Abstract
Histone deacetylases (HDACs) play a critical role in modulating cardiac function and ischemic injury. HDAC4 was found to be elevated and activated in response to injury. However, whether HDAC4 mediates cardiac function is currently unknown. In this study, we created myocyte-specific activated HDAC4 transgenic mice to examine the role of HDAC4 in mediating cardiac function during development and response to infarction. There are no differences in cardiac function and gross phenotype between wild-type and cardiomyocyte-specific HDAC4 transgenic mice at 1 mo of age. However, cardiac dysfunction and vascular growth deficiency were displayed in 6-mo-old HDAC4-transgenic mice compared with wild-type mice. Activation of HDAC4 increased heart and myocyte size, hypertrophic proteins, and interstitial fibrosis in 6-mo-old mice but not in 1-mo-old mice. To further define whether activated HDAC4 in the heart could impact myocardial function and remodeling, myocardial infarction was created in both wild-type and cardiomyocyte-specific HDAC4-transgenic mice. In myocardial infarction, the overexpression of activated HDAC4 exacerbated cardiac dysfunction and augmented cardiac remodeling and interstitial fibrosis, which was associated with the reduction of cardiokines in the heart. These results indicate the activation of HDAC4 as a crucial regulator for cardiac function in development and myocardial infarction.
NEW & NOTEWORTHY We created myocyte-specific activated HDAC4-transgenic mice to examine the function of HDAC4 in mediating cardiac function. HDAC4 overexpression led to cardiac dysfunction, which was associated with increased hypertrophy and myocardial fibrosis. Furthermore, the overexpression of activated HDAC4 exacerbated cardiac dysfunction, augmented remodeling, and increased apoptosis in the infarcted heart. This is the first demonstration that transgenic overexpression of HDAC4 is crucial for modulation of cardiac function and remodeling.
Keywords: cardiac function and injury, heart, histone deacetylase 4
INTRODUCTION
Histone deacetylases (HDACs) are enzymes that affect gene expression through its influence on chromatin modification by controlling the acetylation of the core histones (9). HDACs play a critical role in mediating physiological function to biological responses (4, 7, 15, 16, 18, 21, 25). Ever since the identification of HDAC1 (10), 18 HDACs have been described in mammals and are divided into three distinct classes (22). Class I HDACs consist of HDACs1, -2, -3, and -8, which are predominantly nuclear proteins and ubiquitously expressed. Class II HDACs include IIa (HDAC4, -5, -7, and -9) and IIb (HDAC6 and -10). HDAC4 and HDAC5 are expressed mainly in the heart but have demonstrated little activity (5, 7, 23). Class III HDACs were identified on the basis of sequence similarity with Sir, which includes SIRT1–7 and Sir2.
Recent evidence has revealed the involvement of HDACs in myocardial ischemia and reperfusion injury (19, 27–32), hypertrophy, and skeletal myogenesis (8, 11, 12, 27). The pharmacological inhibition of HDAC was found to prevent myocardial injury (6, 27–32) and increased the resistance of embryonic stem cells in response to oxidant stress in association with HDAC4 protein reduction (3). Myocardial ischemic injury resulted in increases in HDAC4 protein levels and HDAC activity. However, it remains unknown whether this increased HDAC4 in ischemic hearts plays a functional role or only represents an epiphenomenal event. Furthermore, we and others have utilized chemical inhibitors to define the physiological function of HDAC4. However, the lack of gain-of-function analysis for assessment of HDAC4 prevents us from fully understanding the critical role of HDAC4 in the heart. In the present study, we created cardiomyocyte-specific overexpression of HDAC4 transgenic mice to determine how activation of HDAC4 affect cardiac function and myocardial remodeling in infarcted hearts. These results indicate that transgenic overexpression of active HDAC4 resulted in the depression of myocardial function and promoted cardiac remodeling in the infarcted heart. This will provide new insight into understanding the critical role of cardiac HDAC4 in regulation of myocardial function.
MATERIALS AND METHODS
DNA constructs and transgenic mice.
The creation of the mice was carried out in the Boston University Transgenic Core Facility. cDNA encoding-activated human HDAC4 was obtained from Dr. Paola Gallinari (Istituto di Ricerche di Biologia Molecolare, Istituto di Ricerche di Biologia Molecolare P. Angeletti-IRBM-Merck Research Laboratories, Rome, Italy) AND cloned into an expression vector encoding α-myosin heavy chain (α-MHC; promoter 5.4 kb), a cardiomyocyte-specific promoter with multiple cloning sites. After ligation, the construct was purified and verified by restriction enzyme digestion and sequencing. Transgenic mice were produced by microinjection of the MHC-HDAC4 construct into fertilized FVB/n mouse eggs(F1 eggs). Founder mice and transgenic expression of HDAC4 were identified by analysis of genomic DNA with primer A (5-CCTCGTTCCAGCTGTGGT-3), a sense primer specific to MHC promoter exon 2), and antisense primer B (5-AGCGCCAGGAGCTCCTGCTGC-3), which is specific to HDAC4 cDNA. HDAC4 proteins were analyzed in the hearts from transgenic mice. A schematic of the transgenic construct is shown in Fig. 1A. All animal experiments were conducted under a protocol approved by the Institutional Animal Care and Use Committee of Institute at Roger Williams Medical Center that conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996).
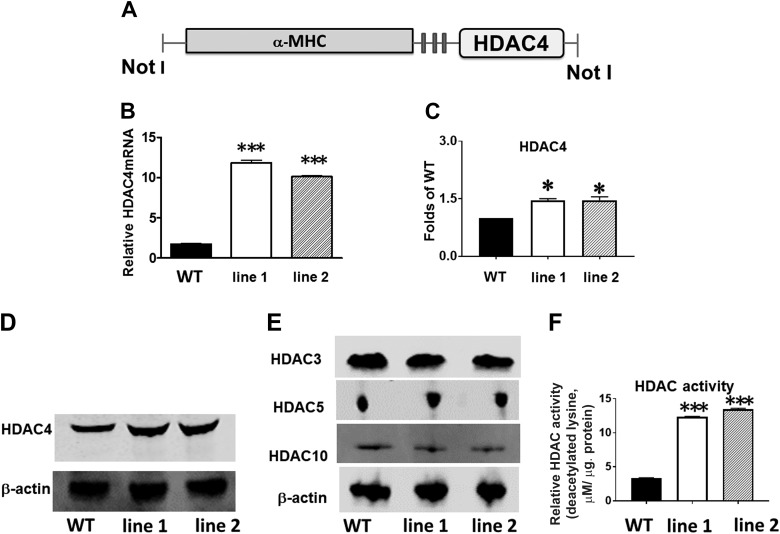
Fig. 1.
