Abstract
Objective:
The objective of the study was to examine a possible relationship between morphometric corpus callosum (CC) measurements, age, and gender characteristics using MR images.
Materials and Methods:
The medical data and MR examinations of 436 consecutive subjects were retrospectively reviewed. The CC thickness from five different sites, and additionally splenium length, height, and total length of the CC, and the splenium index (SI) were measured with a mid-sagittal T1-weighted sequence. Those measurements were compared with age and gender characteristics.
Results:
A weak but statistically significant negative correlation was found between age and thicknesses of genu and all body portions of CC (P = ≤0.001 for all, r = -0.32 for genu, r = -0.317 for B1, r = -0.328 for B2, r = -0.328 for B2, and r = -0.194 for B3 and B4). There was a weak but statistically significant positive correlation between age and the lengths of CC and splenium (P ≤ 0.022 for both, r = 0.112 for CC length and r = 0.11 for splenium length). The second part of the body (B2) was thicker in females (P = 0.014). On the other hand, the CC and splenium lengths were greater in males compared to females (P = 0.029 for both).
Conclusion:
We designed a comprehensive MRI study to investigate a possible relationship between normal morphometric CC measurements in 436 healthy subjects. We preferred splenium length and SI as the main splenium measurements instead of direct splenium thickness, due to discrepancies regarding splenium measurement methods in the medical literature. There was a wide spectrum of results, and we compared those results with existing medical literature.
Keywords: Age, Corpus callosum, Gender, Morphometry, Magnetic resonance imaging
INTRODUCTION
The corpus callosum (CC) is the main interhemispheric commissure of the brain and connects the cerebral hemispheres through more than 200 million fibers.[1-3] The main functions consist of unifying sensory fields and organizing bimanual motor output[4], memory[5], and facilitating language, and auditory functions.[6] CC is a major part of interhemispheric integration, which is very important for creativity and intelligence.[7] Language information for writing and alexia seem to be relatable to the CC.[8] Callosal pathologies may correlate with a wide variety of diseases, such as neurofibromatosis[9], autism, chronic alcoholism, multiple sclerosis,[10,11] cerebrovascular diseases, tumors, chemotherapy, and infection.[1]
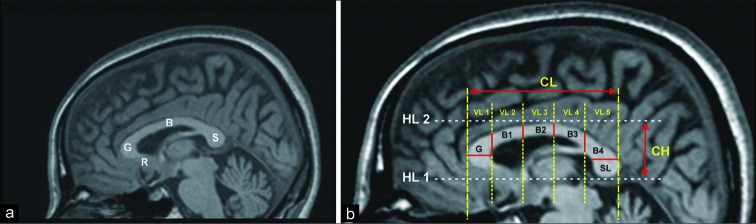
The CC is anatomically divided into four main parts: Rostrum, genu, body, and splenium [Figure 1a]. While the other parts of the CC develop between 8 and 20 weeks of gestation, the rostrum develops at 18–20 weeks postconceptional age.[12] The myelination degree of fibers is the main CC size determinant.[13] The CC shows a massive burst of growth during the first 1–4 years of the postnatal period,[14] and the increase in size may be continued even into the third decade of life.[15,16]
Figure 1:
A 32-year-old man who underwent MRI examination due to refractory frontal headache. (a) Mid-sagittal T1-weighted image shows the main parts of corpus callosum (R: Rostrum, G: Genu, B: Body, S: Splenium). (b) In the same patient, the image shows the morphometric evaluation of the corpus callosum. Reference lines (dashed lines) and the main measurements (solid lines) used in our study are demonstrated on the mid-sagittal T1-weighted image [HL: Horizontal Line, VL: Vertical Line, CL: Callosal Length, CH: Callosal Height, G: Width of genu, B1-to-4: Thickness measurements of body, SL: Splenium Length, Splenium index (SI)= callosal height-to-length of splenium ratio (not shown)].
Numerous studies have been published regarding changes in CC macro-anatomy throughout childhood and adolescence to old age. Among those studies, there is no certain method for specifying the exact CC parts and, hence, the thickness of each of them. In addition, the shape of the CC, especially the splenium, can be different in males and females; however, this fact and its effect on measurement accuracy are still unclear. In medical literature, there is no consensus on how and from where CC measurements should be done, and there are also no comprehensive imaging studies regarding this subject.
