0.8% had brain metastasis (22). Cause-specific survival was reported as 1.3% vs. 29.9% respectively for patients with and without bone metastasis (median 4 months for bone metastasis), while it was 2.3% vs. 28.7% respectively for patients with and without brain metastasis (median 3 months for brain metastasis). Although there is no role for any type of treatment modalities except systemic chemotherapy, some case reports showed benefit from metastasectomy for bone metastasis and from anti-PD1 therapy, stereotactic radiosurgery, and neoadjuvant chemotherapy followed by surgery for brain metastasis (128-130). However, because it is quite rare for gastric cancer to present as isolated bone or brain metastasis, the data are limited, and no treatment options can be suggested with the current knowledge.
Conversion therapy as a novel concept for MGC
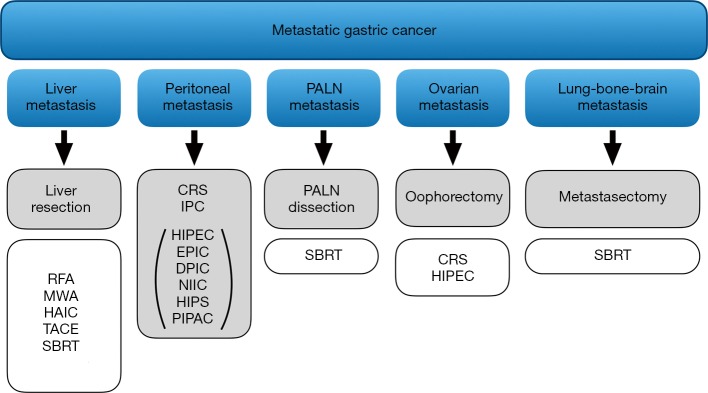
Possible treatment modalities for stage IV gastric cancer are summarized in Figure 2. However, when discussing the treatment options for MGC, room must be made for conversion therapy as well. Increased knowledge on the treatment outcomes for MGC has revealed a novel concept, called conversion surgery or conversion therapy, which has become one of the most commonly studied therapeutic options in the literature for stage IV disease (131,132). Conversion surgery is defined as surgical treatment aiming at R0 resection for originally unresectable or marginally resectable tumors after remarkably good response to the chemotherapy.
Figure 2.
Possible treatment options in patients with metastatic gastric cancer. Modalities that are shown with a gray box indicate that these options are defined as a part of conversion surgery. RFA, radiofrequency ablation; MWA, microwave ablation; HAIC, hepatic artery infusion chemotherapy; TACE, transarterial chemoembolization; SBRT, stereotactic body radiotherapy; CRS, cytoreductive surgery; IPC, intraperitoneal chemotherapy; HIPEC, hyperthermic intraperitoneal chemotherapy; EPIC, early postoperative intraperitoneal chemotherapy; DPIC, delayed postoperative intraperitoneal chemotherapy; NIIC, normothermic intraoperative intraperitoneal chemotherapy; NIPS, neoadjuvant intraperitoneal and systemic chemotherapy; PIPAC, pressurized intraperitoneal aerosol chemotherapy; PALN, para-aortic lymph nodes.
Yoshida described a new biological classification of MGC (131). First, patients were divided into two based on the absence or presence of macroscopic peritoneal dissemination. Patients without macroscopic peritoneal dissemination were further divided into two categories as potentially resectable metastasis (category 1) and marginally resectable metastasis (category 2). Category 1 included patients with a single liver metastasis, patients with positive cytology, or patients with a few metastases to the PALNs (limited to 16a2 and/or 16b1), and patients in this category were treated with surgery (gastrectomy plus metastasectomy) either before or after chemotherapy. For patients in category 2, metastases were considered as technically or oncologically unresectable; therefore, treatment for the patients in category 2 was started with induction chemotherapy; then, if R0 resection was possible, gastrectomy plus metastasectomy was considered based on the treatment response. Category 3 and 4 included patients with macroscopic peritoneal dissemination, and surgery was rarely used in these categories.
In a retrospective series of Yamaguchi and Yoshida, 259 patients were classified into four categories and treated with systemic chemotherapy, among whom 84 patients were further treated with subsequent surgery (133). Seven patients were treated with neoadjuvant intent and the remaining 77 patients were considered for conversion surgery. The overall response rate of the patients was 36.3%, and the disease control rate was 86.1%. For patients who underwent surgery, rates of complete response, partial response, stable disease and progressive disease were 3.6%, 54.7%, 39.3%, and 2.4%, respectively (overall response rate 58.3% and disease control rate 97.6%). The median survival times for the patients who underwent surgery and for the patients who received chemotherapy alone were 31 and 11.3 months, respectively. The median survival times of the patients in category 1, 2, 3, and 4 were 26.3, 14.8, 22.0, and 12.9 months, respectively. The R0 resection rate was 85.7% in category 1, 52.4% in category 2, 50.0% in category 3, and 36.8% in category 4.
