Abstract
A combination of genetic and environmental risk factors has been considered as the pathogenic cause for mental disorders including schizophrenia. Here, we sought to find out whether the abnormality of the dopamine system, coupled with the exposure to modest stress, is sufficient to trigger the manifestation of schizophrenia-like behaviors. We found that exposing dopamine D4 receptor knockout (D4KO) mice with 1-week restraint stress (2 h/d) induced significant deficits in sensorimotor gating, cognitive processes, social engagement, as well as the elevated exploratory behaviors, which are reminiscent to schizophrenia phenotypes. Electrophysiological studies found that GABAergic transmission was significantly reduced in prefrontal cortical neurons from stressed D4KO mice. Additionally, administration of diazepam, a GABA enhancer, restored GABAergic synaptic responses and ameliorated some behavioral abnormalities in stressed D4KO mice. These results have revealed that the combination of 2 key genetic and environmental susceptibility factors, dopamine dysfunction and stress, is a crucial trigger for schizophrenia-like phenotypes, and GABA system in the prefrontal cortex is a downstream convergent target that mediates some behavioral outcomes.
Keywords: schizophrenia, stress, dopamine D4 receptor, GABA
Introduction
Schizophrenia, a devastating neuropsychiatric disorder that affects nearly 1% of the general population, is characterized by 3 core symptoms: positive symptoms (psychosis), negative symptoms (social deficits) and cognitive symptoms (working memory and attention impairment).1 Researchers have been searching for the biological basis that underlies the manifestation of schizophrenia symptoms for decades. One hundred eight schizophrenia-associated genetic loci have been identified in genome-wide studies, and these common alleles of small effect confer genetic risk for this polygenic disease.2 A recent twin study of schizophrenia reinforces the role of genetics in determining risk, suggesting that almost 80% of the likelihood of having schizophrenia may be genetic.3
Several genes involved in dopamine signaling, including those encoding dopamine receptors (DRD2, DRD3, and DRD4) and catecholamines-degrading enzyme catechol-O-methyltransferase (COMT), have been implicated in the etiology of schizophrenia.4 DRD4 is highly enriched in the prefrontal cortex (PFC),5–7 a key brain region significantly affected by schizophrenia.8,9 DRD4 is upregulated in postmortem schizophrenic brain,10 and the uniquely effective antipsychotic drug clozapine has a high affinity to D4 receptors.11,12 Genetic studies have confirmed that D4 gene polymorphisms are highly associated with risk-taking behaviors and attention deficit-hyperactivity disorder.13–16 Genetic ablation of D4 receptors in mice results in supersensitivity to psychomotor stimulants.17 These lines of evidence have implicated DRD4 in schizophrenia and related psychiatric disorders.
Other than genetic factors, environmental factors, such as stress, are also thought to contribute to the risk of developing schizophrenia.18,19 Stressors, such as life adversity, can disrupt PFC function, triggering the development of cognitive deficits observed in schizophrenia.20 Since schizophrenia has the late adolescent/early adulthood onset, a “two-hits” hypothesis of the disease has been proposed.21–24 In this model, genetic factors disrupt central nervous system development at the early stage, and these early disruptions produce long-term vulnerability to a “second hit” that occurs later in life, leading to the onset of schizophrenia symptoms. Consistently, it has been shown that combining a prenatal immune disruptor with unpredictable stress to offspring in their adolescence can trigger behavioral abnormalities reminiscent to some neuropsychiatric disorders.25 Repeated prenatal stress or postnatal social isolation stress also markedly exacerbates schizophrenic endophenotypes in Snap-25 mutant mice26 and an NMDAR hypofunction mouse model,27 as well as induces aggression and hyperactivity in PACAP-deficient mice.28 Development of PFC during the earliest embryonic stage and during the late adolescent stage is thought to represent 2 critical periods of vulnerability for schizophrenia, because cell proliferation and synaptic pruning in these stages may be influenced by environmental factors.29 Moreover, prefrontocortical dopamine response to stress is found to be disrupted in schizophrenia patients.30 In this study, we modeled the “two-hits” scenario by exposing dopamine D4 receptor knockout (D4KO) mice to subchronic restraint stress, and examined the occurrence of schizophrenia-like phenotypes and the underlying mechanisms.
Methods
Animals
Male and female C57BL/6J and D4KO mice17 at the late adolescent (6–7 wk old) stage were used in this study. Animals were maintained in the animal facility under controlled environmental conditions (22°C, 12 h light/dark cycle) with free access to food. All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the State University of New York at Buffalo.
Stress and Drug Treatment
For restraint stress, animals were gently placed in a plastic cylinder and restrained for 2 hours between 9:00 AM and 11:00 AM daily for 7 consecutive days. Diazepam (Sigma-Aldrich) stock solution (10 mg/ml, dissolved in DMSO) was diluted in saline (0.5 mg/ml) before use. Diazepam (5 mg/kg) was injected (i.p.) in a subset of stressed D4KO mice during the 1-week restraint stress procedure (on the 5th, 6th and 7th d, injected after stress exposure each time).
Behavioral Tests
All behavior tests were carried out within 2 days after termination of the restraint stress protocol during the light cycle. The light was adjusted to dim light during all the behavioral experiments. ANY-maze 5.1 software (Stoelting Co.) was used for recording and data analysis. To minimize the interference of previous behavior tests on the upcoming test, at least 2-hour intervals were applied between 2 behavioral assays, and usually, only 2 tests were carried out on the same day. Each animal was subject to 4 behavioral assays within 2 days after stress. One set of mice was tested with locomotion, elevated plus maze (EPM), social approach, and acoustic startle response. Another set of mice was tested with rotarod, temporal order recognition (TOR), forced swimming, and sucrose preference. Details on these behavioral tests are included in supplementary methods.
Electrophysiology
PFC slices from mice (7-wk-old) were prepared as previously described.31 Details on whole-cell voltage-clamp recordings of synaptic currents are included in supplementary methods.
Immunohistochemistry
Details on parvalbumin staining are included in supplementary methods.
Statistics
Clampfit 10.0.7 software (Molecular Devices), Mini Analysis Program 6.0.3 (Synaptosoft) and GraphPad Prism 6.02 (GraphPad Software Inc.) were used for analyzing the results. All data were presented as means ± standard error of the mean (SEM). For statistical significance, experiments with 2 groups were analyzed using 2-tailed Student’s t tests. Experiments with more than 2 groups were subjected to 1-way ANOVA, 2-way ANOVA or 2-way repeated measure ANOVA (rmANOVA), followed by post hoc Bonferroni tests for multiple comparisons.