In the present study, our aim was to investigate the relationship between morphometric CC measurements, age, and gender using magnetic resonance (MR) images of 436 healthy subjects. As far as we know, this is the largest study to investigate the relationship between morphometric CC parameters, gender, and specific age characteristics.
MATERIALS AND METHODS
Subject selection
Review board approval was obtained for this retrospective study, and the procedures followed were in accordance with the Helsinki Declaration of 1975, as revised in 2000. The medical and imaging data of all subjects who underwent brain magnetic resonance imaging (MRI) examination in our radiology department between September 2016 and January 2018 were reviewed. Subjects were excluded from the study if any of the following criteria applied: Extensive gliotic foci, leukoaraiosis or encephalomalacia of any size; history of cerebrovascular accident; congenital brain malformations, intra- or extra-axial brain tumors or findings suggestive of infection in the central nervous system; previous operations for any cranial pathology; and pathology that could affect CC thickness such as demyelinating diseases, schizophrenia, epilepsy, hydrocephalus, or known dementia. Subjects with up to three small, nonspecific, T2-weighted white matter hyperintensities on brain MRI examinations were included. Four hundred and thirty-six consecutive patients [204 males (46.8%) and 232 females (53.2%)] ranging in age from 0 to 89 years who had undergone brain MRI for neurologic problems, whose neuroradiologic reports were free of neuropathologic signs and who met the criteria noted above were enrolled in the study.
Image analysis
We implemented the method used by Weis et al.[17] for morphometric CC assessment with some modifications. Thickness of the CC from five different sites [genu and four measurements from proximal to the distal body: Body1 (B1), body2 (B2), body3 (B3), and body4 (B4)], splenium length, CC height and total length, and the splenium index (SI), which is the ratio of CC height to splenium length, were measured with mid-sagittal T1-weighted sequence images acquired from two different scanners with the same brand (Achieva 3.0T X-series, Philips Medical Systems B.V., Eindhoven, The Netherlands and Intera 1.5T, Philips Medical Systems B.V., Eindhoven).
First, the most inferior points of the rostrum and splenium were connected by a tangent: “Horizontal Line 1” (HL1). Next, a tangent to the superior part of the trunk was drawn: “Horizontal Line 2” (HL2). These two lines served as a reference for the subsequent steps. The callosal height (CH) was defined as the distance between HL1 and HL2 [Figure 1b]. “Vertical Line 1” (VL1) was constructed perpendicular to HL1 and HL2 and tangent to the genu. “Vertical Line 6” (VL6) was constructed a perpendicular to HL1 and HL2 and tangent to the splenium. The callosal length (CL) was defined as the distance between VL1 and VL6 [Figure 1b]. Next, four different vertical lines were drawn (VL2, VL3, VL4, and VL5), which divide the CC into five equal pieces. We defined the first piece to indicate the genu; the second, third, and fourth pieces to indicate the body; and the fifth piece to indicate the splenium. The width of the genu was defined as the maximum extension of the genu parallel to HL1. The body thickness measurements (B1 to B4) were made from the intersections of CC and VL2 to VL5. Due to discrepancies regarding thickness measurement methods in the medical literature[1,12,17,18] and the varying anatomy of the splenium, especially between male and female subjects[19], splenium length and SI were used as the morphometric parameters instead of direct splenium thickness. The splenium was accepted as the last fifth of the CC (posterior 20%) as defined by Weis et al.[17] and splenium length (SL) was measured according to that [Figure 1b]. SI was measured as a ratio of splenium height to splenium length and noted in every subject. Those morphometric parameters were then compared with the age and gender characteristics of the study group.
Statistical analysis
Statistical analysis was done using the Statistical Package for the Social Sciences (SPSS for Mac, Version 11.5. Chicago, SPSS Inc.). Descriptive statistics of nominal samples were expressed with numbers and percentiles. Descriptive statistics of scale samples were expressed as mean ± standard deviation and the median value (minimum–maximum). A Student’s t-test was used to compare the differences between gender group categories of the normal distribution, while the Mann–Whitney U-test was performed if they were not normally distributed. p < 0.05 was accepted as statistically significant.
