Abstract
Objective
Embodied emotions arise from interoceptive and somatosensory processes, and are essential to the development of a stable sense of self. Emotional embodiment is therefore inherently interwoven with our sense of bodily self-awareness, and allows us to navigate complex social situations. Given that the core feature of schizophrenia (SZ) is characterized by the presence of bodily self-disturbances and social-emotional deficits, we hypothesized that embodiment of emotion would be disrupted in SZ.
Method
Twenty-six medicated individuals with SZ and 26 demographically matched controls used a computerized topographical mapping tool (“EmBODY”) to indicate on a body outline where they felt bodily sensations while experiencing an emotion. There were 13 different emotions plus a neutral state. The resulting bodily maps of emotions were quantitatively compared between groups using linear discriminant analysis and similarity scores.
Results
Bodily maps of emotions were anomalous in SZ as indicated by indistinguishable maps across different emotions. Relative to the control group, patients reported less discrete and less clear bodily sensations across emotions. In particular, bodily maps for low-arousal emotions were atypical in comparison with healthy controls.
Conclusions
Anomalous and undifferentiated mapping of embodied emotions in SZ could lead to deficits in linking bodily sensations to conceptual categories of emotions. Disrupted emotional embodiment could also contribute to poor social functioning. Abnormal bodily sensations of emotions might therefore be a promising target for future psychosocial interventions.
Keywords: embodiment, bodily self, self-disturbance, interoception, somatic, schizophrenia
Introduction
I feel disconnected from myself at times”
—Study Participant (2014)
Disrupted sense of self was central to early theories of schizophrenia (SZ).1 Both first-person accounts2 and phenomenological studies highlight the fundamental saliency of bodily self-disturbances in this disorder.3 Anomalous self-experiences are present from premorbid and prodromal stages,4–6 and predict risk for SZ, but not for depression or bipolar disorder.7 Notably, body aberrations in healthy students predict psychotic symptoms at the age of 35.8 Together, these findings underscore the importance of investigating bodily self-disturbances in SZ. In addition, reliable assessment of bodily self-disturbances has practical implications for early identification of those at risk for SZ, and for developing new interventions.
Our sense of bodily self is inseparable from our feelings and emotions.9 Both bodily sensations (eg, temperature, itch, pain, pressure, arousal) and subjective feelings of different emotions (eg, fear, sadness, anger, happiness) are accompanied by discrete patterns of activity in the insula along with the brain stem and diencephalon.10 Recalling an emotional episode also activates the autonomic nervous system in a similar fashion as experiencing that emotion in-the-moment.11 Neuroimaging studies implementing pattern recognition techniques have also established that somatosensory cortices hold a unique neural “fingerprint” for different emotions that link emotion perception to subjective sensations.12
The fundamental role of interoceptive and somatosensory signaling in emotional experience is clearly captured by the common metaphors we use to describe and communicate our internal states.13 Expressions, such as cold feet, hot heads, and butterflies in the stomach might originate from the fact that emotional experiences are accompanied by physiological changes in the body, followed by cognitive categorization of these sensations.14,15 In line with folk knowledge, recent studies have established that different emotions are associated with discernible patterns of autonomic nervous system activity, such as somatosensory and interoceptive changes16 that are also experienced at the subjective level in the body.17 Thus, distinct patterns of autonomic nervous system activity that consistently correspond to different emotions might facilitate the labeling of our own affective states, and help us infer the internal states of others.
Somatomotor simulation and reenacting of emotions allow us to plan for action, facilitate adaptive behavior, and support interpersonal interactions.18,19 The “shared network” hypothesis further suggests that brain regions recruited during the processing of one’s own emotional experiences are also involved in the processing of the emotional experiences of others.20 Thus, our ability to infer the emotional states of others is closely related to our capacity to internally simulate and re-create the bodily states of others. Embodiment of emotion that is intertwined with our sense of bodily self-awareness could therefore help us navigate the complex social world. In sum, our sense of unitary embodied self is essential to overall social functioning, and it depends on the continuous multisensory integration of exteroceptive, interoceptive, and proprioceptive signals against the backdrop of steady-state body representation maintained by homeostasis.9
In the case of SZ, there is a profound alteration of embodiment21 in addition to abnormal perception and expression of emotions. The prevalence of bodily self-disturbances,7,22,23 interoception deficits24 and multisensory integration problems25 suggest that the bodily processing of emotions might be severely disrupted in SZ. The goal of the present study was to directly investigate the bodily sensation of emotions in SZ using a novel topographical method to capture the embodiment of different emotions.14 This mapping task requires participants to color where on their body they feel a change in activity in the context of different emotional experiences. Given the previous findings of bodily-self disturbances, impaired interoception, and disrupted emotion functioning in SZ (for a review, see Kring and Elis26), we hypothesized that bodily maps of emotions would be anomalous in individuals with SZ.
