Abstract
Impairments in basic cognitive processes such as attention and working memory are commonly observed in people with schizophrenia and are predictive of long-term outcome. In this review, we describe a new theory—the hyperfocusing hypothesis—which provides a unified account of many aspects of impaired cognition in schizophrenia. This hypothesis proposes that schizophrenia involves an abnormally narrow but intense focusing of processing resources. This hyperfocusing impairs the ability of people with schizophrenia to distribute attention among multiple locations, decreases the number of representations that can simultaneously be maintained in working memory, and causes attention to be abnormally captured by irrelevant inputs that share features with active representations. Evidence supporting the hyperfocusing hypothesis comes from a variety of laboratory tasks and from both behavioral and electrophysiological measures of processing. In many of these tasks, people with schizophrenia exhibit supranormal effects of task manipulations, which cannot be explained by a generalized cognitive deficit or by nonspecific factors such as reduced motivation or poor task comprehension. In addition, the degree of hyperfocusing in these tasks is often correlated with the degree of impairment in measures of broad cognitive function, which are known to be related to long-term outcome. Thus, the mechanisms underlying hyperfocusing may be a good target for new treatments targeting cognitive deficits in schizophrenia.
Keywords: attention, working memory, useful field of view, attentional capture, ERPs, fMRI
Overview
Although the most distinctive symptoms of schizophrenia are hallucinations, delusions, and thought disorder (positive symptoms), this disorder also involves impairments in basic cognitive processes such as attention and memory.1,2 The degree of cognitive impairment is actually a better predictor of long-term outcome in people with schizophrenia (PSZ) than are positive symptoms.3–5 However, current treatments mainly address the positive symptoms and have minimal impact on cognitive deficits.6 Thus, progress in understanding the nature of the cognitive impairments is vitally important for developing new treatments that can impact long-term functional outcome in schizophrenia. In this paper, we describe a new hypothesis that attempts to provide a unified account of multiple aspects of impaired cognition in PSZ and may provide a new target for treatment development.
This new hypothesis states that schizophrenia involves an aberrant hyperfocusing of processing resources on a small number of representations. In other words, even when the task requires perceiving or remembering multiple objects or locations, PSZ tend to focus intensely but narrowly. Because the ability to simultaneously maintain or process multiple representations is important in a broad range of cognitive tasks and for everyday life functioning, this hypothesis can explain why PSZ exhibit impaired performance across multiple domains. For example, hyperfocusing can explain deficits in tasks that require maintaining multiple representations in working memory (WM) or that require distributing attention broadly across multiple sensory inputs.
Hyperfocusing is often observed in spatial contexts: PSZ tend to focus attention on a narrow spatial region even when the task encourages them to process a broad spatial area. However, hyperfocusing can also occur in the context of nonspatial information, such as representations of color and form. Moreover, hyperfocusing affects not just attention but also WM: If cognitive processing resources are focused more intensely but on a smaller number of representations, this can explain the common finding of reduced WM storage capacity in PSZ.7–9
There are typically many ways to explain impaired performance on a given task, but the hyperfocusing hypothesis also predicts supranormal effects in certain experimental paradigms. For example, PSZ focus their attention more narrowly than healthy control subjects (HCS) on the center of gaze in some situations, leading to enhanced filtering of peripheral distractors.10,11 This sort of supranormal performance is very difficult to explain in terms of nonspecific factors such as reduced motivation and poor task comprehension.
Although PSZ may exhibit supranormal focusing of attention under some circumstances, this does not mean that PSZ will always outperform HCS at narrow focusing. We assume that HCS can focus just as narrowly as PSZ when the task demands it, so the hyperfocusing hypothesis predicts supranormal focusing in PSZ primarily when narrow focusing is not encouraged by the task. In other words, both HCS and PSZ are capable of narrow focusing, but PSZ tend to focus their attention narrowly even when it is unnecessary or counterproductive.
Before describing the evidence for the hyperfocusing hypothesis, we would like to make three key points. First, the hyperfocusing hypothesis appears to be diametrically opposed to the classic idea of impaired filtering in PSZ.12 However, as discussed in a companion paper,13 much of the apparent discrepancy reflects differences in terminology and operationalization. Second, although we posit that hyperfocusing is a core deficit, it is unlikely to be the only factor underlying cognitive dysfunction in schizophrenia and is not intended to explain all cognitive deficits. For example, schizophrenia also appears to involve impaired context processing—a deficit in the ability to use information about the current context, such as a task instruction, to determine the appropriate response to an incoming sensory input.14,15 Finally, the hyperfocusing hypothesis is somewhat abstract at this formative stage in its development, and additional research will be needed to refine it, operationalize it, and link it to specific neurobiological systems.
We now turn to the evidence supporting the hyperfocusing hypothesis. We begin with studies showing an impaired ability of PSZ to distribute their processing across multiple representations. We follow this with more direct evidence that PSZ tend to focus narrowly but intensely. The paper concludes with some speculations about the origins and broader consequences of hyperfocusing.