In a Korean study evaluating the conversion surgery, 101 patients with MGC who were treated with systemic chemotherapy followed by curative intent gastrectomy were analyzed (134). Complete or partial response was observed in 65 (64.4%) patients, and 11 (10.9%) patients received metastasectomy (3 hepatectomy, 6 para-aortic LN dissection, and 3 oophorectomy). Complete macroscopic resection was achieved in 57 (56.4%) patients. The median survival was 26 months, and the best survival was achieved in patients with liver metastasis. An experience from the western centers was also published recently. Eleven of 54 clinically MGC underwent R0 resection (135). The authors concluded that conversion gastrectomy may provide better survival when R0 is achieved in patients with advanced gastric cancer.
Conversion surgery, as a new concept, requires clinical evaluation by prospective studies. The results of a large cohort which is being conducted by several Korean, Japanese, and Chinese collaborative groups will determine the future role of conversion therapy in the management of MGC (131).
Conclusions
MGC is frequently encountered problem due to the aggressive biology of gastric cancer. The standard treatment for MGC is systemic chemotherapy. However, MGC is not a single entity and some perspective changes are needed in the management of gastric cancer to improve the outcomes. Studies, particularly those regarding the liver, peritoneum, and para-aortic lymph node metastasis have shown the benefits of an individualized approach in selected patient groups. Therefore, in the light of the current literature, all patients with MGC should not be considered as hopeless, and, as part of a multidisciplinary approach in experienced centers, possible combined systemic and local treatment options for such patients should be considered on an individual basis.
Acknowledgments
None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kakeji Y, Morita M, Maehara Y. Strategies for treating liver metastasis from gastric cancer. Surg Today 2010;40:287-94. 10.1007/s00595-009-4152-0 [DOI] [PubMed] [Google Scholar]
- 2.Choi YY, An JY, Guner A, et al. Skip lymph node metastasis in gastric cancer: is it skipping or skipped? Gastric Cancer 2016;19:206-15. 10.1007/s10120-015-0472-5 [DOI] [PubMed] [Google Scholar]
- 3.Zhu T, Hu X, Wei P, et al. Molecular background of the regional lymph node metastasis of gastric cancer. Oncol Lett 2018;15:3409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Ng JM, Wong CC, et al. Molecular alterations of cancer cell and tumour microenvironment in metastatic gastric cancer. Oncogene 2018;37:4903-20. 10.1038/s41388-018-0341-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kodera Y. Surgery with curative intent for stage IV gastric cancer: Is it a reality of illusion? Ann Gastroenterol Surg 2018;2:339-47. 10.1002/ags3.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guner A. Recent trends of gastric cancer treatment in Turkey. Transl Gastroenterol Hepatol 2017;2:31. 10.21037/tgh.2017.04.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glimelius B, Ekstrom K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997;8:163-8. 10.1023/A:1008243606668 [DOI] [PubMed] [Google Scholar]
- 8.Pyrhönen S, Kuitunen T, Nyandoto P, et al. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587-91. 10.1038/bjc.1995.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 1993;72:37-41. [DOI] [PubMed] [Google Scholar]
- 10.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. 10.1007/s10120-016-0622-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. 10.6004/jnccn.2016.0137 [DOI] [PubMed] [Google Scholar]
- 12.Keränen I, Kylanpaa L, Udd M, et al. Gastric outlet obstruction in gastric cancer: a comparison of three palliative methods. J Surg Oncol 2013;108:537-41. 10.1002/jso.23442 [DOI] [PubMed] [Google Scholar]
- 13.Müsri FY, Mutlu H, Karaagac M, et al. Primary Tumor Resection and Survival in Patients with Stage IV Gastric Cancer. J Gastric Cancer 2016;16:78-84. 10.5230/jgc.2016.16.2.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He MM, Zhang DS, Wang F, et al. The role of non-curative surgery in incurable, asymptomatic advanced gastric cancer. PLoS One 2013;8:e83921. 10.1371/journal.pone.0083921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto Y, Yoshikawa T, Morinaga S, et al. Significance of volume-reduction surgery for far-advanced gastric cancer during treatment with novel anticancer agents. Int J Clin Oncol 2009;14:225-9. 10.1007/s10147-008-0841-8 [DOI] [PubMed] [Google Scholar]