Generation and characterization of cardiac myocyte-specific histone deacetylase 4 (HDAC4)-transgenic (Tg) mice. A: schematic diagram of transgenic construct used to generate α-myosin heavy chain (α-MHC)-HDAC4 mice. B: quantitative PCR analysis of HDAC4 mRNA from the heart in wild-type (WT) and HDAC4-Tg mice. Results represent means ± SE (n = 5/group); ***P < 0.001. C: densitometric analysis shows the increased HDAC4 protein in HDAC4-Tg mice; *P < 0.05 vs. WT (n = 3/group). D and E: immunoblot detection of HDAC4 and other HDACs in WT and HDAC4-Tg mice. F: HDAC activity increased in HDAC4-Tg and wild type control myocardium; ***P < 0.001 vs. WT (n = 4/group). NotI (restriction enzymes).
Reagents and antibodies.
4,6-Diamidino-2-phenylindole (DAPI) was obtained from Life Technologies (Grand Island, NY). Primary antibodies, including HDAC4 rabbit polyclonal antibody (Cell Signaling Technology (Beverly, MA), α-smooth muscle actin (α-SMA) mouse monoclonal antibody (Sigma, St. Louis, MO), and CD31 rat polyclonal antibody (anti-PECAM-1; Millipore, Billerica, MA) were used. All chemicals for heart perfusion were purchased from Sigma. Atrial natriuretic peptide (ANP) was purchased from Santa Cruz Biotechnology (Dallas, TX).
Immunohistochemistry.
Cardiac tissues and sections were prepared from the paraffin-embedded hearts, as described above. Tissue sections were deparaffinized for 30 min at 70°C and subsequently immersed in xylene and ethanol at decreasing concentrations. Slides were then washed in distilled water. Microvessels were identified by smooth muscle cells, anti-α-smooth muscle actin (α-SMA) monoclonal antibody (Sigma, St. Louis, MO), and endothelial cells by CD31 (anti-PECAM-1) (Millipore, Billerica, MA). The total number of vessels from each group was calculated and normalized to the tissue area. The stained numbers of each section were counted in approximately three to five randomized fields of the tissue sections, which were taken in the middle plane of each heart and contained infarct and border regions.
Histological analysis.
Myocyte cross-sectional area was measured from images captured from the left ventricular (LV) sections obtained mid-distance from the base to the apex. Wheat germ agglutinin (WGA; Sigma) staining was carried out using immunofluorescent staining to measure cell size. Suitable cross-sections were defined as having nearly circular to oval myocyte sections. The outline of myocytes was traced using NIH ImageJ software to determine myocyte cross-sectional area. A value from each heart was calculated by the measurement of ∼400 cells in a remote area from the infarction of an individual heart. To evaluate cardiac fibrosis, LV sections were stained with picrosirius red, and collagen content was quantitated in images taken under a microscope coupled to a computerized morphometry system (Olympus BX41; Olympus, Ontario, NY). Hematoxylin and eosin stain (H & E) and Masson’s trichome staining were carried out as before (27). Interstitial collagen density was expressed as a percentage of myocardial area in each image (27).
Real-time polymerase chain reaction (PCR).
Total RNA was extracted from different groups with Trizol reagent (Life Technologies). cDNA was synthesized from 5 μg of total RNA. The reverse transcribed cDNA (5 μl) was amplified to a final volume of 50 μl by PCR under standard conditions. Real-time PCR experiments were performed on a master cycler realplex4 (Ependorf North America) system using a quantitative PCR kit master mix. (Kapa Biosystems, Boston, MA). Primer sequences used in these studies are as follows: FNDC-5: forward 5-GAA CAA AGA TGA GGT GAC CA-3, reverse 5-ACC ACA ACA ATG ATC AGC A-3; VEGF: forward 5-AGT CCG AAT GCA GAT CCT C-3, reverse 5-TGC ATT CAC ATT GGC TGT G-3. GAPDH was used as the internal control: forward 5-ACC ACA GTC CAT GCC ATC AC-3, reverse 5-TCC ACC ACC CTG TTG CTG TA-3.
Electrophoresis and Western blot analysis.
Heart tissues were homogenized in ice-cold RIPA buffer (Sigma-Aldrich) containing protease inhibitor cocktails (Calbiochem, Billerica, MA). Protein lysates were then obtained after centrifugation at 12,000 g for 15 min at 4°C. Protein concentrations were estimated using a Micro BCA Assay Kit (Thermo Scientific, Rockford, IL). Proteins (50 µg/lane) were separated on reducing SDS polyacrylamide gels and transferred to PVDF membranes. Five percent nonfat dry milk was used to block the membranes at room temperature for 1 h, followed by overnight incubation with primary antibodies at 4°C. The membranes were then incubated in the appropriate horseradish peroxidase-conjugated secondary antibody solution. The blots were incubated with their respective polyclonal antibodies and β-actin (1:1,000) for 2 h and visualized by incubation with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:5,000) for 1 h and developed with ECL chemiluminescence detection reagent (Amersham Pharmacia Biotech).
Measurement of HDAC activity.
Measurement of HDAC activity was carried out by using by using a colorimetric HDAC activity assay kit and expressed as a relative unit (BioVision, Mountain View, CA).
Terminal deoxynucleotidyltransferase nick-end labeling.
Terminal deoxynucleotidyltransferase (TdT) nick-end labeling (TUNEL) was carried out by using a TACS 2-TdT-DAB In Situ Apoptosis Detection Kit (Trevigen, Gaithersburg, MD), following the manufacturer’s instructions. To validate that apoptosis occurred in the cardiomyocytes, immunohistochemical staining of α-sarcomeric actin was carried out with an α-sarcomeric actin antibody (α-Actin) (1:100 dilution, Sigma; St. Louis, MO). Nuclei were identified by staining with DAPI. For each section, the number of TUNEL-positive myocyte nuclei was counted in three to five random selected regions. The index of apoptosis was then determined.
Experimental protocol for myocardial infarction.
Briefly, anesthesia was produced by an intraperitoneal injection of pentobarbital sodium at a dose of 50 mg/kg. During the surgical procedure, the adequacy of anesthesia was monitored with a toe pinch test to check the animal’s reaction. Both wild-type and HDAC4-transgenic male mice aged ∼2 mo old were studied, and they received a subcutaneous injection of buprenorphine (0.03 mg/kg) 2 h before surgery and every 12 h postsurgery for 3 days. Mice were placed in a supine position on the operating table. Mice were kept warm in a warming pad during this procedure. Ventilation was achieved by connecting the endotracheal tube with a rodent ventilator (Harvard, Model 683). The chest was then opened with tenotomy scissors. A 7-0 nylon suture was passed with a tapered needle under the left anterior descending coronary artery. Coronary occlusion was induced by ligation with a nylon suture. Upon completion of ligation, the chest was closed with a 5-0 Tricon suture, with one layer through the chest wall and muscle and a second layer through the skin and subcutaneous material. Sham animals underwent placement of the suture as described above without ligation.
