Abstract
Effective oxygen delivery to active muscle fibers requires that vasodilation initiated in distal arterioles, which control flow distribution and capillary perfusion, ascends the resistance network into proximal arterioles and feed arteries, which govern total blood flow into the muscle. With exercise onset, ascending vasodilation reflects initiation and conduction of hyperpolarization along endothelium from arterioles into feed arteries. Electrical coupling of endothelial cells to smooth muscle cells evokes the rapid component of ascending vasodilation, which is sustained by ensuing release of nitric oxide during elevated luminal shear stress. Concomitant sympathetic neural activation inhibits ascending vasodilation by stimulating α-adrenoreceptors on smooth muscle cells to constrict the resistance vasculature. We hypothesized that compromised muscle blood flow in advanced age reflects impaired ascending vasodilation through actions on both cell layers of the resistance network. In the gluteus maximus muscle of old (24 mo) vs. young (4 mo) male mice (corresponding to mid-60s vs. early 20s in humans) inhibition of α-adrenoreceptors in old mice restored ascending vasodilation, whereas even minimal activation of α-adrenoreceptors in young mice attenuated ascending vasodilation in the manner seen with aging. Conduction of hyperpolarization along the endothelium is impaired in old vs. young mice because of “leaky” membranes resulting from the activation of potassium channels by hydrogen peroxide released from endothelial cells. Exposing the endothelium of young mice to hydrogen peroxide recapitulates this effect of aging. Thus enhanced α-adrenoreceptor activation of smooth muscle in concert with electrically leaky endothelium restricts muscle blood flow by impairing ascending vasodilation in advanced age.
Keywords: adrenoreceptors, aging, ascending vasodilation, endothelium, potassium channels
CONTROL OF MUSCLE BLOOD FLOW
Blood flow throughout the body is governed by the resistance vasculature, which controls the systemic distribution of cardiac output concomitant with blood pressure and perfusion of individual vascular beds. In skeletal muscle, which comprises nearly half of our body mass, resistance vessels supply myofibers via an intricate branching network of arterioles that arise from a parent feed artery (13). Upon entering the muscle, the feed artery transitions into a primary (first-order) arteriole, which branches into smaller, second-order arterioles that give rise to third-order arterioles, and these continue branching into terminal arterioles (Fig. 1A) (84). Each terminal arteriole supplies a group of 15–20 capillaries, collectively known as a microvascular unit (20). Each microvascular unit spans ~1 mm along muscle fibers and encompasses ~0.05 mm3 of tissue volume, with ~20 microvascular units per mm3 (= 1 mg) of muscle that encompasses multiple adjacent muscle fibers (12, 28). Each microvascular unit drains into a collecting venule, and these merge into progressively larger branches comprising venous return. Despite topological differences between muscles of different size and shape (5), branching networks are typical of microvascular architecture in skeletal muscle (4, 31, 46) (Fig. 1A).
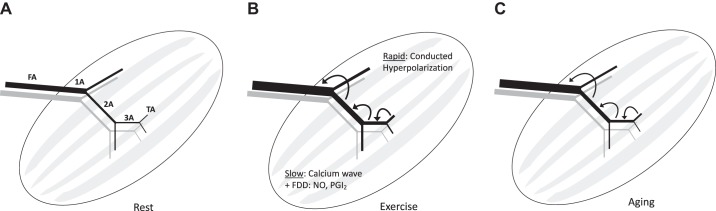
Fig. 1.
Ascending vasodilation in arteriolar networks of skeletal muscle. A: schematic depiction of skeletal muscle microvascular network (black, arterial; gray, venous) beginning with the feed artery (FA) that enters the muscle and transitions into a first-order arteriole (1A). The 1A branches into smaller second-order arterioles (2A), which further branch into third-order arterioles (3A), which ultimately give rise to the terminal arterioles (TA) that control capillary perfusion (not shown). B: in response to skeletal muscle contraction, vasodilation initiated in downstream branches ascends the resistance network into the proximal FA to attain peak increases in muscle blood flow according to the metabolic demand of contracting muscle fibers. The initial rapid component of ascending vasodilation occurs in response to activation of K+ channels, resulting in hyperpolarization that is conducted upstream from cell to cell through gap junctions. The ensuing slower component of ascending vasodilation results from a calcium wave along the endothelium and flow-dependent dilation (FDD) occurring secondary to an increase in luminal shear stress that produces the vasodilator autacoids nitric oxide (NO) and prostacyclin (PGI2). C: with aging, ascending vasodilation is attenuated, thereby limiting muscle blood flow. See Fig. 2 for details of signaling events.
Functional hyperemia reflects dilation of the resistance vasculature in response to contractile activity and serves to enhance the delivery of oxygen and nutrients to active muscle fibers in concert with removing metabolic by-products, thereby coupling local perfusion to metabolic demand. The feed arteries and primary arterioles govern total blood flow entering a muscle, whereas successive branches of arteriolar networks distribute flow according to local requirements of active muscle fibers (2). During intense rhythmic exercise, muscle blood flow can increase 50- to 100-fold (1, 48) owing to a large dilator capacity of the resistance vasculature in concert with an increase in cardiac output and its redistribution effected by the sympathetic nervous system (76). The increase in sympathetic nerve activity during exercise effectively shunts blood away from the viscera and inactive tissues, while contracting skeletal muscle overrides vasoconstriction through “functional sympatholysis” (74, 98). In this manner, dilation of the vascular supply to contracting skeletal muscle is integral to the increase and redistribution of cardiac output during exercise.
In response to a single contraction, there is a near-instantaneous relaxation of the resistance vasculature and a corresponding increase in muscle blood flow (18). This response has become recognized as rapid-onset vasodilation (ROV) and is manifest in humans (19, 103) as well as rodents (58, 89, 108), attributable to hyperpolarization and smooth muscle cell (SMC) relaxation (Fig. 2A). ROV facilitates oxygen delivery upon the initiation of activity during the transition from rest. As shown with the use of intravital microscopy to study mouse gluteus maximus muscle, ROV is initiated in distal branches of the arteriolar network (89) and may well encompass signaling from capillaries (65, 83, 93), i.e., those microvessels most intimately associated with active muscle fibers. Dilation of the smallest arterioles downstream would result in a modest increase in muscle blood flow, as the resistance of upstream branches would limit the volume of blood flowing into the microcirculation. A mechanism that reduces upstream resistance is ascending vasodilation, whereby signals initiated in downstream microvessels travel upstream, opposite the direction of blood flow (Fig. 1B). By manipulating the intensity and duration of muscle contraction, we resolved both rapid and slow components of ascending vasodilation (89). Thus a single tetanic contraction initiates an electrical signal (hyperpolarization) that is conducted rapidly from cell to cell along the resistance network, from distal arterioles into proximal feed arteries. In turn, with sustained rhythmic contractions, flow-dependent dilation of feed arteries is slower in onset and releases nitric oxide in response to elevated luminal shear stress as a consequence of functional vasodilation of downstream arterioles embedded within muscle fibers.