- 16.Sun J, Song Y, Wang Z, et al. Clinical significance of palliative gastrectomy on the survival of patients with incurable advanced gastric cancer: a systematic review and meta-analysis. BMC Cancer 2013;13:577. 10.1186/1471-2407-13-577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tiberio GA, Ministrini S, Gardini A, et al. Factors influencing survival after hepatectomy for metastases from gastric cancer. Eur J Surg Oncol 2016;42:1229-35. 10.1016/j.ejso.2016.03.030 [DOI] [PubMed] [Google Scholar]
- 18.Lasithiotakis K, Antoniou SA, Antoniou GA, et al. Gastrectomy for stage IV gastric cancer. a systematic review and meta-analysis. Anticancer Res 2014;34:2079-85. [PubMed] [Google Scholar]
- 19.Fujitani K, Yang H-K, Mizusawa J, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 2016;17:309-18. 10.1016/S1470-2045(15)00553-7 [DOI] [PubMed] [Google Scholar]
- 20.Zurleni T, Gjoni E, Altomare M, et al. Conversion surgery for gastric cancer patients: A review. World J Gastrointest Oncol 2018;10:398-409. 10.4251/wjgo.v10.i11.398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D'Ugo D, Cananzi FCM, Persiani R, et al. REGATTA trial: a call for the USA and Europe. Lancet Oncol 2016;17:261-2. 10.1016/S1470-2045(15)00619-1 [DOI] [PubMed] [Google Scholar]
- 22.Qiu MZ, Shi SM, Chen ZH, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: A SEER-based study. Cancer Med 2018;7:3662-72. 10.1002/cam4.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004;240:808-16. 10.1097/01.sla.0000143245.28656.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoo CH, Noh SH, Shin DW, et al. Recurrence following curative resection for gastric carcinoma. Br J Surg 2000;87:236-42. 10.1046/j.1365-2168.2000.01360.x [DOI] [PubMed] [Google Scholar]
- 25.Guner A, Son T, Cho I, et al. Liver-directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer 2016;19:951-60. 10.1007/s10120-015-0522-z [DOI] [PubMed] [Google Scholar]
- 26.Hwang JE, Kim SH, Jin J, et al. Combination of percutaneous radiofrequency ablation and systemic chemotherapy are effective treatment modalities for metachronous liver metastases from gastric cancer. Clin Exp Metastasis 2014;31:25-32. 10.1007/s10585-013-9606-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JW, Choi MH, Lee YJ, et al. Radiofrequency ablation for liver metastases in patients with gastric cancer as an alternative to hepatic resection. BMC Cancer 2017;17:185. 10.1186/s12885-017-3156-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Yu XL, Liang P, et al. Microwave ablation is effective against liver metastases from gastric adenocarcinoma. Int J Hyperthermia 2017;33:830-5. [DOI] [PubMed] [Google Scholar]
- 29.Martella L, Bertozzi S, Londero AP, et al. Surgery for Liver Metastases From Gastric Cancer: A Meta-Analysis of Observational Studies. Medicine (Baltimore) 2015;94:e1113. 10.1097/MD.0000000000001113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu SF, Lu CR, Cheng HD, et al. Comparison of Therapeutic Efficacy between Gastrectomy with Transarterial Chemoembolization Plus Systemic Chemotherapy and Systemic Chemotherapy Alone in Gastric Cancer with Synchronous Liver Metastasis. Chin Med J (Engl) 2015;128:2194-201. 10.4103/0366-6999.162497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukami Y, Kaneoka Y, Maeda A, et al. Adjuvant hepatic artery infusion chemotherapy after hemihepatectomy for gastric cancer liver metastases. Int J Surg 2017;46:79-84. 10.1016/j.ijsu.2017.08.578 [DOI] [PubMed] [Google Scholar]
- 32.Yamakado K, Nakatsuka A, Takaki H, et al. Prospective study of arterial infusion chemotherapy followed by radiofrequency ablation for the treatment of liver metastasis of gastric cancer. J Vasc Interv Radiol 2005;16:1747-51. 10.1097/01.RVI.0000188738.84911.3B [DOI] [PubMed] [Google Scholar]
- 33.Lewis GD, Chiang SB, Butler EB, et al. The utility of positron emission tomography/computed tomography in target delineation for stereotactic body radiotherapy for liver metastasis from primary gastric cancer: an illustrative case report and literature review. J Gastrointest Oncol 2017;8:E39-42. 10.21037/jgo.2017.01.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 2010;78:486-93. 10.1016/j.ijrobp.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 35.Cheon SH, Rha SY, Jeung HC, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol 2008;19:1146-53. 10.1093/annonc/mdn026 [DOI] [PubMed] [Google Scholar]
