Abstract
Prior studies using exploratory factor analysis provide evidence that negative symptoms are best conceptualized as 2 dimensions reflecting diminished motivation and expression. However, the 2-dimensional model has yet to be evaluated using more complex mathematical techniques capable of testing structure. In the current study, network analysis was applied to evaluate the latent structure of negative symptoms using a community-detection algorithm. Two studies were conducted that included outpatients with schizophrenia (SZ; Study 1: n = 201; Study 2: n = 912) who were rated on the Brief Negative Symptom Scale (BNSS). In both studies, network analysis indicated that the 13 BNSS items divided into 6 negative symptom domains consisting of anhedonia, avolition, asociality, blunted affect, alogia, and lack of normal distress. Separation of these domains was statistically significant with reference to a null model of randomized networks. There has been a recent trend toward conceptualizing the latent structure of negative symptoms in relation to 2 distinct dimensions reflecting diminished expression and motivation. However, the current results obtained using network analysis suggest that the 2-dimensional conceptualization is not complex enough to capture the nature of the negative symptom construct. Similar to recent confirmatory factor analysis studies, network analysis revealed that the latent structure of negative symptom is best conceptualized in relation to the 5 domains identified in the 2005 National Institute of Mental Health consensus development conference (anhedonia, avolition, asociality, blunted affect, and alogia) and potentially a sixth domain consisting of lack of normal distress. Findings have implications for identifying pathophysiological mechanisms and targeted treatments.
Keywords: anhedonia, avolition, asociality/blunted affect, alogia, negative symptoms, confirmatory factor analysis
Introduction
Negative symptoms have been considered a core symptom of schizophrenia (SZ) since the earliest descriptions of the disorder.1,2 Factor analytic studies are consistent with this notion, indicating that negative symptoms are a dimension of psychopathology that is separate from other symptoms associated with psychotic disorders (eg, positive, disorganization).3–5 This early factor analytic evidence led the field to conceptualize negative symptoms as a unidimensional construct. However, when studies evaluated the factor structure of items within negative symptom scales alone, there was consistent evidence that negative symptoms were multidimensional. The majority of studies provide evidence for 2 distinct dimensions reflecting diminished motivation and pleasure (MAP: anhedonia, avolition, asociality) and diminished expressivity (EXP: blunted affect, alogia).6 The 2-factor structure has been observed across a variety of scales, including the Scale for the Assessment of Negative Symptoms (SANS),7 Schedule for the Deficit Syndrome,8–10 Clinical Assessment Interview for Negative Symptoms (CAINS),11,12 and Brief Negative Symptom Scale (BNSS).13 Translated versions of these scales have also supported a 2-factor structure, suggesting that these dimensions are not culturally bound.14–17 Collectively, these findings have led the field to shift away from a unidimensional conceptualization, in favor of a 2-dimensional conceptualization of negative symptoms.18
However, it is unclear whether the widespread adoption of the 2-dimensional structure is fully statistically or theoretically justified. Prior studies have primarily relied on exploratory factor analysis (EFA), which is ideal for generating hypotheses regarding how many dimensions may be present; however, these analyses are not capable of actually testing competing models regarding the number of dimensions that exist within the negative symptom construct unless formal fit indices are evaluated. To date, such indices have not been adopted when evaluating negative symptom structure via EFA. Studies using confirmatory factor analysis (CFA) can also apply model fit statistics to test competing models regarding the structure of negative symptoms based on theory. However, few CFAs have been conducted on negative symptom scales, and those that have been published do not strongly support the 2-dimensional structure. For example, 2 studies attempted to validate the 2-factor structure using Korean19 and Chinese20 translations of the CAINS. Both concluded that the CAINS was best fit by a 2-factor structure, with dimensions reflecting MAP and EXP. However, the model fit statistics for the 2-factor model in both of these studies did not exceed common conventions for a model to be considered a good fit. In addition, the incorporation of modification indices to account for this poor fit in an exploratory manner flagged many misspecified residual covariances, suggesting that the 2-factor model should not have been supported. Similar findings were reported by a CFA study evaluating the 2-dimensional model in first episode patients who were rated on the SANS, which also found that model statistics fell below threshold for good fit for a 2-factor EXP/MAP model.21
Two recent CFA studies suggest that the failure to support the 2-dimension model may be because this model does not capture the complexity of negative symptoms and there may be additional dimensions of relevance.22,23 Strauss et al22 used CFA to evaluate the latent structure of the SANS,24 BNSS,25 and CAINS.12 Four CFA models were examined. The first was a unidimensional model, which considered whether all items best reflect a single latent negative symptom construct. The second was the 2-dimensional model identified in prior EFA studies,7,11–17 reflecting EXP and MAP factors. The third model was a 5-factor model that specified 1 factor for each of the 5 domains identified in the 2005 National Institute of Mental Health (NIMH) consensus development conference26: anhedonia, avolition, asociality, blunted affect, and alogia. The fourth model was a hierarchical model with 2 second-order factors reflecting EXP and MAP, as well as 5 first-order factors reflecting the 5 consensus domains. Results were consistent across all 3 scales/studies. The 1- and 2-factor models provided poor fit for the data. The 5-factor and hierarchical models provided excellent fit, with the 5-factor model outperforming the hierarchical model. In a follow-up study,23 we evaluated the validity and cross-cultural invariance of the 5-factor model reported by Strauss et al22 The 4 aforementioned models were evaluated across 5 cross-cultural samples, with a total n = 1678 (location/language): Italy/Italian, Spain/Spanish, China/Chinese, Switzerland/German, and United States/English. Results replicated the original study,22 indicating that 1- and 2-factor models provided poor fit for the data, but 5-factor and hierarchical models provided excellent fit. Again, the 5-factor model mathematically outperformed the hierarchical model. It is important to clarify that good fit for the hierarchical model is not simply further support for the 2-dimensional conceptualization. This is because MAP and EXP are secondary dimensions in these hierarchical models and the 5 factors are primary. Because primary dimensions are the ones directly influencing ratings of all negative symptoms in these hierarchical models, this suggests that the 5 domains, not the MAP/EXP dimensions, are most fundamental and best account for negative symptom structure.