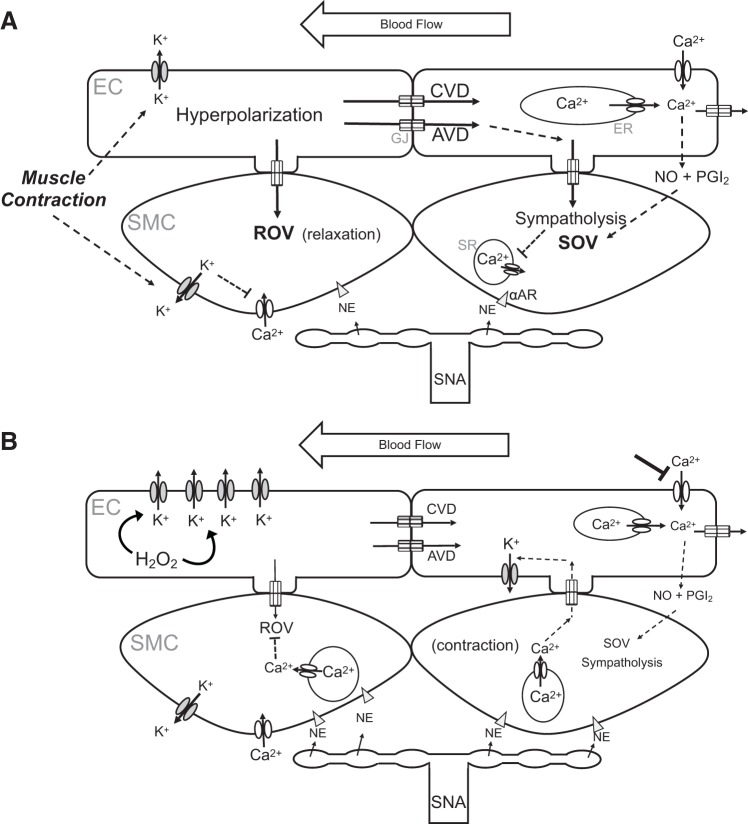
Fig. 2.
Microvascular mechanisms limiting skeletal muscle blood flow with advancing age. A: skeletal muscle contraction (and Ca2+ influx) activates small- and intermediate-conductance K+ channels in endothelial cells (ECs) and inward-rectifying K+ channels of smooth muscle cells (SMCs). The efflux of K+ results in hyperpolarization, thereby inhibiting Ca2+ entry into SMCs through voltage-gated Ca2+ channels and effecting rapid onset vasodilation (ROV). In the endothelium, conducted vasodilation (CVD) and ascending vasodilation (AVD) ensue as hyperpolarization spreads through gap junctions (GJs) from EC to EC and into SMCs through myoendothelial GJs. EC intracellular calcium ion concentration ([Ca2+]i) increases with internal release [from the endoplasmic reticulum (ER)], leading to a Ca2+ wave that spreads from EC to EC, complemented by Ca2+ influx through transient receptor potential channels in response to blood flow (shear stress). The rise in EC [Ca2+]i generates nitric oxide (NO) and prostacyclin (PGI2) that mediate slow onset vasodilation (SOV). These dilator signals override concurrent sympathetic nerve activity (SNA) that releases norepinephrine (NE) onto SMC, attenuating the effects of α-adrenoreceptor (αAR) activation that promote vasoconstriction through release of Ca2+ from the sarcoplasmic reticulum (SR). The net result is functional sympatholysis with elevated blood flow to active skeletal muscle fibers. B: key effects of aging. Elevated hydrogen peroxide (H2O2) in ECs activates K+ channels in the plasma membrane, thereby increasing K+ efflux and attenuating the spread of hyperpolarization and reducing CVD and AVD (as depicted in Fig. 1C) along with sympatholysis. Concurrent increases in SNA result in greater NE release onto SMCs, promoting SMC contraction; the rise in SMC [Ca2+]i promotes Ca2+ diffusion through myoendothelial GJs, further activating K+ channels in ECs to augment signal dissipation. Endothelial dysfunction attenuates the effect of shear stress, reducing the generation of NO and PGI2 and attenuating SOV. A reduction in Ca2+ entry through transient receptor potential channels has a protective effect on the endothelium during oxidative stress.
With advancing age, blood flow to active skeletal muscle is attenuated in humans (22, 73, 109) as well as rodents (3, 66), which underscores a decrease in exercise capacity (39) (Fig. 1C). Understanding this impairment and elucidating the underlying mechanisms provides a foundation for development of strategies to restore and preserve muscle blood flow, thereby supporting the ability to engage in physical activity and maintain the quality of life. The purpose of this minireview is to highlight our advances in elucidating microvascular mechanisms that compromise muscle blood flow in advanced age by exploring how sympathetic activation of smooth muscle interacts with ascending vasodilation along the endothelium (36, 82). As developed below, our studies of the aging microcirculation in mice have revealed subtle increases in the activation of α-adrenergic signaling in conjunction with diminished ability of the endothelium to conduct electrical signals as a consequence of elevated hydrogen peroxide (H2O2). In concert, these events compromise ascending vasodilation and thereby limit muscle blood flow at exercise onset. Endothelial dysfunction further contributes to limiting vasodilation, and thereby oxygen delivery, during sustained activity (Fig. 2B).
SMOOTH MUSCLE CELLS, SYMPATHETIC VASOCONSTRICTION, AND AGING
SMCs encircle all branches of the vascular resistance network; their contraction and relaxation effect changes in vessel diameter to control blood flow magnitude and distribution within the tissue. In addition to spontaneous myogenic tone, SMCs are stimulated by sympathetic nerve activity. Sympathetic axons course along the arterial supply, surrounding feed arteries entering the muscle, and continue along all branches of the arteriolar network (53). The primary neurotransmitter released by sympathetic nerves is norepinephrine, which activates α-adrenoreceptors (αARs) to elicit contraction, vasoconstriction, and a reduction in blood flow. Although both α1AR and α2AR subtypes are present on arteriolar SMCs (Fig. 2A), their functional distribution can vary with branch order between vascular beds (32, 60). At rest, there is a background level of sympathetic nerve activity, and when transitioning to exercise, sympathetic nerve activity increases in proportion to the intensity of skeletal muscle contractions and the muscle mass involved (79, 80).