Results
Stressed D4KO Mice Exhibit Deficits in Sensorimotor Gating, Cognition and Sociability, as well as Elevated Exploratory Behaviors
To test the behavioral abnormalities induced by modest stress (2 h restraint/d, 7 d) in wild-type (WT) and D4KO mice, a variety of behavioral tests that evaluate positive, negative, and cognitive symptoms of schizophrenia were carried out.
We first tested pre-pulse inhibition (PPI), a measurement of sensorimotor gating, in which a weaker acoustic pre-stimulus inhibits the reaction to a subsequent strong startling stimulus. Deficits of PPI reflect the inability to filter out the unnecessary information, which is often present in schizophrenia patients.32
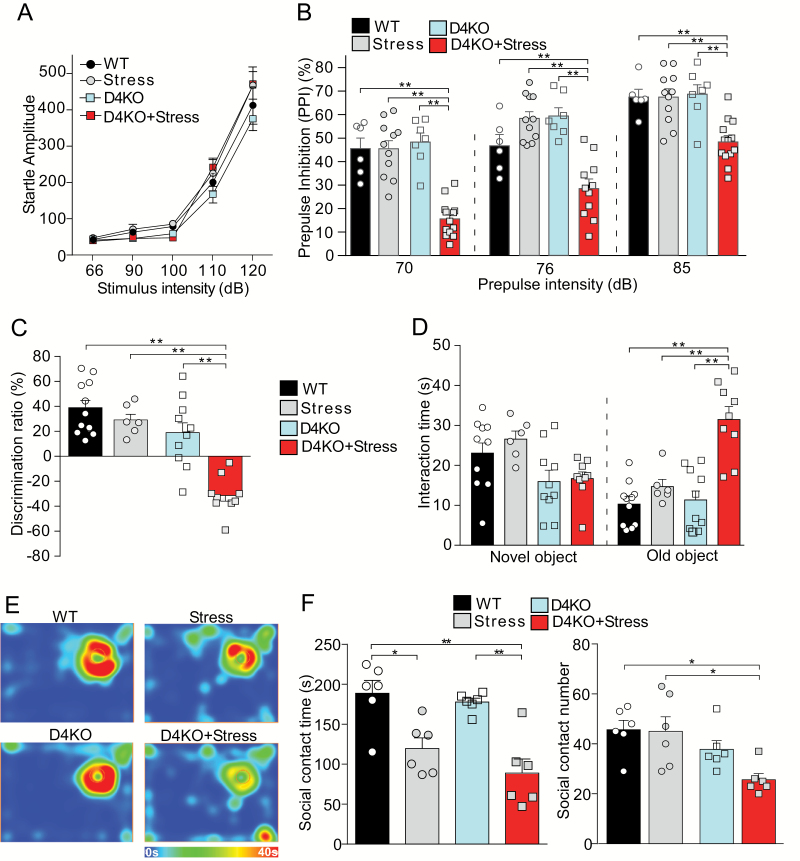
The basal startle responses to white noise background (66 dB) and different acoustic stimulus intensities (90, 100, 110, and 120 dB) were similar in unstressed or stressed WT and D4KO mice (figure 1A, F3,30(group) = 1.55, P = .22; 2-way ANOVA). However, significant PPI deficits were found selectively in the stressed D4KO group at various pre-pulse intensities, compared to the other 3 groups (figure 1B, 70 dB: F1,33(interaction) = 21.73, P < .001; 76 dB: F1, 31(interaction) = 30.95, P < .001; 85 dB: F1,33(interaction) = 8.48, P = .006, 2-way ANOVA). It suggests that D4KO mice are more susceptible to stress in their sensorimotor gating.
Fig. 1.
Stressed D4KO mice exhibit deficits in sensorimotor gating, cognition, and sociability. (A) Plot of the startle response to white noise background (66 dB), and different acoustic stimulus intensities (90, 100, 110, and 120 dB) in non-stressed wild-type (WT) mice (WT, n = 6), WT mice with 7-d restraint stress (Stress, n= 9), non-stressed D4KO mice (D4KO, n = 7), and D4KO mice with 7-d restraint stress (D4KO+Stress, n = 12). (B) Plot of PPI with the pre-pulse intensity of 70, 76 and 85 dB in the 4 mouse groups. (C) Plot of discrimination ratio in TOR tests of WT (n = 11), Stress (n = 6), D4KO (n = 10), and D4KO+Stress (n = 9) mice. (D) Plot of interaction time with novel and old objects in TOR tests of the 4 mouse groups. (E, F) Representative heat maps (E) and plot of social contact time and social approach numbers (F) in the social engagement test of the 4 mouse groups (n = 6 per group). *P < .05, **P < .01, 2-way ANOVA.
Next, we tested the impact of modest stress on cognitive processes in D4KO mice. The TOR memory, a PFC-mediated explicit memory process requiring judgments of the prior occurrence of stimuli based on the relative familiarity information,33 was performed. WT mice without or with stress, as well as non-stressed D4KO mice, all exhibited the preference for the novel (less recent) object, while stressed D4KO mice lost the preference (figure 1C, discrimination ratio, WT: 38.94% ± 6.47%, Stress: 29.13% ± 5.03%, D4KO: 18.99% ± 8.78%, D4KO+Stress: −31.37% ± 5.13%, F1, 32(interaction) = 7.92, P = .008, 2-way ANOVA). Instead of spending more time on the novel object, stressed D4KO mice spent significantly more time on the old familiar (more recent) object (figure 1D, WT: 10.30 ± 1.63 s, Stress: 14.69 ± 1.79 s, D4KO: 11.35 ± 2.36 s, D4KO+Stress: 31.49 ± 3.13 s; F1,32(interaction) = 10.64, P = .003; 2-way ANOVA). It suggests that D4KO mice are more vulnerable to stress in their cognitive function.
Moreover, we performed social engagement tests to evaluate the sociability. Stress exposure significantly reduced the time of social interaction in both WT and D4KO mice (figures 1E and 1F, F1,20(stress) = 32.42, P < .001; 2-way ANOVA), while the significantly decreased number of social approach was only observed in stressed D4KO mice (figure 1F, P < .05, post hoc of 2-way ANOVA). It suggests that D4KO mice exposed to stress are more likely to have social withdrawal.