Support for the 5-factor model across all 3 of the most current negative symptom scales22 and across a diverse range of cultures and languages23 in large representative samples raises the possibility that the recent trend toward conceptualizing negative symptoms in relation to the MAP and EXP dimensions18 does not capture the complexity of the construct, which is best represented by the 5 NIMH consensus domains. Clarifying the latent structure of negative symptoms is important because EFA studies supporting the 2 factors have been influential, informing how researchers search for pathophysiological mechanisms of negative symptoms27–29 and how pharmaceutical companies have recently been approaching targeted treatment development.30 The current focus on the 2 factors precludes the identification of pathophysiological mechanisms or treatment effects that are specific to the 5 domains. Indeed, there is some preliminary evidence for distinct pathophysiological correlates of individual negative symptom domains,31 suggesting that further investigation is warranted.
To further evaluate the latent structure of negative symptoms, the current study applied an alternate mathematical approach, network analysis, to objectively determine the optimal number of negative symptom domains on the BNSS. Network analysis is a mathematical method used to evaluate complex systems, focusing on the interrelatedness of system components, which is ideal for estimating latent structure of a construct. Rather than examining each symptom individually as one effect of a causal disorder, network analysis takes into account the interaction between each symptom and every other symptom, such that there is no examination of one symptom without consideration for the influence of all others.32 Few studies have applied network analysis to evaluate questions related to psychotic disorders. Prior studies have primarily focused on macroscopic network properties, such as network density and characteristic path length, which provide information about the collective properties of the network as a whole. Or they have investigated microscopic network properties using various centrality measures that indicate how individual nodes in the network interact. These approaches were ideal for answering the questions at hand, such as identifying which environmental factors influence the emergence of psychotic symptoms,33 which symptom interactions lead to treatment resistance vs responsiveness,34 and which psychological processes are most central to good vs poor functional outcomes in the community.35 To evaluate the question of interest in the current study (ie, the structure of negative symptoms), we focused on mesoscopic network properties, which are ideal for determining the number of dimensions within a construct. Specifically, a community detection metric was applied, which determines how different subsets of nodes (ie, BNSS items) in the network are clustered together. The nodes that belong to one “community” (ie, negative symptom domain) tend to have a stronger connection with each other whereas having a weaker connection with the nodes in other communities. This notion of communities of symptoms is similar to symptom cluster analysis where a cluster of symptoms is identified using statistical analyses. However, symptom cluster analysis only focuses on the interdependency of symptoms within one cluster, whereas community detection network analysis evaluates how clusters of symptoms interact with each other, to evaluate whether dimensions identified are distinct. Importantly, this network-based approach is not subject to several limitations inherent to CFA (eg, underestimating the number of factors when the correlations between factors are high and when sample size is small) and produces more reliable estimates of latent structure based on heuristic approaches. Network analysis therefore provides an approach that is complementary with CFA, but not redundant, as these 2 analyses can sometimes yield different results.34,36
Data from 2 studies were analyzed using network analysis to evaluate the latent structure of negative symptoms. The first study included an American sample of outpatients with SZ (n = 201), and the second included a larger validation sample of SZ outpatients from the Italian national study (n = 912). On the basis of the results of recent CFAs supporting a 5-factor structure of negative symptoms,22,23 it was hypothesized that the community detection network analysis parameter would indicate that the 13 BNSS items cluster into 5 distinct communities corresponding to the 2005 NIMH consensus domains: anhedonia, avolition, asociality, blunted affect, and alogia. In addition, it was hypothesized that the BNSS lack of normal distress item would produce a sixth community that would be separate from the 5 consensus domains. Although not part of the 5 NIMH consensus domains, lack of normal distress has been consistently demonstrated to be part of the negative symptom construct.37 Using CFA, the lack of normal distress item was found to load on all 5 of the consensus domains, but not highly on any specific domain, potentially indicating that it belongs on a separate dimension or is not part of the negative symptom construct.23 Given the potential for CFA to underestimate the true number of domains in a construct when the correlations between factors are high, network analysis provides a novel means of testing whether lack of normal distress might represent a sixth domain.