Echocardiographic measurement of cardiac function.
Male mice were anesthetized with 1.5% isoflurane, and temperature was maintained at 37°C on a warm pad. Nair lotion (Church & Dwight Canada, Mississauga, ON, Canada) was applied on the precordial region for 3 min to cleanly remove hair, and the region was covered with prewarmed ultrasound transmission gel (Aquasonic; Parker Laboratory, Fairfield, NJ). Transthoracic echocardiography was performed using an Acuson Sequoia C512 system with a 15L8 linear array probe. All images were acquired at a depth setting of 25 mm. Two-dimensional B-mode and M-mode echocardiographic images were obtained at the level of the papillary muscles from the parasternal short-axis view. Wall thickness and chamber dimension were determined from M-mode tracings using cardiac calcs software. All left ventricular (LV) dimensions are presented as the average of measurements using six to nine consecutive selected beats. The measurements include left ventricle internal diameter (LVID), posterior wall thickness (PW), ejection fraction (EF), and fractional shortening (FS).
Data analysis.
All data are expressed as means ± SE. Differences among the groups were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni correction. Student’s unpaired t-test was used for two groups. A probability of P < 0.05 was considered to be a significant difference.
RESULTS
Characterization of cardiac-specific HDAC4 mice.
Mice overexpressing activated HDAC4 in cardiomyocytes via the α-MHC promoter were designated as MHC-HDAC4 mice (Fig. 1A) Two independent lines of MHC-HDAC4 mice were generated in our studies. The HDAC4 mRNA levels in these lines showed a 10- to 11-fold increase relative of wild-type mice (Fig. 1B). The HDAC4 protein levels demonstrated a significant increase relative to wild-type mice (Fig. 1, C and D). There is no significant change in other HDAC isoforms in HDAC4-Tg mice (Fig. 1E). Additionally, HDAC activity was also increased by overexpression of HDAC4 (Fig. 1F). Line 1 of HDAC4 transgenic mice was exclusively used in the following experiments.
HDAC4 promotes progressive cardiac hypertrophy, fibrosis, and myocyte enlargement.
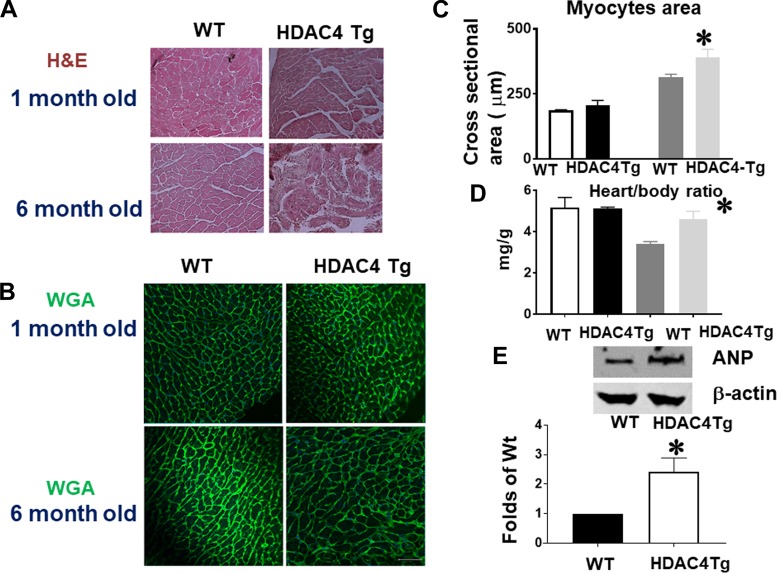
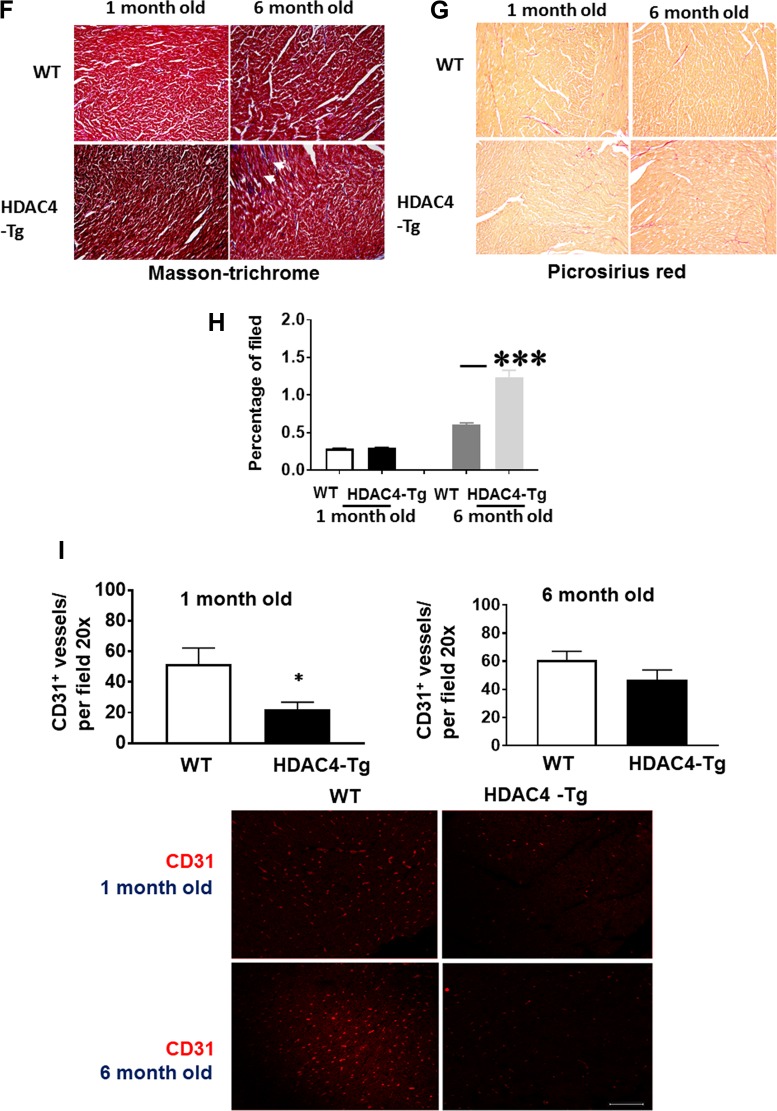
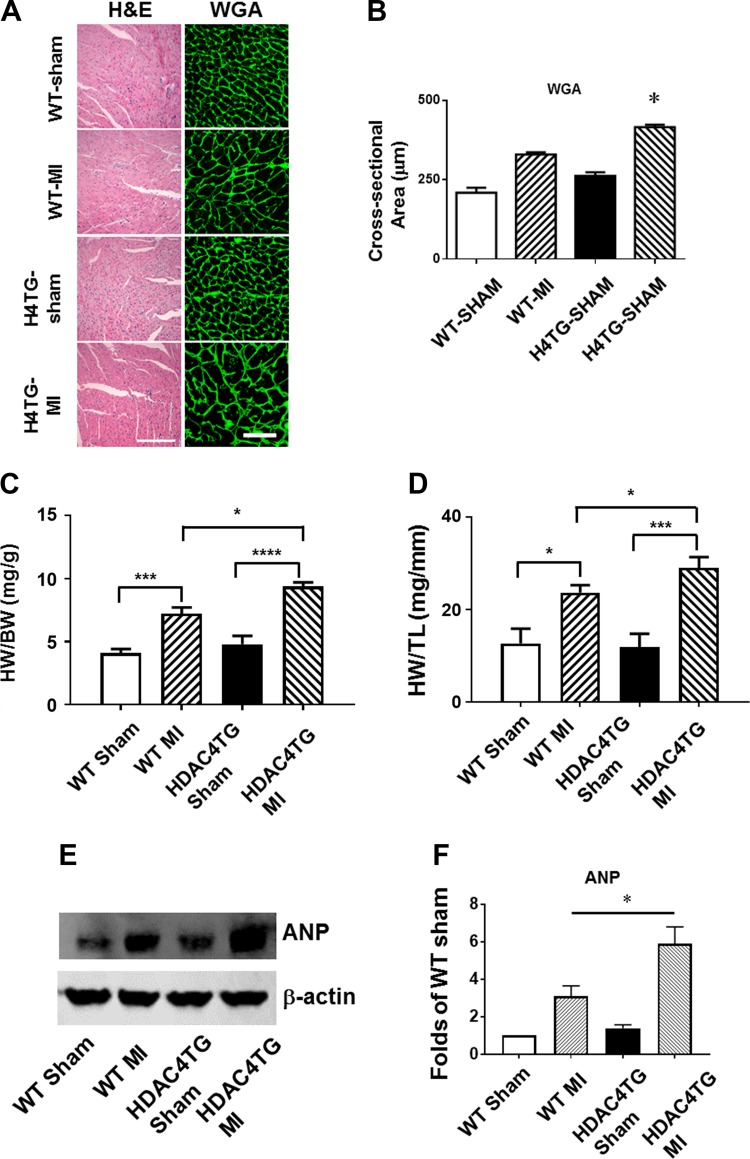