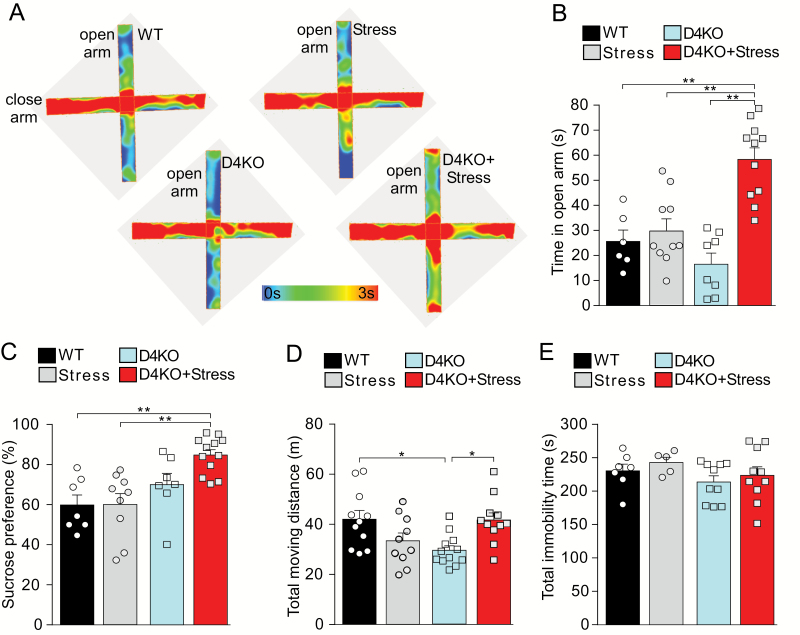
Given the schizophrenia-like behavioral manifestation of stressed D4KO mice, we performed additional tests for anxiety and depression-like behaviors. In the EPM test (figures 2A and 2B), WT mice without or with stress, as well as non-stressed D4KO mice spent relatively less time in the open arm, suggesting the presence of elevation-induced anxiety. However, stressed D4KO mice spent dramatically more time in the open arm (WT: 25.63 ± 4.53 s, Stress: 29.72 ± 4.48 s, D4KO 16.46 ± 4.22 s, D4KO+Stress: 58.36 ± 4.63 s, F1,32(interaction) = 13.00, P = .001, 2-way ANOVA), suggesting that they are less anxious and their exploratory behavior is strongly elevated.
Fig. 2.
Stressed D4KO mice display elevated exploratory behaviors, but no anxiety or depression-like behaviors. (A, B) Representative heat maps (A) and plot of time in the open arm (B) of the elevated plus maze test of wild-type (WT; n = 6), Stress (n = 11), D4KO (n = 8), and D4KO+Stress (n = 11) mice. (C) Plot of the percentage of sucrose preference on day 4 of sucrose preference test of WT (n = 7), Stress (n = 9), D4KO (n = 7), and D4KO+Stress (n = 12) mice. (D) Plot of total moving distance in the locomotion test of WT (n = 11), Stress (n = 10), D4KO (n = 12), and D4KO+Stress (n = 11) mice. (E) Plot of total immobility time in the forced-swimming test of WT (n = 7), Stress (n = 5), D4KO (n = 10), and D4KO+Stress (n = 10) mice. *P < .05, **P < .01, 2-way ANOVA.
In the sucrose preference test of anhedonia, we found that stressed D4KO mice had a significantly higher level of sucrose preference, compared to WT mice (figure 2C, Con: 59.84% ± 4.99%; Stress: 60.04% ± 5.46%; D4KO: 70.03% ± 5.72%, D4KO+Stress: 84.81% ± 2.65%; P < .01, post hoc of 2-way ANOVA), suggesting that stressed D4KO mice display heightened sensitivity to sucrose water cues, which might reflect changes in motivational states.
For the locomotor activity, D4KO mice exhibited hypo-locomotion, compared to WT mice, and stress significantly increased the locomotor activity in D4KO mice, bringing it to the level of WT mice (figure 2D, F1,40(interaction) = 13.51; P < .001, 2-way ANOVA). No significant differences were found on the total immobility in forced-swim tests among the 4 groups (figure 2E, F1,28(interaction) = 0.01; P = .91, 2-way ANOVA), suggesting the lack of depressive behaviors.
Stressed D4KO Mice Show Little Changes in Excitatory Synaptic Signaling, but Significantly Diminished GABAergic Synaptic Inhibition in the PFC
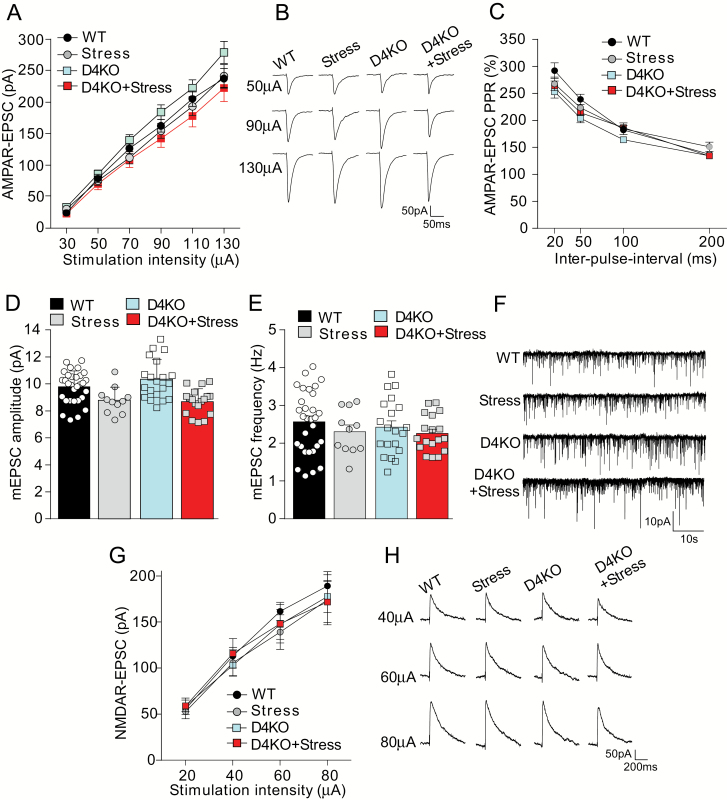
To explore the potential mechanisms that underlie the behavioral changes in stressed D4KO mice, electrophysiological experiments were carried out. We first examined the excitatory synaptic responses mediated by AMPA and NMDA receptors in PFC pyramidal neurons, which are important for PFC-mediated cognitive and emotional processes.34 For AMPAR-EPSC, which evoked by a series of stimulation intensities, the modest restraint stress showed little effect in both WT and D4KO mice (figures 3A and 3B, F3,60(group) = 2.09, P = .11, 2-way ANOVA). The ratio of paired-pulse facilitation of AMPAR-EPSC was similar among the 4 groups (figure 3C, F3,56(group) = 2.18, P = .10, 2-way ANOVA). Miniature EPSC, a synaptic response resulting from the quantal release of single glutamate vesicles, also had little changes in stressed WT or D4KO mice (figures 3D–3F, WT: 9.77 ± 0.22 pA, 2.57 ± 0.15 Hz; Stress: 8.77 ± 0.30 pA, 2.31 ± 0.18 Hz; D4KO: 10.33 ± 0.33 pA, 2.43 ± 0.16 Hz; D4KO+Stress: 8.69 ± 0.33 pA, 2.26 ± 0.11 Hz, Amp: F1,73(interaction) = 1.22, P = .27, Freq: F1,73(interaction) = 0.08, P = .78, 2-way ANOVA). For input-output curves of NMDAR-EPSC, no significant changes were found in WT or D4KO mice without or with stress (figures 3G and 3H, F3,43(group) = 0.11, P = .96, 2-way ANOVA).
