Abstract
Recent evidence suggests that schizophrenia involves hyperfocusing, an unusually narrow but intense focusing of processing resources. This appears to contradict the classic idea that schizophrenia involves an impairment in the ability to focus on relevant information and filter irrelevant information. Here, we review one set of studies suggesting that attentional filtering is impaired in people with schizophrenia and another set of studies suggesting that attentional filtering is unimpaired or even enhanced in these individuals. Considerable evidence supports both conclusions, and we propose 3 potential ways of reconciling the conflicting evidence. First, impaired attentional filtering may occur primarily during periods of active psychosis, with hyperfocusing being a part of the broad pattern of cognitive impairment that persists independent of the level of positive symptoms. Second, schizophrenia may involve hyperfocusing in the visual modality and impaired attentional filtering in the auditory modality. Third, attention may be directed toward irrelevant inputs as a result of impaired executive control, and hyperfocusing on those inputs may be functionally equivalent to a failure of attentional filtering. Given the widespread clinical observations and first-person reports of impaired attentional filtering in schizophrenia, it will be important for future research to test these possibilities.
Keywords: hyperfocusing, control, selective attention, psychosis
We have recently advanced a hyperfocusing hypothesis (described in a companion article [S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data]), which proposes that many aspects of cognitive dysfunction in schizophrenia are a result of an unusually narrow but intense focusing of processing resources. This hypothesis can explain multiple experimental findings and has led to several novel predictions.1–4 However, the hyperfocusing hypothesis seems diametrically opposed to the classic idea that schizophrenia involves impaired attentional filtering.5,6 In this article, we will review studies that address the nature of attentional dysfunction in schizophrenia to address the apparent discrepancy between the hyperfocusing hypothesis and the classic idea of impaired attentional filtering.
Following traditional conceptualizations of attentional dysfunction in schizophrenia,6,7 we will focus on the specific variety of attention called selective attention, which was classically defined by James8: “It is the taking possession by the mind, in clear and vivid form, of one out of what seem several simultaneously possible objects or trains of thought. Focalization, concentration, of consciousness are of its essence. It implies withdrawal from some things in order to deal effectively with others… .” This variety of attention operates by selecting some sources of information for enhanced processing at the expense of other sources, rather than being a global state that waxes and wanes over time.9,10
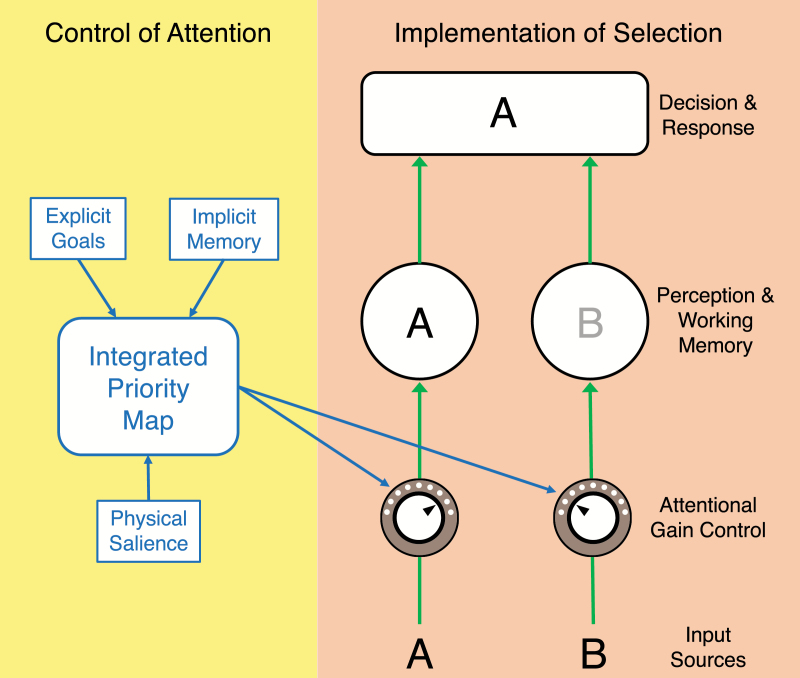
Figure 1 provides a simplified schematic of the operation of attention (based on refs.11–13). It distinguishes between the control of attention (the processes that determine which sources of information will be selected for enhanced processing) and the implementation of selection (the processes that actually enhance representations of the selected information and filter out other sources).14,15 Control mechanisms combine information about explicit goals, implicit memory, and physical salience into a priority map. This map then sends signals into perceptual and WM systems that are used to set the gain on the transmission of information from each individual stimulus within these systems. Thus, the control system determines which inputs have priority, but the actual change in gain occurs within the implementation system.
Fig. 1.
Simplified schematic of the control of attention and implementation of selection. In this example, the gain is increased for the A and decreased for the B.
For example, if an A and a B are presented simultaneously in the left and right visual fields, respectively, and the goal is to identify the item on the left, this goal will lead to greater activity on the left side of the priority map within the attentional control system, which would then increase the gain for the A and/or decrease the gain for the B. This would in turn lead to a stronger representation of the A than the B in perception and WM (effectively filtering out the B). Increasing the gain for relevant stimuli and decreasing the gain for irrelevant stimuli may involve different neural mechanisms,16 but both reduce the relative impact of the irrelevant stimuli and can be considered mechanisms of filtering.
Impaired control processes could lead people with schizophrenia (PSZ) to activate the wrong location in the priority map, resulting in the appearance of impaired filtering even if they are actually hyperfocusing on the information they (erroneously) select. We will therefore distinguish between an impairment in the performance of filtering tasks that arises from a deficit in control processes and an impairment in performance resulting from a deficit in the filtering (gain control) mechanisms. Although both types of deficits may have similar consequences in the daily lives of PSZ, they imply different targets for treatment development because these mechanisms have different neural substrates.10,17
Now that we have defined control and filtering, we turn to the qualitative clinical observations and quantitative experimental evidence that have led to the view that attentional filtering is impaired in PSZ. We will then describe contrasting evidence that attentional filtering mechanisms are intact in PSZ. We end by considering 3 possible explanations for this discrepancy: (1) Differences between acute and chronic phases of the disease; (2) Differences between the visual and auditory modalities; and (3) Differences between control processes and filtering processes.