Advancing age is accompanied by an increase in sympathetic nerve activity under resting conditions in humans (22, 68) and rats (40). Although the release of norepinephrine can increase vasomotor tone and thereby restrict muscle blood flow (73), receptor desensitization with aging in mice (88, 110) as well as humans (21, 23) may mitigate this effect. Nevertheless, increased sympathetic nerve activity will antagonize functional sympatholysis and thereby the ability of active muscle to overcome sympathetic vasoconstriction (Fig. 2B). Indeed, age-related deficits in functional sympatholysis were apparent in early studies of human subjects during cycling exercise (109) and have remained an active area of investigation (24, 43, 45). A recent study found that lifelong physical activity preserves functional sympatholysis in older individuals compared with those having a sedentary lifestyle (61). Thus regular exercise may help maintain exercise capacity by promoting functional hyperemia throughout the life span.
With aging, impaired vasodilator signaling between contracting muscle fibers and the resistance vasculature is exemplified by attenuated ROV (15, 16, 87) (Fig. 2B). Our observations in mouse skeletal (gluteus maximus) muscle are consistent with findings in human subjects, with both approaches implicating a key role for αARs in this impairment. For example, blocking αARs with phentolamine restored ROV in older (~69 yr) vs. younger (~27 yr) male and female human subjects (16) as well as in old (20–24 mo) vs. young (3–4 mo) male mice (41). Coincident with impaired ROV in advanced age is the attenuation of ascending vasodilation (87). This deficit effectively restricts muscle blood flow by limiting the dilation of feed arteries. Studies of the mouse gluteus maximus muscle have shown that conducted vasodilation is also attenuated in advanced age (4) and is not restored by regular exercise [8 wk of voluntary running (3)]. We have found that even minimal activation of αARs (i.e., below that eliciting vasoconstriction) effectively attenuates ROV in response to muscle contraction (41, 87). These data from studies of the intact microcirculation in mice are consistent with results from isolated pressurized feed arteries of hamster skeletal muscle where we demonstrated that increasing sympathetic nerve activity from 2 to 8 Hz (via perivascular nerve stimulation) progressively attenuated conducted vasodilation (36). Collectively, findings from humans and rodents indicate that an increase in sympathetic nerve activity opposes the action(s) of vasodilator metabolites produced by contracting muscle fibers, thereby impairing functional sympatholysis and conducted (ascending) vasodilation and restricting muscle blood flow (Fig. 1C and Fig. 2B).
ENDOTHELIAL CELLS, CONDUCTED VASODILATION, AND AGING
In forming the intima, endothelial cells line the vessel lumen, are in direct contact with the bloodstream, and are subjected to shear stress exerted by the flow of blood while exposed to circulating agents. The endothelium is integral to the regulation of muscle blood flow by eliciting smooth muscle relaxation and vasodilation (Fig. 2A). Intracellular calcium ion concentration ([Ca2+]i) plays a key role in endothelium-dependent vasodilation, with a rise in [Ca2+]i leading to the production of nitric oxide and prostacyclin along with endothelium-dependent hyperpolarization via the opening of small- and intermediate-conductance Ca2+-activated K+ channels (14). The rise in [Ca2+]i reflects its release from intracellular stores (endoplasmic reticulum and mitochondria) complemented by its influx through transient receptor potential channels in the plasma membrane (7, 90, 94, 101). Whereas nitric oxide and prostacyclin are released as vasodilator autacoids and diffuse to surrounding SMCs, hyperpolarization is transmitted directly from endothelial cells to SMCs through myoendothelial gap junctions (29). Moreover, when [Ca2+]i rises in SMCs in response to αAR activation, it can diffuse through myoendothelial gap junctions to activate K+ channels in endothelial cells, providing negative feedback that attenuates sympathetic vasoconstriction (67, 100) (Fig. 2B).
Integral to the ability of resistance networks to coordinate vasomotor responses among downstream and upstream branches is the ability of the endothelium to conduct electrical signals from cell to cell (2, 10, 30). The longitudinal orientation of endothelial cells and their robust coupling to each other through gap junctions underscores this key role in blood flow regulation (35) (Fig. 2A). As the signal (hyperpolarization) is conducted along the intima, it spreads radially into SMCs, resulting in closure of their voltage-gated Ca2+ channels. This conducted response rapidly lowers smooth muscle [Ca2+]i along arterioles and feed arteries to coordinate vasodilation within seconds (104). When feed arteries were stimulated locally with acetylcholine to initiate conducted vasodilation, blocking Ca2+-activated K+ channels (to prevent hyperpolarization) revealed an underlying Ca2+ wave that also spread from endothelial cell to endothelial cell and stimulated the release of nitric oxide, albeit more slowly and over much shorter distances (hundreds of micrometers) than observed for electrical conduction (thousands of micrometers) (25, 97, 104). Selective, localized disruption of the endothelium within a vessel segment abolished conducted vasodilation beyond the site of endothelial cell damage, whereas SMC function remained intact (30, 51, 89). These structural and functional interactions provide a physiological basis supporting the ability of skeletal muscle contraction to initiate ascending vasodilation via the endothelium as developed in ascending vasodilation and its impairment with advanced age.
With advanced age, endothelial cells are characterized by increased levels of reactive oxygen species (ROS) and diminished bioavailability of nitric oxide (62, 81, 96). Increased ROS availability reflects greater production and/or a reduction in their removal (52, 112). Superoxide (which is dismutated to form H2O2) can be produced through mitochondrial respiration, membrane-associated oxidases (e.g., nicotinamide adenine dinucleotide phosphate oxidases, collectively referred to here as NOX) and the uncoupling of nitric oxide synthase (27, 62). Enhanced mitochondrial production of ROS in advanced age has been shown in systemic arteries (105) as well as the myocardium (42) of rats, while greater evolution of H2O2 was manifest in endothelium from resistance arteries in old vs. young mice (90). Additionally, as shown in the rat aorta, aging-related increases in endothelial nitric oxide synthase activity can be accompanied by an increase in superoxide production, with concomitant quenching of nitric oxide to form peroxynitrite (107), thereby reducing the biological availability of nitric oxide in mediating endothelium-dependent dilation (33, 62). Aging increased endothelial nitric oxide synthase-derived ROS production of skeletal muscle arterioles exposed to intraluminal flow (85), while cerebral arterioles from aged rats exhibited increased basal and agonist-induced superoxide production via NOX (56). These findings are consistent with experiments illustrating that inhibiting NOX reversed endothelial dysfunction in soleus muscle feed arteries of aged rats (102).