Methods
Participants
Study 1.
Participants included 201 outpatients meeting Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV)38 criteria for SZ or schizoaffective disorder. Patients were recruited from 2 sites: (1) the outpatient research clinics at the Maryland Psychiatric Research Center and community mental health clinics in the Baltimore metropolitan area (n = 146); (2) the State University of New York at Binghamton, including community outpatient mental health clinics in upstate New York (n = 55). All patients were evaluated during periods of clinical stability as determined by a minimum of 4 weeks of consistent medication dose and type. Consensus diagnosis was established via a best estimate approach based on psychiatric history and subsequently confirmed using the Structured Clinical Interview for DSM-IV (SCID39). The study was approved by the ethics committees of each institution.
The sample was on average 41.6 (12.1) years of age and had 13.2 (2.7) years of parental education; 72.6% were male; and 60.7% were Caucasian, 31.8% African-American, 0.5% Hispanic, 1.5% Asian, 1.0% American Indian/Alaskan Native, and 4.5% Bi-racial. Eighty-nine percent of the sample was prescribed an antipsychotic medication (5.0% first generation, 75% second generation, 9% both, 11% stably unmedicated).
Study 2.
Participants included 912 outpatients from the Italian National Study who were recruited from 26 Italian University psychiatric clinics in the Italian network for Research on Psychoses.40 Participants were 18–65 years of age and met DSM-IV criteria for SZ, which was confirmed with the SCID. Exclusion criteria were history of head trauma with loss of consciousness, history of moderate-to-severe mental retardation or neurological diseases, history of alcohol and/or substance abuse in the last 6 months, current pregnancy or lactation, inability to provide informed consent, and treatment modifications and/or hospitalization due to symptom re-exacerbation in the last 3 months. Participants had a minimum of 3 months of clinical stability as defined by no hospitalization due to symptom exacerbation, and no change in pharmacological treatment (type of drug or dose). The study was approved by the ethics committees of the local institutions.
The sample was on average 40.1 (10.7) years of age, had 11.7 (3.4) years of education, 69.8% were male, and 97% were prescribed an antipsychotic medication (14.1% first generation, 69.1% second generation, 13.8% both, 3% stably unmedicated).
Procedures
In both studies, the BNSS25 was administered as part of larger protocols. Prior to the start of the study, raters at each site were trained via an in-depth review of the BNSS manual, workbook, and scoring procedures. Raters were required to meet minimum reliability standards (inter-rater agreement ≥0.80) on gold-standard training videos prior to conducting assessments. All raters had a bachelor’s degree or higher and 1 or more years of clinical experience.
Measures
Brief Negative Symptom Scale.
The BNSS20 is a 13-item negative symptom rating scale designed in response to recommendations from the 2005 NIMH consensus development conference.26 The BNSS includes 6 subscales: anhedonia, asociality, avolition, blunted affect, alogia, and lack of normal distress. All items are rated on a 7-point (0–6) scale, with anchors generally ranging from absent (0) to severe.6 It has demonstrated excellent psychometric properties in the original and translated versions.17,40–46
Data Analysis
Analyses were conducted using Python (NetworkX package) and MATLAB. Network analysis was used to detect the optimal number of communities among the 13 BNSS items. Complex networks, such as symptom networks in psychiatric disorders, tend to divide into modules (ie, communities). The strength of division of a network into modules is called modularity. Community detection is a problem of finding maximal modularity in the networks. Higher modularity of a network is an indication of dense connections within modules and sparse connections between nodes from different modules. In this study, a community detection algorithm was applied to the BNSS network for determining the number of clusters that the 13 BNSS items divided into.
The BNSS symptom network was constructed using the association between BNSS variables calculated using mutual information (MI). MI is a simple method to calculate both linear and nonlinear relationship between variables using the following equation:
where and are BNSS variables, represents a joint probability distribution between and and are the marginal probability distributions of and respectively.
After calculating the pairwise MI between all BNSS variables, the connectivity matrix was obtained, which is a mathematical representation of a network, where rows and columns are the nodes of the network, and each cell denote the connectivity between nodes and Thus, the nodes of the network represent BNSS variables, and the edges represent the MI value between 2 BNSS variables.