Impaired Performance in Tasks That Require Processing Multiple Objects
WM Capacity
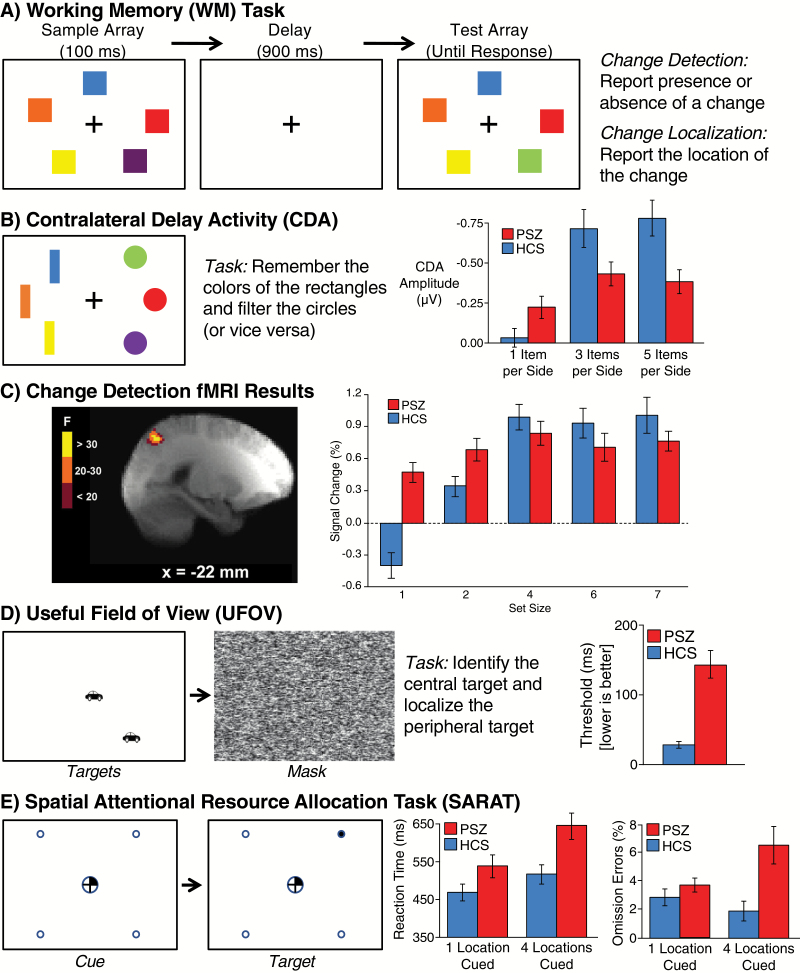
The number of items that can be concurrently held in WM—the storage capacity of WM—places limits on cognitive performance across a range of tasks.16,17 We have assessed WM capacity in PSZ and HCS using variations on the widely used task shown in figure 1A.18 Each trial consists of a sample array containing several to-be-remembered items, followed by a delay interval and then a test array. One item may change between the sample and test arrays, and the participant reports the presence or absence of a change (in change detection tasks) or the location of the change (in change localization tasks).
Fig 1.
Five experimental paradigms that provide evidence of hyperfocusing in people with schizophrenia (PSZ). (A) Change detection/localization task used to measure visual working memory capacity.8 (B) Contralateral delay activity (CDA) experiment.19 (C) Results of an fMRI experiment using a change detection task like that shown in (A). The left side shows the region of posterior parietal cortex in which PSZ and healthy control subjects (HCS) differed in terms of the function relating activity to the number of items stored in memory, and the right side shows the signal change (relative to baseline) within this region for each set size. (D) Useful field of view experiment.20 Participants report whether the central object is a car or truck and the location of the peripheral object. The timing of a mask that follows the targets is adjusted to find the 75% accuracy point (the threshold). (E) Spatial cuing experiment in which a central cue directs attention to between one and 4 potential target locations.21
WM storage capacity in change detection/localization tasks is strongly correlated with broad measures of cognitive ability in healthy individuals.8,22 Several studies have found that WM capacity in these tasks is reduced in PSZ relative to HCS, with a large effect size (typically d > 1).8,23–26 Moreover, individuals with greater capacity reduction exhibit greater overall cognitive impairment.8 The hyperfocusing hypothesis provides a natural explanation for reduced WM capacity in PSZ: If PSZ focus more intensely on fewer representations, then the number of items they can hold in WM would be reduced. Several alternative explanations for the reduced storage capacity have been ruled out.23,24,27
Evidence of more intense focusing by PSZ in the context of WM has been provided by event-related potential (ERP)19 and functional magnetic resonance imaging (fMRI)28 studies using variants of the change detection task. The ERP study took advantage of contralateral delay activity (CDA), a sustained difference in voltage over the hemispheres contralateral versus ipsilateral to the items being maintained in WM that is tightly linked to individual differences in WM capacity.29,30 As illustrated in figure 1B, each display contained rectangles on one side and circles on the other, and participants were instructed to remember the colors of the rectangles and ignore the circles (or vice versa). When each side contained only 1 item, CDA amplitude was actually greater in PSZ than in HCS, providing evidence that PSZ were focusing their WM resources more intensely than HCS on the to-be-remembered item. However, CDA amplitude was smaller in PSZ than in HCS when each side contained 3 or 5 items, consistent with lower average WM capacity.
Similar results were observed using fMRI28 with a task like the one in figure 1A. As in prior studies of typical young adults,31,32 a region of the posterior parietal cortex displayed activity that was linearly related to the number of items actually stored in memory (figure 1C). In this region, greater activity was observed in PSZ than in HCS at set size 1, but activity increased less in PSZ than in HCS as the set size increased (see also Hahn et al33), similar to the pattern of prefrontal cortical activation observed with varying WM control demands.34,35
Greater neural activity at small set sizes can sometimes be explained as being a secondary consequence of reduced WM capacity in PSZ,35 but this possibility was ruled out in both the CDA and fMRI studies. For example, the CDA effects were present even in subgroups of PSZ and HCS who were equated for WM capacity. Thus, these studies show that PSZ focus more intensely than HCS on single items in WM tasks.
