Abstract
The purpose of the present study was to characterize pulmonary vascular stiffness using wave intensity analysis (WIA) in children with pulmonary arterial hypertension (PAH), compare the WIA indexes with catheterization- and MRI-derived hemodynamics, and assess the prognostic ability of WIA-derived biomarkers to predict the functional worsening. WIA was performed in children with PAH (n = 40) and healthy control subjects (n = 15) from phase-contrast MRI-derived flow and area waveforms in the main pulmonary artery (MPA). From comprehensive WIA spectra, we collected and compared with healthy control subjects forward compression waves (FCW), backward compression waves (BCW), forward decompression waves (FDW), and wave propagation speed (c-MPA). There was no difference in the magnitude of FCW between PAH and control groups (88 vs. 108 mm5·s−1·ml−1, P = 0.239). The magnitude of BCW was increased in patients with PAH (32 vs. 5 mm5·s−1·ml−1, P < 0.001). There was no difference in magnitude of indexed FDW (32 vs. 28 mm5·s−1·ml−1, P = 0.856). c-MPA was increased in patients with PAH (3.2 vs. 1.6 m/s, P < 0.001). BCW and FCW correlated with mean pulmonary arterial pressure, right ventricular volumes, and ejection fraction. Elevated indexed BCW [heart rate (HR) = 2.91, confidence interval (CI): 1.18–7.55, P = 0.019], reduced indexed FDW (HR = 0.34, CI: 0.11–0.90, P = 0.030), and increased c-MPA (HR = 3.67, CI: 1.47–10.20, P = 0.004) were strongly associated with functional worsening of disease severity. Our results suggest that noninvasively derived biomarkers of pulmonary vascular resistance and stiffness may be helpful for determining prognosis and monitoring disease progression in children with PAH.
NEW & NOTEWORTHY Wave intensity analysis (WIA) studies are lacking in children with pulmonary arterial hypertension (PAH) partially because WIA, which is necessary to assess vascular stiffness, requires an invasive pressure-derived waveform along with simultaneous flow measurements. We analyzed vascular stiffness using WIA in children with PAH who underwent phase-contrast MRI and observed significant differences in WIA indexes between patients with PAH and control subjects. Furthermore, WIA indexes were predictive of functional worsening and were associated with standard catheterization measures.
Keywords: magnetic resonance imaging, pediatric pulmonary arterial hypertension, vascular stiffness
INTRODUCTION
Pediatric pulmonary arterial hypertension (PAH) contributes to poor long-term clinical outcomes in a wide spectrum of childhood diseases (1, 12, 13). The cardinal feature of PAH is widespread pulmonary arterial remodeling characterized by luminal narrowing and diffuse arterial wall stiffening, which leads to increased pulmonary vascular resistance (PVR) (9, 27). Recent studies evaluating pulmonary vascular stiffness in adult patients with PAH revealed that elevated abnormal wave reflections originating from pulmonary arterial branches contribute to increased right ventricular (RV) afterload (17, 30). Similar studies are lacking in children with PAH partially because wave intensity analysis (WIA), which is necessary to assess vascular stiffness, requires an invasive pressure-derived waveform along with simultaneous flow measurements.
Originally described by Parker and Jones (20), WIA can describe the formation of waves propagating through the arterial system by separating respective pressure and flow waveforms in the time domain rather than in the frequency domain that is traditionally applied for impedance analysis (19, 40). Furthermore, Quail et al. (23) showed that WIA can be performed in pulmonary arteries noninvasively by using phase-contrast MRI-derived flow and area waveforms. Respiratory navigated free-breathing sequences allow for optimal temporal resolution and the generation of high-contrast images for pulmonary artery wall delineation, which enables the ability to perform WIA in children with breathing difficulties or who require anesthesia. This application would be of great benefit in children with PAH because of its noninvasive nature and ability to comprehensively assess the pulmonary vascular stiffness beyond traditional invasive markers of compliance. Importantly, hemodynamic indexes reflective of pulmonary vascular stiffness have been progressively recognized as one of the strongest prognostic markers of clinical outcomes in both adult and pediatric PAH populations (22, 31).
Consequently, the purpose of the present study was to 1) characterize pulmonary vascular stiffness using WIA in children with PAH who underwent phase-contrast MRI and right heart catheterization on the same day, 2) compare WIA indexes with traditional catheterization- and MRI-derived hemodynamics, and 3) assess the ability of WIA indexes to predict functional worsening in children with PAH. We hypothesized that children with PAH will display abnormal wave reflection compared with healthy control subjects and that WIA indexes will reflect traditional cardiac catheterization metrics and functional worsening. Improved understanding of pulmonary vascular stiffness in children with PAH may enhance longitudinal clinical management and aid in future studies of novel therapeutic strategies to reverse pulmonary arterial remodeling.
METHODS
As part of a retrospective study, children followed by the Pulmonary Hypertension Clinic at Children’s Hospital Colorado between January 2010 and December 2017 underwent clinically indicated cardiac MRI followed by right heart catheterization as dictated by their clinical status. The primary diagnosis of PAH was established after evaluation, which included echocardiography and a prior cardiac catheterization per accepted guidelines (1). Children with PAH associated with congenital heart disease who required surgical or cardiac catheterization interventions involving the pulmonary arteries were excluded from the analysis. MRI control subjects were prospectively recruited via a medical campus advertisement and were included if they did not have any known underlying cardiac, pulmonary, or systemic disease. This study was approved by the Colorado Multiple Institutional Review Board, and the legally authorized representative of each subject provided written informed consent.
MRI protocol.
MRI acquisitions were performed using a 1.5- or 3.0-T magnet system (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany; Ingenia, Philips Medical Systems, Best, The Netherlands). The flow hemodynamic evaluation acquisition protocol was performed by applying gradient echo electrocardiographically gated sequence with diaphragmatic respiratory navigation to obtain respective magnitude- and phase velocity-encoding maps. The acquisition plane for the WIA was positioned in the mid main pulmonary artery (MPA) to secure sufficient distance from the pulmonary valve. A typical sequence for free-breathing phase-contrast MRI with Cartesian encoding and retrospective sorting had a temporal resolution of 14–20 ms with 40–50 phases, echo times of 2.2–3.5 ms, matrix 160 × 256, flip angle of 25°, and 100% of the k-space sampling. Depending on patient size and the field of view (128–225 × 210–360 mm), the cross-sectional pixel resolution was found to be 0.82 × 0.82 to 1.56 × 1.56 mm2 with a slice thickness of 5 mm. Final time of acquisition varied on heart rate (HR) and gating efficiency and ranged from 2 to 3 min. Velocity-encoding values were adjusted to avoid aliasing artifact (typical values ranged from 100 to 150 cm/s). The ventricular volumetric and functional analysis was performed using steady-state free precession images with standard short-axis stacks with coverage of the ventricles from the base to apex.
Wave intensity analysis.