Fig. 3.
Stressed D4KO mice show little changes in excitatory synaptic signaling in prefrontal cortex. (A, B, C) Input-output curves of AMPAR-EPSC (A), representative traces (B), and plot of paired-pulse ratio of AMPA-EPSC (C) in prefrontal cortex (PFC) pyramidal neurons from wild-type (WT; n = 20 cells), Stress (n = 15 cells), D4KO (n = 21 cells), and D4KO+Stress (n = 12 cells) mice. (D, E, F) Miniature EPSC amplitude (D), frequency (E) and representative traces (F) in PFC pyramidal neurons from WT (n = 30 cells), Stress (n = 11 cells), D4KO (n = 20 cells), and D4KO+Stress (n = 18 cells) mice. (G, H) Input-output curves (G) and representative traces (H) of evoked NMDAR-EPSC in PFC pyramidal neurons from WT (n = 9 cells), Stress (n = 15 cells), D4KO (n = 14 cells), and D4KO+Stress (n = 8 cells) mice.
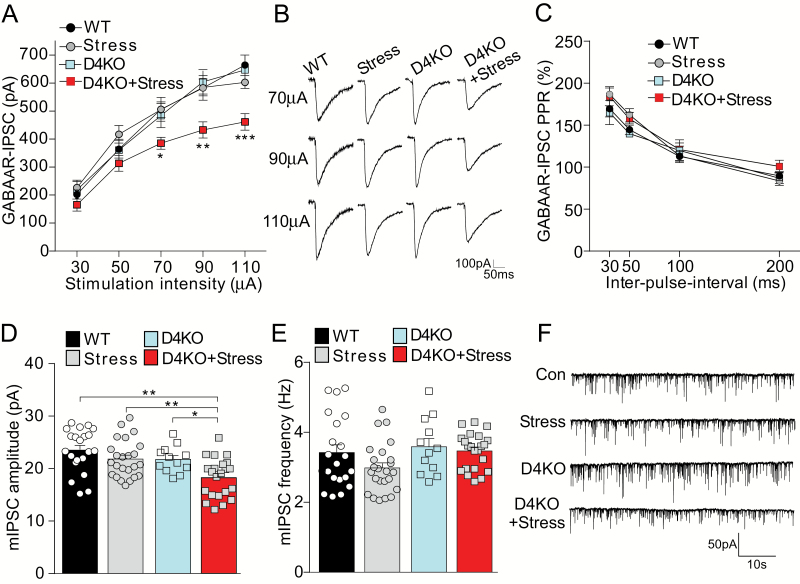
Given the mild effects of stress on glutamatergic responses in D4KO mice, we further examined GABAergic inhibitory transmission in PFC pyramidal neurons. As shown in figures 4A and 4B, stressed D4KO mice had significantly decreased amplitudes of GABAAR-IPSC (~30% reduction) evoked by a series of stimulation intensities, compared to WT or non-stressed D4KO mice (F3,62(group) = 5.13, P = .003, 2-way ANOVA). The ratio of paired-pulse facilitation of GABA-IPSC was not significantly changed among the 4 groups (figure 4C, F3,67(group) = 1.85, P = .15, 2-way ANOVA). The amplitude of miniature IPSC was significantly decreased (~22%) only in stressed D4KO mice (figure 4D and 4F, WT: 23.53 ± 0.90 pA, Stress: 21.83 ± 0.73 pA, D4KO: 21.76 ± 0.74 pA, D4KO+Stress: 18.28 ± 0.79 pA, D4KO+Stress vs WT, P < .01, post hoc of 2-way ANOVA). The mIPSC frequency was not significantly altered by D4 knockout or stress (figure 4E, F1,75(interaction) = 0.75, P = .39, 2-way ANOVA). These results suggest that GABAergic synaptic inhibition is impaired in PFC pyramidal neurons from stressed D4KO mice.
Fig. 4.
Stressed D4KO mice have the significantly diminished GABAergic synaptic inhibition in prefrontal cortex. (A, B, C) Input-output curves of GABAAR-IPSC (A), representative traces (B), and plot of paired-pulse ratio of GABAAR-IPSC (C) in prefrontal cortex (PFC) pyramidal neurons from wild-type (WT; n = 23 cells), Stress (n = 15 cells), D4KO (D4KO, n = 11 cells), and D4KO+Stress (n = 17 cells) mice. (D, E, F) Miniature IPSC amplitude (D), frequency (E) and representative traces (F) in PFC pyramidal neurons from WT (n = 21 cells), Stress (n = 24 cells), D4KO (n = 12 cells), and D4KO+Stress (n = 22 cells) mice. *P < .05, **P < .01, ***P < .001; 2-way ANOVA.