Myocyte size and heart weight were examined in HDAC4-transgenic (Tg) mice and wild-type control at 1 and 6 mo old age. WGA and H & E staining indicates that there are no significant differences in myocyte size at 1 mo of age (Fig. 2, A–C). Likewise, we did not find a difference in heart weight or myocyte size at this age (Figs. 2, C and D). However, for 6-mo-old mice, heart/body ratio increased significantly in HDAC4-Tg mice as compared with wild-type control mice (Fig. 2D). Histological analysis also showed an increase in myocyte size in HDAC4-Tg adult mice at 6 mo of age (Fig. 2, A–C). ANP proteins demonstrated a marked increase in HDAC4-Tg mice as compared with wild-type control mice at 6 mo of age (Fig. 2E). Likewise, interstitial fibrosis in myocardium was increased in HDAC4-Tg mice at 6 mo of age as compared with wild-type control (Fig. 2, F–H). Vascular density, as evidenced by CD31, was decreased in HDAC4-Tg mice as compared with wild-type control mice (Fig. 2I).
Fig. 2.
Histone deacetylase 4 (HDAC4) activation induced cardiac hypertrophy in the adult heart. A and B: HDAC4 overexpression increased myocyte sizes, as indicated by wheat germ agglutinin (WGA) and hematoxylin and eosin (H & E) staining (images taken at ×20 magnification). C: myocyte size from wild-type (WT) and HDAC4-transgenic (Tg) mice. D: heart/body ratios in WT and HDAC4-Tg mice (n = 3–5/group). E: atrial natriuretic peptide (ANP) protein levels in the myocardium of WT and HDAC4-Tg mice (n = 3/group). F: Masson’s trichrome staining indicates interstitial fibrosis in the myocardium (arrows indicate intensive fibrosis). G and H: Picrosirius red staining in WT and HDAC4-Tg mice and statistical analysis (images taken at ×20 magnification). I: immunostaining detection of CD31-positive microvessels from WT and HDAC4-Tg mice. Results represent means ± SE (n = 4–5/group). Scale bar, 50 μm. *P < 0.05 vs. WT; ***P < 0.001 vs WT.
Fig. 2.
—Continued
Activated HDAC4 leads to progressive cardiac dysfunction.
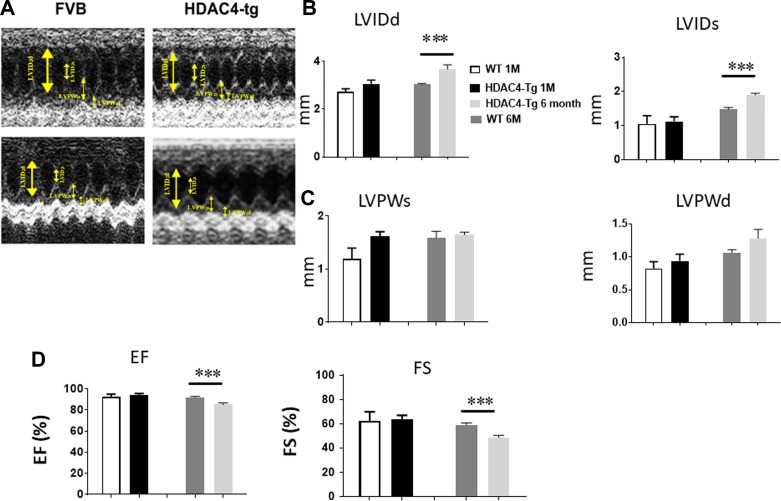
To examine the effect of activation of HDAC4 on cardiac function, we employed echocardiography on both HDAC4-tg mice and wild-type control mice at 1 and 6 mo of age. There is no difference in LV dimension at 1 mo of age between HDAC4-Tg and control wild-type mice (Fig. 3). However, there were profound increases in LV dimension, as indicated by the increases in left ventricle internal diameter at end systole (LVIDs) and end diastole (LVIDd) (Fig. 3, A–C), but there are decreases in FS and EF at the end of 6 mo (Fig. 3D), indicating that overexpression of activated HDAC4 results in a depression in cardiac function at the end of 6 mo. We did not find a difference in heart rates between the groups (not shown).
Fig. 3.