Methods
Participants
Twenty-six medicated individuals diagnosed with SZ were recruited from an outpatient facility in Nashville, TN. Diagnoses were made using the Structured Clinical Interview for DSM-5.27 Symptoms were assessed with the Brief Psychiatric Rating Scale (BPRS28), the Scale for the Assessment of Positive Symptoms (SAPS29), and the Scale for the Assessment of Negative Symptoms (SANS30). Twenty-six control participants (CO) with no history of DSM-5 Axis I disorders were recruited from the same community by advertisements. Exclusion criteria for both groups were substance use or alcohol abuse within the past 6 months, brain injury, and neurological disease. All participants had normal or corrected-to-normal vision. Groups were matched on age and sex. The Schizotypal Personality Questionnaire-Brief (SPQ-B31) was used to assess schizotypy in CO. Participants gave written informed consent, as approved by the Vanderbilt Institutional Review Board, and were paid. Demographic and clinical information are summarized in table 1.
Table 1.
Demographic and Clinical Information for Study Participants
| SZ (N = 26) Mean (SD) |
CO (N = 26) Mean (SD) |
Test Statistic | P | |
|---|---|---|---|---|
| Gender M/F | 14/12 | 16/10 | χ2 = 0.079 | .78 |
| Age | 46.12 (8.49) | 40.92 (11.91) | t = 1.81 | .08 |
| Years of educationa | 13.31 (2.11) | 15.27 (2.07) | t = −3.38 | .001 |
| IQa,b | 102.81 (8.82) | 110.31 (6.72) | t = −3.45 | .001 |
| Medication (mg/day)c | 287.67 (217.9) | N/A | ||
| SAPS | 19.00 (15.96) | |||
| SANS | 29.12 (12.39) | |||
| BPRS | 17.31 (7.58) | |||
| SPQ-B total score | N/A | 4.96 (4.63) | ||
| Perceptual/cognitive | 1.42 (1.53) | |||
| Interpersonal | 2.54 (2.45) | |||
| Disorganized | 1.00 (1.47) |
Procedure
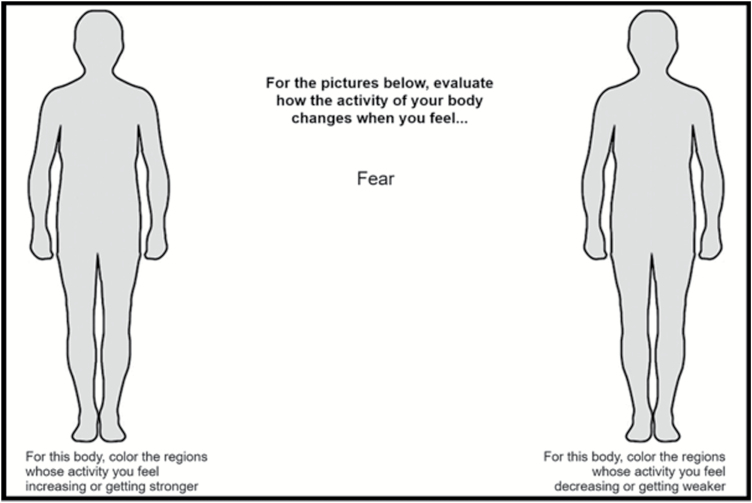
Clinical interviews and psychological assessments were conducted before the administration of the EmBODY task.17 In this task (figure 1), participants were shown human silhouettes on a computer screen with an emotion word between the 2 body outlines. Participants were asked to read the emotion word and use the mouse to color the bodily regions whose activity they felt increasing or getting stronger on the left body, and regions whose activity they felt decreasing or getting weaker on the right body, when experiencing that emotion. Fourteen emotion words (neutral, fear, anger, disgust, sadness, happiness, surprise, anxiety, love, depression, contempt, pride, shame, and jealousy) were presented sequentially, the order being randomized for each participant. Emotion words were used because previous research17 found no significant difference between the body maps generated from presenting emotion words versus evocative stimuli (eg, guided emotional imagery, emotional movie clips, facial expressions.) We provided each participant with a sheet containing the definitions of all 14 words so that they could refer to them if needed.