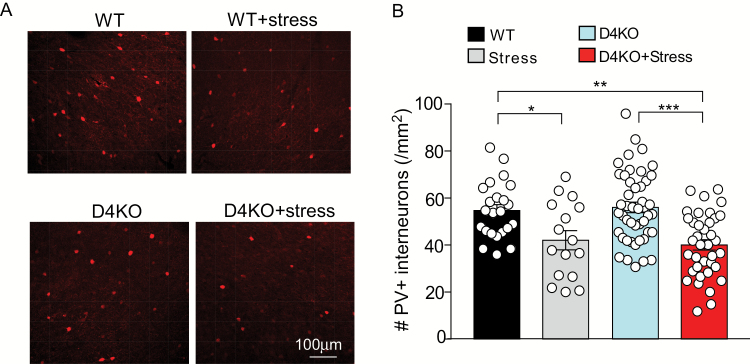
A large amount of literature has shown that parvalbumin (PV)-positive interneurons, which control prefrontal cortical gamma oscillations, a key neural substrate for cognition, are affected in schizophrenia.35–37 The impaired GABA function in stressed D4KO mice prompted us to test whether these mice exhibit the loss of PV staining in PFC. As shown in figures 5A and 5B, compared to non-stressed WT mice, the 7-day restraint stress (2 h daily) induced a modest reduction of the number of PV+ neurons in PFC of WT mice, while a more significant loss of PV+ neurons was observed in stressed D4KO mice (WT: 54.7 ± 2.53, WT+Stress: 42.1 ± 4.06, D4KO: 56.0 ± 2.23, D4KO+Stress: 40.1 ± 2.21, F1,118(stress) = 26.0, P < .0001, 2-way ANOVA). These data provide 1 potential mechanism for the diminished GABAergic inhibition and the schizophrenia-like behavioral phenotypes in stressed D4KO mice.
Fig. 5.
Stressed D4KO mice have the significantly reduced number of parvalbumin (PV)-positive interneurons in the prefrontal cortex. (A) Representative confocal images of PV staining in prefrontal cortex (PFC) slices from wild-type (WT) and D4KO mice without or with 7-d restraint stress. (B) Bar graphs of the number of PV+ interneurons in PFC slices from WT (n = 23 slices), Stress (n = 16 slices), D4KO (D4KO, n = 46 slices), and D4KO+Stress (n = 37 slices) mice (3–4 each group). *P < .05, **P < .01, ***P < .001, 2-way ANOVA.
The GABA Enhancer Diazepam Restores Synaptic Inhibition and Reverses Some Behavioral Abnormalities in Stressed D4KO Mice
To find out whether the decreased GABAergic transmission may underlie the abnormal behaviors of stressed D4KO mice, we injected diazepam (DZ, 5 mg/kg, i.p., 3×), a positive allosteric modulator of GABAA receptors, to elevate GABA signaling, followed by electrophysiological experiments and behavioral tests.
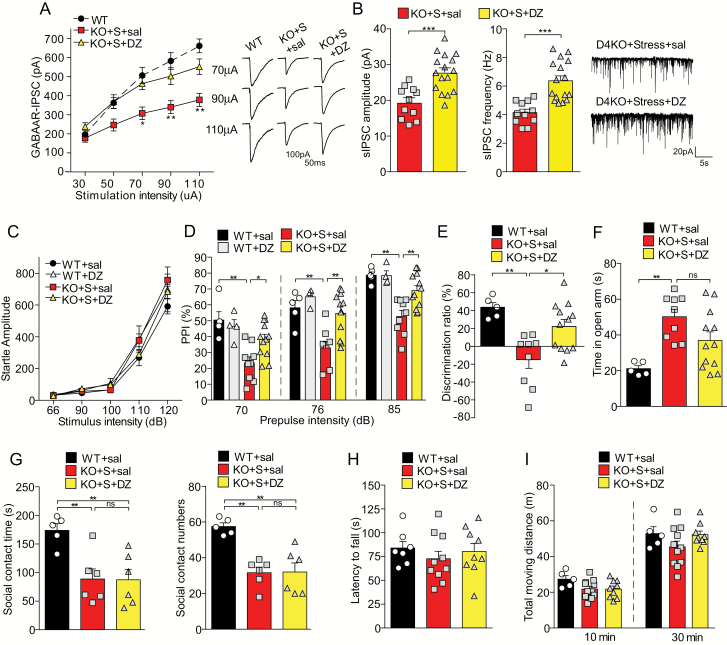
We first examined the effects of diazepam treatment on inhibitory synaptic transmission. As shown in figure 6A, diazepam injections to stressed D4KO mice induced the significant increase (46%–50%) of GABAAR-IPSC evoked by strong stimuli (F2,46(group) = 8.32, P < .001, 2-way ANOVA). Consistently, the spontaneous IPSC amplitude and frequency in PFC neurons from stressed D4KO mice were also significantly increased by diazepam treatment (figure 6B, saline: 19.22 ± 1.34 pA, 4.15 ± 0.24 Hz; DZ: 27.61 ± 1.32 pA, 6.37 ± 0.35 Hz; P < .001, t test).
Fig. 6.
The GABA enhancer diazepam elevates GABAergic responses and reverses some behavioral abnormalities in stressed D4KO mice. (A) Input-output curves of GABAAR-IPSC in prefrontal cortex (PFC) pyramidal neurons from wild-type (WT) or stressed D4KO mice injected with saline (KO+S+sal, n = 15 cells) or diazepam (KO+S+DZ, n = 11 cells). Inset: representative GABAAR-IPSC traces. (B) Bar graphs of spontaneous IPSC amplitude and frequency in PFC pyramidal neurons from stressed D4KO mice injected with saline (KO+S+sal, n = 11 cells) or DZ (KO+S+DZ, n = 16 cells). Inset: representative sIPSC traces. (C) Plot of basal startle response to different acoustic stimulus intensities in WT mice injected with saline (WT+sal, n = 5) or diazepam (WT+DZ, n = 4), or stressed D4KO mice injected with saline (KO+S+sal, n = 9) or diazepam (KO+S+DZ, n = 12). (D) Plot of PPI with the pre-pulse intensity of 70, 76 and 85 dB in the 4 groups. (E) Plot of discrimination ratio in TOR tests of WT+sal (n = 5), KO+S+sal (n = 9), and KO+S+DZ (n = 12) mice. (F) Plot of time in the open arm of elevated plus maze test from WT+sal (n = 5), KO+S+sal (n = 9) and KO+S+DZ (n = 12) mice. (G) Plot of social contact time and social approach numbers in the social engagement test of WT+sal (n = 5), KO+S+sal (n = 6) and KO+S+DZ (n = 6) mice. (H, I) Plot of the latency to fall in the rotarod test (H) and total moving distance within 10 and 30 min of the locomotion test (I) from the 3 groups. (A, D, E, F, G): *P < .05, **P < .01; ANOVA. (B): ***P < .001, unpaired t test.