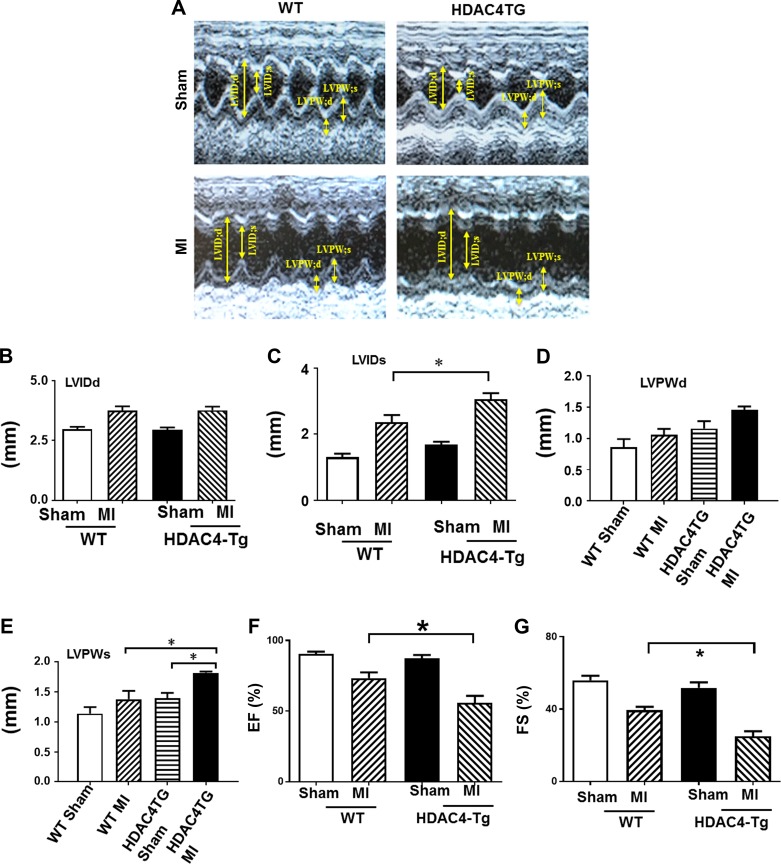
Echocardiographic measurements of ventricular function. Echocardiographic measurements of ventricular functional parameter at 1 and 6 mo age, A: representative images are shown as the M-mode short-axis ultrasound among the groups. B: left ventricle internal diameter at end diastole (LVIDd) and end systole (LVIDs). C: left ventricle posterior wall thickness at end diastole (LVPWd) and end systole (LVPWs). D: ejection fraction (EF) and fraction shortening (FS). Values represent means ± SE (n = 4–5/group); ***P < 0.01.
Activation of HDAC4 attenuated cardiac performance in infarcted hearts.
Echocardiographic measurements were performed every 3 wk in sham and infarcted mice. There were no differences in baseline among wild-type or transgenic mice at 2 mo of age. As shown in Fig. 4, A–E, an increase in left ventricular internal dimension (LVID) and a significant decrease in contractility were observed in all infarcted mice as compared with sham-operated in both control and HDAC4-Tg mice. However, cardiac performance in terms of LVIDd and LVIDs demonstrated progressive left ventricular dilation in HDAC4-Tg mice as compared with wild-type MI mice. On the other hand, genetic activation of HDAC4 caused a ventricular function depression, as indicated by significant decreases in EF and FS (Fig. 4, F and G), suggesting that activated HDAC4 plays an important role in affecting myocardial protection in MI hearts.
Fig. 4.
Histone deacetylase 4 (HDAC4) overexpression suppressed cardiac function recovery in the post-myocardial infarcted (MI) heart. Echocardiographic measurements of ventricular functional parameters at 2-mo-old mice in the post-MI heart. A: representative images are shown as the M-mode short-axis ultrasound among the groups. B and C: left ventricle internal diameter at end diastole (LVIDd) and end systole (LVIDs). D and E: left ventricle posterior wall thickness at end diastole (LVPWd) and end systole (LVPWs). F and G: ejection fraction (EF) and fraction shortening (FS). Values represent means ± SE (n = 5/group). *P < 0.05. Tg, transgenic.
HDAC4 activation enhanced cardiac hypertrophy, remodeling, and interstitial fibrosis following MI.
We measured myocyte size, the heart weight/tibia length, and heart weight/body weight ratios to evaluate the hypertrophic response. As shown in Fig. 5, A and B, the WGA and H & E staining showed that MI led to an increase in the cross-sectional area of cardiomyocytes as compared with sham-operated groups. As compared with wild-type MI mice, overexpression of activated HDAC4 displayed an enlargement in the cross-sectional area of cardiomyocytes as compared with WT MI mice. As shown in Fig. 5, C and D, MI resulted in an increase in the heart weight/body weight and heart weight/tibia length ratios compared with sham control animals although it did not reach a statistical difference. However, overexpression of active HDAC4 exacerbated heart weight/tibia length and heart weight/body weight ratios. In addition, atrial natriuretic peptide was significantly upregulated in HDAC4-Tg mice as compared with wild-type control mice (Fig. 5, E and F).
Fig. 5.
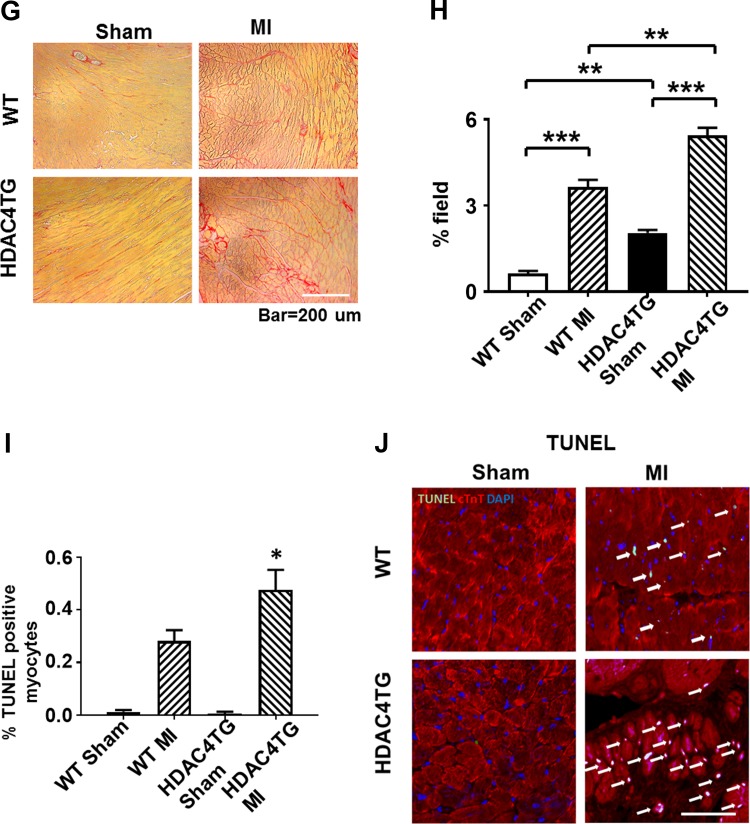
Histone deacetylase 4 (HDAC4) overexpression increased cardiac hypertrophy, interstitial fibrosis, and apoptosis. A and B: wheat germ agglutinin (WGA) and hematoxylin and eosin (H & E) staining showing the cross-sectional area of cardiomyocytes. C and D: heart weight/body weight (HW/BW) and heart weight/tibia length (HW/TL) ratio. Values represent means ± SE (n = 5/group). E and F: Western blot showing the increased atrial natriuretic peptide (ANP) protein in HDAC4-transgenic (Tg) mice in post-myocardial infarcted (MI) heart (n = 3/group). G and H: Picrosirius red staining showing the interstitial fibrosis of myocardium (n = 3/group). Scale bar, 200 μm. I: statistical analysis of apoptotic positive signals among the groups; J: representative images of TUNEL staining from the post-MI hearts of 2-mo-old mice (n = 5/group). Scale, 50 μm in A and 200 μm in J. *P < 0.05, ***P < 0.01, and ****P < 0.001 vs. MI.
