Abstract
Negative symptoms represent a distinct component of psychopathology in schizophrenia (SCZ) and are a stable construct over time. Although impaired frontostriatal connectivity has been frequently described in SCZ, its link with negative symptoms has not been carefully studied. We tested the hypothesis that frontostriatal connectivity at rest may be associated with the severity of negative symptoms in SCZ. Resting state functional connectivity (rsFC) data from 95 mostly medicated patients with SCZ and 139 healthy controls (HCs) were acquired. Negative symptoms were assessed using the Brief Negative Symptom Scale. The study analyzed voxel-wise rsFC between 9 frontal “seed regions” and the entire striatum, with the intention to reduce potential biases introduced by predefining any single frontal or striatal region. SCZ showed significantly reduced rsFC between the striatum and the right medial and lateral orbitofrontal cortex (OFC), lateral prefrontal cortex, and rostral anterior cingulate cortex compared with HCs. Further, rsFC between the striatum and the right medial OFC was significantly associated with negative symptom severity. The involved striatal regions were primarily at the ventral putamen. Our results support reduced frontostriatal functional connectivity in SCZ and implicate striatal connectivity with the right medial OFC in negative symptoms. This task-independent resting functional magnetic resonance imaging study showed that medial OFC–striatum functional connectivity is reduced in SCZ and associated with severity of negative symptoms. This finding supports a significant association between frontostriatal connectivity and negative symptoms and thus may provide a potential circuitry-level biomarker to study the neurobiological mechanisms of negative symptoms.
Keywords: functional connectivity, schizophrenia, negative symptoms
Introduction
Schizophrenia (SCZ) is a severe mental illness characterized by positive and negative symptoms. Negative symptoms, including abnormalities in motivation, sociality, and emotional expressiveness, are considered one of the core components of SCZ and have been increasingly used for assessing treatment outcomes in research studies, clinical trials, and clinical practice.1 Negative symptoms have been shown to be a stable construct over time,2 but unfortunately, they are also among the least treatment-responsive clinical symptoms of SCZ.3,4 There is currently a lack of approved medication specifically targeting negative symptoms,1 and the underlying neural mechanisms responsible for negative symptoms are still poorly understood.
There is growing consensus that the pathophysiology of SCZ goes beyond regional dysfunctions and is at the level of functional networks.5,6 Striatal networks have been implicated in SCZ in postmortem,7 anatomical imaging,8 and functional imaging9 studies. In addition, the basal ganglia network has been associated with familial risks for SCZ.10 Frontostriatal networks serve a wide range of functions from motivational to cognitive control functions,11 and striatal dopaminergic signaling has been shown to modulate goal-directed gating to prefrontal cortex.12,13 Higher-order motivational and social-drive functioning and their deficits in patients have been attributed to frontostriatal connections.14–17 As negative symptoms are closely associated with cognitive, motivational, and social-drive deficits,1,3,4 dysfunctional frontostriatal connectivity may contribute to negative symptoms in SCZ. Although impaired frontostriatal connectivity in SCZ has been correlated with psychosis symptom severity in those with clinical high-risk for psychosis; in first episode, treatment-resistant SCZ and psychotic bipolar disorder18–21; and in auditory verbal hallucinations,22 it remains unknown whether and how frontostriatal resting state functional connectivity (rsFC) is linked to negative symptoms in SCZ.
Frontostriatal networks are also involved in adaptive and motivational behaviors,23 reward learning,24 and social and communicative functions25 under normal conditions. Alexander and Crutcher defined 4 frontal targets from the basal ganglia: motor, oculomotor, limbic, and prefrontal areas.26 Function of frontal limbic areas, especially the medial orbitofrontal cortex (mOFC), is associated with social evaluation, decision making, and affective representation,27,28 impairment of which might have direct implications on diminished social drive and blunted affect observed in patients with negative symptoms.
Abnormal reward-related activation in the striatum correlates with negative symptoms in patients with SCZ and unaffected siblings.29–31 Within the striatum, ventral aspects are implicated more in motivation, whereas central and dorsal aspects are involved in habit formation within the corticostriatal reward circuit.32–35 Diminished motivation and interests are the typical presentations of negative symptoms. Thus, a tenable hypothesis is that ventral (and not central/dorsal) striatum functional communication is associated with negative symptoms.