Next, we examined the impact of diazepam treatment on behaviors in stressed D4KO mice. WT mice were injected with saline as controls. In the test of sensorimotor gating, the basal startle response to different acoustic stimulus intensities was not affected by diazepam treatment (figure 6C, F3,25(group) = 1.78, P = .18, 2-way ANOVA). Diazepam treatment significantly ameliorated the PPI deficits in stressed D4KO mice (figure 6D, 70 dB: P < .05; 76 dB: P < .01; 85 dB: P < .01; D4KO+Stress+saline vs D4KO+Stress+DZ, post hoc of 2-way ANOVA), without affecting PPI in WT mice.
In the test of cognition, diazepam treatment significantly elevated the discrimination ratio of TOR memory in stressed D4KO mice (figure 6E, D4KO+Stress+saline: −14.68% ± 9.86%, D4KO+Stress+DZ: 22.39% ± 7.82%, P < .05, post hoc of 1-way ANOVA). In the elevated plus maze test, stressed D4KO mice with diazepam treatment showed less time in the open arm, but without statistical significance, compared to saline treatment (figure 6F, D4KO+Stress+saline: 50.24 ± 4.12 s, D4KO+Stress+DZ: 36.97 ± 4.78 s, P > .05; post hoc of 1-way ANOVA).
In the social engagement test, diazepam treatment of stressed D4KO mice failed to significantly increase social interaction time (figure 6G, D4KO+Stress+saline: 88.8 ± 17.7 s, D4KO+Stress+DZ: 87.4 ± 18.3 s, P > .05, post hoc of 1-way ANOVA) or social approach numbers (figure 6G, D4KO+Stress+saline: 31.7 ± 3.1, D4KO+Stress+DZ: 32.0 ± 5.1, P > .05, post hoc of 1-way ANOVA).
Diazepam treatment of stressed D4KO mice did not affect movement coordination, as measured by rotarod tests (figure 6H, F2,23 = 0.51, P = .61, 1-way ANOVA). Locomotor activity was also not changed by diazepam (figure 6I, 10 min: F2,22 = 2.47, P = .11; 30 min: F2,22 = 1.92, P = .17, 1-way ANOVA).
Taken together, these results indicate that diazepam treatment of stressed D4KO mice is capable of elevating inhibitory transmission in PFC pyramidal neurons and restoring some behavioral abnormalities, including deficits in sensorimotor gating and cognition, but fails to have a significant impact on other behaviors, such as the reduced sociability and elevated exploratory behaviors.
Discussion
Heredity is the most well-established schizophrenia risk factor.2,3 However, since most people carrying genetic risk factors do not develop the disease, other environmental risk factors during sensitive periods are thought to be involved.21–28,38,39 It is perceived that genetic mutations and environmental insults cause unbalanced excitatory/inhibitory activity, aberrant dopamine system, and reduced signal-to-noise ratio in vulnerable circuitry, leading to network disturbances and system failure, which induces the onset of schizophrenia.8 Here we have found that stress exposure to animals with dopamine dysfunction triggers a variety of schizophrenia-like phenotypes, including deficits in sensorimotor gating, cognition, and sociability, supporting the “two-hits” hypothesis of schizophrenia.21 Concomitant to the behavioral abnormality, diminished GABAergic transmission in PFC is exhibited in these animals with double hits. Elevating GABA signaling ameliorates some aspects of behavioral deficits, highlighting the causal role of the GABA system in certain domains of schizophrenia.
In this study, we have used D4KO mice to model a dysfunctional DA system. These animals exhibit mild phenotypes in basal conditions, but show supersensitivity to psychomotor stimulants,17 reduced exploration of novel stimuli,40 and enhanced reactivity to unconditioned fear.41 We have found very few behavioral changes in D4KO mice. However, exposing these mice to mild stress (7-d restraint, 2 h/d), schizophrenia-like phenotypes are induced. The same stress paradigm exerts a little effect in WT mice, suggesting that abnormal dopamine signaling renders animals more vulnerable to stress. Consistently, a previous study has found that exposing transgenic mice with a putative dominant-negative DISC1 (disrupted in schizophrenia 1) to 3-week isolation stress during adolescence induces long-lasting behavioral changes resembling those of psychotic depression.18
D4 receptor has been suggested to play an important role in PFC-mediated cognitive functions and the pathophysiology of neuropsychiatric disorders.42,43 Our previous studies have found that D4 receptor activation regulates the trafficking and function of AMPA and GABAA receptors in PFC pyramidal neurons and GABAergic interneurons via distinct mechanisms.44–48 The ADHD-linked human dopamine D4 receptor variant induces over-suppression of NMDAR function and aberrant regulation of synchronous network activity in PFC.49,50 Consistent with the general finding of our current study, it has been found that overstimulation of D4 receptors in the PFC induces dysregulation of emotions reminiscent to the effects observed in schizophrenia.51–53 Moreover, disruption of D4 signaling in the PFC renders animals more sensitive to stress-related conditioning memories.54
Compensatory changes have been reported in animals with the ablation of D4 receptors. Dopamine supersensitivity, which correlates with the increased levels of high-affinity D2 receptors, is found in D4 KO mice.55 Increased expression of D1 receptors and NMDA receptors is also found in the striatum of D4R KO mice.56 We did not observe significant changes in the baseline synaptic currents mediated by AMPARs, NMDARs or GABAARs in PFC pyramidal neurons of D4KO mice. However, we have found the selective loss of GABAergic transmission in the PFC of stressed D4KO mice, supporting the concept that dysfunctional GABA system may be a major convergence point for genetic and environmental susceptibility factors for schizophrenia.57 Among the abnormal neurochemical markers for schizophrenia in postmortem brains, the largest portion is associated with developmental/synaptic and GABA systems.58 The reduced expression of glutamic acid decarboxylase (GAD), the key enzyme in GABA synthesis, has been observed in the PFC and hippocampus of schizophrenics.58–60 GABAA receptor dysfunction has also been implicated in cortical excitation/inhibition imbalance in schizophrenia.61,62 In subjects with schizophrenia, GABAAR α1 subunit mRNA expression is significantly (40%) lower in PFC pyramidal cells, but not in GABAergic interneurons, suggesting that pyramidal cell inhibition is specifically reduced in schizophrenia.63 The mRNA expression of GABAAR β2, which preferentially assembles with α1 subunits, is also lower (20%) in dorsolateral PFC of schizophrenia patients.64 The diminished GABAergic inhibition in PFC pyramidal neurons from stressed D4KO mice is likely due to the loss of synaptic GABAA receptors or GABAergic interneurons. In agreement with this, we have found the significantly reduced parvalbumin-positive interneurons in PFC of stressed D4KO mice, consistent with the lower level of parvalbumin mRNA in schizophrenia,35 and the disrupted function of PV+ PFC interneurons in schizophrenia.36
Interestingly, we have found that elevating GABA signaling in stressed D4KO mice with diazepam treatment alleviates the PPI deficits and improves PFC-mediated cognition, pointing to the therapeutic potential of pharmacological compounds that act on GABA function for schizophrenia. Consistently, it has been found that administration of benzodiazepines or diazepam can prevent the development of neuroanatomical and neurophysiological abnormalities associated with schizophrenia.65–67
Overall, our results show that the combination of genetic and environmental risk factors is capable of triggering the manifestation of schizophrenia-like behaviors, which is contributed by the disrupted GABA system. Normalizing GABAergic transmission in PFC provides a promising avenue to treat some schizophrenia-associated symptoms.