Useful Field of View and Span of Apprehension
If reduced WM capacity in PSZ is caused by hyperfocusing, then WM capacity reductions should be correlated with impairments in measures of the ability to distribute attention broadly in other tasks. This was tested in a study that measured both WM capacity and the useful field of view36,37 (UFOV), a well-validated measure of the ability to distribute attention across the visual field that is correlated with real-world outcome measures, such as automobile driving performance.38 As illustrated in figure 1D, the critical condition of the UFOV task requires the participant to discriminate the shape of a central object and simultaneously localize a peripheral object. We found that UFOV performance in this condition was dramatically impaired in PSZ, was strongly correlated with WM capacity in PSZ but not in HCS, and statistically accounted for a significant portion of the shared variance between WM capacity and broad cognitive functioning in PSZ.20
Similar results were obtained many years ago in a series of experiments by Cegalis et al,39–41 who found that PSZ were impaired at discriminating a central stimulus and simultaneously detecting and discriminating peripheral stimuli. Impairments in the classic span of apprehension task, which are present for arrays containing large numbers of items but not for single-item displays, are also consistent with a deficit in distributing attention among multiple objects.42–44
Positive Evidence of Hyperfocusing: Spatial Attention
Performance was impaired in PSZ relative to HCS in the aforementioned examples, raising the possibility that they are simply a consequence of generalized impairments in task comprehension, learning, motivation, etc. We now turn to studies that provide positive evidence of hyperfocusing and in many cases provide evidence of supranormal processing in PSZ. We begin with evidence of spatial hyperfocusing.
Spatial Cuing, Visual Search, and Image Viewing
Direct evidence for spatial hyperfocusing was provided by the spatial cuing experiment shown in figure 1E.21 Each trial began with a central cue that indicated the most likely location(s) of a subsequent peripheral target stimulus. Healthy individuals focus their attention onto the cued location when a single location is cued and distribute their attention broadly when multiple locations are cued, even when gaze remains fixed at the center of the display.45,46 The hyperfocusing hypothesis predicts that PSZ will not be impaired at focusing attention onto a single-cued location (and may even focus more intensely) but will be impaired at distributing their attention broadly when all 4 locations are cued. As a result, the benefit of being cued to a single location (relative to the trials on which all 4 locations are cued) will be greater in PSZ than in HCS.
Indeed, the improvement in target detection latency and omission errors on 1-cued-location relative to 4-cued-locations trials was substantially larger in PSZ than in HCS. This result has been replicated twice by Hahn et al,47,48 and a similar pattern was observed by Spencer et al.49 The effect persisted when controlling for overall slowing of response times, suggesting that it is not secondary to a general reduction in processing resources. These findings indicate that PSZ are able to focus their attention on a single location but are impaired at allocating their attention broadly. In a follow-up study,48 PSZ displayed significantly greater deactivation of the default mode network than HCS when only 1 location was cued, consistent with greater resource allocation.
Evidence of spatial hyperfocusing was also provided by Elahipanah et al50 using a task in which participants searched for a target in a densely packed array. A gaze-contingent stimulus presentation technique was used to estimate the size of the attended region around the currently fixated object. HCS focused their attention more narrowly in difficult search tasks and more broadly in easier tasks, whereas PSZ focused their attention narrowly around the currently fixated object independently of task difficulty. In other words, PSZ focused narrowly even when a broad distribution of attention would have been more efficient.
Analogous results have been found during the scanning of complex images (eg, faces, geometric shapes, natural scenes). Across a variety of conditions, PSZ typically exhibit a restricted scanning pattern characterized by a smaller number of longer-duration fixations and shorter overall scan paths relative to HCS (see review by Beedie et al51). Thus, PSZ spend more time examining a given region (analogous to more intense focusing) but scan less of the overall image (analogous to a narrower focus of attention).
Hyperfocusing on the Center of Gaze
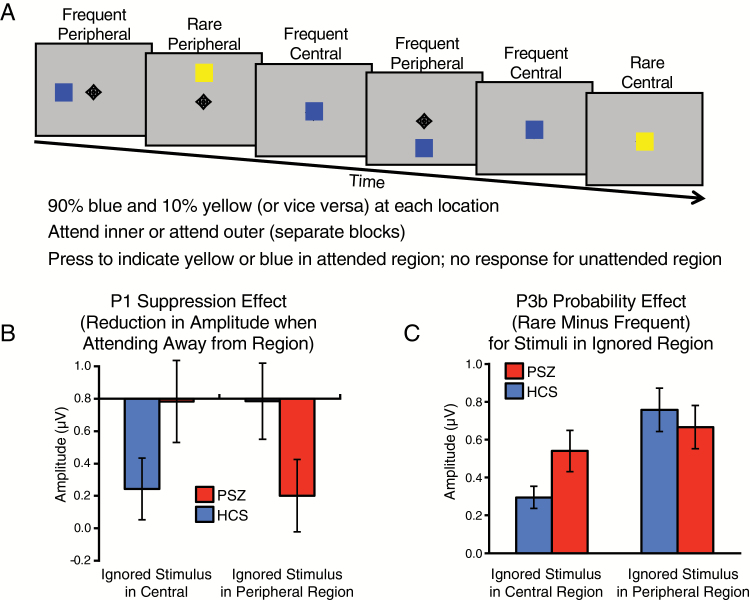
Evidence for spatial hyperfocusing was also obtained in the ERP study illustrated in figure 2A, which demonstrated that PSZ hyperfocus on the center of gaze and have difficulty attending broadly to the periphery.10 Participants were instructed to pay attention to the center of the display in one condition and distribute attention across 4 peripheral locations in another. A random sequence of yellow and blue squares was presented, with each stimulus appearing either at the center or at one of the peripheral locations. One color was rare (10%) and the other was frequent (90%), and the task was to report the color of the stimulus, but only when it appeared in the to-be-attended region. The effects of spatial attention were examined for both early sensory processing (indexed by the P1 wave) and later cognitive processing (indexed by the difference in the P3 wave between the rare and frequent stimuli).
Fig 2.