- 36.Ambiru S, Miyazaki M, Ito H, et al. Benefits and limits of hepatic resection for gastric metastases. Am J Surg 2001;181:279-83. 10.1016/S0002-9610(01)00567-0 [DOI] [PubMed] [Google Scholar]
- 37.Okano K, Maeba T, Ishimura K, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg 2002;235:86-91. 10.1097/00000658-200201000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zacherl J, Zacherl M, Scheuba C, et al. Analysis of hepatic resection of metastasis originating from gastric adenocarcinoma. J Gastrointest Surg 2002;6:682-9. 10.1016/S1091-255X(01)00075-0 [DOI] [PubMed] [Google Scholar]
- 39.Shirabe K, Wakiyama S, Gion T, et al. Hepatic resection for the treatment of liver metastases in gastric carcinoma: review of the literature. HPB (Oxford) 2006;8:89-92. 10.1080/13651820500472168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakamoto Y, Ohyama S, Yamamoto J, et al. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery 2003;133:507-11. 10.1067/msy.2003.147 [DOI] [PubMed] [Google Scholar]
- 41.Koga R, Yamamoto J, Ohyama S, et al. Liver resection for metastatic gastric cancer: experience with 42 patients including eight long-term survivors. Jpn J Clin Oncol 2007;37:836-42. 10.1093/jjco/hym113 [DOI] [PubMed] [Google Scholar]
- 42.Thelen A, Jonas S, Benckert C, et al. Liver resection for metastatic gastric cancer. Eur J Surg Oncol 2008;34:1328-34. 10.1016/j.ejso.2008.01.022 [DOI] [PubMed] [Google Scholar]
- 43.Morise Z, Sugioka A, Hoshimoto S, et al. The role of hepatectomy for patients with liver metastases of gastric cancer. Hepatogastroenterology 2008;55:1238-41. [PubMed] [Google Scholar]
- 44.Garancini M, Uggeri F, Degrate L, et al. Surgical treatment of liver metastases of gastric cancer: is local treatment in a systemic disease worthwhile? HPB (Oxford) 2012;14:209-15. 10.1111/j.1477-2574.2011.00428.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makino H, Kunisaki C, Izumisawa Y, et al. Indication for hepatic resection in the treatment of liver metastasis from gastric cancer. Anticancer Res 2010;30:2367-76. [PubMed] [Google Scholar]
- 46.Miki Y, Fujitani K, Hirao M, et al. Significance of surgical treatment of liver metastases from gastric cancer. Anticancer Res 2012;32:665-70. [PubMed] [Google Scholar]
- 47.Chen L, Song MQ, Lin HZ, et al. Chemotherapy and resection for gastric cancer with synchronous liver metastases. World J Gastroenterol 2013;19:2097-103. 10.3748/wjg.v19.i13.2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiberio GA, Baiocchi GL, Morgagni P, et al. Gastric cancer and synchronous hepatic metastases: is it possible to recognize candidates to R0 resection? Ann Surg Oncol 2015;22:589-96. 10.1245/s10434-014-4018-6 [DOI] [PubMed] [Google Scholar]
- 49.Oki E, Tokunaga S, Emi Y, et al. Surgical treatment of liver metastasis of gastric cancer: a retrospective multicenter cohort study (KSCC1302). Gastric Cancer 2016;19:968-76. 10.1007/s10120-015-0530-z [DOI] [PubMed] [Google Scholar]
- 50.Tatsubayashi T, Tanizawa Y, Miki Y, et al. Treatment outcomes of hepatectomy for liver metastases of gastric cancer diagnosed using contrast-enhanced magnetic resonance imaging. Gastric Cancer 2017;20:387-93. 10.1007/s10120-016-0611-7 [DOI] [PubMed] [Google Scholar]
- 51.Ministrini S, Solaini L, Cipollari C, et al. Surgical treatment of hepatic metastases from gastric cancer. Updates Surg 2018;70:273-8. 10.1007/s13304-018-0536-2 [DOI] [PubMed] [Google Scholar]
- 52.Chen J, Tang Z, Dong X, et al. Radiofrequency ablation for liver metastasis from gastric cancer. Eur J Surg Oncol 2013;39:701-6. 10.1016/j.ejso.2013.03.023 [DOI] [PubMed] [Google Scholar]
- 53.Liao YY, Peng NF, Long D, et al. Hepatectomy for liver metastases from gastric cancer: a systematic review. BMC Surg 2017;17:14. 10.1186/s12893-017-0215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kerkar SP, Kemp CD, Avital I. Liver resections in metastatic gastric cancer. HPB (Oxford) 2010;12:589-96. 10.1111/j.1477-2574.2010.00224.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiberio GA, Roviello F, Donini A, et al. Surgery for liver metastasis from gastric cancer. Transl Gastroenterol Hepatol 2016;1:68. 10.21037/tgh.2016.08.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gadde R, Tamariz L, Hanna M, et al. Metastatic gastric cancer (MGC) patients: Can we improve survival by metastasectomy? A systematic review and meta-analysis. J Surg Oncol 2015;112:38-45. 10.1002/jso.23945 [DOI] [PubMed] [Google Scholar]