Fig. 5.
—Continued
The collagen content was significantly increased after MI in all MI mice. As compared with wild-type MI mice, the overexpression of active HDAC4 resulted in a significant increase in interstitial collagen following MI (Figs. 5, G and H). In addition, MI increased the number of TUNEL-positive nuclei in the mouse myocardium. Overexpression of HDAC4 significantly increased apoptotic signals in MI hearts (Figs. 5, I and J).
Overexpression of HDAC4 decreased vascular density.
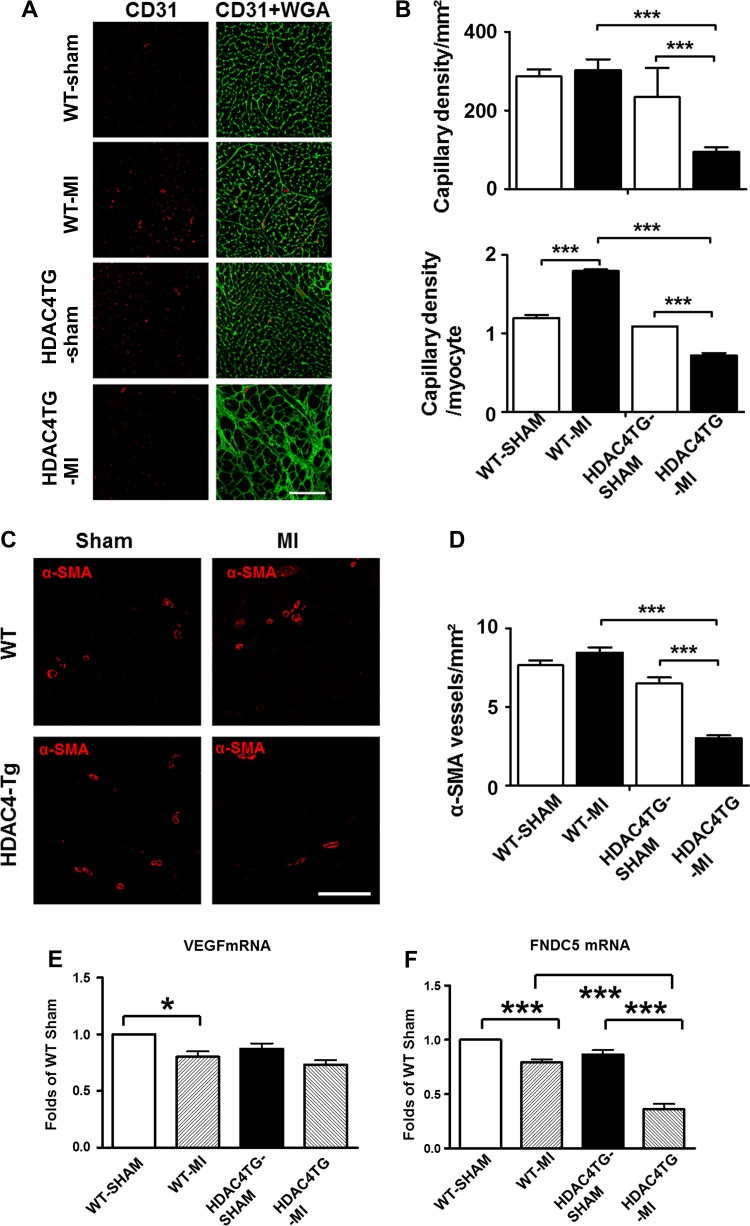
Immunofluorescent staining of CD31 was performed to examine the angiogenetic responses to MI in both wild-type and HDAC4-Tg mice. As shown in Fig. 6, A and B, CD31-positive capillary density in the myocardium was decreased in HDAC4-Tg mice as compared with wild-type mice. Likewise, a significant reduction in the density of α-SMA-positive microvessels was also evident in HDAC4-Tg mice as compared with WT MI groups (Figs. 6, C and D). There was only a mild reduction of VEGF transcription in the MI heart induced by overexpression of HDAC4 as compared with wild-type control, although it did not reach a significant difference. Notably, fibronectin type III domain containing 5 (FNDC5), another cardiokine, displayed a decrease, indicating that HDAC4 activation could attenuate the production of cardiokines (Fig. 6, E and F).
Fig. 6.
Histone deacetylase 4 (HDAC4) overexpression reduced angiogenesis in the post-myocardial infarcted (MI) heart. A and B: statistical analysis of CD31-positive signals among the groups and representative image of CD31 and α-smooth muscle actin (α-SMA) in the MI heart of 2-mo-old mice (n = 4 hearts/group). C and D: statistical analysis of α-SMA-positive signals among the groups and representative images of CD31 in the MI heart (n = 4 hearts/group); Scale, 100 μm. E and F: quantitative PCR analysis of VEGF and fibronectin type III domain containing 5 (FNDC5). The amount of each gene was expressed relative to the amount for GAPDH using the ΔCT method (n = 4/group). Values represent means ± SE. *P < 0.05; ***P < 0.001. Scale bar, 50 μm. HDAC4TG, histone deacetylase transgene; WGA, wheat germ agglutinin./
DISCUSSION
Salient finding.