Unlike task-based paradigms where impaired motivation to perform tasks in SCZ with negative symptoms may confound research findings, resting state functional magnetic resonance imaging (rsfMRI) circumvents the issue by not requiring active participation. Our goal was to use a task-free rsFC approach to understand whether negative symptoms in SCZ are associated with abnormal frontostriatal rsFC independent of specific task performance. This study used a data-driven (voxel-wise) approach to identify patterns of rsFC between 9 frontal seed regions and voxels in the striatum. Consistent with the prior literature, we hypothesized that negative symptoms are associated with functional communication between frontal limbic regions and ventral striatal regions. However, our exploratory voxel-wise approach allowed us to test the alternative hypothesis that negative symptoms are associated with impaired functional communication between a broader set of frontostriatal networks.
Methods
Participants
Patients with SCZ (n = 95, 58 male/37 female) were recruited from the outpatient clinics at the Maryland Psychiatric Research Center and the neighboring mental health clinics. Healthy controls (HCs; n = 139, 87 male/52 female) were recruited through media advertisements. Demographics of the sample are reported in table 1. Diagnoses were confirmed with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) in all participants. Major medical and neurological illnesses, history of head injury with cognitive sequelae, and mental retardation were exclusionary. Other than 18 patients who were not on antipsychotic medication at time of study, all patients were taking antipsychotics, including 64 on atypical, 6 on typical, and 7 taking both an atypical and a typical antipsychotic. Patients were all under outpatient care and were clinically stable. Patients and controls with substance dependence within the past 6 months or current substance abuse (except nicotine) were excluded. Controls had no current DSM-IV Axis I diagnoses and no family history of psychosis in the prior 2 generations. Participants gave written informed consent. The research protocol was approved by the institutional review board of the University of Maryland, Baltimore.
Table 1.
Demographic Data and Clinical Parameters for Schizophrenia (SCZ) and Healthy Control (HC) Groups
| SCZ (n = 95) | HC (n = 139) | Group Comparison | |
|---|---|---|---|
| Mean (STDEV) | Mean (STDEV) | P | |
| Age (y) | 33.4 (13.7) | 31.7 (15.2) | .36 |
| Age range | 11–59 | 10–77 | |
| Gender (M/F) | 58/37 | 87/52 | .81 |
| Education (y) | 12.6 (1.7) | 13.8 (2.2) | .001 |
| BPRS total | 40.3 (11.0) | n/a | n/a |
| BNSS | 18.9 (15.0) | n/a | n/a |
| CPZ | 224.5 (411.6) | n/a | n/a |
Note: BNSS, Brief Negative Symptom Scale; BPRS, Brief Psychiatric Rating Scale; CPZ, chlorpromazine; M, male; F, female; n/a, not applicable.
Clinical Assessments
The Brief Negative Symptom Scale (BNSS) total score was calculated to assess overall negative symptoms.34 The 2 major BNSS factors (motivation/pleasure and emotional expressivity defined by Kirkpatrick et al.36 and replicated by Strauss et al.37) were also used to further explore associations for these distinguished domains.
The BNSS has good validity and is easier to use compared to some other tools,36 important for large sample studies. However, negative symptoms include primary symptoms (refer to negative symptoms that are pathogenic of SCZ) and secondary symptoms (refer to negative symptoms due to positive symptoms, affective symptoms, medication side effects, environmental deprivation, or other factors). Although BNSS does not directly separate these 2 aspects of negative symptoms, previous data suggested that BNSS-assessed negative symptoms do not substantially correlate with psychosis or depression.36 Nevertheless, we will attempt to indirectly assess the specificity question by evaluating whether or not frontostriatal circuitry findings are correlated with other symptom domains.
Overall psychiatric symptoms were assessed with the 20-item Brief Psychiatric Rating Scale (BPRS); the BPRS subscale of withdrawal symptoms was used as a corroborative assessment for aspects of negative symptoms. We also calculated the BPRS psychosis subscale score and anxiety/depression subscale score to measure psychosis and mood symptoms.38
Data Acquisition
All imaging was performed at the University of Maryland Center for Brain Imaging Research using a Siemens 3T TRIO MRI system equipped with a 32-channel phase array head coil. High-resolution structural images were acquired using fast spoiled gradient-recalled sequence (TR: 11.08 ms, TE: 4.3 ms, flip angle: 45°, field of view (FOV): 256 mm, 256 × 256 matrix, 172 slices, 1 mm3 spatial resolution). Resting state functional T2*-weighted images were obtained using a single-shot gradient-recalled, echo-planar pulse sequence (TR: 2 s, TE: 30 ms, flip angle: 90°, FOV: 220 mm, 64 × 64 matrix, 3.4 mm2 in-plane resolution, 3.4 mm slice thickness, 42 axial slices, 15 min scan duration). Participants were asked to keep their eyes closed, to relax, and not to think about anything in particular.