- 57.Petrelli F, Coinu A, Cabiddu M, et al. Hepatic resection for gastric cancer liver metastases: A systematic review and meta-analysis. J Surg Oncol 2015;111:1021-7. 10.1002/jso.23920 [DOI] [PubMed] [Google Scholar]
- 58.Markar SR, Mikhail S, Malietzis G, et al. Influence of Surgical Resection of Hepatic Metastases From Gastric Adenocarcinoma on Long-term Survival: Systematic Review and Pooled Analysis. Ann Surg 2016;263:1092-101. 10.1097/SLA.0000000000001542 [DOI] [PubMed] [Google Scholar]
- 59.Puijk RS, Ruarus AH, Vroomen L, et al. Colorectal liver metastases: surgery versus thermal ablation (COLLISION) - a phase III single-blind prospective randomized controlled trial. BMC Cancer 2018;18:821. 10.1186/s12885-018-4716-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kotewall CN, Cheung TT, She WH, et al. The role of radiofrequency ablation to liver transection surface in patients with close tumor margin of HCC during hepatectomy-a case matched study. Transl Gastroenterol Hepatol 2017;2:33. 10.21037/tgh.2017.03.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Cobelli F, Marra P, Ratti F, et al. Microwave ablation of liver malignancies: comparison of effects and early outcomes of percutaneous and intraoperative approaches with different liver conditions: New advances in interventional oncology: state of the art. Med Oncol 2017;34:49. 10.1007/s12032-017-0903-8 [DOI] [PubMed] [Google Scholar]
- 62.Kim HR, Cheon SH, Lee KH, et al. Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia 2010;26:305-15. 10.3109/02656730903555696 [DOI] [PubMed] [Google Scholar]
- 63.Correa-Gallego C, Fong Y, Gonen M, et al. A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol 2014;21:4278-83. 10.1245/s10434-014-3817-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang K, Liu Y, Dong L, et al. Influence of thermal ablation of hepatic metastases from gastric adenocarcinoma on long-term survival: Systematic review and pooled analysis. Medicine (Baltimore) 2018;97:e13525. 10.1097/MD.0000000000013525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomassen I, van Gestel YR, van Ramshorst B, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer 2014;134:622-8. 10.1002/ijc.28373 [DOI] [PubMed] [Google Scholar]
- 66.Beeharry MK, Liu WT, Yao XX, et al. A critical analysis of the cytoreductive surgery with hyperthermic intraperitoneal chemotherapy combo in the clinical management of advanced gastric cancer: an effective multimodality approach with scope for improvement. Transl Gastroenterol Hepatol 2016;1:77. 10.21037/tgh.2016.08.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desiderio J, Chao J, Melstrom L, et al. The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer. Eur J Cancer 2017;79:1-14. 10.1016/j.ejca.2017.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seshadri RA, Glehen O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J Gastroenterol 2016;22:1114-30. 10.3748/wjg.v22.i3.1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wei J, Wu ND, Liu BR. Regional but fatal: Intraperitoneal metastasis in gastric cancer. World J Gastroenterol 2016;22:7478-85. 10.3748/wjg.v22.i33.7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hasovits C, Clarke S. Pharmacokinetics and pharmacodynamics of intraperitoneal cancer chemotherapeutics. Clin Pharmacokinet 2012;51:203-24. 10.2165/11598890-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 71.Sugarbaker PH. Peritoneal carcinomatosis: natural history and rational therapeutic interventions using intraperitoneal chemotherapy. Cancer Treat Res 1996;81:149-68. 10.1007/978-1-4613-1245-1_13 [DOI] [PubMed] [Google Scholar]
- 72.Sugarbaker PH, Stuart OA. Pharmacokinetic and phase II study of heated intraoperative intraperitoneal melphalan. Cancer Chemother Pharmacol 2007;59:151-5. 10.1007/s00280-006-0238-1 [DOI] [PubMed] [Google Scholar]
- 73.Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol 1989;16:83-97. [PubMed] [Google Scholar]
- 74.González-Moreno S. Peritoneal Surface Oncology: A progress report. Eur J Surg Oncol 2006;32:593-6. 10.1016/j.ejso.2006.03.001 [DOI] [PubMed] [Google Scholar]