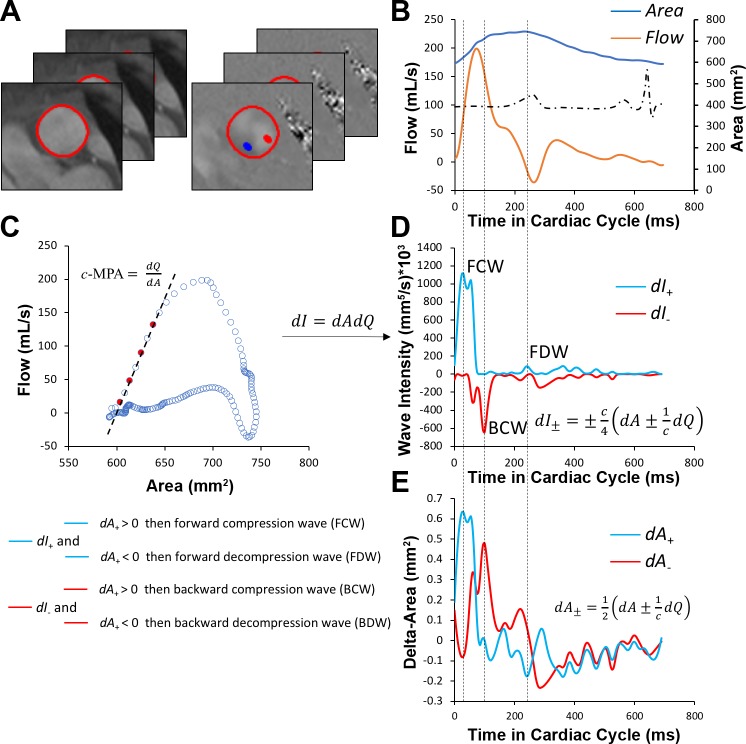
The overall schematic of WIA processing is shown in Fig. 1. First, luminal contours of corresponding magnitude and phase images were segmented using the previously described method applying active contour model, which tracks the edges of the arterial wall initialized by a manual delineation in a single timeframe (10). Segmented images were then exported into an in-house-created MATLAB program (MATLAB 2017a, The MathWorks, Natick, MA) for the generation of flow and area waveforms, which were interpolated using a cubic spline interpolation to achieve 5-ms temporal resolution. Wave propagation speed analogous to pulse wave velocity in the MPA (c-MPA) was then calculated using the previously described flow (Q)-area (A) method (23) as follows:
Fig. 1.
Wave intensity analysis workflow. A and B: segmented magnitude and phase images from acquired phase-contrast MRI (A) were required to create flow and area waveforms (B), which were further interpolated to achieve 5-ms temporal resolution. C: flow-area diagrams were reconstructed to calculate main pulmonary arterial wave speed (pulse wave velocity) by means of linear regression of noninterpolated data points (red) sampled during early systole. D: wave intensity spectra were then generated from separated flow and area waveforms. E: compression or decompression nature of the forward and backward waves was determined from separated area differential waveforms. c-MPA or c, wave speed/pulse wave velocity in the main pulmonary artery; dA+ and dA−, forward-positive and backward-negative components of area differential waveform, respectively; dI+ and dI−, forward-positive and backward-negative components of intensity differential waveform, respectively; dQ, flow differential waveform.
| (1) |
The actual slope of the flow-area diagram was calculated by applying linear regression to time points corresponding to early systole. Only noninterpolated early systole data points were applied for this linear regression analysis to minimize the underestimation from incoming wave reflections. In addition to c-MPA, as a noninvasive marker of pulmonary arterial stiffness, we further measured the relative area change defined as [(Amax − Amin)/Amax] × 100%. To proceed with the WIA, both area and flow waveforms were separated into their respective forward-positive (traveling from the heart) and backward-negative (traveling from the vasculature) components as follows:
| (2) |
| (3) |
The net wave intensity (I) was then calculated as product of flow and area waveform differentials as follows:
| (4) |
Finally, the net wave intensity was separated into its respective forward-positive and backward-negative components as follows:
| (5) |
To classify present intensity waves, respective wave intensity and delta-area waveforms were produced and cross-referenced to assure correct interpretation. All positive waves with a positive dA+ wave were classified as forward compression waves (FCW), whereas all positive waves with negative dA+ were considered as forward decompression waves (FDW). FCW is analogous to RV dP/dt and represents the intensity of ventricular ejection. On the other hand, FDW occurs at the end of systole and is typically associated with diastolic recoil and relaxation. Finally, negative waves with positive dA− were classified as backward compression waves (BCW), whereas the negative waves with negative dA− were described as backward decompression waves (BDW). All magnitudes of carried wave intensities were indexed to ejected stroke volume as previously described (3).
Catheterization.
Right heart catheterization was performed in all patients immediately after MRI acquisition. The cardiac catheterization protocol was performed as recommended by pediatric PAH consensus guidelines (7, 28). Briefly, catheterization was performed using a 5- or 6-Fr Swan-Ganz catheter via right internal jugular or right femoral access for measurement of PAH-typical metrics including mean pulmonary arterial pressure, RV end-diastolic pressure, indexed PVR, and pulmonary arterial wedge pressure. All patients underwent flow evaluation using the Fick principle, and patients without a major intracardiac shunt also underwent thermodilution measurement of cardiac output.
Statistical analysis.
Analyses were performed in JMP (version 13.1 or higher, SAS Institute, Cary, NC). Variables were checked for the distributional assumption of normality using normal plots in addition to Kolmogorov-Smirnov and Shapiro-Wilk tests. Variables that were positively skewed (e.g., c-MPA and WIA indexes) were natural log transformed for the correlative analyses. Demographic and clinical characteristics among children with and without PAH were compared using Student’s t-test for normally distributed continuous variables, Wilcoxon’s rank-sum test for nonnormally distributed variables, and χ2 for categorical variables. Additional group comparisons were performed using Kruskal-Wallis or one-way ANOVA tests between the PAH-specific World Health Organization (WHO) functional class groups. Simple linear regression analyses were used to examine association between the WIA indexes and typical catheterization/MRI indexes of PAH.
All WIA, catheterization, and MRI characteristics were considered for survival univariate analysis. Univariate Cox proportional hazards analysis was applied to assess the predictive ability in all patients with PAH. The composite outcome reflective of functional worsening was defined by an escalation in WHO functional class, PAH-related hospitalization, need for initiation of prostanoid therapy, syncopal event, or hemoptysis. For variables that were found to be significantly associated with survival univariate analysis, Kaplan-Meier survival curves were constructed with specific log-rank test with the population divided by receiver-operating characteristics to find the most optimal cutoff values. All patients were followed up to the particular event or the end of the study (December 2018). Significance was based on an α-level of 0.05.
RESULTS
Patient demographics are shown in Table 1. We identified 40 children with PAH who underwent same-day MRI and right heart catheterization. There were no significant differences in age, body surface area, and sex distribution between patients with PAH and control subjects (n = 15). At the time of MRI evaluation, 13 children were classified as WHO functional class I, 13 children as WHO functional class II, 12 children as WHO functional class III, and 2 children as WHO functional class IV. Twenty patients (50%) were diagnosed with idiopathic PAH, 15 patients (37.5%) had PAH associated with congenital heart disease, and 5 children had PAH due to other causes, including restrictive lung disease (n = 3), schistosomiasis (n = 1), and hereditary PAH (n = 1). The spectrum of congenital heart lesions included atrial septal defect (n = 12), ventricular septal defect (n = 3), patent ductus arteriosus (n = 2), and coarctation of the aorta (n = 1). Out of all congenital heart defects, only two atrial septal defects were unrepaired with minor shunt [pulmonary blood flow-to-systemic blood flow ratio (Qp/Qs) < 1.2 in both cases]. Thirty-eight patients were on phosphodiesterase-5 inhibitors, 28 subjects were receiving endothelin receptor antagonist medications, and 18 patients were on prostanoid therapy.
Table 1.
Patient demographics
| PAH | Control | P Value | |
|---|---|---|---|
| n | 40 | 15 | |
| Age, yr | 13.1 ± 4.9 | 12.4 ± 3.7 | 0.543 |
| Body surface area, m2 | 1.24 ± 0.47 | 1.28 ± 0.28 | 0.719 |
| Sex (female/male participants), n (%) | 25/15 (62.5/37.5) | 10/5 (67/33) | 0.775 |
| World Health Organization functional class | |||
| I n (%) | 13 (32.5) | ||
| II, n (%) | 13 (32.5) | ||
| III, n (%) | 12 (30) | ||
| IV, n (%) | 2 (5) | ||
| PAH diagnosis | |||
| Idiopathic PAH, n (%) | 20 (50) | ||
| PAH-congenital heart disease, n (%) | 15 (37.5) | ||
| Atrial septal defect, n (%) | 12 (30) | ||
| Ventricular septal defect, n (%) | 3 (7.5) | ||
| Patent ductus arteriosus, n (%) | 2 (5) | ||
| Coarctation, n (%) | 1 (2.5) | ||
| Other, n (%) | 5 (12.5) | ||
| Therapy | |||
| Phosphodiesterase-5 inhibitors, % | 38 | ||
| Endothelin receptor antagonist, % | 28 | ||
| Prostanoids, % | 18 |
Values are means ± SD; n is the number of subjects. PAH, pulmonary arterial hypertension.