Funding
National Institutes of Health (DA037618 and MH108842 to Z.Y.) and generous donations from E.F. Trachtman to Yan lab.
Supplementary Material
Acknowledgments
T.T. performed behavioral tests and electrophysiological experiments, analyzed the data, and wrote the draft. W.W., J.W., K.M., and Q.C. performed some electrophysiological or biochemical experiments. Z.Y. designed experiments, supervised the project, and wrote the paper. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Flaum M, Andreasen NC. Diagnostic criteria for schizophrenia and related disorders: options for DSM-IV. Schizophr Bull. 1991;17:133–156. [DOI] [PubMed] [Google Scholar]
- 2. Ripke S, Neale BM, Corvin A, et al. . Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hilker R, Helenius D, Fagerlund B, et al. . Heritability of schizophrenia and schizophrenia spectrum based on the nationwide Danish twin register. Biol Psychiatry. 2018;83:492–498. [DOI] [PubMed] [Google Scholar]
- 4. Abdolmaleky HM, Thiagalingam S, Wilcox M. Genetics and epigenetics in major psychiatric disorders: dilemmas, achievements, applications, and future scope. Am J Pharmacogenomics. 2005;5:149–160. [DOI] [PubMed] [Google Scholar]
- 5. Khan ZU, Gutiérrez A, Martín R, Peñafiel A, Rivera A, De La Calle A. Differential regional and cellular distribution of dopamine D2-like receptors: an immunocytochemical study of subtype-specific antibodies in rat and human brain. J Comp Neurol. 1998;402:353–371. [DOI] [PubMed] [Google Scholar]
- 6. Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. [DOI] [PubMed] [Google Scholar]
- 7. Wedzony K, Chocyk A, Maćkowiak M, Fijał K, Czyrak A. Cortical localization of dopamine D4 receptors in the rat brain–immunocytochemical study. J Physiol Pharmacol. 2000;51:205–221. [PubMed] [Google Scholar]
- 8. Sakurai T, Gamo NJ, Hikida T, et al. . Converging models of schizophrenia–Network alterations of prefrontal cortex underlying cognitive impairments. Prog Neurobiol. 2015;134:178–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Catts VS, Sheedy D, McCrossin T, Kril JJ, Shannon Weickert C. Cortical grey matter volume reduction in people with schizophrenia is associated with neuro-inflammation. Transl Psychiatry. 2016;6:e982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seeman P, Guan HC, Van Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365:441–445. [DOI] [PubMed] [Google Scholar]
- 11. Van Tol HH, Bunzow JR, Guan HC, et al. . Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350:610–614. [DOI] [PubMed] [Google Scholar]
- 12. Kapur S, Remington G. Atypical antipsychotics: new directions and new challenges in the treatment of schizophrenia. Annu Rev Med. 2001;52:503–517. [DOI] [PubMed] [Google Scholar]
- 13. LaHoste GJ, Swanson JM, Wigal SB, et al. . Dopamine D4 receptor gene polymorphism is associated with attention deficit hyperactivity disorder. Mol Psychiatry. 1996;1:121–124. [PubMed] [Google Scholar]
- 14. Rowe DC, Stever C, Giedinghagen LN, et al. . Dopamine DRD4 receptor polymorphism and attention deficit hyperactivity disorder. Mol Psychiatry. 1998;3:419–426. [DOI] [PubMed] [Google Scholar]
- 15. El-Faddagh M, Laucht M, Maras A, Vöhringer L, Schmidt MH. Association of dopamine D4 receptor (DRD4) gene with attention-deficit/hyperactivity disorder (ADHD) in a high-risk community sample: a longitudinal study from birth to 11 years of age. J Neural Transm (Vienna). 2004;111:883–889. [DOI] [PubMed] [Google Scholar]
- 16. Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD). Hum Mol Genet. 2006;15:2276–2284. [DOI] [PubMed] [Google Scholar]
- 17. Rubinstein M, Phillips TJ, Bunzow JR, et al. . Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. [DOI] [PubMed] [Google Scholar]
- 18. Niwa M, Jaaro-Peled H, Tankou S, et al. . Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science. 2013;339:335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Norman RM, Malla AK. Stressful life events and schizophrenia. II: conceptual and methodological issues. Br J Psychiatry. 1993;162:166–174. [DOI] [PubMed] [Google Scholar]
- 20. Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maynard TM, Sikich L, Lieberman JA, LaMantia AS. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr Bull. 2001;27:457–476. [DOI] [PubMed] [Google Scholar]
- 22. Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000;47:210–220. [DOI] [PubMed] [Google Scholar]
- 23. Davis J, Eyre H, Jacka FN, et al. . A review of vulnerability and risks for schizophrenia: beyond the two hit hypothesis. Neurosci Biobehav Rev. 2016;65:185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khan A, Powell SB. Sensorimotor gating deficits in “two-hit” models of schizophrenia risk factors. Schizophr Res. 2018;198:68–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Giovanoli S, Engler H, Engler A, et al. . Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339:1095–1099. [DOI] [PubMed] [Google Scholar]
- 26. Oliver PL, Davies KE. Interaction between environmental and genetic factors modulates schizophrenic endophenotypes in the Snap-25 mouse mutant blind-drunk. Hum Mol Genet. 2009;18:4576–4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K. Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons. Biol Psychiatry. 2013;73:1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishihama T, Ago Y, Shintani N, et al. . Environmental factors during early developmental period influence psychobehavioral abnormalities in adult PACAP-deficient mice. Behav Brain Res. 2010;209:274–280. [DOI] [PubMed] [Google Scholar]
- 29. Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schifani C, Tseng HH, Kenk M, et al. . Cortical stress regulation is disrupted in schizophrenia but not in clinical high risk for psychosis. Brain. 2018;141:2213–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei J, Zhong P, Qin L, Tan T, Yan Z. Chemicogenetic Restoration of the Prefrontal Cortex to Amygdala Pathway Ameliorates Stress-Induced Deficits. Cereb Cortex. 2018;28:1980–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parwani A, Duncan EJ, Bartlett E, et al. . Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662–669. [DOI] [PubMed] [Google Scholar]