Flooding, Selective Attention, and Sensory Gating
Clinical observations and first-person reports support the idea that PSZ have a deficit in filtering. For example, one individual provided the following self-report18: “Ever since I started having problems due to schizophrenia, my senses have been thrown out of whack … I remember one day when I got caught in the rain. Each drop felt like an electric shock and I found it hard to move because of how intense and painful the feeling was.” Beginning in the 1960s,6,19 this kind of sensory flooding was explained in terms of an impairment in selective attention. For example, McGhie and Chapman6 concluded that “patients appear to have lost the ability and freedom to direct their attention focally … the individual finds himself less free to direct his attention at will. Instead, his control of attention is now being increasingly determined for him by concrete changes in the environment.”
Contemporary discussions of sensory flooding in schizophrenia often focus on evidence for impaired sensory gating in the P50 suppression and prepulse inhibition (PPI) paradigms.20,21 In these paradigms, 2 stimuli are presented in rapid succession, and the presentation of the first stimulus leads to a reduction in the response to the second. This sensory gating effect is often reduced in PSZ relative to healthy control subjects (HCS).22,23
However, this kind of sensory gating is not a form of selective attention.24 Sensory gating is a global change in sensory gain for a given modality, not the selection of one source of information at the cost of other concurrent sources. Thus, the kind of global gating examined in P50 and PPI studies is not the same as the selective attention mechanisms that allow us to listen to one person talk while ignoring other voices in the background. PSZ may have a deficit in the type of filtering that is involved in sensory gating without a deficit in the type of filtering that is used to implement selective attention (which we will call attentional filtering).
Evidence for Impaired Attentional Filtering in PSZ
Dichotic Listening
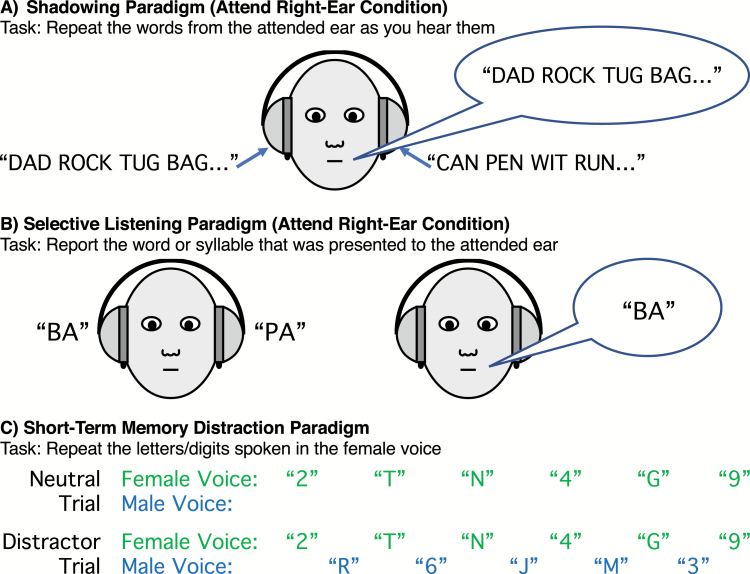
We must often focus attention on the voice of one person and filter out other people who are talking concurrently. This aspect of filtering was classically studied using the dichotic listening paradigm,25–27 in which the participant hears one person speaking in each ear and attempts to understand the voice in one ear while filtering out the other ear. In some experiments, attention is directed to one ear by requiring participants to shadow the voice in that ear (ie, immediately repeat every word spoken in that ear; figure 2A). As reviewed by Spring et al,28 many studies of schizophrenia in the 1970s used this paradigm, comparing shadowing performance in a dichotic listening condition with a no-distraction condition in which no speech is played to the to-be-ignored ear. PSZ typically exhibit impaired shadowing performance relative to HCS in both conditions, but the impairment is typically greater in the dichotic condition than in the no-distraction condition.7,29–31 A more recent study of acute inpatients replicated this classic result.32 These findings are often taken as evidence for impaired attentional filtering in PSZ, but the greater impairment in the dichotic condition could reflect a nonspecific impairment that simply increases with task difficulty.28
Fig. 2.
Paradigms classically used to assess attentional filtering for speech signals. In the shadowing paradigm (A), separate streams of speech are presented concurrently to the 2 ears, and participants repeat each word from that ear as soon as it is presented. In the selective listening paradigm (B), 1 syllable or word is presented to each ear on a given trial, and participants are required to report the syllable from 1 of the 2 ears. In the short-term memory distraction paradigm (C), a sequence of letters and digits is spoken by a female voice on each trial, and participants are required to repeat back these items at the end of the trial, ignoring anything spoken by a male voice.
More direct evidence of a filtering deficit comes from the finding that intrusion errors (ie, repeating a word from the to-be-ignored ear) are more frequent in PSZ than in HCS.7,29,30 In addition, some of these experiments included a memory test to determine how many words were remembered from each ear, and in some cases PSZ remembered more words from the to-be-ignored ear than did HCS.7,30 These findings provide positive evidence of a deficit in attentional filtering.
More recent studies have used a simpler selective listening task (figure 2B), in which participants hear one word or syllable in each ear (simultaneously) and then repeat 1 of the 2 stimuli with no speed pressure. A study of inpatients with treatment-resistant schizophrenia33 found that PSZ were approximately equally likely to report the syllable from the left or right ear no matter which ear they were instructed to attend, whereas HCS predominantly reported the syllable from the to-be-attended ear (see also Bozikas et al34).
A larger study of both inpatients and outpatients found that PSZ were able to filter normally when asked to attend to the right ear but were impaired when instructed to attend to the left ear35 (which is much more difficult in most right-handed individuals). This was interpreted as evidence that the filtering mechanisms were intact in PSZ, allowing them focus on the dominant ear, but that their attentional control systems were unable to direct attention toward the nondominant ear. Thus, although the research reviewed in this section provides evidence that PSZ exhibit impaired performance in filtering tasks, it may be possible to explain these results by means of dysfunctional control mechanisms rather than dysfunctional filtering mechanisms.
Distractibility During Short-Term Memory Encoding
Other classic research on selective attention in PSZ used the short-term memory task shown in figure 2C, in which participants hear a sequence of letters and digits spoken in a female voice and then repeat them back. These stimuli are either presented alone (neutral trials) or interposed with to-be-ignored letters and digits spoken in a male voice (distractor trials). Two studies using this task in the 1960s found that PSZ exhibited nearly normal performance on neutral trials but were strongly impaired on the distractor trials.36,37 Interestingly, they found no evidence of increased distractibility in PSZ in visual versions of this paradigm.