- 75.Aoyama T, Yoshikawa T, Hayashi T, et al. Risk factors for peritoneal recurrence in stage II/III gastric cancer patients who received S-1 adjuvant chemotherapy after D2 gastrectomy. Ann Surg Oncol 2012;19:1568-74. 10.1245/s10434-011-2158-5 [DOI] [PubMed] [Google Scholar]
- 76.Roviello F, Marrelli D, de Manzoni G, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg 2003;90:1113-9. 10.1002/bjs.4164 [DOI] [PubMed] [Google Scholar]
- 77.Koga S, Kaibara N, Iitsuka Y, et al. Prognostic significance of intraperitoneal free cancer cells in gastric cancer patients. J Cancer Res Clin Oncol 1984;108:236-8. 10.1007/BF00402474 [DOI] [PubMed] [Google Scholar]
- 78.Fujimoto S, Shrestha RD, Kokubun M, et al. Positive results of combined therapy of surgery and intraperitoneal hyperthermic perfusion for far-advanced gastric cancer. Ann Surg 1990;212:592-6. 10.1097/00000658-199011000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer 1994;73:2048-52. [DOI] [PubMed] [Google Scholar]
- 80.Koga S, Hamazoe R, Maeta M, et al. Prophylactic therapy for peritoneal recurrence of gastric cancer by continuous hyperthermic peritoneal perfusion with mitomycin C. Cancer 1988;61:232-7. [DOI] [PubMed] [Google Scholar]
- 81.Coccolini F, Cotte E, Glehen O, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol 2014;40:12-26. 10.1016/j.ejso.2013.10.019 [DOI] [PubMed] [Google Scholar]
- 82.Roviello F, Caruso S, Neri A, et al. Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: overview and rationale. Eur J Surg Oncol 2013;39:1309-16. 10.1016/j.ejso.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 83.Xu DZ, Zhan YQ, Sun XW, et al. Meta-analysis of intraperitoneal chemotherapy for gastric cancer. World J Gastroenterol 2004;10:2727-30. 10.3748/wjg.v10.i18.2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mi DH, Li Z, Yang KH, et al. Surgery combined with intraoperative hyperthermic intraperitoneal chemotherapy (IHIC) for gastric cancer: a systematic review and meta-analysis of randomised controlled trials. Int J Hyperthermia 2013;29:156-67. 10.3109/02656736.2013.768359 [DOI] [PubMed] [Google Scholar]
- 85.Yonemura Y, Fujimura T, Nishimura G, et al. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery 1996;119:437-44. 10.1016/S0039-6060(96)80145-0 [DOI] [PubMed] [Google Scholar]
- 86.Fujimoto S, Takahashi M, Mutou T, et al. Improved mortality rate of gastric carcinoma patients with peritoneal carcinomatosis treated with intraperitoneal hyperthermic chemoperfusion combined with surgery. Cancer 1997;79:884-91. [DOI] [PubMed] [Google Scholar]
- 87.Yang XJ, Huang CQ, Suo T, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol 2011;18:1575-81. 10.1245/s10434-011-1631-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:2370-7. 10.1245/s10434-010-1039-7 [DOI] [PubMed] [Google Scholar]
- 89.Shimada S, Tanaka E, Marutsuka T, et al. Extensive intraoperative peritoneal lavage and chemotherapy for gastric cancer patients with peritoneal free cancer cells. Gastric Cancer 2002;5:168-72. 10.1007/s101200200029 [DOI] [PubMed] [Google Scholar]
- 90.Hall JJ, Loggie BW, Shen P, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg 2004;8:454-63. 10.1016/j.gassur.2003.12.014 [DOI] [PubMed] [Google Scholar]
- 91.Glehen O, Schreiber V, Cotte E, et al. Cytoreductive surgery and intraperitoneal chemohyperthermia for peritoneal carcinomatosis arising from gastric cancer. Arch Surg 2004;139:20-6. 10.1001/archsurg.139.1.20 [DOI] [PubMed] [Google Scholar]
- 92.Kuramoto M, Shimada S, Ikeshima S, et al. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg 2009;250:242-6. 10.1097/SLA.0b013e3181b0c80e [DOI] [PubMed] [Google Scholar]
- 93.Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J Surg Oncol 2010;101:457-64. 10.1002/jso.21519 [DOI] [PubMed] [Google Scholar]
- 94.Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84. 10.1002/jso.23633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Magge D, Zenati M, Mavanur A, et al. Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol 2014;21:1448-55. 10.1245/s10434-013-3327-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Topal B, Demey K, Topal H, et al. Cytoreductive surgery and Hyperthermic intra-operative peritoneal chemotherapy with Cisplatin for gastric peritoneal Carcinomatosis Monocentric phase-2 nonrandomized prospective clinical trial. BMC Cancer 2017;17:771. 10.1186/s12885-017-3730-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rihuete Caro C, Manzanedo I, Pereira F, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with gastric cancer and peritoneal carcinomatosis. Eur J Surg Oncol 2018;44:1805-10. 10.1016/j.ejso.2018.06.036 [DOI] [PubMed] [Google Scholar]