(A) Double oddball paradigm.10 (B) Suppression of sensory-evoked P1 when attention was directed away from a given location. A larger bar indicates greater suppression (smaller P1 amplitude). (C) P3b probability effect (rare minus frequent) for stimuli in the ignored region, which indicates the amount of cognitive processing resources devoted to irrelevant stimuli. Here, a larger bar reflects a failure to suppress processing in the to-be-ignored region.
Hyperfocusing should cause PSZ to focus their attention narrowly on the central location when instructed to do so, leading to a suppression of the ERP responses to stimuli presented at the peripheral locations. However, when instructed to distribute attention across the 4 peripheral locations, PSZ should have difficulty suppressing stimuli presented at the to-be-ignored central location. This is exactly what was observed. PSZ were able to suppress the P1 wave for peripheral stimuli when they were attending centrally, and this attentional modulation was significantly greater in PSZ than in HCS (figure 2B). However, PSZ were unable to suppress the P1 for central stimuli when the task required attending broadly in the periphery.
The P3 analyses (figure 2C) focused on the rare-minus-frequent P3 difference at the to-be-ignored location, which reflects the degree to which participants allocated processing resources to irrelevant stimuli. When the task required attending to the central location, the irrelevant peripheral stimuli produced a larger P3 response in HCS than in PSZ, indicating that PSZ were more effective than HCS at filtering the peripheral stimuli. By contrast, when the task required processing the peripheral locations, the irrelevant central stimuli produced a larger P3 response in PSZ than in HCS, indicating that PSZ were impaired at suppressing the irrelevant central stimuli when asked to distribute attention broadly in the periphery.
Evidence that PSZ hyperfocus on the center of gaze has also been observed using behavioral measures. For example, Elahipanah et al52 found that PSZ exhibited no deficit in detecting targets that were close to the center of the display in a visual search task, but they were impaired for targets that were far from the center. Similarly, Leonard et al11 used a task in which distractors were presented at varying distances from a central to-be-attended location and found a steeper falloff of distraction in PSZ than in HCS. Finally, PSZ are impaired at making eye movements away from the current center of gaze53–56; although there may be multiple explanations for these hypometric saccades, they are at least consistent with spatial hyperfocusing.
Positive Evidence of Hyperfocusing: Nonspatial Features
The hypothesis that PSZ focus their attention more intensely and narrowly than HCS is relatively straightforward to operationalize in the domain of spatial attention. However, the hyperfocusing hypothesis goes beyond spatial information, proposing that PSZ focus more intensely on a smaller number of representations, whether these representations are spatial or nonspatial. In particular, this hypothesis attempts to explain reduced WM capacity in PSZ by proposing that PSZ focus their WM resources more narrowly than HCS, leading to more intense representations of fewer items. Substantial evidence shows that PSZ store a smaller number of items in WM compared to HCS,8,57 but it is more difficult to operationalize the idea of greater intensity of representations.
The most direct support for greater intensity of WM representations in PSZ (operationalized in terms of the magnitude of neural activity) comes from the studies described earlier19,28 showing that both ERP and fMRI activity is greater in PSZ than in HCS when only a single object must be stored in WM. However, these findings could instead reflect inefficient fine-tuning of neuronal microcircuits in PSZ, such that greater neural activity is needed to maintain a representation in WM. Thus, to establish the case that WM representations are functionally more intense in PSZ than in HCS, the next section will focus on behavioral studies.
Increased Intensity and the Capture of Attention
We have tested the hypothesis of increased intensity in behavioral paradigms by taking advantage of the automatic capture of attention by items that match task representations (attentional templates). For example, if an individual is looking for red items of a particular shape in a search task, then attention will be attracted to red items of other shapes as well.58–60 If attentional template representations are more intense in PSZ than in HCS, then items that match these representations should capture attention more strongly in PSZ than in HCS. For example, PSZ exhibit exaggerated capture of attention by items that match the current contents of WM.56
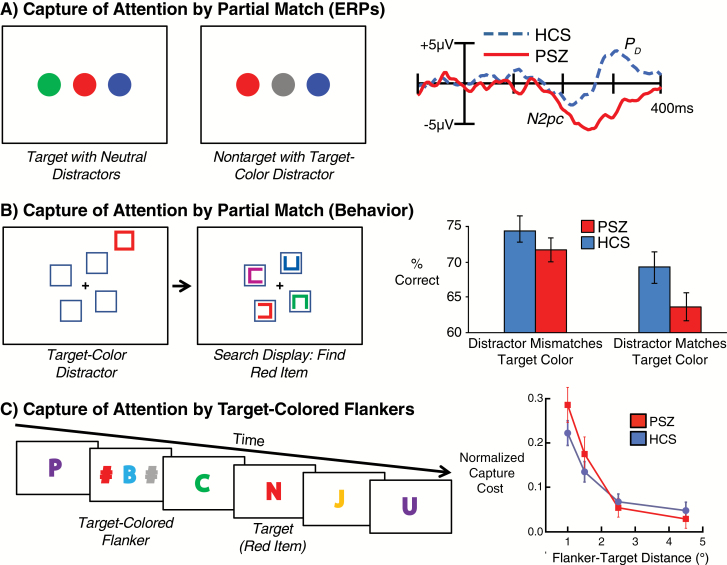
Figure 3A shows an ERP paradigm that was used to determine whether attentional capture by an item that partially matched an attentional template would be greater in PSZ than in HCS.61 Each stimulus display contained a colored disc at fixation along with 2 lateral distractor discs. Participants were instructed to press a button when they detected a particular color (eg, red) at central fixation. One of the lateralized distractors sometimes matched the attended color, and the other was a neutral color, but participants were told to ignore all distractors. In typical college-age participants, the side with the target-colored distractor briefly captures attention, as indexed by the N2pc (N2-posterior-contralateral) ERP component, and this is followed by a period of suppression, as indexed by the PD (distractor positivity) component.62 When we compared PSZ and matched HCS in this paradigm, we found that HCS also showed this N2pc-followed-by-PD pattern, whereas PSZ instead exhibited a large and long-lasting N2pc component, indicating a strong and sustained shift of attention to the target-colored distractor. Moreover, the magnitude of the N2pc effect was robustly correlated with WM in PSZ, with a stronger capture of attention (larger N2pc) in PSZ predicting lower WM capacity. This is consistent with the hypothesis that hyperfocusing on an internal task representation is associated with reduced WM capacity in PSZ.