Fig. 1.
The EmBODY tool. Participants used a computer mouse to color body regions whose activity they felt becoming stronger (left body) or weaker (right body) in the context of a specific emotion (ie, fear). There was no time limit.
Results
Data Analysis and Body Map Generation
The data analysis procedure was modeled after Hietanen et al34 and was adjusted for small samples using nonparametric analyses. First, individual maps were manually screened for anomalous responses (eg, writing or random coloring). Spatial dependencies were observed because a single mouse click reached several hundred pixels. We therefore used a Gaussian disk to smooth the colored areas, thereby incorporating spatial dependency information into the maps to prevent exaggeration of embodiment. From this smoothed activation and deactivation data, body maps of emotions were generated separately for SZ and CO.
To obtain group maps, we took frequency counts for activation and deactivation for each pixel, for each emotion. Our sample size did not allow us to implement mass univariate t-tests to generate the body maps.17 Instead, we converted the smoothed data arrays of shaded pixels into 2 matrices of proportions. Specifically, the number of positive (activation), and negative (deactivation) values were computed for a given pixel per emotion category, across all subjects within a group. These values were divided by the number of subjects in the group, resulting in two 50,364-by-14 matrices; one with the proportion of positive values (P), and one with the proportion of negative values (N). (P + N) represents the number of individuals that colored each pixel in a given group, and (P − N) accounts for the directionality of embodiment (activation vs deactivation) within that group. The product of (P + N) and (P − N) was used as a relative measurement of embodiment in this group. The equation used to obtain the final matrices was therefore: (P − N) × (P + N) = F. This formula accounts for the difference between the proportions of positive to negative values, while also scaling that difference by the proportion of nonzero values. This final matrix (F) containing values from −1 to 1 (corresponding to the color continuum) was used to generate the group maps. To create the color bar range, the absolute values of the maximum and minimum values of F were computed. The highest pixel proportion endorsement differed between SZ (0.53) and CO (0.92), we therefore set the color bar range as [−0.92 to 0.92] for both groups to facilitate visual comparison. In the emotional bodily sensation maps, warm colors represent bodily activation while cold colors show bodily deactivation. We conceptualize “embodiment” to include any sensations associated with emotions. Thus, both activation (eg, feeling stronger) and deactivation (eg, feeling weaker) contribute to embodied emotion.
We also generated difference maps to visualize regions where emotional embodiment differs between SZ and CO. To do so, we computed D, the difference between SZ and CO’s proportion matrices. The process was applied for activation (P) and deactivation (N) separately, resulting in 2 difference matrices (Dactivation and Ddeactivation). These matrices gave rise to 2 difference body maps in which colors represent differences in bodily sensations experienced by SZ and CO.
To test whether different emotions are associated with statistically different bodily sensations in each group, we implemented statistical pattern recognition with linear discriminant analysis (LDA). LDA can discriminate different emotions from one another by using body maps as predictors. To estimate classification accuracy scores, we used stratified 126-fold cross-validation where we trained the classifier to recognize all emotions against all others (complete classification). Given past research indicating abnormal embodiment and interoception in SZ,21,25 we hypothesized that the bodily sensations of emotions reported by SZ and CO would be different. Therefore, we trained the classifier independently for each group. The cross-validation scheme was run iteratively 100 times to yield standard deviations of the classification accuracy scores. To obtain significance thresholds for observed accuracy scores, we generated random data emulating the structure of observed data and analyzed it with the same LDA process. This process was repeated 1000 times and all of the obtained classification accuracies were placed in a single distribution. We then calculated the mean and variance of the resulting random empirical distribution, compared actual data to this random distribution, and obtained significance thresholds from these comparisons by selecting the value corresponding to the 5% cutoff point of the distribution (0.095) and the one for the 1% cutoff point (0.105). These significance thresholds were used to determine whether emotions were associated with unique bodily sensations.