- 98.Kim DW, Park DG, Song S, et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy as Treatment Options for Peritoneal Metastasis of Advanced Gastric Cancer. J Gastric Cancer 2018;18:296-304. 10.5230/jgc.2018.18.e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fujimoto S, Shrestha RD, Kokubun M, et al. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg 1988;208:36-41. 10.1097/00000658-198807000-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yonemura Y, Fujimura T, Fushida S, et al. Hyperthermo-chemotherapy combined with cytoreductive surgery for the treatment of gastric cancer with peritoneal dissemination. World J Surg 1991;15:530-5. 10.1007/BF01675656 [DOI] [PubMed] [Google Scholar]
- 101.Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III Trial Comparing Intraperitoneal and Intravenous Paclitaxel Plus S-1 Versus Cisplatin Plus S-1 in Patients With Gastric Cancer With Peritoneal Metastasis: PHOENIX-GC Trial. J Clin Oncol 2018;36:1922-9. 10.1200/JCO.2018.77.8613 [DOI] [PubMed] [Google Scholar]
- 102.Facchiano E, Scaringi S, Kianmanesh R, et al. Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for the treatment of malignant ascites secondary to unresectable peritoneal carcinomatosis from advanced gastric cancer. Eur J Surg Oncol 2008;34:154-8. 10.1016/j.ejso.2007.05.015 [DOI] [PubMed] [Google Scholar]
- 103.Yonemura Y, Elnemr A, Endou Y, et al. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol 2010;2:85-97. 10.4251/wjgo.v2.i2.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yonemura Y, Endou Y, Shinbo M, et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: Selection for cytoreductive surgery. J Surg Oncol 2009;100:311-6. 10.1002/jso.21324 [DOI] [PubMed] [Google Scholar]
- 105.Girshally R, Demtroder C, Albayrak N, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2016;14:253. 10.1186/s12957-016-1008-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gockel I, Jansen-Winkeln B, Haase L, et al. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) in Gastric Cancer Patients with Peritoneal Metastasis (PM): Results of a Single-Center Experience and Register Study. J Gastric Cancer 2018;18:379-91. 10.5230/jgc.2018.18.e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Canbay E, Torun BC, Torun ES, et al. Evolution of management in peritoneal surface malignancies. Ulus Cerrahi Derg 2015;32:203-7. 10.5152/UCD.2016.3375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. 10.1056/NEJMoa0707035 [DOI] [PubMed] [Google Scholar]
- 109.Baba M, Hokita S, Natsugoe S, et al. Paraaortic lymphadenectomy in patients with advanced carcinoma of the upper-third of the stomach. Hepatogastroenterology 2000;47:893-6. [PubMed] [Google Scholar]
- 110.Roviello F, Pedrazzani C, Marrelli D, et al. Super-extended (D3) lymphadenectomy in advanced gastric cancer. Eur J Surg Oncol 2010;36:439-46. 10.1016/j.ejso.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 111.Yonemura Y, Wu CC, Fukushima N, et al. Randomized clinical trial of D2 and extended paraaortic lymphadenectomy in patients with gastric cancer. Int J Clin Oncol 2008;13:132-7. 10.1007/s10147-007-0727-1 [DOI] [PubMed] [Google Scholar]
- 112.Tokunaga M, Ohyama S, Hiki N, et al. Can superextended lymph node dissection be justified for gastric cancer with pathologically positive para-aortic lymph nodes? Ann Surg Oncol 2010;17:2031-6. 10.1245/s10434-010-0969-4 [DOI] [PubMed] [Google Scholar]
- 113.Fujimura T, Nakamura K, Oyama K, et al. Selective lymphadenectomy of para-aortic lymph nodes for advanced gastric cancer. Oncol Rep 2009;22:509-14. 10.3892/or_00000464 [DOI] [PubMed] [Google Scholar]
- 114.Oyama K, Fushida S, Kinoshita J, et al. Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, and S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J Surg Oncol 2012;105:535-41. 10.1002/jso.22125 [DOI] [PubMed] [Google Scholar]
- 115.Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 2014;101:653-60. 10.1002/bjs.9484 [DOI] [PubMed] [Google Scholar]
- 116.Ito S, Sano T, Mizusawa J, et al. A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer 2017;20:322-31. 10.1007/s10120-016-0619-z [DOI] [PubMed] [Google Scholar]