Cardiac catheterization and MRI hemodynamics are shown in Table 2. Median hemodynamic values included mean pulmonary arterial pressure of 42 ± 17 mmHg, PVR index of 16.3 ± 7.6 WU·m2 (where WU is Wood units), and pulmonary arterial wedge pressure of 8 ± 3 mmHg (means ± SD). Patients with PAH had a median RV end-diastolic pressure of 7 mmHg and elevated RV volumes. Specifically, the median end-diastolic volume index was increased in patients with PAH (108 vs. 86 ml/m2, P < 0.001) along with increased median end-systolic volume index (52 vs. 40 ml/m2, P < 0.001). Patients with PAH also had elevated median indexed stroke volume (53 vs. 45 ml/m2, P = 0.012) and mean RV cardiac index (5.0 vs. 3.6 l·min−1·m−2, P < 0.001). Finally, patients with PAH had decreased RV ejection fraction compared with control subjects (47% vs. 57%, P < 0.001). Mean pulmonary arterial pressure correlated with standard MRI indexes of ejection fraction (β ± SE: −0.31 ± 0.09, R = 0.48, P = 0.002), end-diastolic volume index (β ± SE: 1.28 ± 0.45, R = 0.42, P = 0.007), and end-systolic volume index (β ± SE: 1.23 ± 0.44, R = 0.41, P = 0.009). There were no significant correlations with cardiac index and indexed stroke volume.
Table 2.
Catheterization and MRI hemodynamics
| PAH | Control | P Value | |
|---|---|---|---|
| n | 40 | 15 | |
| Mean pulmonary arterial pressure, mmHg | 42 ± 17 | ||
| Pulmonary vascular resistance index, Wood units·m2 | 16.3 ± 7.6 | ||
| RV end-diastolic pressure, mmHg | 7 (6–9) | ||
| Pulmonary arterial wedge pressure, mmHg | 8 ± 3 | ||
| RV end-diastolic volume index, ml/m2 | 108 (88–131) | 86 (76–96) | <0.001 |
| RV end-systolic volume index, ml/m2 | 52 (42–70) | 40 (28–42) | <0.001 |
| RV stroke volume index, ml/m2 | 53 (44–62) | 45 (41–51) | 0.012 |
| RV ejection fraction, % | 47 ± 11 | 57 ± 4 | <0.001 |
| RV cardiac index, l·min−1·m−2 | 5.0 ± 2.4 | 3.6 ± 0.9 | <0.001 |
Values are means ± SD or medians with the corresponding interquartile ranges in parentheses; n is the number of subjects. PAH, pulmonary arterial hypertension; RV, right ventricular.
Wave intensity analysis.
A summary of WIA performed in the MPA is shown in Table 3. The median wave speed/pulse wave velocity c-MPA was increased in patients with PAH (3.2 vs. 1.6 m/s, P < 0.001). Geometric strain derived by mean of relative area change was decreased in patients with PAH (29% vs. 42%, P < 0.001). There was no difference in median magnitude of indexed FCW [FCW(i)] between PAH and control groups (88 vs. 108 mm5·s−1·ml−1, P = 0.239). The BCW was present in the midsystole in all patients with PAH. Interestingly, seven control subjects revealed mild yet noticeable BCW. The overall median magnitude of indexed BCW [BCW(i)] was increased in patients with PAH (32 vs. 5 mm5·s−1·ml−1, P < 0.001). We did not observe any prominent BDW in patients with PAH or in control subjects. Finally, there was no difference in median magnitude of FDW(i) (32 vs. 28 mm5·s−1·ml−1, P = 0.856). A comparison of WIA spectra of a typical patient with PAH and a control subject is shown in Fig. 2.
Table 3.
Wave intensity analysis
| PAH | Control | P Value | |
|---|---|---|---|
| n | 40 | 15 | |
| FCW(i), mm5·s−1·ml−1 | 88 (56–159) | 108 (88–222) | 0.239 |
| BCW(i), mm5·s−1·ml−1 | 32 (22–69) | 5 (0–12) | <0.001 |
| FDW(i), mm5·s−1·ml−1 | 32 (18–59) | 28 (22–33) | 0.856 |
| c-MPA, m/s | 3.2 (2.0–4.8) | 1.6 (1.3–2.2) | <0.001 |
| RAC, % | 29 ± 11 | 42 ± 9 | <0.001 |
Values are means ± SD or medians with the corresponding interquartile ranges in parentheses; n is the number of subjects. PAH, pulmonary arterial hypertension; FCW(i), indexed forward compression wave; BCW(i), indexed backward compression wave; FDW(i), indexed forward decompression wave; c-MPA, wave speed/pulse wave velocity of the main pulmonary artery; RAC, relative area change.
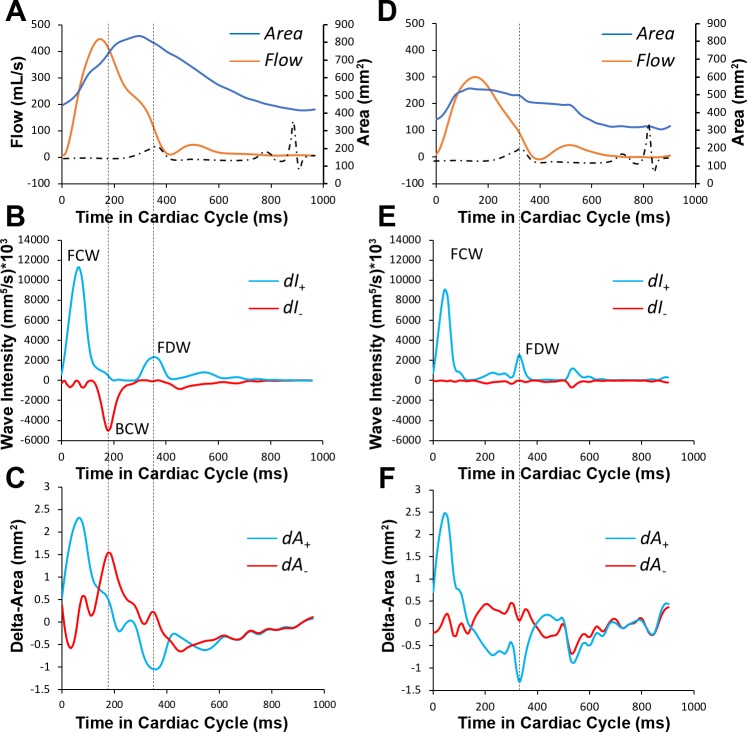
Fig. 2.
Comparison of wave intensity analysis in typical patient with pulmonary arterial hypertension (PAH; A–C) and control subject of similar age and size (D–F). Flow waveform in patient with pulmonary arterial hypertension shows characteristic late systolic notch (A). Wave intensity pattern in patient with PAH shows prominent backward compression wave (BCW; B), which positively identified by parallel analysis of separated differential area waveforms (C). In comparison, wave intensity analysis in control subject does not have backward compression wave (E). Furthermore, wave intensity analysis spectra do not show significant differences between forward compression waveform (FCW) typically associated with dP/dt and right ventricular contraction, and similarly, forward decompression wave (FDW) reflective of right ventricular diastolic function appears to have similar magnitude. dA+ and dA−, forward-positive and backward-negative components of area differential waveform, respectively; dI+ and dI−, forward-positive and backward-negative components of intensity differential waveform, respectively.