RESULTS
A total of 436 consecutive subjects (204 males and 232 females) who underwent brain MRI examination in our radiology department and met the criteria noted in the materials and method section were enrolled for this study. The mean age was 47.05 ± 19.8 years, with a range between 18 and 89. No statistically significant difference was found between gender and general age distribution characteristics or between gender distribution and categorical age groups in our study group (P = 0.87 and 0.92, respectively). CC morphometric measurement parameters are shown in Table 1.
Table 1:
The detailed measurements of CC morphometric parameters in our study group.
| Age | Genu | Body 1 | Body 2 | Body 3 | Body 4 | SL | SI | Height | Length | |
|---|---|---|---|---|---|---|---|---|---|---|
| N | 436 | 436 | 436 | 436 | 436 | 436 | 436 | 436 | 436 | 436 |
| Mean (mm) | 47.05 | 10.7 | 6.25 | 5.81 | 5.14 | 10.3 | 13.6 | 0.56 | 24.7 | 68 |
| SD (mm) | 19.82 | 1.83 | 1.42 | 1.11 | 1.24 | 1.98 | 0.99 | 0.069 | 3.06 | 4.96 |
| Min (mm) | 18 | 6 | 2.9 | 3 | 2.1 | 5.1 | 10.46 | 0.4 | 16 | 52.3 |
| Max (mm) | 89 | 16.3 | 11 | 9.2 | 9.2 | 17 | 16 | 0.79 | 36 | 80 |
N: Number of subjects, SD: Standard Deviation, SL: Splenium Length, SI: Splenium Index (callosal height-to-length of splenium ratio)
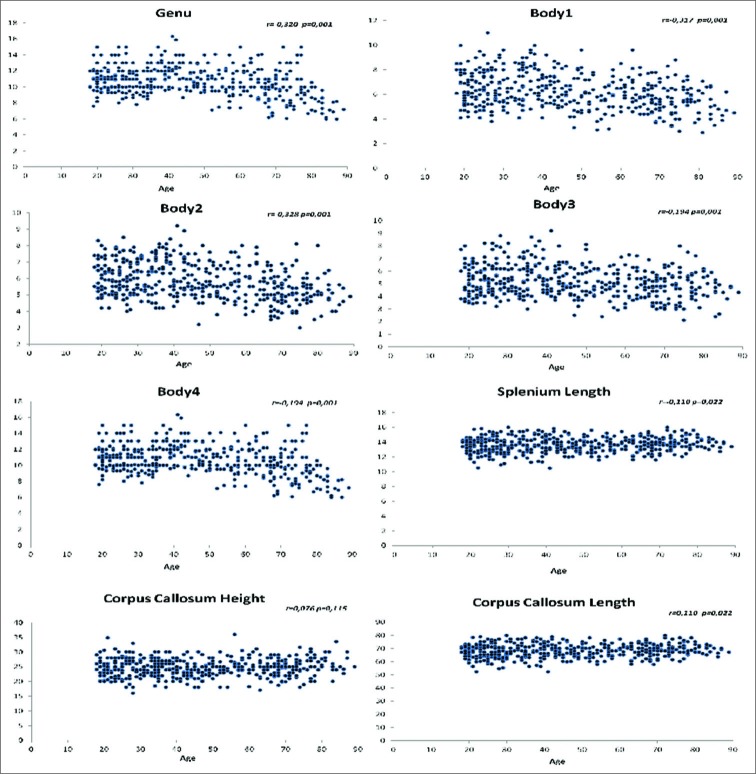
As the main subject of our study, we examined a possible relationship between age and morphometric CC measurements. We detected a weak but statistically significant negative correlation between age and the thicknesses of genu and all the body parts (B1, B2, B3, and B4) (P ≤ 0.001 for all portions and r = -0.320 for genu, r = -0.317 for B1, r = -0.328 for B2, and r = -0.194 for B3 and B4). There was a weak but statistically significant positive correlation between age and the lengths of CC and splenium (P = 0.022 for both, r = 0.112 for CC length, and r = 0.11 for splenium length). There was no statistically significant relationship regarding the SI and CC height (P = 0.902 for SI and P = 0.115 for CC height). The measurements and statistical relationships between age distribution and morphometric CC parameters are shown in Table 2. The distribution of the measurements, which had a statistically significant relationship with age, is demonstrated in Figure 2.