A decade later, Oltmanns et al38–40 raised the possibility that the greater impairment in PSZ for distractor trials than for neutral trials could simply reflect the common finding that impairments are greater for more difficult tasks (which also applies to the early shadowing studies7,29–31). When they controlled task difficulty by adjusting the number of items in each to-be-remembered sequence to match the difficulty of the neutral and distractor trials, they still found evidence of impaired filtering in PSZ (although the pattern of results was complex).39,40
These findings have been replicated and extended more recently by Harvey et al41–45 in studies of acute inpatients. In most of these studies, increased distractibility was associated with higher levels of positive symptoms.42–45 In addition, just like some of the early studies,36,37 PSZ mainly exhibited increased distractibility (and an association with positive symptoms) for the auditory version of the task and not a visual version.43 However, the relationship between increased distractibility and symptoms is complex. For example, increased distractibility sometimes persists during periods of remission in PSZ41 and has been observed in nonsymptomatic children of PSZ.46
Summary of the Evidence for Impaired Attentional Filtering
Consistent with clinical observations and first-person reports, the studies reviewed here provide evidence that—across multiple paradigms—PSZ exhibit poor filtering of task-irrelevant auditory stimuli, reporting fewer stimuli from the to-be-attended source and more stimuli from the to-be-ignored source relative to HCS. However, some limits to this conclusion must be noted. First, the impairment was limited to auditory stimuli; PSZ were unimpaired in visual versions of the selective short-term memory task36,37 and in most of the visual studies described in the next section. Second, most of these studies used inpatients, and many (but not all) of the effects were correlated with measures of positive symptoms, were reduced by antipsychotic medications, or were present only during periods of high positive symptoms.40,47,48 Similarly, the clinical observations and first-person accounts of impaired filtering typically come from acutely psychotic and/or unmedicated patients. Third, as already discussed, many of the effects could potentially be explained by impaired control processes that sometimes direct selective attention to irrelevant stimuli rather than an impairment in the actual filtering mechanisms.
Evidence for Intact Attentional Filtering in PSZ
In this section, we review evidence—mostly from studies of visual attention in medicated outpatients—that attentional filtering is unimpaired in PSZ. Indeed, the hyperfocusing hypothesis proposes that PSZ may focus attention more narrowly and more intensely than HCS under certain conditions (S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data). However, this hypothesis assumes that HCS can focus their attention just as narrowly and intensely as PSZ when the task demands it (S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data), as is the case in most of the paradigms reviewed here. Thus, the studies reviewed in this section mainly demonstrate that attentional filtering is unimpaired in PSZ; evidence for supranormal filtering is reviewed elsewhere (S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data).
The Spatial Cuing Paradigm
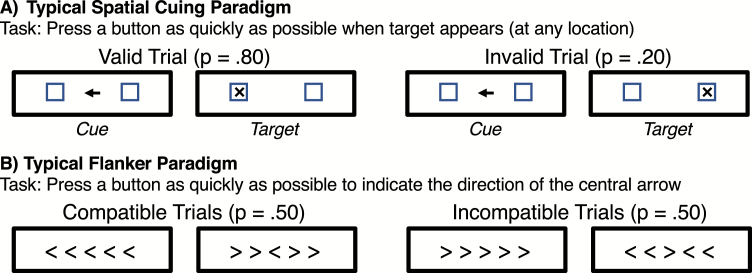
In the spatial cuing paradigm (figure 3A), a cue indicates that a target is likely to occur at one location and unlikely to occur at other locations. If attention is focused on the cued location, then performance should be better when the target appears at that location (valid cue trials) than when the target appears at the uncued location (invalid cue trials). Impaired filtering in this paradigm would manifest as a reduced cuing effect (ie, a reduced difference in performance between valid and invalid trials). Although differences between PSZ and HCS in hemispheric asymmetries have been observed in some cuing studies,49–51 the overall cuing effect is typically just as large (or even larger) in PSZ as in HCS.52–55 Thus, PSZ appear to have no deficit focusing selective attention onto a cued location and filtering stimuli presented at uncued locations in the visual modality.
Fig. 3.
Common paradigms for assessing visual-spatial selective attention. In typical spatial cuing tasks (A), one location is cued on each trial, indicating the likely location of the next target, and participants must respond to the target as quickly as possible. In the flanker paradigm (B), participants must respond on the basis of the central stimulus and ignore the flanking stimuli.
The Flanker Paradigm
The flanker paradigm56 (figure 3B) requires participants to focus on a central stimulus, which indicates which of 2 responses should be made, and ignore flanking stimuli on each side. The flankers are identical to the central stimuli on some trials (compatible trials), and they are associated with opposite responses on other trials (incompatible trials). If the flankers are not completely filtered out, they will lead to slowed response times (interference) on incompatible trials, and greater interference therefore indicates poorer filtering. Although some studies have found more flanker interference in PSZ than in HCS,57,58 most studies have not,59–61 and a meta-analysis based on data from 1029 PSZ and 848 HCS found no significant difference between PSZ and HCS.62 Thus, PSZ are not impaired relative to HCS in filtering irrelevant stimuli around a central target. This contrasts with the greater rate of intrusions from to-be-ignored stimuli in the auditory studies described earlier.