Resting fMRI Data Preprocessing
rsfMRI data processing was carried out using Analysis of Functional NeuroImages (AFNI)39 software. The first 5 time points of each run were discarded because of image instability. Correction for head motion was performed by registering each functional volume to the first time point of the run. No significant group differences were detected for translational motion (0.50 ± 0.07 mm [mean ± SEM] SCZ and 0.52 ± 0.08 mm HC; P = .74) or rotational motion (0.016 ± 0.002 mm SCZ and 0.012 ± 0.002 mm HC; P = .28). The preprocessed data were spatially smoothed with a 4-mm full-width at half maximum Gaussian kernel. Next, multiple linear regression analysis was used to model several types of noise in the functional data, which were then removed as regressors of no interest: the linear trend, 6 motion parameters (3 rotational and 3 translational directions), their 6 temporal derivatives (rate of change in rotational and translational motion) and time courses from the white matter and cerebral spinal fluid. Blood oxygenation level dependent (BOLD) time series were band-pass filtered (0.01–0.1 Hz). Time points with excessive motion (>1.5 mm), estimated as the magnitude of displacement from one time point to the next, including neighboring time points were also censored from statistical analysis. Finally, for group analysis, images were spatially normalized to the Talairach space.40
Frontostriatal rsFC Based on Frontal Seeds
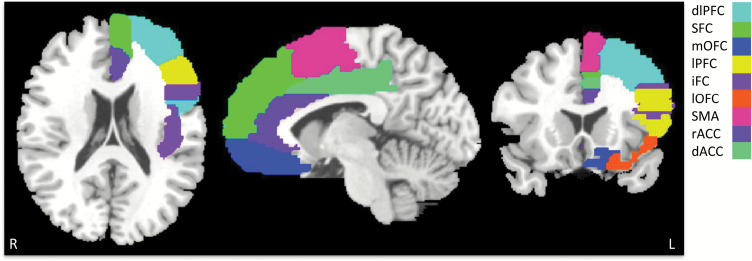
To avoid bias toward any specific frontal region, each major frontal region was included as seed. Binary masks of the following 9 frontal cortical regions were obtained: dorsolateral prefrontal cortex, superior frontal cortex, mOFC, lateral prefrontal cortex (lPFC), inferior frontal cortex, lateral orbitofrontal cortex (lOFC), supplementary motor area, rostral anterior cingulate cortex, and dorsal anterior cingulate cortex in each hemisphere using the standard automated anatomical labeling (AAL) atlas (www.mricro.com) (figure 1). Mean time series were extracted from these seed regions. Connectivity maps were obtained for each frontal seed region by correlating its average time series with every voxel in the striatum. The striatum boundary was defined using the AAL atlas for caudate and putamen, which included all of the ventral striatum.
Fig. 1.
Surface rendering of frontal cortical regions (only left hemisphere shown) used for the functional connectivity driven striatal parcellation. dlPFC, dorsolateral prefrontal cortex; SFC, superior frontal cortex; mOFC, medial orbitofrontal cortex ; lPFC, lateral prefrontal cortex; iFC, inferior frontal cortex; lOFC, lateral orbitofrontal cortex; SMA, supplementary motor area; rACC, rostral anterior cingulate cortex; and dACC, dorsal anterior cingulate cortex.
A seed-based voxel-wise analysis was performed on the entire striatum (seeds correspond to every region of the frontal lobe, analyzed 1 seed at a time). Essentially, we used a data-driven approach to identify patterns of functional connectivity between specific frontal regions and clusters of voxels in the striatum. This method provides a more objective approach to map functional connections between frontal and striatal areas, while exploring potential relevance to negative symptoms. Finally, resulting rsFC maps for each frontal seed were averaged and Fisher’s r-to-z transformations were applied to obtain the normal distribution. Analyses were performed separately for each hemisphere. Only ipsilateral frontostriatal connectivity was considered because the neural circuitry linking the frontal cortex and the striatum primarily runs ipsilaterally, with a few exceptions.41–43
Statistics
rsFC maps from each frontal region were obtained in each participant and then compared between HC and SCZ groups. To examine group differences in functional connectivity between the striatum and frontal cortical regions, voxel-wise 2-sample t tests were performed, using voxel-wise threshold of P < .005 and an extent threshold based on Monte Carlo simulations using AFNI’s updated 3dClustStim with autocorrelation function to achieve corrected P < .05. We then extracted these rsFC values and applied Bonferroni correction for 18 regions (P < .05/18 = .003). Only those rsFC values that were significantly different between groups (based on the earlier 2 statistical thresholds) were used to analyze potential relationships with BNSS scores. Associations between rsFC and BNSS were carried out by Pearson’s correlations, also applying Bonferroni correction for the number of correlations. If a correlation with BNSS total score was significant, post hoc analysis was carried out to explore correlation with the 3 BNSS factor scores (using Bonferroni corrected P = .05/3 subscales = .016). Similarly, if a correlation with BNSS total score was significant, associations between rsFC and BPRS total and subscale scores were carried out by Pearson’s correlations, also applying Bonferroni correction for 4 comparisons (total scores and 3 subscale scores at P = .05/4 = .012). Correlations with chlorpromazine (CPZ) equivalent dosage were not corrected for multiple comparisons.