It is known that myocardial ischemia and reperfusion injury lead to increased HDAC4 protein. Thus, how an increased HDAC4 modulates myocardial function needs to be determined. In this study, we created cardiomyocyte-specific HDAC- transgenic mice to define the effect of HDAC4 overexpression on modulating cardiac function in response to myocardial infarction. We demonstrated that 1) overexpression of activated HDAC4 suppressed ventricular function, increased cardiomyocyte hypertrophy, decreased vascular density, and promoted interstitial fibrosis; 2) in myocardial infarction, activated HDAC4 promoted cardiac dysfunction and cardiac remodeling, increased cardiomyocyte apoptosis, and decreased vascular density in association with attenuated expression of cardiokines, such as FNDC-5. Taken together. This is the first study to demonstrate that cardiomyocyte-specific overexpression of activated HDAC4 exacerbates cardiac injury and attenuates cardiac function.
Activated HDAC4 leads to cardiac hypertrophy and dysfunction.
We are focusing on active HDAC4 because HDAC4 is expressed mainly in the heart but demonstrates little activity. HDAC4-null mice display premature ossification of developing bone (12). In the adult heart, apoptotic myocytes increased significantly, and the cell proliferative rate ceased as compared with the neonatal stage. In our study, overexpression of HDAC4 did not result in changes in cardiomyocyte size, heart size, and fetal genes at 1 mo of age. However, the heart/body ratio and cardiomyocyte sizes were increased in HDAC4-transgenic mice, and this increasing cardiac hypertrophy was documented when mice developed into 6 mo of age. There exists a difference in vascular density of the heart between HDAC4-transgenic mice and wild-type mice, which likely contributes to the developing cardiac hypertrophy. This was in agreement with the previous finding demonstrating that the deficiency in vascular growth caused the cardiac hypertrophy (17). We noticed that HDAC4-transgenic mice demonstrated a mild reduction of VEGF as compared with wild type. An impairment in angiogenesis will accelerate the development of ventricular dysfunction, which may result in the decrease in the vessel/myocyte ratios. This is likely to result in suppression of vascular development and is contributable to cardiac hypertrophy in HDAC4-trasgenic mice. In agreement with this observation, cardiac HDAC3 over-expression transgenic mice demonstrated proliferative defects in cardiomyocytes (20). In addition, it was reported that overexpression of HDAC2 also augmented cardiac hypertrophy (19), which is in agreement with our finding. It is not clear whether HDAC4 and HDAC2 may regulate cardiac function through a commonly unidentified mechanism, which merits future investigation. However, the double deletion of HDAC1 and HDAC2 displayed progressive defects self-renewal in the epidermal progenitor cell population without producing effects on cell apoptosis (13). We found that HDAC4 mRNA in HDAC4-Tg mice demonstrated higher expression levels related to its protein levels, which may be related with the rate of transcription, posttranslational modification, and half-life of proteins. As noted above, we have found that HDAC4 overexpression in the heart resulted in the depression of cardiac function at an older stage. However, it is not clear whether HDAC4 overexpression could regulate cardiogenesis at the embryonic stage, which holds merit for future investigation. In addition, FNDC5 was reported to lead a decrease in myocardial function in myocardial ischemia and reperfusion injury, which is associated with a reduction of HDAC4 to regulate cellular protection (24, 33). It is interesting to identify whether such decrease in FNDC4 could play a critical role for functional decrease in response to HDAC4 overexpression.
HDAC4 activation exacerbates cardiac function and accelerates remodeling.
We and others have previously reported that the inhibition of HDAC activities demonstrated protective effects, suppressed cardiac remodeling, and promoted cardiac repair (27–32). In this observation, the overexpression of activated HDAC4 exacerbated cardiac dysfunction and promoted cardiac remodeling in both MI and older age mice (27). It will be interesting as to whether HDAC4 in the heart regulates ventricular function by an identical or separate pathway in response to MI and aging. The deletion of HDAC4 displayed premature ossification of developing premature bone and early onset chondrocyte hypertrophy, whereas the knockout of HDAC9 showed a normal phenotype (26). It is unclear whether HDAC9 deletion would result in activation or upregulation of HDAC4 in the heart. In support of our results, overexpression of HDAC2 had augmented hypertrophy, but HDAC2 deficiency prevented attenuated cardiac hypertrophy (19). Likewise, transgenic mice with mutant HDAC4 displayed greater left ventricular hypertrophy and a larger cross-sectional area of LV myocytes (1), which is related to the stimulation of cytosolic HDAC4 accumulation. However, the role of nuclear accumulation and cytoplasmic depletion of HDAC4 promoted neurodegeneration in different disease models (14). It is also noted that HDAC4 plays a different role in the regulation of cell survival in other systems (2). The cardiomyocyte-mediated vascular growth was reported to be critical in modulating the progress of cardiac dysfunction. The activation of HDAC4 in cardiomyocytes significantly decreased vascular density in this study, which indicates that HDAC4 activation modulates the interaction of cardiomyocytes and vessels to impact cardiac function and injury.
Summary.
Taken together, our study provides novel evidence showing that activated HDAC4 modulates cardiac development in the postnatal heart but elicits cardiac dysfunction at 6 mo of age. Furthermore, the cardiac-activation of HDAC4 enhanced cardiac dysfunction, promoted cardiac remodeling, and increased apoptosis in the infarcted heart. These studies provide new insight into the understanding of functional role of HDAC4 in mediating cardiac disease. The study also holds promise in developing new therapeutic strategies by specifically targeting HDAC4 to treat heart injury in the future.
GRANTS
The work is supported by National Heart, Lung, and Blood Institute Grants R01-HL-089405 and R01-HL-115265.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.C.Z., S.G.Z., G.Q., and Y.E.C. conceived and designed the study; L.X.Z., J.D., Y.T.Z., and J.W. performed experiments; J.D., Y.T.Z., and J.W. analyzed data; J.W. prepared figures; P.M.D.-S., S.Y.Z., and L.W. interpreted results of experiments; T.C.Z. drafted manuscript; T.C.Z. approved final version of manuscript.