Our studies have resolved ~50% lower vessel wall catalase activity (which decomposes H2O2 into H2O and O2) associated with ~30% greater evolution of H2O2 by the endothelium of superior epigastric arteries isolated from abdominal muscles of old (24 mo) vs. young (4 mo) mice (90). These resistance arteries have a resting diameter of ~150 μm (38) and are similar to larger arterioles studied from other muscles; their lack of branching has underscored their application to development of the endothelial tube preparation (92) for studies of electrical conduction and calcium signaling discussed below. An important adaptation to aging (and likely other conditions associated with oxidative stress) is an increased role for endothelium-derived H2O2 as a vasodilator autacoid, particularly when the bioavailability of nitric oxide is reduced (33, 62, 86, 111). In contrast to the diffusion of a gas (e.g., nitric oxide) through lipid bilayers, H2O2 diffuses through cell membranes via aquaporin channels (11, 99). Furthermore, unlike highly reactive superoxide and peroxynitrite that have extremely short half-lives and thus react in the vicinity of their production, H2O2 is a relatively stable molecule and can therefore serve as signaling intermediate between endothelial cells and SMCs (59, 86). Additionally, by promoting the formation of disulfide bridges within and between thiol groups, H2O2 can modify protein function and thereby alter cellular activity (64), e.g., activation of K+ channels (Fig. 2B). Collectively, aging-related changes in signaling properties of the endothelium contribute to vascular dysfunction and can restrict muscle blood flow by impairing the ability of the endothelium to mediate vasodilation via conduction as well as nitric oxide production (63, 78, 81, 96).
ASCENDING VASODILATION AND ITS IMPAIRMENT WITH ADVANCED AGE
Electrical and chemical signals are activated throughout the resistance network in response to physical forces associated with skeletal muscle contraction, including elevated luminal shear stress during hyperemia and mechanical compression of the vessel (17, 57, 77). In humans (19) and mice (89), muscle contraction activates endothelial K+ channels, resulting in hyperpolarization. Once activated, the electrical signal spreads along the endothelium and into surrounding SMCs as described above for conducted vasodilation. This signaling pathway explains the rapid component of ascending vasodilation that can be evoked in response to a single contraction (19, 89). In turn, the slower component of ascending vasodilation is associated with nitric oxide released in response to elevated shear stress in feed arteries subsequent to metabolic dilation of downstream arterioles during rhythmic contractions (49, 89) (Fig. 2A).
In the mouse gluteus maximus muscle during rhythmic contractions, arteriolar dilations were similar between young and old mice; however, erythrocyte velocity was slower in association with lower blood flow in the old mice (41). This finding implied that blood flow was restricted by the upstream feed arteries supplying the arteriolar networks observed downstream. In recent studies, we found both ROV and ascending vasodilation to be attenuated in old vs. young mice and attributable to the activation of αARs (87). Our direct observations of attenuated ROV in the mouse microcirculation may therefore help to explain the attenuation of ROV and blood flow restriction with aging in limb muscles of human subjects (15, 16).
Using intact, freshly isolated endothelium of the superior epigastric artery (92), we investigated a mechanism for diminished conducted vasodilation with aging. Gently dissociating SMCs from the vessel produces endothelial cell “tubes” that remain structurally intact, retain endothelial cell coupling through gap junctions, and maintain the integrity of Ca2+ and electrical signaling (8, 91). This model therefore provides a powerful experimental approach for studying functional properties intrinsic to microvascular endothelium in the absence of influences from surrounding cells or luminal blood flow. With dual intracellular microelectrodes, current was injected into one endothelial cell while changes in membrane potential were recorded at defined separation distances (9). A key finding was that the change in membrane potential at any given distance was lower in the endothelium of old mice vs. young mice. As alluded to above, H2O2 can alter protein function. When the endothelium from old mice was treated with catalase to decompose H2O2, the change in membrane potential increased at each distance from the site of current injection, indicating that conduction of the electrical signal became more effective. Conversely, application of exogenous H2O2 to the endothelium of young mice diminished electrical conduction in the manner seen with advanced age. We found that inhibition of Ca2+-activated K+ channels restored electrical conduction in old endothelium, while activation of the same channels in young endothelium attenuated electrical conduction (9). These findings led to the conclusion that the impairment in electrical conduction along the endothelium of old mice was attributable to an increase in Ca2+-activated K+ channel function as a consequence of greater H2O2 availability (90). Thus whereas lipid bilayers (i.e., cell membranes) are effective electrical insulators, the opening of ion channels allows current to flow (i.e., leak) through the membrane. The ensuing charge loss dissipates the electrical signal that underlies conducted vasodilation and thereby attenuates the rapid component of ascending vasodilation. Moreover, such an effect can be exacerbated by increasing the adrenergic activation of SMCs, which, through the myoendothelial coupling discussed above, further stimulates Ca2+-activated K+ channels (67, 100) and dissipates the electrical signal underlying conducted vasodilation (Fig. 2B).
In vivo studies have demonstrated competition between conducted vasodilation and sympathetic vasoconstriction in arterioles (47) and feed arteries (37), further suggesting a functional interaction between these signaling pathways (82). Thus ascending vasodilation initiated downstream within the active muscle interacts with sympathetic vasoconstriction propagating along perivascular nerves, with feed arteries serving as a key site of integrating respective signals (82). Our studies of feed arteries isolated from hamster skeletal muscle illustrate that increasing sympathetic nerve activity progressively attenuated conducted vasodilation (36), lending further support to the concept of competition between ascending vasodilation and sympathetic vasoconstriction first shown in vivo (37). With respect to the slower component of conducted vasodilation (25) and ascending vasodilation (89), a potential effect of aging on endothelial cell function that remains to be elucidated centers on the reduced bioavailability of nitric oxide. Thus, even if the spread of a calcium wave along the endothelium (or shear stress exerted by luminal flow) persists, reducing the amount of nitric oxide produced should inhibit the slow component of ascending vasodilation and attenuate muscle blood flow during sustained activity (Fig. 2B). As discussed above, however, this may be compensated for by a greater role for H2O2 in mediating endothelium-dependent vasodilation (33, 62, 86, 111).