Next, the modularity of the constructed BNSS network was calculated as follows47:
where is the link weight between nodes and and are degrees of nodes and and are the communities that node and belong to; and m equals to The δ-function equals to 1 if and 0 if Finding the communities in a network can be formulated as an optimization problem. In this study, a greedy optimization method known as the Louvain method was used to find the optimal community structures.47
The statistical significance of the detected communities was tested by comparing the intra-community connectivity of the individual communities with those in randomized networks.48 The 2 comparison criteria used here are internal average degree of communities and sum of the degrees of nodes in a community which are defined as follows48:
where represents the detected community using Louvain method; is the total number of nodes in community and are the nodes within that community, and is the MI value between nodes and
Results
Study 1
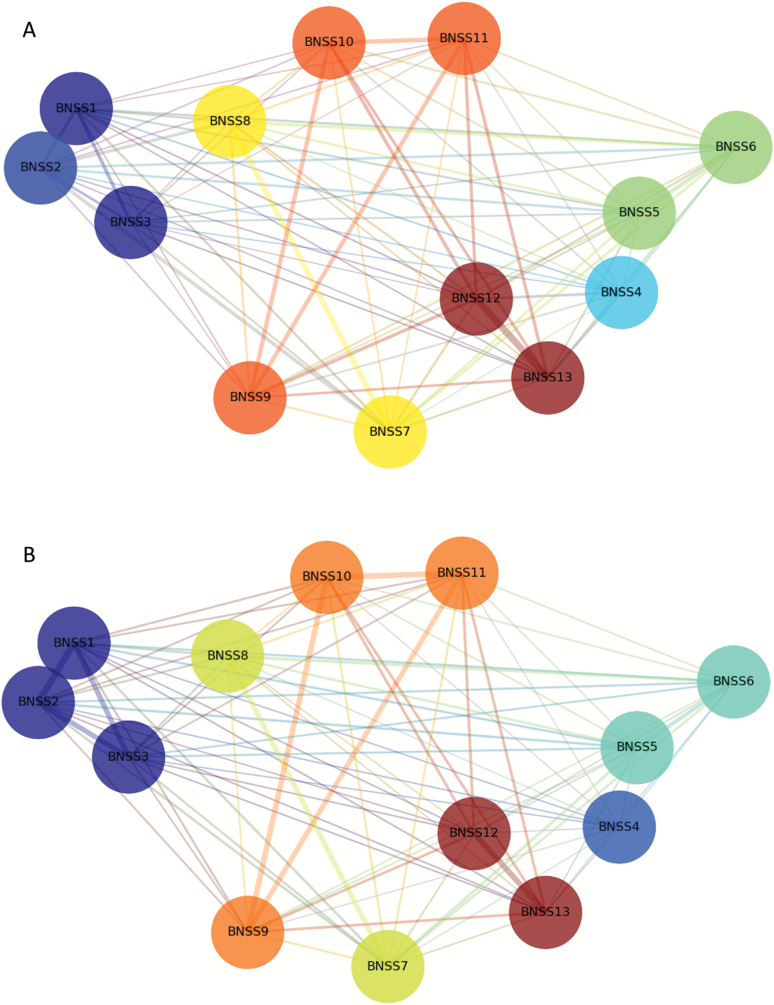
Figure 1 displays a topographic map of the community detection findings resulting from network analysis. As shown in figure 1A, the 13 BNSS items divided into 7 communities reflecting avolition, asociality, blunted affect, alogia, lack of normal distress, anhedonia intensity of pleasure, and anhedonia frequency of pleasure. Separation of these communities was demonstrated statistically by estimating the distribution of a quality function for randomized networks. Results indicated that each of the communities was significantly larger than communities of the same size detected in randomized networks, suggesting that the probability of identifying each community is greater than chance (see table 1).
Fig. 1.
Network analysis community detection topographic map. The nodes in the network represent the 13 Brief Negative Symptom Scale (BNSS) items; the node colors represent detected communities by the Louvain method; and the edge width represents the strength of the MI value. BNSS items: 1 = intensity of pleasure during activities; 2 = frequency of pleasurable activities; 3 = intensity of expected pleasure from future activities; 4 = lack of normal distress; 5 = asociality behavior; 6 = asociality internal experience; 7 = avolition behavior; 8 = avolition internal experience; 9 = facial expression; 10 = vocal expression; 11 = expressive gestures; 12 = quantity of speech; 13 = spontaneous elaboration. The 6 negative symptom domains identified by the network analysis are: anhedonia = BNSS items 1–3; lack of normal distress = 4; asociality = 5–6; avolition = 7–8; blunted affect = 9–11; alogia = 12–13. (A) American Sample (Study 1: n = 201); (B) Italian Sample (Study 2: n = 912). For color, please see the figure online.