Table 2:
Measurements and statistical relationships between age distribution and morphometric CC parameters.
| Genu | Body 1 | Body 2 | Body 3 | Body 4 | SL | SI | Height | Length | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | CC | −0.32 | −0.317 | −0.328 | −0.194 | −0.194 | 0.11 | −0.006 | 0.076 | 0.112 |
| *P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.022 | 0.902 | 0.115 | 0.022 | |
CC: Correlation Coefficient, SL: Splenium Length, SI: Splenium Index (callosal height-to-length of splenium ratio). *Mann–Whitney U-Test
Figure 2:
The charts show the distribution of the mean values of CC morphometric parameters according to the age in our study group. The related correlation coefficients and P values can be seen in the upper right corner of each chart.
Some morphometric parameters were different in males than they were in females. The B2 was thicker in females (mean thickness of 5.67 ± 1.1 mm in males and 5.93 ± 1.11 mm, P = 0.014). CC length was greater in males (mean height 68.56 ± 4.98 mm in males and 67.53 ± 4.9 mm, P = 0.029). On the other hand, splenium length was also greater in males compared to females (mean length 13.71 ± 0.99 mm in males and 13.5 ± 0.98 mm, P = 0.029). No statistically significant relationship was detected in the other parameters (P > 0.05). The measurements and statistical relationships between gender distribution and morphometric CC parameters are shown in Table 3.
Table 3:
Measurements and statistical relationships between gender distribution and morphometric CC parameters.
| Genu | Body 1 | Body 2 | Body 3 | Body 4 | SL | SI | Height | Length | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | N | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 | 204 |
| Mean (mm) | 10.6 | 6.18 | 5.67 | 5.13 | 10.2 | 13.7 | 0.55 | 25 | 68.6 | |
| Sd (mm) | 1.97 | 1.43 | 1.1 | 1.26 | 2 | 0.99 | 0.072 | 3.25 | 4.98 | |
| Min (mm) | 6 | 3 | 3 | 2.1 | 5.1 | 10.46 | 0.4 | 18 | 53 | |
| Max (mm) | 15 | 11 | 8.4 | 8.8 | 15.3 | 16 | 0.78 | 36 | 80 | |
| Female | N | 232 | 232 | 232 | 232 | 232 | 232 | 232 | 232 | 232 |
| Mean (mm) | 10.8 | 6.3 | 5.93 | 5.14 | 10.4 | 13.5 | 0.56 | 24.5 | 67.5 | |
| Sd (mm) | 1.7 | 1.42 | 1.11 | 1.23 | 1.94 | 0.98 | 0.066 | 2.86 | 4.9 | |
| Min (mm) | 6 | 2.9 | 3 | 2.2 | 5.1 | 10.5 | 0.41 | 16 | 52.3 | |
| Max (mm) | 16.3 | 9.6 | 9.2 | 9.2 | 17 | 16 | 0.79 | 31.6 | 80 | |
| *P value | 0.19 | 0.339 | 0.014 | 0.938 | 0.189 | 0.029 | 0.756 | 0.069 | 0.029 | |
N: Number of subjects, Sd: Standard deviation, SL: Splenium Length, SI: Splenium Index (callosal height-to-length of splenium ratio). *Mann–Whitney U-Test
While we assessed the CC and its specific morphometric parameters, we detected some shape differences. Bulbous- shaped splenium seen in the female patients was the most prominent one and detected in 106 out of 232 (45.7%) females in our study group. However, due to we did not assess the splenium thickness as a parameter, due to the discrepancies regarding this measurement in the medical literature. Another shape difference that we detected was the presence of depression on the superior surface of the posterior part of the CC body. This shape abnormality was detected in 27 patients (6.2%).