To understand the nature of pulmonary arterial remodeling between different types of PAH, we compared WIA indexes between patients with idiopathic PAH and patients with PAH associated with congenital heart disease. A summary of all subanalyses is shown in Table 4. There were no differences in median FCW(i) magnitudes between both considered groups (88 vs. 102 mm5·s−1·ml−1, P = 0.972), nor were there differences in median magnitudes of BCW(i) (32 vs. 32 mm5·s−1·ml−1, P = 0.880) or FDW(i) (35 vs. 32 mm5·s−1·ml−1, P = 0.413) or median c-MPA (3.3 vs. 3.0 m/s, P = 0.765). Furthermore, we investigated whether the WIA indexes were associated with present WHO functional class. We combined groups I + II and III + IV for comparative analysis and found that there were no differences between considered WIA. Specifically, there were no differences in median FCW(i) magnitudes (87 vs. 101 mm5·s−1·ml−1, P = 0.640), median BCW(i) magnitudes (29 vs. 33 mm5·s−1·ml−1, P = 0.223), median FDW(i) magnitudes (31 vs. 44 mm5·s−1·ml−1, P = 0.149), or median c-MPA (3.3 vs. 3.0 m/s, P = 0.869).
Table 4.
Subgroup analysis
| Idiopathic PAH | PAH- congenital heart disease | P Value | World Health Organization Classes I + II | World Health Organization Classes III + IV | P Value | |
|---|---|---|---|---|---|---|
| n | 20 | 15 | 26 | 14 | ||
| FCW(i), mm5·s−1·ml−1 | 88 (75–154) | 102 (54–165) | 0.880 | 87 (52–158) | 101 (76–152) | 0.6407 |
| BCW(i), mm5·s−1·ml−1 | 32 (25–57) | 32 (17–72) | 0.972 | 31 (17–44) | 44 (27–110) | 0.1499 |
| FDW(i), mm5·s−1·ml−1 | 35 (20–61) | 32 (21–56) | 0.413 | 29 (18–59) | 33 (21–50) | 0.2235 |
| c-MPA, m/s | 3.3 (2.0–4.5) | 3.0 (2.5–3.7) | 0.765 | 3.0 (2.0–4.3) | 3.4 (2.2–5.6) | 0.869 |
Values are medians with the corresponding interquartile ranges in parentheses; n is the number of subjects. PAH, pulmonary arterial hypertension; FCW(i), indexed forward compression wave; BCW(i), indexed backward compression wave; FDW(i), indexed forward decompression wave; c-MPA, wave speed/pulse wave velocity of the main pulmonary artery.
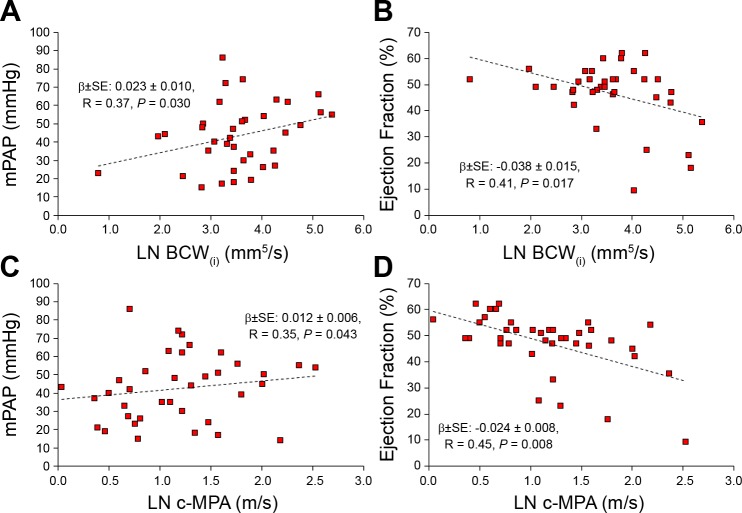
Finally, to investigate the relationship between WIA indexes and standard catheterization and MRI hemodynamic indexes associated with PAH, we performed simple linear regression analyses between the aforementioned markers. A summary of correlative analyses is shown in Table 5. FCW(i) correlated with the indexed end-diastolic (β ± SE: 0.007 ± 0.002, R = 0.42, P = 0.013) and end-systolic volumes (β ± SE: 0.007 ± 0.003, R = 0.42, P = 0.014). BCW(i) correlated with mean pulmonary arterial pressure (β ± SE: 0.023 ± 0.010, R = 0.37, P = 0.030), indexed end-diastolic volume (β ± SE: 0.010 ± 0.003, R = 0.55, P = 0.001), indexed end-systolic volume (β ± SE: 0.010 ± 0.003, R = 0.53, P = 0.002), and RV ejection fraction (β ± SE: −0.038 ± 0.015, R = 0.41, P = 0.017). Finally, c-MPA correlated with mean pulmonary arterial pressure (β ± SE: 0.012 ± 0.006, R = 0.35, P = 0.043), indexed end-diastolic volume (β ± SE: 0.004 ± 0.002, R = 0.40, P = 0.019), indexed end-systolic volume (β ± SE: 0.004 ± 0.002, R = 0.37, P = 0.026), and RV ejection fraction (β ± SE: −0.024 ± 0.008, 0.45, P = 0.008). Correlations with mean pulmonary arterial pressure and RV ejection fraction are shown in Fig. 3.
Table 5.
Correlative analysis
| ln [FCW(i)] | ln [BCW(i)] | ln [c-MPA] | |
|---|---|---|---|
| Main pulmonary arterial pressure | −0.010 ± 0.009, −0.20, 0.270 | 0.023 ± 0.010, 0.37, 0.030* | 0.012 ± 0.006, 0.35, 0.043* |
| pulmonary vascular resistance index | −0.013 ± 0.019, −0.10, 0.496 | 0.033 ± 0.021, 0.26, 0.138 | 0.009 ± 0.013, 0.10, 0.5103 |
| RV end-diastolic pressure | 0.032 ± 0.058, 0.10, 0.5845 | 0.032 ± 0.058, 0.10, 0.584 | 0.056 ± 0.039, 0.24, 0.160 |
| RV end-diastolic volume index | 0.007 ± 0.002, 0.42, 0.013* | 0.010 ± 0.003, 0.55, 0.001* | 0.004 ± 0.002, 0.40, 0.019* |
| RV end-systolic volume index | 0.007 ± 0.003, 0.42, 0.014* | 0.010 ± 0.003, 0.53, 0.002* | 0.004 ± 0.002, 0.37, 0.026* |
| RV ejection fraction | −0.018 ± 0.014, −0.22, 0.214 | −0.038 ± 0.015, 0.41, 0.017* | −0.024 ± 0.008, 0.45, 0.008* |
Correlations are β-values ± SE with corresponding R values and P values. FCW(i), indexed forward compression wave; BCW(i), indexed backward compression wave; c-MPA, wave speed/pulse wave velocity of the main pulmonary artery; RV, right ventricular.
P < 0.05.
Fig. 3.
Graphical summary of observed correlations between indexed indexed backward compression wave [BCW(i)] magnitude with catheterization-derived mean pulmonary arterial pressure (mPAP; A) and right ventricular ejection fraction (B). Similarly, wave speed (pulse wave velocity) measured in the main pulmonary artery (c-MPA) correlated with mPAP (C) and right ventricular ejection fraction (D).
Prognostic analysis.