Visual Search and Span of Apprehension
Attention is also commonly studied using visual search and span of apprehension tasks, in which participants search for a target in an array of multiple objects. Many studies have found that PSZ are impaired in these tasks,63–66 but this could reflect a deficit in the ability to distribute attention among multiple objects (S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data) or an impairment in the control of attention1,65 rather than a deficit in filtering. Studies that more directly assessed the filtering process during visual search tasks have found no evidence of impaired filtering.67,68 Other studies found that PSZ were impaired relative to HCS in search tasks only to the extent that top-down control mechanisms were essential to performance.1,65
Selective Attention in Working Memory
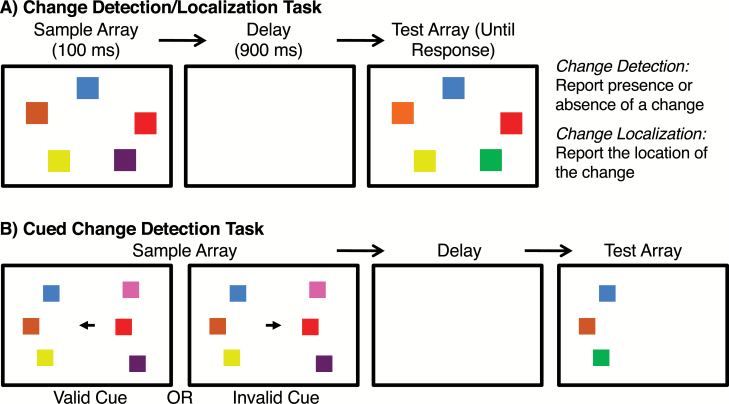
PSZ exhibit reduced working memory (WM) storage capacity relative to HCS in a variety of experimental paradigms (S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data),69 including the visual change detection/localization paradigm shown in figure 4A.70–72 A filtering impairment could easily explain the reduced storage capacity in this task: if PSZ fail to filter out task-irrelevant information from WM, this would leave less capacity for storing the task-relevant stimuli. Indeed, filtering ability is correlated with WM capacity among typical young adults.73,74
Fig. 4.
(A) Basic change detection/localization task. On each trial, the sample and test arrays are either identical or differ in the color of one item (shown as differences in lightness in the print version of the article). Participants either report the presence or absence of a changed color (change detection) or the location of the change (change localization). (B) Cued version of the change detection task. A cue arrow on each trial indicates the side that is likely to be tested.
However, no evidence of impaired filtering in PSZ was obtained in a series of 4 WM experiments.75 In the first experiment (figure 4B), participants were shown an array containing 3 colored squares on each side of the display along with a cue arrow. The test array was usually presented on the cued side (valid trials), but it was occasionally presented on the uncued side (invalid trials). In either case, participants reported whether the colors in the test array were the same as or different from the colors on that same side in the sample array. If PSZ are impaired at focusing on the cued side and filtering out the uncued side, they should be impaired relative to HCS on valid trials but they should exhibit better memory than HCS on the invalid trials (just as PSZ exhibit more intrusions from the unattended ear in dichotic listening experiments). However, PSZ and HCS exhibited large and nearly identical effects of cuing in this experiment. The same pattern was observed in follow-up experiments that involved more demanding filtering tasks. Similar results have also been reported in a task that required focusing on words of one color and filtering words of another color76 and in a task in which the stimuli were sequentially presented photos of natural objects and the to-be-remembered stimuli were cued by a tone.77 Thus, PSZ appear to be unimpaired at preferentially encoding relevant items and filtering out irrelevant items from WM.
Challenging Attentional Control With Highly Salient Stimuli
One way to “rescue” the hypothesis of an attention deficit in the visual modality is to propose that filtering fails in PSZ only when attentional control is challenged by the need to overcome the prepotent tendency to allocate attention to highly salient stimuli.15,78 This would be consistent with the proposal of McGhie and Chapman6 that, in PSZ, “the individual finds himself less free to direct his attention at will. Instead, his control of attention is now being increasingly determined for him by concrete changes in the environment.” This possibility has been tested in several ways, and a deficit in overcoming distraction by highly salient stimuli has been observed mainly under conditions that strongly activate the magnocellular visual pathway.79–82
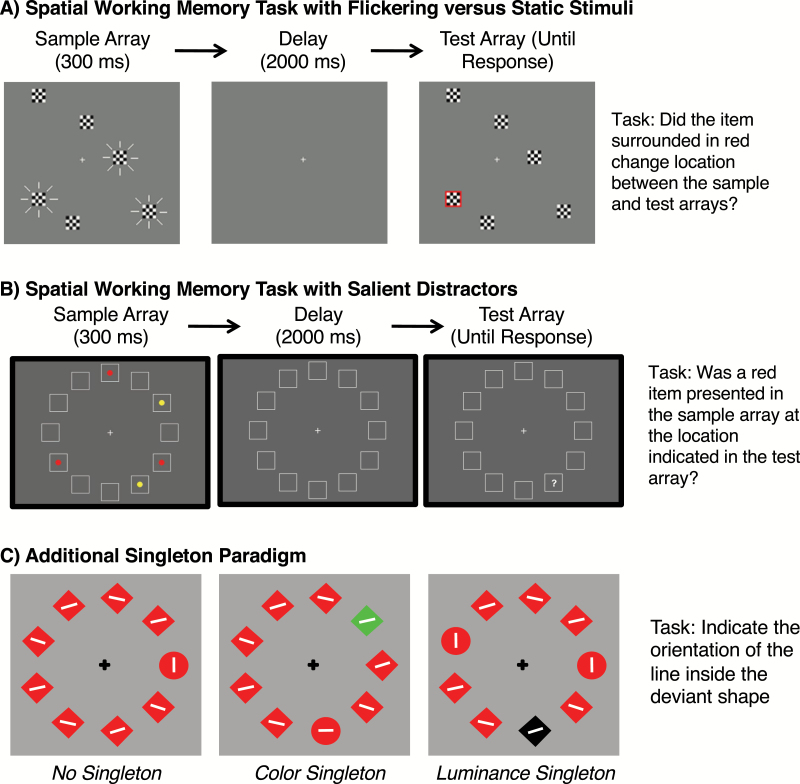
In the experiment illustrated in figure 5A,81 each display consisted of 3 highly salient flickering items and 3 less salient nonflickering items. The task was to remember the locations of the items. In the key condition, the nonflickering items were more likely to be tested than the flickering items, motivating participants to selectively store the less salient nonflickering items in WM. HCS were able to remember the nonflickering items better than the flickering items in this condition, whereas PSZ had no performance advantage for the cued nonflickering items. However, PSZ exhibited normal selective attention when the flickering items were more likely to be tested than the nonflickering items. This pattern suggests that PSZ have difficulty filtering only when this requires overcoming the intrinsic tendency to focus on physically salient sensory inputs, consistent with impaired top-down control over attention (and not an impairment in the actual filtering mechanisms, as defined in figure 1).
Fig. 5.