ENDOTHELIAL CELL PROTECTION WITH AGING
Studies of the aging vasculature typically indicate a progressive loss of function, even during healthy aging. However, the news is not all bad! In studying endothelial tubes from young vs. old mice, we found that when exposed to acute oxidative stress (200 µM H2O2 for 20 min) endothelium from young mice exhibited a fourfold greater increase in [Ca2+]i vs. endothelium from old mice, attributed to greater Ca2+ influx through transient receptor potential channels in the plasma membrane (Fig. 2B) (90). Extending H2O2 exposure to 60 min resulted in a fivefold greater death of endothelial cells in the endothelium of young (35%) vs. old (7%) mice (90). These findings suggest an adaptation associated with aging whereby endothelial cells continuously exposed to ROS during advanced age become protected from oxidative stress-induced injury and death. As suggested in the discussion above, when faced with chronically elevated levels of ROS the endothelium adapts and is able to use ROS in signaling under physiological conditions when nitric oxide bioavailability is reduced (95, 106, 112). Studies in humans and mice have demonstrated that H2O2 derived from endothelial cells can hyperpolarize SMCs to produce vasodilation (54, 55), e.g., through activating large-conductance calcium-activated channels (BKCa) in SMCs (50). These studies further indicate an adaptive shift in the aged endothelium from a reliance on nitric oxide as the primary vasodilator to a greater influence of ROS (particularly H2O2) in the regulation of muscle blood flow. Moreover, this adaptation occurs in association with protective mechanisms to ensure cell survival and the maintenance of vasomotor control. Nevertheless, repeated incidences of acute oxidative stress lead to irreversible cellular damage with accumulation of senescent cells and increased pathology characteristic of aging (115).
INTEGRATION AND FUTURE DIRECTIONS
In addition to the reduction in nitric oxide bioavailability and endothelial dysfunction discussed above, there are increases in vasoconstrictors including endothelin-1 (34) and angiotensin II (113) as well as alterations in the composition of skeletal muscle as a tissue (71). Thus a multitude of mechanisms and changes in the regulation of key signaling pathways contribute to decrements in muscle blood flow (or lack thereof via compensatory adaptations) reported in advanced age (26, 44, 70, 72, 73). In addition to the nature of exercise performed (e.g., tasks involving multiple muscle groups vs. contractions of isolated muscle groups), training status (6, 61) and sex (69) can influence the effect of aging on muscle blood flow in a manner that can differ between limbs (114). Moreover, attenuated muscle blood flow with aging may be explained by a reduction in vascular conductance (e.g., vascular remodeling), such that the resistance to blood flow in skeletal muscle is greater in older vs. younger subjects (72, 75) irrespective of vasoconstrictor or vasodilator signaling.
Within the microcirculation, integrated findings suggest that endothelial cell hyperpolarization initiated via local activation of potassium channels spreads along the endothelium and into surrounding SMCs by electrical coupling through gap junction channels. Thus, once initiated, conducted vasodilation enables the rapid component of ascending vasodilation from downstream arterioles into their feed arteries located upstream, external to the muscle. In turn, the flow-dependent component of ascending vasodilation is slower in onset and occurs secondary to an increase in luminal shear stress that stimulates the release of autacoids (e.g., nitric oxide) to relax SMCs. With advancing age conducted vasodilation is impaired, as reflected in diminished ascending vasodilation; the resistance of proximal vessels thereby limits muscle blood flow. This situation is exacerbated by attenuated functional sympatholysis, which enables greater sympathetic vasoconstriction. Respective adaptations are adverse, as they collectively impair the ability to transition from rest to activity or to sustain elevated workloads. Impaired conduction of electrical signals along the endothelium can be explained by the activation of ion channels by H2O2, which thereby dissipates the electrical signal via charge loss through “leaky” cell membranes. This effect may be compounded by the activation of αARs on SMCs, which leads to further activation of ion channels (and signal dissipation) in both cell layers. Nevertheless, aging exerts a beneficial effect, whereby the endothelium adapts during aging by acquiring resilience to oxidative stress-induced damage and transitions to greater reliance on H2O2 as a signaling modality in regulating muscle blood flow.
In light of adaptations that sustain blood flow regulation during aging, several key questions are apparent. Foremost in our current research is resolving whether aging has a protective effect on SMCs in the manner shown for endothelial cells. Identifying specific adaptations in the plasma membrane in respective cell types that protect the integrity of the lipid bilayer as well as the regulation of constitutive proteins (e.g., ion channels) and within the cytosol (e.g., ROS scavengers and antioxidants) is paramount to understanding the mechanism(s) of adaptation. Complementary questions center on the regulation of smooth muscle function through alterations in sympathetic nerve activity, adrenoreceptor expression, and downstream signaling events. Supporting studies are needed to understand how smooth muscle and endothelium interact with each other to maintain the functional integrity of the resistance vasculature so as to orchestrate the regulation of muscle blood flow through myoendothelial coupling. Gaining such insight opens new directions for developing selective therapeutic interventions designed to preserve muscle blood flow and maintain the quality of life by supporting physical activity in advanced age.
GRANTS
The authors’ research contributing to this minireview was supported by National Heart, Lung, and Blood Institute Grants R37 HL-041026 and R01 HL-086483 to S. S. Segal and F32 HL-107050 to M. J. Socha.
DISCLAIMERS
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.S. and S.S.S. interpreted results of experiments; M.J.S. and S.S.S. prepared figures; M.J.S. drafted manuscript; M.J.S. and S.S.S. edited and revised manuscript; M.J.S. and S.S.S. approved final version of manuscript.