To obtain similarity scores for maps between groups, we compared FSZ and FCO, the final matrices of proportions described above. Hietanan et al34 used the Euclidean distance between the matrices to compare maps between groups. However, our data followed an exponential distribution due to the high number of uncolored pixels. Spearman rank correlations were therefore used to measure similarity of bodily sensation maps for each emotion across the 2 groups. To correct for multiple testing, a FDR correction was applied. This process yielded a correlation coefficient per emotion, quantifying the similarity between the topography of emotion between the 2 groups. Bootstrapping was used to compute 95% confidence intervals around these similarity estimates.
Body Maps of SZ Vs CO
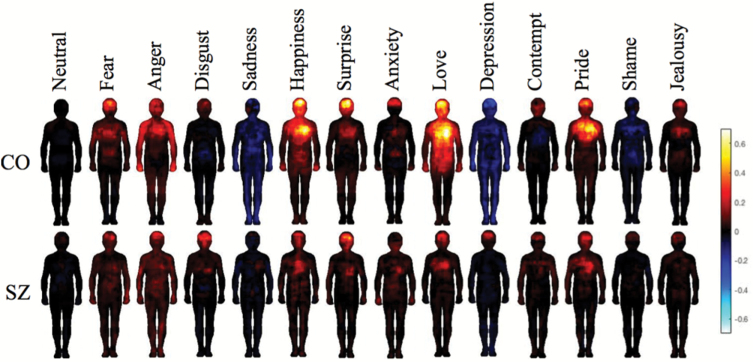
Figure 2 shows the bodily sensation maps associated with each emotion for CO and SZ. The color bar indicates the proportion of endorsement at each pixel. For the CO group, the highest proportion of endorsement was 0.92 whereas it was 0.53 for the SZ group. In both groups, the emotions are associated with distributions of body areas where activity was felt to change. In the CO group, the uniqueness of the emotion maps is more evident and in accordance with previous results,17 whereas the maps of the SZ group show more similarity across different emotions (eg, activation in the head and chest across emotions). To test these qualitative observations, we then implemented statistical tests to compare the group maps of SZ and CO.
Fig. 2.
Bodily topography of emotions in CO and SZ. Topographical maps of bodily sensations associated with 13 emotions and a neutral state for the CO (top) and SZ (bottom). Areas of increased/stronger activity (warm colors) or decreased/weaker sensations (cool colors) for each emotion are mapped. The color bar indicates the proportion of endorsement at each pixel ranging from −1 to 1 (1 represents absolute group consensus on the activation of a pixel, and −1 represents group consensus for deactivation).
Classification with LDA confirmed the independence of body maps across emotion categories in CO only. The overall prediction accuracy was higher for CO than SZ. When comparing the prediction accuracy scores to the thresholds of significance generated from random data, we found that average classification accuracy exceeded the significance threshold (0.095) in CO (0.1082 [0.1077, 0.1087], P < .01). In contrast, the classification accuracy was below the significance threshold in SZ (0.0871 [0.0867, 0.0874], P > .05). Following the original analysis described in Hietanen et al,34 we included all 14 emotion conditions as separate categories, which explains the low prediction accuracy scores reported here.