- 33. Barker GR, Bird F, Alexander V, Warburton EC. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuen EY, Liu W, Karatsoreos IN, et al. . Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci. 2009;106:14075–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. [DOI] [PubMed] [Google Scholar]
- 36. Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. [DOI] [PubMed] [Google Scholar]
- 37. Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. French L, Gray C, Leonard G, et al. . Early cannabis use, polygenic risk score for schizophrenia and brain maturation in adolescence. JAMA Psychiatry. 2015;72:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 40. Dulawa SC, Grandy DK, Low MJ, Paulus MP, Geyer MA. Dopamine D4 receptor-knock-out mice exhibit reduced exploration of novel stimuli. J Neurosci. 1999;19:9550–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Falzone TL, Gelman DM, Young JI, Grandy DK, Low MJ, Rubinstein M. Absence of dopamine D4 receptors results in enhanced reactivity to unconditioned, but not conditioned, fear. Eur J Neurosci. 2002;15:158–164. [DOI] [PubMed] [Google Scholar]
- 42. Oak JN, Oldenhof J, Van Tol HH. The dopamine D(4) receptor: one decade of research. Eur J Pharmacol. 2000;405:303–327. [DOI] [PubMed] [Google Scholar]
- 43. Rondou P, Haegeman G, Van Craenenbroeck K. The dopamine D4 receptor: biochemical and signalling properties. Cell Mol Life Sci. 2010;67:1971–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhong P, Yan Z. Distinct physiological effects of dopamine D4 receptors on prefrontal cortical pyramidal neurons and fast-spiking interneurons. Cereb Cortex. 2016;26:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang X, Zhong P, Yan Z. Dopamine D4 receptors modulate GABAergic signaling in pyramidal neurons of prefrontal cortex. J Neurosci. 2002;22:9185–9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Graziane NM, Yuen EY, Yan Z. Dopamine D4 receptors regulate GABAA receptor trafficking via an actin/cofilin/myosin-dependent mechanism. J Biol Chem. 2009;284:8329–8336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yuen EY, Yan Z. Cellular mechanisms for dopamine D4 receptor-induced homeostatic regulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. J Biol Chem. 2011;286:24957–24965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yuen EY, Zhong P, Yan Z. Homeostatic regulation of glutamatergic transmission by dopamine D4 receptors. Proc Natl Acad Sci U S A. 2010;107:22308–22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qin L, Liu W, Ma K, et al. . The ADHD-linked human dopamine D4 receptor variant D4.7 induces over-suppression of NMDA receptor function in prefrontal cortex. Neurobiol Dis. 2016;95:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhong P, Liu W, Yan Z. Aberrant regulation of synchronous network activity by the attention-deficit/hyperactivity disorder-associated human dopamine D4 receptor variant D4.7 in the prefrontal cortex. J Physiol. 2016;594:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kwon OB, Lee JH, Kim HJ, et al. . Dopamine regulation of amygdala inhibitory circuits for expression of learned fear. Neuron. 2015;88:378–389. [DOI] [PubMed] [Google Scholar]
- 52. Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lauzon NM, Laviolette SR. Dopamine D4-receptor modulation of cortical neuronal network activity and emotional processing: implications for neuropsychiatric disorders. Behav Brain Res. 2010;208:12–22. [DOI] [PubMed] [Google Scholar]
- 54. Jing Li J, Szkudlarek H, Renard J, Hudson R, Rushlow W, Laviolette SR. Fear memory recall potentiates opiate reward sensitivity through dissociable dopamine D1 versus D4 receptor-dependent memory mechanisms in the prefrontal cortex. J Neurosci. 2018;38:4543–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Seeman P, Weinshenker D, Quirion R, et al. . Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci U S A. 2005;102:3513–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gan L, Falzone TL, Zhang K, Rubinstein M, Baldessarini RJ, Tarazi FI. Enhanced expression of dopamine D(1) and glutamate NMDA receptors in dopamine D(4) receptor knockout mice. J Mol Neurosci. 2004;22:167–178. [DOI] [PubMed] [Google Scholar]
- 57. Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology. 2015;40:190–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Torrey EF, Barci BM, Webster MJ, Bartko JJ, Meador-Woodruff JH, Knable MB. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–260. [DOI] [PubMed] [Google Scholar]
- 59. Akbarian S, Huntsman MM, Kim JJ, et al. . GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995;5:550–560. [DOI] [PubMed] [Google Scholar]
- 60. Benes FM, Lim B, Matzilevich D, Walsh JP, Subburaju S, Minns M. Regulation of the GABA cell phenotype in hippocampus of schizophrenics and bipolars. Proc Natl Acad Sci U S A. 2007;104:10164–10169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Jonge JC, Vinkers CH, Hulshoff Pol HE, Marsman A. GABAergic mechanisms in schizophrenia: linking postmortem and in vivo studies. Front Psychiatry. 2017;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gonzalez-Burgos G, Fish KN, Lewis DA. GABA neuron alterations, cortical circuit dysfunction and cognitive deficits in schizophrenia. Neural Plast. 2011;2011:723184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor α1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology. 2011;36:2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gill KM, Lodge DJ, Cook JM, Aras S, Grace AA. A novel α5GABA(A)R-positive allosteric modulator reverses hyperactivation of the dopamine system in the MAM model of schizophrenia. Neuropsychopharmacology. 2011;36:1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dold M, Li C, Gillies D, Leucht S. Benzodiazepine augmentation of antipsychotic drugs in schizophrenia: a meta-analysis and Cochrane review of randomized controlled trials. Eur Neuropsychopharmacol. 2013;23:1023–1033. [DOI] [PubMed] [Google Scholar]
- 67. Du Y, Grace AA. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology. 2013;38:1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.