Results
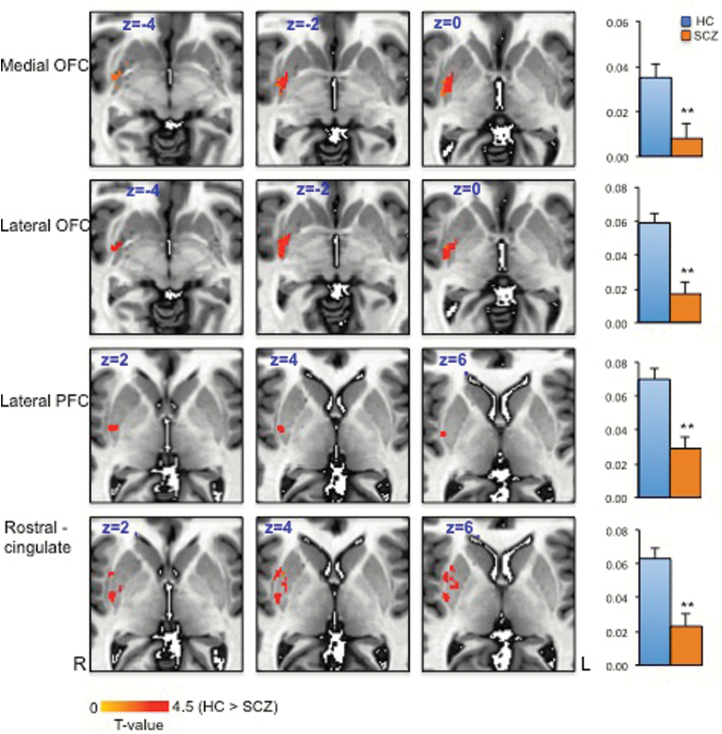
SCZ and HC did not differ in age and sex (table 1). Functional connectivity between each voxel of the striatum and predefined frontal cortical regions was performed to examine overall frontostriatal network disturbances in SCZ. After correction for multiple comparisons, significantly reduced rsFC between 4 frontal cortical regions and the striatum were found in patients with SCZ compared to controls: specifically between striatum and mOFC, lOFC, lPFC, and rostral cingulate seeds in the right hemisphere (corrected P < .05) (figure 2). The exact striatal areas varied in each case, but interestingly, effects were mainly observed in ventral areas of the putamen (figure 2).
Fig. 2.
Significant group differences in the functional connectivity between the striatum and frontal cortical regions: medial orbitofrontal cortex (OFC), lateral OFC, lateral prefrontal cortex (PFC), and rostral cingulate. z score comparisons between healthy control (HC) and schizophrenia (SCZ) groups are also shown (corrected P < .05).
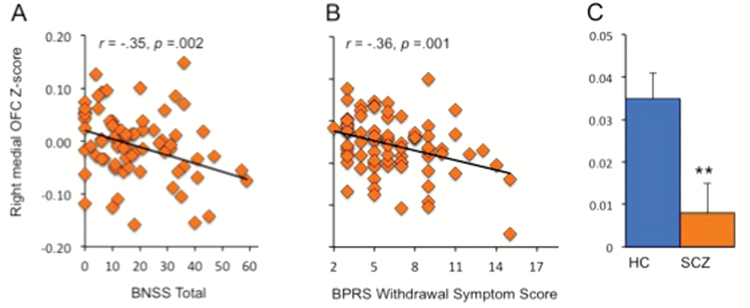
Among the 4 frontal regions, BNSS total was significantly correlated only with mean rsFC z score of the right mOFC–ventral putamen (r = −.35, P = .002, significant after Bonferroni correction for 3 correlations) (figure 3A and C). Post hoc analyses revealed that right mOFC–ventral putamen connectivity was significantly correlated with BNSS motivation/pleasure factor (r = −.32, P = .005) and the emotional expressivity factor (r = −.33, P = .004). Both of these correlations remained significant after Bonferroni correction, whereas the correlation between right mOFC–ventral putamen connectivity and distress was not significant (r = −.03, P = .80).