Fig 3.
Three experimental paradigms in which people with schizophrenia (PSZ) exhibit greater capture of attention to stimuli that partially match a relevant color. (A) When participants are asked to detect a specified color (eg, red) at fixation, a target-color distractor at a lateral location elicits an initial shift of attention (indexed by the N2pc component). In healthy control subjects (HCS), this is rapidly followed by suppression of the target-color distractor (indexed by the PD component), but attention remains focused on the target-color distractor in PSZ.61 (B) When participants search for an item of a particular color (eg, red), a distractor captures attention when it matches this color, impairing target discrimination accuracy, and this is exaggerated in PSZ.26 (C) When participants monitor a rapid stream of foveal letters for a target of a particular color (eg, red), a flanker of this color captures attention, especially when the flankers are close to the target. PSZ show more of this capture than HCS when the flankers are close to the target, but less when the flankers are farther away.11 Neither group exhibited distraction by a neutral-colored distractor (not shown here).
An analogous behavioral effect was observed by Mayer et al.26 As shown in figure 3B, participants searched for a target of a particular color at 1 of 4 locations surrounding the fixation point. Prior to the onset of the search array, a task-irrelevant flanker object was presented adjacent to 1 of the 4 task-relevant locations, and this flanker could either match or mismatch the target color. Both HCS and PSZ were impaired at correctly reporting the target when the task-irrelevant flanker matched the target color, but this effect was larger in PSZ than in HCS. The larger effect in PSZ is consistent with a more intense WM representation of the target color in PSZ than in HCS, leading to greater capture by a target-colored distractor. Moreover, longer-lasting capture of attention was associated with lower WM capacity.
Another analogous behavioral effect was reported by Leonard et al11 (figure 3C). A rapid stream of letters was presented in the center of the display, and participants were instructed to report the identity of the one letter presented in a specified color. On a subset of trials, task-irrelevant flankers were presented shortly before the target, and one of these flankers sometimes matched the target color. In both groups, a nonmatching distractor had no effect, but a matching distractor near the central target location drew attention away from the target (as has been observed in healthy young adults63). This distraction effect was larger in PSZ than in HCS when the flankers were close to the target, and the drop-off with distance was sharper in PSZ than in HCS. This result is consistent with both spatial hyperfocusing—because the spatial drop-off of the distractor effect was sharper in PSZ than in HCS—and nonspatial hyperfocusing—because the distraction produced by target-colored distractors (but not by neutral distractors) was larger in PSZ than in HCS.
Could the results of the studies shown in figure 3 simply reflect greater distractibility in PSZ than in HCS? There are 2 lines of evidence against this possibility. First, as reviewed in the companion paper,13 we have repeatedly failed to find evidence of greater general distractibility in PSZ relative to HCS (although PSZ exhibit greater distraction than HCS under certain specific conditions). Second, all 3 of the studies shown in figure 3 contained conditions in which a nontarget color was presented, and PSZ exhibited no evidence of being more distracted than HCS by the nontarget color. That is, PSZ exhibited exaggerated distraction only by items that matched the attentional template. In addition, these effects cannot be explained by impaired context maintenance or a generalized deficit. For example, if the task required searching for a red item, and PSZ failed to store or maintain this task representation, then they should have exhibited reduced rather than increased capture of attention by red stimuli.
Possible Origins of Hyperfocusing
Now that we have reviewed the evidence supporting the hyperfocusing hypothesis, we turn to the more difficult question of why PSZ hyperfocus. Is hyperfocusing a cause or a consequence of cognitive impairment? For example, a reduction in cognitive resources might lead PSZ to hyperfocus as a compensatory response. Although we cannot rule out this possibility, it does not easily explain why PSZ exhibit more intense focusing and not just a narrower focusing of processing resources. Moreover, hyperfocusing can be observed even after controlling for differences in WM capacity or processing speed (as in the experiments shown in figures 1B, C, and E).
It is also worth considering whether hyperfocusing is a side effect of deficits in learning.64,65 Learning is certainly important for optimal performance of cognitive tasks,66 but it is not clear how the specific pattern documented here (intense but narrow focusing) would result from impaired learning. Moreover, recent evidence suggests that impaired reinforcement learning may, in part, be a consequence rather than a cause of reduced WM capacity in PSZ.67 An impaired ability to adjust resource allocation according to task demands68,69 could explain the finding of greater delay period activity in PSZ when a single item must be held in WM.19,28 However, we do not simply find a general failure to adjust: PSZ seem to be “stuck” with a narrow but intense focusing of resources. Thus, the evidence to date suggests that hyperfocusing is not a secondary consequence of other aspects of impaired cognition.