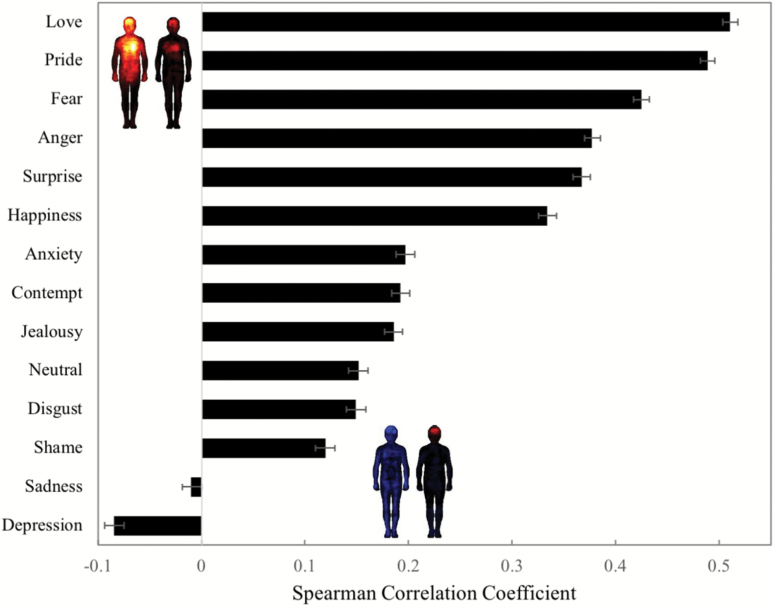
The maps (Figure 2) also suggest a more specific alteration in bodily deactivation in the context of emotions in SZ. This was quantitatively confirmed by computing the similarity (Spearman correlation) between SZ and CO for each emotion. We found the highest correlations between the maps of SZ and CO among high-arousal emotions (love rs = .511 [0.504, 0.512], P < .001; pride rs = .489 [0.482, 0.496], P < .001; fear rs = .425 [0.418, 0.432], P < .001) while the maps for low-arousal emotions had correlations close to zero (sadness rs = −.010 [−0.019, 0.000], P = .11; depression rs = −.084 [−0.093, −0.075], P < .001). We note that although the correlations between the maps of SZ and CO are strongly significant for high-arousal emotions (P < .001), their effect sizes are in the moderate range (.43 < rs < .51). This discrepancy between significance and size of the observed effect can be explained by the large number of observations (N = 50364 pixels) used to compute each correlation coefficient. We therefore exercise caution in interpreting the magnitude of the relationship between the maps of SZ and CO. Instead, we focus our subsequent discussion of similarity scores on the general trend observed. Figure 3 shows the similarity between emotion-wise bodily sensation of SZ and CO.
Fig. 3.
Similarity between the emotion-wise bodily sensation of SZ and CO. Spearman correlation coefficient between the bodily sensation maps of SZ and CO per emotion. The maps shown represent the bodily sensation reported by CO (left) and SZ (right) for the most (love), and least (depression) similar emotions. The error bars represent 95% confidence intervals.
To investigate the clarity of bodily sensations of emotions reported by SZ and CO, we computed the number of pixels where both activation and deactivation were endorsed for a single emotion. Because participants could color the same bodily region for activation and deactivation, we computed each participant’s number of “mixed pixels” (ie, a pixel colored both as activation and deactivation for a given emotion) across emotions. These individual “mixed pixel” counts were compared between the 2 groups. We found significantly more mixed pixels in SZ (M = 12043.35, SD = 18547.08) than CO (M = 9098.846, SD = 20467.74), (U = 225.5, P = .037, r = .29), indicating more blurred bodily sensation of emotions in SZ compared with CO.
Next, we addressed the question of the magnitude of reported bodily sensations. To quantify the net amount of bodily sensations felt, we computed the total number of pixels colored (ie, both activation and deactivation) across all emotions for each individual. This was done to test whether the observed results simply reflect a smaller number of responses, rather than different spatial organization of the responses, in the SZ group. No significant group difference was found between the number of pixels colored by SZ (M = 7573.60, SD = 5556.50) and CO (M = 9819.095, SD = 6943.14), t(50) = −1.29, P = .20. Overall, the 2 groups do not differ in the magnitude of sensations reported, and that SZ and CO were equally engaged in the task.