Fig. 3.
Pearson correlations between Brief Negative Symptom Scale (BNSS) total score (A) and Brief Psychiatric Rating Scale (BPRS) withdrawal symptom subscale (B), and the mean functional connectivity z score for the right medial orbitofrontal cortex (OFC) and the right striatum. Between-group comparison for the right medial OFC–right striatum functional connectivity z score is also shown (C).
We also assessed the BPRS subscale withdrawal score and found that a significant correlation was also present between right mOFC–ventral putamen rsFC z score and withdrawal symptoms (r = −.36, P = .001, significant after Bonferroni correction) (figure 3B). In comparison, BPRS total score, psychosis, or depression/anxiety subscale scores were not significantly correlated with the right mOFC–ventral putamen connectivity (all Ps > .05), suggesting the specificity of the finding with negative symptoms.
To examine whether age and sex contribute to the main findings, we used age and sex as covariates in group comparisons and found that age (F = 4.7, P = .03) but not sex (F = 2.2, P = .11) was a nominally significant covariate in patient–control difference on the mOFC–ventral putamen rsFC, although the group differences were essentially the same as without (F = 25.9, P < .001) vs with age and sex as covariates (F = 24.5, P < .001). Using age and sex as control variables in partial correlations, the mOFC–ventral putamen rsFC and BNSS remain significantly correlated (partial r = −.32, P = .005) as compared to without age and sex as control variables (r = −0.35, P = .002); CPZ dosage did not correlate with rsFC z scores (all Ps > .03) or with BNSS total (P = .7) after Bonferroni correction of multiple comparisons. To further reduce potential medication confounds, we selected only the subgroup on atypical antipsychotics (n = 64). Here, right mOFC–ventral putamen connectivity was still significantly lower in SCZ compared with controls (P < .001). After repeating the correlation analyses between right mOFC–ventral putamen connectivity and BNSS in SCZ on atypical antipsychotic medications with CPZ included as a covariate, we found that the correlation remained significant (partial r = −0.29, P = .024).
Discussion
Although dopamine dysregulation in the striatum is considered a general feature of SCZ pathology,44,45 negative symptoms in SCZ are thought to be related to abnormal stimulation of dopamine type 1 receptors in the prefrontal cortex.46–48 Dopaminergic dysfunction at the level of the prefrontal cortex could lead to prefrontal-dependent deficits including negative symptoms as observed in patients with SCZ.9 This study used a data-driven, voxel-wise rsFC approach to identify specific patterns of functional connectivity between frontal and striatal regions that were associated with negative symptoms. The findings indicate compromised frontostriatal connectivity in SCZ between several frontal and striatal regions, which are consistent with, but also expand upon, previous reports of striatal abnormalities49,50 as well as compromised function of frontostriatal pathways51,52 in SCZ. Furthermore, there was a significant, inverse association between the right mOFC–striatal connectivity and negative symptoms, such that reduced rsFC was associated with more severe negative symptoms in SCZ.
Frontostriatal circuits have previously been implicated in the negative symptoms of SCZ using task-based fMRI, magnetic resonance spectroscopy, diffusion imaging, and positron emission tomography (PET) research techniques,53–55 and it has been posited that this relationship is driven by deficits in representing and using neural resources to support flexible and goal-directed decision-making.56 In one PET study, hypoperfusion in the right OFC was linked to development of severe negative symptoms in SCZ patients with deficit syndrome.57 Findings of this study extend this prior finding and suggest that right OFC-based frontostriatal circuitry may modulate negative symptoms in SCZ. Although BPRS withdrawal symptom scale does not directly measure negative symptoms (eg, the rating assesses some aspects of negative symptoms through a different tool), the finding of a similar correlation between BPRS withdrawal symptoms and the same OFC–striatal rsFC lends additional support to the BNSS-based finding. However, it remains unclear whether the pathophysiology of negative symptoms stems from the OFC, from the striatum, or from OFC–striatal connectivity.
The association of negative symptoms with rsFC between the striatum and OFC—but not other frontal regions—is interesting in light of what is currently known about the unique functional contributions of OFC.58 OFC has been functionally separated from other frontal areas for its unique role in decoding and linking positive or negative reinforcers of pleasure, pain, taste, and smell59 to more abstract reinforcers such as economic value60; OFC is also critical for activating or inhibiting reward-related, punishment-related, goal-directed, and emotional behaviors.59,60 Impairments in the reinforcement functions associated with striatal reward-related signaling may lead to dampening of interests and motivations in a variety of personal and social situations and may plausibly be linked to negative symptoms.