REFERENCES
- 1.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol 366: 233–249, 1985. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagher P, Segal SS. Regulation of blood flow in the microcirculation: role of conducted vasodilation. Acta Physiol (Oxf) 202: 271–284, 2011. doi: 10.1111/j.1748-1716.2010.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bearden SE, Linn E, Ashley BS, Looft-Wilson RC. Age-related changes in conducted vasodilation: effects of exercise training and role in functional hyperemia. Am J Physiol Regul Integr Comp Physiol 293: R1717–R1721, 2007. doi: 10.1152/ajpregu.00827.2006. [DOI] [PubMed] [Google Scholar]
- 4.Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol 561: 535–545, 2004. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bearden SE, Segal SS. Neurovascular alignment in adult mouse skeletal muscles. Microcirculation 12: 161–167, 2005. doi: 10.1080/10739680590904964. [DOI] [PubMed] [Google Scholar]
- 6.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation 100: 1085–1094, 1999. doi: 10.1161/01.CIR.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 7.Behringer EJ, Segal SS. Impact of aging on calcium signaling and membrane potential in endothelium of resistance arteries: a role for mitochondria. J Gerontol A Biol Sci Med Sci 72: 1627–1637, 2017. doi: 10.1093/gerona/glx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Behringer EJ, Segal SS. Membrane potential governs calcium influx into microvascular endothelium: integral role for muscarinic receptor activation. J Physiol 293: 4531–4548, 2015. doi: 10.1113/JP271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behringer EJ, Shaw RL, Westcott EB, Socha MJ, Segal SS. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+-activated K+ channel activation. Arterioscler Thromb Vasc Biol 33: 1892–1901, 2013. doi: 10.1161/ATVBAHA.113.301514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behringer EJ, Socha MJ, Polo-Parada L, Segal SS. Electrical conduction along endothelial cell tubes from mouse feed arteries: confounding actions of glycyrrhetinic acid derivatives. Br J Pharmacol 166: 774–787, 2012. doi: 10.1111/j.1476-5381.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bienert GP, Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys Acta 1840: 1596–1604, 2014. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Bloch EH, Iberall AS. Toward a concept of the functional unit of mammalian skeletal muscle. Am J Physiol Regul Integr Comp Physiol 242: R411–R420, 1982. doi: 10.1152/ajpregu.1982.242.5.R411. [DOI] [PubMed] [Google Scholar]
- 13.Blomfield LB. Intramuscular vascular patterns in man. Proc R Soc Med 38: 617–618, 1945. [PMC free article] [PubMed] [Google Scholar]
- 14.Busse R, Edwards G, Félétou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci 23: 374–380, 2002. doi: 10.1016/S0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 15.Carlson RE, Kirby BS, Voyles WF, Dinenno FA. Evidence for impaired skeletal muscle contraction-induced rapid vasodilation in aging humans. Am J Physiol Heart Circ Physiol 294: H1963–H1970, 2008. doi: 10.1152/ajpheart.01084.2007. [DOI] [PubMed] [Google Scholar]
- 16.Casey DP, Joyner MJ. Influence of α-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol (1985) 113: 1201–1212, 2012. doi: 10.1152/japplphysiol.00734.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB, Jasperse JL. Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. J Physiol 572: 561–567, 2006. doi: 10.1113/jphysiol.2005.099507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corcondilas A, Koroxenidis GT, Shepherd JT. Effect of a brief contraction of forearm muscles on forearm blood flow. J Appl Physiol 19: 142–146, 1964. doi: 10.1152/jappl.1964.19.1.142. [DOI] [PubMed] [Google Scholar]
- 19.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delashaw JB, Duling BR. A study of the functional elements regulating capillary perfusion in striated muscle. Microvasc Res 36: 162–171, 1988. doi: 10.1016/0026-2862(88)90016-7. [DOI] [PubMed] [Google Scholar]
- 21.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm postjunctional α-adrenergic vasoconstriction in healthy men. Circulation 106: 1349–1354, 2002. doi: 10.1161/01.CIR.0000028819.64790.BE. [DOI] [PubMed] [Google Scholar]
- 22.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation 100: 164–170, 1999. doi: 10.1161/01.CIR.100.2.164. [DOI] [PubMed] [Google Scholar]
- 23.Dinenno FA, Joyner MJ. α-Adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation 13: 329–341, 2006. doi: 10.1080/10739680600618843. [DOI] [PubMed] [Google Scholar]
- 24.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic α-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol 579: 175–186, 2007. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- 27.Drouin A, Thorin-Trescases N, Hamel E, Falck JR, Thorin E. Endothelial nitric oxide synthase activation leads to dilatory H2O2 production in mouse cerebral arteries. Cardiovasc Res 73: 73–81, 2007. doi: 10.1016/j.cardiores.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Emerson GG, Segal SS. Alignment of microvascular units along skeletal muscle fibers of hamster retractor. J Appl Physiol (1985) 82: 42–48, 1997. doi: 10.1152/jappl.1997.82.1.42. [DOI] [PubMed] [Google Scholar]
- 29.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 87: 474–479, 2000. doi: 10.1161/01.RES.87.6.474. [DOI] [PubMed] [Google Scholar]
- 30.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res 86: 94–100, 2000. doi: 10.1161/01.RES.86.1.94. [DOI] [PubMed] [Google Scholar]
- 31.Engelson ET, Skalak TC, Schmid-Schönbein GW. The microvasculature in skeletal muscle. I. Arteriolar network in rat spinotrapezius muscle. Microvasc Res 30: 29–44, 1985. doi: 10.1016/0026-2862(85)90035-4. [DOI] [PubMed] [Google Scholar]
- 32.Faber JE. In situ analysis of α-adrenoceptors on arteriolar and venular smooth muscle in rat skeletal muscle microcirculation. Circ Res 62: 37–50, 1988. doi: 10.1161/01.RES.62.1.37. [DOI] [PubMed] [Google Scholar]
- 33.Félétou M. Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol 156: 545–562, 2009. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goettsch W, Lattmann T, Amann K, Szibor M, Morawietz H, Münter K, Müller SP, Shaw S, Barton M. Increased expression of endothelin-1 and inducible nitric oxide synthase isoform II in aging arteries in vivo: implications for atherosclerosis. Biochem Biophys Res Commun 280: 908–913, 2001. doi: 10.1006/bbrc.2000.4180. [DOI] [PubMed] [Google Scholar]
- 35.Haas TL, Duling BR. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res 53: 113–120, 1997. doi: 10.1006/mvre.1996.1999. [DOI] [PubMed] [Google Scholar]
- 36.Haug SJ, Segal SS. Sympathetic neural inhibition of conducted vasodilatation along hamster feed arteries: complementary effects of α1- and α2-adrenoreceptor activation. J Physiol 563: 541–555, 2005. doi: 10.1113/jphysiol.2004.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haug SJ, Welsh DG, Segal SS. Sympathetic nerves inhibit conducted vasodilatation along feed arteries during passive stretch of hamster skeletal muscle. J Physiol 552: 273–282, 2003. doi: 10.1113/jphysiol.2003.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hayoz S, Pettis J, Bradley V, Segal SS, Jackson WF. Increased amplitude of inward rectifier K+ currents with advanced age in smooth muscle cells of murine superior epigastric arteries. Am J Physiol Heart Circ Physiol 312: H1203–H1214, 2017. doi: 10.1152/ajpheart.00679.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hearon CM Jr, Dinenno FA. Regulation of skeletal muscle blood flow during exercise in ageing humans. J Physiol 594: 2261–2273, 2016. doi: 10.1113/JP270593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito K, Sato A, Sato Y, Suzuki H. Increases in adrenal catecholamine secretion and adrenal sympathetic nerve unitary activities with aging in rats. Neurosci Lett 69: 263–268, 1986. doi: 10.1016/0304-3940(86)90491-X. [DOI] [PubMed] [Google Scholar]