Table 1.
Results of Significance Testing Calculated by Comparing the Intra-Community Connectivity of the Individual Communities With Those in Randomized Networks
| Study 1 (American sample: n = 201) | |||||||
| Community | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Node in the community (BNSS item) | 1, 3 | 2 | 4 | 5, 6 | 7, 8 | 9, 10, 11 | 12, 13 |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Study 2 (Italian sample: n = 912) | |||||||
| Community | 1 | 2 | 3 | 4 | 5 | 6 | |
| Node in the community (BNSS item) | 1, 2, 3 | 4 | 5, 6 | 7, 8 | 9, 10, 11 | 12, 13 | |
| P value | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 | |
Note: Separation of communities was demonstrated by estimating the distribution of a quality function for randomized networks. Significant P values indicate that each of the communities was significantly larger than communities of the same size detected in randomized networks. Findings suggest that the probability of identifying each community is greater than chance. BNSS items: 1 = intensity of pleasure during activities; 2 = frequency of pleasurable activities; 3 = intensity of expected pleasure from future activities; 4 = lack of normal distress; 5 = asociality behavior; 6 = asociality internal experience; 7 = avolition behavior; 8 = avolition internal experience; 9 = facial expression; 10 = vocal expression; 11 = expressive gestures; 12 = quantity of speech; 13 = spontaneous elaboration. BNSS, Brief Negative Symptom Scale.
Study 2
Results obtained for the Italian national sample largely replicated those of the American sample (see figure 1B). The BNSS items divided into 6 communities reflecting anhedonia, avolition, asociality, blunted affect, alogia, lack of normal distress. Unlike Study 1, the 3 anhedonia items fell into 1 community, rather than separate communities for intensity and frequency. The quality function indicated that each of the 6 communities was significantly larger than communities of the same size detected in randomized networks (see table 1).
To evaluate replicability of the networks in both studies, we randomly eliminated 5%, 10%, 20%, 30%, and 40% of the cases and repeated the network analysis. This approach resulted in the same community structure. We also evaluated whether alternate community detection methods produced comparable results to the Louvain method, including the Fast Newman, Danon, and Martelot’s methods.49–51 These methods all led to results supporting the 5 domain structure in both American and Italian samples, although there were slight differences in whether item 2 produced its own community or aligned with items 1 and 2 to produce an aggregate anhedonia community (see Supplementary Materials).
Discussion
The current study used network analysis to evaluate the latent structure of negative symptoms across 2 experiments with large samples of outpatients with SZ. Findings were largely consistent across both studies. In Study 1, the community detection method revealed 7 distinct communities of symptoms that the 13 BNSS items fell into avolition, asociality, blunted affect, alogia, lack of normal distress, anhedonia intensity of pleasure, and anhedonia frequency of pleasure. In Study 2, 6 communities were detected, reflecting avolition, asociality, blunted affect, alogia, lack of normal distress, and anhedonia. The 3 anhedonia items fell into a single community, rather than separate communities for intensity and frequency in Study 2. We doubt these differences in the functioning of item 2 are meaningful given that in both the American and Italian samples, alternative network methods presented in Supplementary Materials (Fast Newman, Danon, and Martelot’s methods) indicated that item 2 sometimes clustered with items 1 and 3 and at other times formed its own community. Despite these minor differences across samples and network methods, these findings are consistent with the results of 2 recent CFA papers.22,23 These papers found that 1- and 2-factor models of negative symptoms were a poor fit, whereas a 5-factor model provided an excellent fit. Collectively, results from these 2 CFA papers and the current network analysis findings support the 5-domain conceptualization of negative symptoms, and indicate that conclusions regarding latent structure are not scale dependent, culturally restricted, or specific to a singular mathematical approach.
Findings also extend the prior CFAs by identifying a potential sixth domain for consideration: lack of normal distress. This domain reflects the pathological reduction in the intensity or frequency of negative emotional experience. It has been demonstrated to be a hallmark feature of patients with deficit SZ who display primary and enduring negative symptoms37 and was part of the original descriptions of negative symptom pathology by early clinicians.1,2 The 2005 NIMH consensus conference26 indicated that there are at least 5 core negative symptom domains and listed the following 5 domains for consideration: anhedonia, avolition, asociality, blunted affect, and alogia. Lack of normal distress represents a strong candidate for a sixth domain to be considered.26 Future studies could use network analysis to test whether lack of normal distress is part of the negative symptom construct by constructing networks that include items from other symptom constructs (eg, hostility, depression, guilt, anxiety) and examining whether lack of normal distress falls into communities with negative symptom items, rather than communities related to these other negative emotion constructs.