Tasks that have been used to assess the ability of people with schizophrenia to avoid focusing attention on highly salient stimuli. (A) Spatial working memory task with flickering stimuli.81 In the key condition, participants were motivated to attend to the nonflickering items by testing them more often than the flickering items. (B) Working memory task in which participants were instructed to remember the locations of the red items and ignore the yellow items (which appear as dark and light circles, respectively, in the print version of the article).80 (C) Additional singleton paradigm,82 in which participants made a buttonpress response to indicate the orientation of the line inside the deviant shape.
Converging evidence was obtained with the paradigm shown in figure 5B, in which participants were required to remember the locations of the red items and ignore the yellow items. Previous research had shown that neurological patients are impaired at filtering the yellow items,83 demonstrating the sensitivity of the paradigm, but there was no evidence that PSZ were impaired at filtering the yellow items.80 A follow-up experiment included trials with rotating distractors, which were much more salient, and these highly salient distractors disrupted PSZ somewhat more than they disrupted HCS. Again, this provided evidence that the basic filtering mechanisms are intact in PSZ and that evidence of impaired attentional selection is observed only when control mechanisms must overcome the prepotent tendency to shift attention to highly salient objects (see also Bansal et al79).
Filtering of Highly Salient Stimuli in Visual Search
The experiments in the previous section suggest that attentional control mechanisms are impaired in PSZ, leaving them vulnerable to distraction from highly salient stimuli. An alternative account however, is that the salient stimuli used in those experiments strongly activated the magnocellular pathway, which may exhibit aberrant processing in PSZ and underlie some of the sensory abnormalities observed in this disorder.84 This pathway plays a key role in motion perception, the processing of low spatial frequencies, and the detection of low-contrast stimuli. PSZ exhibit reduced neural responses and contrast sensitivity for stimuli that activate this pathway,85–87 but they also show greater masking by such stimuli,88–90 suggesting a complex dysregulation of magnocellular processing. We have proposed that PSZ have a specific impairment in controlling attention in the face of strong magnocellular activation rather than a general deficit in attentional control or attentional filtering.82
This hypothesis was tested in an experiment using the additional singleton paradigm82 (figure 5C). Participants searched for a target defined by a unique shape (a circle among diamonds or a diamond among circles), and they reported the orientation of a line inside the target (horizontal vs vertical). On a subset of trials, a highly salient color singleton was present, which slows target processing if it captures selective attention.91 However, this singleton was isoluminant with the rest of the items in the display, making it indiscriminable from the other items from the perspective of the magnocellular stream (which is largely colorblind92). The experiment also included luminance singletons, which should have strongly activated the magnocellular pathway.
The color and luminance singletons used in this experiment were previously shown to be approximately equal in salience.93 Consequently, if PSZ are generally less able to ignore salient distractors than HCS, then both color and luminance singletons should have distracted PSZ more than HCS. However, color singletons produced no more distraction in PSZ than in HCS, and whereas HCS were slightly more distracted by color singletons than by luminance singletons, PSZ were more distracted by the luminance singletons than by the color singletons.82 Moreover, PSZ and HCS were approximately equally likely to fixate the color singleton, but PSZ were more likely than HCS to fixate the luminance singleton (see also Bansal et al79). These results suggest that PSZ are not generally more distractible by salient items than HCS, but are particularly susceptible to distraction by stimuli that strongly activate the magnocellular pathway.
Reconciling the Evidence For and Against Impaired Selective Attention
How can we reconcile the overwhelming evidence of intact or even supranormal selective attention in PSZ in these recent studies with the clinical observations, first-person reports, and older studies indicating that PSZ are impaired at filtering irrelevant information? Here, we discuss 3 possibilities (which are not mutually exclusive).
One possibility is that sensory flooding and poor selectivity are present during periods of acute psychosis but are not part of the stable pattern of cognitive dysfunction that persists independently of the level of positive symptoms. Most of the studies finding evidence of attentional dysfunction in PSZ involved inpatients with fairly high levels of positive symptoms (eg, mean BPRS total scores of 50–7033,35), whereas most of the studies finding no evidence of attentional dysfunction involved stable outpatients with low to moderate levels of positive symptoms (eg, mean BPRS total scores of approximately 3554,77,80). However, some studies have found evidence of impaired selective attention without high levels of psychosis.41,46
Another possibility is that filtering impairments in PSZ are modality-specific. Much of the evidence for intact selective attention (and virtually all of the evidence for hyperfocusing [S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data]) comes from visual paradigms, whereas most of the reports of flooding and impaired filtering come from other modalities, especially the auditory modality. Consistent with this possibility, studies of the P3 ERP component—which is closely linked with the controlled allocation of high-level processing resources94—have found a substantial reduction of P3 amplitude in PSZ relative to HCS for auditory tasks and a much weaker reduction in visual tasks.95 Thus, the neuropathology of schizophrenia may impact attention differently in the auditory and visual modalities.
A third possibility is that attentional control may be impaired in PSZ, so that they often focus on the wrong sources of information. This would be even more problematic if, as proposed by the hyperfocusing hypothesis (S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data), PSZ then process these erroneously selected stimuli with unusual intensity.
The possibility of an attentional control impairment could explain the conflicting evidence regarding selective attention in PSZ by proposing that impairments are observed only under conditions that strongly challenge control mechanisms. That is, no impairment should be observed when control is easy (eg, when a cue indicates that a single location should be attended52,54,75), and impairments should emerge when control is made more difficult (eg, when participants are instructed to attend to stimuli in the nondominant ear that are presented simultaneously with stimuli in the dominant ear35). The dichotic listening paradigms used in many of the studies showing impaired attention in PSZ7,29–31 are certainly very demanding. Spatial segregation of simultaneous inputs is weaker in the auditory than in the visual system, which may make attentional selection fundamentally more challenging in typical auditory attention situations (eg, focusing on 1 of 2 voices) than in typical visual attention situations (eg, focusing on 1 of 2 faces).
To test such an explanation, however, it would be necessary to have an independent measure of the difficulty of attentional control. Moreover, in the visual modality, PSZ appear to have a specific problem suppressing stimuli that strongly activate the magnocellular pathway rather than a general deficit in attentional control. More research is needed to assess the presence and nature of attentional control deficits in schizophrenia in the auditory and visual modalities.