- 41.Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol 588: 2269–2282, 2010. doi: 10.1113/jphysiol.2010.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Judge S, Jang YM, Smith A, Hagen T, Leeuwenburgh C. Age-associated increases in oxidative stress and antioxidant enzyme activities in cardiac interfibrillar mitochondria: implications for the mitochondrial theory of aging. FASEB J 19: 419–421, 2005. doi: 10.1096/fj.04-2622fje. [DOI] [PubMed] [Google Scholar]
- 43.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Modulation of postjunctional α-adrenergic vasoconstriction during exercise and exogenous ATP infusions in ageing humans. J Physiol 589: 2641–2653, 2011. doi: 10.1113/jphysiol.2010.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol 551: 337–344, 2003. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koller A, Dawant B, Liu A, Popel AS, Johnson PC. Quantitative analysis of arteriolar network architecture in cat sartorius muscle. Am J Physiol Heart Circ Physiol 253: H154–H164, 1987 10.1152/ajpheart.1987.253.1.H154. [DOI] [PubMed] [Google Scholar]
- 47.Kurjiaka DT, Segal SS. Interaction between conducted vasodilation and sympathetic nerve activation in arterioles of hamster striated muscle. Circ Res 76: 885–891, 1995. doi: 10.1161/01.RES.76.5.885. [DOI] [PubMed] [Google Scholar]
- 48.Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol Heart Circ Physiol 243: H296–H306, 1982. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- 49.Lie M, Sejersted OM, Kiil F. Local regulation of vascular cross section during changes in femoral arterial blood flow in dogs. Circ Res 27: 727–737, 1970. doi: 10.1161/01.RES.27.5.727. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 93: 573–580, 2003. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 51.Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol (1985) 97: 1152–1158, 2004. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- 52.Mansouri A, Muller FL, Liu Y, Ng R, Faulkner J, Hamilton M, Richardson A, Huang TT, Epstein CJ, Van Remmen H. Alterations in mitochondrial function, hydrogen peroxide release and oxidative damage in mouse hind-limb skeletal muscle during aging. Mech Ageing Dev 127: 298–306, 2006. doi: 10.1016/j.mad.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Marshall JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol 332: 169–186, 1982. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 290: 909–913, 2002. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- 55.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 106: 1521–1530, 2000. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles: role of oxidative stress. Microcirculation 15: 225–236, 2008. doi: 10.1080/10739680701641421. [DOI] [PubMed] [Google Scholar]
- 57.Michel T, Vanhoutte PM. Cellular signaling and NO production. Pflugers Arch 459: 807–816, 2010. doi: 10.1007/s00424-009-0765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mihok ML, Murrant CL. Rapid biphasic arteriolar dilations induced by skeletal muscle contraction are dependent on stimulation characteristics. Can J Physiol Pharmacol 82: 282–287, 2004. doi: 10.1139/y04-016. [DOI] [PubMed] [Google Scholar]
- 59.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 92: e31–e40, 2003. doi: 10.1161/01.RES.0000054200.44505.AB. [DOI] [PubMed] [Google Scholar]
- 60.Moore AW, Jackson WF, Segal SS. Regional heterogeneity of α-adrenoreceptor subtypes in arteriolar networks of mouse skeletal muscle. J Physiol 588: 4261–4274, 2010. doi: 10.1113/jphysiol.2010.194993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590: 6227–6236, 2012. doi: 10.1113/jphysiol.2012.240093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller-Delp JM, Gurovich AN, Christou DD, Leeuwenburgh C. Redox balance in the aging microcirculation: new friends, new foes, and new clinical directions. Microcirculation 19: 19–28, 2012. doi: 10.1111/j.1549-8719.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muller-Delp JM, Spier SA, Ramsey MW, Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol 283: H1662–H1672, 2002. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- 64.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13, 2009. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murrant CL, Lamb IR, Novielli NM. Capillary endothelial cells as coordinators of skeletal muscle blood flow during active hyperemia. Microcirculation 24: e12348, 2017. doi: 10.1111/micc.12348. [DOI] [PubMed] [Google Scholar]
- 66.Musch TI, Eklund KE, Hageman KS, Poole DC. Altered regional blood flow responses to submaximal exercise in older rats. J Appl Physiol (1985) 96: 81–88, 2004. doi: 10.1152/japplphysiol.00729.2003. [DOI] [PubMed] [Google Scholar]
- 67.Nausch LW, Bonev AD, Heppner TJ, Tallini Y, Kotlikoff MI, Nelson MT. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am J Physiol Heart Circ Physiol 302: H594–H602, 2012. doi: 10.1152/ajpheart.00773.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension 21: 498–503, 1993. doi: 10.1161/01.HYP.21.4.498. [DOI] [PubMed] [Google Scholar]
- 69.Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J Appl Physiol (1985) 104: 655–664, 2008. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- 70.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. doi: 10.1152/ajpheart.00790.2002. [DOI] [PubMed] [Google Scholar]
- 71.Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of V̇o2max in trained older subjects. J Appl Physiol (1985) 82: 1411–1415, 1997. doi: 10.1152/jappl.1997.82.5.1411. [DOI] [PubMed] [Google Scholar]
- 72.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol (1985) 95: 1963–1970, 2003. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- 73.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol (1985) 85: 68–75, 1998. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- 74.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res 11: 370–380, 1962. doi: 10.1161/01.RES.11.3.370. [DOI] [PubMed] [Google Scholar]
- 75.Richards JC, Luckasen GJ, Larson DG, Dinenno FA. Role of α-adrenergic vasoconstriction in regulating skeletal muscle blood flow and vascular conductance during forearm exercise in ageing humans. J Physiol 592: 4775–4788, 2014. doi: 10.1113/jphysiol.2014.278358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev 54: 75–159, 1974. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 77.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol 250: H1145–H1149, 1986. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 78.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007. doi: 10.1113/jphysiol.2006.124313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seals DR. Influence of muscle mass on sympathetic neural activation during isometric exercise. J Appl Physiol (1985) 67: 1801–1806, 1989. doi: 10.1152/jappl.1989.67.5.1801. [DOI] [PubMed] [Google Scholar]