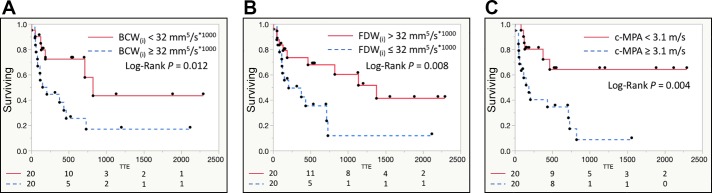
To investigate the prognostic ability of noninvasively derived WIA indexes to detect future functional class worsening, we performed univariate proportional hazard analysis between all considered WIA metrics and, in comparison, also standard catheterization and MRI PAH-specific hemodynamic markers. From all considered patients, we recorded 19 clinical events indicative of functional worsening; a forest plot summarizing our findings is shown in Fig. 4. Among the clinical outcomes indicating functional worsening were WHO functional class escalation (n = 12), syncopal event (n = 3), PAH-related hospitalization (n = 2), and need for initiation of prostanoid therapy (n = 2). We found that elevated BCW(i) [HR = 2.91, confidence interval (CI): 1.18–7.55, P = 0.019] is prognostic of functional worsening along with reduced FDW(i) (HR = 0.34, CI: 0.11–0.90, P = 0.030). Furthermore, increased c-MPA was prognostic of functional worsening (HR = 3.67, CI: 1.47–10.20, P = 0.004). In comparison, prognostic catheterization-derived indexes were mean pulmonary arterial pressure (HR = 4.13, CI: 1.60–11.52, P = 0.004), indexed PVR (HR = 3.84, CI: 1.49–11.01, P = 0.007), and RV end-diastolic pressure (HR = 2.88, CI: 1.19–7.39, P = 0.021). The only MRI hemodynamic index prognostic of functional worsening was RV ejection fraction (HR = 0.28, CI: 0.11–0.66, P = 0.004). To further illustrate the prognostic potential of noninvasive WIA indexes, we constructed Kaplan-Meier curves for WIA metrics, which were shown to be significant in Cox proportional analysis (Fig. 5). Specifically, BCW(i) magnitude ≥32 mm5·s−1·ml−1 was shown to be prognostic of functional worsening along with FDW(i) magnitude ≤ 32 mm5·s−1·ml−1 and c-MAP ≥ 3.1 m/s.
Fig. 4.
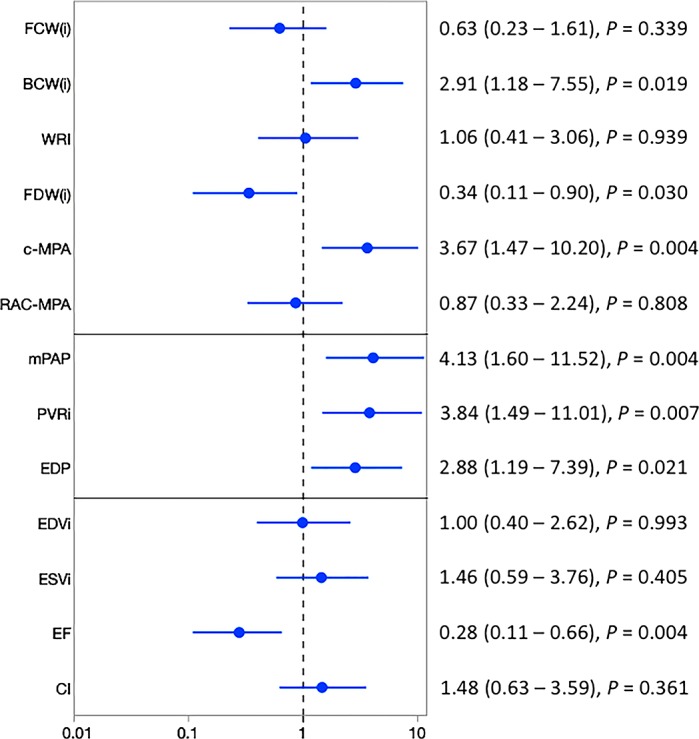
Forest plot summarizing the univariate Cox proportional hazard analysis investigating the prognostic ability of wave intensity analysis, catheterization, and MRI hemodynamic indexes to depict clinical functional worsening. Increased indexed backward compression wave [BCW(i)] and elevated wave speed/pulse wave velocity measured in the main pulmonary artery (c-MPA) were prognostic of functional deterioration. Additionally, reduced indexed forward decompression wave [FDW(i)] was prognostic of functional worsening. All typical catheterization markers and MRI-derived right ventricular ejection fraction (EF) were prognostics of functional worsening. CI, cardiac index; EDP, end-diastolic pressure; EDVi, end-diastolic volume index; ESVi, end-systolic volume index; FCW(i), indexed forward compression wave; mPAP, mean pulmonary arterial pressure; PVRi, pulmonary vascular resistance index; RAC-MPA, relative area change of the main pulmonary artery; WRI, wave reflection index.
Fig. 5.
Kaplan-Meier curves depicting the prognostic potential of noninvasive wave intensity analysis metrics. Indexed backward compression wave [BCW(i), A], indexed forward decompression wave [FDW(i), B], and wave speed propagation (pulse wave velocity) measured in the main pulmonary artery (c-MPA, C). TTE, time to event.
DISCUSSION
In this study, we have shown that children with PAH have increased wave propagation speed along the MPA (c-MPA) and have incidental systolic BCW [BCW(i)], both of which are indicative of elevated pulmonary arterial stiffness. Furthermore, c-MPA and BCW(i) were associated with typical catheterization and MRI indexes known to be strongly associated with poor clinical outcomes in both adult and pediatric populations. Most importantly, WIA indexes were prognostic of clinical functional worsening suggesting that noninvasive MRI indexes should be considered as a component of clinical follow-up in pediatric PAH. Intrinsic pulmonary arterial wall stiffness and backward arterial wave reflections significantly contribute to RV afterload (8, 16). The more recently appreciated concept of proximal pulmonary vascular remodeling (27, 36) with the strong predictive role of noninvasively measured compliance metrics in adult patients with PAH (8, 31, 33) opens a new route of comprehensive PAH analysis using WIA in children.
Wave propagation speed and WIA measurements in the pulmonary circulation using noninvasive imaging are scarce. WIA is typically conducted by means of simultaneous right heart catheterization and Doppler imaging resulting in pressure-velocity-based analysis, the so-called “P-U method.” With regard to incident wave reflection, the flow-area (Q-A) method applied in this study has been previously shown to underestimate wave propagation speed measurements, whereas the P-U method typically overestimates them (29). However, Quail et al. showed that the degree of overestimation for a given incident wave is greater by the P-U method than the degree of underestimation by the Q-A method (23). The noninvasive standard for wave speed analysis is the wave propagation method, which requires measurement of flow hemodynamic waveform at two different locations sufficiently apart and requires low temporal resolution (39). Unfortunately, this method is more applicable to the systemic vasculature, where sufficient length between aortic segments can be guaranteed, and is thus not practical in the pediatric setting per se in the pulmonary vasculature.
WIA in pediatric PAH.
The concept of WIA has been widely applied in studies of central aortic stiffness and systemic hypertension (24, 40). With regard to the pulmonary arterial vasculature, WIA has been typically conducted using combined pressure and velocity waveforms derived from simultaneous right heart catheterization and echocardiography. This approach has been shown to be useful for characterization of adult patients with PAH and chronic thromboembolic pulmonary hypertension (17, 30) but is less applicable in children with PAH because of its complexity and invasive nature. More recently, Quail et al. (23) introduced noninvasive WIA methodology applicable to pulmonary arteries applying high-resolution phase-contrast MRI-derived area and flow waveforms to characterize different adult PAH populations. All aforementioned adult studies described abnormal WIA indexes typically characterized by reduced FCW suggestive of diminished RV contractile function, elevated/present BCW indicative of elevated stiffness of the pulmonary arterial vasculature, and reduced FDW typically associated with reduced RV compliance and poor diastolic function. Furthermore, both invasive and noninvasively derived WIA differentiated between idiopathic PAH and thromboembolic pulmonary hypertension (23, 30).