Within the OFC, mOFC responses are particularly associated with reward outcomes, whereas lOFC responses are more related to negative outcomes.61 The observed mOFC-specific association with negative symptoms in this study is consistent with the idea that patients with SCZ appear to have intact response to negative outcomes but show deficits in learning positive reward associations, with these deficits more pronounced in those with high negative symptoms.62,63 In turn, the reduced rsFC between mOFC and putamen in SCZ may reflect a functional disconnect that serves as a predisposition for impaired reward responsivity and adaptive behavior, which may manifest as negative symptoms. Finally, mOFC is also a core “default mode network” hub, which is involved in emotion processing64 and is affected in SCZ.57 Although, this study focused on links between frontostriatal connectivity and negative symptoms of SCZ, abnormalities in the frontostriatal circuit found in this study may reflect other commonly observed cognitive and functional deficits in SCZ, which should be further studied.
The OFC–striatal rsFC findings with negative symptoms are primarily at the ventral putamen (figure 2). In fact, results between patients and controls suggested an overlapping area in the ventral to ventral–medial putamen, which has abnormal connectivity to many parts of the frontal lobe (figure 2). The putamen is part of the sensorimotor loop; ventral aspects are implicated more in motivation, whereas central and dorsal aspects are involved in habit formation within the corticostriatal circuit.32–35 We found that connectivity between the anterior cingulate cortex and central putamen was also reduced in SCZ compared with controls, but its association with negative symptoms was not significant. In comparison, the aberrant connectivity significantly associated with negative symptoms was between OFC and ventral putamen (figure 2), suggesting the critical role of the circuitry for motivational and goal-directed behaviors in negative symptoms.
There are several limitations of this study. First, the effect of medication on BOLD fMRI remains unknown. Findings on the effects of medication on functional connectivity are mixed showing both increased65 and attenuated66 functional connectivity associated with antipsychotic medication. Although CPZ dosage did not correlate with rsFC measures, cumulative medication effects cannot be entirely ruled out. The sample included SCZ patients under age 18 years whose frontal circuitries may still be under development. However, we included controls under age 18, and correlation analyses between rsFC and BNSS showed no meaningful differences with under-aged subjects removed. Functional connectivity analysis lacks information about the causality as it gives information only about whether the BOLD signal in 1 seed region is more or less synchronized with the BOLD signal in another region. Also, our study focused on ipsilateral frontostriatal connections; therefore, any possible abnormalities in the contralateral hemispheric connectivity in SCZ may have gone undetected in our study. Finally, the analysis of different categories of negative symptoms using BNSS subscales shows that the correlations with the mOFC–striatal rsFC were similar across most of the subscale measures, suggesting that mOFC–striatal rsFC may be associated with a broad range of negative symptoms. Notably, distress has been distinguished as a different aspect from the rest of the BNSS measures.34 Therefore, the lack of a significant association with distress, and also the lack of significant associations with other BPRS subscale symptoms, provides some support to the specificity of the finding in relationship to BNSS.
To summarize, this task-independent resting fMRI study showed that mOFC–striatal functional connectivity is reduced in SCZ and is associated with severity of negative symptoms. The finding may provide a potential circuitry-level biomarker to study the neurobiological mechanisms of negative symptoms.
Funding
Support was received from the National Institutes of Health grants (U01MH108148, R01MH112180, P50MH103222, and T32MH067533) and a State of Maryland contract (M00B6400091). L.E.H. has received or plans to receive research funding or consulting fee on research projects from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho, Heptares, Pfizer, Regeneron, and Takeda.
Acknowledgment
All other authors declare no conflict of interest.