- 80.Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol (1985) 66: 2472–2478, 1989. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- 81.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 120: 357–375, 2011. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Segal SS. Integration of blood flow control to skeletal muscle: key role of feed arteries. Acta Physiol Scand 168: 511–518, 2000. doi: 10.1046/j.1365-201x.2000.00703.x. [DOI] [PubMed] [Google Scholar]
- 83.Segal SS. Microvascular recruitment in hamster striated muscle: role for conducted vasodilation. Am J Physiol Heart Circ Physiol 261: H181–H189, 1991. doi: 10.1152/ajpheart.1991.261.1.H181. [DOI] [PubMed] [Google Scholar]
- 84.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation 12: 33–45, 2005. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 85.Sindler AL, Delp MD, Reyes R, Wu G, Muller-Delp JM. Effects of ageing and exercise training on eNOS uncoupling in skeletal muscle resistance arterioles. J Physiol 587: 3885–3897, 2009. doi: 10.1113/jphysiol.2009.172221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sindler AL, Reyes R, Chen B, Ghosh P, Gurovich AN, Kang LS, Cardounel AJ, Delp MD, Muller-Delp JM. Age and exercise training alter signaling through reactive oxygen species in the endothelium of skeletal muscle arterioles. J Appl Physiol (1985) 114: 681–693, 2013. doi: 10.1152/japplphysiol.00341.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sinkler SY, Fernando CA, Segal SS. Differential α-adrenergic modulation of rapid onset vasodilatation along resistance networks of skeletal muscle in old versus young mice. J Physiol 594: 6987–7004, 2016. doi: 10.1113/JP272409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sinkler SY, Segal SS. Aging alters reactivity of microvascular resistance networks in mouse gluteus maximus muscle. Am J Physiol Heart Circ Physiol 307: H830–H839, 2014. doi: 10.1152/ajpheart.00368.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sinkler SY, Segal SS. Rapid versus slow ascending vasodilatation: intercellular conduction versus flow-mediated signalling with tetanic versus rhythmic muscle contractions. J Physiol 595: 7149–7165, 2017. doi: 10.1113/JP275186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Socha MJ, Boerman EM, Behringer EJ, Shaw RL, Domeier TL, Segal SS. Advanced age protects microvascular endothelium from aberrant Ca2+ influx and cell death induced by hydrogen peroxide. J Physiol 593: 2155–2169, 2015. doi: 10.1113/JP270169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Socha MJ, Hakim CH, Jackson WF, Segal SS. Temperature effects on morphological integrity and Ca2+ signaling in freshly isolated murine feed artery endothelial cell tubes. Am J Physiol Heart Circ Physiol 301: H773–H783, 2011. doi: 10.1152/ajpheart.00214.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Socha MJ, Segal SS. Isolation of microvascular endothelial tubes from mouse resistance arteries. J Vis Exp 2013: e50759, 2013. doi: 10.3791/50759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song H, Tyml K. Evidence for sensing and integration of biological signals by the capillary network. Am J Physiol Heart Circ Physiol 265: H1235–H1242, 1993. doi: 10.1152/ajpheart.1993.265.4.H1235. [DOI] [PubMed] [Google Scholar]
- 94.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336: 597–601, 2012. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Suvorava T, Kojda G. Reactive oxygen species as cardiovascular mediators: lessons from endothelial-specific protein overexpression mouse models. Biochim Biophys Acta 1787: 802–810, 2009. doi: 10.1016/j.bbabio.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. doi: 10.1161/01.CIR.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 97.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 101: 1300–1309, 2007. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 98.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol (1985) 97: 731–738, 2004. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- 99.Tie L, Wang D, Shi Y, Li X. Aquaporins in cardiovascular system. Adv Exp Med Biol 969: 105–113, 2017. doi: 10.1007/978-94-024-1057-0_6. [DOI] [PubMed] [Google Scholar]
- 100.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol 302: C1226–C1242, 2012. doi: 10.1152/ajpcell.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tran QK, Watanabe H. Calcium signalling in the endothelium. Handb Exp Pharmacol 176: 145–187, 2006. doi: 10.1007/3-540-32967-6_5. [DOI] [PubMed] [Google Scholar]
- 102.Trott DW, Seawright JW, Luttrell MJ, Woodman CR. NAD(P)H oxidase-derived reactive oxygen species contribute to age-related impairments of endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol (1985) 110: 1171–1180, 2011. doi: 10.1152/japplphysiol.01037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol (1985) 96: 639–644, 2004. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- 104.Uhrenholt TR, Domeier TL, Segal SS. Propagation of calcium waves along endothelium of hamster feed arteries. Am J Physiol Heart Circ Physiol 292: H1634–H1640, 2007. doi: 10.1152/ajpheart.00605.2006. [DOI] [PubMed] [Google Scholar]
- 105.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-κB activation in aged rat arteries. Am J Physiol Heart Circ Physiol 293: H37–H47, 2007. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 106.Ungvari Z, Wolin MS, Csiszar A. Mechanosensitive production of reactive oxygen species in endothelial and smooth muscle cells: role in microvascular remodeling? Antioxid Redox Signal 8: 1121–1129, 2006. doi: 10.1089/ars.2006.8.1121. [DOI] [PubMed] [Google Scholar]
- 107.van der Loo B, Labugger R, Skepper JN, Bachschmid M, Kilo J, Powell JM, Palacios-Callender M, Erusalimsky JD, Quaschning T, Malinski T, Gygi D, Ullrich V, Lüscher TF. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med 192: 1731–1744, 2000. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.VanTeeffelen JW, Segal SS. Rapid dilation of arterioles with single contraction of hamster skeletal muscle. Am J Physiol Heart Circ Physiol 290: H119–H127, 2006. doi: 10.1152/ajpheart.00197.2005. [DOI] [PubMed] [Google Scholar]
- 109.Wahren J, Saltin B, Jorfeldt L, Pernow B. Influence of age on the local circulatory adaptation to leg exercise. Scand J Clin Lab Invest 33: 79–86, 1974. doi: 10.3109/00365517409114201. [DOI] [PubMed] [Google Scholar]
- 110.Westcott EB, Segal SS. Ageing alters perivascular nerve function of mouse mesenteric arteries in vivo. J Physiol 591: 1251–1263, 2013. doi: 10.1113/jphysiol.2012.244483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Widlansky ME, Gutterman DD. Regulation of endothelial function by mitochondrial reactive oxygen species. Antioxid Redox Signal 15: 1517–1530, 2011. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 296: H539–H549, 2009. doi: 10.1152/ajpheart.01167.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wray DW, Nishiyama SK, Harris RA, Richardson RS. Angiotensin II in the elderly: impact of angiotensin II type 1 receptor sensitivity on peripheral hemodynamics. Hypertension 51: 1611–1616, 2008. doi: 10.1161/HYPERTENSIONAHA.108.111294. [DOI] [PubMed] [Google Scholar]
- 114.Wray DW, Richardson RS. Aging, exercise, and limb vascular heterogeneity in humans. Med Sci Sports Exerc 38: 1804–1810, 2006. doi: 10.1249/01.mss.0000230342.86870.94. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y, Unnikrishnan A, Deepa SS, Liu Y, Li Y, Ikeno Y, Sosnowska D, Van Remmen H, Richardson A. A new role for oxidative stress in aging: the accelerated aging phenotype in Sod1−/− mice is correlated to increased cellular senescence. Redox Biol 11: 30–37, 2017. doi: 10.1016/j.redox.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]