When viewed in relation to the 2 recent CFA papers,22,23 the current network analysis findings have important theoretical implications regarding the latent structure of negative symptoms. Recent trends toward conceptualizing negative symptoms in relation to 1- or 2-dimensional models, which primarily resulted from conclusions drawn from EFA studies,11–17 are not fully statistically justified. These models do not adequately capture the complexity of the negative symptom construct. The latent structure of negative symptoms is best conceptualized in relation to the 5 domains identified in the 2005 NIMH Consensus Development Conference: anhedonia, avolition, asociality, alogia, and blunted affect. A sixth domain, lack of normal distress, should also be considered if future studies support its separation from the other 5 domains. Finding evidence for these 5 domains from a network perspective also extends the CFA findings, indicating that not only do items within these domains cluster together, but also that they have minimal interactions one another. This suggests that constructs (ie, items) within these domains are more likely to influence and be influenced by one another than they are from negative symptom constructs outside of that domain. Future studies should extend the current results by evaluating macroscopic properties, such as density and average shortest path length. These properties will offer insight into questions such as whether negative symptoms respond to treatment in patients whose networks are more vs less densely connected. It is conceivable for treatments to either benefit networks that are very densely connected (ie, because successful treatment of one domain will have a cascading effect on the others, which interact with the treated domain, resulting in global improvement) or not densely connected (ie, having an effect on one domain, which has little interaction with others and therefore does not result in interactions with other domains and global improvement). Microscopic properties, such as various measures of centrality, should also be examined because these will shed light onto whether any specific domains are more likely to influence others, and therefore the most appropriate targets for treatment. For example, Foussias and Remington52 proposed that avolition is the most central negative symptom domain, influencing all other domains to become more severe when it is present (ie, when low motivation is present, patients are also less likely to seek out rewarding activities, social interactions, speak frequently, or express emotion). Such a hypothesis could be directly tested using network analysis.
These findings also have several practical implications. First, based primarily on prior EFA results,11–17 the DSM-5 based its description of negative symptoms around the 2 broad MAP and EXP dimensions. In future iterations of the DSM-5, a revision of these descriptions should be considered and the 5 consensus domains should be defined and considered individually. Second, the current focus on the 2 factors precludes the identification of pathophysiological mechanisms or treatment effects that are specific to the 5 domains. Indeed, there is some preliminary evidence for distinct pathophysiological correlates driving individual symptoms,31 suggesting that investigating mechanisms with greater granularity holds promise. The NIMH research domain criteria initiative has delineated neurobiological processes associated with aspects of “negative valence systems” relevant to lack of normal distress, “positive valence systems” relevant to avolition and anhedonia, and “social processes” that are relevant to asociality. Using such a framework, pathophysiology mechanisms associated with each domain should be evaluated to promote targeted treatment development. Finally, current procedures for scoring negative symptom scales as a singular total score or MAP/EXP dimension scores may be inadequate. Modern scales (CAINS, BNSS, SANS) should calculate scores for each of the 5 domains separately (for specific scoring suggestions see Strauss et al22).
Finally, certain limitations should be considered. First, there was overlap between the 2 current samples and those included in our prior CFA studies22,23. Second, although results were replicated across multiple samples and cultures, only a single negative symptom measure was evaluated. It is unclear whether these findings generalize to other measures. Third, our patients were in the chronic phase of illness, and it is unclear whether these results generalize to earlier phases. Fourth, it is unclear how certain clinical (eg, antipsychotics, functional outcome, diagnosis) and demographic (eg, sex) factors might influence clusters obtained. Future studies should replicate these findings in other datasets, using large representative samples of patients from multiple phases of illness, using multiple negative symptom scales that adequately cover the 5 domains according to modern conceptualizations (ie, BNSS, CAINS, SANS). Despite these limitations, findings add to a growing body of literature indicating that recent trends toward conceptualizing negative symptoms in relation to MAP and EXP factors do not adequately capture the complexity of the negative symptom construct; rather, negative symptoms appear to be best conceptualized in relation to the 5 consensus domains. It is possible that as negative symptom measurement evolves beyond clinical rating scales and tools with better temporal and spectral resolution become available (eg, ecological momentary assessment), these 5 domains will indeed be found to be even more complex and granular.
Supplementary Material
Supplementary data are available at Schizophrenia Bulletin online.
Funding
The American study was supported by National Institute of Mental Health (K23MH092530 to G.P.S.). The Italian study was supported by Italian Ministry of Education, the Italian Society of Psychopathology (SOPSI), the Italian Society of Biological Psychiatry (SIPB), Roche, Lilly, Astra-Zeneca, Lundbeck, and Bristol-Myers Squibb.