References
- 1. Marder SR, Kirkpatrick B. Defining and measuring negative symptoms of schizophrenia in clinical trials. Eur Neuropsychopharmacol. 2014;24:737–743. [DOI] [PubMed] [Google Scholar]
- 2. Ring N, Tantam D, Montague L, Morris J. Negative symptoms in chronic schizophrenia. relationship to duration of illness. Br J Psychiatry. 1991;159:495–499. [DOI] [PubMed] [Google Scholar]
- 3. Stahl SM, Buckley PF. Negative symptoms of schizophrenia: a problem that will not go away. Acta Psychiatr Scand. 2007;115:4–11. [DOI] [PubMed] [Google Scholar]
- 4. Carpenter WT, Blanchard JJ, Kirkpatrick B. New standards for negative symptom assessment. Schizophr Bull. 2016;42:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmitt A, Hasan A, Gruber O, Falkai P. Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci. 2011;261(suppl 2):S150–S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Potkin SG, Ford JM. Widespread cortical dysfunction in schizophrenia: the FBIRN imaging consortium. Schizophr Bull. 2009;35:15–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. [DOI] [PubMed] [Google Scholar]
- 8. Gaser C, Nenadic I, Buchsbaum BR, Hazlett EA, Buchsbaum MS. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am J Psychiatry. 2004;161:154–156. [DOI] [PubMed] [Google Scholar]
- 9. Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Solé-Padullés C, Castro-Fornieles J, de la Serna E, et al. Altered cortico-striatal connectivity in offspring of schizophrenia patients relative to offspring of bipolar patients and controls. PLoS One. 2016;11:e0148045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casey BJ, Epstein JN, Buhle J, et al. Frontostriatal connectivity and its role in cognitive control in parent–child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. [DOI] [PubMed] [Google Scholar]
- 12. Stelzel C, Basten U, Montag C, Reuter M, Fiebach CJ. Frontostriatal involvement in task switching depends on genetic differences in d2 receptor density. J Neurosci. 2010;30:14205–14212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer-Lindenberg A, Straub RE, Lipska BK, et al. Genetic evidence implicating DARPP-32 in human frontostriatal structure, function, and cognition. J Clin Invest. 2007;117:672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pantelis C, Barnes TR, Nelson HE, et al. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain. 1997;120 (pt 10):1823–1843. [DOI] [PubMed] [Google Scholar]
- 15. Achterberg M, Peper JS, van Duijvenvoorde AC, Mandl RC, Crone EA. Frontostriatal white matter integrity predicts development of delay of gratification: a longitudinal study. J Neurosci. 2016;36:1954–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quidé Y, Morris RW, Shepherd AM, Rowland JE, Green MJ. Task-related fronto-striatal functional connectivity during working memory performance in schizophrenia. Schizophr Res. 2013;150:468–475. [DOI] [PubMed] [Google Scholar]
- 17. James A, Joyce E, Lunn D, et al. Abnormal frontostriatal connectivity in adolescent-onset schizophrenia and its relationship to cognitive functioning. Eur Psychiatry. 2016;35:32–38. [DOI] [PubMed] [Google Scholar]
- 18. Fornito A, Harrison BJ, Goodby E, et al. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–1151. [DOI] [PubMed] [Google Scholar]
- 19. Lin F, Zhou Y, Du Y, et al. Aberrant corticostriatal functional circuits in adolescents with internet addiction disorder. Front Hum Neurosci. 2015;9:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. White TP, Wigton R, Joyce DW, Collier T, Fornito A, Shergill SS. Dysfunctional striatal systems in treatment-resistant schizophrenia. Neuropsychopharmacology. 2016;41:1274–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kindler J, Schultze-Lutter F, Hauf M, et al. Increased striatal and reduced prefrontal cerebral blood flow in clinical high risk for psychosis. Schizophr Bull. 2018;44:182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cui LB, Liu K, Li C, et al. Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Res. 2016;173:13–22. [DOI] [PubMed] [Google Scholar]
- 23. Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. [DOI] [PubMed] [Google Scholar]
- 24. Haber SN. Corticostriatal circuitry. Dialogues Clin Neurosci. 2016;18:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y, Liu WH, Li Z, et al. Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychol Med. 2016;46:125–135. [DOI] [PubMed] [Google Scholar]
- 26. Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. [DOI] [PubMed] [Google Scholar]
- 27. Noonan MP, Sallet J, Rudebeck PH, Buckley MJ, Rushworth MF. Does the medial orbitofrontal cortex have a role in social valuation?Eur J Neurosci. 2010;31:2341–2351. [DOI] [PubMed] [Google Scholar]
- 28. Burke KA, Franz TM, Miller DN, Schoenbaum G. The role of the orbitofrontal cortex in the pursuit of happiness and more specific rewards. Nature. 2008;454:340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Radua J, Schmidt A, Borgwardt S, et al. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiatry. 2015;72:1243–1251. [DOI] [PubMed] [Google Scholar]