We can also speculate about the circuit-level neural abnormalities that might lead to hyperfocusing. Here, we point to computational neuroscience studies of cortical microcircuitry70,71 that could underlie hyperfocusing. These studies have shown that the D1 and D2 classes of dopamine receptors in the prefrontal cortex interact with N-Methyl-D-aspartate (NMDA) and gamma-Aminobutyric acid (GABA)-mediated processes to produce 2 competing attractor states: a D1-dominated state with deep basins of attraction that lead to exaggerated winner-take-all dynamics; and a D2-dominated state with shallow basins of attraction that promote rapid updating of representations. Optimal cognitive processing requires the ability to flexibly switch between the D1- and D2-dominated states depending on current task demands. If PSZ were biased toward the D1-dominated state, this would lead to a smaller number of more intense representations. Some evidence suggests that long-term changes in dopaminergic activity in PSZ lead to an upregulation of prefrontal D1 receptors.72 However, other studies suggest an upregulation of D2-related activity in PSZ.73,74 This issue is particularly difficult to study in medicated patients, for whom it is difficult to dissociate the causes from the consequences of the medications. Note, however, that we have typically found little or no correlation between antipsychotic medication dosage and our measures of hyperfocusing. Because medication effects may be nonlinear, however, further research research—including studies of unmedicated patients and the development of animal models75—is needed to determine how D1-and D2-mediated states operate in PSZ and whether these states are related to hyperfocusing.
Conclusions
The hyperfocusing hypothesis can explain many aspects of impaired cognition in PSZ, and it has led to several counterintuitive predictions that have been confirmed, including several findings of supranormal effects in PSZ. It is easy to imagine that an inability to distribute attention among multiple representations could impair cognitive performance in many natural contexts. For example, hyperfocusing could lead to impaired ability in real-world perceptual-motor tasks such as searching for ingredients while making a meal and in more complex social tasks such as making appraisals of the self and others.76,77 Hyperfocusing could also operate at the level of task selection, leading to perseveration and a limited range of activities.
However, many questions remain unanswered. First, although hyperfocusing can explain a broad set of laboratory effects, it is not yet known whether all of these effects truly reflect a single underlying mechanism. A few studies have shown that different hyperfocusing-related effects are correlated,8,20,61,78 but a larger-scale effort is needed to determine whether hyperfocusing is a unitary factor that accounts for substantial variance across a broad range of tasks. A second important issue is whether the degree of hyperfocusing predicts daily function or long-term outcome. Third, all of the research described here has focused on chronic, medicated PSZ, and additional research is needed to determine whether hyperfocusing is also present without medications, in unaffected relatives, in the prodromal period, or shortly after the onset of psychosis. Finally, although measures associated with hyperfocusing are typically correlated with broad cognitive ability, evidence that measures of hyperfocusing are correlated with measures of current psychotic symptoms has been rare.11 This may reflect the fact that the existing hyperfocusing studies have been conducted in stable, medicated outpatients, or it may indicate that cognitive deficits are a separate group of symptoms with independent underlying neuropathology. Additional research will be needed to determine whether hyperfocusing is related to positive symptoms in the absence of medications, to negative symptoms, or to the onset of psychosis.
If additional research confirms that hyperfocusing is a unitary factor and underlies multiple aspects of cognitive impairment in schizophrenia, hyperfocusing will provide a target for new treatments. Some cognitive training procedures already focus on increasing the breadth of attention,79,80 and these procedures may prove effective at countering the negative consequences of hyperfocusing in PSZ. In addition, if hyperfocusing is related to a D1-dominated cortical state, this may suggest novel pharmacological approaches.
Funding
Preparation of this manuscript and many of the studies reviewed here were made possible by the National Institutes of Health (R01MH065034 to Dr Gold and Dr Luck).
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Green MF. Schizophrenia From a Neurocognitive Perspective. Boston, MA: Allyn & Bacon; 1998. [Google Scholar]
- 2. Keefe RSE, Harvey PD. Cognitive Impairment in Schizophrenia. In: Geyer MA, Gross G, eds. Novel Antischizophrenia Treatments. Handbook of Experimental Pharmacology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012:11–37. doi: 10.1007/978-3-642-25758-2_2 [DOI] [PubMed] [Google Scholar]
- 3. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–330. [DOI] [PubMed] [Google Scholar]
- 4. Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72(1):41–51. [DOI] [PubMed] [Google Scholar]
- 5. Tan BL. Profile of cognitive problems in schizophrenia and implications for vocational functioning. Aust Occup Ther J. 2009;56(4):220–228. [DOI] [PubMed] [Google Scholar]
- 6. Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res. 2010;122(1-3):1–23. [DOI] [PubMed] [Google Scholar]
- 7. Barch DM, Moore H, Nee DE, Manoach DS, Luck SJ. CNTRICS imaging biomarkers selection: working memory. Schizophr Bull. 2012;38(1):43–52. doi: 10.1093/schbul/sbr160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johnson MK, McMahon RP, Robinson BM, et al. The relationship between working memory capacity and broad measures of cognitive ability in healthy adults and people with schizophrenia. Neuropsychology. 2013;27(2):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114(4):599–611. [DOI] [PubMed] [Google Scholar]
- 10. Kreither J, Lopez-Calderon J, Leonard CJ, et al. Electrophysiological evidence for hyperfocusing of spatial attention in schizophrenia. J Neurosci. 2017;37(14):3813–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leonard CJ, Robinson BM, Hahn B, Luck SJ, Gold JM. Altered spatial profile of distraction in people with schizophrenia. J Abnorm Psychol. 2017;126(8):1077–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mcghie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34:103–116. [DOI] [PubMed] [Google Scholar]