Conflict of Interest
G.P.S. and B.K. are original developers of the Brief Negative Symptom Scale (BNSS) and receive royalties and consultation fees from ProPhase LLC in connection with commercial use of the BNSS and other professional activities. All other authors have no relevant conflicts of interest to report.
References
- 1. Bleuler E. [Dementia praecox or the group of schizophrenias]. Vertex. 2010;21:394–400. [PubMed] [Google Scholar]
- 2. Kraepelin E.In: Robertson MR, ed. Dementia Praecox and Paraphrenia. New York, NY: Krieger Publishing; 1919. [Google Scholar]
- 3. Andreasen NC, Arndt S, Miller D, Flaum M, Nopoulos P. Correlational studies of the scale for the assessment of negative symptoms and the scale for the assessment of positive symptoms: an overview and update. Psychopathology. 1995;28:7–17. [DOI] [PubMed] [Google Scholar]
- 4. Arndt S, Alliger RJ, Andreasen NC. The distinction of positive and negative symptoms. The failure of a two-dimensional model. Br J Psychiatry. 1991;158:317–322. [DOI] [PubMed] [Google Scholar]
- 5. Grube BS, Bilder RM, Goldman RS. Meta-analysis of symptom factors in schizophrenia. Schizophr Res. 1998;31:113–120. [DOI] [PubMed] [Google Scholar]
- 6. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kelley ME, van Kammen DP, Allen DN. Empirical validation of primary negative symptoms: independence from effects of medication and psychosis. Am J Psychiatry. 1999;156:406–411. [DOI] [PubMed] [Google Scholar]
- 8. Kimhy D, Yale S, Goetz RR, McFarr LM, Malaspina D. The factorial structure of the schedule for the deficit syndrome in schizophrenia. Schizophr Bull. 2006;32:274–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakaya M, Ohmori K. A two-factor structure for the Schedule for the Deficit Syndrome in schizophrenia. Psychiatry Res. 2008;158:256–259. [DOI] [PubMed] [Google Scholar]
- 10. Strauss GP, Horan WP, Kirkpatrick B, et al. Deconstructing negative symptoms of schizophrenia: avolition-apathy and diminished expression clusters predict clinical presentation and functional outcome. J Psychiatr Res. 2013;47:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Horan WP, Kring AM, Gur RE, Reise SP, Blanchard JJ. Development and psychometric validation of the clinical assessment interview for negative symptoms (CAINS). Schizophr Res. 2011;132:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kring AM, Gur RE, Blanchard JJ, Horan WP, Reise SP. The clinical assessment interview for negative symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strauss GP, Hong LE, Gold JM, et al. Factor structure of the brief negative symptom scale. Schizophr Res. 2012;142:96–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Engel M, Fritzsche A, Lincoln TM. Validation of the German version of the clinical assessment interview for negative symptoms (CAINS). Psychiatry Res. 2014;220:659–663. [DOI] [PubMed] [Google Scholar]
- 15. Valiente-Gómez A, Mezquida G, Romaguera A, et al. Validation of the Spanish version of the clinical assessment for negative symptoms (CAINS). Schizophr Res. 2015;166:104–109. [DOI] [PubMed] [Google Scholar]
- 16. Chan RC, Shi C, Lui SS, et al. Validation of the Chinese version of the clinical assessment interview for negative symptoms (CAINS): a preliminary report. Front Psychol. 2015;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chieffi M, Galderisi S, Mucci A, et al. The brief negative symptom scale: convergent/discriminant validity and factor structure in a large sample of outpatients with schizophrenia. European Psychiatry 2015;30:246. [DOI] [PubMed] [Google Scholar]
- 18. Marder SR, Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16:14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jung SI, Woo J, Kim YT, Kwak SG. Validation of the Korean-version of the Clinical Assessment Interview for Negative Symptoms of Schizophrenia (CAINS). J Korean Med Sci. 2016;31:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie DJ, Shi HS, Lui SSY, et al. Cross cultural validation and extension of the clinical assessment interview for negative symptoms (CAINS): evidence from a spectrum perspective. Schiz. Bull. 2018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lyne J, Renwick L, Grant T, et al. Scale for the assessment of negative symptoms structure in first episode psychosis. Psychiatry Res. 2013;210(3):1191–1197. [DOI] [PubMed] [Google Scholar]
- 22. Strauss GP, Nuñes A, Ahmed AO, et al. The latent structure of negative symptoms in schizophrenia. JAMA Psychiatry. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahmed AO, Kirkpatrick B, Galderisi S, et al. Cross-Cultural validation of the five-factor structure of negative symptoms in schizophrenia. Schizophr. Bull. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 25. Kirkpatrick B, Strauss GP, Nguyen L, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirkpatrick B, Fenton WS, Carpenter WT Jr, Marder SR. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Messinger JW, Trémeau F, Antonius D, et al. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin Psychol Rev. 2011;31:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strauss GP, Cohen AS. A transdiagnostic review of negative symptom phenomenology and etiology. Schizophr Bull. 2017;43:712–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kring AM, Barch DM. The motivation and pleasure dimension of negative symptoms: neural substrates and behavioral outputs. Eur Neuropsychopharmacol. 2014;24:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marder SR, Kirkpatrick B. Defining and measuring negative symptoms of schizophrenia in clinical trials. Eur Neuropsychopharmacol. 2014;24:737–743. [DOI] [PubMed] [Google Scholar]