DISCUSSION
The CC is a topographically organized cranial structure and the major transverse commissure connecting the two cerebral hemispheres.[12,20] It provides for interhemispheric integration and plays an important role in cognitive, sensory, and motor functions between homologous regions in the hemispheres. [7,12,21,22] The CC may be anatomically separated into four main parts from rostral to caudal: Rostrum, genu, body, and splenium. However, the functional topography of the callosal fibers is still not clearly understood.[1]
In medical literature, there are many studies regarding gross anatomy, age- and gender-related morphometric changes, the relationship between CC morphometry and academic performance, and anatomical CC changes in various diseases such as schizophrenia, depression, and epilepsy. However, the use of rating and measurement scales are questionable and results in discrepancies due to examiner subjectivity measurement methods.[21] Precise subdivisions of the CC are also not clear, and authors mention either 4 or 5 parts.[23-26] There is still a need for a detailed morphometric measurement method. To obtain accurate measurements of this important neural structure, further large-scale and comprehensive studies are still needed.
In the current study, we investigated a possible relationship between morphometric CC measurements, age, and gender using MR images of a large healthy-sample group. We found a weak but statistically significant negative correlation between age and thicknesses of genu and all body portions (B1-to-B4) of the CC. There was also a weak but statistically significant positive correlation between age, total CC, and splenium lengths. No statistically significant relationship was seen between age, SI, and CC height in our study group. Weis et al.[17] reported in their MR study with 46 healthy subjects that genu width had no age- specific changes; whereas, the anterior trunk height (the mean height of the second fifth of the CC) underwent a significant decrease during aging. In addition, they did not find any significant relationship between the CH, CL, and age. They used a “rotatory diameter measurement” for measuring the splenial width and did not find significantly decreased diameter measurements. Karakas et al.[16] reported mean values for the genu, body, and splenium widths, maximum CC heights and lengths and various third and lateral ventricle measurements in normal adults and tried to provide documentation of the normal values obtained from 52 MR scans. We found lower values for genu and body widths and similar results for CC heights as previous studies in the medical literature. However, due to the dissimilarity of measurement methods, splenium measurements in previous studies are totally different from those in our study.
The B2 was thicker in females, while CC and splenium lengths were greater in males. Karakas et al.[16] did not find any gender difference in callosal width measurements. Sullivan et al.[23] showed that callosal size did not correlate with either age or gender in their 92-case MRI study. Ng et al.[1] also did not detect a significant gender difference in CC height and length, total callosal area, or thickness of different CC portions.
There are many studies in the medical literature focusing on the CC morphometry. Undoubtfully, it would be great to be able to make a clinical interpretation only by looking at the morphology of the CC. However, studies that highlighted the clinical use of this information are limited. Ng et al.[1] reported the association of academic performance with the thickness of the posterior part of the CC body in their study consisting of 100 primary school children. In the same study, this CC portion was also associated with language and mathematics performance. On the other hand, Chavarria et al.[2] showed that higher puberty scores in the children with thicker CC. In addition, there are some studies[27-29] in the medical literature regarding the relationship between myelination and CC size. However, further studies are needed on the potential clinical use of CC morphometric features.
Our study has some limitations. Not performing a volume study and measuring CC thickness using only one mid- sagittal image are the primary limitations. However, this method was the most commonly used way to perform those kinds of CC measurements in previous studies, so we also chose it. Using images acquired from two different MRI scanners were also a limitation. However, the same program was used for all measurements, regardless of the scanner used. The choice of levels where thickness measurements were obtained may also have been a limitation. We mainly utilized the same method as Weis et al.,[17] but we made some modifications to it. However, because of the discrepancies regarding splenium thickness measurements in other studies, we used SL and SI instead of direct measurement of splenium thickness. Subjects in the study older than 70 years may be problematic due to the confounding effects of age-related callosal atrophy. However, we did not exclude them from the study so we could monitor possible changes in CC measurements in this age group. We included those subjects greater than 70 with three or fewer small, nonspecific T2-weighted white matter hyperintensities.