Conclusions
The present review indicates that there is substantial evidence that failures of selective attention can be observed in PSZ, especially during periods of acute psychosis and in the auditory modality. This is consistent with clinical observations and first-person reports of impaired attention, which also mainly derive from periods of acute psychosis. However, these failures of focusing may reflect a deficit in the attentional control mechanisms that determine which sources of information should be attended rather than a deficit in the attentional filtering mechanisms. Indeed, a combination of directing attention to irrelevant sources of information and then hyperfocusing on these sources may provide an excellent account of some of the phenomenology of schizophrenia.
In PSZ who are not experiencing high levels of positive symptoms, there is less evidence of a general impairment in attentional control and very little evidence of an impairment in the filtering mechanisms, especially in the visual modality. But even in such individuals, hyperfocusing may create the appearance of distractibility. Unusually intense WM representations or task representations may cause attention to be captured by objects that partially match these representations but are not actually relevant (S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data),3,4 and hyperfocusing on these objects would lead to greater functional distraction.
Although a deficit in filtering and hyperfocusing on irrelevant information might be functionally very similar, they imply very different targets for treatment development. Specifically, the hyperfocusing hypothesis suggest that new treatments should not attempt to increase the effectiveness of the filtering process itself, which might actually increase the negative consequences of hyperfocusing. However, it may be valuable to target the circuits underlying the control mechanisms that determine where attention should be focused,96,97 as well as the circuits that may underlie hyperfocusing (S. J. Luck, B. Hahn, C. J. Leonard, J. M. Gold, unpublished data).
Funding
This article was made possible by the National Institutes of Health (R01MH065034 to J.M.G. and S.J.L.).
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Gold JM, Robinson B, Leonard CJ, et al. . Selective attention, working memory, and executive function as potential independent sources of cognitive dysfunction in schizophrenia. Schizophr Bull. 2018;44:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kreither J, Lopez-Calderon J, Leonard CJ, et al. . Electrophysiological evidence for hyperfocusing of spatial attention in schizophrenia. J Neurosci. 2017;37:3813–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luck SJ, McClenon C, Beck VM, et al. . Hyperfocusing in schizophrenia: evidence from interactions between working memory and eye movements. J Abnorm Psychol. 2014;123:783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sawaki R, Kreither J, Leonard CJ, et al. . Hyperfocusing of attention on goal-related information in schizophrenia: evidence from electrophysiology. J Abnorm Psychol. 2017;126:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frith CD. Consciousness, information processing and schizophrenia. Br J Psychiatry. 1979;134:225–235. [DOI] [PubMed] [Google Scholar]
- 6. Mcghie A, Chapman J. Disorders of attention and perception in early schizophrenia. Br J Med Psychol. 1961;34: 103–116. [DOI] [PubMed] [Google Scholar]
- 7. Payne RW, Hochberg AC, Hawks DV. Dichotic stimulation as a method of assessing disorder of attention in overinclusive schizophrenic patients. J Abnorm Psychol. 1970;76:185–193. [DOI] [PubMed] [Google Scholar]
- 8. James W. The Principles of Psychology. New York: Holt; 1890. [Google Scholar]
- 9. Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. [DOI] [PubMed] [Google Scholar]
- 10. Luck SJ, Vecera SP. Attention. In: Yantis S, ed. Stevens’ Handbook of Experimental Psychology: Vol. 1: Sensation and Perception. 3rd ed.New York: Wiley; 2002:235–286. [Google Scholar]
- 11. Awh E, Belopolsky AV, Theeuwes J. Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends Cogn Sci. 2012;16:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. [DOI] [PubMed] [Google Scholar]
- 13. Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27:611–647. [DOI] [PubMed] [Google Scholar]
- 14. Beck DM, Kastner S. Neural systems for spatial attention in the human brain: evidence from neuroimaging in the framework of biased competition. Oxf Handb Atten. 2014:253–288. [Google Scholar]
- 15. Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luck SJ. Multiple mechanisms of visual-spatial attention: recent evidence from human electrophysiology. Behav Brain Res. 1995;71:113–123. [DOI] [PubMed] [Google Scholar]
- 17. Posner MI, DiGirolamo GJ. Attention in cognitive neuroscience: An overview. In: Gazzaniga MS, ed. The New Cognitive Neurosciences. Vol 2 Cambridge, MA: MIT Press; 2000:623–632. [Google Scholar]
- 18. Djinn (pseudonym). Sensory Overload. Schizophrenia.com https://forum.schizophrenia.com/t/sensory-overload/4910. Published April 14, 2014. Accessed January 4, 2019.