- 30. Waltz JA, Schweitzer JB, Ross TJ, et al. Abnormal responses to monetary outcomes in cortex, but not in the basal ganglia, in schizophrenia. Neuropsychopharmacology. 2010;35:2427–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Leeuw M, Kahn RS, Vink M. Fronto-striatal dysfunction during reward processing in unaffected siblings of schizophrenia patients. Schizophr Bull. 2015;41:94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. [DOI] [PubMed] [Google Scholar]
- 33. Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal–ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. [DOI] [PubMed] [Google Scholar]
- 34. Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. [DOI] [PubMed] [Google Scholar]
- 35. Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull. 2011;37:300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strauss GP, Keller WR, Buchanan RW, et al. Next-generation negative symptom assessment for clinical trials: validation of the Brief Negative Symptom Scale. Schizophr Res. 2012;142:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- 39. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 40. Talairach J, Tournoux P.. Co-Planar Stereotaxic Atlas of the Human Brain. New York, NY: Thieme Medical Publishers; 2009. [Google Scholar]
- 41. Rosell A, Giménez-Amaya JM. Anatomical re-evaluation of the corticostriatal projections to the caudate nucleus: a retrograde labeling study in the cat. Neurosci Res. 1999;34:257–269. [DOI] [PubMed] [Google Scholar]
- 42. Shepherd GM. Corticostriatal connectivity and its role in disease. Nat Rev Neurosci. 2013;14:278–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parkes SL, Bradfield LA, Balleine BW. Interaction of insular cortex and ventral striatum mediates the effect of incentive memory on choice between goal-directed actions. J Neurosci. 2015;35:6464–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Perez-Costas E, Melendez-Ferro M, Roberts RC. Basal ganglia pathology in schizophrenia: dopamine connections and anomalies. J Neurochem. 2010;113:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1:179–186. [DOI] [PubMed] [Google Scholar]
- 46. Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(suppl 1):S1–S5. [DOI] [PubMed] [Google Scholar]
- 47. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patel NH, Vyas NS, Puri BK, Nijran KS, Al-Nahhas A. Positron emission tomography in schizophrenia: a new perspective. J Nucl Med. 2010;51:511–520. [DOI] [PubMed] [Google Scholar]
- 49. van Tol MJ, van der Meer L, Bruggeman R, Modinos G, Knegtering H, Aleman A. Voxel-based gray and white matter morphometry correlates of hallucinations in schizophrenia: the superior temporal gyrus does not stand alone. Neuroimage Clin. 2014;4:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gleich T, Deserno L, Lorenz RC, et al. Prefrontal and striatal glutamate differently relate to striatal dopamine: potential regulatory mechanisms of striatal presynaptic dopamine function?J Neurosci. 2015;35:9615–9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cui LB, Liu K, Li C, et al. Putamen-related regional and network functional deficits in first-episode schizophrenia with auditory verbal hallucinations. Schizophr Res. 2016;173:13–22. [DOI] [PubMed] [Google Scholar]
- 52. Koch K, Rus OG, Reeß TJ, et al. Functional connectivity and grey matter volume of the striatum in schizophrenia. Br J Psychiatry. 2014;205:204–213. [DOI] [PubMed] [Google Scholar]
- 53. Millan MJ, Fone K, Steckler T, Horan WP. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24:645–692. [DOI] [PubMed] [Google Scholar]
- 54. Lee JS, Jung S, Park IH, Kim JJ. Neural basis of anhedonia and amotivation in patients with schizophrenia: the role of reward system. Curr Neuropharmacol. 2015;13:750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sigmundsson T, Suckling J, Maier M, et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158:234–243. [DOI] [PubMed] [Google Scholar]
- 56. Deserno L, Heinz A, Schlagenhauf F. Computational approaches to schizophrenia: a perspective on negative symptoms. Schizophr Res. 2017;186:46–54. [DOI] [PubMed] [Google Scholar]
- 57. Kanahara N, Sekine Y, Haraguchi T, et al. Orbitofrontal cortex abnormality and deficit schizophrenia. Schizophr Res. 2013;143:246–252. [DOI] [PubMed] [Google Scholar]
- 58. Stalnaker TA, Cooch NK, Schoenbaum G. What the orbitofrontal cortex does not do. Nat Neurosci. 2015;18:620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn. 2004;55:11–29. [DOI] [PubMed] [Google Scholar]
- 60. Padoa-Schioppa C, Conen KE. Orbitofrontal cortex: a neural circuit for economic decisions. Neuron. 2017;96:736–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Elliott R, Agnew Z, Deakin JF. Hedonic and informational functions of the human orbitofrontal cortex. Cereb Cortex. 2010;20:198–204. [DOI] [PubMed] [Google Scholar]
- 62. Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biol Psychiatry. 2011;69:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: further evidence of orbitofrontal dysfunction. Schizophr Res. 2007;93:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. [DOI] [PubMed] [Google Scholar]
- 65. Honey GD, Suckling J, Zelaya F, et al. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. [DOI] [PubMed] [Google Scholar]