CONCLUSION
We designed a comprehensive study to determine normal morphometric CC measurements using MRI images of 436 healthy subjects. We utilized mostly the same methods used in the previous studies for CC measurements, except for the splenium. SL and SI were used as the main splenium measurements instead of direct splenium thickness because there were discrepancies with the measurement method in previous studies. Some of our results were similar to previous studies, and some were different. As far as we know, this study is the most comprehensive imaging study investigating these relationships using such a large population. However, further large-scale studies are needed to determine the most accurate CC size measurement method and to specify the relationship of CC size with human age and gender characteristics.
Footnotes
How to cite this article: Arda KN, Akay S. The relationship between corpus callosum morphometric measurements and age/gender characteristics: A comprehensive MR imaging study. J Clin Imaging Sci 2019;9:33.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ng WH, Chan YL, Au KS, Yeung KW, Kwan TF, To CY, et al. Morphometry of the corpus callosum in chinese children: Relationship with gender and academic performance. Pediatr Radiol. 2005;35:565–71. doi: 10.1007/s00247-004-1336-z. [DOI] [PubMed] [Google Scholar]
- 2.Chavarria MC, Sánchez FJ, Chou YY, Thompson PM, Luders E. Puberty in the corpus callosum. Neuroscience. 2014;265:1–8. doi: 10.1016/j.neuroscience.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Individual differences in brain asymmetries and fiber composition in the human corpus callosum. Brain Res. 1992;598:154–61. doi: 10.1016/0006-8993(92)90178-C. [DOI] [PubMed] [Google Scholar]
- 4.Zaidel D, Sperry RW. Some long-term motor effects of cerebral commissurotomy in man. Neuropsychologia. 1977;15:193–204. doi: 10.1016/0028-3932(77)90028-8. [DOI] [PubMed] [Google Scholar]
- 5.Zaidel D, Sperry RW. Memory impairment after commissurotomy in man. Brain. 1974;97:263–72. doi: 10.1093/brain/97.1.263. [DOI] [PubMed] [Google Scholar]
- 6.Schaltenbrand G. The effects on speech and language of stereotactical stimulation in thalamus and corpus callosum. Brain Lang. 1975;2:70–7. doi: 10.1016/S0093-934X(75)80055-1. [DOI] [PubMed] [Google Scholar]
- 7.Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, et al. Development of the human corpus callosum during childhood and adolescence: A longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:571–88. doi: 10.1016/S0278-5846(99)00017-2. [DOI] [PubMed] [Google Scholar]
- 8.Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- 9.Dubovsky EC, Booth TN, Vezina G, Samango-Sprouse CA, Palmer KM, Brasseux CO, et al. MR imaging of the corpus callosum in pediatric patients with neurofibromatosis type 1. AJNR Am J Neuroradiol. 2001;22:190–5. [PMC free article] [PubMed] [Google Scholar]
- 10.Pozzilli C, Bastianello S, Padovani A, Passafiume D, Millefiorini E, Bozzao L, et al. Anterior corpus callosum atrophy and verbal fluency in multiple sclerosis. Cortex. 1991;27:441–5. doi: 10.1016/S0010-9452(13)80039-1. [DOI] [PubMed] [Google Scholar]
- 11.Gean-Marton AD, Vezina LG, Marton KI, Stimac GK, Peyster RG, Taveras JM, et al. Abnormal corpus callosum: A sensitive and specific indicator of multiple sclerosis. Radiology. 1991;180:215–21. doi: 10.1148/radiology.180.1.2052698. [DOI] [PubMed] [Google Scholar]
- 12.Karakas P, Koç Z, Koç F, Gülhal Bozkir M. Morphometric MRI evaluation of corpus callosum and ventricles in normal adults. Neurol Res. 2011;33:1044–9. doi: 10.1179/1743132811Y.0000000030. [DOI] [PubMed] [Google Scholar]