- 13. Luck SJ, Leonard CJ, Hahn B, Gold JM. Is selective attention impaired in schizophrenia? Schizophr Bull. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacDonald AW III, Carter CS, Kerns JG, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162(3):475–484. [DOI] [PubMed] [Google Scholar]
- 15. Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99(1):45–77. [DOI] [PubMed] [Google Scholar]
- 16. Cowan N, Elliott EM, Scott Saults J, et al. On the capacity of attention: its estimation and its role in working memory and cognitive aptitudes. Cogn Psychol. 2005;51(1):42–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short-term memory, and general fluid intelligence: a latent-variable approach. J Exp Psychol Gen. 1999;128(3):309–331. [DOI] [PubMed] [Google Scholar]
- 18. Luck SJ, Vogel EK. Visual working memory capacity: from psychophysics and neurobiology to individual differences. Trends Cogn Sci. 2013;17(8):391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leonard CJ, Kaiser ST, Robinson BM, et al. Toward the neural mechanisms of reduced working memory capacity in schizophrenia. Cereb Cortex. 2013;23(7):1582–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gray BE, Hahn B, Robinson B, et al. Relationships between divided attention and working memory impairment in people with schizophrenia. Schizophr Bull. 2014;40(6):1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hahn B, Robinson BM, Harvey AN, et al. Visuospatial attention in schizophrenia: deficits in broad monitoring. J Abnorm Psychol. 2012;121(1):119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukuda K, Vogel E, Mayr U, Awh E. Quantity, not quality: the relationship between fluid intelligence and working memory capacity. Psychon Bull Rev. 2010;17(5):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erickson MA, Hahn B, Leonard CJ, et al. Impaired working memory capacity is not caused by failures of selective attention in schizophrenia. Schizophr Bull. 2015;41(2):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115(4):658–673. [DOI] [PubMed] [Google Scholar]
- 25. Gold JM, Wilk CM, McMahon RP, Buchanan RW, Luck SJ. Working memory for visual features and conjunctions in schizophrenia. J Abnorm Psychol. 2003;112(1):61–71. [PubMed] [Google Scholar]
- 26. Mayer JS, Fukuda K, Vogel EK, Park S. Impaired contingent attentional capture predicts reduced working memory capacity in schizophrenia. PLoS One. 2012;7(11):e48586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erickson M, Hahn B, Leonard C, Robinson B, Luck S, Gold J. Enhanced vulnerability to distraction does not account for working memory capacity reduction in people with schizophrenia. Schizophr Res Cogn. 2014;1(3):149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hahn B, Robinson BM, Leonard CJ, Luck SJ, Gold JM. Posterior parietal cortex dysfunction is central to working memory storage and broad cognitive deficits in schizophrenia. J Neurosci. 2018;37:8378–8387. doi: 10.1523/JNEUROSCI.0913-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428(6984):748–751. [DOI] [PubMed] [Google Scholar]
- 30. Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438(7067):500–503. [DOI] [PubMed] [Google Scholar]
- 31. Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428(6984):751–754. [DOI] [PubMed] [Google Scholar]
- 32. Todd JJ, Marois R. Posterior parietal cortex activity predicts individual differences in visual short-term memory capacity. Cogn Affect Behav Neurosci. 2005;5(2):144–155. [DOI] [PubMed] [Google Scholar]
- 33. Hahn B, Harvey AN, Gold JM, Ross TJ, Stein EA. Load-dependent hyperdeactivation of the default mode network in people with schizophrenia. Schizophr Res. 2017;185:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Glahn DC, Ragland JD, Abramoff A, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60(2-3):285–298. [DOI] [PubMed] [Google Scholar]
- 36. Ball K, Owsley C. The useful field of view test: a new technique for evaluating age-related declines in visual function. J Am Optom Assoc. 1993;64(1):71–79. [PubMed] [Google Scholar]
- 37. Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34(11):3110–3123. [PubMed] [Google Scholar]
- 38. Clay OJ, Wadley VG, Edwards JD, Roth DL, Roenker DL, Ball KK. Cumulative meta-analysis of the relationship between useful field of view and driving performance in older adults: current and future implications. Optom Vis Sci. 2005;82(8):724–731. [DOI] [PubMed] [Google Scholar]
- 39. Cegalis JA, Deptula D. Attention in schizophrenia. Signal detection in the visual periphery. J Nerv Ment Dis. 1981;169(12):751–760. [PubMed] [Google Scholar]
- 40. Cegalis JA, Leen D, Solomon EJ. Attention in schizophrenia: an analysis of selectivity in the functional visual field. J Abnorm Psychol. 1977;86(5):470–482. [DOI] [PubMed] [Google Scholar]
- 41. Cegalis JA, Tegtmeyer PF. Visual selectivity in schizophrenia. J Nerv Ment Dis. 1980;168(4):229–235. [DOI] [PubMed] [Google Scholar]
- 42. Asarnow RF, Granholm E. The contribution of cognitive psychology to vulnerability models. Search Causes Schizophr. 1990;2:205–220. [Google Scholar]
- 43. Asarnow RF, Granholm E, Sherman T. Span of apprehension in schizophrenia. In: Steinhauer SR, Gruzelier JH, Zubin J, eds. Handbook of Schizophrenia, Vol. 5. Neuropsychology, Psychophysiology, and Information Processing. New York: Elsevier Science; 1991. [Google Scholar]
- 44. Granholm E, Asarnow RF, Marder SR. Display visual angle and attentional scanpaths on the span of apprehension task in schizophrenia. J Abnorm Psychol. 1996;105(1):17–24. [DOI] [PubMed] [Google Scholar]
- 45. Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. [DOI] [PubMed] [Google Scholar]
- 46. Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL. Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J Exp Psychol Hum Percept Perform. 1994;20(4):887–904. [DOI] [PubMed] [Google Scholar]