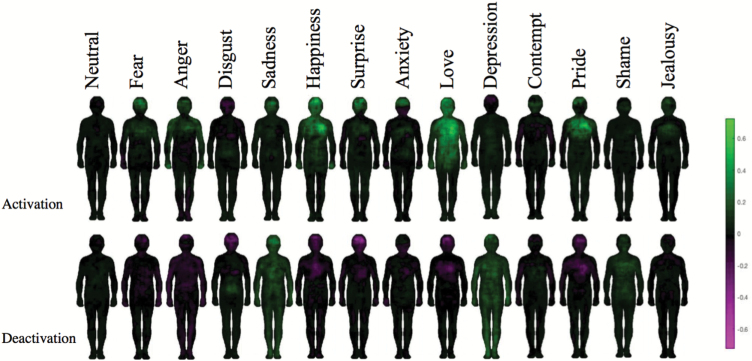
We then examined potential differences in the spatial patterns for activation maps vs deactivation maps. Figure 4 illustrates group differences in reported bodily sensations of activation (top) and deactivation (bottom). Green hues indicate greater bodily sensation reported by CO, magenta hues represent greater bodily sensations reported by SZ. When the activation maps are compared, CO show greater bodily sensations, especially for high-arousal emotions (eg, fear, anger, happiness, pride). When deactivation maps are compared, SZ show greater embodied sensations than CO for 6 out of 14 emotion categories. Interestingly, these emotions (pride, love, anxiety, surprise, happiness, anger) are all associated with increased arousal in the general population.17 We note that greater reported deactivation of SZ is not noticeable in the group maps of emotions (Figure 2), likely due to the high variability of reported bodily sensation of emotions reported in SZ. In sum, CO report more congruent bodily sensation of emotions (ie, activation for high-arousal emotions, deactivation for low-arousal emotions) than SZ.
Fig. 4.
Group difference maps for bodily sensation of emotions. Green represents regions where CO consistently reported more activation (top), or deactivation (bottom) than SZ. Magenta represents regions where SZ consistently reported more activation (top), or deactivation (bottom) than CO. The color bar represents the difference between SZ and CO in pixel values.
There was no correlation between medication dose (CPZ) and the number of pixels colored in SZ (r = .04, P = 0.85), suggesting that antipsychotic drugs do not alter embodied emotions.
Discussion
The topography of bodily sensations of emotions in SZ was qualitatively different from that of CO. Emotion categories were associated with distinct spatial maps in CO but not in SZ. This suggests that interoceptive signals might be less differentiated across emotions in SZ, even though the magnitude of felt sensations does not differ between the 2 groups, as indicated by the lack of group difference in the total pixels painted. The low similarity score between the CO and SZ maps of low-arousal emotions (eg, depression) suggests a specific alteration in embodiment of low-arousal emotions in SZ. It is possible that the difference between CO and SZ for low-arousal emotions reflect an abnormality in subjectively felt arousal in SZ.35
In addition to being less distinct, embodied emotions were more blurred and less precisely localized in SZ. Across emotions, SZ reported significantly more mixed bodily sensation than CO. In other words, SZ were more likely to feel activation and deactivation simultaneously and in the same location for an emotion. The individual variability of embodied emotions was also higher in SZ than CO, and less agreement over bodily sensation of emotions was observed within the SZ group compared with CO. The difference maps (Figure 4) further suggest that SZ report feeling more deactivation for high-arousal emotions. In the general population, low-arousal emotions are primarily associated with bodily deactivation, while high-arousal emotions are associated bodily activation.17 This result suggests a more incongruous bodily sensation of emotions in SZ.
Because the EmBODY task inherently relies on the ability of an individual to recall and simulate his/her past emotional experiences, our finding of anomalous bodily sensation of emotions in patients may be tied to weakened simulation,36 and autobiographical memory deficits37 in SZ. In control individuals, bodily sensations felt during an emotion correspond well with recalled sensations.17 Simulation deficit in SZ36 could prevent the encoding of emotional experiences, as well as the retrieval of autobiographical memory and associated bodily sensations. As such, impaired mental simulation and reenacting of emotions are likely to impact one’s ability to access personal memories, thereby altering one’s sense of self. Alternatively, abnormal bodily sensation of emotions could arise from anomalous reactions to emotional stimuli caused by alterations in spatiotemporal features of the resting state activity in SZ.38 These hypotheses remain to be empirically tested.
Previous studies have established that physiological states associated with emotions may not correspond to explicit awareness of emotions in SZ.39 In a previous study, individuals with SZ did not differ from controls in the labeling of emotions evoked by visual stimuli or the self-reported arousal ratings, but the tonic galvanic skin response was significantly greater in the patient group, suggesting a differential awareness, or calibration of internal states in SZ.39 The EmBODY task provides a nonverbal tool to communicate internal sensations associated with emotional experiences. We do not believe the awareness of internal sensations per se is reduced in SZ because the magnitude of pixel counts in the EmBODY task did not differ between SZ and CO. However, we observed that the internal sensations associated with emotion categories are not sufficiently distinct across different emotions in SZ. Embodiment theories of emotion postulate that emotion processing relies on somatosensory feedback.40 Future work should aim at mapping the actual physiological changes during experimentally induced emotions to understand how underlying physiological activity during emotional experiences leads to categorization of emotions.