- 19. Venables PH. Input dysfunction in schizophrenia. Prog Exp Pers Res. 1964;72:1–47. [PubMed] [Google Scholar]
- 20. Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. [DOI] [PubMed] [Google Scholar]
- 21. Freedman R, Adler LE, Gerhardt GA, et al. . Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 1987;13:669–678. [DOI] [PubMed] [Google Scholar]
- 22. Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl). 2001;156:234–258. [DOI] [PubMed] [Google Scholar]
- 23. Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophr Res. 2004;70:315–329. [DOI] [PubMed] [Google Scholar]
- 24. Jin Y, Bunney WE Jr, Sandman CA, et al. . Is P50 suppression a measure of sensory gating in schizophrenia? Biol Psychiatry. 1998;43:873–878. [DOI] [PubMed] [Google Scholar]
- 25. Broadbent DE. Perception and Communication. New York: Pergamon; 1958. [Google Scholar]
- 26. Cherry EC. Some experiments on the recognition of speech with one and with two ears. J Acoust Soc Am. 1953;25:975–979. [Google Scholar]
- 27. Treisman AM. Contextual cues in selective listening. Q J Exp Psychol. 1960;12:242–248. [Google Scholar]
- 28. Spring B, Weinstein L, Freeman R, Thompson S. Selective attention in schizophrenia. In: Neuropsychology, Psychophysiology, and Information Processing. Handbook of schizophrenia, Vol. 5 New York, NY, US: Elsevier Science; 1991:371–396. [Google Scholar]
- 29. Wahl O. Schizophrenic patterns of dichotic shadowing performance. J Nerv Ment Dis. 1976;l63:401–407. [DOI] [PubMed] [Google Scholar]
- 30. Wishner J, Wahl O. Dichotic listening in schizophrenia. J Consult Clin Psychol. 1974;42:538–546. [DOI] [PubMed] [Google Scholar]
- 31. Schneider SJ. Selective attention in schizophrenia. J Abnorm Psychol. 1976;85:167–173. [DOI] [PubMed] [Google Scholar]
- 32. Wielgus MS, Harvey PD. Dichotic listening and recall in schizophrenia and mania. Schizophr Bull. 1988;14:689–700. [DOI] [PubMed] [Google Scholar]
- 33. Løberg EM, Hugdahl K, Green MF. Hemispheric asymmetry in schizophrenia: a “dual deficits” model. Biol Psychiatry. 1999;45:76–81. [DOI] [PubMed] [Google Scholar]
- 34. Bozikas VP, Kosmidis MH, Giannakou M, et al. . Controlled shifting of attention in schizophrenia and bipolar disorder through a dichotic listening paradigm. Compr Psychiatry. 2014;55:1212–1219. [DOI] [PubMed] [Google Scholar]
- 35. Hugdahl K, Rund BR, Lund A, et al. . Attentional and executive dysfunctions in schizophrenia and depression: evidence from dichotic listening performance. Biol Psychiatry. 2003;53:609–616. [DOI] [PubMed] [Google Scholar]
- 36. Lawson JS, McGhie A, Chapman J. Distractibility in schizophrenia and organic cerebral disease. Br J Psychiatry. 1967;113:527–535. [DOI] [PubMed] [Google Scholar]
- 37. Mcghie A, Chapman J, Lawson JS. The effect of distraction on schizophrenic performance. 1. perception and immediate memory. Br J Psychiatry. 1965;111:383–390. [DOI] [PubMed] [Google Scholar]
- 38. Oltmanns TF. Selective attention in schizophrenic and manic psychoses: the effect of distraction on information processing. J Abnorm Psychol. 1978;87:212–225. [DOI] [PubMed] [Google Scholar]
- 39. Oltmanns TF, Neale JM. Schizophrenic performance when distractors are present: attentional deficit or differential task difficulty? J Abnorm Psychol. 1975;84:205–209. [DOI] [PubMed] [Google Scholar]
- 40. Oltmanns TF, Ohayon J, Neale JM. The effect of anti-psychotic medication and diagnostic criteria on distractibility in schizophrenia. J Psychiatr Res. 1978;14:81–91. [DOI] [PubMed] [Google Scholar]
- 41. Harvey PD, Docherty NM, Serper MR, Rasmussen M. Cognitive deficits and thought disorder: II. An 8-month followup study. Schizophr Bull. 1990;16:147–156. [DOI] [PubMed] [Google Scholar]
- 42. Harvey PD, Earle-Boyer EA, Levinson JC. Cognitive deficits and thought disorder: a retest study. Schizophr Bull. 1988;14:57–66. [DOI] [PubMed] [Google Scholar]
- 43. Harvey PD, Pedley M. Auditory and visual distractibility in schizophrenia. Clinical and medication status correlations. Schizophr Res. 1989;2:295–300. [DOI] [PubMed] [Google Scholar]
- 44. Serper MR, Davidson M, Harvey PD. Attentional predictors of clinical change during neuroleptic treatment in schizophrenia. Schizophr Res. 1994;13:65–71. [DOI] [PubMed] [Google Scholar]
- 45. Walker E, Harvey P. Positive and negative symptoms in schizophrenia: attentional performance correlates. Psychopathology. 1986;19:294–302. [DOI] [PubMed] [Google Scholar]
- 46. Harvey P, Winters K, Weintraub S, Neale JM. Distractibility in children vulnerable to psychopathology. J Abnorm Psychol. 1981;90:298–304. [DOI] [PubMed] [Google Scholar]
- 47. Green MF, Hugdahl K, Mitchell S. Dichotic listening during auditory hallucinations in patients with schizophrenia. Am J Psychiatry. 1994;151:357–362. [DOI] [PubMed] [Google Scholar]
- 48. Hugdahl K, Løberg EM, Falkenberg LE, et al. . Auditory verbal hallucinations in schizophrenia as aberrant lateralized speech perception: evidence from dichotic listening. Schizophr Res. 2012;140:59–64. [DOI] [PubMed] [Google Scholar]
- 49. Carter CS, Robertson LC, Chaderjian MR, Celaya LJ, Nordahl TE. Attentional asymmetry in schizophrenia: controlled and automatic processes. Biol Psychiatry. 1992;31:909–918. [DOI] [PubMed] [Google Scholar]
- 50. Maruff P, Hay D, Malone V, Currie J. Asymmetries in the covert orienting of visual spatial attention in schizophrenia. Neuropsychologia. 1995;33:1205–1223. [DOI] [PubMed] [Google Scholar]
- 51. Posner MI, Early TS, Reiman E, Pardo PJ, Dhawan M. Asymmetries in hemispheric control of attention in schizophrenia. Arch Gen Psychiatry. 1988;45:814–821. [DOI] [PubMed] [Google Scholar]
- 52. Elshaikh AA, Sponheim SR, Chafee MV, MacDonald AW. Spatial attentional control is not impaired in schizophrenia: dissociating specific deficits from generalized impairments. J Abnorm Psychol. 2015;124:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gold JM, Hahn B, Strauss GP, Waltz JA. Turning it upside down: areas of preserved cognitive function in schizophrenia. Neuropsychol Rev. 2009;19:294–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hahn B, Robinson BM, Harvey AN, et al. . Visuospatial attention in schizophrenia: deficits in broad monitoring. J Abnorm Psychol. 2012;121:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Spencer KM, Nestor PG, Valdman O, Niznikiewicz MA, Shenton ME, McCarley RW. Enhanced facilitation of spatial attention in schizophrenia. Neuropsychology. 2011;25:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in nonsearch task. Percept Psychophys. 1974;16:143–149. [Google Scholar]
- 57. Gooding DC, Braun JG, Studer JA. Attentional network task performance in patients with schizophrenia-spectrum disorders: evidence of a specific deficit. Schizophr Res. 2006;88:169–178. [DOI] [PubMed] [Google Scholar]