- 117.Yoshikawa T, Rino Y, Yukawa N, et al. Neoadjuvant chemotherapy for gastric cancer in Japan: a standing position by comparing with adjuvant chemotherapy. Surg Today 2014;44:11-21. 10.1007/s00595-013-0529-1 [DOI] [PubMed] [Google Scholar]
- 118.Yoshikawa T, Sasako M, Yamamoto S, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 2009;96:1015-22. 10.1002/bjs.6665 [DOI] [PubMed] [Google Scholar]
- 119.Kinoshita T, Sasako M, Sano T, et al. Phase II trial of S-1 for neoadjuvant chemotherapy against scirrhous gastric cancer (JCOG 0002). Gastric Cancer 2009;12:37-42. 10.1007/s10120-008-0496-1 [DOI] [PubMed] [Google Scholar]
- 120.Cheong JH, Hyung WJ, Chen J, et al. Surgical management and outcome of metachronous Krukenberg tumors from gastric cancer. J Surg Oncol 2004;87:39-45. 10.1002/jso.20072 [DOI] [PubMed] [Google Scholar]
- 121.Yu P, Huang L, Cheng G, et al. Treatment strategy and prognostic factors for Krukenberg tumors of gastric origin: report of a 10-year single-center experience from China. Oncotarget 2017;8:82558-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu LC, Shao YY, Hsu CH, et al. Metastasectomy of Krukenberg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res 2012;32:3397-401. [PubMed] [Google Scholar]
- 123.Yan D, Du Y, Dai G, et al. Management Of Synchronous Krukenberg Tumors From Gastric Cancer: a Single-center Experience. J Cancer 2018;9:4197-203. 10.7150/jca.25593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosa F, Marrelli D, Morgagni P, et al. Krukenberg Tumors of Gastric Origin: The Rationale of Surgical Resection and Perioperative Treatments in a Multicenter Western Experience. World J Surg 2016;40:921-8. 10.1007/s00268-015-3326-8 [DOI] [PubMed] [Google Scholar]
- 125.Iijima Y, Akiyama H, Atari M, et al. Pulmonary Resection for Metastatic Gastric Cancer. Ann Thorac Cardiovasc Surg 2016;22:230-6. 10.5761/atcs.oa.16-00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yoshida Y, Imakiire T, Yoneda S, et al. Ten cases of resected solitary pulmonary metastases arising from gastric cancer. Asian Cardiovasc Thorac Ann 2014;22:578-82. 10.1177/0218492313513777 [DOI] [PubMed] [Google Scholar]
- 127.Aurello P, Petrucciani N, Giulitti D, et al. Pulmonary metastases from gastric cancer: Is there any indication for lung metastasectomy? A systematic review. Med Oncol 2016;33:9. 10.1007/s12032-015-0718-4 [DOI] [PubMed] [Google Scholar]
- 128.Yang Y, Pei X, Yang M. Combination of apatinib and continuous nutritional support for a gastric cancer patient with brain metastasis prolongs survival. J Clin Pharm Ther 2018;43:726-9. 10.1111/jcpt.12708 [DOI] [PubMed] [Google Scholar]
- 129.Ahn MJ, Lee K, Lee KH, et al. Combination of anti-PD-1 therapy and stereotactic radiosurgery for a gastric cancer patient with brain metastasis: a case report. BMC Cancer 2018;18:173. 10.1186/s12885-017-3906-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choi YJ, Kim DH, Han HS, et al. Long-term survival after gastrectomy and metastasectomy for gastric cancer with synchronous bone metastasis. World J Gastroenterol 2018;24:150-6. 10.3748/wjg.v24.i1.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yoshida K, Yamaguchi K, Okumura N, et al. Is conversion therapy possible in stage IV gastric cancer: the proposal of new biological categories of classification. Gastric Cancer 2016;19:329-38. 10.1007/s10120-015-0575-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamaguchi K, Yoshida K, Tanaka Y, et al. Conversion therapy for stage IV gastric cancer-the present and future. Transl Gastroenterol Hepatol 2016;1:50. 10.21037/tgh.2016.05.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamaguchi K, Yoshida K, Tanahashi T, et al. The long-term survival of stage IV gastric cancer patients with conversion therapy. Gastric Cancer 2018;21:315-23. 10.1007/s10120-017-0738-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beom SH, Choi YY, Baek SE, et al. Multidisciplinary treatment for patients with stage IV gastric cancer: the role of conversion surgery following chemotherapy. BMC Cancer 2018;18:1116. 10.1186/s12885-018-4998-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morgagni P, Solaini L, Framarini M, et al. Conversion surgery for gastric cancer: A cohort study from a western center. Int J Surg 2018;53:360-5. 10.1016/j.ijsu.2018.04.016 [DOI] [PubMed] [Google Scholar]