- 31. Shaffer JJ, Peterson MJ, McMahon MA, et al. Neural correlates of schizophrenia negative symptoms: distinct subtypes impact dissociable brain circuits. Mol Neuropsychiatry. 2015;1:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borsboom D. A network theory of mental disorders. World Psychiatry. 2017;16(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Isvoranu AM, Borsboom D, van Os J, Guloksuz S. A network approach to environmental impact in psychotic disorder: brief theoretical framework. Schizophr Bull. 2016;42(4):870–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esfahlani FZ, Sayama H, Visser KF, Strauss GP. Sensitivity of the positive and negative syndrome scale (PANSS) in detecting treatment effects via network analysis. Innov Clin Neurosci. 2017;14(11–12):59–67. [PMC free article] [PubMed] [Google Scholar]
- 35. Galderisi S, Rucci P, Kirkpatrick B, et al. ; Italian Network for Research on Psychoses Interplay among psychopathologic variables, personal resources, context-related factors, and real-life functioning in individuals with schizophrenia: a network analysis. JAMA Psychiatry. 2018;75(4):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Esfahlani FZ, Visser K, Strauss GP, Sayama H. A network-based classification framework for predicting treatment response of schizophrenia patients. Expert Systems with Applications. 2018;109:152–161. [Google Scholar]
- 37. Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. [DOI] [PubMed] [Google Scholar]
- 38. American Psychiatric Association. In: American Psychiatric Association, eds. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., revised. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 39. First MB, Gibbon M, Spitzer RL, et al. User’s Guide for the Structured Clinical Interview for DSM-IV Research Version. New York, NY: American Psychiatric Publishing; 1996. [Google Scholar]
- 40. Mucci A, Galderisi S, Merlotti E, et al. ; Italian Network for Research on Psychoses The brief negative symptom scale (BNSS): independent validation in a large sample of Italian patients with schizophrenia. Eur Psychiatry. 2015;30:641–647. [DOI] [PubMed] [Google Scholar]
- 41. Mané A, García-Rizo C, Garcia-Portilla MP, et al. Spanish adaptation and validation of the brief negative symptoms scale. Compr Psychiatry. 2014;55:1726–1729. [DOI] [PubMed] [Google Scholar]
- 42. Strauss GP, Keller WR, Buchanan RW, et al. Next-generation negative symptom assessment for clinical trials: validation of the brief negative symptom scale. Schizophr Res. 2012;142:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Garcia-Portilla MP, Garcia-Alvarez L, Mané A, et al. The negative syndrome of schizophrenia: three -underlying components are better than two. Schizophr Res. 2015;166:115–118. [DOI] [PubMed] [Google Scholar]
- 44. Merlotti E, Mucci A, Bucci P, Nardi A, Galderisi S. Italian version of the “Brief Negative Symptom Scale”. J Psychopathol. 2014;20:199–215. [Google Scholar]
- 45. Bischof M, Obermann C, Hartmann MN, et al. The brief negative symptom scale: validation of the German translation and convergent validity with self-rated anhedonia and observer-rated apathy. BMC Psychiatry. 2016;16:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Polat Nazlı I, Ergül C, Aydemir Ö, Chandhoke S, Üçok A, Gönül AS. Validation of Turkish version of brief negative symptom scale. Int J Psychiatry Clin Pract. 2016;20:265–271. [DOI] [PubMed] [Google Scholar]
- 47. Blondel VD, Guillaume JL, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J Stat Mech. 2008;(10)P10008. [Google Scholar]
- 48. Kojaku S, Masuda N. A generalised significance test for individual communities in networks. Sci Rept 2018;8:7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Newman ME. Fast algorithm for detecting community structure in networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;69:066133. [DOI] [PubMed] [Google Scholar]
- 50. Danon L, Díaz-Guilera A, Arenas A. The effect of size heterogeneity on community identification in complex networks. J Stat Mech. 2006;(11):P11010. [Google Scholar]
- 51. Le Martelot E, Hankin C. Fast multi-scale detection of relevant communities in large-scale networks. Comp Jl. 2013;56(9):1136–1150. [Google Scholar]
- 52. Foussias G, Remington G. Negative symptoms in schizophrenia: avolition and Occam’s razor. Schizophr Bull. 2010;36(2):359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.