- 58. Wang K, Fan J, Dong Y, Wang CQ, Lee TM, Posner MI. Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophr Res. 2005;78:235–241. [DOI] [PubMed] [Google Scholar]
- 59. Ettinger U, Aichert DS, Wöstmann N, Dehning S, Riedel M, Kumari V. Response inhibition and interference control: effects of schizophrenia, genetic risk, and schizotypy. J Neuropsychol. 2018;12:484–510. [DOI] [PubMed] [Google Scholar]
- 60. Kopp B, Mattler U, Rist F. Selective attention and response competition in schizophrenic patients. Psychiatry Res. 1994;53:129–139. [DOI] [PubMed] [Google Scholar]
- 61. Yücel M, Volker C, Collie A, et al. . Impairments of response conflict monitoring and resolution in schizophrenia. Psychol Med. 2002;32:1251–1260. [DOI] [PubMed] [Google Scholar]
- 62. Westerhausen R, Kompus K, Hugdahl K. Unaffected control of distractor interference in schizophrenia: a meta-analysis of incompatibility slowing in flanker tasks. J Psychiatr Res. 2013;47:246–251. [DOI] [PubMed] [Google Scholar]
- 63. Asarnow RF, Granholm E. The contribution of cognitive psychology to vulnerability models. Search Causes Schizophr. 1990;2:205–220. [Google Scholar]
- 64. Asarnow RF, Granholm E, Sherman T. Span of apprehension in schizophrenia. In: Steinhauer SR, Gruzelier JH, Zubin J, eds. Handbook of Schizophrenia, Vol. 5. Neuropsychology, Psychophysiology, and Information Processing. New York: Elsevier Science; 1991. [Google Scholar]
- 65. Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. J Abnorm Psychol. 2006;115:266–275. [DOI] [PubMed] [Google Scholar]
- 66. Granholm E, Asarnow RF, Marder SR. Display visual angle and attentional scanpaths on the span of apprehension task in schizophrenia. J Abnorm Psychol. 1996;105:17–24. [DOI] [PubMed] [Google Scholar]
- 67. Gray BE, Hahn B, Robinson B, et al. . Relationships between divided attention and working memory impairment in people with schizophrenia. Schizophr Bull. 2014;40:1462–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Luck SJ, Fuller RL, Braun EL, Robinson B, Summerfelt A, Gold JM. The speed of visual attention in schizophrenia: electrophysiological and behavioral evidence. Schizophr Res. 2006;85:174–195. [DOI] [PubMed] [Google Scholar]
- 69. Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. [DOI] [PubMed] [Google Scholar]
- 70. Gold JM, Hahn B, Zhang WW, et al. . Reduced capacity but spared precision and maintenance of working memory representations in schizophrenia. Arch Gen Psychiatry. 2010;67:570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Gold JM, Wilk CM, McMahon RP, Buchanan RW, Luck SJ. Working memory for visual features and conjunctions in schizophrenia. J Abnorm Psychol. 2003;112:61–71. [PubMed] [Google Scholar]
- 72. Johnson MK, McMahon RP, Robinson BM, et al. . The relationship between working memory capacity and broad measures of cognitive ability in healthy adults and people with schizophrenia. Neuropsychology. 2013;27:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. [DOI] [PubMed] [Google Scholar]
- 74. Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. [DOI] [PubMed] [Google Scholar]
- 75. Gold JM, Fuller RL, Robinson BM, McMahon RP, Braun EL, Luck SJ. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115:658–673. [DOI] [PubMed] [Google Scholar]
- 76. Smith EE, Eich TS, Cebenoyan D, Malapani C. Intact and impaired cognitive-control processes in schizophrenia. Schizophr Res. 2011;126:132–137. [DOI] [PubMed] [Google Scholar]
- 77. Hahn B, Hollingworth A, Robinson BM, et al. . Control of working memory content in schizophrenia. Schizophr Res. 2012;134:70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Maruff P, Pantelis C, Danckert J, Smith D, Currie J. Deficits in the endogenous redirection of covert visual attention in chronic schizophrenia. Neuropsychologia. 1996;34:1079–1084. [DOI] [PubMed] [Google Scholar]
- 79. Bansal S, Robinson BM, Leonard CJ, Hahn B, Luck SJ, Gold JM. Failures in top-down control in schizophrenia revealed by patterns of saccadic eye movements. J Abnorm Psychol. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Erickson MA, Hahn B, Leonard CJ, et al. . Impaired working memory capacity is not caused by failures of selective attention in schizophrenia. Schizophr Bull. 2015;41:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hahn B, Robinson BM, Kaiser ST, et al. . Failure of schizophrenia patients to overcome salient distractors during working memory encoding. Biol Psychiatry. 2010;68:603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leonard CJ, Robinson BM, Hahn B, Gold JM, Luck SJ. Enhanced distraction by magnocellular salience signals in schizophrenia. Neuropsychologia. 2014;56:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Baier B, Karnath HO, Dieterich M, Birklein F, Heinze C, Müller NG. Keeping memory clear and stable—the contribution of human basal ganglia and prefrontal cortex to working memory. J Neurosci. 2010;30:9788–9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Butler PD, Martinez A, Foxe JJ, et al. . Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130(Pt 2):417–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Butler PD, Zemon V, Schechter I, et al. . Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schechter I, Butler PD, Zemon VM, et al. . Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Butler PD, DeSanti LA, Maddox J, et al. . Visual backward-masking deficits in schizophrenia: relationship to visual pathway function and symptomatology. Schizophr Res. 2003;59:199–209. [DOI] [PubMed] [Google Scholar]
- 89. Cadenhead KS, Serper Y, Braff DL. Transient versus sustained visual channels in the visual backward masking deficits of schizophrenia patients. Biol Psychiatry. 1998;43:132–138. [DOI] [PubMed] [Google Scholar]
- 90. Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch Gen Psychiatry. 1994;51:945–951. [DOI] [PubMed] [Google Scholar]
- 91. Theeuwes J. Perceptual selectivity for color and form. Percept Psychophys. 1992;51:599–606. [DOI] [PubMed] [Google Scholar]
- 92. Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. [DOI] [PubMed] [Google Scholar]
- 93. Leonard CJ, Luck SJ. The role of magnocellular signals in oculomotor attentional capture. J Vis. 2011;11:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Polich J. Neuropsychology of P300. In: Luck SJ, Kappenman ES, eds. Oxford Handbook of Event-Related Potential Components. New York: Oxford University Press; 2012:159–188. [Google Scholar]
- 95. Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. [DOI] [PubMed] [Google Scholar]
- 96. Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]