In this study, we applied noninvasive phase-contrast MRI to characterize the pulmonary arterial system in children with PAH. Compared with adult studies, we have not observed significantly reduced FCW and FDW compared with the healthy population of the same age category. This may be partially explained by better RV functional reserve in children compared with adult patients with PAH and different disease mechanisms responsible for vascular remodeling (2). The most prominent feature of WIA spectra in our patient population was the presence of BCW, which has been previously reported in adult studies and appears to be a characteristic feature of PAH (17, 23, 30). The additive impact of BCW on ventricular afterload has been demonstrated in both systemic and pulmonary circulations and is typically associated with ventricular hypertrophy, eccentric remodeling, and worsening ventricular-vascular coupling ratio (6, 24, 40). BCW represents the primary reflection of the FCW generated by the ventricle and seems to originate primarily in large bifurcations corresponding on the pulmonary side to secondary and tertiary pulmonary artery branches (11, 21). However, incident wave reflections experienced by the RV do not arise from a single reflection site but instead are a composition of waves originating from the entire pulmonary arterial tree including distal resistant arterioles. Consequently, it is impossible to exactly determine whether the BCW observed in our study arise predominantly from proximal or distal vessel branches. Serial measurements along the pulmonary arterial tree would have to be conducted to better understand the relationship between experienced incident wave reflections and their respective origins. Previous work by Davies et al. applying serial catheterizations and numerical modeling emphasized that reflection sites more proximal to the location of measurement contribute greatly to the wave magnitude, with distal sites contributing considerably less (5). Similarly to the study conducted by Quail et al., we have failed to observe a relationship between WIA markers and steady-state measures (PVR index) reflective of the hemodynamic state of the entire pulmonary arterial system (23). Overall, these findings would correlate with the present observations highlighting the role of structural remodeling of proximal pulmonary arteries in PAH, as characterized by cellular proliferation and altered extracellular matrix composition (36). These present and previous findings support the importance of developing novel therapeutic aims targeting proliferative and remodeling pathways in pulmonary arterial endothelial cells rather than treatments focusing on pulmonary vasodilation (27).
Elevated wave speed propagation or pulse wave velocity measured in the MPA has been previously described in adult patients with PAH (17, 23, 30) and in association with RV afterload (6) but has not previously been described in children with PAH. In this study, we report elevated c-MPA as measured in the mid-MPA using a flow-area method that is more sensitive to locally measured stiffness properties. This method might be preferable in children over more conventional wave propagation methods, which require two flow hemodynamic waveforms that are sufficiently temporarily separated from each other (39). Furthermore, flow hemodynamic forces acting on endothelial surfaces can promote vascular remodeling through mechanisms that might differ in the MPA and branch pulmonary arteries because of different degrees of stiffness at these locations (25). Unfortunately, our imaging protocol did not uniformly apply branch pulmonary artery imaging, which limited our ability to assess the WIA and stiffness evaluation in more distal locations. Future longitudinal studies considering serial WIA evaluations with respect to RV functional evaluation are required to assess the direct relationship between ventricular and vascular function in PAH.
WIA and RV function.
Abnormal wave reflections with elevated MPA stiffness have been previously associated with RV function in both adult and pediatric PAH populations (18, 26, 34, 38). Our study showed that FCW, BCW, and c-MPA are each reflective of RV volumes that have been previously associated with mortality in pediatric PAH (18). The relationship between RV function and pulmonary arterial compliance has been thoroughly evaluated using ventricular-vascular coupling studies, which have been shown to be prognostic of pulmonary vasoreactivity in children with PAH (37). However, abnormal wave reflections can significantly contribute to RV afterload and thus perpetuate RV remodeling and dilation, yet the exact pathophysiological mechanics remain unknown. We speculate that BCW might be decelerating the stroke bolus propagating through proximal pulmonary arteries, which exposes the RV to additional mechanical stress requiring more energetic demand. This would be supported by observed association between RV ejection fraction with BCW and c-MPA. Interestingly, the WIA indexes were not associated with PVR index, further suggesting that measured BCW is more reflective of proximal pulmonary arterial stiffening. This additional augmentation of afterload should appear with disease progression when altered flow and pulmonary arterial dilation can induce proximal pulmonary arterial remodeling toward stiffer characters and accelerate the mechanical decoupling of the RV and pulmonary arteries. These findings have been previously described from animal and in vitro studies that demonstrated extensive arterial remodeling in the extracellular matrix as characterized by increased collagen deposition within proximal pulmonary arteries and loss of elastin-dominant behavior during systolic luminal expansion (15, 35). Altered intrinsic tissue characteristics can reduce the elastic buffering function of “proximal elastic arteries,” leading to altered vessel wall biomechanical properties with abnormal WIA and elevated pulse wave velocity. These findings suggest that drugs that can inhibit extracellular matrix remodeling in large pulmonary arteries may alleviate the increases in afterload with progressive PAH.
WIA and functional worsening.
Functional deterioration is a hallmark of progressive PAH typically requiring an escalation of therapy and more frequent clinical followup (1). The search for novel noninvasive indexes prognostic of clinical worsening is of utmost importance to prevent dramatic clinical consequences such as need for atrial septostomy, creation of Pott’s shunt, or lung transplantation. Moledina et al. (18) have previously described the strong prognostic role of MRI-derived typical ventricular functional and volumetric indexes in predicting need for lung transplantation and death in children with PAH. More recently, Swift et al. (31) described the most comprehensive noninvasive MRI study in adult patients with PAH revealing the independent prognostic roles of RV volumes and relative area change measured in the MPA. These findings suggest that both RV function and proximal pulmonary arterial stiffness may play important roles during clinical followup and might be considered part of routine clinical evaluation. In this study, we showed that noninvasively derived WIA indexes are predictive of functional worsening in the child population, which typically precedes more dramatic clinical outcomes. Specifically, elevated c-MPA and magnitude of BCW have been shown to be associated with functional worsening. Although our results were significant, we recognize that the relatively small number of patients and corresponding clinical events limits the broad application of our results. Pediatric PAH studies are typically challenged with limited recruitment and usually require multicenter collaborations to achieve sufficient prognostic power (1, 13, 41). We speculate that early noninvasive detection of WIA abnormalities and elevated pulmonary arterial stiffness may lead to therapeutic escalation and aid with overall clinical management.
Furthermore, we have identified the prognostic role of FDW typically associated with RV compliance and diastolic function. Indeed, previous echocardiographic studies assessing the diastolic strain and strain rate have described a significant prognostic role of diastolic dysfunction and its related indexes in children with PAH (4, 14). Biventricular myocardial remodeling has been described using delayed gadolinium studies and mechanical deformation analyses in both the adult and pediatric PAH population (32). The limited ability of a stiff RV to generate sufficient preload leads to elevated systemic venous pressure, abnormal right atrial function, reduced RV stroke volume, and decreased pulmonary perfusion. Further evaluation of diastolic dysfunction using more specific ventricular mechanical and flow analysis techniques should be performed to better understand the progressive role of PAH diastolic dysfunction in pediatric patients with PAH.
Limitations.
We acknowledge several limitations associated with our study. First, we did not perform WIA in branch pulmonary arteries, which would have enhanced our understanding of the nature of backward wave reflections and possible heterogeneity of proximal pulmonary vascular stiffness. Second, some of our patients were receiving anesthesia or sedation during MRI acquisition that potentially could alter flow hemodynamics. However, as previously stated, this is largely unavoidable because the vast majority of pediatric catheterizations at our institution are performed under general anesthesia, and within this study, only eight patients with PAH (<8 yr) required anesthesia for both MRI and catheterization. Another limitation of this study is related to the heterogenous composition of our patient population. Although all patients have met hemodynamic criteria for PAH, different disease etiologies were present within our patient population. Future studies will focus on the nature of vascular stiffness and remodeling in specific PAH pathologies. Finally, our study subjects underwent phase-contrast MRI acquisition on two different scanning systems. Ideally, the same acquisition sequence and vendor system should have been applied to every subject. Furthermore, given that both systems operate on different magnetic field strengths, intersystem variability is an important consideration.
Conclusions.