Some limitations should be noted. First, our patients were chronically medicated. Side effects of antipsychotic medications include numbness and social withdrawal,41 which could alter interoception. However, when we examined the number of pixels colored by SZ compared with CO, there was no overall group difference, which suggests that the stark difference in body maps does not stem from reduced sensations per se but from the increased variability of colored areas in the SZ group. Furthermore, medication dosage did not correlate with measures of emotional embodiment in our sample. Second, we did not control for previous exposure to computer technology. However, all SZ were able to complete the task and their ability to color was similar to that of CO. Therefore, it seems unlikely that group differences were due to unfamiliarity with computers. Third, we did not collect physiological data (eg, heart rate variability, skin conductance) during the EmBODY task, which required participants to use the computer mouse extensively, thereby creating modest amounts of physical activity and preventing concurrent acquisition of reliable arousal data. Finally, we did not independently assess interoceptive accuracy. Therefore, we cannot determine if the abnormal mapping of bodily sensations of SZ is related to weakened interoceptive signals, reduced awareness of sensations or an inability to accurately match internal sensations (eg, fast heart rate) to verbal labels (eg, “I am anxious.”)
To summarize, we showed that reliable quantification of bodily sensations of emotions is made possible with a visual mapping task and that bodily sensations of emotions are abnormal in SZ. Whereas bodily “fingerprints” of distinct emotions were clearly separable in CO, they were undifferentiated in SZ. Furthermore, the embodied emotions reported by SZ were less precisely mapped and more incongruous than those reported by CO. Undifferentiated and nebulous bodily sensations that cannot easily be categorized and communicated to others could lead to social difficulties. These findings point to the importance of embodiment in social and functional outcome of SZ.
Funding
This work was supported in part by the Gertrude Conaway Vanderbilt Endowment.
Acknowledgments
We thank Andy Tomarken for his statistical advice, and our participants for their dedication to research.
References
- 1. Bleuler E. Dementia Praecox or the Group of Schizophrenias. Translated by Zinkin J. New York, NY: International Universities Press; 1911. [Google Scholar]
- 2. Kean C. Silencing the self: schizophrenia as a self-disturbance. Schizophr Bull. 2009;35:1034–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson B, Parnas J, Sass LA. Disturbance of minimal self (ipseity) in schizophrenia: clarification and current status. Schizophr Bull. 2014;40:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sass LA, Parnas J. Schizophrenia, consciousness, and the self. Schizophr Bull. 2003;29:427–444. [DOI] [PubMed] [Google Scholar]
- 5. Nelson B, Thompson A, Yung AR. Basic self-disturbance predicts psychosis onset in the ultra high risk for psychosis “prodromal” population. Schizophr Bull. 2012;38:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brent BK, Seidman LJ, Thermenos HW, Holt DJ, Keshavan MS. Self-disturbances as a possible premorbid indicator of schizophrenia risk: a neurodevelopmental perspective. Schizophr Res. 2014;152:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lenzenweger MF, Loranger AW. Detection of familial schizophrenia using a psychometric measure of schizotypy. Arch Gen Psychiatry. 1989;46:902–907. [DOI] [PubMed] [Google Scholar]
- 8. Lenzenweger MF. Schizotypy and Schizophrenia: The View from Experimental Psychopathology. New York, London: Guilford Press;2011. [Google Scholar]
- 9. Damasio AR. Feelings of emotion and the self. Ann N Y Acad Sci. 2003;100:253–261. [DOI] [PubMed] [Google Scholar]
- 10. Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3:1049–1056. [DOI] [PubMed] [Google Scholar]
- 11. Levenson RW. Autonomic specificity and emotion. Handb Affect Sci. 2003;2:212–224. [Google Scholar]
- 12. Saarimäki H, Gostopoulos A, Jääskeläinen IP, et al. Discrete neural signatures of basic emotions. Cereb Cortex. 2016;6:2563–2573. [DOI] [PubMed] [Google Scholar]