REFERENCES
- 1.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell 133: 978–993, 2008. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 2.Chen B, Cepko CL. HDAC4 regulates neuronal survival in normal and diseased retinas. Science 323: 256–259, 2009. doi: 10.1126/science.1166226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen HP, Denicola M, Qin X, Zhao Y, Zhang L, Long XL, Zhuang S, Liu PY, Zhao TC. HDAC inhibition promotes cardiogenesis and the survival of embryonic stem cells through proteasome-dependent pathway. J Cell Biochem 112: 3246–3255, 2011. doi: 10.1002/jcb.23251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell 103: 263–271, 2000. doi: 10.1016/S0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- 5.Fischle W, Emiliani S, Hendzel MJ, Nagase T, Nomura N, Voelter W, Verdin E. A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1p. J Biol Chem 274: 11713–11720, 1999. doi: 10.1074/jbc.274.17.11713. [DOI] [PubMed] [Google Scholar]
- 6.Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J 22: 3549–3560, 2008. doi: 10.1096/fj.08-108548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grozinger CM, Hassig CA, Schreiber SL. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc Natl Acad Sci USA 96: 4868–4873, 1999. doi: 10.1073/pnas.96.9.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet 10: 32–42, 2009. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen JC, Tse C, Wolffe AP. Structure and function of the core histone N-termini: more than meets the eye. Biochemistry 37: 17637–17641, 1998. doi: 10.1021/bi982409v. [DOI] [PubMed] [Google Scholar]
- 10.Hassig CA, Tong JK, Fleischer TC, Owa T, Grable PG, Ayer DE, Schreiber SL. A role for histone deacetylase activity in HDAC1-mediated transcriptional repression. Proc Natl Acad Sci USA 95: 3519–3524, 1998. doi: 10.1073/pnas.95.7.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kee HJ, Sohn IS, Nam KI, Park JE, Qian YR, Yin Z, Ahn Y, Jeong MH, Bang YJ, Kim N, Kim JK, Kim KK, Epstein JA, Kook H. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 113: 51–59, 2006. doi: 10.1161/CIRCULATIONAHA.105.559724. [DOI] [PubMed] [Google Scholar]
- 12.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, Hill JA. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation 113: 2579–2588, 2006. doi: 10.1161/CIRCULATIONAHA.106.625467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeBoeuf M, Terrell A, Trivedi S, Sinha S, Epstein JA, Olson EN, Morrisey EE, Millar SE. Hdac1 and Hdac2 act redundantly to control p63 and p53 functions in epidermal progenitor cells. Dev Cell 19: 807–818, 2010. doi: 10.1016/j.devcel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Chen J, Ricupero CL, Hart RP, Schwartz MS, Kusnecov A, Herrup K. Nuclear accumulation of HDAC4 in ATM deficiency promotes neurodegeneration in ataxia telangiectasia. Nat Med 18: 783–790, 2012. doi: 10.1038/nm.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389: 251–260, 1997. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 16.McKinsey TA. Therapeutic potential for HDAC inhibitors in the heart. Annu Rev Pharmacol Toxicol 52: 303–319, 2012. doi: 10.1146/annurev-pharmtox-010611-134712. [DOI] [PubMed] [Google Scholar]
- 17.Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, Colucci WS, Walsh K. Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure. J Clin Invest 115: 2108–2118, 2005. doi: 10.1172/JCI24682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strahl BD, Allis CD. The language of covalent histone modifications. Nature 403: 41–45, 2000. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 19.Trivedi CM, Luo Y, Yin Z, Zhang M, Zhu W, Wang T, Floss T, Goettlicher M, Noppinger PR, Wurst W, Ferrari VA, Abrams CS, Gruber PJ, Epstein JA. Hdac2 regulates the cardiac hypertrophic response by modulating Gsk3 beta activity. Nat Med 13: 324–331, 2007. doi: 10.1038/nm1552. [DOI] [PubMed] [Google Scholar]
- 20.Trivedi CM, Lu MM, Wang Q, Epstein JA. Transgenic overexpression of Hdac3 in the heart produces increased postnatal cardiac myocyte proliferation but does not induce hypertrophy. J Biol Chem 283: 26484–26489, 2008. doi: 10.1074/jbc.M803686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turner BM. Histone acetylation and an epigenetic code. BioEssays 22: 836–845, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 22.Verdin E, Dequiedt F, Kasler HG. Class II histone deacetylases: versatile regulators. Trends Genet 19: 286–293, 2003. doi: 10.1016/S0168-9525(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang AH, Bertos NR, Vezmar M, Pelletier N, Crosato M, Heng HH, Th’ng J, Han J, Yang XJ. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol Cell Biol 19: 7816–7827, 1999. doi: 10.1128/MCB.19.11.7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Zhao YT, Zhang S, Dubielecka PM, Du J, Yano N, Chin YE, Zhuang S, Qin G, Zhao TC. Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J Cell Physiol 232: 3775–3785, 2017. doi: 10.1002/jcp.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter M, Moser MA, Meunier D, Fischer C, Machat G, Mattes K, Lichtenberger BM, Brunmeir R, Weissmann S, Murko C, Humer C, Meischel T, Brosch G, Matthias P, Sibilia M, Seiser C. Divergent roles of HDAC1 and HDAC2 in the regulation of epidermal development and tumorigenesis. EMBO J 32: 3176–3191, 2013. doi: 10.1038/emboj.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang CL, McKinsey TA, Chang S, Antos CL, Hill JA, Olson EN. Class II histone deacetylases act as signal-responsive repressors of cardiac hypertrophy. Cell 110: 479–488, 2002. doi: 10.1016/S0092-8674(02)00861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Chen B, Zhao Y, Dubielecka PM, Wei L, Qin GJ, Chin YE, Wang Y, Zhao TC. Inhibition of histone deacetylase-induced myocardial repair is mediated by c-kit in infarcted hearts. J Biol Chem 287: 39338–39348, 2012. doi: 10.1074/jbc.M112.379115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L, Qin X, Zhao Y, Fast L, Zhuang S, Liu P, Cheng G, Zhao TC. Inhibition of histone deacetylases preserves myocardial performance and prevents cardiac remodeling through stimulation of endogenous angiomyogenesis. J Pharmacol Exp Ther 341: 285–293, 2012. doi: 10.1124/jpet.111.189910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang LX, Zhao Y, Cheng G, Guo TL, Chin YE, Liu PY, Zhao TC. Targeted deletion of NF-kappaB p50 diminishes the cardioprotection of histone deacetylase inhibition. Am J Physiol Heart Circ Physiol 298: H2154–H2163, 2010. doi: 10.1152/ajpheart.01015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao TC, Cheng G, Zhang LX, Tseng YT, Padbury JF. Inhibition of histone deacetylases triggers pharmacologic preconditioning effects against myocardial ischemic injury. Cardiovasc Res 76: 473–481, 2007. doi: 10.1016/j.cardiores.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Zhao TC, Du J, Zhuang S, Liu P, Zhang LX. HDAC inhibition elicits myocardial protective effect through modulation of MKK3/Akt-1. PLoS One 8: e65474, 2013. [Correction in: PLoS One 8: 2013. 10.1371/annotation/29be7977-2e22-40cd-8a71-b3fbb99de9d5] doi: 10.1371/journal.pone.0065474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao TC, Zhang LX, Cheng G, Liu JT. gp-91 mediates histone deacetylase inhibition-induced cardioprotection. Biochim Biophys Acta 1803: 872–880, 2010. doi: 10.1016/j.bbamcr.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao YT, Wang H, Zhang S, Du J, Zhuang S, Zhao TC. Irisin Ameliorates Hypoxia/Reoxygenation-Induced Injury through Modulation of Histone Deacetylase 4. PLoS One 11: e0166182, 2016. doi: 10.1371/journal.pone.0166182. [DOI] [PMC free article] [PubMed] [Google Scholar]