- 13.de Lacoste MC, Kirkpatrick JB, Ross ED. Topography of the human corpus callosum. J Neuropathol Exp Neurol. 1985;44:578–91. doi: 10.1097/00005072-198511000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Hayakawa K, Konishi Y, Matsuda T, Kuriyama M, Konishi K, Yamashita K, et al. Development and aging of brain midline structures: Assessment with MR imaging. Radiology. 1989;172:171–7. doi: 10.1148/radiology.172.1.2740500. [DOI] [PubMed] [Google Scholar]
- 15.Pujol J, Vendrell P, Junqué C, Martí-Vilalta JL, Capdevila A. When does human brain development end? Evidence of corpus callosum growth up to adulthood. Ann Neurol. 1993;34:71–5. doi: 10.1002/ana.410340113. [DOI] [PubMed] [Google Scholar]
- 16.Rauch RA, Jinkins JR. Analysis of cross-sectional area measurements of the corpus callosum adjusted for brain size in male and female subjects from childhood to adulthood. Behav Brain Res. 1994;64:65–78. doi: 10.1016/0166-4328(94)90119-8. [DOI] [PubMed] [Google Scholar]
- 17.Weis S, Kimbacher M, Wenger E, Neuhold A. Morphometric analysis of the corpus callosum using MR: Correlation of measurements with aging in healthy individuals. AJNR Am J Neuroradiol. 1993;14:637–45. [PMC free article] [PubMed] [Google Scholar]
- 18.Sullivan EV, Rosenbloom MJ, Desmond JE, Pfefferbaum A. Sex differences in corpus callosum size: Relationship to age and intracranial size. Neurobiol Aging. 2001;22:603–11. doi: 10.1016/S0197-4580(01)00232-9. [DOI] [PubMed] [Google Scholar]
- 19.Allen LS, Richey MF, Chai YM, Gorski RA. Sex differences in the corpus callosum of the living human being. J Neurosci. 1991;11:933–42. doi: 10.1523/JNEUROSCI.11-04-00933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salat D, Ward A, Kaye JA, Janowsky JS. Sex differences in the corpus callosum with aging. Neurobiol Aging. 1997;18:191–7. doi: 10.1016/S0197-4580(97)00014-6. [DOI] [PubMed] [Google Scholar]
- 21.Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, et al. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage. 2006;31:1445–52. doi: 10.1016/j.neuroimage.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Hofer S, Frahm J. Topography of the human corpus callosum revisited comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–94. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 23.DeLacoste-Utamsing C, Holloway RL. Sexual dimorphism in the human corpus callosum. Science. 1982;216:1431–2. doi: 10.1126/science.7089533. [DOI] [PubMed] [Google Scholar]
- 24.Byne W, Bleier R, Houston L. Variations in human corpus callosum do not predict gender: A study using magnetic resonance imaging. Behav Neurosci. 1988;102:222–7. doi: 10.1037/0735-7044.102.2.222. [DOI] [PubMed] [Google Scholar]
- 25.Elster AD, DiPersio DA, Moody DM. Sexual dimorphism of the human corpus callosum studied by magnetic resonance imaging: Fact, fallacy and statistical confidence. Brain Dev. 1990;12:321–5. doi: 10.1016/S0387-7604(12)80314-7. [DOI] [PubMed] [Google Scholar]
- 26.Clarke S, Kraftsik R, Van der Loos H, Innocenti GM. Forms and measures of adult and developing human corpus callosum: Is there sexual dimorphism? J Comp Neurol. 1989;280:213–30. doi: 10.1002/cne.902800205. [DOI] [PubMed] [Google Scholar]
- 27.Langmeier M, Pokorný J, Mares J, Mares P, Trojan S. Effect of prolonged hypobaric hypoxia during postnatal development on myelination of the corpus callosum in rats. J Hirnforsch. 1987;28:385–95. [PubMed] [Google Scholar]
- 28.Lai M, Lewis PD. Effects of undernutrition on myelination in rat corpus callosum. J Comp Neurol. 1980;193:973–82. doi: 10.1002/cne.901930410. [DOI] [PubMed] [Google Scholar]
- 29.Wiggins RC, Bissell AC, Durham L, Samorajski T. The corpus callosum during postnatal undernourishment and recovery: A morphometric analysis of myelin and axon relationships. Brain Res. 1985;328:51–7. doi: 10.1016/0006-8993(85)91321-6. [DOI] [PubMed] [Google Scholar]