We found that children with PAH have abnormal WIA patterns along with elevated pulse wave velocity, which reflect increased proximal pulmonary arterial stiffness. The WIA indexes correlated with typical catheterization-derived indexes of PVR and were prognostic of clinical functional worsening. Our results suggest that noninvasively derived biomarkers of PVR and stiffness may be helpful during routine clinical follow-up of children with PAH. Furthermore, additional therapeutic targets aimed at proximal pulmonary vascular remodeling should be considered beyond the use of pulmonary vasodilation.
GRANTS
This research was supported in part by the Jayden de Luca Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S., N.W., D.D.I., L.P.B., and U.T. conceived and designed research; M.S., R.I., L.P.B., G.M., M.R., D.M., B.F., M.D.M., K.S.H., and U.T. performed experiments; M.S., N.W., R.I., S.A., G.M., M.R., D.M., A.J.B., B.F., M.D.M., K.S.H., and U.T. analyzed data; M.S., N.W., D.D.I., R.I., S.A., L.P.B., G.M., M.R., D.M., A.J.B., B.F., M.D.M., K.S.H., and U.T. interpreted results of experiments; M.S. and N.W. prepared figures; M.S., N.W., D.D.I., S.A., L.P.B., D.M., A.J.B., and U.T. drafted manuscript; M.S., N.W., D.D.I., R.I., S.A., L.J.B., G.M., M.R., D.M., A.J.B., B.F., M.D.M., K.S.H., and U.T. edited and revised manuscript; M.S., N.W., D.D.I., R.I., S.A., L.P.B., G.M., M.R., D.M., A.J.B., B.F., M.D.M., K.S.H., and U.T. approved final version of manuscript.
REFERENCES
- 1.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Surgery and Anesthesia; American Thoracic Society . Pediatric pulmonary hypertension: guidelines from the American Heart Association and American Thoracic Society. Circulation 132: 2037–2099, 2015. [Erratum in Circulation 133: e368, 2016. doi: 10.1161/CIR.0000000000000363.] doi:. [DOI] [PubMed] [Google Scholar]
- 2.Barst RJ, Ertel SI, Beghetti M, Ivy DD. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 37: 665–677, 2011. doi: 10.1183/09031936.00056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biglino G, Schievano S, Steeden JA, Ntsinjana H, Baker C, Khambadkone S, de Leval MR, Hsia TY, Taylor AM, Giardini A; Modeling of Congenital Hearts Alliance (MOCHA) Collaborative Group . Reduced ascending aorta distensibility relates to adverse ventricular mechanics in patients with hypoplastic left heart syndrome: noninvasive study using wave intensity analysis. J Thorac Cardiovasc Surg 144: 1307–1314, 2012. doi: 10.1016/j.jtcvs.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Burkett DA, Slorach C, Patel SS, Redington AN, Ivy DD, Mertens L, Younoszai AK, Friedberg MK. Impact of pulmonary hemodynamics and ventricular interdependence on left ventricular diastolic function in children with pulmonary hypertension. Circ Cardiovasc Imaging 9: e004612, 2016. doi: 10.1161/CIRCIMAGING.116.004612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies JE, Alastruey J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, Aguado-Sierra J, Willson K, Foale RA, Malik IS, Hughes AD, Parker KH, Mayet J. Attenuation of wave reflection by wave entrapment creates a “horizon effect” in the human aorta. Hypertension 60: 778–785, 2012. doi: 10.1161/HYPERTENSIONAHA.111.180604. [DOI] [PubMed] [Google Scholar]
- 6.Dawes TJ, Gandhi A, de Marvao A, Buzaco R, Tokarczuk P, Quinlan M, Durighel G, Diamond T, Monje Garcia L, de Cesare A, Cook SA, O’Regan DP. Pulmonary artery stiffness is independently associated with right ventricular mass and function: a cardiac MR imaging study. Radiology 280: 398–404, 2016. doi: 10.1148/radiol.2016151527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Cerro MJ, Moledina S, Haworth SG, Ivy D, Al Dabbagh M, Banjar H, Diaz G, Heath-Freudenthal A, Galal AN, Humpl T, Kulkarni S, Lopes A, Mocumbi AO, Puri GD, Rossouw B, Harikrishnan S, Saxena A, Udo P, Caicedo L, Tamimi O, Adatia I. Cardiac catheterization in children with pulmonary hypertensive vascular disease: consensus statement from the Pulmonary Vascular Research Institute, Pediatric and Congenital Heart Disease Task Forces. Pulm Circ 6: 118–125, 2016. doi: 10.1086/685102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gan CT, Lankhaar JW, Westerhof N, Marcus JT, Becker A, Twisk JW, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 132: 1906–1912, 2007. doi: 10.1378/chest.07-1246. [DOI] [PubMed] [Google Scholar]
- 9.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54, Suppl: S10–S19, 2009. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Heiberg E, Sjögren J, Ugander M, Carlsson M, Engblom H, Arheden H. Design and validation of Segment: freely available software for cardiovascular image analysis. BMC Med Imaging 10: 1, 2010. doi: 10.1186/1471-2342-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollander EH, Wang JJ, Dobson GM, Parker KH, Tyberg JV. Negative wave reflections in pulmonary arteries. Am J Physiol Heart Circ Physiol 281: H895–H902, 2001. doi: 10.1152/ajpheart.2001.281.2.H895. [DOI] [PubMed] [Google Scholar]
- 12.Hopper RK, Abman SH, Ivy DD. Persistent challenges in pediatric pulmonary hypertension. Chest 150: 226–236, 2016. doi: 10.1016/j.chest.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivy DD, Abman SH, Barst RJ, Berger RMF, Bonnet D, Fleming TR, Haworth SG, Raj JU, Rosenzweig EB, Schulze Neick I, Steinhorn RH, Beghetti M. Pediatric pulmonary hypertension. J Am Coll Cardiol 62, Suppl: D117–D126, 2013. doi: 10.1016/j.jacc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 14.Jone PN, Schäfer M, Li L, Craft M, Ivy DD, Kutty S. Right atrial deformation in predicting outcomes in pediatric pulmonary hypertension. Circ Cardiovasc Imaging 10: e006250, 2017. doi: 10.1161/CIRCIMAGING.117.006250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lammers SR, Kao PH, Qi HJ, Hunter K, Lanning C, Albietz J, Hofmeister S, Mecham R, Stenmark KR, Shandas R. Changes in the structure-function relationship of elastin and its impact on the proximal pulmonary arterial mechanics of hypertensive calves. Am J Physiol Heart Circ Physiol 295: H1451–H1459, 2008. doi: 10.1152/ajpheart.00127.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lankhaar JW, Westerhof N, Faes TJ, Marques KM, Marcus JT, Postmus PE, Vonk-Noordegraaf A. Quantification of right ventricular afterload in patients with and without pulmonary hypertension. Am J Physiol Heart Circ Physiol 291: H1731–H1737, 2006. doi: 10.1152/ajpheart.00336.2006. [DOI] [PubMed] [Google Scholar]
- 17.Lau EM, Abelson D, Dwyer N, Yu Y, Ng MK, Celermajer DS. Assessment of ventriculo-arterial interaction in pulmonary arterial hypertension using wave intensity analysis. Eur Respir J 43: 1804–1807, 2014. doi: 10.1183/09031936.00148313. [DOI] [PubMed] [Google Scholar]
- 18.Moledina S, Pandya B, Bartsota M, Mortensen KH, McMillan M, Quyam S, Taylor AM, Haworth SG, Schulze-Neick I, Muthurangu V. Prognostic significance of cardiac magnetic resonance imaging in children with pulmonary hypertension. Circ Cardiovasc Imaging 6: 407–414, 2013. doi: 10.1161/CIRCIMAGING.112.000082. [DOI] [PubMed] [Google Scholar]