- 13. Lakoff G, Johnson M. The metaphorical structure of the human conceptual system. Cogn Sci. 1980;4:195–208. [Google Scholar]
- 14. Damasio AR. The Feeling of What Happens. New York, NY: Harcourt; 1999. [Google Scholar]
- 15. Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annu Rev Psychol. 2007;58:373–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kreibig SD. Autonomic nervous system activity in emotion: a review. Biol Psychol. 2010;84:394–421. [DOI] [PubMed] [Google Scholar]
- 17. Nummenmaa L, Glerean E, Hari R, Hietanen JK. Bodily maps of emotions. Proc Natl Acad Sci USA. 2014;111:646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100. [DOI] [PubMed] [Google Scholar]
- 19. Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11:417–428. [DOI] [PubMed] [Google Scholar]
- 20. Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30:855–863. [DOI] [PubMed] [Google Scholar]
- 21. Parnas J, Handest P. Phenomenology of anomalous self-experience in early schizophrenia. Compr Psychiatry. 2003;44:121–134. [DOI] [PubMed] [Google Scholar]
- 22. Michael J, Park S. Anomalous bodily experiences and perceived social isolation in schizophrenia: an extension of the social deafferentation hypothesis. Schizophr Res. 2016;176:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferri F, Frassinetti F, Mastrangelo F, Salone A, Ferro FM, Gallese V. Bodily self and schizophrenia: the loss of implicit self-body knowledge. Conscious Cogn. 2012;21:1365–1374. [DOI] [PubMed] [Google Scholar]
- 24. Ardizzi M, Ambrosecchia M, Buratta L, et al. Interoception and positive symptoms in schizophrenia. Front Hum Neurosci. 2016;10:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Postmes L, Sno HN, Goedhart S, van der Stel J, Heering HD, de Haan L. Schizophrenia as a self-disorder due to perceptual incoherence. Schizophr Res. 2014;152:41–50. [DOI] [PubMed] [Google Scholar]
- 26. Kring AM, Elis O. Emotion deficits in people with schizophrenia. Annu Rev Clin Psychol. 2013;9:409–433. [DOI] [PubMed] [Google Scholar]
- 27. First MB, Williams JBW, Karg RS, Spitzer RL.. Structured Clinical Interview for DSM-5—Research Version (SCID-5-RV). Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- 28. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 29. Andreasen NC. Scale for the Assessment of Positive Symptoms. Iowa City: University of Iowa; 1984. [Google Scholar]
- 30. Andreasen NC. Scale for the Assessment of Negative Symptoms. Iowa City: University of Iowa; 1983. [Google Scholar]
- 31. Raine A, Benishay D. The SPQ-B: a brief screening instrument for schizotypal personality disorder. J. Personality Disord. 1995;9:346–355. [Google Scholar]
- 32. Uttl B. North American adult reading test: age norms, reliability, and validity. J Clin Exp Neuropsychol. 2002;24:1123–1137. [DOI] [PubMed] [Google Scholar]
- 33. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hietanen JK, Glerean E, Hari R, Nummenmaa L. Bodily maps of emotions across child development. Dev Sci. 2016;19:1111–1118. [DOI] [PubMed] [Google Scholar]
- 35. Llerena K, Strauss GP, Cohen AS. Looking at the other side of the coin: a meta-analysis of self-reported emotional arousal in people with schizophrenia. Schizophr Res. 2012;142:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thakkar KN, Peterman JS, Park S. Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am J Psychiatry. 2014;171:539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McLeod HJ, Wood N, Brewin CR. Autobiographical memory deficits in schizophrenia. Cogn Emot. 2006;20:536–547. [DOI] [PubMed] [Google Scholar]
- 38. Northoff G, Duncan NW. How do abnormalities in the brain’s spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Prog Neurobiol. 2016;145–146:26–45. [DOI] [PubMed] [Google Scholar]
- 39. Peterman JS, Bekele E, Bian D, Sarkar N, Park S. Complexities of emotional responses to social and non-social affective stimuli in schizophrenia. Front Psychol. 2015;6:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Niedenthal PM, Barsalou LW, Winkielman P, Krauth-Gruber S, Ric F. Embodiment in attitudes, social perception, and emotion. Pers Soc Psychol Rev. 2005;9:184–211. [DOI] [PubMed] [Google Scholar]
- 41. Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]