- 47. Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol Psychiatry. 2013;74(6):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hahn B, Harvey AN, Gold JM, Fischer BA, Keller WR, Ross TJ, Stein EA. Hyperdeactivation of the default mode network in people with schizophrenia when focusing attention in space. Schizophr Bull. 2016;42:1158–11 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spencer KM, Nestor PG, Valdman O, Niznikiewicz MA, Shenton ME, McCarley RW. Enhanced facilitation of spatial attention in schizophrenia. Neuropsychology. 2011;25(1):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Elahipanah A, Christensen BK, Reingold EM. Controlling the spotlight of attention: visual span size and flexibility in schizophrenia. Neuropsychologia. 2011;49(12):3370–3376. [DOI] [PubMed] [Google Scholar]
- 51. Beedie SA, Benson PJ, St Clair DM. Atypical scanpaths in schizophrenia: evidence of a trait- or state-dependent phenomenon? J Psychiatry Neurosci. 2011;36(3):150–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Elahipanah A, Christensen BK, Reingold EM. Visual search performance among persons with schizophrenia as a function of target eccentricity. Neuropsychology. 2010;24(2):192–198. [DOI] [PubMed] [Google Scholar]
- 53. Everling S, Krappmann P, Preuss S, Brand A, Flohr H. Hypometric primary saccades of schizophrenics in a delayed-response task. Exp Brain Res. 1996;111(2):289–295. [DOI] [PubMed] [Google Scholar]
- 54. Hutton SB, Cuthbert I, Crawford TJ, Kennard C, Barnes TR, Joyce EM. Saccadic hypometria in drug-naive and drug-treated schizophrenic patients: a working memory deficit? Psychophysiology. 2001;38(1):125–132. [PubMed] [Google Scholar]
- 55. Leonard CJ, Robinson BM, Kaiser ST, et al. Testing sensory and cognitive explanations of the antisaccade deficit in schizophrenia. J Abnorm Psychol. 2013;122(4):1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Luck SJ, McClenon C, Beck VM, et al. Hyperfocusing in schizophrenia: evidence from interactions between working memory and eye movements. J Abnorm Psychol. 2014;123(4):783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gold JM, Hahn B, Zhang WW, et al. Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Arch Gen Psychiatry. 2010;67(6):570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 1992;18(4):1030–1044. [PubMed] [Google Scholar]
- 59. Eimer M, Kiss M. Involuntary attentional capture is determined by task set: evidence from event-related brain potentials. J Cogn Neurosci. 2008;20(8):1423–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee J, Leonard CJ, Luck SJ, Geng JJ. Dynamics of feature-based attentional selection during color-shape conjunction search. J Cogn Neurosci. 2018;30:1773–1787. [DOI] [PubMed] [Google Scholar]
- 61. Sawaki R, Kreither J, Leonard CJ, Kaiser ST, Hahn B, Gold JM, Luck SJ. Hyperfocusing on goal-related information in schizophrenia: Evidence from electrophysiology. J Abnorm Psychol. 2017;126:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sawaki R, Geng JJ, Luck SJ. A common neural mechanism for preventing and terminating the allocation of attention. J Neurosci. 2012;32(31):10725–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Leonard CJ, Balestreri A, Luck SJ. Interactions between space-based and feature-based attention. J Exp Psychol Hum Percept Perform. 2015;41(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dowd EC, Frank MJ, Collins A, Gold JM, Barch DM. Probabilistic reinforcement learning in patients with schizophrenia: relationships to anhedonia and avolition. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1(5):460–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28(1):7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nassar MR, Helmers JC, Frank MJ. Chunking as a rational strategy for lossy data compression in visual working memory. Psychol Rev. 2018;125(4):486–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Collins AGE, Albrecht MA, Waltz JA, Gold JM, Frank MJ. Interactions among working memory, reinforcement learning, and effort in value-based choice: a new paradigm and selective deficits in schizophrenia. Biol Psychiatry. 2017;82(6):431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jones MT, Deckler E, Laurrari C, Jarskog LF, Penn DL, Pinkham AE, Harvey PD. Confidence, performance, and accuracy of self-assessment of social cognition: a comparison of schizophrenia patients and healthy controls. Schizophr Res Cogn. 2019. doi: 10.1016/j.scog.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cornacchio D, Pinkham AE, Penn DL, Harvey PD. Self-assessment of social cognitive ability in individuals with schizophrenia: appraising task difficulty and allocation of effort. Schizophr Res. 2017;179:85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-o-methyltransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64(9):739–749. [DOI] [PubMed] [Google Scholar]
- 71. Rolls ET, Loh M, Deco G, Winterer G. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci. 2008;9(9):696–709. [DOI] [PubMed] [Google Scholar]
- 72. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22(9):3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97(14):8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Howes OD, Kambeitz J, Kim E, Stahl D, Slifstein M, Abi-Dargham A, Kapur S. The nature of dopamine dysfunction in schizophrenia and what this means for treatment: meta-analysis of imaging studies. Arch Gen Psychiatry. 2012;69(8):776–786. doi: 10.1001/archgenpsychiatry.2012.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hahn B, Reneski CH, Pocivavsek A, Schwarcz R. Prenatal kynurenine treatment in rats causes schizophrenia-like broad monitoring deficits in adulthood. Psychopharmacology (Berl). 2018;235(3):651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. James AV, Hasson-Ohayon I, Vohs J, et al. Metacognition moderates the relationship between dysfunctional self-appraisal and social functioning in prolonged schizophrenia independent of psychopathology. Compr Psychiatry. 2016;69:62–70. [DOI] [PubMed] [Google Scholar]
- 77. Moritz S, Balzan RP, Bohn F, et al. Subjective versus objective cognition: evidence for poor metacognitive monitoring in schizophrenia. Schizophr Res. 2016;178(1-3):74–79. [DOI] [PubMed] [Google Scholar]
- 78. Gold JM, Robinson B, Leonard CJ, et al. Selective attention, working memory, and executive function as potential independent sources of cognitive dysfunction in schizophrenia. Schizophr Bull. 2018;44(6):1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Anguera JA, Boccanfuso J, Rintoul JL, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Green CS, Bavelier D. Action video game training for cognitive enhancement. Curr Opin Behav Sci. 2015;4:103–108. doi: 10.1016/j.cobeha.2015.04.012 [DOI] [Google Scholar]