- 19.Parker KH. An introduction to wave intensity analysis. Med Biol Eng Comput 47: 175–188, 2009. doi: 10.1007/s11517-009-0439-y. [DOI] [PubMed] [Google Scholar]
- 20.Parker KH, Jones CJ. Forward and backward running waves in the arteries: analysis using the method of characteristics. J Biomech Eng 112: 322–326, 1990. doi: 10.1115/1.2891191. [DOI] [PubMed] [Google Scholar]
- 21.Penny DJ, Mynard JP, Smolich JJ. Aortic wave intensity analysis of ventricular-vascular interaction during incremental dobutamine infusion in adult sheep. Am J Physiol Heart Circ Physiol 294: H481–H489, 2008. doi: 10.1152/ajpheart.00962.2006. [DOI] [PubMed] [Google Scholar]
- 22. Ploegstra MJ, Brokelman JG, Roos-Hesselink JW, Douwes JM, van Osch-Gevers LM, Hoendermis ES, van den Bosch AE, Witsenburg M, Bartelds B, Hillege HL, Berger RM. Pulmonary arterial stiffness indices assessed by intravascular ultrasound in children with early pulmonary vascular disease: prediction of advanced disease and mortality during 20-year follow-up. Eur Heart J Cardiovasc Imaging 19: 216–224, 2018. doi: 10.1093/ehjci/jex015. [DOI] [PubMed] [Google Scholar]
- 23.Quail MA, Knight DS, Steeden JA, Taelman L, Moledina S, Taylor AM, Segers P, Coghlan GJ, Muthurangu V. Noninvasive pulmonary artery wave intensity analysis in pulmonary hypertension. Am J Physiol Heart Circ Physiol 308: H1603–H1611, 2015. doi: 10.1152/ajpheart.00480.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quail MA, Short R, Pandya B, Steeden JA, Khushnood A, Taylor AM, Segers P, Muthurangu V. Abnormal wave reflections and left ventricular hypertrophy late after coarctation of the aorta repair. Hypertension 69: 501–509, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schäfer M, Barker AJ, Kheyfets V, Stenmark KR, Crapo J, Yeager ME, Truong U, Buckner JK, Fenster BE, Hunter KS. Helicity and vorticity of pulmonary arterial flow in patients with pulmonary hypertension: quantitative analysis of flow formations. J Am Heart Assoc 6: e007010, 2017. doi: 10.1161/JAHA.117.007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schäfer M, Ivy DD, Barker AJ, Kheyfets V, Shandas R, Abman SH, Hunter KS, Truong U. Characterization of CMR-derived haemodynamic data in children with pulmonary arterial hypertension. Eur Heart J Cardiovasc Imaging 18: 424–431, 2017. doi: 10.1093/ehjci/jew152. [DOI] [PubMed] [Google Scholar]
- 27.Schäfer M, Myers C, Brown RD, Frid MG, Tan W, Hunter K, Stenmark KR. Pulmonary arterial stiffness: toward a new paradigm in pulmonary arterial hypertension pathophysiology and assessment. Curr Hypertens Rep 18: 4, 2016. doi: 10.1007/s11906-015-0609-2. [DOI] [PubMed] [Google Scholar]
- 28.Schäfer M, Truong U, Browne LP, Morgan GJ, Ross M, Ing R, Hunter KS, Kheyfets VO, Abman SH, Ivy DD, Wilson N. Measuring flow hemodynamic indices and oxygen consumption in children with pulmonary hypertension: a comparison of catheterization and phase-contrast MRI. Pediatr Cardiol 39: 268–274, 2018. doi: 10.1007/s00246-017-1751-1. [DOI] [PubMed] [Google Scholar]
- 29.Segers P, Swillens A, Taelman L, Vierendeels J. Wave reflection leads to over- and underestimation of local wave speed by the PU- and QA-loop methods: theoretical basis and solution to the problem. Physiol Meas 35: 847–861, 2014. doi: 10.1088/0967-3334/35/5/847. [DOI] [PubMed] [Google Scholar]
- 30.Su J, Manisty C, Parker KH, Simonsen U, Nielsen-Kudsk JE, Mellemkjaer S, Connolly S, Lim PB, Whinnett ZI, Malik IS, Watson G, Davies JE, Gibbs S, Hughes AD, Howard L. Wave intensity analysis provides novel insights into pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. J Am Heart Assoc 6: e006679, 2017. doi: 10.1161/JAHA.117.006679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swift AJ, Capener D, Johns C, Hamilton N, Rothman A, Elliot C, Condliffe R, Charalampopoulos A, Rajaram S, Lawrie A, Campbell MJ, Wild JM, Kiely DG. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med 196: 228–239, 2017. doi: 10.1164/rccm.201611-2365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swift AJ, Rajaram S, Capener D, Elliot C, Condliffe R, Wild JM, Kiely DG. LGE patterns in pulmonary hypertension do not impact overall mortality. JACC Cardiovasc Imaging 7: 1209–1217, 2014. doi: 10.1016/j.jcmg.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Swift AJ, Rajaram S, Condliffe R, Capener D, Hurdman J, Elliot C, Kiely DG, Wild JM. Pulmonary artery relative area change detects mild elevations in pulmonary vascular resistance and predicts adverse outcome in pulmonary hypertension. Invest Radiol 47: 571–577, 2012. doi: 10.1097/RLI.0b013e31826c4341. [DOI] [PubMed] [Google Scholar]
- 34.Swift AJ, Rajaram S, Hurdman J, Hill C, Davies C, Sproson TW, Morton AC, Capener D, Elliot C, Condliffe R, Wild JM, Kiely DG. Noninvasive estimation of PA pressure, flow, and resistance with CMR imaging: derivation and prospective validation study from the ASPIRE registry. JACC Cardiovasc Imaging 6: 1036–1047, 2013. doi: 10.1016/j.jcmg.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Tan Y, Tseng PO, Wang D, Zhang H, Hunter K, Hertzberg J, Stenmark KR, Tan W. Stiffening-induced high pulsatility flow activates endothelial inflammation via a TLR2/NF-κB pathway. PLoS One 9: e102195, 2014. doi: 10.1371/journal.pone.0102195. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Thompson AA, Lawrie A. Targeting vascular remodeling to treat pulmonary arterial hypertension. Trends Mol Med 23: 31–45, 2017. doi: 10.1016/j.molmed.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Truong U, Patel S, Kheyfets V, Dunning J, Fonseca B, Barker AJ, Ivy D, Shandas R, Hunter K. Non-invasive determination by cardiovascular magnetic resonance of right ventricular-vascular coupling in children and adolescents with pulmonary hypertension. J Cardiovasc Magn Reson 17: 81, 2015. doi: 10.1186/s12968-015-0186-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, Naeije R, Newman J, Oudiz RJ, Provencher S, Torbicki A, Voelkel NF, Hassoun PM. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 62, Suppl: D22–D33, 2013. doi: 10.1016/j.jacc.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Wentland AL, Grist TM, Wieben O. Review of MRI-based measurements of pulse wave velocity: a biomarker of arterial stiffness. Cardiovasc Diagn Ther 4: 193–206, 2014. doi: 10.3978/j.issn.2223-3652.2014.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Westerhof N, Segers P, Westerhof BE. Wave separation, wave intensity, the reservoir-wave concept, and the instantaneous wave-free ratio: presumptions and principles. Hypertension 66: 93–98, 2015. doi: 10.1161/HYPERTENSIONAHA.115.05567. [DOI] [PubMed] [Google Scholar]
- 41.Zijlstra WM, Douwes JM, Rosenzweig EB, Schokker S, Krishnan U, Roofthooft MT, Miller-Reed K, Hillege HL, Ivy DD, Berger RM. Survival differences in pediatric pulmonary arterial hypertension: clues to a better understanding of outcome and optimal treatment strategies. J Am Coll Cardiol 63: 2159–2169, 2014. doi: 10.1016/j.jacc.2014.02.575. [DOI] [PubMed] [Google Scholar]